Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation (original) (raw)
Significance
This study demonstrates that antiangiogenic therapy increases tumor blood perfusion in a subset of newly diagnosed glioblastoma patients, and that it is these patients who survive longer when this expensive and potentially toxic therapy is combined with standard radiation and chemotherapy. This study provides fresh insights into the selection of glioblastoma patients most likely to benefit from antiangiogenic treatments.
Keywords: brain tumor, personalized treatment
Abstract
Antiangiogenic therapy has shown clear activity and improved survival benefit for certain tumor types. However, an incomplete understanding of the mechanisms of action of antiangiogenic agents has hindered optimization and broader application of this new therapeutic modality. In particular, the impact of antiangiogenic therapy on tumor blood flow and oxygenation status (i.e., the role of vessel pruning versus normalization) remains controversial. This controversy has become critical as multiple phase III trials of anti-VEGF agents combined with cytotoxics failed to show overall survival benefit in newly diagnosed glioblastoma (nGBM) patients and several other cancers. Here, we shed light on mechanisms of nGBM response to cediranib, a pan-VEGF receptor tyrosine kinase inhibitor, using MRI techniques and blood biomarkers in prospective phase II clinical trials of cediranib with chemoradiation vs. chemoradiation alone in nGBM patients. We demonstrate that improved perfusion occurs only in a subset of patients in cediranib-containing regimens, and is associated with improved overall survival in these nGBM patients. Moreover, an increase in perfusion is associated with improved tumor oxygenation status as well as with pharmacodynamic biomarkers, such as changes in plasma placenta growth factor and sVEGFR2. Finally, treatment resistance was associated with elevated plasma IL-8 and sVEGFR1 posttherapy. In conclusion, tumor perfusion changes after antiangiogenic therapy may distinguish responders vs. nonresponders early in the course of this expensive and potentially toxic form of therapy, and these results may provide new insight into the selection of glioblastoma patients most likely to benefit from anti-VEGF treatments.
Despite advances in surgical, radiation and medical therapies, survival of patients with glioblastoma (GBM) is typically <2 y and <10% of patients survive >5 y (1). A pathophysiological hallmark of GBM is the elevated expression of VEGF and other proangiogenic cytokines that stimulate endothelial cell proliferation, migration, and survival (2). This process leads to the formation of a highly abnormal tumor vasculature characterized by hyperpermeable vessels, increased vessel diameter, and abnormally thickened basement membranes. The abnormal vascular network not only promotes tumor progression but may also limit the efficacy of radiation and chemotherapy—the current standard of care (3)—by enhancing tumor hypoxia and compromising tumor blood flow and concomitant delivery of chemotherapeutics (4–6).
Antiangiogenic agents target the abnormal tumor vasculature but their mechanism of action is incompletely understood. The classic hypothesis is that antiangiogenic therapy—through vessel pruning and reduced blood perfusion—starves the tumor of oxygen and essential nutrients, halting the tumor’s uncontrolled growth (7). However, a logical consequence of diminished tumor blood perfusion following antiangiogenic therapy might be reduced delivery of concurrent chemotherapy. In fact, bevacizumab rapidly reduced blood perfusion in a small study of 10 lung cancer patients, resulting in a decreased influx rate of concurrent docetaxel (8). However, the relationship between antiangiogenic therapies and chemotherapy delivery is complex and may vary depending on underlying patient characteristics, different tumor types, or class and dose of antiangiogenic treatments. Given the recent failure of bevacizumab to extend overall survival when combined with chemoradiation in phase III trials in newly diagnosed GBM (nGBM), further investigation is essential to improve understanding of why antiangiogenic agents may be effective in certain patients but not in others (9).
Preclinical and clinical studies in a number of solid tumors, including recurrent GBM (rGBM), have demonstrated that after the administration of antiangiogenic therapies a subset of rGBM patients experience a transient period of vascular normalization characterized by increased perfusion, reduced vessel diameter and permeability, specific circulating biomarker changes, reduction in tumor interstitial pressure, and improved tumor oxygenation (10–16). These parameters can be used to generate a “vascular normalization index” to help identify rGBM patients most likely to benefit from antiangiogenic therapy (17, 18). However, whether tumor perfusion and oxygenation increase during combined antiangiogenic and cytotoxic treatments and whether this increased perfusion improves overall survival are not known. Increased tumor perfusion may enhance delivery of concurrently administered anticancer therapeutics, reduce hypoxia, and improve patient outcomes. Here, we demonstrate that cediranib, an oral pan-VEGF receptor tyrosine kinase (RTK) inhibitor, in combination with chemotherapy and radiation, increases perfusion and oxygenation in 50% of patients with nGBM, and that these patients survive 9-mo longer than those whose perfusion does not increase. In contrast, tumor perfusion increased in only 1 of 14 nGBM patients from a contemporary control group treated with chemotherapy and radiation without cediranib.
Results
Cediranib with Chemoradiation Normalizes nGBM Vasculature.
Forty-six patients with histologically confirmed nGBM were enrolled in a prospective phase Ib (n = 6)/II (n = 40) clinical trial of cediranib in combination with radiation and temozolomide (NCT00662506). All patients had at least 1 cm of measurable, residual contrast-enhancing tumor after surgery, as gross total tumor resection was an exclusion criterion. Patients received 6 wk of fractionated radiation along with daily temozolomide, the current standard of care, and cediranib was administered daily (Table 1 and Fig. S1). The median progression-free survival (PFS) was 15.6 mo [95% confidence interval (CI): 9.3, 26.0 mo] and median overall survival (OS) was 20.2 mo (95% CI: 16.7, 29.4 mo) for all 46 study subjects. With the exception of one patient who experienced biopsy-proven early disease progression, all patients responded to therapy with durable reductions in volume of contrast enhancement, volume of vasogenic edema [estimated by abnormal fluid-attenuated inversion recovery (FLAIR) hyperintensity and diffusion imaging], tumor vascular permeability (estimated by _K_trans), and tumor vessel size (Fig. 1 and Fig. S2). These markers of vascular normalization persisted throughout the 6 wk of combined antiangiogenic and chemoradiation therapy. Moreover, 26 of 30 patients who were taking corticosteroids at the start of chemoradiation—to control vasogenic edema—were able to reduce or discontinue these antiedema drugs.
Table 1.
Patient characteristics
| Variable | Cediranib + chemoradiation (n = 46) | Chemoradiation alone (n = 14) | Perfusion increased* (n = 20) | Perfusion stable/reduced* (n = 20) |
|---|---|---|---|---|
| Age | 57 (22–74) | 60 (35–70) | 59 (22–74) | 57 (41–67) |
| KPS | 90 (60–100) | 90 (60–100) | 100 (90–100) | 90 (60–100) |
| EOR | 36 subtotal resection | 10 subtotal resection | 16 subtotal resection | 16 subtotal resection |
| 10 biopsy | 4 biopsy | 4 biopsy | 4 biopsy | |
| Sex | 14 women | 8 women | 8 women | 5 women |
| 32 men | 6 men | 12 men | 15 men | |
| MGMT | 19 methylated | 2 methylated | 8 methylated | 7 methylated |
| 18 unmethylated | 8 unmethylated | 9 unmethylated | 10 unmethylated | |
| 9 unable to determine† | 4 unable to determine† | 3 unable to determine† | 3 unable to determine† |
Fig. 1.
MRI changes during treatment with cediranib and chemoradiation in nGBM patients. Combination treatment induces a decrease in contrast enhancing (CE) tumor volume, FLAIR volume, vessel caliber, and permeability measured by MRI. Error bars represent SEM.
Patients with Increased Perfusion Have Improved Oxygenation and Survival.
Twenty of 40 patients (50%) who underwent advanced imaging experienced a durable and consistent increase in microvessel tumor perfusion (Fig. 2_A_ and Table S1), 10 patients (25%) experienced stable perfusion, and 10 patients (25%) experienced a decrease in perfusion. These perfusion changes were noted as early as day 1 of chemoradiation and the three groups remained stable and distinct during the 6 wk of combination treatment (Fig. 2_B_ and Table S1). A similar durable increase in tumor perfusion was observed in only 1 of 14 contemporary patients with nGBM who underwent MRI at similar time points and were treated with radiation and temozolomide but not cediranib (NCT00756106). After stratifying patients based on O6-methyl guanine methyl transferase (MGMT) gene-promoter methylation status and performance status, established prognostic factors in nGBM, the median OS in patients with increased perfusion was 789 d (26.3 mo) compared with 510 d (17.0 mo) in those with stable or decreased perfusion (P < 0.05) (Fig. 2_C_ and Table S1). Patients with MGMT methylation status or other favorable prognostic factors were not overrepresented in the increased perfusion group or underrepresented in the stable of decreased perfusion groups (Table 1). Patients in the standard treatment cohort had a median OS of 431.5 d (14.2 mo) with 95% CI from 340 d (11.2 mo) to 881 d (28.9 mo), which was comparable to the decreased/stable perfusion group (Table S2).
Fig. 2.
Increased tumor perfusion. (A) Representative anatomic MRI showing decrease in contrast-enhanced tumor area. (B) Perfusion maps demonstrating increased perfusion. (C) Histogram analysis of enhancing tumor region (red line) showing increased and subsequent normalization of perfusion compared with reference tissue (black line). (D) Perfusion increased in 20 patients, decreased in 10 patients, and remained stable in 10 patients during combination therapy. (E) Kaplan–Meier OS distributions in these groups, after adjustment for MGMT status and Karnofsky performance status (KPS).
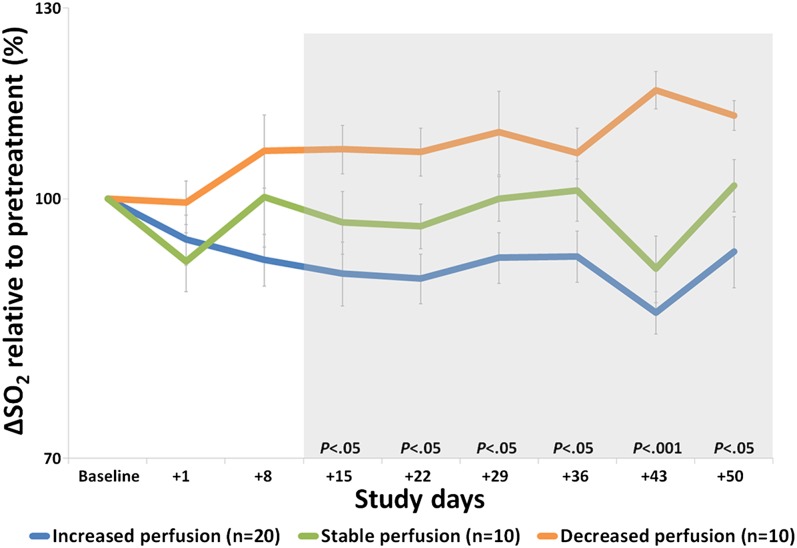
Vessel architectural imaging (VAI), which reflects the relative difference between arteriole and venule oxygen saturation levels (∆SO2) and thus tissue oxygen consumption, demonstrated that the average tumor ∆SO2 levels before treatment onset (day –1) were higher than normal-appearing reference tissue (P < 0.05). A higher value implies impaired delivery of oxygen to the tumor (19). During cediranib therapy and compared with baseline, abnormal ∆SO2 levels collectively decreased from day +15 through day +50 for patients with increased microvessel perfusion, reflecting a return of ∆SO2 levels to that of healthy brain tissue, consistent with improved delivery of oxygen to the tumor (Fig. 3, and Figs. S3 and S4). This finding was supported by normalization of abnormal arteriole ∆SO2 levels (Fig. S5_A_) and abnormal venule ∆SO2 levels (Fig. S5_B_). In contrast, patients with stable and decreased microvessel perfusion had stable or increased tumor ∆SO2 levels, reflecting a lack of improved oxygenation. There was no significant association between ∆SO2 and survival.
Fig. 3.
Relative arteriole-to-venule oxygen saturation (∆SO2) levels. In patients with increased tumor perfusion, ∆SO2 decreases suggesting improved oxygenation, whereas ∆SO2 increased in patients with decreased perfusion suggesting impaired delivery of oxygen to the tumor.
Blood Biomarker Changes After Cediranib with Chemoradiation and Their Impact on Outcomes.
A number of potential pharmacodynamic, response, and resistance biomarkers have emerged from studies of antiangiogenic therapies in rGBM, including angiogenic and inflammatory factors (10, 20, 21). We examined here the changes in angiogenic and inflammatory biomarkers after cediranib with chemoradiation in nGBM. We observed that combination therapy decreased plasma basic FGF, sVEGFR1, sVEGFR2, and Ang-2, and increased plasma VEGF, placental growth factor (PlGF), stromal cell-derived factor 1α (SDF1α), IL-6, IL-8, TNF-α, and carbonic anhydrase IX (CAIX) (Table S3). The changes in plasma PlGF and sVEGFR2 were significantly different in patients treated with cediranib and chemoradiation compared with patients treated with chemoradiation alone (Tables S4 and S5).
No baseline or early change in biomarkers showed consistent association with outcomes. However, high levels of plasma sVEGFR1 at day 29 correlated with poor PFS [hazard ratio (HR) = 1.84 (1.02, 3.32), n = 40] and OS [HR = 1.90 (1.01, 3.58), n = 40] (both P < 0.05). Similarly, high levels of IL-8 at day 43 (end of combination therapy) correlated with poor PFS [HR = 2.74 (1.57, 4.80), n = 38; P < 0.001] and showed a nonsignificant trend for association with poor OS [HR = 1.48 (0.93, 2.35), n = 38; P = 0.09]. No other proangiogenic or inflammatory protein showed correlations with outcome. Of note, an increase in blood perfusion was significantly associated with greater increases in plasma PlGF and with greater decreases in sVEGFR2 during treatment, but not with their baseline values (Fig. 4 and Table S6).
Fig. 4.
Kinetics of VEGF family proteins in nGBM patients treated with cediranib and chemoradiation. (A) Circulating PlGF. (B) Circulating sVEGFR2. Data are shown as median levels (pg/mL) in patients with increased, stable, or decreased perfusion during combination therapy.
Impact of RTK Amplification Profile on Survival.
To investigate whether amplification of EGFR, PDGFRA, and MET genes predicts response or resistance to cediranib, we evaluated initial diagnostic samples of these patients for EGFR, PDGFRA, and MET amplification. EGFR was tested in 45 cases, and PDGFRA and MET could be tested in 31 samples (Table S7). The presence of EGFR, MET or PDFGRA amplification showed no association with response to combination therapy or survival. Interestingly, we observed that amplification of EGFR was associated with stable or decreased perfusion (P < 0.05) (Table S8).
Discussion
Cediranib, a small-molecule tyrosine kinase inhibitor of all three VEGF receptors as well as platelet derived growth factor receptor (PDGFR) and c-kit, showed promising activity in nGBM. These results in our overall study population of nGBM patients undergoing only biopsy or subtotal resection compared favorably to historic controls treated with radiation and temozolomide alone, as well as preliminary data from a phase III trial of patients with nGBM treated with bevacizumab and chemoradiation (3, 9). However, as observed in other trials of antiangiogenic therapies in GBM, the beneficial clinical impact is primarily on PFS and disease progression is typically rapid after conventional radiographic progression, accounting for the more modest improvement in OS (9, 22, 23).
Our physiological MRI evaluations showed that the addition of cediranib to chemoradiation improved vascular integrity and perfusion in 20 out of 40 (50%) patients, compared with only 1 out of 14 (7%) patients treated with conventional chemoradiation alone. Temozolomide may have mild antiangiogenic properties when given at a low daily dose (i.e., a “metronomic” schedule), which might explain the elevated perfusion in this one patient (24). However, the higher prevalence of increased perfusion in patients treated with cediranib suggests that antiangiogenic therapy with cediranib contributed to the elevated blood perfusion. The impact of increased perfusion was evident with stratification based on MGMT methylation status, so even patients with unmethylated MGMT seemed to benefit from increased tumor perfusion. Although a randomized control group would be ideal for comparative purposes, this is not feasible in a single institution study using advanced imaging and comprehensive blood biomarker analyses. Moreover, the successful replication of advanced MRI techniques and protocols, similar to those used in this study, across multiple institutions and imaging platforms has yet to be achieved in the randomized trial setting in the glioblastoma patient population. Therefore, although imperfect, the inclusion of a contemporary control group with similar study eligibility criteria from within our own institution offered the best opportunity for comparisons.
To further explore the link between perfusion and oxygenation, we recently developed a technique (VAI) to measure ∆SO2 (the fractional saturation of hemoglobin with oxygen) in tumors (19). The ∆SO2 parameter, as measured by MRI, is sensitive to changes in deoxygenated blood and reflects the relative difference between arteriole and venule oxygen saturation levels, and thus tissue oxygen consumption. Using VAI, we demonstrated that the same rGBM patients who respond to cediranib with increased perfusion also have improved delivery of oxygen to the tumor. The current study in nGBM patients mirrors this finding.
Specifically, we observed that nGBM patients with improved tumor blood perfusion after cediranib treatment had improved OS, controlling for the established prognostic markers in this type of brain cancer. Because this marker of vascular normalization was observed in a subset of nGBM patients in the trial, despite almost all patients experiencing a decrease in contrast enhancement and brain edema, the improvement in survival could not solely be attributed to the antipermeability and antiedema effect of cediranib. We hypothesize that the critical factor is improved vascular function, which (i) enhanced tumor oxygenation and potentially sensitized tumor cells to the cytotoxic actions of chemoradiation, and (ii) potentially improved tumor delivery of temozolomide (25, 26). A study in a murine U87 GBM model demonstrated that intratumoral concentrations of temozolomide were higher when administered with cediranib (27). Furthermore, cediranib has also been reported to have its own tumoricidal effects (18, 28). Thus, improved tumor delivery of this agent through normalization also might have benefited patients through a direct cytotoxic effect in both rGBM and nGBM patients. Finally, vascular normalization after antiangiogenic therapy can reprogram the immunosuppressive microenvironment that is characteristic of tumors and inhibits the body’s native defenses against cancer development. As a result, there may be enhanced native antitumor immune responses (29, 30).
To date, no validated blood biomarkers of tumor vascular normalization have been identified in cancer patients treated with antiangiogenic therapies. Circulating VEGF level has been a natural candidate biomarker for response. However, we observed no association of circulating VEGF with outcomes after cediranib with chemoradiation in this trial, consistent with a lack of correlation with outcomes seen in many studies of anti-VEGF agents (21). Furthermore, consistent with the results from a study of cediranib in rGBM (10), we detected an association between sVEGFR1 levels after 4 wk of treatment and PFS after cediranib/chemoradiation in nGBM. We previously proposed that sVEGFR1—a negative regulator of the VEGF pathway—is a potential resistance biomarker to anti-VEGF therapy (31). However, because cediranib significantly decreases sVEGFR1 levels in circulation, future studies should determine if the associations observed are because of the biological effects of sVEGFR1 or because of “pharmacodynamic” changes in its level as a result of treatment. Moreover, cediranib with chemoradiation—but not chemoradiation alone—consistently increased PlGF and decreased plasma sVEGFR2. We and others have demonstrated that anti-VEGFR therapy is invariably associated with increases in PlGF and decreases in plasma sVEGFR2 across different anti-VEGF tyrosine kinase inhibitors (e.g. cediranib, sorafenib, vandetanib, sunitinib) and different cancers (10, 31–36). Thus, data from our study support the notion that PlGF and sVEGFR2 should be further explored as pharmacodynamic biomarkers (i.e., surrogate biomarkers of biological activity). The biological activity of anti-VEGF agents has been assumed to be an antivascular effect reflected by a decrease in tumor vascular density and decreased blood perfusion. However, our data show that a greater increase in PlGF or decrease in sVEGFR2 is actually associated with an improvement in blood perfusion in nGBM. Future studies should validate this concept and establish whether PlGF—the increase of which is largely a result of systemic effects of anti-VEGF therapy—has any direct role in mediating vascular function in nGBM (37).
Inflammatory factors have been linked with resistance to anti-VEGF therapy in multiple studies in glioblastoma (10, 38). Here, we detected increases in multiple cytokines in circulation during and after cediranib with chemoradiation, which were concomitant with plasma elevations in the hypoxia marker CAIX. However, only plasma levels of IL-8 after treatment showed an association with poor survival outcomes and warrants further exploration as an escape biomarker in larger studies.
Finally, we examined if genetic heterogeneity in nGBMs may account for differences in response to cediranib with chemoradiation. EGFR, PDGFRA, and MET are the most frequently amplified genes in GBM, and PDGFRA is often coamplified with KIT and VEGFR2 because all three are located on chromosome 4q12 (39–41). PDGFRA, KIT, and VEGFR2 RTK activity is directly inhibited by cediranib, whereas that of EGFR and MET is not. We observed no correlation between the presence of EGFR, MET, or PDFGRA amplification and survival after treatment. This result may be because of the low number of PDGFRA or MET amplified GBM cases or to concomitant EGFR or MET amplification in two tumors, which also harbored PDGFRA amplification. The importance of these concurrent subclones is currently unknown but highlights the complexity of treating these tumors with single targeted drugs. Of note, we observed a correlation between EGFR amplification and a lack of increase in perfusion after treatment. Future studies should determine whether EGFR amplification is causally related to changes in vascular function after anti-VEGF therapy or is merely a poor prognostic variable (42).
In conclusion, we provide direct evidence that improved tumor perfusion—a consequence of vascular normalization—is associated with improved oxygenation and longer OS in nGBM patients treated with combination antiangiogenic and cytotoxic therapy. These results are consistent with our finding that rGBM patients treated with cediranib monotherapy had improved OS in a subset of subjects with increased perfusion (18). Taken together, these observations suggest that anti-VEGF agents improve OS only in the subset of GBM patients who experience increased tumor perfusion and oxygenation and not in all patients. This finding may account for the failure of cediranib and bevacizumab to extend OS in randomized phase III trials conducted in unselected nGBM and rGBM patient populations (9, 43, 44). We also identified tissue markers, as well as circulating blood proteins that may serve as biomarkers of changes in tumor perfusion and oxygenation during anti-VEGF therapy. This finding indicates that vascular normalization—and not vessel pruning—is a potential mechanism of benefit for GBM patients.
Materials and Methods
Study Design.
Data were obtained from two prospective, concurrent clinical studies (NCT00662506 and NCT00756106) performed at the Massachusetts General Hospital Cancer Center and the Dana Farber Cancer Institute (SI Materials and Methods).
Analyses of RTK Gene Amplification in nGBM Tissue Specimens.
Evaluation of the three most commonly amplified RTK genes in glioblastoma was performed in all cases with sufficient tissue using FISH, as previously described (39) (SI Materials and Methods).
Circulating Biomarkers.
Peripheral blood was obtained from all patients with advanced imaging studies (n = 40 from NCT00662506 and n = 14 from NCT00756106) and evaluated as previously described (11) (SI Materials and Methods).
MRI.
MRI studies were performed before and at multiple time points after treatment. MRI scans were obtained weekly during the 6 wk of concurrent chemoradiation and cediranib, then monthly. MRI scans included scout, pre- and postcontrast T1-weighted images, FLAIR, dynamic susceptibility contrast imaging, dynamic contrast-enhanced imaging, diffusion tensor imaging, and VAI analysis (SI Materials and Methods).
Statistical Analysis.
Groups were compared using exact Mann–Whitney and Fisher tests (for comparisons of circulating biomarkers and genotypes), as well as stratified log-rank test and Wald test in Cox regression analysis with log-transformed covariates (for survival data). Biomarker changes were expressed as ratios, reported as median with interquartile intervals, and tested using exact paired Wilcoxon test. P values of less than 0.05 were considered statistically significant.
Supplementary Material
Supporting Information
Acknowledgments
We thank A. Khachatryan, C. Koppel, O. Pulluqi, and C. Smith for outstanding technical support for biomarker studies. This work was funded by National Institutes of Health Grants R01CA129371 and K24CA125440A (to T.T.B.), P01CA080124 and R01CA163815 (to R.K.J.), N01CM-2008-00060C and 5R01NS060918 (to A.G.S.), 1U01CA154601 (to B.R.R.), and R01CA159258 (to D.G.D.); the Proton Beam/Federal Share Program (R.K.J and D.G.D.); the National Foundation for Cancer Research (R.K.J.); Merck (A.G.S. and E.R.G.); Norwegian Research Council Grant 191088/V50 and South-Eastern Norway Regional Health Authority Grant 2013069 (to K.E.E.); and the Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 8UL1TR000170-05 and financial contributions from Harvard University and its affiliated academic health care centers).
Footnotes
Conflict of interest statement: T.T.B. received consulting honoraria from Merck & Co., Roche, Kirin Pharmaceuticals, Spectrum, Amgen, and Novartis; has provided CME lectures and material for Up to Date, Inc., Robert Michael Educational Institute LLC, Educational Concepts Group, Research to Practice, Oakstone Medical Publishing, American Society of Hematology, and Imedex; has consulted for Champions Biotechnology, Advance Medical; and received research support from Pfizer, Astra Zeneca, and Millennium. E.R.G. received research support from Merck. D.G.D. served as a consultant for Hexal/Sandoz. R.K.J. received research grants from MedImmune and Roche; received consultant fees from Enlight, Noxxon, and Zyngenia; owns equity in Enlight, SynDevRx, and XTuit; and serves on the Board of Directors of XTuit and Boards of Trustees of H&Q Healthcare Investors and H&Q Life Sciences Investors.
References
- CBTRUS (2012) CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004-2008 (March 23, 2010 Revision) (Central Brain Tumor Registry of the United States, Hinsdale, IL). Available at www.cbtrus.org. Accessed October 1, 2013.
- 2.Sitohy B, Nagy JA, Dvorak HF. Anti-VEGF/VEGFR therapy for cancer: Reassessing the target. Cancer Res. 2012;72(8):1909–1914. doi: 10.1158/0008-5472.CAN-11-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain RK. Normalizing tumor microenvironment to treat cancer: Bench to bedside to biomarkers. J Clin Oncol. 2013;31(17):2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folkman J. Anti-angiogenesis: New concept for therapy of solid tumors. Ann Surg. 1972;175(3):409–416. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van der Veldt AA, et al. Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: Implications for scheduling of anti-angiogenic drugs. Cancer Cell. 2012;21(1):82–91. doi: 10.1016/j.ccr.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert MR, et al. RTOG 0825: Phase III double-blind placebo-controlled trial evaluating bevacizumab (Bev) in patients (Pts) with newly diagnosed glioblastoma (GBM) J Clin Oncol. 2013;31(suppl; abstr 1) [Google Scholar]
- 10.Batchelor TT, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28(17):2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batchelor TT, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chae SS, et al. Angiopoietin-2 interferes with anti-VEGFR2-induced vessel normalization and survival benefit in mice bearing gliomas. Clin Cancer Res. 2010;16(14):3618–3627. doi: 10.1158/1078-0432.CCR-09-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamoun WS, et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol. 2009;27(15):2542–2552. doi: 10.1200/JCO.2008.19.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willett CG, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10(2):145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willett CG, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: A multidisciplinary phase II study. J Clin Oncol. 2009;27(18):3020–3026. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkler F, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Sorensen AG, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69(13):5296–5300. doi: 10.1158/0008-5472.CAN-09-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorensen AG, et al. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72(2):402–407. doi: 10.1158/0008-5472.CAN-11-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emblem KE, et al. Vessel architectural imaging identifies cancer patient responders to anti-angiogenic therapy. Nat Med. 2013;19(9):1178–1183. doi: 10.1038/nm.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucio-Eterovic AK, Piao Y, de Groot JF. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res. 2009;15(14):4589–4599. doi: 10.1158/1078-0432.CCR-09-0575. [DOI] [PubMed] [Google Scholar]
- 21.Jain RK, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6(6):327–338. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quant EC, et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol. 2009;11(5):550–555. doi: 10.1215/15228517-2009-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai A, et al. Phase II pilot study of bevacizumab in combination with temozolomide and regional radiation therapy for up-front treatment of patients with newly diagnosed glioblastoma multiforme: Interim analysis of safety and tolerability. Int J Radiat Oncol Biol Phys. 2008;71(5):1372–1380. doi: 10.1016/j.ijrobp.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 24.Kurzen H, Schmitt S, Näher H, Möhler T. Inhibition of angiogenesis by non-toxic doses of temozolomide. Anticancer Drugs. 2003;14(7):515–522. doi: 10.1097/00001813-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Lee CG, et al. Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 2000;60(19):5565–5570. [PubMed] [Google Scholar]
- 26.Chakravarti A, et al. Temozolomide-mediated radiation enhancement in glioblastoma: A report on underlying mechanisms. Clin Cancer Res. 2006;12(15):4738–4746. doi: 10.1158/1078-0432.CCR-06-0596. [DOI] [PubMed] [Google Scholar]
- 27.Grossman R, et al. Microdialysis measurement of intratumoral temozolomide concentration after cediranib, a pan-VEGF receptor tyrosine kinase inhibitor, in a U87 glioma model. Cancer Chemother Pharmacol. 2013;72(1):93–100. doi: 10.1007/s00280-013-2172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H, et al. Serial magnetic resonance spectroscopy reveals a direct metabolic effect of cediranib in glioblastoma. Cancer Res. 2011;71(11):3745–3752. doi: 10.1158/0008-5472.CAN-10-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci USA. 2012;109(43):17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73(10):2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duda DG, et al. Plasma soluble VEGFR-1 is a potential dual biomarker of response and toxicity for bevacizumab with chemoradiation in locally advanced rectal cancer. Oncologist. 2010;15(6):577–583. doi: 10.1634/theoncologist.2010-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyerhardt JA, et al. Phase I study of cetuximab, irinotecan, and vandetanib (ZD6474) as therapy for patients with previously treated metastastic colorectal cancer. PLoS ONE. 2012;7(6):e38231. doi: 10.1371/journal.pone.0038231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tolaney SM, et al. A phase II study of preoperative (preop) bevacizumab (bev) followed by dose-dense (dd) doxorubicin (A)/cyclophosphamide (C)/paclitaxel (T) in combination with bev in HER2-negative operable breast cancer (BC) J Clin Oncol. 2012;30(suppl; abstr 1026) [Google Scholar]
- 34.Zhu AX, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: A phase II study. J Clin Oncol. 2009;27(18):3027–3035. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raut CP, et al. Effects of sorafenib on intra-tumoral interstitial fluid pressure and circulating biomarkers in patients with refractory sarcomas (NCI protocol 6948) PLoS ONE. 2012;7(2):e26331. doi: 10.1371/journal.pone.0026331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu AX, et al. Efficacy, safety, pharmacokinetics, and biomarkers of cediranib monotherapy in advanced hepatocellular carcinoma: A phase II study. Clin Cancer Res. 2013;19(6):1557–1566. doi: 10.1158/1078-0432.CCR-12-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu L, et al. Direct evidence that bevacizumab, an anti-VEGF antibody, up-regulates SDF1alpha, CXCR4, CXCL6, and neuropilin 1 in tumors from patients with rectal cancer. Cancer Res. 2009;69(20):7905–7910. doi: 10.1158/0008-5472.CAN-09-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu-Emerson C, et al. Increase in tumor-associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastoma. Neuro-oncol. 2013;15(8):1079–1087. doi: 10.1093/neuonc/not082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snuderl M, et al. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell. 2011;20(6):810–817. doi: 10.1016/j.ccr.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Puputti M, et al. Amplification of KIT, PDGFRA, VEGFR2, and EGFR in gliomas. Mol Cancer Res. 2006;4(12):927–934. doi: 10.1158/1541-7786.MCR-06-0085. [DOI] [PubMed] [Google Scholar]
- 41.Joensuu H, Puputti M, Sihto H, Tynninen O, Nupponen NN. Amplification of genes encoding KIT, PDGFRalpha and VEGFR2 receptor tyrosine kinases is frequent in glioblastoma multiforme. J Pathol. 2005;207(2):224–231. doi: 10.1002/path.1823. [DOI] [PubMed] [Google Scholar]
- 42.Shinojima N, et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63(20):6962–6970. [PubMed] [Google Scholar]
- 43.Henriksson R, et al. Progression-free survival (PFS) and health-related quality of life (HRQoL) in AVAglio, a phase III study of bevacizumab (Bv), temozolomide (T), and radiotherapy (RT) in newly diagnosed glioblastoma (GBM) J Clin Oncol. 2013;31(suppl; abstr 2005) [Google Scholar]
- 44.Batchelor TT, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. doi: 10.1200/JCO.2012.47.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information