TGF-β regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes (original) (raw)
Abstract
CD4+CD25+ regulatory T cells are essential in the protection from organ-specific autoimmune diseases. In the pancreas, they inhibit actions of autoreactive T cells and thereby prevent diabetes progression. The signals that control the generation, the maintenance, or the expansion of regulatory T cell pool in vivo remain poorly understood. Here we show that a transient pulse of transforming growth factor β (TGF-β) in the islets during the priming phase of diabetes is sufficient to inhibit disease onset by promoting the expansion of intraislet CD4+CD25+ T cell pool. Approximately 40–50% of intraislet CD4+ T cells expressed the CD25 marker and exhibited characteristics of regulatory T cells including small size, high level of intracellular CTLA-4, expression of Foxp3, and transfer of protection against diabetes. Results from in vivo incorporation of BrdUrd revealed that the generation of a high frequency of regulatory T cells in the islets is due to in situ expansion upon TGF-β expression. Thus, these findings demonstrate a previously uncharacterized mechanism by which TGF-β inhibits autoimmune diseases via regulation of the size of the CD4+CD25+ regulatory T cell pool in vivo.
Type I diabetes is an autoimmune disease that results from the failure of tolerance to beta-cell antigens (1). The mechanisms that have evolved to ensure discrimination between self and nonself are highly complex and not foolproof. Models of passive tolerance, such as thymic deletion of autoreactive T cells or nonreponsiveness in the periphery because of anergy or ignorance, cannot account for the presence of autoreactive T cells in healthy individuals despite the absence of the development of organ-specific autoimmune diseases. T cells endowed with suppressive function to control actions of autoreactive T cells were described decades ago and were thought originally to be a specialized T cell population the effect of which would be mediated by secreted antigen-specific factors (2). Nowadays, suppressor T cells (also referred to as regulatory T cells) are delineated into two cell subsets of natural regulatory (CD4+CD25+) cells that emerge from the thymus (3, 4) and adaptive regulatory (CD4+CD25–) cells induced in the periphery to develop suppressive activity (5–7). However, this concept of dichotomous thymic CD25+ versus adaptive CD25– regulatory T cells has been challenged by several reports, supporting evidence for the peripheral generation of CD25+ regulatory T cells in vivo and in vitro (8–12). The finding that suppressive functions are instructively programmed by the expression of Foxp3 finally provided the basis for integrating a unified model of regulatory T cell diversity (13–15). Forced expression of Foxp3 in CD4+CD25– nonregulatory T cells, either by retroviral expression or in transgenic mice, showed acquisition of suppressive activity in vitro and inhibition of disease in vivo, inducing in a substantial proportion of Foxp3-bearing cells the expression of CD25 and GITR markers indicating that expression of Foxp3 within peripheral T cells may convert nonregulatory CD25– cells into CD25+ regulatory cells. The fact that the expression of the Foxp3 gene during thymic development alone was not sufficient to protect otherwise Foxp3-null animal from disease (16) works in favor of this hypothesis. The point of interest here is that continuous Foxp3 expression in peripheral tissues seems to be necessary either for the maintenance or the function of regulatory T cells extrathymically. The signals that control regulatory T cell pool in the periphery, however, remain to be determined.
We believe that potential candidates lie within the inflammatory milieu of autoimmune diseases. Several studies have demonstrated that CD4+CD25+ regulatory T cells produce elevated levels of transforming growth factor β (TGF-β) in both mice and humans. A key to understanding the cell contact-dependent immunosuppression by these cells was the recognition that CD4+CD25+ T cells express surface membrane-bound TGF-β (17). Not only do CD4+CD25+ regulatory T cells express latent TGF-β (18), but they also bear TGF-β in its active configuration on the cell surface (19). The fact that enhanced TGF-β signaling receptors reside on the membrane of CD4+CD25+ regulatory T cells underscores the potential for autocrine and/or paracrine receptor-ligand interactions in these cells. In this study, we provide direct evidence that TGF-β is a positive regulator of CD4+CD25+ regulatory T cell expansion in vivo. Expression of TGF-β in the pancreatic cells by using a tetracycline on/off system shows that a short pulse of TGF-β is sufficient to inhibit the development of type I diabetes by promoting the expansion of CD4+CD25+ T cells. Detailed analysis with an adoptive transfer system identified the profile of regulatory T cells, including their small size, high level of intracellular CTLA-4, expression of Foxp3, and transfer of protection against diabetes. This finding provides a significant step forward in our understanding of the mechanisms by which suppressive function can be maintained in peripheral tissues.
Materials and Methods
Mice. Plasmid pTet-TTA that encodes the tetracycline-responsive repressor was described previously (20). pTet-TTA was cleaved at the _Sac_I–_Hin_dIII sites and hCMV promoter was replaced with the rat insulin promoter type II, creating pRIP-TTA (RIP, rat insulin promoter), which carries the RIP-TTA element (Fig. 1_A_). Plasmid _p_-TRE-TGF-β (TRE, tetracycline-responsive element) that encodes an active form of TGF-β1 under the control of the TRE was generated by substituting the RIP component of a RIP-TGF-β plasmid (21) with the TRE element described in detail previously for tumor necrosis factor expression (22). Both transgene fragments were purified by using Elutip purification columns (Schleicher & Schuell) and coinjected to make double transgenic mice in the nonobese diabetic (NOD) background. Transgenic founders were screened by using PCR and further selected based on TGF-β expression in pancreas. The primers used for screening transgenic mice and RT-PCR were as follows: TGF-β, 5′-GACCTCCATAGAAGACACC-3′ and 5′-AACCCGTTGATGTCCACTTGC-3′; and TTA, 5′-GTCGCTAAAGAA GAAAGGGAAACAC-3′ and 5′-TTCCAAGGGCATCGGTAAACATCTG-3′. Doxycycline-supplemented food (20 mg/kg) was purchased from Bioserv (Frenchtown, NJ), and TGF-β expression was repressed by replacing normal chow with doxycycline-supplemented chow and initiated by changing back to normal diet. When TGF-β expression was repressed from birth, the breeding mothers were fed with supplemented food since pregnancy. Urine Glucose level was monitored by using Diastyx sticks (Bayer) on weekly basis. Animals that had values of >250 mg/dl on two consecutive occasions were counted as diabetic and confirmed by One-Touch strips (Lifescan, Milpitas, CA). All mice were maintained under specific pathogen-free conditions.
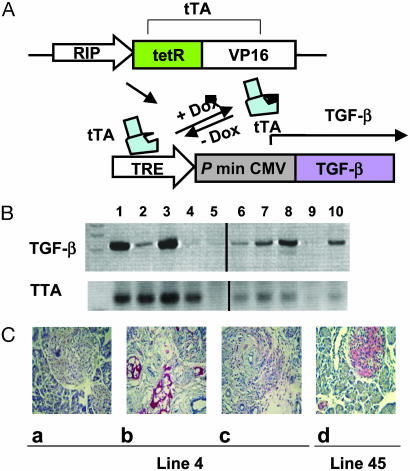
Fig. 1.
Regulated TGF-β expression in TTA/TGF-β NOD mice. (A) TTA regulatory system. Under the control of a rat insulin promoter (RIP), tetracycline-controlled transactivator (TTA) is expressed specifically in insulin-producing cells. In the presence of tetracycline or its analogue doxycycline (Dox), TTA (+Dox) is unable to bind the TRE and TGF-β transcription is inactive, whereas in the absence of doxycycline (–Dox) TTA binds TRE and induces TGF-β expression via the cytomegalovirus minimal promoter (P min CMV). DNA fragments RIP-TTA and TRE-TGF-β were coinjected to generate double transgenic mice in NOD background. (B) Regulated TGF-β transcription. NOD Transgenic mice were fed with normal or doxycycline-supplemented food. Total RNA was isolated from pancreatic tissue. The transcripts of TGF-β (Upper) and TTA (Lower) were amplified by using RT-PCR. Lanes 1 and 3, constitutively on for 5 weeks since birth; lanes 2 and 4, constitutively on for 5 weeks followed by 1-week turn-off; lane 5, transgene negative control; lane 6, constitutively off for 2 months since birth; lanes 7, 8, and 10, constitutively off for 2 month since birth followed by a turn-on for 4 days (lanes 7 and 10) or 1 week (lane 8); lane 9, spleen RNA controls. Each lane represents a different mouse. (C) Regulated TGF-β protein expression. Pancreatic tissues were isolated from line 4 (a_–_c) and line 45 (d), then fixed and stained for TGF-β protein (red, counterstained by hematoxylin/eosin). (a) Constitutively off for 8 weeks since birth. (b) Constitutively on for 5 weeks since birth. (c) Constitutively on for 5 weeks since birth followed by 1 week turn-off. (d) Constitutively on for 8 weeks since birth.
RT-PCR and Real-Time PCR. Total pancreatic RNA was prepared by guanidine thiocyanate extraction (23); for all other tissues, RNA was prepared by using TRIzol reagent (GIBCO/BRL). cDNA was synthesized with random primers (GIBCO/BRL). TGF-β and TTA transcripts were amplified with the primers described above. The expression of Foxp3 was measured by the real-time RT-PCR with the primers 5′-GGCCCTTCTCCAGGACAGA-3′ and 5′-GCTGATCATGGCTGGGTTGT-3′ at a final concentration of 500 nM, and the internal TaqMan probe 5′-FAM-ACTTCATGCATCAGCTCTCCACTGTGGAT-BHQ-1–3′ at a final concentration of 200 nM. Hypoxanthine phosphoribosyltransferase (HPRT) was used as an internal reference and measured by using the primers 5′-CTGGTGAAAAGGACCTCTCG-3′ and 5′-TGAAGTACTCATTATAGTCAAGGGCA-3′ at a final concentration of 200 nM, and the TaqMan probe 5′-FAM-TGTTGGATACAGGCCAGACTTTGTTGGAT-BHQ-1–3′ at a final concentration of 200 nM. Relative expression of Foxp3 normalized to HPRT levels was presented.
Cell and Tissue Preparation. Pancreatic tissues were collected and digested with Liberase RI (Roche Applied Science) at 37°C for 15–20 min. Dissociated islets were hand-picked under dissecting microscope. To release intraislet lymphocytes, islets were digested with Trypsin-EDTA for 10 min, followed by treatment with cell dissociation buffer (GIBCO/BRL) for 15 min at 37°C. Digested islets were incubated at 37°Cfor6–7 h to recover the expression of cell-surface receptors in RPMI medium 1640 with 10% FCS. Subsequently, lymphocytes were purified with lympholyte (Cederlane Laboratories).
Fluorescence-Activated Cell Sorting (FACS) and Histology. Expression of surface markers were analyzed by using anti-CD4 (H129.19), anti-CD8 (53.67), anti-CD25 (PC61), and Vβ4 (KT4) (BD Pharmingen). Intracellular staining of CTLA-4 was performed by using anti-CTLA-4 (UC10–4F10-11). For intracellular cytokine staining, intraislet lymphocytes were first stimulated with phorbol 12-myristate 13-acetate (50 ng/ml) and ionomycin (1 μM) for 6 h in the presence of Golgi Stop (BD Pharmingen). Subsequently, cells were surface-labeled, fixed, and permeabilized, then stained with anti-IFN-γ (XMG1.2) and anti-IL-4 (11B11) antibodies (BD Pharmingen).
For tissue expression of TGF-β, pancreas were fixed in 1% paraformaldehyde and passed through sucrose gradients before freezing in Tissue-Tek OCT (Bayer). Five- to 7-μm frozen sections were permeated with Triton X-100. Intracellular TGF-β expression was detected by using biotinylated anti-TGF-β (A75-7.1, BD Pharmingen), followed by phosphatase streptavidin and 5-bromo-4-chloro-3-indolyl phosphate (BCIP)/nitroblue tetrazolium (NBT) substrate (Zymed). For histology, tissues were prepared by fixation in 15% formaldehyde, and 5- to 7-μm sections of paraffin-embedded tissue were stained with hematoxylin and eosin.
Adoptive Transfers. Spleen cells from 6- to 7-month-old NOD transgenic mice were pooled from each group, and 10 × 106 cells were transferred to NOD–severe combined immunodeficient (SCID) recipient mice. Development of diabetes was monitored every 3 days after transfer for 12 weeks.
When indicated, islets were isolated from transgenic mice and lymphocytes were prepared as previously described. Transgenic intraislet lymphocytes (105) were cotransferred with 20 × 106 diabetogenic splenocytes isolated from NOD mice into NOD-SCID mice together. Development of diabetes was monitored for 9 weeks.
Splenic naive BDC2.5 T cells (CD62LhighCD25low) were selected by using magnetic beads (Miltenyi Biotec, Auburn, CA) and labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE). Cells (2 × 106) were transferred into transgenic or control mice. Pancreatic lymph nodes were collected 3 days posttransfer and analyzed by FACS to monitor the intensity of CFSE.
BrdUrd Detection. Eight week-old control and transgenic mice were treated (i.p.) with 1 mg of BrdUrd (Sigma) for 4 consecutive days. Subsequently, mice were killed and islets and several lymphoid organs were removed. Cell suspensions were surface stained, fixed, and permeabilized in PBS containing 1% paraformaldehyde plus 0.01% Tween 20 for 48 h at 4°C, then treated with 250 units/ml DNase I (Sigma) for 30 min at 37°C. BrdUrd incorporation was revealed with anti-BrdUrd mAb (Becton Dickinson).
Results
**Transient Expression of TGF-**β in the Islets Is Sufficient to Suppress Diabetes. The development of diabetes in NOD mice occurs as a result of the early development of lymphoid cell infiltration around the islets at 3 weeks of age and a late event wherein beta-cell destruction occurs several months later, suggesting that the antiislet immune response is countered by regulatory mechanisms (24). Protection conferred by constitutive expression of TGF-β was previously reported (21, 25), but constitutive expression of TGF-β in the islets led to fibrosis that compromised investigations of the mechanisms underlying immune suppression. To overcome this limitation, we generated mice in which TGF-β expression can be induced temporally by the tetracycline regulatory system (Fig. 1_A_) (22). We developed two transgenic lines expressing high or low levels of TGF-β in the pancreas, referred to as line 4 and line 45, respectively. Data from RT-PCR and histochemistry studies showed that TGF-β gene and protein expression in both lines can be turned on and off in the islets efficiently within 1 week after changing of the diet to a doxycycline-containing food source (Fig. 1 B and C).
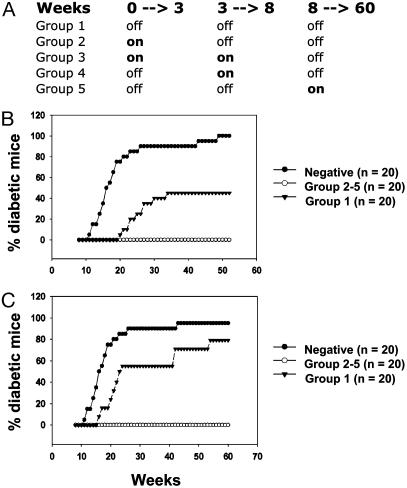
Using this system, we were able to control and target the expression of the transgene at specific stages of diabetes development (Fig. 2_A_). This includes stages from birth to 3 weeks (preinsulitis; group 2), from birth to 8 weeks (pre- and postinsulitis; group 3), from 4 to 8 weeks (priming phase of disease; group 4), and from 8 to 60 weeks (beta-cell destruction phase; group 5). Transgenic mice from groups 1–5 were monitored for diabetes development in comparison to transgene-negative control littermates during 60 weeks. We found that TGF-β expression significantly inhibited the development of diabetes in NOD transgenic mice (groups 2–5), indicating that a short pulse of TGF-β in the islets during either the priming or effector phase of the disease was sufficient to provide protection (Fig. 2 B and C). Histological analysis did not reveal any significant difference in the progression of insulitis between different groups of transgenic mice suggesting that TGF-β was unlikely to inhibit diabetes by down-regulating lymphocyte infiltration (data not shown). To determine effects of TGF-β on the development of antiislet T cell repertoire, splenic T cells from 6- to 7-month-old nondiabetic transgenic mice were transferred to NOD-SCID recipient mice, and diabetes development was monitored during 12 weeks posttransfer (Table 1). Mice that had received T cells from control transgenic mice in which TGF-β expression had been repressed for entire time period (group 1) developed diabetes within 3–6 weeks after transfer. In contrast, we found that spleen cells from transgenic mice with transient expression of TGF-β in the islets (groups 2–5) were less efficient in inducing diabetes, indicating that TGF-β inhibits the development of an antiislet repertoire.
Fig. 2.
Transient expression of TGF-β inhibits diabetes. (A) Summary of the different groups (1–5) of transgenic mice with turning on and off period time of the expression of TGF-β. Development of diabetes was monitored in line 4 (B) and line 45 (C) transgenic mice during 60 weeks. Filled circle, transgene negative; open circle, TGF-β turned on and off (groups 2–5); filled triangle, TGF-β constitutively turned off (group 1).
Table 1. Transfer of diabetes.
| Time posttransfer, weeks | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transgenic mice group | n | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| 1 | 10 | 0/10 | 0/10 | 6/10 | 9/10 | 9/10 | 10/10 | ||||||
| 2 | 9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 1/9 | 1/9 | 1/9 | 4/9 | 8/9 |
| 3 | 9 | 0/9 | 0/9 | 0/9 | 0/9 | 2/9 | 8/9 | 8/9 | 8/9 | 8/9 | 9/9 | ||
| 4 | 10 | 0/10 | 0/10 | 0/10 | 1/10 | 1/10 | 2/10 | 2/10 | 3/10 | 4/10 | 4/10 | 4/10 | 4/10 |
| 5 | 10 | 0/10 | 0/10 | 2/10 | 2/10 | 2/10 | 2/10 | 3/10 | 3/10 | 3/10 | 4/10 | 4/10 | 4/10 |
Because TGF-β-induced fibrosis was significant in line 4 but minimal in line 45 (Fig. 1_C_), and because constitutive transgene repression was more efficient in line 45 than in line 4 (Fig. 2 B and C), further investigation of mechanisms of protection by TGF-β were performed by using line 45.
**Neither Antigen Presentation nor Immune Deviation Is Affected by TGF-**β Expression in the Islets. Several mechanisms might account for the immunoregulatory action of TGF-β in protecting against autoreactive T cells in NOD mice. The first event of the autoagressive immune response is initiated by immature dendritic cells that take up beta-cell antigens and migrate into pancreatic lymph nodes for antigenic presentation to naïve T cells (26). To determine whether expression of TGF-β influences antigen presentation by antigen-presenting cells, CFSE-labeled naïve BDC2.5 T cells were adoptively transferred into transgenic mice. BDC2.5 T cells are reactive to self beta-cell antigen(s); on encountering their specific antigens in the pancreatic lymph nodes, these cells are primed and activated (27). Their proliferation therefore reflects the antigen presentation capacity of antigen-presenting cells in islets. Four days posttransfer, pancreatic lymph nodes were isolated and the cell division profile was investigated. We found that proliferation of BDC2.5 T cells was not reduced and, if anything, was more efficient in transgenic mice compared to control group 1 mice (Fig. 3 A and B), indicating that antigen presentation is not affected by the expression of TGF-β.
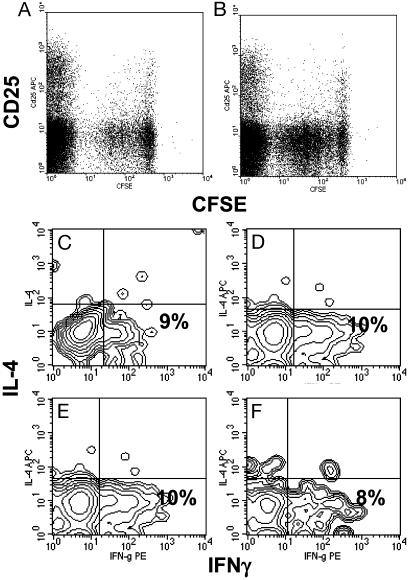
Fig. 3.
TGF-β expression does not affect intraislet antigen-presenting function or T cell differentiation profile. CFSE-labeled purified naïve BDC2.5 T cells were injected into 4-week-old transgenic mice (line 45) in which TGF-β was either turned off (A) or on (B) from birth to 4 weeks of age. Four days posttransfer, pancreatic lymph node cells were collected and lymphocytes were analyzed by FACS. Dot plots represent CFSE intensity on gated CD4+ T cells. The results are a representative of eight experiments. Similar results were obtained from line 4. Intraislet T cells were pooled from five transgenic mice (line 45) and stimulated with phorbol 12-myristate 13-acetate plus ionomycin for 6 h. Contour plots represent the distribution of IL-4-versus IFN-γ-expressing cells among gated CD4+ T cells isolated from mice with TGF-β constitutively off for 10 weeks since birth (C), with TGF-β off for 8 weeks since birth and turned on for the next 5 weeks (D), with TGF-β on for 8 weeks since birth (E), or with TGF-β on for 16 weeks since birth (F).
Next we considered suppression of T helper (Th) 1 function through immune deviation toward a Th2 profile as previously reported from mouse models of constitutive expression of TGF-β in NOD mice (25). In these mice, TGF-β modified the profile of antigens presented by antigen-presenting cells and caused beta-cell-specific splenic T cells to shift from IFN-γ-producing Th1 cells to IL-4-secreting Th2 cells. Accordingly, we isolated T cells from islets and analyzed their effector phenotype, Th1 versus Th2, after a 6-h stimulation with phorbol 12-myristate 13-acetate and ionomycin (Fig. 3 C_–_F). We found no significant changes in the distribution of IFNγ-versus IL-4-expressing T cells in mice with or without expression of TGF-β. Approximately 10% of the CD4+ T cells expressed IFN-γ, whereas IL-4-expressing T cells were barely detectable, indicating no effect of TGF-β on Th1/Th2 differentiation of intraislet effector T cells.
**TGF-**β Promotes Expansion of the Foxp3-Expressing CD4+CD25+ Regulatory T Cell Pool. CD4+CD25+ regulatory T cells account for 5–10% of the CD4+ T cells infiltrating NOD islets, the effect of which might be responsible for the slow development of diabetes. To investigate whether TGF-β regulates the homeostasis of the regulatory T cell pool, the frequency of intraislet CD4+CD25+ T cells was determined (Fig. 4 A_–_D). We found that exposure to TGF-β for the first 8 weeks (group 3, Fig. 4_C_) dramatically enhanced the frequency of CD4+CD25+ T cells among total intraislet CD4+ T cells by 4-fold when compared to control transgenic mice (group 1, Fig. 4_A_). Accumulation of intraislet CD4+CD25+ T cells was observed at 8 weeks of age and further persisted up to 16 weeks of age (Fig. 4_D_). However, this phenomenon was not observed if the expression of TGF-β was initiated after the priming phase that is between 3 and 8 weeks of age (Fig. 4_B_). Thus, the expression of TGF-β in the islets during the priming phase of diabetes is associated with the generation of CD4+CD25+ T cells in the islets and the protection against diabetes.
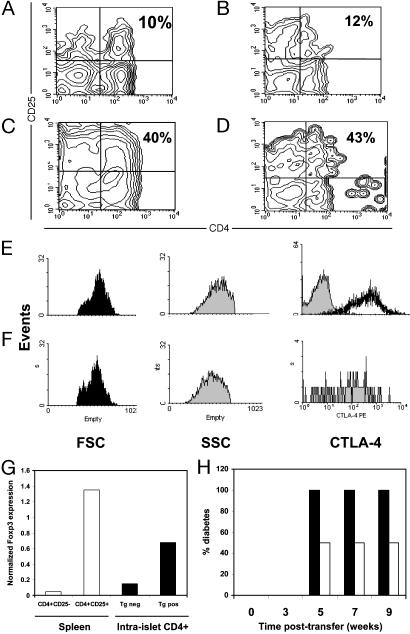
Fig. 4.
TGF-β induces high frequency of intraislet CD4+CD25+ regulatory T cells Intraislet T cells were pooled from five transgenic mice (line 45) and then stained for the expression of CD4 and CD25 markers. Contour plots represents results from mice with TGF-β constitutively off for 10 weeks since birth (A), TGF-β off for 8 weeks since birth and turned on for the next 5 weeks (B), TGF-β on for 8 weeks since birth (C), or TGF-β on for 16 weeks since birth (D). Gated CD4+CD25+ T cells from pancreatic lymph nodes (E) or islets (F) isolated from transgenic line 45 with TGF-β on for 8 weeks since birth were compared for intracellular expression of CTLA-4 as well as cell size (FSC) and cell granulosity (SSC). (G) Intraislet CD4+ T cells were purified from transgenic line 45 with TGF-β on for 8 weeks since birth (Tg pos) or from transgenic negative mice (Tg neg), and levels of mRNA of Foxp3 expression were determined by real-time quantitative PCR (filled bars). Splenic CD4+CD25+ and CD4+CD25– T cells from NOD mice were used as controls (open bars). (H) Intraislet T cells were purified from transgenic line 45 with TGF-β on for 8 weeks since birth, and 105 cells were transferred to NOD-SCID recipient mice together with 20 × 106 splenic cells from diabetic NOD mice (open bars). As control, 20 × 106 splenic cells from diabetic NOD mice were transferred alone to recipient mice (filled bars). Diabetes development was monitored for 10 weeks posttransfer.
Regulatory T cells (CD4+CD25+) are most commonly characterized as small cells expressing several activation markers including OX40, GITR, CD62L, and CTLA-4. We found that intraislet infiltrating CD4+CD25+ T cells exhibited the typical phenotypes of regulatory T cells compared to control regulatory T cells isolated from pancreatic lymph nodes, as indicated by small size and high levels of CTLA-4 expression (Fig. 4 E and F).
Recent studies have demonstrated that Foxp3 is selectively expressed in CD4+CD25+ regulatory T cells and is essential for the development and function of these cells (13, 14). To investigate the expression of Foxp3, intraislet CD4+ T cells were purified and levels of Foxp3 mRNA expression were determined by using real-time quantitative PCR analysis (Fig. 4_G_). Sorted splenic CD4+CD25+ and CD4+CD25– T cells were used as controls in this experiment. As previous reported, levels of Foxp3 mRNA showed 30-fold higher expression in CD4+CD25+ T cells compared to the CD4+CD25– T cells purified from the spleen of NOD mice (Fig. 4_G_). Consistent with the increased frequency of CD4+CD25+ regulatory T cells in the transgenic islets (Fig. 4_C_), Foxp3 mRNA expression showed a 4-fold increase among transgenic intraislet CD4+ T cells compared to the control islet T cells (Fig. 4_G_). These observations provide direct evidence that expression of TGF-β results in the accumulation of Foxp3-expressing CD4+CD25+ regulatory T cells in the islet.
To determine whether these cells with a regulatory T cell phenotype are endowed with suppressive activity in vivo, an adoptive transfer model was used that is commonly applied toward this end (28). Intraislet T cells were isolated from transgenic or control mice and then cotransferred with splenic cells from diabetic NOD mice into NOD-SCID recipient mice (Fig. 4_H_). Transfer of 20 × 106 diabetogenic splenic cells alone induced severe onset of diabetes in 100% of recipient mice by 5 weeks posttransfer. In contrast, cotransfer of transgenic intraislets T cells with diabetogenic splenic cells significantly reduced the development of diabetes and conferred protection on >50% of recipient mice up to 9 weeks posttransfer (Fig. 4_H_). This finding is consistent with the hypothesis that TGF-β leads to the generation of a T cell population endowed with suppressive activity.
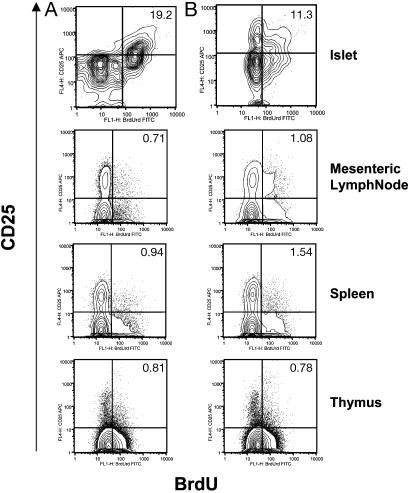
TGF-β is best known for its ability to inhibit rather than promote T cell proliferation. One exception comes from the work of Horwitz et al. (29), indicating that in the presence of sufficient IL-2 to counteract its suppressive effects on T cell proliferation, TGF-β showed positive effects on the proliferation and survival of human T cells that develop potent suppressive activity (29). Accordingly, the potential of different intraislet T cell subsets to proliferate in vivo was investigated by using an in vivo BrdUrd incorporation approach. After 4 days of continuous administration of BrdUrd, lymphocytes were isolated from islets, spleen, mesenteric lymph nodes, and thymus, then stained for BrdUrd together with CD4 and CD25 antibodies (Fig. 5). Among the thymic, splenic, and lymph node CD4+ T cell populations, the distribution of BrdUrd-positive cells between CD25+ and CD25– cells showed no difference in transgenic compared to control mice (Fig. 5). In contrast, a higher frequency of proliferating cells was found among transgenic intraislet CD4+CD25+ T cells in comparison to T cells from transgenic negative controls (Fig. 5). Consistent with the expected potent suppressive effects of CD4+CD25+ T cells, intraislet CD4+CD25– T cells showed a complete lack of proliferation in transgenic mice, whereas intermediate levels of proliferation were observed in cells from nontransgenic mice (Fig. 5). It is possible, however, that lack of proliferation of intraislet CD4+CD25– T cells in TGF-β transgenic mice is a combined effect of direct exposure to islet TGF-β expression and suppressor activity of CD4+CD25+ regulatory T cells present at high frequency. Nevertheless, these observations demonstrate a previously uncharacterized function for TGF-β in inducing the proliferation of CD4+CD25+ regulatory T cells in vivo.
Fig. 5.
TGF-β induces proliferation of intraislet CD4+CD25+ T cells. Control NOD mice (B) and transgenic line 45 with TGF-β on for 8 weeks since birth (A) were injected for 4 consecutive days with 1 mg of BrdUrd. Lymphocytes were isolated from islets, mesenteric lymph nodes, spleen, and thymus, and stained with anti-CD4 and anti-CD25 and anti-BrdUrd antibodies. Contour plots show the distribution of BrdUrd versus CD25 expression on gated CD4+ T cells. The results are representative of four pooled mice.
Discussion
High levels of membrane-bound TGF-β on the surface of regulatory T cells had linked TGF-β with regulatory T cell suppressive function. The relationship between both components has been proposed to function in a paracrine pathway in which regulatory T cells produce TGF-β that inhibits T cell activation. Here we focused on the potential effects of TGF-β on the regulatory T cell compartment and provided direct evidence for the expansion of Foxp3-expressing regulatory T cells involved in the protection against diabetes.
One consideration is that the timing and duration of TGF-β expression in the islets influences the accumulation of intraislet CD4+CD25+ T cells. If expression of TGF-β is initiated after 8 weeks of age, CD4+CD25+ regulatory T cells no longer accumulate. Nonetheless, TGF-β still confers protection against diabetes. Studies on the dynamics of pathogenic and suppressor T cells in NOD mice reported that progression of diabetes occurs along with progressive modulation of T cell activity reflected in reduced suppressor activity of CD4+CD25+ T cells and increased pathogenicity of CD4+CD25– T cells (30). Indeed, CD4+CD25+ regulatory T cells from 8-week-old, but not from 16-week-old, NOD mice delayed the onset of diabetes in adoptive transfer models and showed decreased production of immunoregulatory factors such as IL-10. In contrast, the profile of T cell differentiation of CD4+CD25– T cells revealed a higher frequency of Th1 cells in the pancreas of 16-week-old NOD mice compared to 8-week-old NOD mice (30). Consistent with this, our analysis of BrdUrd incorporation in CD4+CD25+ versus CD4+CD25– intraislets T cells indicates that TGF-β can rebalance the dynamic of these cell subsets by promoting expansion of the regulatory T cells and the suppression of pathogenic T cells. Indeed, expression of TGF-β in the islets led to a diminished repertoire of autoaggressive T cells in the spleens of transgenic mice. As a result, adoptive transfer of these splenic cells did not yield rapid diabetes in recipient mice that was found by using spleen cells from control nontransgenic mice. However, the frequency of CD4+CD25+ T cells was not increased in the spleen, in contrast to the high levels observed in the islets. We think that splenocytes from the transgenic mice show a reduced potential for the induction of type I diabetes because of the inhibitory effects of regulatory T cells in situ in the islets on the clonal expansion and activation of autoaggressive T cells. In the normal nontransgenic animal, such autoaggressive cells expand in the islets and migrate systemically, and can be recovered in spleen. Nevertheless, we cannot exclude differential effects of TGF-β on autoaggressive T cell differentiation at later time points, and further experimentation will be required to elucidate the fate of autoaggressive cells under these circumstances. The point of interest here is that TGF-β, a factor known to be a negative regulator of CD4+CD25– T cells, is conversely a positive regulator of CD4+CD25+ regulatory T cell pool in vivo.
Convincing evidence that CD4+CD25+ T cells can develop in the thymus does not rule out alternative mechanisms for the generation of regulatory T cells in the periphery (8–12). It is unlikely, at least in humans, that the thymus can replenish the peripheral pool of CD4+CD25+ regulatory T cells throughout the human lifespan. As a result of thymic involution, the number of CD4+CD25+ regulatory T cells should decrease over time, and yet evidence suggests that this is not the case, indicating that thymically derived regulatory T cells must be efficiently maintained in the periphery. Our data revealed a critical role of TGF-β in the expansion of the Foxp3-expressing CD4+CD25+ regulatory T cell pool. Although regulatory T cells are most commonly known to be anergic and prone to apoptosis (31–33), several lines of evidence indicate that they can vigorously proliferate. Anergic CD4+CD25+ T cells proliferate efficiently when transferred to lymphopenic hosts, and regulatory T cells generated ex vivo in the presence of IL-2 and TGF-β can proliferate when transferred to nonlymphopenic syngeneic mice (15, 34). How TGF-β drives proliferation of regulatory T cells in the islets remains to be determined, but multiple factors are known to impact on TGF-β effects. With sufficient IL-2 to counteract its suppressive effects, TGF-β enhances the proliferation and survival of human T cells that develop potent suppressive activities (29). Moreover, engagement of GITR on CD4+CD25+ regulatory T cells seems able to lead to an intermediate step of reversal anergy where IL-2-induced proliferation may occur (35, 36). Engagement of both GITR and TGF-β engagement may therefore allow proliferation of regulatory T cells while preserving their suppressive activity.
After this manuscript was prepared, a paper was published showing that TGF-β could convert peripheral CD4+CD25– T cells into Foxp3-expressing CD4+CD25+ regulatory T cells in vitro (37). Accordingly, we cannot exclude the possibility of conversion of a similar subset of CD4+CD25– T cells to the regulatory intraislet CD4+CD25+ T cells by expression of TGF-β in the pancreas. Nonetheless, our study provided direct evidence for a previously uncharacterized function of TGF-β in the expansion of regulatory T cells in vivo that prevents the development of autoimmune diabetes.
Acknowledgments
We thank Drs. Li Wen and F. Suzan Wong for helpful discussions, Debby Butkus and Cindy Hughes for generating transgenic mice, Tony Ferrandino and Joanne Daugherty for helpful assistance, and Fran Manzo for help with manuscript preparation. This work was supported in part by Diabetes Endocrinology Research Center Award NIH DK45735. FACS analysis was supported by National Institutes of Health Program Project AI36529 (to R.A.F.). Y.P. is supported by the Juvenile Diabetes Foundation. Y.L. is supported by the American Diabetes Association (research grant to R.A.F.) and was previously supported by an American Diabetes Association Mentor-Based Post-doctoral Fellowship (to R.A.F.). M.O.L. is a postdoctoral fellow of the American Cancer Society. R.A.F. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations: CFSE, carboxyfluorescein diacetate succinimidyl ester; NOD, nonobese diabetic; RIP, rat insulin promoter; SCID, severe combined immunodeficient; TGF-β, transforming growth factor β; Th, T helper; TRE, tetracycline-responsive element; FACS, fluorescence-activated cell sorter.
References
- 1.Bach, J. F. & Chatenoud, L. (2001) Annu. Rev. Immunol. 19**,** 131–161. [DOI] [PubMed] [Google Scholar]
- 2.Gershon, R. K. (1975) Transplant Rev. 26**,** 170–185. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. (1995) J. Immunol. 155**,** 1151–1164. [PubMed] [Google Scholar]
- 4.Shevach, E. M. (2001) J. Exp. Med. 193**,** F41–F46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groux, H., O'Garra, A., Bigler, M., Rouleau, M., Antonenko, S., de Vries, J. E. & Roncarolo, M. G. (1997) Nature 389**,** 737–742. [DOI] [PubMed] [Google Scholar]
- 6.Levings, M. K. & Roncarolo, M. G. (2000) J. Allergy Clin. Immunol. 106**,** S109–S112. [DOI] [PubMed] [Google Scholar]
- 7.Weiner, H. L. (2001) Microbes Infect. 3**,** 947–954. [DOI] [PubMed] [Google Scholar]
- 8.Thorstenson, K. M. & Khoruts, A. (2001) J. Immunol. 167**,** 188–195. [DOI] [PubMed] [Google Scholar]
- 9.Asano, M., Toda, M., Sakaguchi, N. & Sakaguchi, S. (1996) J. Exp. Med. 184**,** 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang, X., Izikson, L., Liu, L. & Weiner, H. L. (2001) J. Immunol. 167**,** 4245–4253. [DOI] [PubMed] [Google Scholar]
- 11.Jonuleit, H., Schmitt, E., Schuler, G., Knop, J. & Enk, A. H. (2000) J. Exp. Med. 192**,** 1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregori, S., Casorati, M., Amuchastegui, S., Smiroldo, S., Davalli, A. M. & Adorini, L. (2001) J. Immunol. 167**,** 1945–1953. [DOI] [PubMed] [Google Scholar]
- 13.Hori, S., Nomura, T. & Sakaguchi, S. (2003) Science 299**,** 1057–1061.12522256 [Google Scholar]
- 14.Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. (2003) Nat. Immunol. 4**,** 330–336. [DOI] [PubMed] [Google Scholar]
- 15.Khattri, R., Cox, T., Yasayko, S. A. & Ramsdell, F. (2003) Nat. Immunol. 4**,** 337–342. [DOI] [PubMed] [Google Scholar]
- 16.Khattri, R., Kasprowicz, D., Cox, T., Mortrud, M., Appleby, M. W., Brunkow, M. E., Ziegler, S. F. & Ramsdell, F. (2001) J. Immunol. 167**,** 6312–6320. [DOI] [PubMed] [Google Scholar]
- 17.Green, E. A., Gorelik, L., McGregor, C. M., Tran, E. H. & Flavell, R. A. (2003) Proc. Natl. Acad. Sci. USA 100**,** 10878–10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura, K., Kitani, A. & Strober, W. (2001) J. Exp. Med. 194**,** 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen, W. & Wahl, S. M. (2003) Cytokine Growth Factor Rev. 14**,** 85–89. [DOI] [PubMed] [Google Scholar]
- 20.Shockett, P., Difilippantonio, M., Hellman, N. & Schatz, D. G. (1995) Proc. Natl. Acad. Sci. USA 92**,** 6522–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grewal, I. S., Grewal, K. D., Wong, F. S., Wang, H., Picarella, D. E., Janeway, C. A., Jr., & Flavell, R. A. (2002) J. Autoimmun. 19**,** 9–22. [DOI] [PubMed] [Google Scholar]
- 22.Green, E. A. & Flavell, R. A. (2000) Immunity 12**,** 459–469. [DOI] [PubMed] [Google Scholar]
- 23.Chomczynski, P. & Sacchi, N. (1987) Anal. Biochem. 162**,** 156–159. [DOI] [PubMed] [Google Scholar]
- 24.Delovitch, T. L. & Singh, B. (1997) Immunity 7**,** 727–738. [DOI] [PubMed] [Google Scholar]
- 25.King, C., Davies, J., Mueller, R., Lee, M. S., Krahl, T., Yeung, B., O'Connor, E. & Sarvetnick, N. (1998) Immunity 8**,** 601–613. [DOI] [PubMed] [Google Scholar]
- 26.Spatz, M., Eibl, N., Hink, S., Wolf, H. M., Fischer, G. F., Mayr, W. R., Schernthaner, G. & Eibl, M. M. (2003) Cell Immunol. 221**,** 15–26. [DOI] [PubMed] [Google Scholar]
- 27.Hoglund, P., Mintern, J., Waltzinger, C., Heath, W., Benoist, C. & Mathis, D. (1999) J. Exp. Med. 189**,** 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee, R., Chaturvedi, P., Qin, H. Y. & Singh, B. (2003) J. Autoimmun. 21**,** 221–237. [DOI] [PubMed] [Google Scholar]
- 29.Yamagiwa, S., Gray, J. D., Hashimoto, S. & Horwitz, D. A. (2001) J. Immunol. 166**,** 7282–7289. [DOI] [PubMed] [Google Scholar]
- 30.Gregori, S., Giarratana, N., Smiroldo, S. & Adorini, L. (2003) J. Immunol. 171**,** 4040–4047. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi, T., Kuniyasu, Y., Toda, M., Sakaguchi, N., Itoh, M., Iwata, M., Shimizu, J. & Sakaguchi, S. (1998) Int. Immunol. 10**,** 1969–1980. [DOI] [PubMed] [Google Scholar]
- 32.Itoh, M., Takahashi, T., Sakaguchi, N., Kuniyasu, Y., Shimizu, J., Otsuka, F. & Sakaguchi, S. (1999) J. Immunol. 162**,** 5317–5326. [PubMed] [Google Scholar]
- 33.Kuniyasu, Y., Takahashi, T., Itoh, M., Shimizu, J., Toda, G. & Sakaguchi, S. (2000) Int. Immunol. 12**,** 1145–1155. [DOI] [PubMed] [Google Scholar]
- 34.Gavin, M. A., Clarke, S. R., Negrou, E., Gallegos, A. & Rudensky, A. (2002) Nat. Immunol. 3**,** 33–41. [DOI] [PubMed] [Google Scholar]
- 35.McHugh, R. S., Whitters, M. J., Piccirillo, C. A., Young, D. A., Shevach, E. M., Collins, M. & Byrne, M. C. (2002) Immunity 16**,** 311–323. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu, J., Yamazaki, S., Takahashi, T., Ishida, Y. & Sakaguchi, S. (2002) Nat. Immunol. 3**,** 135–142. [DOI] [PubMed] [Google Scholar]
- 37.Chen, W., Jin, W., Hardegen, N., Lei, K. J., Li, L., Marinos, N., McGrady, G. & Wahl, S. M. (2003) J. Exp. Med. 198**,** 1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]