Microglia/macrophage polarization dynamics in white matter after traumatic brain injury (original) (raw)
Abstract
Mononuclear phagocytes are a population of multi-phenotypic cells and have dual roles in brain destruction/reconstruction. The phenotype-specific roles of microglia/macrophages in traumatic brain injury (TBI) are, however, poorly characterized. In the present study, TBI was induced in mice by a controlled cortical impact (CCI) and animals were killed at 1 to 14 days post injury. Real-time polymerase chain reaction (RT–PCR) and immunofluorescence staining for M1 and M2 markers were performed to characterize phenotypic changes of microglia/macrophages in both gray and white matter. We found that the number of M1-like phagocytes increased in cortex, striatum and corpus callosum (CC) during the first week and remained elevated until at least 14 days after TBI. In contrast, M2-like microglia/macrophages peaked at 5 days, but decreased rapidly thereafter. Notably, the severity of white matter injury (WMI), manifested by immunohistochemical staining for neurofilament SMI-32, was strongly correlated with the number of M1-like phagocytes. In vitro experiments using a conditioned medium transfer system confirmed that M1 microglia-conditioned media exacerbated oxygen glucose deprivation–induced oligodendrocyte death. Our results indicate that microglia/macrophages respond dynamically to TBI, experiencing a transient M2 phenotype followed by a shift to the M1 phenotype. The M1 phenotypic shift may propel WMI progression and represents a rational target for TBI treatment.
Keywords: inflammation, macrophage, microglia, polarization, white matter injury
Introduction
Traumatic brain injury (TBI) is a neurologic disorder with growing prevalence among young adults. Traumatic brain injury poses a significant financial burden to society as well as physical and emotional burden to victims and caregivers. Neurologic morbidities after TBI are common and include cognitive impairments, dementia, epilepsy, and depression.1, 2, 3, 4 The impact of TBI on brain tissue has been studied for many decades; from these studies, TBI is known to damage gray matter and elicit neuronal death. However, TBI can also elicit severe white matter injury (WMI) that correlates with long-term deficits in motor and cognitive function.5, 6 Complications after TBI involve more than the initial tissue damage induced directly by mechanical trauma.1, 7, 8 Delayed secondary events, such as ischemia, lipid degradation, free radical formation, excitotoxicity, and protease release can be equally devastating.9, 10, 11 For example, secondary events can lead to demyelination, axonal degeneration, neuronal death, cavitation, and glial scarring around the area of the initial damage.9, 10, 11 Inflammation, including the activation of resident microglial cells, is thought to have an important role in these secondary changes.3, 9, 10, 12
Microglia/macrophages are the primary mediators of the immune defense system in the central nervous system (CNS) and are integral to subsequent inflammatory responses.12, 13 Resident microglia and peripheral macrophages are rapidly mobilized to the site of injury and initiate the release of effector molecules and recruitment of other immune cells.14, 15 An increasing number of studies now agree that microglia/macrophages are highly plastic cells that can assume diverse phenotypes and engage different functional programs in response to specific microenvironmental signals.13, 15 The dual roles of distinctly polarized microglia/macrophage populations have been reported in several CNS disorders, including stroke,16 multiple sclerosis,17 and spinal cord injury.18 However, the specific roles of polarized microglia/macrophages in the pathophysiology of TBI have not been explored.
The goal of the present study was to analyze the temporal kinetics of microglia/macrophage polarization after TBI using a well-established murine model of controlled cortical impact (CCI). Based on our previous observations in ischemic injury models,16 we tested the hypothesis that microglia/macrophages respond dynamically to mechanical trauma, switching from a transient M2 phenotype to a sustained M1 phenotype. In support of this hypothesis, we found that microglia/macrophages expressed the early M2 phenotype after TBI, but that this was gradually replaced by the M1 phenotype at the site of injury. Notably, the severity of WMI was strongly correlated with activation of the M1 phenotype. In vitro experiments further revealed that M1 microglia-conditioned media exacerbated oxygen glucose deprivation (OGD) -induced oligodendrocyte death. Taken together, our in vivo and in vitro findings support the notion that the M1 subtype of differentially activated microglia exacerbates WMI after TBI.
Materials and Methods
Animals
Young male C57BL/6J mice (10- to 12-week-old) were obtained from Jackson Laboratory (Bar Harbor, Maine, USA). Animals were housed in groups of four per cage in a temperature- and humidity-controlled animal facility with a 12-hour light–dark cycle. Food and water were available ad libitum. All animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and the number of animals killed.
Traumatic Brain Injury
Traumatic brain injury was induced in C57/BL6 mice by a CCI, as described previously.11 Briefly, all animals were randomly assigned into experimental groups through the use of a lottery drawing box. Mice were anesthetized with 1.5% isoflurane in a 30% O2/68.5% N2O mixture under spontaneous breathing conditions. Using aseptic techniques and principles consistent with IACUC surgical policy, an approximately 4-mm craniotomy was performed over the right parietotemporal cortex using a motorized drill. The CCI was centered 2 mm lateral to the midline and 1.0 mm anterior to the bregma and produced with a pneumatically driven CCI device (Precision Systems and Instrumentation, Fairfax, VA, USA) using a 3 mm flat-tip impounder (velocity, 3.5 m/second; duration, 150 milliseconds; depth, 1.5 mm). Immediately after injury, the bone flap was replaced and sealed with Koldmount cement (Vernon Benshoff, Albany, NY, USA), and the scalp was sutured shut. Rectal temperature was controlled at 37°C±0.5°C during surgery and up to 30 minutes after TBI using a temperature-regulated heating pad. Sham animals were subjected to all aspects of the protocol (surgery, anesthesia, craniotomy, recovery) except for CCI.
Immunohistochemistry and Cell Counting
After killing by an anesthetic overdose and perfusion with cold saline followed by formaldehyde (4% in phosphate-buffered saline), brains were removed and cryoprotected in 30% sucrose in phosphate-buffered saline. Serial sections (25-_μ_m thickness; between 0.38 mm and 0.26 mm from the bregma) were cut on a freezing microtome and subjected to immunohistochemistry. Primary antibodies included goat anti-CD206 (R&D Systems, Minneapolis, MN, USA), rat anti-CD16/32 (BD, Franklin Lakes, NJ, USA), rabbit anti-MBP (myelin basic protein) (Abcam, Cambridge, MA, USA), mouse anti-non-phosphorylated neurofilaments (SMI-32, Abcam) and rabbit anti-Iba1 (Wako, Richmond, VA, USA). All images were processed with ImageJ software (National Institutes of Health) for automated cell counting. Mean cell counts were calculated from three random microscopic fields at × 200 × magnification in the cortex, striatum, and corpus callosum (CC) of each section respectively, and three consecutive sections were analyzed for each brain. Data from these nine images per brain region are expressed as mean numbers of cells per square millimeter. Counts were made by an investigator who was masked to experimental group assignment (_n_=4 to 5 animals per group).
Fluorescence Quantification
All fluorescence values were generated using confocal microscopy (FV1000, Olympus, Tokyo, Japan) and analyzed semi-quantitatively with ImageJ software. Fluorescence intensity of SMI-32 in the CC area affected by CCI was contrasted with the CC of sham animals. The region of interest for the CC area was drawn close to the lesion cavity, whereas a mirror image of this outline was drawn over the contralateral CC in the contralateral hemisphere. Similar regions of interest were drawn in sham animals in the same anatomic regions. The analysis was done by an investigator masked to experimental group.
Real-Time Polymerase Chain Reaction
Total RNA was isolated from sham and injured brains using the RNeasy Mini Kit (Qiagen, Germantown, MD, USA) according to the manufacturer's instructions. Five micrograms of RNA was used to synthesize the first strand of cDNA using the Superscript First-Strand Synthesis System (Invitrogen, Grand Island, NY, USA) for reverse-transcription polymerase chain reaction (RT–PCR). Polymerase chain reaction was performed with the Opticon 2 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA), and SYBR gene PCR Master Mix (Invitrogen) as described previously.16 The cycle time values were normalized to glyceraldehyde 3-phosphate dehydrogenase messenger RNA levels in the same sample. The expression levels of the messenger RNA were then reported as fold changes versus sham controls. The sequences of the primer pairs for M1 phenotype genes are as follows (in pairs, sense, and antisense): (1) iNOS: CAAGCACCTTGGAAGAGGAG and 5′-AAGGCCAAACACAGCATACC-3′ (2) CD32: 5′-AATCCTGCCGTTCCTACTGATC-3′ and 5′-GTGTCACCGTGTCTTCCTTGAG-3′ (3) CD16: 5′-TTTGGACACCCAGATGTTTCAG-3′ and 5′-GTCTTCCTTGAGCACCTGGATC-3′ (4) CD86: 5′-GACCGTTGTGTGTGTTCTGG-3′ and 5′-GATGAGCAGCATCACAAGGA-3′ (5) CD11b: 5′-CCAAGACGATCTCAGCATCA-3′ and 5′-TTCTGGCTTGCTGAATCCTT-3′. The sequences of the primer pairs for M2 phenotype genes are as follows: CD206: 5′-CAAGGAAGGTTGGCATTTGT-3′ and 5′-CCTTTCAGTCCTTTGCAAGC-3′ (2) Arg1: 5′-TCACCTGAGCTTTGATGTCG-3′ and 5′-CTGAAAGGAGCCCTGTCTTG-3′ (3) IL-10: 5′-CCAAGCCTTATCGGAAATGA-3′ and 5′-TTTTCACAGGGGAGAAATCG-3′ (4) CCL-22: 5′-CTGATGCAGGTCCCTATGGT-3′ and 5′-GCAGGATTTTGAGGTCCAGA-3′ (5) TGF-b: 5′-TGCGCTTGCAGAGATTAAAA-3′ and 5′-CGTCAAAAGACAGCCACTCA-3′ (6) Ym1/2: 5′-CAGGGTAATGAGTGGGTTGG-3′ and 5′-CACGGCACCTCCTAAATTGT-3′.
Primary Microglia and Oligodendrocyte Cultures
Primary microglia and oligodendrocytes were prepared from mixed glial cultures of 1-day-old mouse brains, as described previously.16, 19 Briefly, microglia were isolated by shaking flasks containing mixed glia for 1 hour at 180 g, collecting and seeding microglia at 3 × 106/well in 6-well plates. For microglial M1 induction, lipopolysaccharide (100 ng/mL) and interferon-gamma 20 ng/mL) were added to the microglial cultures for 48 hours. For microglial M2 induction, interleukin 4 (IL-4; 20 ng/mL) was added to the cultures for 48 hours. Conditioned medium was collected and concentrated 100-fold using Amicon ultra centrifuge filters (Millipore, Billerica, MA, USA). For oligodendrocyte preparation, fresh media was added to the flasks after microglia removal and flasks were shaken overnight at 200 g. Oligodendrocyte precursors were collected and seeded at 1 × 105/mL in 96- or 24-well plates. To culture oligodendrocytes, basal chemically defined medium containing 15 nmol/L triiodothyronine, 1 ng/mL ciliary neurotrophic factor and 1 × _N_-acetyl-L-cysteine were used. After 3 days in culture, oligodendrocytes were subjected to 120-minute oxygen-glucose deprivation (OGD) followed by the addition of conditioned media from either unstimulated microglia, M1 microglia, or M2 microglia. Oligodendrocytes were collected 24 hours later for the MTT viability assay (see below) and immunocytochemical staining for MBP. Media was also collected at this time for measurements of lactate dehydrogenase release through damaged membranes (see below).
Measurements of Cell Damage
Metabolic viability was assessed with the MTT method. The MTT solution (50 _μ_L) was added into each well (final concentration 1 g.L−1). After incubation at 37°C for 2 hours, media was carefully removed and 100 _μ_L dimethylformamide was added to each well to dissolve the resultant dark blue crystals for 1 hours. Absorbance was measured at 570 nm (OD 570) with a Universal Microplate Reader (Elx800, BioTek Instruments, Winooski, VT, USA). As a second measure of cell damage, the activity of LDH released into the media was assayed spectrophotometrically by monitoring the reduction of NAD+ at 340 nm at 25°C in the presence of lactate (Pointe Scientific, Lincoln Park, MI, USA).
Phagocytosis Assay
For analysis of phagocytosis, microglia (1 × 105 cells/well) were plated into 8-well chamber slides (Nunc, ThermoScientific, Pittsburgh, PA, USA) as described previously.16 Nile red fluorescent microspheres (Invitrogen) were solubilized to a concentration of 0.03% solids. Cells were incubated with or without microspheres for 3 hours. Cells were then rinsed with PBS and fixed in 4% paraformaldehyde. Alexa Fluor 488 phalloidin (Invitrogen) was added and incubated at room temperature in the dark for 1 hour. Images were captured with an Olympus confocal microscope.
Statistical Analyses
All values are presented as mean±standard error of the mean. Data with two groups were analyzed with the Student's _t_-test (non-directional), and data with repeated groups were analyzed with one-way analysis of variance and the Student Newman–Keuls test for post hoc comparisons. The Pearson product linear regression analysis was used to correlate the M1 or M2 phenotype with SMI-32 intensity. Differences were considered statistically significant at _P_⩾0.05.
Results
Dynamic Changes in Messenger RNA Expression of M1 and M2 Polarization Markers
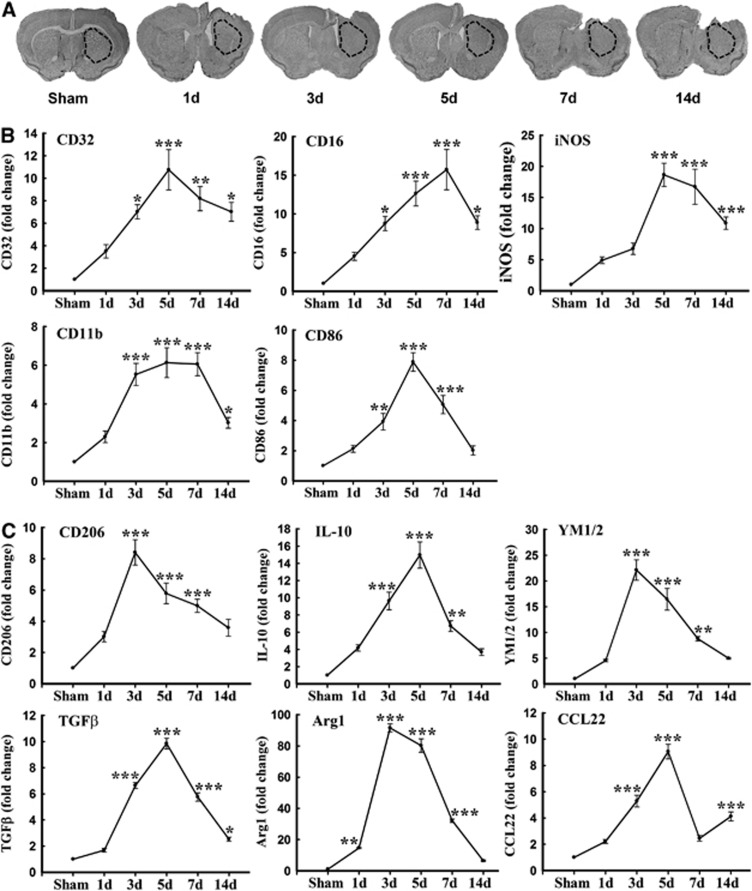
Polarized microglia/macrophages can be distinguished by surface marker expression and cytokine/chemokine production. Real-time polymerase chain reaction was performed using total RNA extracted from ipsilateral striatum at 1, 3, 5, 7, and 14 days after TBI or from sham-operated brains (Figure 1A). Levels of M1-type genes (iNOS, CD11b, CD16, CD32, and CD86) gradually increased over time from day 3 onward and peaked at day 5 or 7 (Figure 1B). Furthermore, all but CD86 were maintained at relatively high levels for at least 14 days after TBI.
Figure 1.
Traumatic brain injury (TBI) induced changes in messenger RNA (mRNA) expression of M1 and M2 polarization markers in injured tissue. (A) Reverse-transcription polymerase chain reaction (RT–PCR) was performed using total RNA extracted from ipsilateral striatum at 1, 3, 5, 7, and 14 days after TBI or after sham operation. The dotted black lines outline the area of striatal dissections. (B) Messenger RNA expression of M1 markers; (C) mRNA expression of M2 markers. Data are expressed as fold change versus sham-operated controls and presented as mean±standard error; _n_=6 per group; *_P_⩽0.05, **_P_⩽0.01, ***_P_⩽0.001 versus sham. TGF-β, transforming growth factor β.
In contrast to M1 markers, the messenger RNA expression of M2 markers, including CD206, Arg1, CCL-22, Ym1/2, IL-10, and transforming growth factor-β, was induced beginning 1 to 3 days after TBI and peaked by 3 to 5 days post injury. The majority of M2-type genes began to decrease at 7 days and returned to pre-injury levels by day 14 (Figure 1C). These real-time RT–PCR results support a rapid rise and fall in the M2 phenotype and a sustained rise in the M1 phenotype after mechanical trauma.
Dynamic Changes in Microglia/Macrophages with M1 or M2 Phenotypes
M1 and M2 signature genes are not only expressed in microglia/macrophages but also in other CNS cells or infiltrating immune cells. The real-time PCR data on striatal tissue therefore reflect a mixture of cell types. To specifically evaluate the polarization state of microglia/macrophages after TBI, representative M1-associated or M2-associated marker proteins were double labeled with the microglia/macrophage marker Iba1 in cortex, striatum, and CC near the lesion cavity, and in their corresponding contralateral regions (Figures 2A and 3A). The lack of a specific antibody for either microglia or macrophages precluded the distinction between local microglia and circulating macrophages recruited after injury into the brain. Thus, immunofluorescence for M1 and M2 markers in Iba1+ cells may reflect both microglia and macrophages.
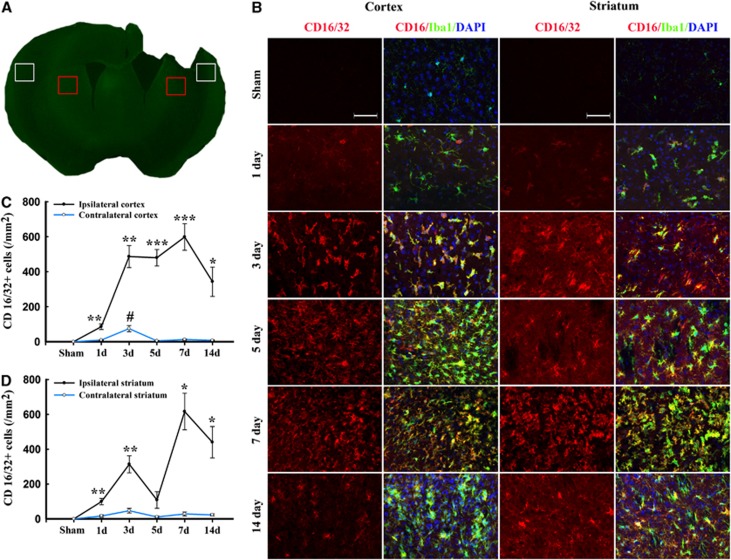
Figure 2.
Temporal changes in microglia/macrophages expressing the M1 phenotype in cortex and striatum after traumatic brain injury (TBI). (A) Iba1 immunostaining in a coronal section of the mouse brain after TBI. The bilateral regions of interest are parietotemporal cortex (white square) and striatum (red square); (B) representative triple staining for CD16/32, Iba1, and 4',6-diamidino-2-phenylindole (DAPI) on brain sections obtained from mice at 1, 3, 5, 7, and 14 days after TBI or sham operation. Scale bar, 50 _μ_m. Time course of bilateral CD16/32 expression in the cortex (C) and striatum (D). _n_=4 to 5 animals per group. *_P_⩽0.05, **_P_⩽0.01, ***_P_⩽0.001 versus ipsilateral side of sham animals; #_P_⩽0.05 versus contralateral side of sham animals.
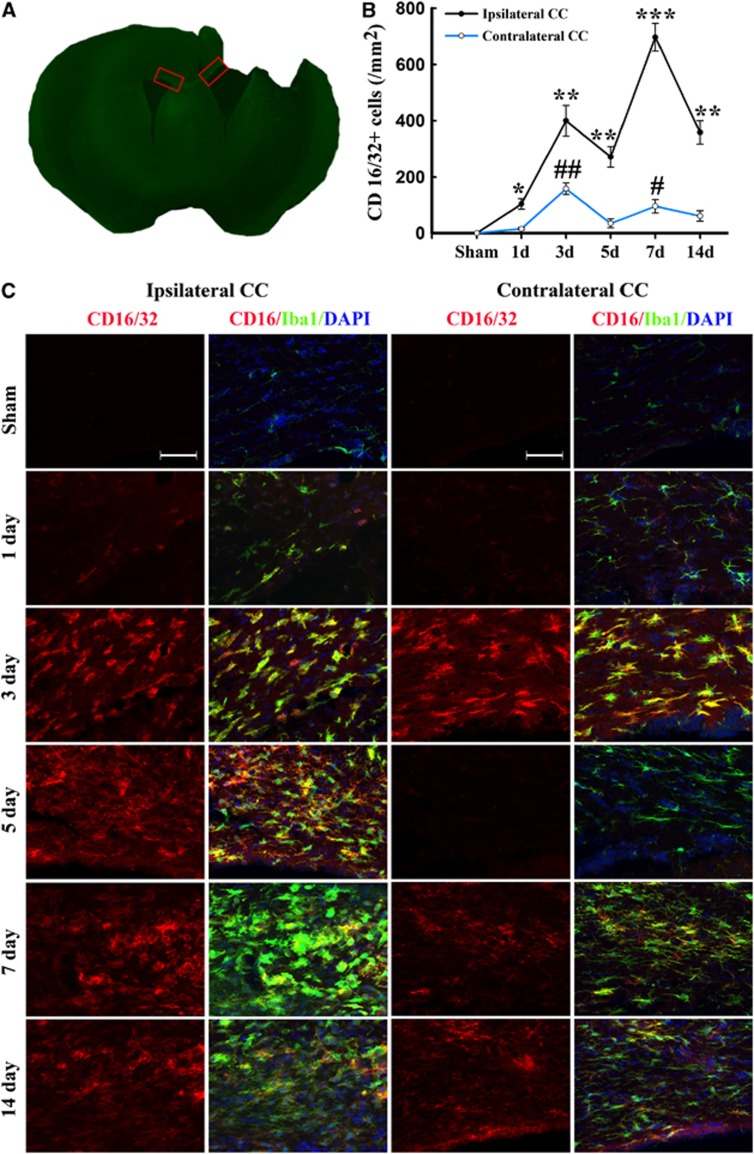
Figure 3.
Temporal changes in microglia/macrophages expressing the M1 phenotype in corpus callosum (CC) after traumatic brain injury (TBI). (A) Iba1 immunostaining in a coronal section of the mouse brain after TBI. The bilateral regions of interest are the CC areas (red square); (B) time course of bilateral CD16/32 expression in the CC; (C) representative triple staining for CD16/32, Iba1, and 4',6-diamidino-2-phenylindole (DAPI) in brain sections obtained from mice at 1, 3, 5, 7, and 14 days after TBI or sham operation. Scale bar, 50 _μ_m. _n_=4 to 5 animals per group. *_P_⩽0.05, **_P_⩽0.01, ***_P_⩽0.001 versus ipsilateral side of sham animals; #_P_⩽0.05 versus contralateral side of sham animals.
Expression of the M1 marker CD16/32 slightly increased in cortex at day 1 after TBI and was significantly raised thereafter until day 14 (Figures 2B and C). This finding was consistent with the real-time PCR data. Interestingly, the number of CD16/32-positive microglia/macrophages in the contralateral cortex 3 days after TBI was higher than in sham-operated mice (Figure 2B). In the CC areas and ipsilateral striatum, CD16/32 expression peaked bimodally at 3 and 7 days after TBI (Figures 2B, 2D and 3B–C). CD16/32-positive cells were co-localized with Iba1, indicating that the majority of CD16/32 expression was associated with microglia/macrophages.
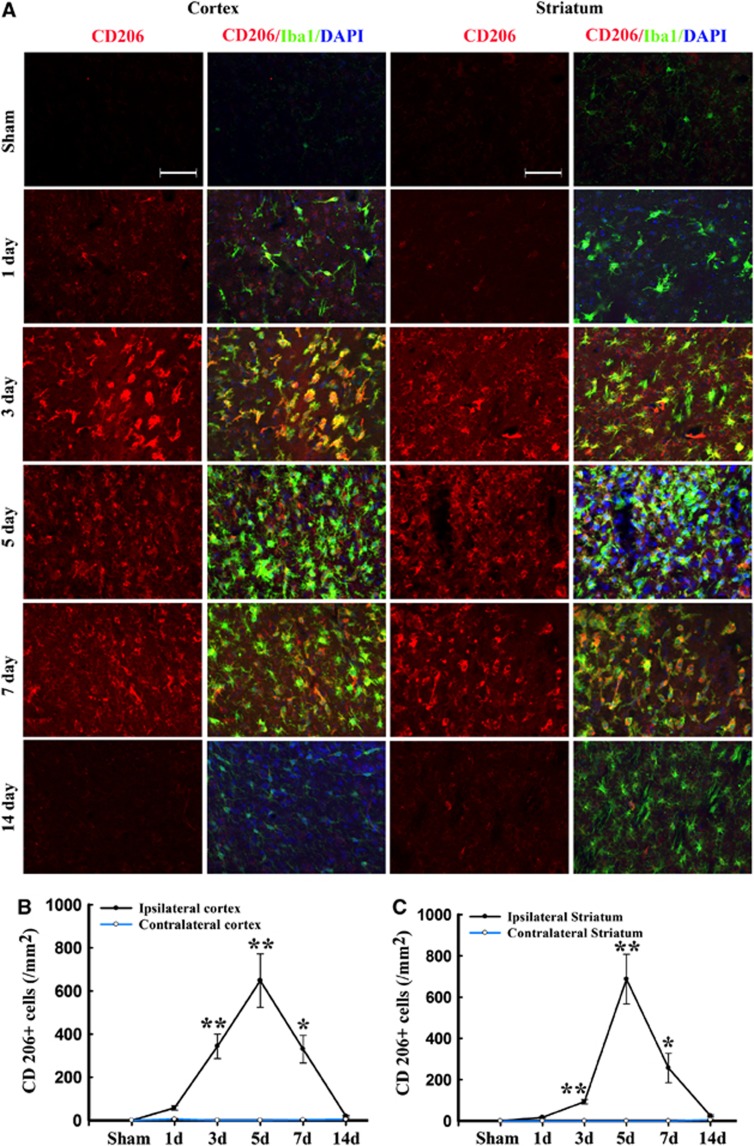
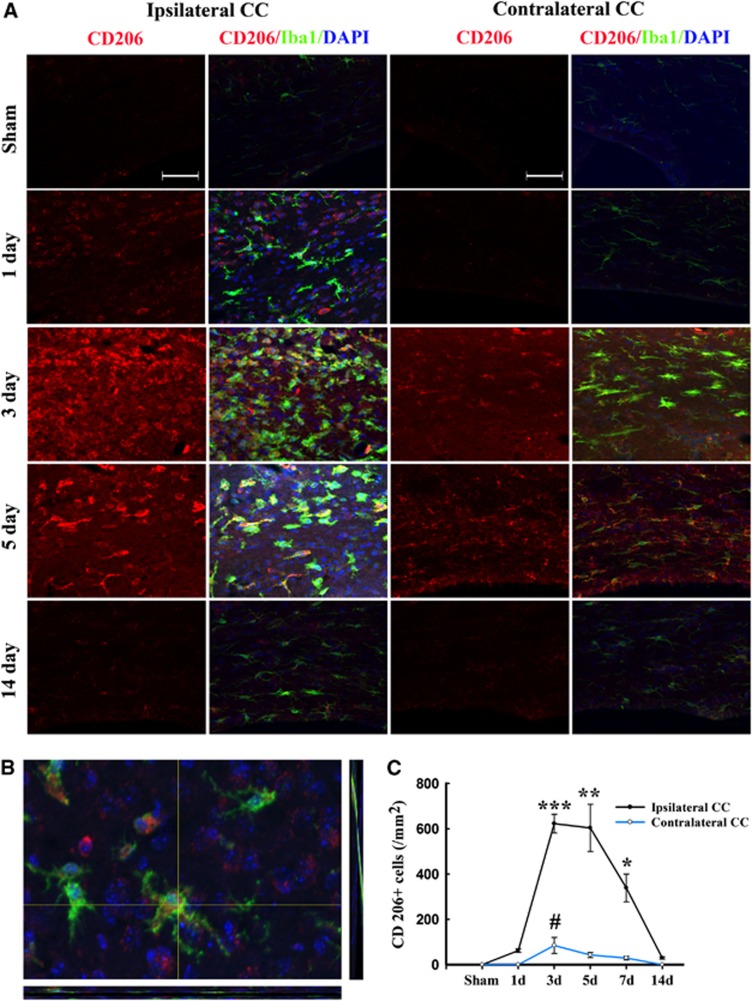
Immunofluorescence for the M2 marker CD206 increased significantly over control levels in the ipsilateral cortex and striatum at 3 days after TBI, peaking on day 5, and then decreasing back to baseline on day 14 (Figure 4). In contrast to the striatal and cortical expression of CD206, the ipsilateral CC area exhibited a peak in CD206 expression from day 3 onwards until day 5, followed by a gradual decrease to control levels (Figures 5A and C). Finally, CD206 was slightly increased after TBI in the contralateral CC, but only on day 3. Taken together, these findings reveal a temporal shift in microglial phenotype from transient M2 to sustained M1 in the 14 days after mechanical trauma.
Figure 4.
Temporal changes in microglia/macrophages expressing the M2 phenotype in cortex and striatum after traumatic brain injury (TBI). (A) Representative triple staining for CD206, Iba1, and 4',6-diamidino-2-phenylindole (DAPI) on brain sections obtained from mice at 1, 3, 5, 7, and 14 days after TBI or sham operation. Scale bar, 50 _μ_m.Time course of bilateral CD206 expression in the cortex (B) and striatum (C). _n_=4 to 5 animals per group. *_P_⩽0.05, **_P_⩽0.01 versus sham.
Figure 5.
Temporal changes in microglia/macrophages expressing the M2 phenotype in corpus callosum (CC) after traumatic brain injury (TBI). (A) Representative triple staining for CD206, Iba1 and 4',6-diamidino-2-phenylindole (DAPI) on sections from mice at 1, 3, 5, and 14 days after TBI or sham operation. Scale bar, 50 _μ_m. (B) The higher magnification photograph shows that CD206-positive cells contain the Iba1 marker. (C) Time course of bilateral CD206 expression in the CC area. _n_=4 to 5 animals per group. *_P_⩽0.05, **P<0.01, ***P<0.001 versus ipsilateral side of sham animals; #_P_⩽0.05 versus contralateral side of sham animals.
White Matter Damage after Traumatic Brain Injury
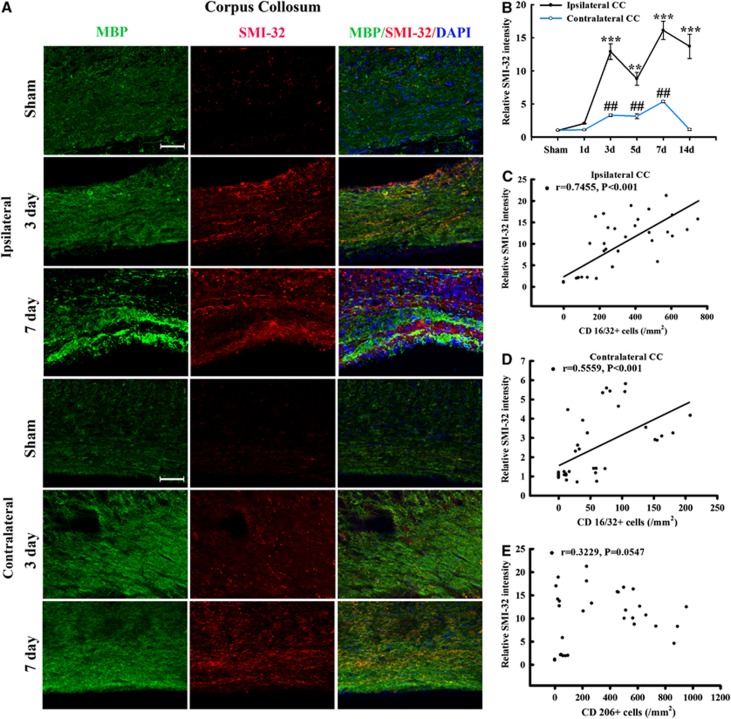
To verify the presence of WMI in this TBI model, we performed co-immunostaining for MBP and non-phosphorylated neurofilament (SMI-32) in the CC area. Neurofilaments in healthy myelinated axons are heavily phosphorylated. Dephosphorylation of a neurofilament H epitope specifically recognized by the SMI-32 antibody, is a known marker of axonal damage.20 As expected, SMI-32 immunoreactivity was rarely seen in normal-appearing white matter from sham-treated brains (Figure 6A). At 3 and 7 days after TBI, abundant SMI-32 immunoreactivity was detected within the CC (Figure 6B). The SMI-32 staining appeared as thin lines and small dots, reflecting the morphology of damaged axons (Figure 6A). We also found that MBP staining decreased early (day 3) after TBI and then increased by day 7, as compared with the contralateral hemisphere. It has been reported previously that the levels of MBP fragments change over time after TBI, with an early reduction (day 1 to 3) followed by dramatic increases by day 5 after TBI.21 Thus our results are largely consistent with these previous findings and suggest that the late increase of MBP may represent an attempt by the brain to compensate for myelin loss from the initial injury. However, the anatomic distribution of MBP immunoreactivity remained irregular and discontinuous through day 7 after TBI (Figure 6A), suggesting ongoing impairments in axon myelination. Thus, the compensatory increases in MBP appear to be insufficient to fully restore the integrity of white matter after TBI.
Figure 6.
Temporal changes in white matter injury after traumatic brain injury (TBI). (A) Representative triple staining for myelin basic protein (MBP), SMI-32, and 4',6-diamidino-2-phenylindole (DAPI) in bilateral corpus callosum (CC) area in mice at 3 and 7 days after TBI or sham operation. Scale bar, 50 _μ_m. (B) Time course of bilateral SMI-32 expression in the CC area. _n_=4 to 5 animals per group. *_P_⩽0.05, **_P_⩽0.01, ***_P_⩽0.001 versus ipsilateral side of sham animals; #_P_⩽0.05 versus contralateral side of sham animals. There was a direct positive correlation between SMI-32 intensity and numbers of CD16/32+ in both ipsilateral (C) and contralateral CC (D) (all _P_⩽0.001) from mice at 1, 3, 5, and 14 days after TBI or sham operation. (E) There was no significant correlation between relative SMI-32 intensity and CD206-positive cells in ipsilateral CC (_P_=0.0547).
Remarkably, there was a direct positive correlation of SMI-32 intensity with the number of CD16/32+cells for all animals in both the ipsilateral and contralateral CC (all P<0.001, Figures 6C and 6D). This correlation suggests that an increase in M1-type microglia/macrophages may underlie trauma-induced WMI. In contrast, SMI-32 intensity was not correlated with CD206-positive cells (_P_>0.05). This suggests that the M2 phenotype is not correlated with WMI after TBI.
Effect of M1 and M2 Microglia on Post-Oxygen Glucose-Deprived Oligodendrocyte Survival
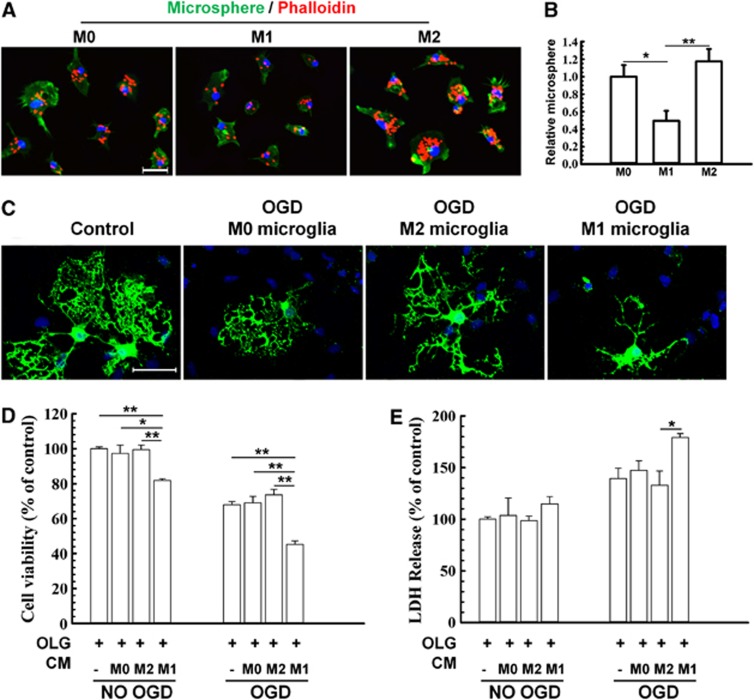
Central nervous system mechanical trauma and ischemic or hemorrhagic stroke share common detrimental features such as reduced blood flow and energy failure, and all three insults directly result in cell death and tissue loss. These common features can be mimicked by exposing cells to hypoxic conditions in glucose-free medium in vitro. The principal cellular target of WMI after trauma is the oligodendrocyte. We therefore used a conditioned medium transfer system to elucidate the effect of microglial phenotype on oligodendrocyte survival under OGD conditions. First we induced M1 or M2 microglia polarization in vitro using lipopolysaccharide (100 ng/mL) with either interferon-gamma (20 ng/mL) or IL-4 (20 ng/mL), respectively, for 48 hours as previously described.16 M1 microglia exhibited less phagocytic activity than M0 (unstimulated microglia), whereas M2 microglia exhibited a 2- to 3-fold greater phagocytic capacity than M1 microglia (Figures 7A and B).
Figure 7.
Effect of M1 and M2 microglia/macrophages on oxygen glucose deprivation (OGD)-induced injury in oligodendrocytes. (A)The M1 or M2 phenotypes were induced in microglial cultures using lipopolysaccharide (100 ng/mL) plus interferon-gamma (20 ng/mL) or IL-4 (20 ng/mL), respectively, for 48 hours. Microglial cells were stained with phalloidin to visualize F-actin. Scale bar, 50 _μ_m. (B) Phagocytotic activity of unstimulated (M0), M1, and M2 microglia. Phagocytosis was quantified by counting the number of phagocytosed beads in each cell. _n_=5 to 6 per group. (C) Myelin basic protein and Hoechst staining of non-OGD or OGD oligodendrocytes co-cultured with conditioned media from microglia of different phenotypes. Scale bar, 40 _μ_m. Conditioned media from microglia of different phenotypes were applied to non-OGD or post-OGD oligodendrocyte cultures for 24 hours. Neuronal survival was quantified by the MTT assay (D) and cell death was quantified by lactate dehydrogenase release (E). _n_=6 per group. All images are representative of three independent experiments. *_P_⩽0.05, **_P_⩽0.01.
Conditioned medium was collected from M1 polarized, M2 polarized, or non-polarized microglia and applied to non-OGD or post-OGD oligodendrocyte cultures (Figures 7C, D, and E). Myelin basic protein fluorescence was relatively stable in these mature oligodendrocytes in control conditions. However, OGD evoked rapid and severe loss of processes in media from unstimulated microglia (Figure 7C). This loss of processes was further exacerbated by M1 media but alleviated by M2 media (Figure 7C). Lactate dehydrogenase release and MTT assays both showed that the addition of M1 microglia-conditioned media reduced oligodendrocyte survival after OGD compared with all other groups (Figures 7D and E).
Discussion
Although WMI is a frequent complication of TBI and an important determinant of cognitive impairment, the underlying pathophysiology remains poorly understood.22 The present study characterizes the dynamic microglia/macrophage response to TBI and its relation to WMI. Microglia/macrophages are the primary mediators of the innate immune response to injury and disease in the CNS.13, 16 The functions of microglia in the injured CNS are under intense scrutiny, and recent research provides strong support for dual microglial roles, both beneficial and destructive, as well as differential activation of these immune cells into one of at least two phenotypes, M1 or M2.13, 18, 23 On one hand, microglia/macrophage activation is thought to benefit the injured brain by removing cellular debris and restoring tissue integrity.24, 25 However, a large number of studies have also shown that microglia release proinflammatory mediators that contribute to neuronal dysfunction and cell death.13, 26, 27 In other words, microglia can either promote delayed cell damage by generating proinflammatory cytokines or participate in regenerative processes by scavenging necrotic tissue.28, 29 Thus, microglial activation in white matter after cerebral trauma may reflect either or both of these processes.30 An improved understanding of the dynamic equilibrium between beneficial and destructive microglia/macrophages may advance our knowledge of post-trauma recovery and help ameliorate WMI in trauma victims.
In the present study, we described the temporal kinetics of microglia/macrophage polarization in both gray and white matter after TBI. It is evident from our findings that the dynamic changes of microglia/macrophages are quite different in white and gray matter. For example, a clear M2–to-M1 transit is apparent in gray matter in response to TBI, whereas the changes in white matter seem to be bimodal. The majority of microglia/macrophages migrating or infiltrating into the lesioned white matter areas assume the M1 phenotype at day 3, as evidenced by the increased expression of CD16/32 protein in Iba+ cells. Subsequently, an alternatively activated subset of microglia/macrophage (M2) occupied the leading position at day 5 after TBI. The present study as well as previous reports16 further indicate that M2 microglia/macrophages are healthier cells with enhanced phagocytic activity and reduced production of inflammatory mediators. Therefore, the recruitment of M2 microglia/macrophages into the cortex, striatum, and CC may represent an endogenous effort to clean injured tissue and restrict brain damage. Previous studies also suggest that expression of M2 microglial factors promote CNS repair while limiting secondary inflammation-mediated injury after spinal cord injury.18 Maintaining the M2 microglia/macrophage phenotype could therefore benefit the injured brain in multiple ways.16 However, the present study reveals that the M2 phagocyte responses in both gray and white matter are transient and phased out within 7 days after injury. During these changes, M1 microglia/macrophages, which are characterized by reduced phagocytosis and increased secretion of proinflammatory mediators, begin to dominate the injured landscape. These M1 phagocytes may exacerbate nerve cell demise through proinflammatory cytokines (tumor necrosis factor-α and IL-1_β_), potent ROS (superoxide radicals(O2−), nitric oxide NO), and reductions in neurotrophic factors.16 Moreover, M1 microglia/macrophages have been shown to impair axon regrowth.31 It is thus conceivable that the M2-to-M1 shift during chronic inflammation after TBI expands WMI and reduces self-restorative abilities. A similar M2-to-M1 shift has already been reported in models of stroke16 and spinal cord injury,31 suggesting that the microglia/macrophage phenotypic changes may be a common pathologic mechanism underlying multiple types of CNS injuries. Thus, preventing the M2-to-M1 transition with microglia-directed therapies may present an attractive opportunity to benefit not only victims of TBI but also other neurologic disorders.
Interestingly, the M1 microglial phenotype was also transiently activated at low levels in the contralateral cortex and CC area. Previous studies have revealed the presence of damage in contralateral brain areas after TBI.32, 33 The direct injury on the ipsilateral hemisphere may stress remote areas through transcallosal or transhemispheric diaschisis,34 resulting in alterations in electrical activity, cerebral blood flow, and metabolites in the contralateral hemisphere. Inflammatory processes are also known to contribute to the contralateral responses after TBI. As part of the neuroimmune response, a cascade of pro- and anti-inflammatory cytokines are released and can be detected at the site of injury as well as contralateral regions after brain injury.35 Our present study further suggests that the M1 microglia/macrophage phenotype is also related to the extent of WMI in the contralateral brain. Further studies of the effect of microglia/macrophage phenotype on white matter alterations in both hemispheres may guide us toward a more complete understanding of TBI and help us develop therapeutic interventions.
Our results indicate that microglia/macrophages respond dynamically to TBI, experiencing a transient M2 phenotype followed by a shift to the M1 phenotype in both white and gray matter. The current study focused on WMI after TBI because the severity of WMI, manifested by immunohistochemical staining for neurofilament SMI-32, was strongly correlated with the number of M1-like phagocytes. In contrast, there was poor correlation between microglia/macrophage dynamics and the number of surviving neurons, as revealed by NeuN staining, in this model (data not shown). Given the importance of WMI to the long-term deficiencies in motor and cognitive functions in TBI patients, our study not only identifies a novel mechanism of WMI after TBI, but also provides a potential target for TBI treatment.
The findings of the present study support the hypothesis that the M1 phenotype exacerbates WMI. First, we found a strong positive correlation between the extent of WMI and activation of M1 (but not M2) microglia on both the ipsilateral and contralateral sides. Second, in vitro experiments showed that M1 cells engage in less phagocytic activity and may therefore be unable to remove cellular debris. Third, M1 microglia-conditioned media exacerbated OGD-induced oligodendrocyte cell death. It remains to be determined whether microglia/macrophage dynamics mediate oligodendrocytic changes in vivo.
As mentioned earlier, neuroinflammation in the CNS predominantly involves microglia and macrophages, and is believed to elicit secondary expansion of injury after trauma.3, 9, 13 As a result, there is significant interest in developing novel anti-inflammatory agents to ameliorate or even prevent CNS inflammation.9, 36 However, classic anti-inflammatory agents such as steroids and nonsteroidal anti-inflammatory drugs have not had a major role in the management of CNS inflammatory conditions.36 Among the potential factors that might have prevented their use in inflammatory conditions is that broad suppression of microglia/macrophages may deprive the brain of their normal phagocytic roles and cause the buildup of cellular debris. This notion is supported by observations that selective depletion of proliferative microglia exacerbate brain injuries24, 37 and, conversely, that injections of exogenous microglia into the brain ameliorate CNS injuries.38, 39 Many authors have concluded that acute inflammation serves several protective functions,16 whereas chronic inflammation exacerbates injury.5, 6 In keeping with this traditional view of chronic inflammation, the results of our study demonstrate that microglia/macrophages experience a sustained rise in the destructive M1 phenotype within 1 week after TBI.
It is important to note here that mononuclear phagocytes exhibit extensive heterogeneity and plasticity in response to different physiologic and pathologic stimuli. Indeed, there are likely to be additional activation states, as well as transitional stages between M1 and M2.40, 41 Microglia/macrophages present during TBI are therefore not just comprising 1 or 2 discrete subpopulations but may include a spectrum of diverse activation states. The concept of M1 and M2 microglia/macrophage polarization is a simplified framework that represents two extremes along a continuum.41 This framework is nevertheless useful to help understand the different functional status of phagocytes during injury progression and to explore therapeutic strategies targeting microglia/macrophage responses. One caveat to using signature markers, even in combination, to characterize phagocyte polarization is that many established M1 and M2 markers are not specific for phagocytes or for the polarization processes. To this end, further analyses of microglia/macrophage functions, including migration, phagocytosis, and protein production, are warranted to conclusively define the functional states of these cells in different disease stages. Furthermore, whether microglia and macrophages have similar activation patterns in humans is still under debate. For example, some in vitro studies have suggested that human microglia develop polarization responses that are distinct from macrophages and show a tendency to maintain M2 properties under stimulation.42 Validation of these observations in vivo in TBI is, however, difficult at the present time because of the paucity of specific markers to distinguish microglia from macrophages.
In conclusion, a comprehensive analysis of white matter changes in the mouse brain after TBI suggests that mechanical trauma induces both acute and chronic local inflammatory changes in microglia/macrophages. The transient M2 response is replaced by a chronic M1 response and the M1 response is correlated with the extent of WMI. Our results suggest that the goals of anti-inflammatory therapy for TBI should be shifted from blanketed microglia/macrophage suppression towards a more specific titration of the inflammatory response away from the destructive M1 phenotype. This more nuanced approach may avoid disruptions in beneficial phagocyte responses while reducing the secondary expansion of gray and WMI in trauma victims.
The authors declare no conflict of interest.
Footnotes
This research is supported in part by the Special Research Funds from Chinese Ministry of Science and Technology to State Key laboratories (to GH Wang, Y Gao and J Chen), National Institutes of Health/NINDS grants NS36736, NS43802 and NS45048 (to J Chen), VA Career Scientist Award (J Chen), and Chinese Natural Science Foundation grants No 30870794, No 81020108021, No 81171149, and No 81150110494 (to YG), and No 81000497 (to GW).
References
- Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- Fork M, Bartels C, Ebert AD, Grubich C, Synowitz H, Wallesch CW. Neuropsychological sequelae of diffuse traumatic brain injury. Brain Inj. 2005;19:101–108. doi: 10.1080/02699050410001726086. [DOI] [PubMed] [Google Scholar]
- Stoica BA, Faden AI. Cell death mechanisms and modulation in traumatic brain injury. Neurotherapeutics. 2010;7:3–12. doi: 10.1016/j.nurt.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umschweif G, Alexandrovich AG, Trembovler V, Horowitz M, Shohami E. Hypoxia-inducible factor 1 is essential for spontaneous recovery from traumatic brain injury and is a key mediator of heat acclimation induced neuroprotection. J Cereb Blood Flow Metab. 2013;33:524–531. doi: 10.1038/jcbfm.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen KM, Greenwood R, Powell JH, Leech R, Hawkins PC, Bonnelle V, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134:449–463. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130:2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JV, Maas AI, Bragge P, Morganti-Kossmann MC, Manley GT, Gruen RL. Early management of severe traumatic brain injury. Lancet. 2012;380:1088–1098. doi: 10.1016/S0140-6736(12)60864-2. [DOI] [PubMed] [Google Scholar]
- Wang GH, Zhang XG, Jiang ZL, Li X, Peng LL, Li YC, et al. Neuroprotective effects of hyperbaric oxygen treatment on traumatic brain injury in the rat. J Neurotrauma. 2010;27:1733–1743. doi: 10.1089/neu.2009.1175. [DOI] [PubMed] [Google Scholar]
- Wang GH, Jiang ZL, Li YC, Li X, Shi H, Gao YQ, et al. Free-radical scavenger edaravone treatment confers neuroprotection against traumatic brain injury in rats. J Neurotrauma. 2011;28:2123–2134. doi: 10.1089/neu.2011.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgens RB, Liu-Snyder P. Understanding secondary injury. Q Rev Biol. 2012;87:89–127. doi: 10.1086/665457. [DOI] [PubMed] [Google Scholar]
- Wang G, Jiang X, Pu H, Zhang W, An C, Hu X, et al. Scriptaid, a novel histone deacetylase inhibitor, protects against traumatic brain injury via modulation of pten and AKT pathway: scriptaid protects against TBI via AKT. Neurotherapeutics. 2013;10:124–142. doi: 10.1007/s13311-012-0157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan C, Chrzaszcz M, Choi N, Wainwright MS. Chronic upregulation of activated microglia immunoreactive for galectin-3/Mac-2 and nerve growth factor following diffuse axonal injury. J Neuroinflammation. 2010;7:32. doi: 10.1186/1742-2094-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7:366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Ishii H, Bai Z, Itokazu T, Yamashita T. Temporal changes in cell marker expression and cellular infiltration in a controlled cortical impact model in adult male C57BL/6 mice. PLoS One. 2012;7:e41892. doi: 10.1371/journal.pone.0041892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- Mikita J, Dubourdieu-Cassagno N, Deloire MS, Vekris A, Biran M, Raffard G, et al. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult Scler. 2011;17:2–15. doi: 10.1177/1352458510379243. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassmann CM, Quintes S, Rietdorf J, Mobius W, Sereda MW, Nientiedt T, et al. A role for myelin-associated peroxisomes in maintaining paranodal loops and axonal integrity. FEBS Lett. 2011;585:2205–2211. doi: 10.1016/j.febslet.2011.05.032. [DOI] [PubMed] [Google Scholar]
- Lovas G, Szilagyi N, Majtenyi K, Palkovits M, Komoly S. Axonal changes in chronic demyelinated cervical spinal cord plaques. Brain. 2000;123:308–317. doi: 10.1093/brain/123.2.308. [DOI] [PubMed] [Google Scholar]
- Liu MC, Akle V, Zheng W, Kitlen J, O'Steen B, Larner SF, et al. Extensive degradation of myelin basic protein isoforms by calpain following traumatic brain injury. J Neurochem. 2006;98:700–712. doi: 10.1111/j.1471-4159.2006.03882.x. [DOI] [PubMed] [Google Scholar]
- Hellyer PJ, Leech R, Ham TE, Bonnelle V, Sharp DJ. Individual prediction of white matter injury following traumatic brain injury. Ann Neurol. 2013;73:489–499. doi: 10.1002/ana.23824. [DOI] [PubMed] [Google Scholar]
- Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics. 2010;7:354–365. doi: 10.1016/j.nurt.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Das A, Ray SK, Banik NL. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2012;87:10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polazzi E, Monti B. Microglia and neuroprotection: from in vitro studies to therapeutic applications. Prog Neurobiol. 2010;92:293–315. doi: 10.1016/j.pneurobio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- Wieloch T, Nikolich K. Mechanisms of neural plasticity following brain injury. Curr Opin Neurobiol. 2006;16:258–264. doi: 10.1016/j.conb.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Shitaka Y, Tran HT, Bennett RE, Sanchez L, Levy MA, Dikranian K, et al. Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J Neuropathol Exp Neurol. 2011;70:551–567. doi: 10.1097/NEN.0b013e31821f891f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchkremer-Ratzmann I, August M, Hagemann G, Witte OW.Electrophysiological transcortical diaschisis after cortical photothrombosis in rat brain Stroke 1996271105–1109.discussion 1109-1111. [DOI] [PubMed] [Google Scholar]
- Patterson ZR, Holahan MR. Understanding the neuroinflammatory response following concussion to develop treatment strategies. Front Cell Neurosci. 2012;6:58. doi: 10.3389/fncel.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo AJ, Vink R. Recent patents in CNS drug discovery: the management of inflammation in the central nervous system. Recent Pat CNS Drug Discov. 2009;4:86–95. doi: 10.2174/157488909788452997. [DOI] [PubMed] [Google Scholar]
- Faustino JV, Wang X, Johnson CE, Klibanov A, Derugin N, Wendland MF, et al. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. J Neurosci. 2011;31:12992–13001. doi: 10.1523/JNEUROSCI.2102-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai F, Suzuki H, Oda J, Ninomiya T, Ono K, Sano H, et al. Neuroprotective effect of exogenous microglia in global brain ischemia. J Cereb Blood Flow Metab. 2007;27:488–500. doi: 10.1038/sj.jcbfm.9600362. [DOI] [PubMed] [Google Scholar]
- Girard S, Brough D, Lopez-Castejon G, Giles J, Rothwell NJ, Allan SM. Microglia and macrophages differentially modulate cell death after brain injury caused by oxygen-glucose deprivation in organotypic brain slices. Glia. 2013;61:813–824. doi: 10.1002/glia.22478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boche D, Perry VH, Nicoll JA. Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol. 2013;39:3–18. doi: 10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, et al. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717–727. doi: 10.1002/glia.22298. [DOI] [PubMed] [Google Scholar]