Nocturnal Continuous Glucose and Sleep Stage Data in Adults with Type 1 Diabetes in Real-World Conditions (original) (raw)
Abstract
Background
Sleep plays an important role in health, and poor sleep is associated with negative impacts on diabetes management, but few studies have objectively evaluated sleep in adults with type 1 diabetes mellitus (T1DM). Nocturnal glycemia and sleep characteristics in T1DM were evaluated using body-worn sensors in real-world conditions.
Methods
Analyses were performed on data collected by the Diabetes Management Integrated Technology Research Initiative pilot study of 17 T1DM subjects: 10 male, 7 female; age 19–61 years; T1DM duration 14.9 ± 11.0 years; hemoglobin A1c (HbA1c) 7.3% ± 1.3% (mean ± standard deviation). Each subject was equipped with a continuous glucose monitor and a wireless sleep monitor (WSM) for four nights. Sleep stages [rapid eye movement (REM), light, and deep sleep] were continuously recorded by the WSM. Nocturnal glycemia (mg/dl) was evaluated as hypoglycemia (<50 mg/dl), low (50–69 mg/dl), euglycemia (70–120 mg/dl), high (121–250 mg/dl), and hyperglycemia (>250 mg/dl) and by several indices of glycemic variability. Glycemia was analyzed within each sleep stage.
Results
Subjects slept 358 ± 48 min per night, with 85 ± 27 min in REM sleep, 207 ± 42 min in light sleep, and 66 ± 30 min in deep sleep (mean ±standard deviation). Increased time in deep sleep was associated with lower HbA1c (R2 = 0.42; F = 9.37; p < .01). Nocturnal glycemia varied widely between and within subjects. Glycemia during REM sleep was hypoglycemia 5.5% ± 18.1%, low 6.6% ± 18.5%, euglycemia 44.6% ± 39.5%, high 37.9% ± 39.7%, and hyperglycemia 5.5% ± 21.2%; glycemia during light sleep was hypoglycemia 4.8% ± 12.4%, low 7.3% ± 12.9%, euglycemia 42.1% ± 33.7%, high 39.2% ± 34.6%, and hyperglycemia 6.5% ± 20.5%; and glycemia during deep sleep was hypoglycemia 0.5% ± 2.2%, low 5.8% ± 14.3%, euglycemia 48.0% ± 37.5%, high 39.5% ± 37.6%, and hyperglycemia 6.2% ± 19.5%. Significantly less time was spent in the hypoglycemic range during deep sleep compared with light sleep (p = .02).
Conclusions
Increased time in deep sleep was associated with lower HbA1c, and less hypoglycemia occurred in deep sleep in T1DM, though this must be further evaluated in larger subsequent studies. Furthermore, the consumer-grade WSM device was useful for objectively studying sleep in a real-world setting.
J Diabetes Sci Technol 2013;7(5):1337–1345
Keywords: hypoglycemia, real-world monitoring, sleep, type 1 diabetes mellitus
Introduction
Sleep plays an important role in human health, with implications for memory1 and cognitive function,2 depression and anxiety, 3 abuse of alcohol4 and drugs,5 cardiovascular outcomes,6 and more. A meta-analysis showed that people who neither oversleep nor undersleep have the lowest mortality risk.7 Laboratory and epidemiologic evidence suggests that sleep loss and poor sleep quality may promote the development of obesity and diabetes and exacerbate these conditions.8,9
In the face of the many other challenges faced by people with type 1 diabetes mellitus (T1DM), sleep health is not often considered in the course of an individual’s diabetes management and care. The research community has only just begun to explore sleep characteristics in adults with T1DM. Controlled studies with small sample sizes showed that adults with T1DM have altered neuroendocrine sleep architecture10 and that insulin sensitivity is affected by sleep deprivation.11 A larger subjective study reported that adults with long-standing T1DM have disturbed sleep quality and a higher risk for obstructive sleep apnea, while finding no significant association between individual sleep characteristics and hemoglobin A1c (HbA1c) values.12 Many individuals in this study reported frequent sleep disturbance from hypoglycemia.
Hypoglycemia during sleep represents a great health risk to people with T1DM.13 As reviewed by Jauch-Chara and Schultes,14 nocturnal hypoglycemia is common but often undetected. In fact, people with T1DM frequently fail to wake up during nocturnal hypoglycemia, which in some cases leads to coma and even death. Frequently asymptomatic, nocturnal hypoglycemia can impair wellbeing on the subsequent day and negatively impact awareness of and response to subsequent hypoglycemic episodes.
To date, only a few studies have explored T1DM nocturnal glycemia during sleep.10,15–17 As most sleep research is conducted in a controlled laboratory setting, the expense of collecting objective sleep data from patients with diabetes has constrained large-scale studies in this domain, thus limiting the application of sleep health knowledge in diabetes care. The advent of ubiquitous body-worn sensing technologies has contributed to the availability of affordable, user-friendly wireless sleep monitors (WSMs) that can be employed to collect objective sleep data comparable to polysomnography, the gold standard of sleep recording.18–20 These new WSM devices may provide a means to expand our knowledge about real-world sleep characteristics in the broader T1DM population.
In this study, we analyzed nocturnal glycemia and sleep stages in patients with T1DM in an unsupervised real-world setting. Our results reveal new knowledge about the relationship between glycemia and sleep stages and provide evidence for the feasibility of collecting objective sleep data outside the controlled laboratory environment.
Methods
Data were collected as part of the Diabetes Management Integrated Technology Research Initiative (DMITRI) pilot study, a community research partnership between the University of California, San Diego, and Insulindependence.21 The DMITRI pilot study was exploratory in nature and not powered to evaluate specific outcomes. The study included 17 subjects with T1DM: 10 males and 7 females; ages 19–61 years; T1DM duration 14.9 ± 11.0 years; HbA1c 7.3% ± 1.3% (mean ± standard deviation). Trait daytime sleepiness was assessed by the Epworth sleepiness scale (ESS), a validated questionnaire to assess daytime sleepiness.22 Subjects were free to roam the university campus and surrounding area over a study period of four days and slept for four nights in unsupervised dormitories with shared bedrooms.
The study employed continuous glucose monitors (CGMs; SEVEN PLUS, Dexcom Inc., San Diego, CA), worn by each subject at all times, and consumer-grade WSMs (Bedside Sleep Manager, Zeo Inc., Newton, MA), worn from bedtime until morning waking. The WSM device consists of a lightweight sensor headband that continuously records electroencephalogram (EEG) data that are transmitted wirelessly to a bedside receiver unit, where an algorithm processes the data and provides a sleep stage value for each 30 s interval. These time-stamped values are stored on a memory card in the receiver for subsequent retrieval and analysis.20 Sleep is a highly structured set of processes separated into four stages [N1, N2, N3, and rapid eye movement (REM) sleep],23 each demonstrating stereotypic electrical and neuromodulatory activity in the brain.24 The WSM algorithm employed in the present study categorizes the EEG data into one of three sleep stages, namely, REM, light (i.e., N1 and N2), and deep (i.e., N3).
Following the study period, data were retrieved from each device for preprocessing and analysis (R, Matlab, Excel). The WSM data were collected for a total of 52 subject-nights. Because the WSM sampled data at a higher rate than the CGM (two samples per minute versus one sample per 5 min), WSM data were binned into 5 min intervals synchronized with the CGM data, and the mode sleep stage for each interval was calculated to generate a comparable epoch length for subsequent analysis. Sleep start and end times were determined based on WSM data, as the devices automatically start and stop collecting data when the headband is attached to or removed from the head, and times of sleep onset and waking are evident in the data. A subset of subjects (n = 10) wore an additional monitoring device (Actiwatch-64, Philips-Respironics, Andover, MA) that was used only to determine sleep start and end times for nocturnal glycemia analyses (as in Tables 1 and 2) in the absence of reliable WSM data (user error or device malfunction). Subject-nights without WSM data were excluded from any analyses of sleep characteristics (as in Table 3 and Figure 1). For analysis of glycemia during sleep stages, WSM data from each subject-night were normalized to correct for differences in duration of each sleep stage [specifically, for each subject-night, duration in each sleep stage was converted to percentage of total sleep time (TST) to enable comparison of multiple subject-nights regardless of TST]. Differences in glycemic range per sleep stage were assessed with Wilcoxon rank-sum test.
Table 1.
Percentage of Nocturnal Time Spent in Glycemic Rangesa_a_
| Subject | Nights | Hypoglycemia, % | Low, % | Euglycemia, % | High, % | Hyperglycemia, % | H bA1c |
|---|---|---|---|---|---|---|---|
| 101 | 4 | 4.2 (8.3) | 6.6 (11.0) | 49.0 (14.9) | 40.2 (29.1) | 0.0 | 6.2 |
| 102 | 2 | 0.0 | 0.0 | 79.7 (27.0) | 20.3 (27.0) | 0.0 | 7.1 |
| 103 | 2 | 2.4 (1.3) | 16.7 (4.7) | 49.0 (31.7) | 31.9 (35.0) | 0.0 | 6.6 |
| 104 | — | — | — | — | — | — | 7.2 |
| 105 | 3 | 0.0 | 0.0 | 58.6 (50.9) | 41.4 (50.9) | 0.0 | 5.6 |
| 106 | 3 | 4.1 (7.1) | 6.6 (7.9) | 15.7 (17.0) | 46.8 (28.3) | 26.8 (26.7) | 6.8 |
| 107 | 3 | 7.8 (13.5) | 6.5 (11.2) | 40.1 (30.3) | 45.6 (47.5) | 0.0 | 6.3 |
| 108 | 4 | 1.9 (2.2) | 6.7 (8.0) | 51.0 (37.1) | 38.0 (37.9) | 2.5 (2.9) | 7.5 |
| 109 | 2 | 0.0 | 0.0 | 5.5 (7.7) | 44.5 (63.0) | 50.0 (70.7) | 8.2 |
| 111 | — | — | — | — | — | — | 6.1 |
| 112 | 1 | 0.0 | 0.0 | 0.0 | 0.0 | 100 | 8.5 |
| 113 | 3 | 0.0 | 0.9 (1.5) | 43.6 (51.2) | 55.6 (50.9) | 0.0 | 7.6 |
| 114 | — | — | — | — | — | — | 7.3 |
| 115 | 4 | 0.0 | 0.0 | 63.4 (42.4) | 36.6 (42.4) | 0.0 | 6.4 |
| 116 | 4 | 17.2 (34.3) | 0.6 (1.2) | 21.9 (15.4) | 51.2 (29.2) | 9.1 (18.3) | 10.9 |
| 117 | 4 | 0.6 (1.3) | 18.4 (15.6) | 44.7 (37.8) | 25.6 (26.6) | 10.6 (20.4) | 8.9 |
| 118 | 4 | 0.0 | 0.9 (1.9) | 54.6 (23.4) | 44.4 (22.0) | 0.0 | 7 |
| Average | 3.2 (11.2) | 4.8 (8.8) | 43.7 (33.2) | 39.7 (33.0) | 8.6 (23.6) | 7.3 (1.3) |
Table 2.
Nocturnal Glycemic Variabilitya_a_
| Subject | Nights | Mean_b_ | Standard deviation_b_ | Mean amplitude of glucose excursions_b_ | Mean absolute glucose_b_ | J index | Low blood glucose index | High blood glucose index | Glycemic risk assessment in diabetes equation | M value |
|---|---|---|---|---|---|---|---|---|---|---|
| 101 | 4 | 113.5 (25.0) | 22.7 (7.8) | 30.7 (35.8) | 67.6 (42.1) | 19.1 (7.5) | 3.2 (4.4) | 1.5 (1.5) | 2.4 (1.8) | 6.0 (9.9) |
| 102 | 2 | 114.0 (9.8) | 16.3 (15.4) | 9.5 (13.4) | 22.4 (3.8) | 17.3 (6.6) | 0.5 (0.4) | 1.4 (1.8) | 1.1 (0.2) | 0.6 (0.6) |
| 103 | 2 | 107.3 (23.5) | 34.9 (14.8) | 55.3 (78.2) | 75.4 (29.0) | 20.9 (10.9) | 7.2 (2.5) | 2.7 (2.0) | 2.8 (3.1) | 9.6 (0.2) |
| 105 | 3 | 117.4 (26.2) | 13.9 (2.4) | 18.8 (16.3) | 46.0 (27.9) | 17.6 (6.7) | 0.8 (0.8) | 1.4 (1.1) | 2.1 (2.9) | 1.1 (0.4) |
| 106 | 3 | 173.6 (47.0) | 64.9 (24.6) | 0.0 | 77.4 (14.0) | 59.8 (30.0) | 8.1 (8.8) | 13.7 (9.8) | 11.4 (8.3) | 22.0 (16.7) |
| 107 | 3 | 116.7 (45.9) | 22.3 (1.8) | 38.5 (4.4) | 51.7 (23.1) | 20.8 (13.4) | 4.9 (6.0) | 2.2 (3.0) | 3.9 (4.7) | 11.4 (15.7) |
| 108 | 4 | 131.6 (58.1) | 32.5 (20.2) | 37.3 (26.5) | 86.5 (28.0) | 29.5 (21.4) | 3.6 (3.4) | 7.4 (7.8) | 4.5 (7.4) | 11.0 (7.5) |
| 109 | 2 | 215.5 (93.8) | 18.7 (7.7) | 0.0 | 29.5 (8.2) | 58.4 (40.2) | 0.7 (1.0) | 16.7 (18.3) | 14.2 (11.5) | 26.5 (34.7) |
| 112 | 1 | 363.0 | 34.8 | 0.0 | 105.4 | 157.9 | 0.0 | 48.5 | 30.3 | 111.9 |
| 113 | 3 | 137.1 (41.3) | 18.0 (11.7) | 14.3 (12.4) | 29.0 (8.5) | 25.1 (12.4) | 1.8 (2.3) | 3.6 (4.3) | 5.6 (5.1) | 3.3 (2.9) |
| 115 | 4 | 125.6 (29.8) | 15.0 (5.0) | 13.3 (19.9) | 40.4 (13.4) | 20.6 (10.5) | 0.4 (0.4) | 1.9 (2.9) | 3.3 (4.2) | 1.5 (2.0) |
| 116 | 4 | 139.8 (66.7) | 42.3 (22.1) | 33.6 (42.7) | 59.9 (28.2) | 38.3 (34.8) | 9.3 (15.1) | 7.3 (9.4) | 16.5 (18.7) | 28.9 (37.3) |
| 117 | 4 | 129.0 (74.9) | 41.9 (38.4) | 2.9 (5.8) | 32.3 (18.5) | 38.3 (44.4) | 5.9 (4.9) | 7.9 (10.2) | 7.0 (7.2) | 18.3 (13.8) |
| 118 | 4 | 120.8 (11.3) | 19.8 (10.2) | 29.4 (27.1) | 49.2 (20.2) | 20.0 (5.1) | 1.3 (1.7) | 1.6 (1.2) | 2.3 (1.6) | 1.1 (1.2) |
| Average | 142.6 (67.3) | 28.7 (20.5) | 21.2 (27.6) | 55.0 (29.7) | 35.0 (35.7) | 3.6 (5.9) | 7.1 (11.6) | 7.0 (9.5) | 15.4 (28.8) |
Table 3.
Percentage of Sleep in Stagea_a_
| Subject | Nights | TST, min | REM, % | Light, % | Deep, % | ESS |
|---|---|---|---|---|---|---|
| 101 | 3 | 316 (15) | 20.1 (4.2) | 57.8 (2.6) | 22.3 (6.9) | 9 |
| 102 | 1 | 384.0 | 13.3 | 68.0 | 18.8 | 7 |
| 103 | 4 | 351 (42) | 30.6 (4.6) | 41.0 (4.1) | 28.7 (6.5) | 5 |
| 104 | 4 | 364 (16) | 33.4 (7.6) | 54.9 (6.0) | 11.8 (2.4) | 5 |
| 105 | 2 | 373 (23) | 19.9 (3.5) | 66.0 (7.4) | 14.3 (3.9) | 14 |
| 106 | 3 | 418 (41) | 24.6 (4.0) | 49.5 (5.6) | 26.0 (3.6) | 5 |
| 107 | 4 | 385 (27) | 21.0 (2.5) | 54.4 (3.7) | 24.6 (2.0) | 9 |
| 108 | 4 | 351 (66) | 15.7 (8.8) | 63.1 (8.9) | 21.5 (3.3) | 2 |
| 109 | 2 | 365 (23) | 24.8 (4.2) | 63.1 (5.0) | 12.2 (0.6) | 7 |
| 111 | — | — | — | — | — | 8 |
| 112 | — | — | — | — | — | 8 |
| 113 | 4 | 333 (18) | 24.8 (3.1) | 66.0 (2.3) | 9.4 (1.1) | 4 |
| 114 | 2 | 376 (21) | 16.9 (1.5) | 62.2 (3.3) | 20.9 (4.8) | 9 |
| 115 | 4 | 319 (45) | 21.4 (6.3) | 56.3 (9.7) | 22.3 (5.1) | 18 |
| 116 | 4 | 370 (75) | 26.0 (3.0) | 68.3 (2.8) | 5.8 (0.6) | 12 |
| 117 | 3 | 318 (83) | 19.8 (4.0) | 65.6 (3.0) | 14.9 (4.4) | 10 |
| 118 | 4 | 381 (48) | 29.1 (3.3) | 49.7 (4.3) | 21.3 (2.6) | 10 |
| Average | 358 (48) | 23.7 (6.8) | 58.0 (9.3) | 18.4 (7.6) | 8.4 (3.9) |
Figure 1.
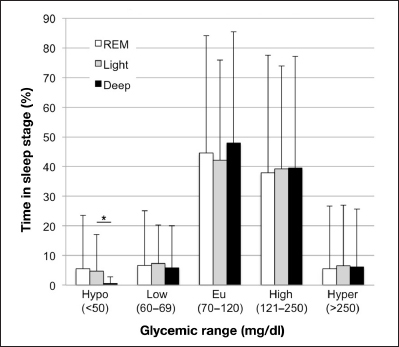
Glycemia during sleep stages. The percentage of time spent in the various glycemic ranges (hypoglycemia, low, euglycemia, high, hyperglycemia) was calculated for each sleep stage (REM, light, deep). Analysis was performed on 39 subject-nights with ≥4 h of WSM data and corresponding CGM data, with sleep start and end times determined by WSM. *Significantly less time is spent in hypoglycemia (<50 mg/dl) during deep sleep compared with light sleep (p = .02, Wilcoxon rank-sum test).
Results
Characterization of Nocturnal Glycemia
We calculated the nocturnal time spent in different ranges of glycemia, designated as hypoglycemia (<50 mg/dl), low (50–69 mg/dl), euglycemia (70–120 mg/dl), high (121–250 mg/dl), and hyperglycemia (>250 mg/dl). Calculations were performed on 43 subject-nights with ≥48 CGM data points (i.e., ≥4 h). As shown in Table 1, T1DM nocturnal dysglycemia observed here differs from the tightly controlled nocturnal glycemia measured in healthy adults in other studies.10,25 Substantial variability was observed between individuals’ nocturnal glycemia. Marked intraindividual variability was observed from night to night, in agreement with previous findings.26 Nocturnal hypoglycemia (<50 mg/dl) was observed for seven subjects.
We further characterized nocturnal glycemia using different measures of glycemic variability (GV).27–29 Nocturnal CGM data were analyzed with previously developed software to compute GV for each subject-night.29 As shown in Table 2 , we observed substantially increased nocturnal GV in the study cohort as compared with reference values in healthy individuals.29 These findings are in agreement with other observations of increased nocturnal GV in professional cyclists with T1DM as compared with their nondiabetic counterparts.30 Substantial variability was observed between individuals, and marked intraindividual variability was observed from night to night.
Characterization of Sleep
Subjects in this study had higher levels of trait daytime sleepiness (8.4 ± 3.9, mean ± standard deviation) than previously reported for T1DM (5.9 ± 4.0; p < . 0 2),12 with five subjects scoring ≥10 on the ESS, usually interpreted as hypersomnolence (excessive sleepiness). From 48 subject-nights with ≥4 h of WSM data, we calculated the subjects’ average TST to be slightly less than 6 h (Table 3), less than the 7.2 h previously self-reported for people with T1DM12 and less than the United States national average of approximately 7 h in adults over the age of 19 years.31
To characterize the time spent in different sleep stages in T1DM, we calculated the percentage of time in REM, light, and deep sleep for each subject-night. Table 3 shows sleep stage values for each subject and the cohort average. Rapid eye movement sleep accounted for 23.7% of TST, and light and deep sleep accounted for 58.0% and 18.4% of sleep time, respectively. These sleep stage proportions are similar to those reported in a study in nondiabetic adults with the same WSM employed in our study (REM, 24.1%; light, 60.6%; deep, 15.3%),20 but different from previous findings for REM and light sleep in adults with T1DM (REM, 13.9%; light, 69.8%; deep 14.7%) using polysomnography instrumentation.10
Notably, we observed a negative correlation between time spent in deep sleep and HbA1c (_R_2 = 0.42; F = 9.37; p < .01), indicating that increased time in deep sleep is associated with lower HbA1c. This is in agreement with previous findings suggesting a role for deep sleep in glycemic control.32–34 No significant relationships were observed between HbA1c or ESS and any other sleep traits in Table 3.
Glycemia during Different Sleep Stages
We assessed glycemia as it occurs within each sleep stage, calculating the time spent in each glycemic range during REM, light, and deep sleep for 39 subject-nights with ≥4 h of WSM data and corresponding CGM data (missing CGM data ≤15 min were linearly interpolated). As shown in Figure 1, while glycemia appears to be similar between sleep stages, we observed significantly less time spent in hypoglycemia during deep sleep compared with light sleep (p = .02, Wilcoxon rank-sum test). This trend is intriguing in light of our observation that increased time in deep sleep is related to lower HbA1c, as it suggests that decreased HbA1c is not due simply to hypoglycemia in deep sleep. The apparent difference in hypoglycemia between deep and REM sleep is not statistically significant. With respect to the other glycemic ranges, most (38–48%) REM, light, and deep sleep was characterized by euglycemia and high glycemia, and much less (5.5–7.4%) of each sleep stage was spent in low glycemia and hyperglycemia (Figure 1), with no significant differences in glycemic range between sleep stages.
Discussion
We report decreased time spent in hypoglycemia during deep sleep relative to light sleep in T1DM and an association between increased deep sleep and lower HbA1c. These results suggest associations between glycemia and sleep stage that should be considered in diabetes care to reduce the risk of nocturnal hypoglycemia through improved sleep health and continuous sleep monitoring. Prior studies have demonstrated a role for slow wave sleep (SWS; i.e., deep sleep) in glucose maintenance and insulin sensitivity.33,34 Diabetes patients spend less time in SWS compared with N1, N2 (light sleep)10,35 and REM.36 Compared with age-matched controls, children with T1DM spent more time in light sleep than SWS, and more time in light sleep was associated with higher average daily glucose values, more hyperglycemia, and higher HbA1c levels.32 Behaviorally, increased time in light sleep was associated with parental reports of emotional and behavioral difficulties, reduced diabetes quality of life, lower grades, sleep–wake behavior problems, depressive mood, daytime poor sleep quality, sleepiness, and worse math performance, while increased deep sleep was associated with better outcomes.32 The current results suggest that the beneficial effect of deep sleep may be due to decreased nocturnal hypoglycemia. Taken together, understanding of a causal relationship between sleep stages and blood glucose dynamics will be an important direction for future studies.
The data analyzed in this study were collected from subjects in real-world conditions, yet not in the familiar environment of their homes (or in their normal time zones, in some cases), which may impact sleep quality. It is also possible that sleep quality and traits could be affected by sleeping in shared dormitory rooms or changed by the absence of a normal sleep companion, such as a spouse or pet. The time spent in REM and light sleep by our subjects differs from previous findings.10 The basis for the observed differences remains unclear but could arise from the different sleep monitoring technology employed or from the study sleep environment (clinical sleep laboratory versus real-world dormitory). Further, the experience of wearing a WSM, however unobtrusive, may require some acclimation time greater than the short study period evaluated here. Subsequent at-home observational studies, conducted over longer periods of time, may shed additional light on the relationship between typical sleep and nocturnal glycemia in the individual with T1DM. Collection of data from larger cohorts will also permit more fine-tuned analyses on the sleep quality effects of age, duration of diabetes, and other clinical characteristics, as the subjects in this exploratory study were diverse in these respects.
Research has explored the capacity of algorithmically processed EEG data to identify and signal the onset of hypo-glycemia, including during sleep, with applications in patient-alarm systems.37–40 These strategies currently employ technologies more commonly associated with the clinical environment and have not, to our knowledge, been adapted to the EEG sensors found in consumer-grade devices such as the WSM used in the present study. It is quite possible, however, that improvements in EEG data collection and processing will enable consumer-grade WSM-based hypoglycemia prediction, of particular value during sleep when T1DM patients’ ability to respond is compromised.15 Further, more macro-level evaluations of sleep quality and duration may inform changes to daily insulin regimens, as partial sleep restriction has been shown to decrease insulin sensitivity in people with T1DM.11 The use of body-worn sensor data in the automated altering of insulin delivery has precedent in insulin pump systems with the low glucose suspend feature, which has been shown to decrease nocturnal hypoglycemia.41 Perhaps such features can be even more effective in the context of sleep health data.
While a Food and Drug Administration-approved clinical decision-support process (open or closed loop) would likely require more sophisticated, validated sleep measurements than are presently available in consumer-grade sleep-monitoring technology, user-friendly devices such as the WSM employed here are being joined by a proliferation of smartphone applications that also purport to monitor sleep health.42,43 Self-monitoring trends in the population, such as “citizen science” and the quantified self, suggest that the popularity of these devices and applications will continue to grow in the foreseeable future.44,45 Further, data collected by and for the individual with diabetes lend themselves to evaluation by the increasingly discussed “n-of-1” paradigm for personalized health.46–48
Regardless of the means by which sleep health is assessed in T1DM individuals and the population, one might envision that the availability of affordable real-world sleep monitoring technologies will offer insight and improvements for personal sleep management akin to the improved diabetes control imparted by use of CGM and insulin pumps. Digital systems that integrate and display data from these devices in meaningful ways are likely to be adopted into diabetes self-management and forward-looking clinical practice.
Conclusions
This exploratory analysis revealed that increased deep sleep is associated with lower HbA1c and that less hypoglycemia occurs in deep sleep, though this relationship is not yet understood. Follow-up studies with greater numbers of patients over longer periods of time in real-world and clinical settings are needed. We found that a consumer-grade WSM is useful for collecting objective sleep data in an unsupervised setting and anticipate that this technology may be more broadly adopted in future research.
Glossary
(CGM)
continuous glucose monitor
(DMITRI)
Diabetes Management Integrated Technology Research Initiative
(EEG)
electroencephalogram
(ESS)
Epworth sleepiness scale
(GV)
glycemic variability
(HbA1c)
hemoglobin A1c
(SWS)
slow wave sleep
(T1DM)
type 1 diabetes mellitus
(TST)
total sleep time
(WSM)
wireless sleep monitor
Funding
Support for this study was provided by National Institutes of Health Grant Numbers U54HL108460 and K01MH080992, and National Library of Medicine Grant Number T15LM011271. The WSM devices were provided by Zeo Inc.
References
- 1.Mednick SC, Cai DJ, Shuman T, Anagnostaras S, Wixted JT. An opportunistic theory of cellular and systems consolidation. Trends Neurosci. 2011;34(10):504–514. doi: 10.1016/j.tins.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson AM, Feyer AM. Moderate sleep deprivation produces impairments in cognitive and motor performance equivalent to legally prescribed levels of alcohol intoxication. Occup Environ Med. 2000;57(10):649–655. doi: 10.1136/oem.57.10.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28(11):1457–1464. doi: 10.1093/sleep/28.11.1457. [DOI] [PubMed] [Google Scholar]
- 4.Roehrs T, Roth T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev. 2001;5(4):287–297. doi: 10.1053/smrv.2001.0162. [DOI] [PubMed] [Google Scholar]
- 5.Mednick SC, Christakis NA, Flowler JH. The spread of sleep loss influences drug use in adolescent social networks. PLoS One. 2010;5(3):e9775. doi: 10.1371/journal.pone.0009775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. In: Eur Heart J, editor. 12. Vol. 32. 2011. pp. 1484–1492. [DOI] [PubMed] [Google Scholar]
- 7.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5(5):253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jauch-Chara K, Schmid SM, Hallschmid M, Born J, Schultes B. Altered neuroendocrine sleep architecture in patients with type 1 diabetes. Diabetes Care. 2008;31(6):1183–1188. doi: 10.2337/dc07-1986. [DOI] [PubMed] [Google Scholar]
- 11.Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers GJ, van Kralingen K, Hoogma RP, Corssmit EP, Romijn JA. Partial sleep restriction decreases insulin sensitivity in type 1 diabetes. Diabetes Care. 2010;33(7):1573–1577. doi: 10.2337/dc09-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Dijk M, Donga E, van Dijk JG, Lammers GJ, van Kralingen KW, Dekkers OM, Corssmit EP, Romijn JA. Disturbed subjective sleep characteristics in adult patients with long-standing type 1 diabetes mellitus. Diabetologia. 2011;54(8):1967–1976. doi: 10.1007/s00125-011-2184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cryer PE. Hypoglycemia in type 1 diabetes mellitus. Endocrinol Metab Clin North Am. 2010;39(3):641–654. doi: 10.1016/j.ecl.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jauch-Chara K, Schultes B. Sleep and the response to hypoglycaemia. Best Pract Res Clin Endocrinol Metab. 2010;24(5):801–815. doi: 10.1016/j.beem.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Banarer S, Cryer PE. Sleep-related hypoglycemia-associated autonomic failure in type 1 diabetes: reduced awakening from sleep during hypoglycemia. Diabetes. 2003;52(5):1195–1203. doi: 10.2337/diabetes.52.5.1195. [DOI] [PubMed] [Google Scholar]
- 16.Pillar G, Schuscheim G, Weiss R, Malhotra A, McCowen KC, Shlitner A, Peled N, Shehadeh N. Interactions between hypoglycemia and sleep architecture in children with type 1 diabetes mellitus. J Pediatr. 2003;142(2):163–168. doi: 10.1067/mpd.2003.66. [DOI] [PubMed] [Google Scholar]
- 17.Matyka KA, Crawford C, Wiggs L, Dunger DB, Stores G. Alterations in sleep physiology in young children with insulin-dependent diabetes mellitus: relationship to nocturnal hypoglycemia. J Pediatr. 2000;137(2):233–238. doi: 10.1067/mpd.2000.107186. [DOI] [PubMed] [Google Scholar]
- 18.Shambroom J, Fabregas SE. Age related changes in objectively measured sleep observed in a large population in the home. Sleep. 2010;33:A348. [Google Scholar]
- 19.Fabregas SE, Shambroom JR. Objective in-home weekday vs weekend sleep in a sample of the US population. Sleep. 2010;33:214. [Google Scholar]
- 20.Shambroom JR, Fábregas SE, Johnstone J. Validation of an automated wireless system to monitor sleep in healthy adults. J Sleep Res. 2012;21(2):221–230. doi: 10.1111/j.1365-2869.2011.00944.x. [DOI] [PubMed] [Google Scholar]
- 21.Heintzman ND, Chen B, Dave T, Ohno-Machado L. The DMITRI pilot study: exploring the diabetes data ecosystem. To be submitted [Google Scholar]
- 22.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 23.Iber C, Ancoli-Israel S, Chesson A, Quan SF. 1st ed. Westchester: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification. [Google Scholar]
- 24.Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Rev Neurosci. 2002;3(9):679–693. doi: 10.1038/nrn915. [DOI] [PubMed] [Google Scholar]
- 25.Spiegel K, Tasali E, Leproult R, Scherberg N, Van Cauter E. Twenty-four-hour profiles of acylated and total ghrelin: relationship with glucose levels and impact of time of day and sleep. J Clin Endocrinol Metab. 2011;96(2):486–493. doi: 10.1210/jc.2010-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vervoort G, Goldschmidt HM, van Doorn LG. Nocturnal blood glucose profiles in patients with type 1 diabetes mellitus on multiple (> or = 4) daily insulin injection regimens. Diabet Med. 1996;13(9):794–799. doi: 10.1002/(SICI)1096-9136(199609)13:9<794::AID-DIA185>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 27.Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA. 2006;295(14):1707–1708. doi: 10.1001/jama.295.14.1707. [DOI] [PubMed] [Google Scholar]
- 28.Rodbard D. New and improved methods to characterize glycemic variability using continuous glucose monitoring. Diabetes Technol Ther. 2009;11(9):551–565. doi: 10.1089/dia.2009.0015. [DOI] [PubMed] [Google Scholar]
- 29.Hill NR, Oliver NS, Choudhary P, Levy JC, Hindmarsh P, Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther. 2011;13(9):921–928. doi: 10.1089/dia.2010.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frias PF, Frias JP, Heintzman ND. Bethesda, MD: Diabetes Technology Meeting; 2012. Glycemic variability during road cycling in elite athletes with type 1 diabetes. [Google Scholar]
- 31.National Sleep Foundation. Washington DC: National Sleep Foundation; 2011. 2011 sleep in America poll. [Google Scholar]
- 32.Perfect MM, Patel PG, Scott RE, Wheeler MD, Patel C, Griffin K, Sorensen ST, Goodwin JL, Quan SF. Sleep, glucose, and daytime functioning in youth with type 1 diabetes. Sleep. 2012;35(1):81–88. doi: 10.5665/sleep.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105(3):1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dijk DJ. Slow-wave sleep, diabetes, and the sympathetic nervous system. Proc Natl Acad Sci U S A. 2008;105(4):1107–1108. doi: 10.1073/pnas.0711635105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serrano Ríos M, Navascués I, Ordóñez A, Sabán J, Núnez A, Sánchez M, García Austt E. Nocturnal growth hormone surges in type 1 diabetes mellitus are both sleep- and glycemia-dependent: assessment under continuous sleep monitoring. Diabetes Res Clin Pract. 1990;10(1):1–8. doi: 10.1016/0168-8227(90)90075-5. [DOI] [PubMed] [Google Scholar]
- 36.Pallayova M, Donic V, Gresova S, Peregrim I, Tomori Z. Do differences in sleep architecture exist between persons with type 2 diabetes and nondiabetic controls? J Diabetes Sci Technol. 2010;4(2):344–352. doi: 10.1177/193229681000400215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juhl CB, Højlund K, Elsborg R, Poulsen MK, Selmar PE, Holst JJ, Christiansen C, Beck-Nielsen H. Automated detection of hypoglycemia-induced EEG changes recorded by subcutaneous electrodes in subjects with type 1 diabetes--the brain as a biosensor. Diabetes Res Clin Pract. 2010;88(1):22–28. doi: 10.1016/j.diabres.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Snogdal LS, Folkestad L, Elsborg R, Remvig LS, Beck-Nielsen H, Thorsteinsson B, Jennum P, Gjerstad M, Juhl CB. Detection of hypoglycemia associated EEG changes during sleep in type 1 diabetes mellitus. Diabetes Res Clin Pract. 2012;98(1):91–97. doi: 10.1016/j.diabres.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen LB, Ling SS, Jones TW, Nguyen HT. Identifcation of hypoglycemic states for patients with T1DM using various parameters derived from EEG signals. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:2760–2763. doi: 10.1109/IEMBS.2011.6090756. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen LB, Nguyen AV, Ling SH, Nguyen HT. An adaptive strategy of classifcation for detecting hypoglycemia using only two EEG channels. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:3515–3518. doi: 10.1109/EMBC.2012.6346724. [DOI] [PubMed] [Google Scholar]
- 41.Choudhary P, Shin J, Wang Y, Evans ML, Hammond PJ, Kerr D, Shaw JA, Pickup JC, Amiel SA. Insulin pump therapy with automated insulin suspension in response to hypoglycemia: reduction in nocturnal hypoglycemia in those at greatest risk. Diabetes Care. 2011;34(9):2023–2025. doi: 10.2337/dc10-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Connor A. New York Times; 2012. Jul 10, New technologies aim to help you sleep better. http://well.blogs.nytimes.com/2012/07/10/new-technologies-aim-at-sounder-sleep/. Accessed March 15 2013. [Google Scholar]
- 43.Quantifed Self: Guide to Self-Tracking Tools. 34 tools tagged “sleep.”. 2013. http://quantifedself.com/guide/tag/sleep. Accessed March 15.
- 44.Quantifed Self. 2013. http://quantifedself.com/. Accessed March 15.
- 45.Swan M. Crowdsourced health research studies: an important emerging complement to clinical trials in the public health research ecosystem. J Med Internet Res. 2012;14(2):e46. doi: 10.2196/jmir.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lillie EO, Patay B, Diamant J, Issell B, Topol EJ, Schork NJ. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Per Med. 2011;8(2):161–173. doi: 10.2217/pme.11.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smarr L. Quantifying your body: a how-to guide from a systems biology perspective. Biotechnol J. 2012;7(8):980–991. doi: 10.1002/biot.201100495. [DOI] [PubMed] [Google Scholar]
- 48.Hood L, Flores M. A personal view on systems medicine and the emergence of proactive P4 medicine: predictive, preventive, personalized and participatory. N Biotechnol. 2012;29(6):613–624. doi: 10.1016/j.nbt.2012.03.004. [DOI] [PubMed] [Google Scholar]