Chimeric antigen receptor T cells with dissociated signaling domains exhibit focused anti-tumor activity with reduced potential for toxicity in vivo (original) (raw)
. Author manuscript; available in PMC: 2014 Jul 1.
Abstract
Adoptive immunotherapy using lymphocytes genetically-modified to express a chimeric antigen receptor (CART) holds considerable promise for the treatment of cancer. However, CAR-based therapies may involve on-target toxicity against normal tissues expressing low amounts of the targeted tumor-associated antigen (TAA). To specify T cells for robust effector function that is selective for tumor but not normal tissue, we developed a trans-signaling CAR strategy whereby T cell activation signal 1 (CD3ζ) is physically dissociated from costimulatory signal 2 (CD28) in two CARs of differing antigen specificity; mesothelin and a-folate receptor (FRa). Human T cells were genetically modified to co-express signal 1 (Anti-Meso scFv-CD3ζ) and signal 2 (Anti-FRa scFv-CD28) CARs in trans. Trans-signaling CART cells showed weak cytokine secretion against target cells expressing only one TAA in vitro, similar to first generation CART cells bearing CD3ζ only, but demonstrated enhanced cytokine secretion upon encountering natural or engineered tumor cells co-expressing both antigens, equivalent to that of second generation CART cells with dual signaling in cis. CART cells with dual specificity also showed potent anti-cancer activity and persistence in vivo which was superior to first generation CART cells and equivalent to second generation CARs. Importantly, second generation CART cells exhibited potent activity against cells expressing mesothelin alone, recapitulating normal tissue, whereas trans-signaling CART cells did not. Thus, a dual specificity, trans-signaling CAR approach can potentiate the therapeutic efficacy of CART cells against cancer while minimizing parallel reactivity against normal tissues bearing single antigen.
INTRODUCTION
Genetic redirection of T cells with chimeric antigen receptors (CARs) that link an antigen-specific single-chain antibody fragment (scFv) to intracellular signaling domains is at the forefront of cancer immunotherapy (1, 2). CARs functionally redirect T cells with high specificity to various surface antigens on tumor cells independent of MHC restriction and antigen processing, and therefore bypass major mechanisms by which tumors escape immune recognition. T cells bearing a first generation CAR having only the T cell CD3ζ intracellular signaling domain either fail to persist or become anergic since tumor cells frequently lack requisite ligands for costimulation (3). This incomplete activation of CART cells in vivo appears to limit their persistence, and has thus hampered their efficacy in clinical trials for lymphoma (4), neuroblastoma (5), ovarian cancer (6) or renal cell cancer (7). To overcome these limitations, second generation CART cells were developed that incorporate the intracellular domain of various costimulatory molecules such as CD28, 4-1BB, OX-40, and CD27 leading to improved expansion, persistence and activity of the CART cells in preclinical mouse models (8, 9) and in clinical studies (2, 10, 11). Still, the enhanced potency of these CARs can be associated with autoimmunity due to on-target toxicities against normal tissues expressing lower levels of the TAAs. For instance, administration of high numbers of T cells bearing an anti-ErbB2 CAR comprising the CD28 and 4-1BB costimulatory domains to a lymphodepleted patient with metastatic colon cancer resulted in rapid onset of pulmonary toxicity with lung infiltrates and a “cytokine storm” followed by cardiac arrest and death (12). Clearly, the development of strategies limiting potential early or late phase toxicity is of importance. We have previously generated a fully human anti-mesothelin CAR capable of conferring potent in vitro and in vivo effector functions to primary T cells against mesothelin-expressing tumors (13). Mesothelin-redirected CART cells also hold the potential to inflict damage against normal mesothelial cells lining the pleura, peritoneum as well as epithelial cells of the trachea, tonsils, fallopian tube and the rete testis which express low levels of mesothelin (14, 15). To limit “on target” toxicity and improve tumor-focused targeting and attack, we have developed and tested the concept of a trans-signaling CAR strategy where the T cell activation signal 1 (CD3ζ module) is physically dissociated from the costimulatory signal 2 (CD28 module). Since mesothelin and FRa are TAAs co-expressed in the majority of epithelial ovarian cancers, but expressed differentially and at low levels in normal tissues (14, 16–19), two independent CARs of distinct specificity were utilized; a signal 1 CAR (Meso-CD3ζ only), and a signal 2 CAR (FRa-CD28 only) using pre-validated scFvs (13, 20). In this fashion, T cells transduced to co-express both CARs exhibit potent in vitro and in vivo effector functions that are driven by tumor encounter and coupled with diminished damage to normal tissues.
MATERIALS AND METHODS
CAR constructs
The F-28 CAR was constructed by using lentiviral vector backbone constructs previously described (20). CAR construction and lentivirus production are detailed in Supplementary Materials and Methods.
Recombinant lentivirus production
High-titer replication-defective lentiviral vectors were produced and concentrated as previously described (13).
Human T cell transduction
Primary human T cells, purchased from the Human Immunology Core at University of Pennsylvania, were isolated from healthy volunteer donors following leukapheresis by negative selection. All specimens were collected under a University Institutional Review Board-approved protocol, and written informed consent was obtained from each donor. T cell activation and lentiviral transduction was performed as previously described (13).
Functional assays
Cytokine release assays were carried out using an IFN-γ ELISA Kit (Biolegend). 51Cr-release and CD107 degranulation assays of cytolysis were carried out as previously described (13, 21). The Cytometric Bead Array and apoptosis assay were carried out according to manufacturer’s instructions (BD Biosciences). Functional assays are further detailed described in Supplementary Materials and Methods.
Xenograft model of ovarian cancer
Mouse studies were carried out as detailed in Supplementary Materials and Methods.
Immunohistochemistry
Fresh frozen tumor samples were sectioned for immunohistochemical analysis as described in Supplementary Materials and Methods.
Statistical analysis
Statistical evaluation was performed using 2-tailed Student’s t-test. GraphPad Prism 4.0 (GraphPad Software) was used for the statistical calculations. P-values less than 0.05 were considered significant.
RESULTS
CAR construction
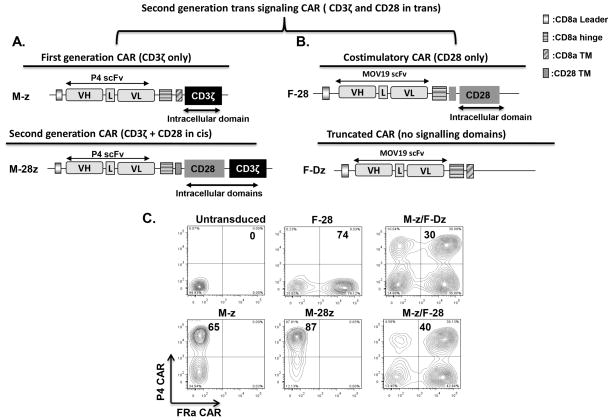
Anti-mesothelin CAR constructs comprised of the P4 scFv linked to a CD8α hinge and transmembrane region, followed by a CD3ζ signaling moiety alone (referred to as M-z) or in tandem with the CD28 intracellular signaling motif (M-28z) were previously shown to confer specific mesothelin-redirected activity in vitro and in vivo (13)(Fig. 1A). The costimulatory anti-FRa CAR (F-28) construct comprised the MOv-19 scFv linked to a CD8α hinge and the CD28 transmembrane region and intracellular signaling motif; the signaling deficient F-Dz construct lacks a functional signaling domain. F-28 CAR expression was monitored through the GFP transgene which is included in the CAR lentiviral construct with its expression driven from the same EF1a promoter which regulates CAR expression. GFP signal was highly correlated with protein-L binding to the mouse scFv of the CAR highlighting the reliability of this reporter gene expression (Fig. S1). Primary human T cells were efficiently transduced with the two CAR-encoding lentiviral vectors with >40% dual-transduced T cells reproducibly expressing both CARs (Fig. 1B). CART cell populations were adjusted to equivalent frequencies of anti-mesothelin CART cells (60–70%) by adding untransduced T cells for all functional assays.
Figure 1. Generation and expression of mesothelin and FRa specific CARs.
A. Schemata of the anti-mesothelin (P4) based chimeric antigen receptor (CAR) constructs containing the CD3ζ endodomain alone (M-z) or in combination with the CD28 costimulatory module (M-28z). B. Schemata of the anti-FRa (F) based CAR constructs containing the scFv (MOV-19) fused through the CD8a hinge and CD28 TM to the CD28 costimulatory module (F-28). A truncated anti-FRa CAR with no signaling domains is shown (F-Dz). P4, anti-mesothelin scFv; VL, variable L chain; L, Linker; VH, variable H chain; TM, transmembrane region. C. P4 CAR expression detected on human CD3-gated cells via recombinant mesothelin protein staining after transduction with lentivirus compared to untransduced T cells. T cells bearing the F-28 CAR were detected via GFP transgene expression. Transduction efficiencies are indicated with the percentage of CAR-expressing cells within the transduced populations.
Trans-signaling CART cells exert superior antigen-specific cytokine secretion in vitro
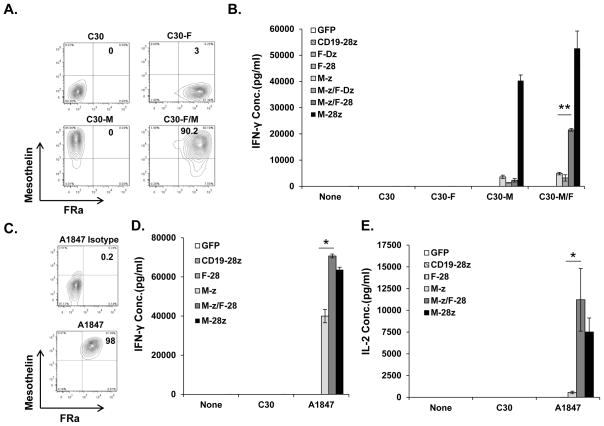
To evaluate the in vitro effector functions of CART cells in response to cells that express mesothelin alone versus tumor cells that co-express mesothelin and FRa, TAA-negative C30 cancer cell lines which lack endogenous CD80/86 costimulatory ligands were engineered to over-express either one or both of these antigens (Fig. 2A). T cells engineered to express the M-z CAR recognized and secreted similar levels of IFN-γ when co-cultured with C30 cells expressing either mesothelin (C30M) or mesothelin and FRa (C30M/F). By comparison, second generation M-28z CART cells exerted superior IFN-γ secretion against all C30 cell variants expressing mesothelin. In contrast, trans-signaling M-z/F-28 CART cells produced low levels of IFN-γ against C30M, similar to M-z CART cells, but exerted enhanced IFN-γ production when exposed to C30M/F tumor cells (Fig. 2B). M-z/F-28 CAR activity against C30M/F cells surpassed that of M-z CART cells demonstrating that expression of both antigens by tumor cells promotes the costimulation of M-z/F-28 CART cells through the F-28 CAR (Fig. 2B). Consistent with this notion, co-expression of the M-z and the signaling-deficient F-Dz CAR in T cells did not enhance their response against C30M/F cells. M-28z CART cells produced more IFN-g than M-z/F-28 CART cells using this artificial target cell system where cells are engineered to express antigens at supraphysiological levels. T cells expressing the F-28 CAR alone in did not produce any IFN-γ, consistent with a lack of CD3-z signaling. Moreover, control T cells transduced to express green fluorescent protein (GFP) or an anti-CD19 CAR containing CD3ζ with CD28 signaling motifs in tandem (CD19-28z) (22) did not produce cytokines after stimulation with CD19-negative C30 cells, illustrating the need for antigen-specificity (Fig. 2B).
Figure 2. Trans-signaling CART cells exert superior antigen-specific cytokine secretion in vitro compared with first generation CART cells.
A. Detection of surface mesothelin and/or FRa protein expression in genetically modified C30 tumor cells by flow cytometry. B. Primary human T cells transduced with M-z and F-28 CARs exert superior IFN-γ secretion compared to M-z CART cells. Mean IFN-γ concentration ± SEM (pg/ml) is shown. **P<0.01 comparing M-z and M-z/F-28 CART cells against C30-M/F cancer cells. C. Co-expression of mesothelin and FRa by A1847 ovarian cancer cell line. D. Trans-signaling CART cells secrete higher levels of IFN-γ in response to A1847. Mean IFN-γ concentration ± SEM (pg/ml) is shown. *P<0.05 comparing M-z and M-z/F-28 CART cells against A1847 cancer cells. E. Trans-signaling CART cells secrete more IL-2 (pg/ml) in response to A1847 than M-z CART. Values represent the mean ± SEM concentration of triplicate wells.
We next tested trans-signaling CART cell activity against an ovarian cancer cell line that naturally expresses both mesothelin and FRa, A1847 (Fig. 2C). M-z/F-28 CART cells secreted IFN-γ in response to A1847 that was significantly higher than that secreted by M-z CART cells but similar to cis-signaling M-28z CART cells, showing that the natural expression of both antigens on tumor cells is capable of inducing costimulatory effects in trans-signaling CART cells (Fig. 2D). IL-2 secretion from the dual transduced M-z/F-28 CART cells was also similar to second generation M-28z CART cells and higher than that of M-z CART cells indicating that integrated delivery of signal 1 and 2 in trans or cis can enhance production of IL-2 cytokine (Fig. 2E).
Trans-signaling CART cells show in vitro cytolytic potency
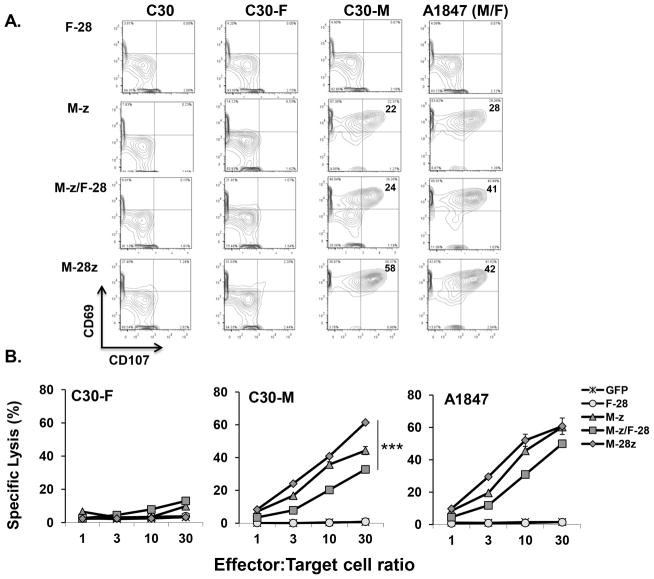
Degranulation is a quantitative indicator of lytic function by T cells (21). M-z and M-z/F-28 CART cell degranulation was accompanied by upregulation of surface co-expression of mobilized CD107 (Lysosomal-associated membrane protein 1) and the activation-associated marker CD69 in response to C30M, but not when stimulated with either C30 or C30F cells (Fig. 3A). Consistent with active costimulation, M-28z CART cells displayed a superior cytolytic phenotype against C30M compared with M-z and M-z/F-28 CART cells. However, exposure to A1847 (M+/F+) tumor cells with both antigens led to enhanced degranulation by both M-28z and M-z/F-28 CART cells, relative to M-z CART cells (Fig. 3A). Anti-CD19 CART cells did not degranulate in response to C30 or A1847 cells (Fig. S2). In short-term chromium release assays, all of the various mesothelin-directed CART cell populations (M-28z, M-z and M-z/F-28) showed cytolytic activity against single antigen-expressing C30-M target cells with M-28z CART cells exhibiting greater cytolytic activity than M-z/F-28 and M-z cells at all ratios tested. A1847 target cells were also lysed by all anti-mesothelin CART groups yet M-28z and M-z CART showed statistically comparable and higher lysis than M-z/F-28 CART cells at E:T ratios ≥10:1 (Fig. 3B). At lower E:T ratios, M-28z CART cells lysed dual antigen A1847 cells more efficiently than both M-z and M-z/F-28 groups suggesting that in tandem costimulatory signaling may permit more efficient killing in the short term. Control GFP and F-28 CART cells did not exhibit cytolytic function against either cell target.
Figure 3. Trans-signaling CART cells show in vitro cytolytic potency.
A. Dual CART cells exert superior degranulation and express T cell activation markers in response to specific stimulation. Detectable CD107a, b and CD69 expression in CART cells during co-culture with the indicated tumor target or control cell line by flow cytometry. B. Cytolytic function of dual CART cells. Primary human T cells transduced to express M-z, M-z/F-28, M-28z, F-28 or GFP were co-cultured with 51Cr-labeled native C30-F, C30-M or A1847 cancer cell lines for 4 hrs at the indicated effector to target ratio. Error bars indicate standard deviation. ***P < 0.001 comparing M-z/F-28 and M-28z CART cells against C30-M cancer cells.
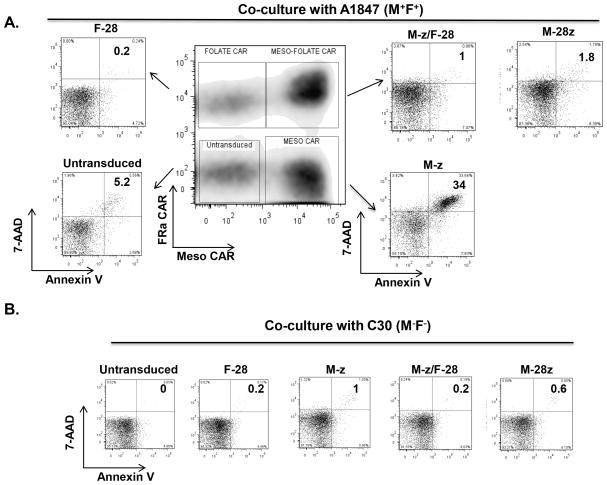
Trans-signaling CART cells resist AICD
Incorporation of costimulatory domains into CARs can increase resistance of CART cells to apoptosis upon activation by tumors (23). To investigate if provision of CD28 costimulation in trans protects T cells from antigen-induced cell death (AICD), single or dual CAR bearing T cells were measured for their rate of apoptosis following co-culture with A1847 (M+/F+) tumor cells. Apoptosis (7-AAD+ Annexin V+) was elevated in M-z T cells exposed to A1847 (34%) for three days but reduced in trans-signaling M-z/F-28 CART cells (0.5–1%; Fig. 4A). Consistent with past studies (24), cis-signaling M-28z CART cells were also resistant to AICD. Control F-28 or CD19-28z CART cells displayed no AICD in the absence of specific antigenic stimulation (Fig. 4B).
Figure 4. Trans-signaling CART cells resist AICD upon antigen specific stimulation.
A. Apoptosis in mixed CART cell population containing dual CAR transduced T cells (M-z/F-28), single CAR transduced T cells (M-z or F-28) and untransduced T cells that were stimulated with A1847(M+/F+) cancer cells for three days. Cis M-28z CART cells were co-cultured in parallel with A1847 for the same time period. After 3 days, apoptosis by antigen-induced cell death (AICD) was quantified using annexin V and 7-AAD staining. B. AICD is not observed in CART cells stimulated with antigen-negative cancer cells (C30; M−/F−) for 3 days.
Dual-specific CART cells possess enhanced anti-tumor potency and persistence in vivo
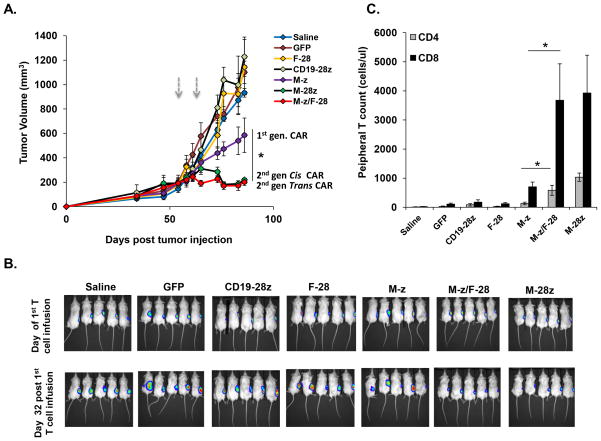
The capability of trans-signaling CART cells to inhibit human tumor outgrowth was evaluated in vivo in immunodeficient NOD/SCID/IL-2R-γcnull (NSG) mice inoculated s.c. with 1 × 106 firefly luciferase-expressing A1847 cells. Mice with established tumors (150–200 mm3) received intravenous injections of CART cells. Four weeks after the first T cell dose, tumor growth was inhibited modestly in mice receiving M-z CART cells (p=0.043), compared to saline, CD19-28z CART cells, F-28 CART cells or GFP-T cell control groups (Fig. 5A). Compared to M-z CART cells, transfer of M-28z or M-z/F-28 CART cells mediated even more potent inhibition of tumor outgrowth (p= 0.028) indicating that incorporation of CD28 signaling domain in cis or in trans enhances anti-tumor activity in vivo against tumors co-expressing both TAAs (Fig. 5A). Bioluminescence imaging results from treated mice confirmed the lower tumor burden in those treated with the trans-signaling CART cells compared with first generation CART cells. Tumors exhibited similar low levels of bioluminescence regardless of whether the mice were treated with trans- or cis-signaling CART cells (Fig. 5B). Two weeks after first T cell dose, peripheral blood CD8+ T and CD4+ T cell counts from mice injected with M-28z or M-z/F-28 CART cells were similar and higher than in the M-z group (p<0.05; Fig. 5C). No substantial human T cell persistence was observed in mice treated with CD19-28z CART, F-28 CART or GFP-T cells.
Figure 5. Trans-signaling CART cells exert superior anti-tumor effector functions in vivo compared with first generation CART cells.
A. In vivo inhibition of large pre-established tumors by M-z/F-28 CART cells: effect of the CD28 costimulatory signaling domain in trans. NSG mice bearing established s.c. tumors were treated with two i.v. injections of 7.5 × 106 M-z, M-z/F-28 or M-28z CART cells or control anti-CD19-28z and GFP T cells or saline on days 55 and 59 post-tumor inoculation (arrows). Tumor growth was assessed by caliper measurement. Mean tumor volume (mm3 ± SEM) is shown with n=5 for all groups. *P < 0.05 comparing M-z CART-treated mice with M-z/F-28 or M-28z CART-treated mice. B. A1847 fLuc+ bioluminescence signal is similar in M-z/F-28 and M-28z CART-treated mice and less than in M-z treated mice 4 weeks after first T cell dose. Control groups show no decrement in the bioluminescence signal. C. Stable persistence of CD28 cis- or trans-costimulated CART T cells in vivo. Peripheral blood was collected 2 weeks after the first T cell infusion and quantified for the absolute number of human CD4+ and CD8+ T cells/ul of blood. Mean cell count ± SEM is shown with n = 5 for all groups. *P < 0.05 comparing M-z CART-treated mice with M-z/F-28 CART-treated mice.
Trans-, but not cis-, signaling CART cells exhibit more limited activity against cells bearing single antigen in vivo
FRa-deficient A1847 cells (A1847M+/F−) were generated via transduction with lentiviral particles encoding for a FRa-specific shRNA, as a surrogate for normal human mesothelial cells expressing only mesothelin for use in modeling in vivo. Fluorescence-activated cell sorting resulted in an enriched cancer cell population (~98%) which lacked surface FRa expression (Fig. S3A). FRa expression was unaltered after engineering cells with control shRNA (A1847M+/F+). Aside from the lack of FRa expression, the two lines appeared identical by all measures. No difference in in vitro growth kinetic or viability of A1847M+/F+ and A1847M+/F− cells was observed (Fig. S4) and control anti-FRa (F-28z) CART cells produced minimal IFN-γ in response to stimulation with A1847 cells after FRa silencing; similar to levels seen in response to PEO-1 ovarian cancer cells, which have undetectable surface FRa expression via flow cytometry (9) (Fig. S5A, B). In co-culture assays, IFN-γ secretion by trans M-z/F-28 CART cells was significantly reduced in response to A1847M+/F− compared with A1847M+/F+, a confirmation of potent effector function only upon engagement of both antigens (Fig. S6). Additionally, M-z/F-28 and M-z CART cells reacted equivalently against A1847M+/F− cells. As expected, cis M-28z CART cells secreted significantly higher amounts of IFN-γ against A1847M+/F−, similar to the IFN-γ levels achieved against A1847M+/F+.
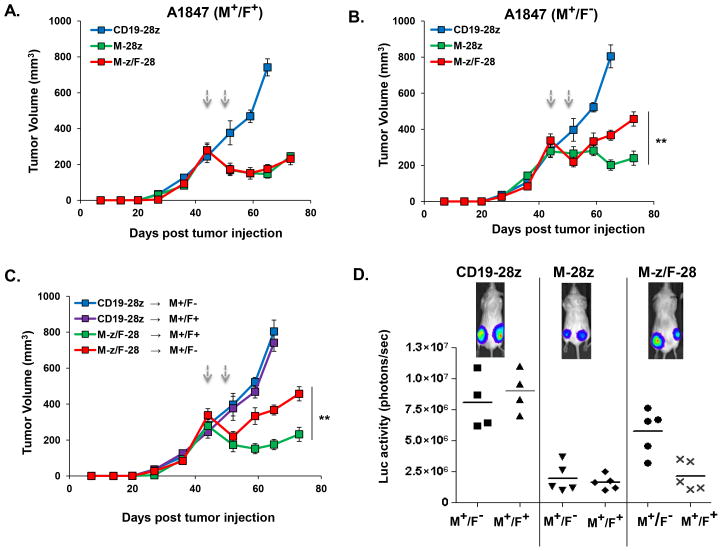
To evaluate the in vivo potency of trans- or cis-signaling T cells against A1847 cells co-expressing or lacking FRa, A1847M+/F+ and A1847M+/F− cells were inoculated s.c. separately in the same NSG mice on opposite hind flanks. A1847M+/F+ and A1847M+/F− tumors were established comparably and grew similarly in vivo (Fig. 1A, B). Mice bearing two established A1847 (≥330mm3) tumors received tail vein injections of CART cells and tumor outgrowth was monitored. The efficiency of A1847M+/F+ tumor outgrowth inhibition was identical between trans M-z/F-28 CART and cis M-28z CART cell groups (Fig. 6A). However, inhibition of A1847M+/F− outgrowth was significantly attenuated in the trans M-z/F-28 CART cell group compared with the cis M-28z group (p=0.0045; Fig. 6B). Notably, trans-signaling CART cells were less effective in inhibiting the outgrowth of A1847M+/F−, compared with their activity against the A1847M+/F+ tumor in the same mice (p=0.0001; Fig. 6C). Bioluminescence imaging of the tumors confirmed these results (Fig. 6D).
Figure 6. _Cis_-, but not _trans_-, signaling CART cells exhibit enhanced in vivo activity against cells bearing a single antigen.
In vivo anti-tumor efficacy of trans- or cis-signaling T cells against A1847 cells expressing or lacking FRa. 7.5 × 106 A1847(M+/F+) and A1847(M+/F−) cells were inoculated s.c. separately in the same NSG mice on opposite hind flanks. Mice with two established A1847 tumors (≥330mm3) received tail vein injections of CART cells on days 45 and 49 post-tumor inoculation (arrows). Tumor growth was assessed by caliper measurement. A. The efficiency of A1847(M+/F+) tumor outgrowth inhibition was identical between trans M-z/F-28 CART and cis-signaling M-28z CART cell groups. B. Inhibition of A1847(M+/F−) outgrowth in the trans M-z/F-28 CART group was reduced compared to the cis M-28z group (**P < 0.01 in M-z/F-28 versus M-28z). C. Trans-signaling CART cells were not as effective in inhibiting the outgrowth of A1847(M+/F−) as in inhibiting the A1847(M+/F+) tumor in the same mice. Mean tumor volume (mm3 ± SEM) is reported with n=5 for all groups in A–C. **P < 0.01 comparing tumor growth of A1847(M+F+) versus A1847(M+F−) in mice treated with M-z/F-28 CART. D. FLuc+ bioluminescence signal from A1847(M+/F+) tumors is dramatically lower compared with the signal derived from A1847(M+/F−) masses in the same mice treated with M-z/F-28 CART cells 4 weeks after first T cell dose. In the cis M-28z group, M+F+ and M+F− A1847 tumors exhibit similarly low levels of FLuc+ bioluminescence signal. The CD19-28z CART treatment group showed no decrement in bioluminescence signal.
Preferential accumulation of trans-signaling CART cells in tumor in vivo
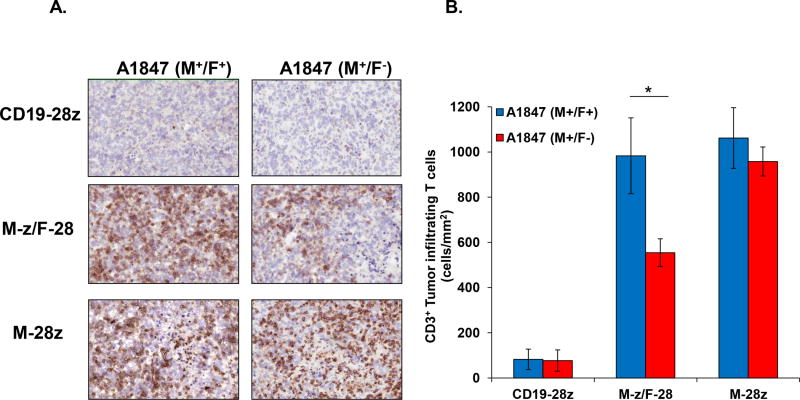
The accumulation of trans- and cis-signaling CART cells in regressing tumors from treated and euthanized mice with established masses in both flanks was measured via immunohistochemical analysis for the detection of human CD3+ T cells (Fig. 7). The abundance of T cells was equally high in dual (A1847M+/F+) or single antigen expressing tumors (A1847M+/F−) from mice treated with cis M-28z CART cells. In contrast, in mice treated with trans M-z/F-28 CART cells, a significant increase in T cell accumulation was observed in tumors expressing both antigens with lower numbers observed in single antigen tumors, illustrating selectivity. Few CD3+ T cells were detected in tumors resected from mice that received CD19-28z CART cells. Thus, the survival and accumulation of trans-signaling CART cells into tumor sites is dependent on the engagement of the FRa antigen to the FRa specific-CAR, an interaction that triggers optimal costimulation.
Figure 7. Trans-signaling CART cells preferentially localize to dual antigen-expressing tumors in vivo.
A. NSG mice with s.c. established A1847 tumors expressing or not FRa in opposite flanks received i.v. injections of 7.5 × 106 CART cells expressing CD19-28z (top), M-z/F-28 (middle), or M-28z (bottom) on days 45 and 49 post-tumor inoculation. Mice were euthanized after four weeks, and tumors were collected and stained for human CD3 expression (brown) or isotype control. Representative sections for M+F+ and M+F− A1847 tumor derivatives are shown (10X magnifications). B. Quantification of CD3+ T cells within A1847(M+/F+) and A1847(M+/F−) tumors in the differentially treated groups. CD3+ T cells were counted in 10 randomly selected intratumoral fields of each slide at 20X magnification. Data are expressed as the means ± SE with n=5 for all groups. *P < 0.05 comparing the tumor T cell infiltration in A1847(M+/F+) versus A1847(M+/F−) in M-z/F-28 CART-treated mice.
DISCUSSION
Adoptive immunotherapy involving genetic modification of T cells with antigen-specific, chimeric, single-chain receptors is a promising approach for the treatment of cancer (25). However, the therapeutic value of adoptively transferred, gene-engineered T cells may be compromised by extensive autoimmune damage to normal tissues expressing the target antigen (26). This adverse side-effect has been encountered in clinical trials using T cells genetically modified to express a tumor antigen-specific CAR (27). Mild to severe autoimmune toxicity has been reported following transfer of tumor-reactive T cells including liver toxicity upon adoptive transfer of anti-CAIX CART cells (28) due to CAIX expression on bile duct epithelium. Serious adverse events (SAEs) involving death of a patient has been observed in two distinct clinical trials following adoptive transfer of CART cells redirected against ErbB2(12) or CD19(29), though the role for modified T cells in the latter SAE remains unclear. Collectively, studies in preclinical mouse models and in patients have indicated that the number of T cells administered, the expression levels and localization of antigen in normal tissues, the type of signaling domains incorporated into chimeric receptors and the level of immune preconditioning used are parameters that must be carefully considered in the design of safe engineered T-cell therapeutic strategies (26, 30–35).
CARs with one or more costimulatory signals confer a new potential to respond to target antigen with sustained proliferation and cytotoxicity, and resistance to AICD and regulatory T-cell suppression (20, 36). However, powerful costimulation raises a potential danger for cross-reactivity against normal host tissues expressing low levels of the target TAA. One approach to forestall this problem involves the physical separation of the signal 1 module (CD3ζ) from the signal 2 module (costimulation) through their incorporation into two distinct CARs specific for two different antigens, to recapitulate natural T cell biology and function. In this way, dual CART cells may selectively traffic, survive and exert sustained proliferation within the tumor microenvironment since synergistic signals would be delivered to T-cells preferentially at that location. Hence, the potential for “on-target” toxicity should be reduced commensurately. Indeed, CARs can be engineered to provide co-stimulation alone (37). For example, Jurkat cells engineered to co-express hapten-specific CD3zeta- and CD28-based CARs, triggered complementary signaling leading to IL-2 production (38). So far, early attempts to preferentially redirect CART cells against tumors expressing multiple antigens in order to limit potential toxicity were not successful enough to hold promise for clinical application. In one study, CART were outfitted with two first generation CARs specific for ErbB2 and a-folate receptor to increase specificity for tumor. These dual-transduced CART cells expressed similar amounts of total surface CARs as mono-transduced T cells, that is each individual CAR was expressed at a lower level(39). Thus, the dual-transduced T cells cross-reacted considerably less avidly with target cells expressing only a single antigen than with tumor cells expressing both antigens. Since these CART cells exhibited no costimulation activity due to the lack of any costimulatory domains in either of the CARs, they were unlikely to survive, persist and clear tumors in vivo. Another recent study proposed dual targeting of ErbB2 and MUC1 in breast cancer by generating dual CART cells transduced with both a CD28 containing MUC1 CAR and a CD3ζ containing ErbB2 CAR (40). These dual CART cells were even less efficient than first generation CART cells and failed to secrete IL-2 in response to trans-signaled costimulation upon encounter with a second antigen, thus rendering the system unsuitable for further pre-clinical investigation.
Here, we utilized two distinct CARs, redirected against mesothelin and FRa, to test the concept of combinatorial CAR signaling for highly selective anti-tumor activity. The anti-mesothelin CAR contained the CD3ζ signaling motif alone whereas the anti-FRa CAR included only the intracellular domain of the CD28 costimulatory molecule. Proper costimulation of these engineered T cells relies upon the co-expression of both FRa and mesothelin on the tumor cell surface. In comparisons between trans-signaling CART cells with conventional first and second generation anti-mesothelin CART cells, trans-signaling CART cells showed similar in vitro potency to cis-signaling CART cells and were capable of producing increased levels of Th1 cytokines compared to first generation CART cells. Unlike the findings of Wilkie and colleagues(40), tumor-induced costimulation through the anti-FRa-28 CAR triggered enhanced secretion of IL-2, a cytokine known to promote T cell proliferation and persistence in vivo(41, 42). Furthermore, costimulation delivered in trans was sufficient to protect those CART cells from AICD, similar to the effects seen in cis-signaling CART cells (9, 24). The cytolytic potential of trans-signaling CART cells in vitro was similar to first and second generation CART cells. This is consistent with previous studies demonstrating no statistically significant difference in specific tumor lysis by CART cells that include or lack incorporated costimulatory domains (11, 20, 43).
The significance of a trans-signaling CAR approach is best tested in preclinical models where the targeting of both human tumor cells and representative normal tissue cells by CART cells can be evaluated simultaneously. Ovarian tumors generally overexpress both FRa (90%) and mesothelin (70%), rendering ovarian cancer an attractive study paradigm (17, 44). Moreover, the pattern of mesothelin and FRa expression on normal tissues is largely non-overlapping. Mesothelin is expressed by mesothelial cells lining the pleura and peritoneum and at low levels by epithelial cells of the trachea, tonsils, fallopian tube and the testis (14, 15). FRa expression is limited to the apical surface of the kidney proximal tubules, choroid plexus, lung epithelium, thyroid and intestinal brush border epithelial cells (45, 46). In our study, in vivo anti-tumor activity of trans-signaling CART cells was initially tested by adoptively transferring the different CART cell populations into immunodeficient mice with established ovarian cancer, using the A1847 human ovarian cancer cell line expressing both mesothelin and FRa. Trans-signaling CART cells exhibited potent antitumor activity against ovarian cancer in vivo, equivalent to that achieved using conventional cis-signaling CART cells. More importantly, the finding that trans-signaling CART cells are more selective, potent and localized within cancers furnished with both antigens, but spare normal cells bearing a single antigen, may have important translational ramifications. Indeed, tumor localization appears to be a key factor for the success of CART cell therapy (6). For example, mesothelin redirected T cells co-cexpressing an anti-mesothelin CAR and a chemokine receptor (CCR2) showed increased localization in malignant pleural mesotheliomas secreting the chemokine CCL2 and thus improved the therapeutic outcome in pre-clinical mouse models(47).
Upon trafficking to the normal tissues expressing mesothelin alone, trans-signaling CART cells receive only signal 1 upon antigen contact. This may render the designer T cells susceptible to AICD or drive them into anergy (24, 36, 48) and consequently restrict their potential for long-term persistence and tumor elimination when applied to patients. Alternatively, recognition of FRa alone and singular transmission of CD28 signal, in the absence of mesothelin-directed CD3ζ-signals, does not activate trans-signaling CART cells. Importantly, equal regression of tumors was observed in our model using cis- or trans-signaling CART cells, illustrating the idea that a dual CART cell approach can modestly improve safety for clinical application without significantly diminishing the antitumor potential of the second generation CAR approach. Dual CART cells which receive combined CD3ζ and costimulatory signals upon antigen-specific interactions with tumor did not appear to be rendered costimulation-independent and endowed with the capacity to react strongly and systemically against the tissue expressing single antigen. This was apparent in our preclinical model where there was a significant difference in the control of tumor bearing two antigens versus the more rapid growth of cells with only one antigen following treatment with M-z/F-28 CART cells. This was further supported by the statistically higher infiltration and accumulation of trans-signaling CART cells into the tumor sites where both antigens were expressed.
Our preclinical findings extend beyond the in vitro study of Wilkie and colleagues(40) and were rationalized by the finding that our dual CAR T cells are more favorable effector cells for adoptive cell transfer than first generation CART cells due to their resistance to AICD and ability to secrete elevated levels of Th1 cytokine including IL-2 in response to trans-signaled costimulation upon encounter with a second antigen. Since the submission of our manuscript, an additional study of has now confirmed these enhanced effector properties of dual signaling CAR T cells upon secondary antigen encounter in vitro(49). Using CARs specific for PSMA and PSCA, this report confirmed the preferential killing of tumor cells bearing both antigens in vivo, compared to single antigen target cells, thus reducing the potential for harmful side effects(49). The factor(s) accounting for the reduced effector cell efficiency and IL-2 production by dual CAR T cells stimulated with both antigens in vitro observed in the Wilkie study (40) remain unclear. Differences in specificity, affinity and stoichiometry of CARs, as well as different model systems may be influencing factors however the observation of high level IL-2 production by T cells bearing a ErbB2-specific CAR with no costimulatory endodomain in this study (40) is unusual, not observed in multiple other studies (8, 13, 43) and suggests uniqueness of this particular first generation CAR.
Trans-signaling CARs represent a novel approach to focus CART cells to tumor cells and reduce their impact on single antigen-expressing cells; however this method is highly dependent upon identification of two antigens which are nearly uniformly expressed in a particular cancer type, with relatively low and non-overlapping expression in normal tissues. Accordingly, the identification and application of alternative approaches to limit CART-mediated autoimmune effects remains warranted. One approach to limit such toxicity is the careful design of a dose-escalation strategy to better define the optimal T cell dose (26). Some of the potential side effects of non-tumor cell recognition by CART cells can be overcome by the co-expression of conditional suicide genes such as such as incorporation of HSV-TK, the cytoplasmic domain of Fas or an inducible caspase into gene-engineered T cells to abort aberrant T-cell responses(30–33). Indeed, the iCasp9 cell-suicide system has been shown to increase the safety of cellular therapies in patients that received T cells depleted of allo-reactive progenitor cells (34). Alternatively, electroporation of T cells with optimized RNAs for transient CAR expression has been effective in preclinical mouse models and might bypass the associated safety concerns of integrating gene vectors(50). These approaches toward cell product safety are not mutually exclusive and future application of dual CART cells engineered in combination with suicide switches as described above may better permit preferential CART cell accumulation and activity within the tumor microenvironment and in a safe manner.
Supplementary Material
1
2
3
4
5
6
7
Acknowledgments
We gratefully acknowledge Drs. Steven Albelda, Gwenn Danet-Desnoyers, Michael Kalos, Michael Milone, Edmund Moon and Nathalie Scholler, and Denarda Dangaj, for helpful suggestions, discussions and reagents. This work was supported by grants from the W.W. Smith Charitable Trust, the Sandy Rollman Ovarian Cancer Foundation, the Ovarian Cancer Research Fund (PPD-Penn-01.12), NIH RO1-CA168900 and the Joint Fox Chase Cancer Center and University of Pennsylvania Ovarian Cancer SPORE (P50 CA083638).
References
- 1.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989;86:10024–8. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inman BA, Frigola X, Dong H, Kwon ED. Costimulation, coinhibition and cancer. Curr Cancer Drug Targets. 2007;7:15–30. doi: 10.2174/156800907780006878. [DOI] [PubMed] [Google Scholar]
- 4.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–71. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–15. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–2. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 8.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song DG, Ye Q, Poussin M, Harms GM, Figini M, Powell DJ., Jr CD27 costimulation augments the survival and anti-tumor activity of redirected human T cells in vivo. Blood. 2011 doi: 10.1182/blood-2011-03-344275. [DOI] [PubMed] [Google Scholar]
- 10.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–6. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanitis E, Poussin M, Hagemann IS, Coukos G, Sandaltzopoulos R, Scholler N, et al. Redirected antitumor activity of primary human lymphocytes transduced with a fully human anti-mesothelin chimeric receptor. Mol Ther. 2012;20:633–43. doi: 10.1038/mt.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang K, Pastan I, Willingham MC. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer. 1992;50:373–81. doi: 10.1002/ijc.2910500308. [DOI] [PubMed] [Google Scholar]
- 15.Ordonez NG. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod Pathol. 2003;16:192–7. doi: 10.1097/01.MP.0000056981.16578.C3. [DOI] [PubMed] [Google Scholar]
- 16.Kalli KR, Oberg AL, Keeney GL, Christianson TJ, Low PS, Knutson KL, et al. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol Oncol. 2008;108:619–26. doi: 10.1016/j.ygyno.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toffoli G, Cernigoi C, Russo A, Gallo A, Bagnoli M, Boiocchi M. Overexpression of folate binding protein in ovarian cancers. Int J Cancer. 1997;74:193–8. doi: 10.1002/(sici)1097-0215(19970422)74:2<193::aid-ijc10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 18.Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338:284–93. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 19.Mantovani LT, Miotti S, Menard S, Canevari S, Raspagliesi F, Bottini C, et al. Folate binding protein distribution in normal tissues and biological fluids from ovarian carcinoma patients as detected by the monoclonal antibodies MOv18 and MOv19. Eur J Cancer. 1994;30A:363–9. doi: 10.1016/0959-8049(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 20.Song DG, Ye Q, Carpenito C, Poussin M, Wang LP, Ji C, et al. In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T cells is enhanced by costimulatory signaling through CD137 (4-1BB) Cancer Res. 2011;71:4617–27. doi: 10.1158/0008-5472.CAN-11-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 22.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hombach A, Abken H. Costimulation tunes tumor-specific activation of redirected T cells in adoptive immunotherapy. Cancer Immunol Immunother. 2007;56:731–7. doi: 10.1007/s00262-006-0249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther. 2010;18:413–20. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plaimauer B, Zimmermann K, Volkel D, Antoine G, Kerschbaumer R, Jenab P, et al. Cloning, expression, and functional characterization of the von Willebrand factor-cleaving protease (ADAMTS13) Blood. 2002;100:3626–32. doi: 10.1182/blood-2002-05-1397. [DOI] [PubMed] [Google Scholar]
- 26.Ertl HC, Zaia J, Rosenberg SA, June CH, Dotti G, Kahn J, et al. Considerations for the clinical application of chimeric antigen receptor T cells: observations from a recombinant DNA Advisory Committee Symposium held June 15, 2010. Cancer Res. 2011;71:3175–81. doi: 10.1158/0008-5472.CAN-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heslop HE. Safer CARS. Mol Ther. 2010;18:661–2. doi: 10.1038/mt.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feugier P, Labouyrie E, Djeridane M, Jenabian A, Dubruille V, Berthou C, et al. Comparison of initial characteristics and long-term outcome of patients with lymphocyte-predominant Hodgkin lymphoma and classical Hodgkin lymphoma at clinical stages IA and IIA prospectively treated by brief anthracycline-based chemotherapies plus extended high-dose irradiation. Blood. 2004;104:2675–81. doi: 10.1182/blood-2004-02-0567. [DOI] [PubMed] [Google Scholar]
- 29.Brentjens R, Yeh R, Bernal Y, Riviere I, Sadelain M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18:666–8. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Straathof KC, Pule MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–54. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen JL, Boyer O, Thomas-Vaslin V, Klatzmann D. Suicide gene-mediated modulation of graft-versus-host disease. Leuk Lymphoma. 1999;34:473–80. doi: 10.3109/10428199909058474. [DOI] [PubMed] [Google Scholar]
- 32.Thomis DC, Marktel S, Bonini C, Traversari C, Gilman M, Bordignon C, et al. A Fas-based suicide switch in human T cells for the treatment of graft-versus-host disease. Blood. 2001;97:1249–57. doi: 10.1182/blood.v97.5.1249. [DOI] [PubMed] [Google Scholar]
- 33.Tey SK, Dotti G, Rooney CM, Heslop HE, Brenner MK. Inducible caspase 9 suicide gene to improve the safety of allodepleted T cells after haploidentical stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:913–24. doi: 10.1016/j.bbmt.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–83. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuo JS, Zhao Y, Schiro PG, Ng L, Lim DS, Shelby JP, et al. Deformability considerations in filtration of biological cells. Lab Chip. 2010;10:837–42. doi: 10.1039/b922301k. [DOI] [PubMed] [Google Scholar]
- 36.Emtage PC, Lo AS, Gomes EM, Liu DL, Gonzalo-Daganzo RM, Junghans RP. Second-generation anti-carcinoembryonic antigen designer T cells resist activation-induced cell death, proliferate on tumor contact, secrete cytokines, and exhibit superior antitumor activity in vivo: a preclinical evaluation. Clin Cancer Res. 2008;14:8112–22. doi: 10.1158/1078-0432.CCR-07-4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krause A, Guo HF, Latouche JB, Tan C, Cheung NK, Sadelain M. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. J Exp Med. 1998;188:619–26. doi: 10.1084/jem.188.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarez-Vallina L, Hawkins RE. Antigen-specific targeting of CD28-mediated T cell co-stimulation using chimeric single-chain antibody variable fragment-CD28 receptors. Eur J Immunol. 1996;26:2304–9. doi: 10.1002/eji.1830261006. [DOI] [PubMed] [Google Scholar]
- 39.Duong CP, Westwood JA, Berry LJ, Darcy PK, Kershaw MH. Enhancing the specificity of T-cell cultures for adoptive immunotherapy of cancer. Immunotherapy. 2011;3:33–48. doi: 10.2217/imt.10.81. [DOI] [PubMed] [Google Scholar]
- 40.Wilkie S, van Schalkwyk MC, Hobbs S, Davies DM, van der Stegen SJ, Pereira AC, et al. Dual Targeting of ErbB2 and MUC1 in Breast Cancer Using Chimeric Antigen Receptors Engineered to Provide Complementary Signaling. J Clin Immunol. 2012 doi: 10.1007/s10875-012-9689-9. [DOI] [PubMed] [Google Scholar]
- 41.Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66:10995–1004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 42.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–23. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hombach A, Sent D, Schneider C, Heuser C, Koch D, Pohl C, et al. T-cell activation by recombinant receptors: CD28 costimulation is required for interleukin 2 secretion and receptor-mediated T-cell proliferation but does not affect receptor-mediated target cell lysis. Cancer Res. 2001;61:1976–82. [PubMed] [Google Scholar]
- 44.Hassan R, Kreitman RJ, Pastan I, Willingham MC. Localization of mesothelin in epithelial ovarian cancer. Appl Immunohistochem Mol Morphol. 2005;13:243–7. doi: 10.1097/01.pai.00000141545.36485.d6. [DOI] [PubMed] [Google Scholar]
- 45.Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski VR, Jr, et al. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992;52:3396–401. [PubMed] [Google Scholar]
- 46.Holm J, Hansen SI, Sondergaard K, Hoier-Madsen M. The high-affinity folate binding protein in normal and malignant mammary gland tissue. Adv Exp Med Biol. 1993;338:757–60. doi: 10.1007/978-1-4615-2960-6_158. [DOI] [PubMed] [Google Scholar]
- 47.Moon EK, Carpenito C, Sun J, Wang LC, Kapoor V, Predina J, et al. Expression of a Functional CCR2 Receptor Enhances Tumor Localization and Tumor Eradication by Retargeted Human T cells Expressing a Mesothelin-Specific Chimeric Antibody Receptor. Clin Cancer Res. 2011;17:4719–30. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berry LJ, Moeller M, Darcy PK. Adoptive immunotherapy for cancer: the next generation of gene-engineered immune cells. Tissue Antigens. 2009;74:277–89. doi: 10.1111/j.1399-0039.2009.01336.x. [DOI] [PubMed] [Google Scholar]
- 49.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2012;31:71–5. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9053–61. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2
3
4
5
6
7