Fluoxetine Ameliorates Behavioral and Neuropathological Deficits in a Transgenic Model Mouse of α-synucleinopathy (original) (raw)
. Author manuscript; available in PMC: 2014 Jan 21.
Abstract
The term α-synucleinopathies refers to a group of age-related neurological disorders including Parkinson’s disease (PD), Dementia with Lewy Bodies (DLB) and Multiple System Atrophy (MSA) that display an abnormal accumulation of alpha-synuclein (α-syn). In contrast to the neuronal α-syn accumulation observed in PD and DLB, MSA is characterized by a widespread oligodendrocytic α-syn accumulation. Transgenic mice expressing human α-syn under the oligodendrocyte-specific myelin basic protein promoter (MBP1-hαsyn tg mice) model many of the behavioral and neuropathological alterations observed in MSA.
Fluoxetine, a selective serotonin reuptake inhibitor, has been shown to be protective in toxin-induced models of PD, however its effects in an in vivo transgenic model of α-synucleinopathy remain unclear. In this context, this study examined the effect of fluoxetine in the MBP1-hαsyn tg mice, a model of MSA.
Fluoxetine adminstration ameliorated motor deficits in the MBP1-hαsyn tg mice, with a concomitant decrease in neurodegenerative pathology in the basal ganglia, neocortex and hippocampus. Fluoxetine adminstration also increased levels of the neurotrophic factors, GDNF (glial-derived neurotrophic factor) and BDNF (brain-derived neurotrophic factor) in the MBP1-hαsyn tg mice compared to vehicle-treated tg mice. This fluoxetine-induced increase in GDNF and BDNF protein levels was accompanied by activation of the ERK signaling pathway. The effects of fluoxetine adminstration on myelin and serotonin markers were also examined.
Collectively these results indicate that fluoxetine may represent a novel therapeutic intervention for MSA and other neurodegenerative disorders.
INTRODUCTION
The term α-synucleinopathies collectively refers to a recently recognized group of heterogeneous neurodegenerative disorders with parkinsonism, autonomic dysfunction and non-motor deficits (Marti, et al., 2003). In Parkinson’s disease (PD) and Dementia with Lewy bodies (DLB) alpha-synuclein (α-syn) tends to accumulate mostly in neurons while in Multiple System Atrophy (MSA) there is extensive accumulation of α-syn in oligodendroglial cells and to a lesser extent in neurons (Lantos and Papp, 1994, Wakabayashi and Takahashi, 2006, Yoshida, 2007). The characteristic oligodendrocytic neuropathological lesions are known as glial cytoplasmic inclusions (GCI) (Arima, et al., 1998, Tu, et al., 1998, Wakabayashi, et al., 1998, Wakabayashi, et al., 1998). In MSA, the characteristic motor, autonomic and non-motor deficits are not responsive to conventional anti-parkinsonian treatments and currently there are no therapies available for MSA. Despite the primarily oligodendrocytic accumulation of α-syn, MSA patients also display considerable neuronal loss in the striatum, cerebellum, brainstem and cortex, accompanied by astrogliosis, microgliosis and myelin loss (Wakabayashi and Takahashi, 2006, Yoshida, 2007). Comparable neurodegenerative alterations have been observed in transgenic (tg) mice over expressing human α-syn (h-αsyn) under the control of an oligodendrocytic-specific promoter (MBP-myelin basic protein) (Shults, et al., 2005); these tg mice develop oligodendrocytic accumulations of α-syn and display behavioral deficits associated with MSA including motor abnormalities and olfactory alterations (Ubhi, et al., 2010). Similarly, tg mice over expressing h-αsyn under the proteolipid protein (PLP) (Kahle, et al., 2002) and the 2,' 3'-cyclic nucleotide 3'-phosphodiesterase (CNP) promoter (Yazawa, et al., 2005) develop MSA-like pathology.
Fluoxetine, a selective serotonin reuptake inhibitor (SSRI), is a commonly prescribed anti-depressant. In addition to its activity on the serotonergic system, fluoxetine has also been reported to have pro-proliferative activity (Chang, et al., 2010, Clark, et al., 2006, Wang, et al., 2011) and to effect levels of neurotrophic factors (NTFs) such as glial-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF) (Allaman, et al., 2011, Chang, et al., 2010, Mercier, et al., 2004). A number of recent studies have also reported that fluoxetine can protect against 6-OHDA (6-hydroxydopamine) (Suzuki, et al., 2010) and MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) (Chung, et al., 2011) induced damage in toxin-induced models of PD. However, the effects of fluoxetine in an in vivo transgenic models of α-synucleinopathy remain unclear, in this context, this study examined the effect of fluoxetine in the MBP1-hαsyn tg mice, a model of MSA (Shults, et al., 2005).
We show that fluoxetine administration ameliorated motor behavioral and neuropathological deficits in the MBP1-hαsyn tg mice and that this amelioration was associated with increased levels of the NTFs GDNF and BDNF and activation of the ERK signaling pathway in the fluoxetine-treated mice.
These results support the hypothesis that, in addition to its antidepressant activity, fluoxetine may have a neuroprotective function in the MBP1-hαsyn tg mice, which may be mediated via the elevation of NTF levels. These results suggest that fluoxetine may have a therapeutic role in the treatment of α-synucleinopathies such as MSA.
MATERIALS AND METHODS
Animals and Fluoxetine Administration
Mice expressing human α-syn under the control of the MBP promoter (MBP-hαsyn tg) were generated as previously described (Shults, et al., 2005); specifically, this study used the MBP1 line. The MBP-hαsyn line 1 mice (MBP1-hαsyn tg mice) were chosen for this study as they express an intermediate level of α-syn expression compared with the other lines; they are able to be assessed behaviorally and are breedable. In contrast, other higher expressing lines are less viable and more aggressive, making them less suitable for this study. The MBP1-hαsyn tg mice have previously been shown to accumulate α-syn in oligodendrocytes from 3 months of age and to display neuropathological alterations including myelin loss and astrogliosis and behavioral deficits (Shults, et al., 2005). These mice have also been shown to display alteration in specific NTFs (Ubhi, et al., 2010).
A total of 24 mice (6 month old), were used for this study and received either 18mg/kg of fluoxetine by gavage daily for 28 days (MBP1-hαsyn tg mice, n=6 and age-matched non-tg mice, n=6) or saline daily for 28 days (MBP1-hαsyn tg mice, n=6 and age-matched non-tg mice, n=6).
Motor behavior assessment using the Round Beam Test
The Round Beam test allows the assessment of gait and balance impairments through distance traveled in an allotted amount of time over a round beam placed horizontally. As previously described (Ubhi, et al., 2010), three consecutive trials, 1min each, are run in one day. The total forward distance traveled is recorded, as are the number of foot slippages. Speed on the beam is calculated as ‘distance traveled/time taken’ and errors on the beam are calculated as ‘foot slips/distance traveled’.
Total Activity and Thigmotaxis
Briefly, as previously described (Ubhi, et al., 2010), total activity was calculated as total beam breaks in a 10-minute period. The test was conducted for 4 trials each day for a period of 4 days. Thigmotaxis was calculated as the % of time spent in the periphery.
Tissue processing
Following NIH guidelines for the humane treatment of animals, under anesthesia mice were sacrificed and brains removed. The right hemibrain was immersion-fixed in 4% paraformaldehyde in PBS pH 7.4 and serially sectioned at 40 µm with the Vibratome (Leica, Deerfield, IL) for subsequent analysis of neurodegeneration. The left hemibrain was kept at −80°C for biochemical analysis.
Immunohistochemistry
As previously described (Ubhi, et al., 2010), vibratome sections (40µm) were immunolabeled overnight with antibodies against GDNF (rabbit polyclonal, 1:250, Abcam), NeuN (mouse monoclonal, 1:1000, Millipore), GFAP (rabbit polyclonal, 1:500, Millipore), TH (rabbit polyclonal, 1:200; Millipore), doublecortin (goat polyclonal, 1:500, Santa Cruz), PCNA (mouse monoclonal, 1:500, Millipore), tryptophan hydroxylase (5HT-OH) (sheep polyclonal, 1:500, Millipore), serotonin (5HT) (mouse monoclonal, 1:500, Millipore) and α-syn (rabbir polyclonal, 1:500; Millipore) followed by incubation with species-appropriate secondary antibodies (1:2000, Vector Laboratories). Sections stained with GDNF, NeuN, GFAP, TH, doublecortin, PCNA and 5HT-OH were reacted with 3,3'-Diaminobenzidine (DAB) and imaged on the Olympus BX54 brightfield digital microscope. Sections stained with 5HT and α-syn were reacted with FITC-tagged secondary antibodies and were analyzed with the laser scanning confocal microscope (LSCM) (MRC1024, BioRad). Sections were transferred to SuperFrost slides (Fisher Scientific, Tustin, CA) and mounted under glass coverslips with anti-fading media (Vector Laboratories). Additional sections were stained with Luxol Fast Blue (LFB) in order to visualize the myelin layers and imaged on the Olympus BX54 brightfield digital microscope.
As previously described (Ubhi, et al., 2010) stereological estimates of density of NeuN and raphe nucleus 5HT-OH immunoreactive cells was conducted with the Stereo-Investigator Software system (MBF Biosciences) for which images were randomly collected utilizing the optical disector method and expressed as cells per unit volume. The levels of TH, GFAP and GDNF and 5HT-OH fiber immunoreactivity were assessed by obtaining optical density measurements using the Image Quant 1.43 program (NIH), corrected against background levels and are presented as ‘corrected optical density’ where the background signal has been subtracted from optical density measurements.
Immunoblotting
Briefly as previously described (Ubhi, et al., 2010), twenty micrograms of protein per mouse, from the cytosolic fraction of mouse brain homogenates, was loaded onto 4–12% Bis-Tris (Invitrogen) SDS-PAGE gels, transferred onto Immobilon membranes, washed and blocked in BSA. After an overnight incubation with antibodies against either GDNF (rabbit polyclonal, 1:1000, Abcam), BDNF (rabbit polyclonal, 1:1000, Millipore), total α-syn (rabbit polyclonal, 1:1000, Millipore), PCNA (1:1000, Millipore), phosphorylated ERK (pERK, 1:1000, Millipore) or total ERK (tERK, 1:1000, Millipore), membranes were incubated in appropriate secondary antibodies, reacted with ECL and developed on a VersaDoc gel-imaging machine (Bio-Rad, Hercules, CA). Anti-beta-actin (mouse monoclonal, 1:1000; Sigma) antibody was used to confirm equal loading. For each antibody, lysates from all 24 samples across the experimental groups were analyzed in triplicate, images presented are representative of the replicates however only contain four samples from each groups for the purposes of clarity. Quantitative analyses presented were performed with results from all the samples tested.
Tissue Culture and Fluoxetine Administration
As previously described (Ubhi, et al., 2010), the rat CG4 line (Louis, et al., 1992) was differentiated to an oligodendrocytic lineage by incubation in 70% (DMEM with 10ng/ml biotin and 100ug penicillin) 30% B104 conditioned media at 37°C. Cells were allowed to differentiate for four days without additional feeding and were infected with LV-α-syn (Spencer, et al., 2009) at a multiplicity of infection (MOI) of 30 and left for an additional three days. Following differentiation and infection, cells were treated with 25uM Fluoxetine (LKT Labs) for 24 hours. Cells were then lysed in TNE (20mM Tris–HCl, 150mM NaCl, 1mM EDTA, 1% NP-40, 5mM 2-mercaptoethanol, 1× protease inhibitor cocktail (Calbiochem) and 1× phosphatase inhibitor cocktail (Calbiochem) containing sodium orthovanadate) with 1% Triton-X 100 for immunoblot analysis as described above. Appropriate differentiation into an oligodendrocytic lineage was assessed using the oligodendrocytic marker myelin basic protein (MBP, mouse monoclonal, 1:1000, Abcam), the astroglial marker GFAP (rabbit polyclonal, 1:1000, Millipore) and the neuronal marker Tuj1 (mouse monoclonal, 1:2000, Covance). The effect of fluoxetine treatment was assessed using antibodies against GDNF (rabbit polyclonal, 1:1000, Abcam), total α-syn (rabbit polyclonal, 1:1000, Millipore), phosphorylated ERK (mouse monoclonal, pERK, 1:1000, Millipore) or total ERK (mouse monoclonal, tERK, 1:1000, Millipore). Anti-beta-actin (mouse monclonal, 1:1000; Sigma) antibody was used to confirm equal loading.
Determination of GDNF protein content by ELISA
As previously described (Ubhi, et al., 2010), the concentration of GDNF protein in mouse whole brain homogenate tissue was determined by ELISA (Promega, Madison, WI), briefly the samples were sonicated in a homogenization buffer (20 mM Tris, pH 8, 137 mM NaCl, 1% Nonidet P-40, 1,7 µg/ml phenylmethylsulfonyl fluoride, 10 µg/ml aprotinin, 1 µg/ml leupeptin and 0.5 mM sodium vanadate) and centrifuged at 100K for 1 hour (Ultracentrifuge, Beckkam Coulter) to obtain cytosolic (soluble) and particulate (insoluble, membrane-bound) fractions. The cytosolic fraction was used for ELISA analysis. Levels of GDNF were determined in homogenates by ELISA according to the supplier's protocol.
RNA Extraction and Quantification of mRNA by Real Time-PCR (RT-PCR) analysis
Total RNA was extracted from MBP-hαsyn tg mice and non-tg mice using the RNAeasy kit (QIAGEN) as per manufacturer's instructions. All the samples were treated with DNAse I to eliminate genomic DNA contamination. RNA quantification was determined by spectrophotometer readings. The ratio of OD260/OD280 was used to evaluate the purity of the nucleic acid samples and the quality of the extracted total RNA was determined using agarose gel electrophoresis.
For cDNA synthesis, 1 µg total RNA was reverse transcribed using iScript cDNA Synthesis kit (BioRad). RT-PCR experiments were performed using the iQ5 Detection System (BioRad). Amplification was performed on a cDNA amount equivalent to 25 ng total RNA with 1× iQ SYBRGreen Supermix (BioRad) containing dNTPs, MgCl2, Taq DNA polymerase, and forward and reverse primers. PCR reactions were performed on three independent sets of template. Experimental samples and no-template controls were all run in triplicate. The PCR cycling parameters were: 50 °C for 2 min, 95 °C for 10 min, and 40 cycles of 94 °C for 15 s, 60 °C for 1 min. Finally, a dissociation protocol was also performed at the end of each run to verify the presence of a single product with the appropriate melting point temperature for each amplicon. The amount of studied cDNA in each sample was calculated by the comparative threshold cycle method and expressed using mouse actin as an internal control. RT-PCR primers for GDNF (forward): CGA TAT TGC AGC GGT TCC TG, GDNF (reverse): CTG CAA CAT GCC TGG CCT AC, BDNF (forward): TCA TAC TTC GGT TGC ATG AAG G and BDNF (reverse): AGA CCT CTC GAA CCT GCC C.
Statistical Analysis
Differences between groups were tested using one and two-way analysis of variance (ANOVA) with Fisher PLSD posthoc tests. All results are expressed as mean +/− SEM.
RESULTS
Fluoxetine ameliorates motor behavioral deficits in a transgenic model of MSA
MSA, particularly the parkinsonian subtype, is characterized by motor abnormalities such as tremor, rigidity and gait and limb ataxia; many of these features are replicated in our MBP1-hαsyn tg mice (Shults, et al., 2005). In order to investigate the effects of fluoxetine on motor behavior in the MBP1-hαsyn tg mice, saline and fluoxetine-treated mice were examined on the Round Beam test to examine gait and balance impairments and in the Open Field test to assess effects on spontaneous motor behavior.
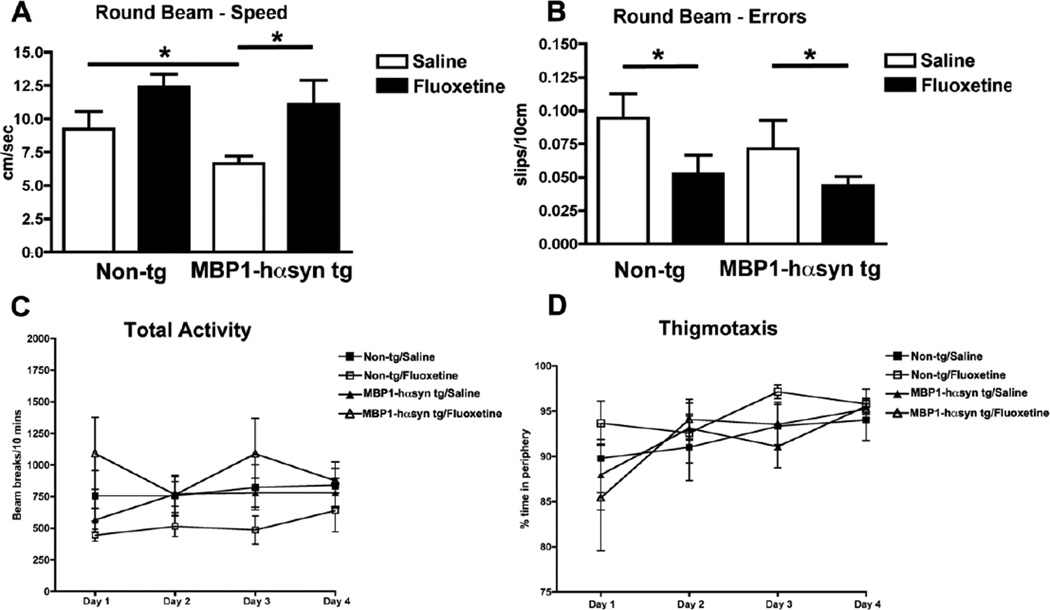
Saline-treated MBP1-hαsyn tg mice were slower at traversing the beam in comparison to the saline-treated non-tg mice (Figure 1A) indicative of gait and balance deficits. Fluoxetine significantly increased the speed with which the MBP1-hαsyn tg mice were able to travel the beam (Figure 1A), with the time taken by fluoxetine-treated MBP1-hαsyn tg mice to travel the beam being comparable to that of the saline-treated non-tg mice. Fluoxetine had no significant effect on the speed of the non-tg mice. Whilst the error-rate (foot slips/10cm) did not differ significantly between saline-treated MBP1-hαsyn tg and non-tg mice, fluoxetine was able to significantly decrease the number of errors made by both the MBP1-hαsyn tg (treated=0.047±0.007 vs untreated 0.72±0.035) and non-tg (treated=0.051±0.013 vs untreated 0.92±0.02) mice in comparison to their saline-treated littermates (Figure 1B, p<0.05). Fluoxetine had no effect on levels of total spontaneous activity or thigmotaxis in either group (Figure 1C, D), indicating that its effect on motor behavior is not a reflection of non-specific hyperactivity. These results indicate that fluoxetine is able to ameliorate motor deficits in the MBP1-hαsyn tg mice by improving the gait, balance and motor coordination of these mice.
Figure 1. Fluoxetine ameliorates motor deficits in the MBP1-hαsyn transgenic model of MSA.
The effect of fluoxetine administration on motor behavior was examined using the Round Beam Test. (A) Speed and (B) Error rate were recorded. (C) Total activity and (D) thigmotaxis were also examined. Error bars represent mean ± SEM. * Indicates a significant difference (p< 0.05) between the groups indicated by the bar analyzed by one-way ANOVA and Dunnett’s post hoc test.
Fluoxetine ameliorates neurodegenerative pathology in a transgenic model of MSA
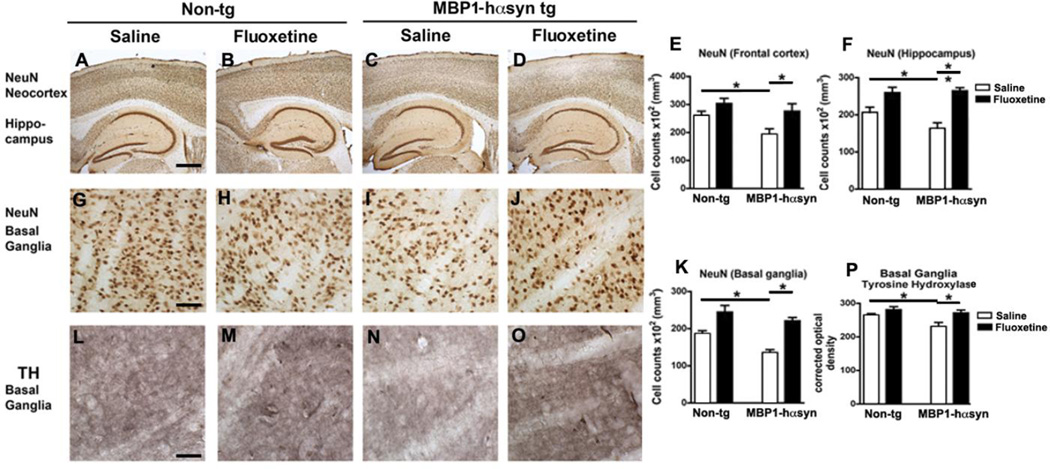
In MSA patients there is widespread neurodegeneration (Lantos and Papp, 1994, Wakabayashi and Takahashi, 2006), which is recapitulated in the MBP1-hαsyn tg mice (Shults, et al., 2005). Consistent with previous reports (Shults, et al., 2005, Ubhi, et al., 2010), analysis of NeuN immunoreactivity in the saline-treated MBP1-hαsyn tg demonstrated decreased neuronal density in the frontal cortex, hippocampus (most notable in the CA3 subfield) (Figure 2A, C, E, F) and basal ganglia (Figure 2G, I, K) in comparison to the saline-treated non-tg mice (p<0.05). In contrast, treatment with fluoxetine restored the levels of neuronal density in the frontal cortex and hippocampus of the MBP1-hαsyn tg mice in comparison to saline-treated MBP1-hαsyn tg mice (Figure 2C–F, p<0.05). In line with the improved motor behavior, fluoxetine treatment restored neuronal counts in the basal ganglia of the MBP1-hαsyn tg mice compared to saline-treated MBP1-hαsyn tg mice (Figure 2I–K, p<0.05). Fluoxetine had no effect on NeuN stereological cell counts in the non-tg mice (Figure 2A, B, E, F) and fluoxetine-treated MBP1-hαsyn tg mice displayed levels of NeuN immunoreactivity comparable to those observed in the non-tg mice (Figure 2A, B, D, E, F).
Figure 2. Fluoxetine adminstration ameliorates neuropathological deficits in neuronal density and TH alterations in the MBP1-hαsyn transgenic model of MSA.
The effect of fluoxetine administration on neuronal density was examined using an antibody against the neuronal marker NeuN. (A–D) NeuN immunoreactivity in the neocortex and hippocampus of saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. (E) Analysis of neocortical NeuN immunoreactivity across the experimental groups. (F) Analysis of hippocampal NeuN immunoreactivity across the experimental groups. (G–J) NeuN immunoreactivity in the basal ganglia of saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. (K) Analysis of basal ganglia NeuN immunoreactivity across the experimental groups. (L–O) The effect of fluoxetine administration on TH level was in the basal ganglia of saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. (P) Analysis of TH immunoreactivity in the basal ganglia across the experimental groups. Error bars represent mean ± SEM. * Indicates a significant difference (p< 0.05) between the groups indicated by the bar analyzed by one-way ANOVA and Dunnett’s post hoc test. Scale bar (AD) = 200µM, (G–J and L–O) = 50µM.
In MSA the parkinsonian features have been related to the loss of dopaminergic input to the basal ganglia (Halliday, 2007). Similarly in the saline-treated MBP1-hαsyn tg mice there is a loss of tyrosine hydroxylase (TH) immunoreactive fibers in the caudo-putamen region in comparison to the saline-treated non-tg mice (Figure 2L, N, P, p<0.05). Fluoxetine treatment restored TH immunoreactivity in the MBP1-hαsyn tg mice to levels comparable with saline-treated non-tg mice (Figure 2L, O, P, p<0.05). Fluoxetine had no effect on basal ganglia TH levels in the non-tg mice (Figure 2L, M, P) or on levels of TH in the substantia nigra of MBP1-hαsyn or non-tg mice (data not shown).
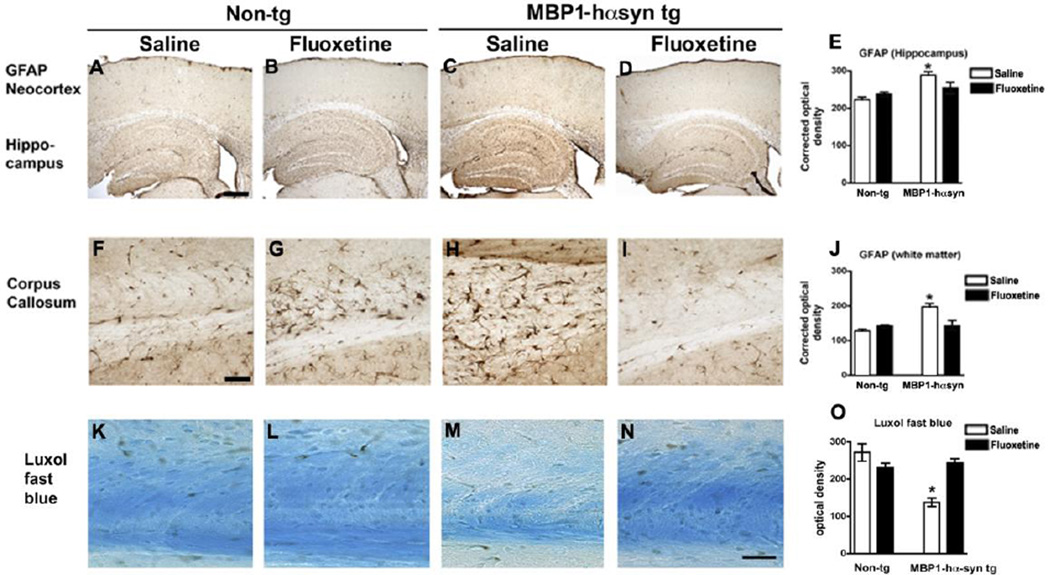
In the MBP1-hαsyn tg mice the neurodegenerative pathology is accompanied by astrogliosis in the white matter tracts, hippocampus and basal ganglia (Shults, et al., 2005). Immunohistochemical analysis of levels of astrogliosis by GFAP immunoreactivity demonstrated an increase in astrogliosis in the hippocampus (Figure 3A, C, E, p<0.05) and corpus callosum (Figure 3F, H, J, p<0.05) of the saline-treated MBP1-hαsyn tg mice in comparison to the saline-treated non-tg mice. Fluoxetine treatment significantly reduced GFAP immunoreactivity in the corpus callosum of the fluoxetine-treated MBP1-hαsyn tg mice in comparison to saline-treated MBP1-hαsyn tg mice (Figure 3H–J, p<0.05). Fluoxetine had no effect on GFAP immunoreactivity in the non-tg mice (Figure 3A, B, E, F, G, J) and fluoxetine-treated MBP1-hαsyn tg mice displayed GFAP levels comparable to those observed in the non-tg mice (Figure 3A, D, E, F, I, J). Fluoxetine had no effect on GFAP immunoreactivity in the frontal cortex of non-tg or MBP1-hαsyn tg mice (Figure 3A–E).
Figure 3. Fluoxetine adminstration does not alter levels of astrogliosis in the MBP1-hαsyn a transgenic model of MSA.
The effect of fluoxetine administration on astrogliosis was examined using an antibody against the astrocytic marker GFAP. (A–D) GFAP immunoreactivity in the neocortex and hippocampus of saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. (E) Analysis of neocortical GFAP immunoreactivity across the experimental groups. (F–I) GFAP immunoreactivity in the corpus callosum of saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. (J) Analysis of GFAP immunoreactivity in the corpus callosum across the experimental groups. (K–N) Luxol fast blue (LFB) stained myelin in the corpus callosum of saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. (O) Analysis of LFB in the corpus callosum across the experimental groups. Error bars represent mean ± SEM. * Indicates a significant difference (p< 0.05) between the groups indicated by the bar analyzed by one-way ANOVA and Dunnett’s post hoc test. Scale bar (A–D) = 150µM, (F–I) = 50µM and (K–N) = 5µM.
In order to examine the effect of fluoxetine on myelination in the MBP1-hαsyn tg mice LFB staining was performed in the corpus callosum and in line with previous studies in these mice (Shults, et al., 2005), the MBP1-hαsyn tg mice displayed reduced levels of staining with LFB in the corpus callosum in comparison to non-tg controls (Figure 3K, M, O), indicative of myelin disruption in these mice. Treatment with fluoxetine was able to ameliorate this loss of myelin and returned LFB staining levels in the MBP1-hαsyn tg mice to those observed in the control mice (Figure 3M–O). Fluoxetine had no effect on LFB staining in the non-tg mice (Figure 3K, L, O).
Together, these results indicate that the improved behavioral deficits observed in the fluoxetine-treated MBP1-hαsyn tg mice may be associated with an amelioration in neurodegenerative pathology as reflected by analysis of NeuN, TH and GFAP and that fluoxetine may be able to ameliorate myelination deficits in the corpus callosum of the MBP1-hαsyn tg mice as evidenced by LFB.
Fluoxetine ameliorates deficits in neurogenesis in a transgenic model of MSA
Neurodegeneration has traditionally been viewed in the light of cellular loss in certain neuronal populations in the brain, however emerging research has suggested that in addition to increased cell death there is also a concomitant reduction in levels of neurogenesis (Enciu, et al., 2011, Winner, et al., 2011) such that is possible to view increased cellular loss and reduced neurogenesis as two contributing factors to the neurodegenerative process.
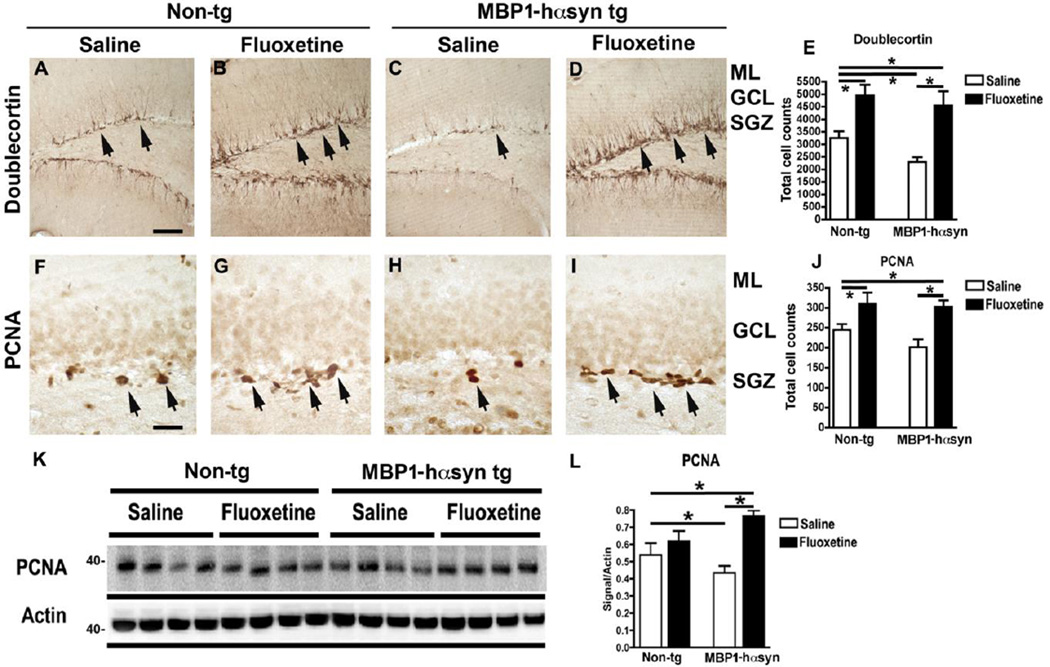
Recent work in mouse models of α-synucleinopathy has reported reduced/altered neurogenesis in the hippocampus and sub-ventricular zone of these animals (Crews, et al., 2008, Marxreiter, et al., 2009, Winner, et al., 2011, Winner, et al., 2004, Winner, et al., 2008). Fluoxetine has been reported to increase levels of neurogenesis (Chang, et al., 2010, Li, et al., 2009, Marcussen, et al., 2008, Perera, et al., 2011, Wang, et al., 2011) therefore we examined the effect of fluoxetine in the hippocampus sub-granular zone of the MBP1-hαsyn tg mice. Saline-treated MBP1-hαsyn tg mice display significantly reduced levels of doublecortin, a marker of neuroblasts (Figure 4A, C, E, p<0.05) and PCNA, a marker of proliferating cells in the sub-granular zone of the dentate gyrus (Figure 4F, H, J, p<0.05) in comparison to saline-treated non-tg mice, indicating reduced neurogenesis in the tg mice. Fluoxetine treatment resulted in an increase in the number of doublecortin (Figure 4B, D, E, p<0.05) and PCNA (Figure 4G, I, J, p<0.05) immunoreactive cells in the sub-granular zone of both the MBP1-hαsyn tg and non-tg mice. Consistent with the immunohistochemical results, immunoblot analysis showed reduced PCNA immunoreactivity in the saline-treated MBP1-hαsyn tg mice compared to saline-treated non-tg mice (Figure 4K, L, p<0.05). Fluoxetine treatment resulted in a significant increase in PCNA levels in the MBP1-hαsyn tg mice compared to saline-treated and non-tg mice (Figure 4K, L, p<0.05). These results indicate that the MBP1-hαsyn tg mice displayed altered levels of neurogenesis in comparison to the non-tg control mice and that administration of fluoxetine is able to ameliorate the deficits in neurogenesis by increasing the proliferation of neuroblasts.
Figure 4. Fluoxetine ameliorates deficits in neurogenesis in the MBP1-hαsyn transgenic model of MSA.
The effect of fluoxetine administration on neurogenesis was examined in the MBP1-hαsyn tg mice using markers of neurogenesis. (A–D) Doublecortin immunoreactivity in the dentate gyrus of saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively, arrows indicate doublecortin positive cells. (E) Analysis of dentate gyrus doublecortin levels across the experimental groups. (F–I) PCNA immunoreactivity in the subgranular zone of saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively, arrows indicate PCNA positive cells. (J) Analysis of dentate gyrus doublecortin levels across the experimental groups. (K) Immunoblot of levels PCNA in saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. (L) Analysis of PCNA in saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. Beta-actin was used as loading control. Error bars represent mean ± SEM. * Indicates a significant difference (p< 0.05) between the groups indicated by the bar analyzed by one-way ANOVA and Dunnett’s post hoc test. Scale bar (A–D) = 100µM, (F–I) = 50µM.
Fluoxetine alters levels of white matter α-syn in a transgenic model of MSA
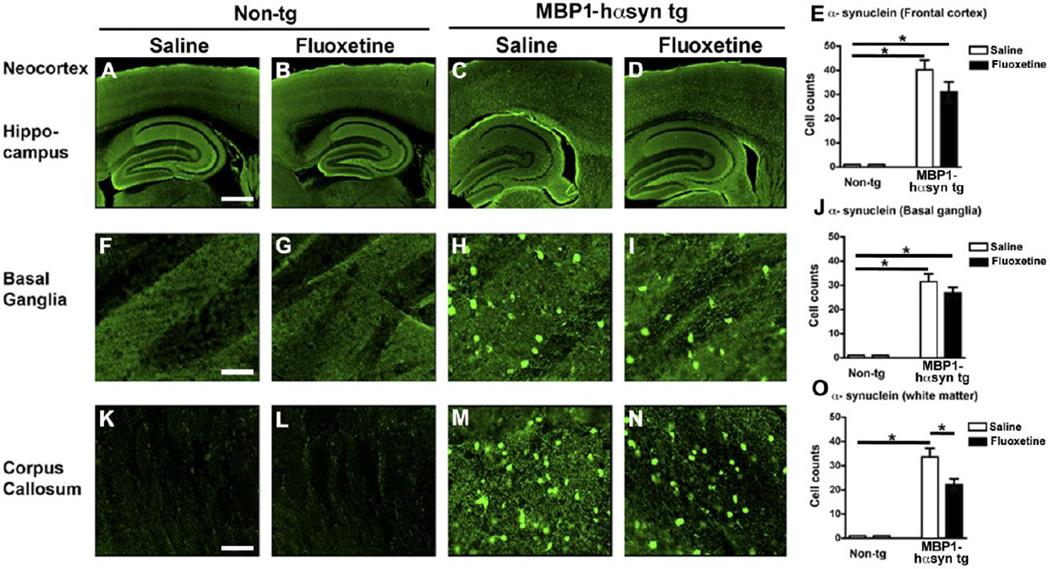
In MSA there is an extensive oligodendroglial neuropathological accumulation of α-syn, a feature that is recapitulated in the MBP1-hαsyn tg mice (Shults, et al., 2005). As previously reported (Shults, et al., 2005, Ubhi, et al., 2010) the saline-treated MBP1-hαsyn tg mice displayed oligodendroglial accumulation of α-syn in the frontal cortex (Figure 5A, C and E), basal ganglia (Figure 5F, H and J) and corpus callosum (Figure 5K, M, O). Interestingly, fluoxetine treatment resulted in a decreased accumulation of α-syn in the corpus callosum in treated MBP1-hαsyn tg mice compared to saline-treated MBP1-hαsyn tg mice (Figure 5M–O, p<0.05), but had no significant effect in the other areas examined. These results indicate that fluoxetine treatment may have a regionally specific effect on oligodendrocytic α-syn load in the white matter of the corpus callosum.
Figure 5. Fluoxetine selectively alters αsyn load in the corpus callosum of the MBP1-hαsyn transgenic model of MSA.
The effect of fluoxetine administration on αsyn protein levels was examined using immunohistochemistry and confocal microscopy. (A–D) Confocal images of αsyn immunoreactivity in the neocortex and hippocampus of saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. (E) Analysis of frontal αsyn immunoreactivity across the experimental groups. (F–I) Confocal images of αsyn immunoreactivity in the basal ganglia of saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. (J) Analysis of αsyn immunoreactivity in the basal ganglia across the experimental groups. (K–N) Confocal images of αsyn immunoreactivity in the corpus callosum of saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. (O) Analysis of αsyn immunoreactivity in the corpus callosum across the experimental groups. Error bars represent mean ± SEM. * Indicates a significant difference (p< 0.05) between the groups indicated by the bar analyzed by one-way ANOVA and Dunnett’s post hoc test. Scale bar (A–D) = 200µM, (F–I, and K–N) = 50µM.
Fluoxetine alters levels of GDNF and BDNF in a transgenic model of MSA
Fluoxetine has been shown to increase levels of NTFs (Allaman, et al., 2011, Hisaoka, et al., 2001, Mercier, et al., 2004), therefore in order obtain a mechanistic understanding of the pathways underlying the neuroprotective effect of fluoxetine in the MBP1-hαsyn tg mice we investigated whether fluoxetine treatment could enhance levels of GDNF and BDNF in the MBP1-hαsyn tg mice.
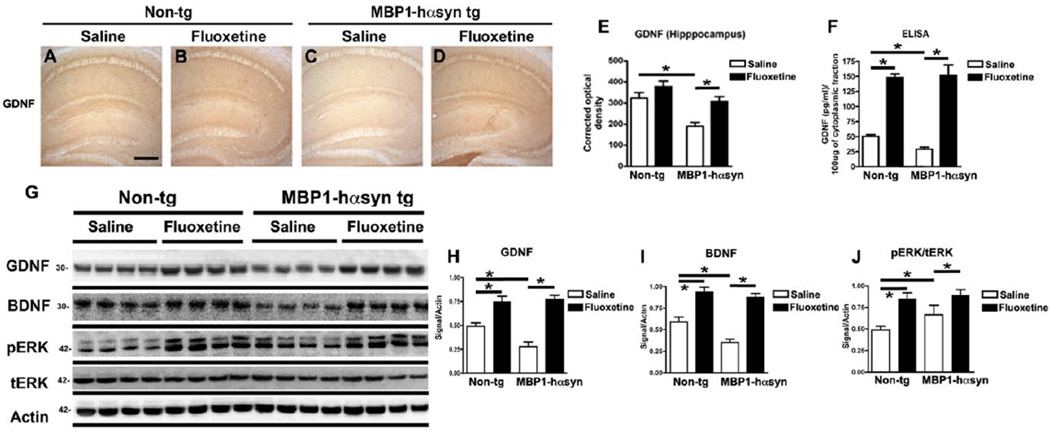
Consistent with previous reports (Ubhi, et al., 2010), the saline-treated MBP1-hαsyn tg mice displayed a significant reduction in the hippocampal levels of GDNF immunoreactivity, in comparison to the saline-treated non-tg mice, (Figure 6A, C, E, p<0.05). Fluoxetine treatment resulted in a significant increase in hippocampal levels of GDNF in the treated MBP1-hαsyn tg mice compared to saline-treated MBP1-hαsyn tg mice (Figure 6C–E, p<0.05). In agreement with the immunohistochemistry, analysis of GDNF levels by ELISA also demonstrated a robust increase in levels of GDNF following fluoxetine administration (Figure 6F, p<0.05). This effect was observed in both the non-tg and MBP1-hαsyn tg mice (Figure 6F).
Figure 6. Fluoxetine enhances GDNF and BDNF protein levels and activates the ERK signaling pathway in the MBP1-hαsyn transgenic model of MSA.
The effect of fluoxetine administration on protein levels of GDNF was examined using immunohistochemistry and ELISA. (A–D) GDNF immunoreactivity in the hippocampus of saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. (E) Analysis of hippocampal GDNF immunoreactivity across the experimental groups. (F) GDNF levels in the cytosolic fraction (containing soluble proteins) from whole-brain homogenates from saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. Error bars represent mean ± SEM. * Indicates a significant difference (p< 0.05) between the groups indicated by the bar analyzed by one-way ANOVA and Dunnett’s post hoc test. Scale bar (A–D) = 150µM
The effect of fluoxetine administration on GDNF and BDNF protein levels and the ERK signaling pathway was also examined by immunoblot. (G) Immunoblot analysis of levels GDNF, BDNF, phospho-ERK (pERK) and total ERK (tERK) in the cytosolic fraction from whole-brain homogenates from saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. (H) Analysis of GDNF levels in saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. (I) Analysis of BDNF levels saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. (J) Analysis of ERK activation (taken as a ratio between pERK/tERK) in saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. Beta-actin was used as loading control. Error bars represent mean ± SEM. * Indicates a significant difference (p< 0.05) between the groups indicated by the bar analyzed by one-way ANOVA and Dunnett’s post hoc test.
The immunohistochemical (Figure 6A–E) and ELISA (Figure 6F) results were confirmed by a third independent method, namely immunoblot analysis (Figure 6G). As anticipated, there was a significant reduction in the levels of GDNF (Figure 6G, H, p<0.05) and BDNF (Figure 6G, I, p<0.05) in the saline-treated MBP1-hαsyn tg mice compared to saline-treated non-tg controls. Treatment with fluoxetine significantly increased GDNF and BDNF levels in the MBP1-hαsyn tg mice and in the non-tg mice in comparison to their saline-treated cohorts (Figure 6G–I, p<0.05).
Given that the expression of NTFs triggered by fluoxetine has been reported to involve the ERK signaling pathway (Li, et al., 2010, Li, et al., 2008, Mercier, et al., 2004) we examined the ratio of pERK to tERK as a surrogate marker of pathway activation. We observed significantly increased the levels of pERK to tERK in the fluoxetine-treated MBP1-hαsyn tg and non-tg mice compared to the respective saline groups (Figure 6G, J, p<0.05). These results indicate the fluoxetine is able to significantly increase protein levels of GDNF (and BDNF) and activate the ERK signaling pathway in both non-tg and MBP1-hαsyn tg mice.
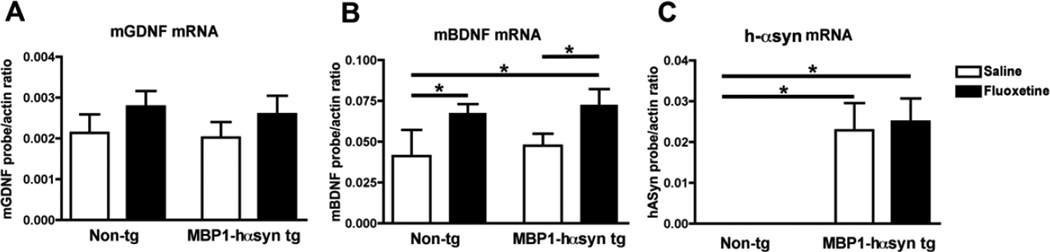
In order to determine whether the effects of fluoxetine on NTF levels is regulated at the transcriptional level, mRNA levels of GDNF, BDNF and hα-syn in the MBP1-hαsyn tg mice were examined by qPCR (Figure 7). No differences in levels of GDNF or BDNF mRNA were observed between saline-treated non-tg and MBP1-hαsyn tg mice (Figure 7A, B) and fluoxetine-treatment had no effect on levels of GDNF mRNA in these mice (Figure 7A). In contrast, fluoxetine treatment resulted in a significant increase in BDNF mRNA levels in both non-tg and MBP1-hαsyn tg mice (Figure 7B, p<0.05). Finally fluoxetine treatment had no detectable effect on levels of hαsyn mRNA in the MBP1-hαsyn tg mice (Figure 7C). Collectively, these results indicate that fluoxetine is capable of promoting the expression of GDNF protein and BDNF protein and mRNA in the MBP1-hαsyn tg mice and suggest that this NTF-enhancement by fluoxetine may have to the ability to ameliorate motor and neuropathological alterations observed in the MBP1-hαsyn tg mice (Shults, et al., 2005).
Figure 7. Differential effect of fluoxetine on GDNF, BDNF and hα-syn mRNA levels in the MBP1-hαsyn transgenic model of MSA.
Quantitative-PCR was conducted to examine the effect of fluoxetine treatment on mRNA levels of GDNF, BDNF and hα-syn. (A) GDNF mRNA levels in saline and fluoxetine-treated non-tg and MBP1-hαsyn tg mice. (B) BDNF mRNA levels in saline and fluoxetine-treated non-tg and MBP1-hαsyn tg mice. (C) h-αsyn mRNA levels in saline and fluoxetine-treated non-tg and MBP1-hαsyn tg mice. All results are presented as mean mean ± SEM of the ratio of the probe to murine actin. * Indicates a significant difference (p< 0.05) between the groups indicated by the bar analyzed by one-way ANOVA and Dunnett’s post hoc test.
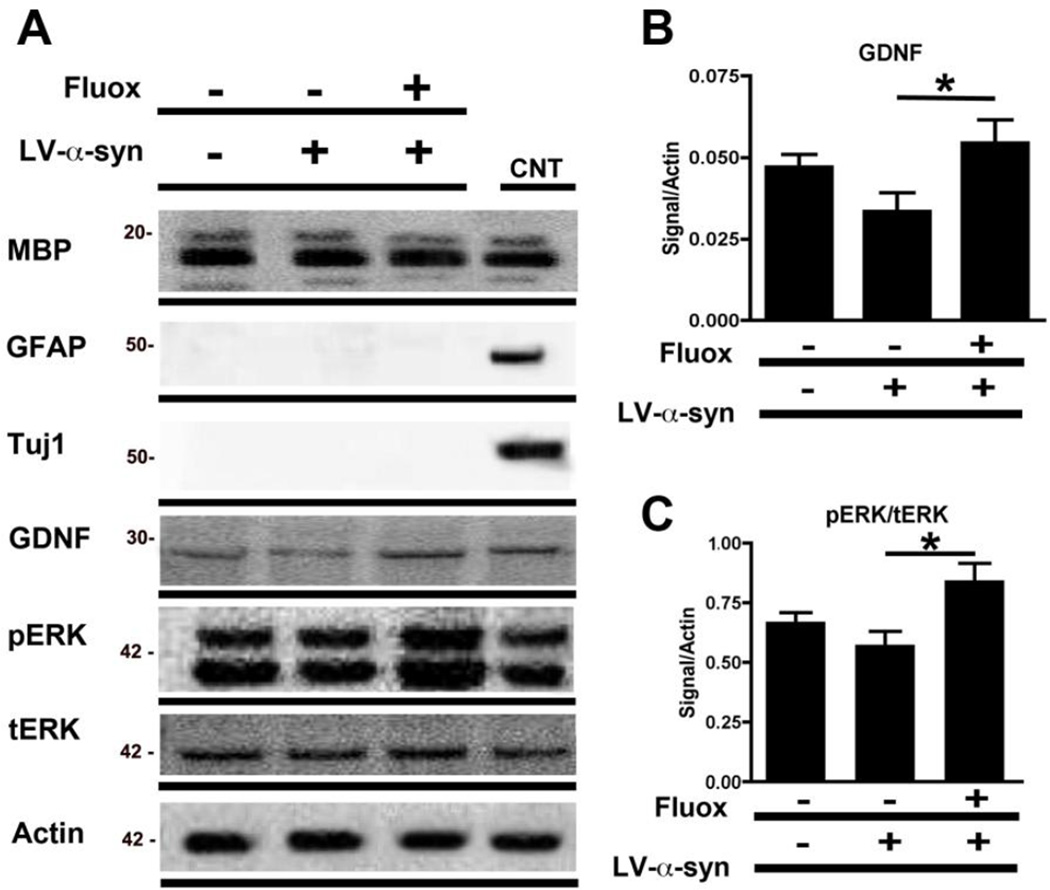
Fluoxetine has been shown in vitro to increase astroglial expression of GDNF and BDNF (Allaman, et al., 2011) however its effect on these NTFs, specifically GDNF, in oligodendrocytes, remains unexamined. To investigate this, and to further verify by an independent method the effects of fluoxetine on oligodendrocytes in vitro, the CG4 rat cell line was used. These cells were differentiated to an oligodendrocytic phenotype, infected with LV-α-syn and treated with 25uM fluoxetine for 24 hours. Consistent with the studies in the MBP1-hαsyn tg mice, immunoblot analysis of the cell lysates demonstrated a significant increase in the levels of GDNF in the fluoxetine-treated, LV-α-syn infected differentiated CG4 cells in comparison to LV-α-syn infected differentiated CG4 cells alone (Figure 8A, B, p<0.05). The increase in GDNF protein levels was accompanied by increased ERK activation as evidenced by an increased ratio of pERK to tERK (Figure 8A, C, p<0.05). Taken together, these studies support the notion that the neurotrophic effects of fluoxetine might be mediated in part by the increased expression of GDNF by oligodendroglial cells.
Figure 8. In vitro fluoxetine adminstration elevates GDNF levels and ERK signaling in an oligodendrocytic cell line.
In order to examine the effect of fluoxetine on oligodendroglial levels of GDNF the CG4 rat cell line was differentiated to an oligodendrocytic phenotype, infected with LV-α-syn and treated with 25µM fluoxetine for 24 hours. (A) Immunoblot analysis of oligodendroglial differentiation in cell lysates from CG-4 using oligodendroglial, astrocytic and neuronal markers (MBP, GFAP and Tuj-1), levels of GDNF and phospho-ERK (pERK) and total ERK (tERK). Brain homogenate from a non-tg mouse was used as a positive control for antibody signal and beta-actin was used as a loading control. (B) Analysis of GDNF levels in fluoxetine-treated oligodendroglial CG-4 cells. (C) Analysis of ERK activation (taken as a ratio between pERK/tERK) in oligodendroglial CG-4 cells. Error bars represent mean ± SEM. * Indicates a significant difference (p< 0.05) between the groups indicated by the bar analyzed by one-way ANOVA and Dunnett’s post hoc test.
Effect of Fluoxetine on the serotonergic system in a transgenic model of MSA
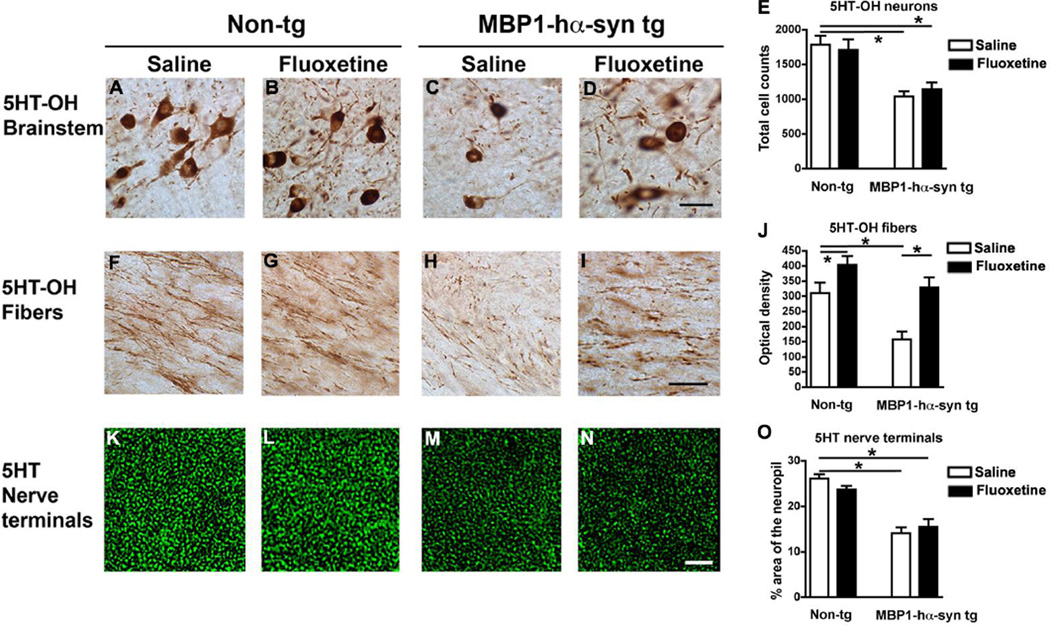
In order to examine the serotonergic system in the MBP1-hαsyn tg mice we performed immunohistochemistry with an antibody against tryptophan hydroxylase (5HT-OH), an enzyme involved in the synthesis of serotonin, in neurons in the brainstem raphe nucleus and the projecting fibers. Additional analysis of serotonergic terminals in the hippocampus was performed using an antibody against serotonin (5HT).
In comparison to saline-treated non-tg mice, saline-treated MBP1-hαsyn tg mice display a decrease in levels of 5HT-OH immunoreactivity in neurons in the brainstem (Figure 9A, C, E), decreased 5HT-OH immunoreactive fibers (Figure F, H, J). Fluoxetine treatment increased 5-HT-OH immunoreactive fibers in the MBP1-hαsyn tg mice (Figure 9H–J) but had no effect on brainstem neurons (Figure 9C–E)
Figure 9. Characterization of the serotonergic system in the MBP1-hαsyn transgenic model of MSA and the effects of fluoxetine.
The effect of fluoxetine administration on the serotonergic system was examined using immunohistochemistry. (A–D) Representative images of tryptophan hydroxylase (5HT-OH) immunoreactivity in the brainstem raphe nucleus of saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. (E) Analysis of 5HT-OH in the raphe nucleus across the experimental groups. (F–I) Representative images of 5HT-OH immunoreactivity in fibers projecting from the raphe nucleus of saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. (J) Analysis of 5HT-OH in projecting fibers across the experimental groups. (K–N) Confocal images of 5HT immunoreactivity in hippocampal nerve terminals of saline-treated non-tg, fluoxetine-treated non-tg, saline-treated MBP1-hαsyn tg and fluoxetine-treated MBP1-hαsyn tg mice respectively. (O) Analysis of 5HT-immunoreactive hippocampal nerve terminals across the experimental groups. Error bars represent mean ± SEM. * Indicates a significant difference (p< 0.05) between the groups indicated by the bar analyzed by one-way ANOVA and Dunnett’s post hoc test. Scale bar (A–D) = 20µM, (E–H) = 20µM and (I–L) = 10µM.
Saline-treated MBP1-hαsyn tg mice also display a decrease in levels of 5HT immunoreactivity in hippocampal nerve terminals (Figure 9K, M, O) and fluoxetine treatment had no effect on levels of 5HT immunoreactivity in these nerve terminals (Figure 9M–O).
DISCUSSION
The present study sought to investigate the hypothesis that administration of the antidepressant fluoxetine may ameliorate deficits in the MBP1-hαsyn tg mice, a tg model of MSA. The results demonstrate that fluoxetine is indeed able to reduce motor behavioral deficits and neurodegenerative pathology in the MBP1-hαsyn tg mice. Furthermore, we suggest that the neuroprotective effects of fluoxetine in this model of α-synucleinopathy are mediated by its ability to increase the expression of GDNF and BDNF.
NTFs are altered in a number of neurodegenerative disorders (Siegel and Chauhan, 2000, Zuccato and Cattaneo, 2009) and specific alterations in the levels of GDNF and BDNF have been reported in neurodegenerative disorders including Alzheimer’s disease (AD) and PD (Hong, et al., 2008, Peterson and Nutt, 2008, Ramaswamy, et al., 2009, Saragovi, et al., 2009, Siegel and Chauhan, 2000, Yasuhara, et al., 2007, Zuccato and Cattaneo, 2009). Recently we reported a selective reduction in the expression of GDNF in patients with MSA, a feature that is recapitulated in the MBP1-hαsyn tg mice (Ubhi, et al., 2010). We have also shown that infusion of GDNF rescued the motor behavioral and neuropathological deficits in these tg mice (Ubhi, et al., 2010).
NTF replacement strategies have been widely investigated in a number of neurodegenerative disorders (Siegel and Chauhan, 2000, Zuccato and Cattaneo, 2009). There has been a great deal of interest in GDNF replacement therapies in relation to PD in particular and many of these have progressed to the clinical trial stage however there have been mixed reports regarding the success of these methods, many of which are highly invasive (Hong, et al., 2008, Nutt, et al., 2003, Peterson and Nutt, 2008, Yasuhara, et al., 2007). Therefore, there is a real need for an alternative method to augment GDNF levels in MSA patients. A non-invasive approach such as fluoxetine administration may fulfill this need and it may indeed prove more efficacious than GDNF replacement alone given that it also affects BDNF levels.
In support of this possibility, we showed that fluoxetine administration is able to recapitulate many of the neuroprotective effects of GDNF infusion observed in our earlier study (Ubhi, et al., 2010) and that it may have a disease-modifying effect on regional levels of α-syn aggregation. The effects of fluoxetine observed in this study are consistent with studies in which fluoxetine has been shown to reduce the loss of dopaminergic neurons in MPTP (Chung, et al., 2011) and 6-OHDA (Suzuki, et al., 2010) models of PD and LPS induced degeneration of the nigral system (Chung, et al., 2010), moreover our study expands the therapeutic application of fluoxetine to MSA, particularly in relation to its ability to augment levels of GDNF.
The effects of fluoxetine have been widely investigated in astrocytes in vitro (Allaman, et al., 2011, Li, et al., 2009, Li, et al., 2010, Li, et al., 2008, Mercier, et al., 2004) and rat C6 glioblastoma cells (Hisaoka, et al., 2001) however the present study is the first to report fluoxetine-induced increases in GDNF in oligodendrocytes, the primary cell type affected in MSA. Whilst the oligodendroglial cell culture data does not exclude the possibility that the effects of fluoxetine might target in the MBP1-hαsyn tg mice cells other than oligodendrocytes it does provide important information regarding the ability of fluoxetine to affect this cell type. Moreover, given that both oligodendrocytes and astrocytes have been reported to express GDNF (Du and Dreyfus, 2002, Schaar, et al., 1993, Wilkins, et al., 2003), the most parsimonious explanation for the effect of fluoxetine in the MBP1-hαsyn tg mice is that a combinatorial effect on these cell types is responsible for the motor and neuropathological improvements observed.
In addition to reducing the neurodegenerative pathology in the neocortex and nigrostriatal system, treatment with fluoxetine also rescued the alterations in neurogenesis observed in the MBP1-hαsyn tg mice. Previous studies have shown that adult neurogenesis is defective in models of α-synucleinopathy (Crews, et al., 2008, Marxreiter, et al., 2009, Winner, et al., 2004, Winner, et al., 2008) and it has been suggested that defects in neurogenesis might be responsible for non-motor deficits in these disorders (Winner, et al., 2004, Winner, et al., 2008). Our findings in the fluoxetine-treated MBP1-hαsyn tg mice are in agreement with previous studies showing that fluoxetine potentiates neurogenesis in human stem cells and hippocampal cells (Chang, et al., 2010, Suzuki, et al., 2010) and with studies of neurogenesis in the APPsw (Tg2576) mutant mice model of AD (Dong, et al., 2004) and the Ts65Dn mouse model of Down syndrome (Bianchi, et al., 2010, Clark, et al., 2006).
The mechanisms through which fluoxetine might enhance GDNF and BDNF levels to promote neurogenesis and rescue the neurodegenerative pathology in our tg MSA model remain unclear. However, a number of studies indicate that fluoxetine treatment may enhance transactivation of serotonin receptors including the 5-HT2B and 5-HT2C receptors (Li, et al., 2009, Li, et al., 2010, Li, et al., 2008) resulting in ERK phosphorylation and activation of the ERK signaling pathway (Li, et al., 2009, Li, et al., 2008, Mercier, et al., 2004). This is consistent with the increased pERK/tERK ratio we observe in the fluoxetine-treated mice and in the in vitro study. Moreover, increased expression of GDNF by fluoxetine is likely to be ERK-dependent as it is blocked by U0126, a mitogen-activated protein kinase (MAPK)-ERK kinase inhibitor (Hisaoka, et al., 2001, Mercier, et al., 2004). In contrast, fluoxetine-induced BDNF expression is not blocked by U0126, suggestive of an ERK-independent pathway (Mercier, et al., 2004). One possible alternative pathway by which fluoxetine may enhance BDNF expression is via the transactivation of the BDNF receptor TrkB (Rantamaki, et al., 2011, Saarelainen, et al., 2003, Wyneken, et al., 2006) followed by the binding of BDNF to the TrkB receptor resulting in the internalization of the BDNF-TrkB complex which then undergoes retrograde transport from spines to cell bodies where it activates the synthesis of more BDNF (Wyneken, et al., 2006).
The present study is the first to demonstrate alterations in the serotonergic system in the MBP1-hαsyn tg mice. We show a decrease in serotonin raphe nucleus brainstem neurons, fibers and nerve terminals in these mice compared to controls, these results are consistent with a previous study in humans (Tada, et al., 2009) and with studies that have shown that BDNF and GDNF may subserve a supporting function for serotonergic neurons (Djalali, et al., 2005). We show that fluoxetine administration in the MBP1-hαsyn tg mice is able to increase 5HT-OH immunoreactive projecting fibers, without effecting numbers of neurons or nerve terminals suggesting that, rather that increasing the actual numbers of serotonergic neurons or terminals, fluoxetine may play a role in increasing the transport of serotonin from the soma to the axons in these mice.
We also show that fluoxetine administration was able to ameliorate the myelin deficits observed in the corpus callosum of MBP1-hαsyn tg mice, again these results are in line with the reported involvement of NTFs such as BDNF and GDNF on myelination (Hoke, et al., 2003, Notterpek, 2003, Tada, et al., 2009, Tolwani, et al., 2004, Xiao, et al., 2009, Xiao, et al., 2010, Zhang, et al., 2009). This effect on oligodendrocytes in the corpus callosum is particularly interesting in light of the regional effect of fluoxetine at lowering α-syn immunoreactivity in the corpus callosum, however further, more detailed studies, are necessary on order to fully determine the precise mechanisms underlying this observation. In addition to it effects on serotonin, NTF levels and neurogenesis, fluoxetine is reported to effect inflammatory processes (Kubera, et al., 2004, Roumestan, et al., 2007) and mitochondrial activity (Abdel-Razaq, et al., 2011, Lee, et al., 2010). Further investigation of these alternative pathways is also necessary in order to fully define the mechanisms underlying the neuroprotective effects of fluoxetine.
It is also important to note that our particular interest in fluoxetine was precipitated by previous reports of is neuroprotective activity (Chung, et al., 2010, Chung, et al., 2011, Suzuki, et al., 2010) and that fact that it is currently in a Phase II trial for depressive symptoms in MSA (NCT01146548). However, it is possible that other SSRIs or psychopharmacological agents may have a similar effect in the MBP1-hαsyn tg mice or other models of α-synucleinopathy and/or neurodegeneration, for example, paroxetine, another SSRI, has been reported to prevent loss of nigrostriatal dopaminergic neurons by inhibiting brain inflammation and oxidative stress in the MPTP mouse model of PD (Chung, et al., 2010). Indeed, there is a whole body of evidence to suggest a neuroprotective effect of psychopharmacological treatments (Hu, et al., 2011, Lauterbach, et al., 2010, Machado-Vieira, et al., 2009) and many of these drugs have been reported to effect levels of NTFs (Gonzalez-Pinto, et al., 2010, Heberlein, et al., 2010, Hunsberger, et al., 2009, Lee, et al., 2011, Nishimura, et al., 2008, Parikh, et al., 2004, Quiroz, et al., 2010).
Antidepressants and other psychopharmacological agents have traditionally been prescribed to patients with neurodegenerative disorders in order to help them cope with symptoms such as depression and anxiety (Aboukhatwa, et al., 2010). It is only in the last few decades that these drugs have been examined more closely to assess other effects that they may be having (Lauterbach, et al., 2010). The most well documented example of this kind of multifactorial effect involves lithium, a commonly prescribed mood stabilizer treatment for bipolar depression, which has been shown to effect levels of amyloid beta (Aβ) and tau in AD. The effects of lithium on Aβ and tau are related to its ability to inhibit the activity of GSK-3β a key kinase involved in the transcriptional regulation of amyloid precursor protein (APP, the precursor of Aβ) and in the phosphorylation of tau. Antipsychotics such as haloperidol and chlorpromazine have also reported to effect levels of Aβ and tau phosphorylation (Lauterbach, et al., 2010).
The effects of psychopharmacological agents are not restricted to AD-related proteins, valproic acid, used to treat certain types of seizures or episodes of mania in patients with bipolar disorder, has been shown to increase α-syn expression and prevent its nuclear translocation (Leng and Chuang, 2006), whilst the antioxidant melatonin has been reported to reduce α-syn aggregation in vitro (Ishido, 2007, Klongpanichapak, et al., 2007, Klongpanichapak, et al., 2008). It is clear that drugs traditionally prescribed for disorders such as depression or schizophrenia may have more pleiotropic effects than previously anticipated and further investigation of these effects is necessary in order to fully appreciate the therapeutic potential of these drugs in neurodegenerative disorders such as MSA.
In conclusion, we demonstrate that the SSRI fluoxetine is able to ameliorate motor behavioral and neuropathological alterations in the MBP1-hαsyn tg model of MSA, these results suggest that fluoxetine, a well characterized and FDA approved drug, may potentially have a therapeutic application for MSA patients and for other disorders that display alterations in NTF expression.
Highlights.
- We examined the effect of fluoxetine in the MBP1-hαsyn tg mice, a model of MSA
- Fluoxetine ameliorated motor deficits in the MBP1-hαsyn tg mice.
- Fluoxetine decreased neurodegenerative pathology and increased levels of NTFs
- Fluoxetine promoted proliferation and reduced corpus callosum accumulation of α-syn
- Fluoxetine may represent a novel therapeutic intervention for MSA
Acknowledgements
This work was supported by NIH grants AG 18440, NS 044233, AG 10435 and AG 022074 and the Donner Canadian Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdel-Razaq W, Kendall DA, Bates TE. The effects of antidepressants on mitochondrial function in a model cell system and isolated mitochondria. Neurochem Res. 2011;36:327–338. doi: 10.1007/s11064-010-0331-z. [DOI] [PubMed] [Google Scholar]
- Aboukhatwa M, Dosanjh L, Luo Y. Antidepressants are a rational complementary therapy for the treatment of Alzheimer's disease. Molecular neurodegeneration. 2010;5:10. doi: 10.1186/1750-1326-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaman I, Fiumelli H, Magistretti PJ, Martin JL. Fluoxetine regulates the expression of neurotrophic/growth factors and glucose metabolism in astrocytes. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2190-y. [DOI] [PubMed] [Google Scholar]
- Arima K, Ueda K, Sunohara N, Arakawa K, Hirai S, Nakamura M, Tonozuka-Uehara H, Kawai M. NACP/alpha-synuclein immunoreactivity in fibrillary components of neuronal and oligodendroglial cytoplasmic inclusions in the pontine nuclei in multiple system atrophy. Acta Neuropathol. 1998;96:439–444. doi: 10.1007/s004010050917. [DOI] [PubMed] [Google Scholar]
- Bianchi P, Ciani E, Guidi S, Trazzi S, Felice D, Grossi G, Fernandez M, Giuliani A, Calza L, Bartesaghi R. Early pharmacotherapy restores neurogenesis and cognitive performance in the Ts65Dn mouse model for Down syndrome. J Neurosci. 2010;30:8769–8779. doi: 10.1523/JNEUROSCI.0534-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EA, Beyhan Z, Yoo MS, Siripattarapravat K, Ko T, Lookingland KJ, Madhukar BV, Cibelli JB. Increased cellular turnover in response to fluoxetine in neuronal precursors derived from human embryonic stem cells. Int J Dev Biol. 2010;54:707–715. doi: 10.1387/ijdb.092851ec. [DOI] [PubMed] [Google Scholar]
- Chung ES, Chung YC, Bok E, Baik HH, Park ES, Park JY, Yoon SH, Jin BK. Fluoxetine prevents LPS-induced degeneration of nigral dopaminergic neurons by inhibiting microglia-mediated oxidative stress. Brain Res. 2010;1363:143–150. doi: 10.1016/j.brainres.2010.09.049. [DOI] [PubMed] [Google Scholar]
- Chung YC, Kim SR, Jin BK. Paroxetine prevents loss of nigrostriatal dopaminergic neurons by inhibiting brain inflammation and oxidative stress in an experimental model of Parkinson's disease. Journal of immunology. 2010;185:1230–1237. doi: 10.4049/jimmunol.1000208. [DOI] [PubMed] [Google Scholar]
- Chung YC, Kim SR, Park JY, Chung ES, Park KW, Won SY, Bok E, Jin M, Park ES, Yoon SH, Ko HW, Kim YS, Jin BK. Fluoxetine prevents MPTP-induced loss of dopaminergic neurons by inhibiting microglial activation. Neuropharmacology. 2011;60:963–974. doi: 10.1016/j.neuropharm.2011.01.043. [DOI] [PubMed] [Google Scholar]
- Clark S, Schwalbe J, Stasko MR, Yarowsky PJ, Costa AC. Fluoxetine rescues deficient neurogenesis in hippocampus of the Ts65Dn mouse model for Down syndrome. Exp Neurol. 2006;200:256–261. doi: 10.1016/j.expneurol.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Crews L, Mizuno H, Desplats P, Rockenstein E, Adame A, Patrick C, Winner B, Winkler J, Masliah E. Alpha-synuclein alters Notch-1 expression and neurogenesis in mouse embryonic stem cells and in the hippocampus of transgenic mice. J Neurosci. 2008;28:4250–4260. doi: 10.1523/JNEUROSCI.0066-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djalali S, Holtje M, Grosse G, Rothe T, Stroh T, Grosse J, Deng DR, Hellweg R, Grantyn R, Hortnagl H, Ahnert-Hilger G. Effects of brain-derived neurotrophic factor (BDNF) on glial cells and serotonergic neurones during development. Journal of neurochemistry. 2005;92:616–627. doi: 10.1111/j.1471-4159.2004.02911.x. [DOI] [PubMed] [Google Scholar]
- Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127:601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Du Y, Dreyfus CF. Oligodendrocytes as providers of growth factors. J Neurosci Res. 2002;68:647–654. doi: 10.1002/jnr.10245. [DOI] [PubMed] [Google Scholar]
- Enciu AM, Nicolescu MI, Manole CG, Muresanu DF, Popescu LM, Popescu BO. Neuroregeneration in neurodegenerative disorders. BMC neurology. 2011;11:75. doi: 10.1186/1471-2377-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Pinto A, Mosquera F, Palomino A, Alberich S, Gutierrez A, Haidar K, Vega P, Barbeito S, Ortiz A, Matute C. Increase in brain-derived neurotrophic factor in first episode psychotic patients after treatment with atypical antipsychotics. Int Clin Psychopharmacol. 2010;25:241–245. doi: 10.1097/yic.0b013e328338bc5a. [DOI] [PubMed] [Google Scholar]
- Halliday G. Clinicopathological aspects of motor parkinsonism. Parkinsonism Relat Disord. 2007;13(Suppl 3):S208–S210. doi: 10.1016/S1353-8020(08)70003-8. [DOI] [PubMed] [Google Scholar]
- Heberlein A, Lenz B, Muschler M, Frieling H, Buechl R, Groschl M, Kornhuber J, Bleich S, Hillemacher T. BDNF plasma levels decrease during benzodiazepine withdrawal in patients suffering from comorbidity of depressive disorder and benzodiazepine dependence. Psychopharmacology (Berl) 2010;209:213–215. doi: 10.1007/s00213-010-1781-3. [DOI] [PubMed] [Google Scholar]
- Hisaoka K, Nishida A, Koda T, Miyata M, Zensho H, Morinobu S, Ohta M, Yamawaki S. Antidepressant drug treatments induce glial cell line-derived neurotrophic factor (GDNF) synthesis and release in rat C6 glioblastoma cells. J Neurochem. 2001;79:25–34. doi: 10.1046/j.1471-4159.2001.00531.x. [DOI] [PubMed] [Google Scholar]
- Hoke A, Ho T, Crawford TO, LeBel C, Hilt D, Griffin JW. Glial cell line-derived neurotrophic factor alters axon schwann cell units and promotes myelination in unmyelinated nerve fibers. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:561–567. doi: 10.1523/JNEUROSCI.23-02-00561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Mukhida K, Mendez I. GDNF therapy for Parkinson's disease. Expert Rev Neurother. 2008;8:1125–1139. doi: 10.1586/14737175.8.7.1125. [DOI] [PubMed] [Google Scholar]
- Hu X, Zhou H, Zhang D, Yang S, Qian L, Wu HM, Chen PS, Wilson B, Gao HM, Lu RB, Hong JS. Clozapine Protects Dopaminergic Neurons from Inflammation-Induced Damage by Inhibiting Microglial Overactivation. J Neuroimmune Pharmacol. 2011 doi: 10.1007/s11481-011-9309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsberger J, Austin DR, Henter ID, Chen G. The neurotrophic and neuroprotective effects of psychotropic agents. Dialogues Clin Neurosci. 2009;11:333–348. doi: 10.31887/DCNS.2009.11.3/jhunsberger. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishido M. Melatonin inhibits maneb-induced aggregation of alpha-synuclein in rat pheochromocytoma cells. Journal of pineal research. 2007;42:125–130. doi: 10.1111/j.1600-079X.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Spooren W, Fuss B, Mallon B, Macklin WB, Fujiwara H, Hasegawa M, Iwatsubo T, Kretzschmar HA, Haass C. Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Rep. 2002;3:583–588. doi: 10.1093/embo-reports/kvf109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klongpanichapak S, Phansuwan-Pujito P, Ebadi M, Govitrapong P. Melatonin protects SK-N-SH neuroblastoma cells from amphetamine-induced neurotoxicity. Journal of pineal research. 2007;43:65–73. doi: 10.1111/j.1600-079X.2007.00444.x. [DOI] [PubMed] [Google Scholar]
- Klongpanichapak S, Phansuwan-Pujito P, Ebadi M, Govitrapong P. Melatonin inhibits amphetamine-induced increase in alpha-synuclein and decrease in phosphorylated tyrosine hydroxylase in SK-N-SH cells. Neuroscience letters. 2008;436:309–313. doi: 10.1016/j.neulet.2008.03.053. [DOI] [PubMed] [Google Scholar]
- Kubera M, Kenis G, Bosmans E, Kajta M, Basta-Kaim A, Scharpe S, Budziszewska B, Maes M. Stimulatory effect of antidepressants on the production of IL-6. Int Immunopharmacol. 2004;4:185–192. doi: 10.1016/j.intimp.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Lantos PL, Papp MI. Cellular pathology of multiple system atrophy: a review. J Neurol Neurosurg Psychiatry. 1994;57:129–133. doi: 10.1136/jnnp.57.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterbach EC, Victoroff J, Coburn KL, Shillcutt SD, Doonan SM, Mendez MF. Psychopharmacological neuroprotection in neurodegenerative disease: assessing the preclinical data. J Neuropsychiatry Clin Neurosci. 2010;22:8–18. doi: 10.1176/jnp.2010.22.1.8. [DOI] [PubMed] [Google Scholar]
- Lee AH, Lange C, Ricken R, Hellweg R, Lang UE. Reduced brain-derived neurotrophic factor serum concentrations in acute schizophrenic patients increase during antipsychotic treatment. J Clin Psychopharmacol. 2011;31:334–336. doi: 10.1097/JCP.0b013e31821895c1. [DOI] [PubMed] [Google Scholar]
- Lee CS, Kim YJ, Jang ER, Kim W, Myung SC. Fluoxetine induces apoptosis in ovarian carcinoma cell line OVCAR-3 through reactive oxygen species-dependent activation of nuclear factor-kappaB. Basic Clin Pharmacol Toxicol. 2010;106:446–453. doi: 10.1111/j.1742-7843.2009.00509.x. [DOI] [PubMed] [Google Scholar]
- Leng Y, Chuang DM. Endogenous alpha-synuclein is induced by valproic acid through histone deacetylase inhibition and participates in neuroprotection against glutamate-induced excitotoxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:7502–7512. doi: 10.1523/JNEUROSCI.0096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Zhang S, Li M, Hertz L, Peng L. Chronic treatment of astrocytes with therapeutically relevant fluoxetine concentrations enhances cPLA2 expression secondary to 5-HT2B-induced, transactivation-mediated ERK1/2 phosphorylation. Psychopharmacology (Berl) 2009;207:1–12. doi: 10.1007/s00213-009-1631-3. [DOI] [PubMed] [Google Scholar]
- Li B, Zhang S, Li M, Hertz L, Peng L. Serotonin increases ERK1/2 phosphorylation in astrocytes by stimulation of 5-HT2B and 5-HT2C receptors. Neurochem Int. 2010;57:432–439. doi: 10.1016/j.neuint.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Li B, Zhang S, Zhang H, Nu W, Cai L, Hertz L, Peng L. Fluoxetine-mediated 5-HT2B receptor stimulation in astrocytes causes EGF receptor transactivation and ERK phosphorylation. Psychopharmacology (Berl) 2008;201:443–458. doi: 10.1007/s00213-008-1306-5. [DOI] [PubMed] [Google Scholar]
- Li WL, Cai HH, Wang B, Chen L, Zhou QG, Luo CX, Liu N, Ding XS, Zhu DY. Chronic fluoxetine treatment improves ischemia-induced spatial cognitive deficits through increasing hippocampal neurogenesis after stroke. J Neurosci Res. 2009;87:112–122. doi: 10.1002/jnr.21829. [DOI] [PubMed] [Google Scholar]
- Louis JC, Magal E, Muir D, Manthorpe M, Varon S. CG-4, a new bipotential glial cell line from rat brain, is capable of differentiating in vitro into either mature oligodendrocytes or type-2 astrocytes. J Neurosci Res. 1992;31:193–204. doi: 10.1002/jnr.490310125. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Manji HK, Zarate CA., Jr The role of lithium in the treatment of bipolar disorder: convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord. 2009;11(Suppl 2):92–109. doi: 10.1111/j.1399-5618.2009.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcussen AB, Flagstad P, Kristjansen PE, Johansen FF, Englund U. Increase in neurogenesis and behavioural benefit after chronic fluoxetine treatment in Wistar rats. Acta Neurol Scand. 2008;117:94–100. doi: 10.1111/j.1600-0404.2007.00910.x. [DOI] [PubMed] [Google Scholar]
- Marti MJ, Tolosa E, Campdelacreu J. Clinical overview of the synucleinopathies. Mov Disord. 2003;18(Suppl 6):S21–S27. doi: 10.1002/mds.10559. [DOI] [PubMed] [Google Scholar]
- Marxreiter F, Nuber S, Kandasamy M, Klucken J, Aigner R, Burgmayer R, Couillard-Despres S, Riess O, Winkler J, Winner B. Changes in adult olfactory bulb neurogenesis in mice expressing the A30P mutant form of alpha-synuclein. Eur J Neurosci. 2009;29:879–890. doi: 10.1111/j.1460-9568.2009.06641.x. [DOI] [PubMed] [Google Scholar]
- Mercier G, Lennon AM, Renouf B, Dessouroux A, Ramauge M, Courtin F, Pierre M. MAP kinase activation by fluoxetine and its relation to gene expression in cultured rat astrocytes. J Mol Neurosci. 2004;24:207–216. doi: 10.1385/JMN:24:2:207. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Ishima T, Iyo M, Hashimoto K. Potentiation of nerve growth factor-induced neurite outgrowth by fluvoxamine: role of sigma-1 receptors, IP3 receptors and cellular signaling pathways. PLoS One. 2008;3:e2558. doi: 10.1371/journal.pone.0002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notterpek L. Neurotrophins in myelination: a new role for a puzzling receptor. Trends in neurosciences. 2003;26:232–234. doi: 10.1016/S0166-2236(03)00099-7. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Jr, Lozano AM, Penn RD, Simpson RK, Jr, Stacy M, Wooten GF. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- Parikh V, Terry AV, Khan MM, Mahadik SP. Modulation of nerve growth factor and choline acetyltransferase expression in rat hippocampus after chronic exposure to haloperidol, risperidone, and olanzapine. Psychopharmacology (Berl) 2004;172:365–374. doi: 10.1007/s00213-003-1669-6. [DOI] [PubMed] [Google Scholar]
- Perera TD, Dwork AJ, Keegan KA, Thirumangalakudi L, Lipira CM, Joyce N, Lange C, Higley JD, Rosoklija G, Hen R, Sackeim HA, Coplan JD. Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLoS One. 2011;6:e17600. doi: 10.1371/journal.pone.0017600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AL, Nutt JG. Treatment of Parkinson's disease with trophic factors. Neurotherapeutics. 2008;5:270–280. doi: 10.1016/j.nurt.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz JA, Machado-Vieira R, Zarate CA, Jr, Manji HK. Novel insights into lithium's mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010;62:50–60. doi: 10.1159/000314310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Soderstrom KE, Kordower JH. Trophic factors therapy in Parkinson's disease. Prog Brain Res. 2009;175:201–216. doi: 10.1016/S0079-6123(09)17514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantamaki T, Vesa L, Antila H, Di Lieto A, Tammela P, Schmitt A, Lesch KP, Rios M, Castren E. Antidepressant drugs transactivate TrkB neurotrophin receptors in the adult rodent brain independently of BDNF and monoamine transporter blockade. PLoS One. 2011;6:e20567. doi: 10.1371/journal.pone.0020567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumestan C, Michel A, Bichon F, Portet K, Detoc M, Henriquet C, Jaffuel D, Mathieu M. Anti-inflammatory properties of desipramine and fluoxetine. Respir Res. 2007;8:35. doi: 10.1186/1465-9921-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, Ernfors P, Castren E. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saragovi HU, Hamel E, Di Polo A. A Neurotrophic Rationale for the Therapy of Neurodegenerative Disorders. Curr Alzheimer Res. 2009 doi: 10.2174/156720509789207912. [DOI] [PubMed] [Google Scholar]
- Schaar DG, Sieber BA, Dreyfus CF, Black IB. Regional and cell-specific expression of GDNF in rat brain. Exp Neurol. 1993;124:368–371. doi: 10.1006/exnr.1993.1207. [DOI] [PubMed] [Google Scholar]
- Shults CW, Rockenstein E, Crews L, Adame A, Mante M, Larrea G, Hashimoto M, Song D, Iwatsubo T, Tsuboi K, Masliah E. Neurological and neurodegenerative alterations in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. J Neurosci. 2005;25:10689–10699. doi: 10.1523/JNEUROSCI.3527-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer's and Parkinson's disease brain. Brain Res Brain Res Rev. 2000;33:199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, Adame A, Wyss-Coray T, Masliah E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson's and Lewy body diseases. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Okada K, Wakuda T, Shinmura C, Kameno Y, Iwata K, Takahashi T, Suda S, Matsuzaki H, Iwata Y, Hashimoto K, Mori N. Destruction of dopaminergic neurons in the midbrain by 6-hydroxydopamine decreases hippocampal cell proliferation in rats: reversal by fluoxetine. PLoS One. 2010;5:e9260. doi: 10.1371/journal.pone.0009260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Kakita A, Toyoshima Y, Onodera O, Ozawa T, Morita T, Nishizawa M, Takahashi H. Depletion of medullary serotonergic neurons in patients with multiple system atrophy who succumbed to sudden death. Brain : a journal of neurology. 2009;132:1810–1819. doi: 10.1093/brain/awp110. [DOI] [PubMed] [Google Scholar]
- Tolwani RJ, Cosgaya JM, Varma S, Jacob R, Kuo LE, Shooter EM. BDNF overexpression produces a long-term increase in myelin formation in the peripheral nervous system. Journal of neuroscience research. 2004;77:662–669. doi: 10.1002/jnr.20181. [DOI] [PubMed] [Google Scholar]
- Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VM. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44:415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- Ubhi K, Rockenstein E, Mante M, Inglis C, Adame A, Patrick C, Whitney K, Masliah E. Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors. J Neurosci. 2010;30:6236–6246. doi: 10.1523/JNEUROSCI.0567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Hayashi S, Kakita A, Yamada M, Toyoshima Y, Yoshimoto M, Takahashi H. Accumulation of alpha-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol. 1998;96:445–452. doi: 10.1007/s004010050918. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H. Cellular pathology in multiple system atrophy. Neuropathology. 2006;26:338–345. doi: 10.1111/j.1440-1789.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H. Alpha-synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett. 1998;249:180–182. doi: 10.1016/s0304-3940(98)00407-8. [DOI] [PubMed] [Google Scholar]
- Wang Y, Neumann M, Hansen K, Hong SM, Kim S, Noble-Haeusslein LJ, Liu J. Fluoxetine increases hippocampal neurogenesis and induces epigenetic factors but does not improve functional recovery after traumatic brain injury. J Neurotrauma. 2011;28:259–268. doi: 10.1089/neu.2010.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins A, Majed H, Layfield R, Compston A, Chandran S. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J Neurosci. 2003;23:4967–4974. doi: 10.1523/JNEUROSCI.23-12-04967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Kohl Z, Gage FH. Neurodegenerative disease and adult neurogenesis. The European journal of neuroscience. 2011;33:1139–1151. doi: 10.1111/j.1460-9568.2011.07613.x. [DOI] [PubMed] [Google Scholar]
- Winner B, Kohl Z, Gage FH. Neurodegenerative disease and adult neurogenesis. Eur J Neurosci. 2011;33:1139–1151. doi: 10.1111/j.1460-9568.2011.07613.x. [DOI] [PubMed] [Google Scholar]
- Winner B, Lie DC, Rockenstein E, Aigner R, Aigner L, Masliah E, Kuhn HG, Winkler J. Human wild-type alpha-synuclein impairs neurogenesis. J Neuropathol Exp Neurol. 2004;63:1155–1166. doi: 10.1093/jnen/63.11.1155. [DOI] [PubMed] [Google Scholar]
- Winner B, Rockenstein E, Lie DC, Aigner R, Mante M, Bogdahn U, Couillard-Despres S, Masliah E, Winkler J. Mutant alpha-synuclein exacerbates age-related decrease of neurogenesis. Neurobiol Aging. 2008;29:913–925. doi: 10.1016/j.neurobiolaging.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyneken U, Sandoval M, Sandoval S, Jorquera F, Gonzalez I, Vargas F, Falcon R, Monari M, Orrego F. Clinically relevant doses of fluoxetine and reboxetine induce changes in the TrkB content of central excitatory synapses. Neuropsychopharmacology. 2006;31:2415–2423. doi: 10.1038/sj.npp.1301052. [DOI] [PubMed] [Google Scholar]
- Xiao J, Wong AW, Willingham MM, Kaasinen SK, Hendry IA, Howitt J, Putz U, Barrett GL, Kilpatrick TJ, Murray SS. BDNF exerts contrasting effects on peripheral myelination of NGF-dependent and BDNF-dependent DRG neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:4016–4022. doi: 10.1523/JNEUROSCI.3811-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Wong AW, Willingham MM, van den Buuse M, Kilpatrick TJ, Murray SS. Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neuro-Signals. 2010;18:186–202. doi: 10.1159/000323170. [DOI] [PubMed] [Google Scholar]
- Yasuhara T, Shingo T, Date I. Glial cell line-derived neurotrophic factor (GDNF) therapy for Parkinson's disease. Acta Med Okayama. 2007;61:51–56. doi: 10.18926/AMO/32888. [DOI] [PubMed] [Google Scholar]
- Yazawa I, Giasson BI, Sasaki R, Zhang B, Joyce S, Uryu K, Trojanowski JQ, Lee VM. Mouse model of multiple system atrophy alpha-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron. 2005;45:847–859. doi: 10.1016/j.neuron.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Yoshida M. Multiple system atrophy: alpha-synuclein and neuronal degeneration. Neuropathology. 2007;27:484–493. doi: 10.1111/j.1440-1789.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ma Z, Smith GM, Wen X, Pressman Y, Wood PM, Xu XM. GDNF-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia. 2009;57:1178–1191. doi: 10.1002/glia.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5:311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]