Protein profiling of formalin fixed paraffin embedded tissue: Identification of potential biomarkers for pediatric brainstem glioma (original) (raw)
. Author manuscript; available in PMC: 2014 Jan 30.
Published in final edited form as: Proteomics Clin Appl. 2008 Jun;2(6):915–924. doi: 10.1002/prca.200780061
Abstract
Little is known about the molecular characteristics of pediatric brainstem gliomas (BSG), which continue to have a dismal prognosis. Targeted molecular strategies are limited due to rarity of biopsy BSG specimen coupled with obstacles associated with the analyses of formalin-fixed paraffin- embedded (FFPE) autopsies. The objective of this study was to develop methodologies to successfully identify the proteome profile from these archived FFPE specimens. Peptides were extracted from both tumor and adjacent normal FFPE brainstem specimen and quantified using 18O proteolytic labeling strategy and LC-MS/MS analysis. The ingenuity pathway analysis software was used to elucidate interactions amongst differentially expressed proteins. We identified 188 proteins of which 54 (29%) were found up-regulated (≥1.5-fold) in BSG compared to normal sections. Of these, 15 (28%) proteins have previously been reported as potential biomarkers for supratentorial malignant gliomas, while the rest appear to be exclusive to pediatric BSG. Because the majority of differentially expressed proteins are unique to BSG, we conclude that pediatric BSG is distinct from supratentorial gliomas. To the best of our knowledge, this is the first proteome profile of pediatric BSG, which may facilitate discovery of novel therapeutic targets for early diagnostics and improving prognostics.
Keywords: Formalin fixed paraffin embedded, Ingenuity pathway analysis, Pediatric brainstem
1 Introduction
Pediatric brainstem glioma (BSG) is the most difficult childhood cancer to treat, largely due to the inoperability and diffuse nature of the lesion. Apart from conventional radiation therapy, which typically results in only transient symptomatic relief, other treatments have failed to improve outcome, including high doses (up to 78 Gy) of radiation [1] and the addition of chemotherapy that has been effective for other types of brain tumors [2–4]. In fact, for diffused gliomas, despite numerous clinical research trials over the last three decades investigating the use of experimental chemotherapy, radiation and biologic therapy, the overall survival rate of approximately 10% has remained unchanged [5, 6].
BSG predominantly occurs in children between the ages of six to nine years and accounts for 10–20% of all pediatric CNS tumors [7, 8]. The pons is the most common brainstem location of BSG; however, it can also arise in the cervicomedullary junction, midbrain, or the tectum. BSG are categorized into two main groups: diffusely infiltrative and focal [9, 10]. Diffusely infiltrative BSG spreads throughout the pons with occasional dissemination to other parts of the brainstem. Focal brainstem gliomas are, however, localized tumors typically occurring in the midbrain or medulla.
Recent advances in the treatment of other refractory cancers have been accomplished through drug targeting of identified biomarkers that are specific to the type of tumor. To date, however, there are no known biomarkers specific to BSG. Despite advancement of proteomics and genomics applications, little progress has been made towards understanding the molecular constituents of BSG primarily because of the relative lack of biological samples for conducting research studies of this kind. Because this tumor has a typical appearance on magnetic resonance imaging (MRI), and because it is often difficult to safely perform biopsies of BSG, obtaining tumor tissue for histological confirmation of the diagnosis is generally not clinically warranted. Thus, there is a lack of available fresh-frozen tissue for the molecular characterization of these tumors. Furthermore, the complexities associated with protein profiling of formalin-fixed paraffinembedded (FFPE) autopsy specimens have similarly limited performing analyses for biomarker discovery. New methodologies allowing for the identification of BSG-specific biomarkers are therefore greatly needed.
Formalin fixation has been long used to study molecular interactions. Histone-histone and histone-DNA complexes for example, were studied by formation and then reversal of formalin cross-links [11–13]. Cross-linking experiments have also been insightful into the interaction of anticancer drugs and DNA molecule [14, 15]. Formalin-fixed autopsy samples are an excellent resource for genomic and proteomic analyses. During the past decades, methods for reversing cross-links of FFPE samples have improved, specifically for antibody retrieval in immunohistochemistry assays [16–20]. Recent advances in proteomics field have also led to the successful extraction and analysis of peptides from FFPE tissues [21–23]. In an attempt to discover potential BSG markers for therapeutic targeting, as well as define the molecular signature of the tumor, we used FFPE pediatric BSG archival tissues for protein identification and quantification. Our analyses show the feasibility of profiling proteins from archival FFPE brain tissues and obtaining qualitative and quantitative molecular insights into BSG. Here, we have successfully conducted comparative protein profiling of normal versus BSG brainstem area obtained from the same FFPE patient sample and generated a list of 54 differentially expressed proteins. Thus, we present the first proteome profile of BSG, suggesting potential biomarkers and molecular signatures of disease progression for possible therapeutic intervention as well as describe a technique that may be utilized broadly for all FFPE cancer specimens.
2 Materials and methods
2.1 Human subjects
FEPE BSG autopsy samples were provided by the Children’s National Medical Center. Institutional Review Board (IRB) was obtained to perform evaluations on these samples. All identifiers were removed from the samples prior to evaluation.
2.2 Removal of formalin and partial reversal of cross-links
Two FFPE BSG autopsy samples were analyzed. Hematoxylin and eosin (H&E)-stained FFPE sections (5 µm thick), were examined by a neuropathologist using light microscopy to identify the area of the brainstem involved by tumor versus the adjacent normal areas. All the cases selected were astrocytoma. For each case, the best possible representative area of tumor was highlighted by the neuropathologist with a marker such that the identical area on the unstained slide could be located by overlapping the H&E-stained section with the unstained section. The unstained sections were all 7 µm in thickness and were prepared on Superfrost Plus microscope glass slides (Fisher Scientific, Houston, TX). Ten slides were used to extract tumor and normal areas for each experiment. Slides were heated in a bench-top oven at 65°C for 60 min to remove excess wax. To ensure maximum wax removal, slides were washed for two rounds of xylene (5 min each), two rounds of 100% fresh ethanol (5 min each), two rounds of 95% ethanol (3 min each), two rounds of 90% ethanol (3 min each), two rounds of 75% ethanol (3 min each) and then rehydrated in water for 30 s.
Delineated areas of interest were then removed directly from the slides as explained here. To remove tissue sections, 10–15 µL of extraction buffer (100 mM ammonium bicarbonate and 30% ACN) was directly deposited on marked tumor or normal areas. Hydrated tissue was then scraped off using the pipette tip, suctioned off the slide, and deposited into 1.5- mL polypropylene tubes (Eppendorf, Westbury, NY). The tumor tissue subsequently removed by pipetting resulted in the recovery of the most representative area of tumor for analysis. To reverse cross-links, tubes containing the tissue were placed in a bench-top oven at 95°C for 30 min followed by 2-min incubation on ice and 3 h at 65°C. Samples were immediately placed in vacuum centrifuge and completely dehydrated.
2.3 Proteolytic 18O-labeling and peptide extraction
Differential labeling by stable isotope was performed using 18O proteolytic labeling strategy [24]. Briefly, dehydrated tissue extracts were digested by trypsin either in 18O-enriched (97%) water (Sigma-Aldrich, St. Louis, MO) or in 16O-water. Two micrograms of horse cytochrome C (CYC; Sigma-Aldrich) was added in each sample as an internal standard. For data confirmation and validation, reverse labeling was also performed where normal extracts were digested in the presence of heavy 18O and tumor extracts were digested in the presence of light 16O-water. All digestions were performed overnight in a water bath set at 37°C in the presence of 4.5 µg MS grade trypsin (Promega, Madison, WI). To ensure a complete digestion and incorporation of 18O atoms in the C termini of tryptic peptides, additional trypsin (3.5 µg) was added next day and digestion was continued for an additional 5 h.
2.4 Quality control of peptides extracted from FFPE tissue and of 18O labeling
After digestion, samples were spun in a bench top centrifuge at maximum speed to pellet cellular debris. Supernatant was then removed and stored at −80°C until further processing. Two microliters of supernatants from each sample was desalted using C18 ZipTip micropipette tips (Millipore, Bedford, MA) following the manufacturer’s User Guide. The peptides were eluted from the ZipTip in 10 µL of ACN/0.1% TFA (70:30 v/v). Peptide solution (0.3 µL) was mixed with 0.3 µL of matrix solution (50 mM CHCA in ACN/0.1% TFA 70:30 v/v) and spotted on the MALDI plate. MS analysis was performed on a 4700 ABI TOF-TOF mass spectrometer (Applied Biosystems, Foster City, CA) in a positive reflectron mode. A mixture of standard peptides was used to externally calibrate the instrument. The high resolution of the ABI-4700 MALDITOF- TOF and its sensitivity helped verification of both the quality of extracted peptides and the incorporation rate of 18O atoms at the C termini of each tryptic peptide. Inspected samples were then completely dried using a vacuum centrifuge, dissolved in 4 µL of 0.1% TFA and immediately analyzed by LC-MS/MS as described below.
2.5 Nanoflow LC-MS/MS
Differentially labeled peptides from normal and tumor sections were mixed at a 1:1 ratio (4 µL each) and an aliquot (6 µL) was analyzed by LC-MS/MS using an LC-Packing system (DIONEX Ulti-Mate™ Capillary/Nano LC System, Dionex, Sunnyvale, CA) connected to a Linear IT (LTQ) mass spectrometer (Thermo Electron, San Jose, CA). Each sample was injected via an autosampler and loaded onto a C18 trap column (300 µm×1 mm, LC Packing) for 6 min at a flow rate of 10 µL/min. The sample was subsequently separated by a C18 RP column (75 µm × 15 cm, Vydac, Columbia, MD) at a flow rate of 300 nL/min. The mobile phases consisted of water with 0.1% formic acid (A) and 90% ACN with 0.1% formic acid (B), respectively. A 240-min linear gradient from 5 to 50% B was typically employed. Separated peptides were introduced into the mass spectrometer via a 10-mm silica tip (New Objective, Ringoes, NJ) adapted to a nanoelectrospray source (Thermo Electron). The spray voltage was set at 1.7 kV and the heated capillary at 160°C. The LTQ was operated in data-dependent mode in which one cycle of experiments consisted of one full-MS survey and subsequently three sequential pairs of intercalated zoom scans and MS/ MS experiments. The targeted ion counts in the IT during full-MS, zoom scan, and MS/MS were 30 000, 3000 and, 10 000, respectively. Peptides were fragmented in the trap using CID with the collision gas (helium) pressure set at 1.3 mTorr and the normalized collision energy value set at 35%.
2.6 Protein database search
Protein identification was performed with the BioWorks 3.2 software (Thermo Electron, Waltham, MA). Briefly, each file was searched against the human Swiss-Prot database (version 52.0, release date of March 2007), which contained 15 945 protein sequences. Horse CYC FASTA sequence (Swiss-Prot ID P00004) was added manually to the Swiss-Prot library, with the following possible protein modifications: 16-Da shift for oxidized Met and 4-Da Cterminal modification (18O incorporation). The acceptance criteria for peptide identification were set as follows: a DeltaCn (DCn).0.1, a variable threshold of Xcorr versus charge state (Xcorr = 1.9 for z = 1, Xcorr = 2.5 for z = 2, and Xcorr = 3.5 for z =3) and a peptide probability based score with a p value <0.001. False discovery rate (FDR) was determined at the protein level by searching a reversed FASTA format of human Swiss-Prot database using the same filtration criteria. FDR was calculated to be less than 3.2% using the following formula: FDR = [2 × (Reverse Database Protein Hits)/(Reverse + Forward Database Protein Hits)] × 100.
2.7 ZoomQuant analysis
To measure the intensity ratios of labeled and unlabeled peptide pairs, we used the ZoomQuant software developed by Halligan and colleagues [25]. The generated SEQUEST output files were loaded into the Epitomize filter to generate a colon-delimited text file that contained the list of identified proteins, the sequence and atomic composition of the identified peptides with scan number, charge state, Xcorr, and mass data. This file was then loaded into the ZoomQuant software along with a file containing zoom scans that were extracted from the corresponding raw data file using a visual basic RawBitz script. Zoom scan information was then matched to the SEQUEST-identified peptides, after which labeled and unlabeled pairs were detected based on their amino acid composition and theoretical masses. Zoom- Quant software takes into account partial (one 18O molecule) as well as full (two 18O molecules) incorporation of heavy oxygen. Ratios were determined from the peak areas of the isotope clusters of the labeled and unlabeled peptides. Obtained ratios were then normalized to the horse CYC ratios as described here.
2.8 Data normalization and assessment of peptide concentration
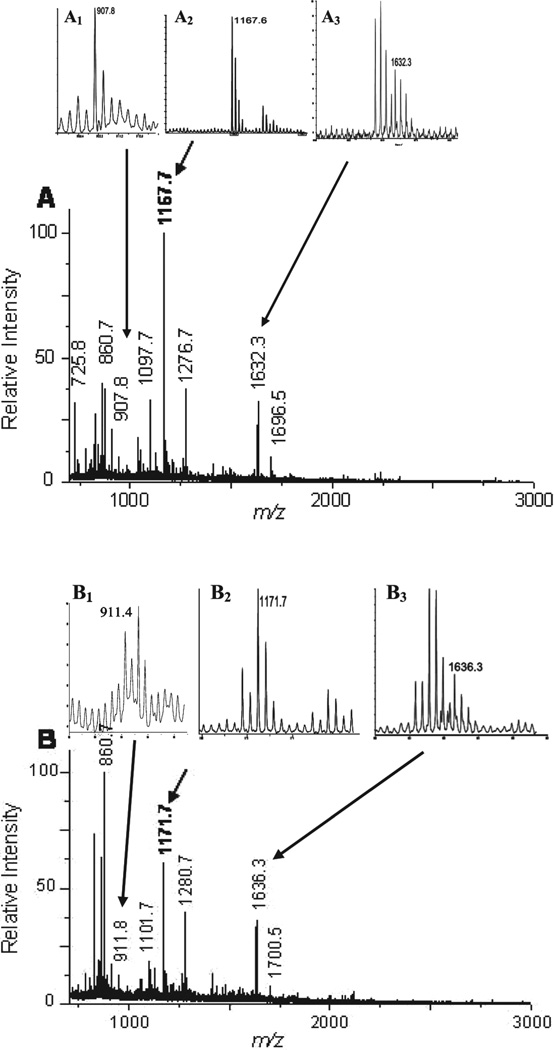
Horse CYC was used as the internal control. Two micrograms of purified horse CYC was added to each sample prior to proteolytic assay. Peptide concentration estimation was done using spectra generated by MALDI-TOF for both the normal and tumor regions prior to mixing the samples. Consequent to analyzing samples with MALDI-TOF, generated spectra were searched and the following three peptide peaks of CYC were selected: MIFAGIKK (m/z = 907.8), TGPNLHGLFGR (m/z = 1168.1), and IFVQKCAQCHTVEK (m/z = 1633.3) for 16O-labeled (Fig. 2A, Insets A1, A2, and A3) and 18O-labeled peptides (Fig. 2B, Insets B1, B2, and B3). Relative peptide concentrations were estimated by dividing the total ion count value of all observed peaks by the average ion count of the three CYC peaks. These relative concentrations were then used to determine the normalization factor we used to adjust for the ZoomQuant (tumor to normal) ratios. Average fold change was calculated based on values generated by the two samples as well as reverse labeling experiments.
Figure 2.
Analyses of FFPE extracted peptides using MALDI TOF-MS. Aliquots of 16O- and 18O-labeled peptides extracted from FFPE samples were separately analyzed by MALDI TOF-MS to verify the efficiency of peptide extraction (Panels A and B). Relative amount of extracted peptides in the presence of either 16O (A), or 18O (B) were estimated by dividing the TIC value of all observed peaks by the average ion count of the three CYC peptide peaks: MIFAGIKK (m/z = 907.8, Inset A1), TGPNLHGLFGR (m/z = 1168.1, Inset A2), and IFVQKCAQCHTVEK (m/z = 1633.3, Inset A3). Compared to 16O (Insets A1, A2, and A3) incorporation, labeling with 18O resulted in a shift of molecular masses of the three CYC peptides (B, insets B1, B2, and B3).
3 Results
3.1 Assessment of peptide extraction from (FFPE) tissue and of 18O-labeling efficiency
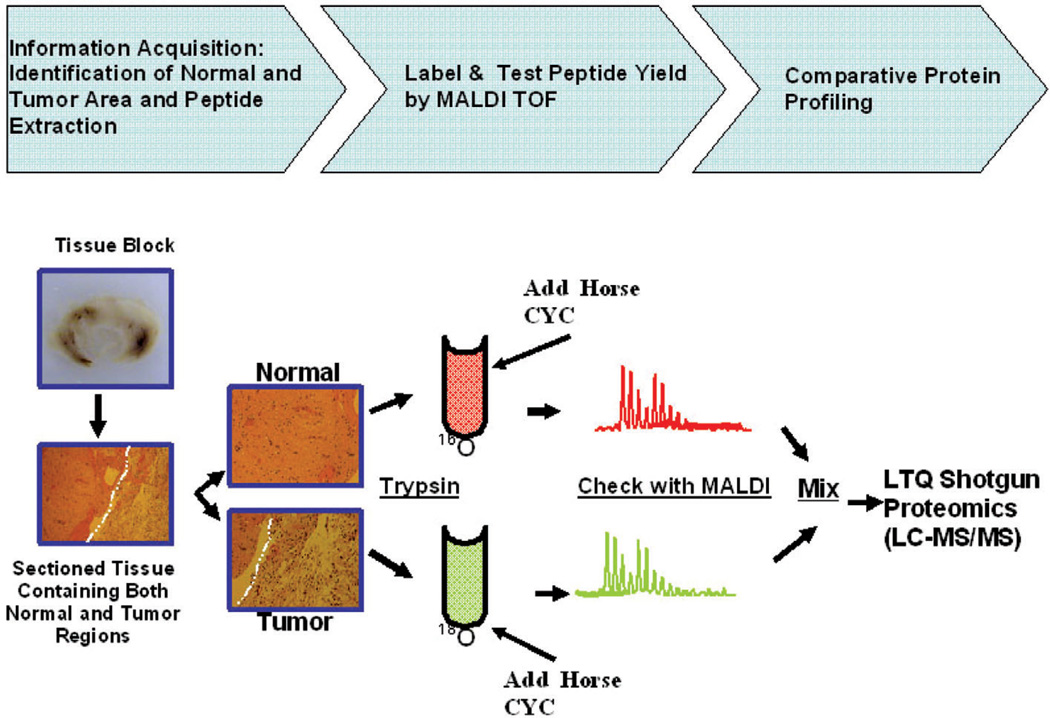
We have implemented and optimized a method for the extraction of peptides from FFPE BSG samples (Fig. 1). This improved protocol includes three main steps, (i) peptide extraction, (ii) stable isotope labeling using enriched 18O water, and (iii) proteome profiling. Each step of this process was checked for quality control (QC) to ensure the validity of the process.
Figure 1.
Flowchart of the implemented methodology for the extraction and the analyses of peptides from BSG FFPE samples. The overall methodology consists of three major steps: (i) removal of paraffin by heating samples and a series of washes followed by extraction of tryptic peptides (information acquisition); (ii) addition of 2 mg horse CYC to each sample as internal control followed by 18O or 16O proteolytic labeling and control quality by MALDI-TOF analysis; (iii) in the last step, peptides extracted from the tumor and the normal regions are mixed and subjected to LC-MS/ MS analysis.
Before mixing 18O-labeled and unlabeled samples for proteome profiling, we checked both the yield of peptide extraction from FFPE tissue and the efficiency of 18O incorporation during proteolysis (see Section 2). Each sample was spiked with 2 µg horse CYC before proteolysis. Following the completion of proteolysis, 2 µL of tryptic digested peptides from 16O- (Fig. 2A) and 18O-labeled peptides (Fig. 2B) were separately analyzed by MALDI-TOF (Fig. 2). The relative higher intensity of peaks with lower m/z in Fig. 2B compared to A is due to the interference of matrix background. Because the absolute peptide extract, including CYC, is lower in the tumor (Fig. 2B) compared to the normal (Fig. 2A), the relative intensity of the background seems higher. Peak 860.7 (Figs. 2A and B) is a good indicator of matrix noise, as it is not 18O labeled (Fig. 2B). Generated spectra were inspected and three predominant CYC peptide peaks were selected (see Section 2) (Fig. 2, Insets A1, A2, A3, B1, B2, and B3). The relative amount of peptides extracted from normal and adjacent tumor FFPE brainstem regions was estimated using the CYC peaks as internal control (see Section 2). To account for protein concentration differences, protein ratios generated by ZoomQuant were normalized to the internal control (horse CYC) following ZoomQuant analyses (see Section 2). Furthermore, spectra generated by MALDI-TOF were inspected to assess the efficiency of 18O labeling. Majority of inspected peaks (>95%) showed 4-Da shift due to incorporation of two 18O molecules as well as 2-Da shift due to the incorporation of a single 18O molecule. ZoomQuant software was used to account for single as well as double incorporation of 18O as described below.
3.2 Proteome profiling and identification of differentially expressed proteins in BSG FFPE tissues
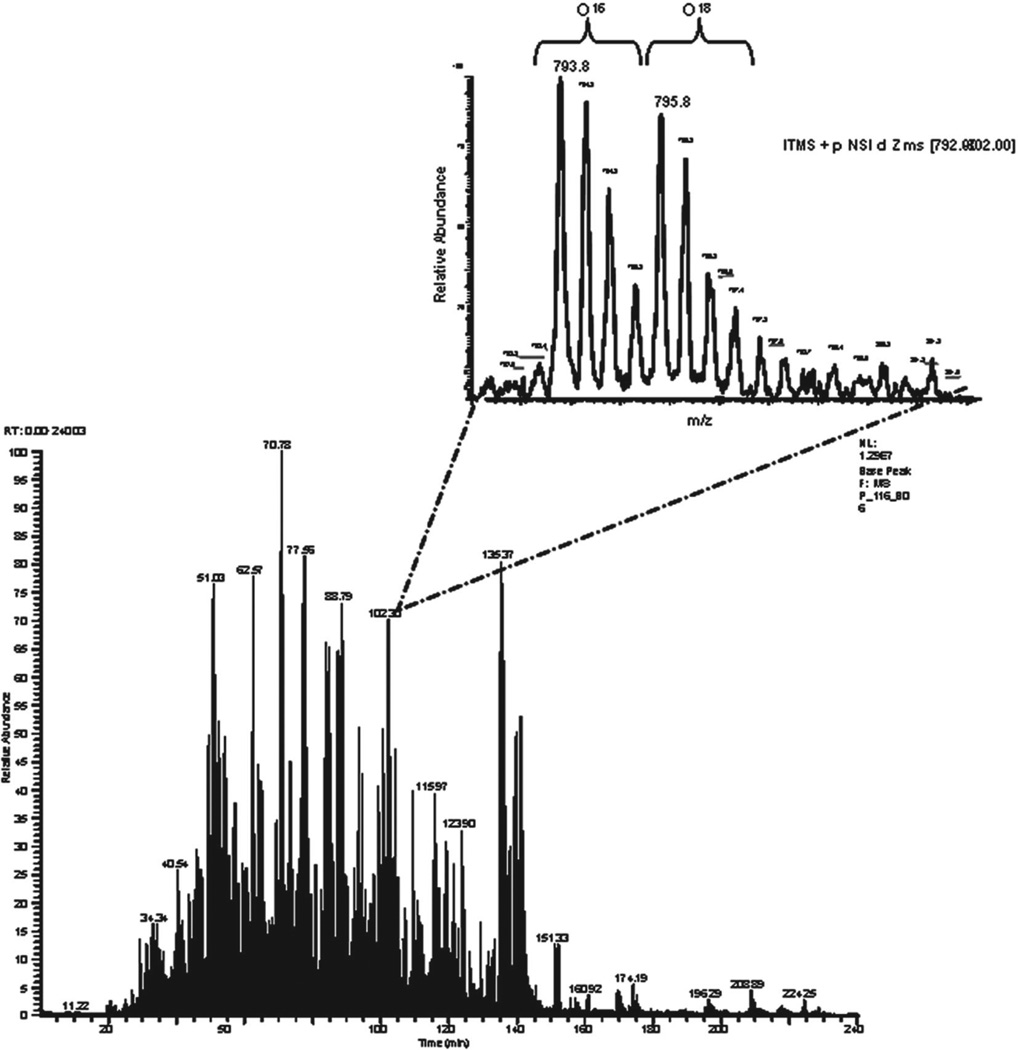
Once efficient peptide extraction and isotopic labeling were confirmed, labeled and unlabeled sample pairs were mixed at 1:1 ratio and subjected to LC-MS/MS analysis as described in Section 2. To increase proteome coverage, we increased the peptide elution time from the most commonly used 90 to 240 min. This significantly increased the number of identified peptides when compared to samples that were processed for only 90 min (data not shown). We performed two sets of experiments, one, in which peptides extracted from normal sections were labeled with 16O and tumor with 18O and a reverse experiment, where peptides from tumour sections were labeled with 16O while peptides from normal section were labeled with 18O. Figure 3 shows a based peak chromatogram and a zoom scan of co-eluting pairs of labeled and unlabeled peptides with m/z of 795.8 and 793.8, respectively. These doubly charged ions show a 4-Da mass difference, indicating incorporation of two 18O atoms. A low intensity peak, however, was detected between the two isotopic clusters of the peptide pairs, corresponding to species with single 18O atom incorporation. We used ZoomQuant software, developed by Halligan and colleagues [25], to measure ratios between labeled and unlabeled peptides (Fig. 4). Briefly, after protein identification using SEQUEST search engine, both identified peptide list and the raw MS run were uploaded into the ZoomQuant software where zoom scans were extracted and ratios between labeled and unlabeled peptide pairs were measured (Fig. 4). Ratios were determined from the peak areas of isotope clusters of the labeled and unlabeled peptide pairs (Fig. 4) using the following equation L/U = [A1 + A2]/A0 where A0 represents the cluster area of the unlabeled peak, A1 the cluster area of the peak with a single 18O incorporation and A2 with a double 18O incorporation. Obtained ratios were then normalized to the ratios of internal standard (horse CYC) as described below.
Figure 3.
LC trace of a mixture of total extracted peptides from normal and control tissue. Shown is the LC trace of peptides eluted during a 4-h run. Peptides from tumor and normal areas were extracted, labeled by 18O and 16O, respectively, then mixed and analyzed by LC-MS/MS as described in Section 2. Inset shows a co-eluted labeled and unlabeled peptide pair generated from the same protein that is present equally in tumor and normal tissue.
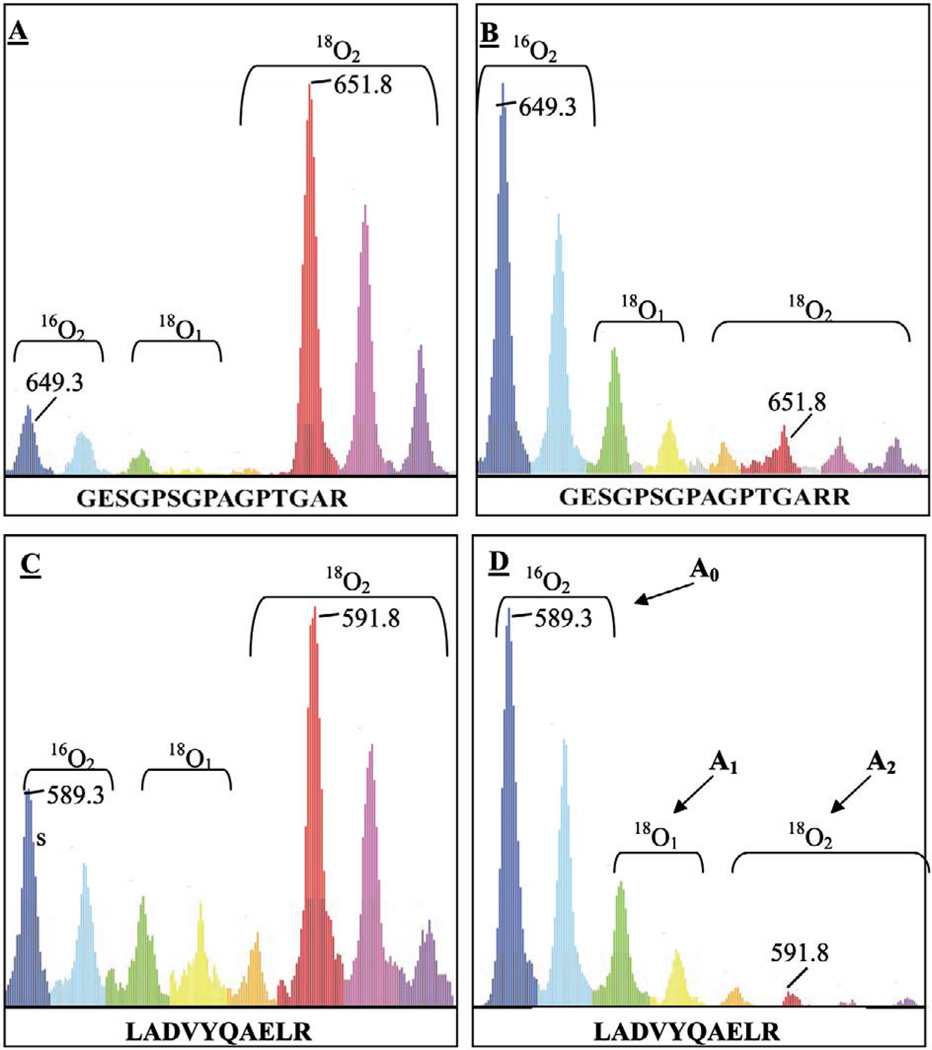
Figure 4.
Schematic representation of 18O-labeled and unlabeled peaks by ZoomQuant software. Shown are 18O-labeled and unlabeled peaks for different peptides visualized by ZoomQuant software. A doubly charged peptide (GESGPSGPAGPTGARR) of collagen alpha 1(I) (Swiss-Prot ID P02452) (A) and a doubly charged peptide (LADVYQAELR) of glial fibrillary acidic protein (Swiss-Prot ID P14136) (C) were measured to be 1.8- and 2.8-fold up-regulated, respectively, in tumor versus normal. Reverse experiments, in which normal tissue was labeled with 18O, confirmed the observation (B and D). ZoomQuant software uses both single and double incorporation of 18O atoms in a given peptide to calculate the ratios between 16O- and 18O-labeled samples using the following equation: L/U = [A1 + A2]/A0 where A0 represents the cluster area of the unlabeled peak, A1 the cluster area of the peak with a single 18O incorporation and A2 with double 18O incorporation (D).
3.3 Analyses of identified proteins
The combined analyses of both forward and reverse 18O labeling identified and quantified 188 proteins. Initially, labeled and unlabeled peptide ratios (tumor to normal) levels were normalized to internal standard horse CYC and then relative expression levels of each protein was calculated (see Section 2). Our analyses showed that the expression levels of 134 proteins (71%) were not changed while 54 proteins (29%) showed up-regulation of ≥1.5-fold in tumor compared to the normal brain tissue and these are listed in Table 1. We then inspected this list for known markers of tumor formation, metastasis and cell invasion. We found that 15 (28%) of these 54 differentially expressed proteins have been previously reported as molecules with a potential role in tumor formation (Table 1A) and six of these proteins were indicative of angiogenesis or associated with various tumor types (Table 1B) while the remaining 33 could be potential novel components of pediatric BSG (Table 1C).
Table 1.
Potential pediatric brainstem glioma biomarkers
| Protein | gi | SP_ID | AverageFC | Standarderror | Peptidecount |
|---|---|---|---|---|---|
| A. Proteins previously associated with adult tissue/cell lines of supratentorial gliomas | |||||
| Glial fibrillary acidic protein, astrocyte | 121135 | P14136 | 2.86 | 1.00 | 25 |
| Vimentin | 55977767 | P08670 | 2.64 | 1.10 | 17 |
| Heat-shock protein beta-1 | 19855073 | P04792 | 2.56 | 0.01 | 2 |
| Peroxiredoxin-6 | 1718024 | P30041 | 2.45 | 1.10 | 17 |
| Tubulin beta-2C chain | 55977480 | P68371 | 2.41 | 0.10 | 2 |
| Neurofilament light polypeptide | 62511894 | P07196 | 2.13 | 0.45 | 6 |
| Dihydropyrimidinase-related protein 2 (DRP-2) | 3122051 | Q16555 | 2.07 | 1.10 | 3 |
| Superoxide dismutase 2 | 134665 | P04179 | 1.97 | 0.75 | 2 |
| Annexin A5 | 113960 | P08758 | 1.90 | 0.10 | 6 |
| Glucose-6-phosphate isomerase | 17380385 | P06744 | 1.86 | 0.20 | 2 |
| Fructose-bisphosphate aldolase C | 113613 | P09972 | 1.82 | 0.56 | 5 |
| Serum albumin precursor | 113576 | P02768 | 1.74 | 0.02 | 7 |
| Alpha crystalline B chain | 117385 | P02511 | 1.68 | 0.28 | 2 |
| L-lactate dehydrogenase B chain | 126041 | P07195 | 1.67 | 0.20 | 2 |
| Pyruvate kinase isozymes M1/M2 | 20178296 | P14618 | 1.64 | 0.45 | 6 |
| B. Proteins previously associated with angiogenesis or various tumor types | |||||
| Periostin precursor | 93138709 | Q15063 | 2.42 | 0.16 | 3 |
| Hemoglobin subunit alpha | 57013850 | P69905 | 2.26 | 0.25 | 4 |
| Hemoglobin subunit beta | 56749856 | P68871 | 1.98 | 0.95 | 2 |
| Fibrinogen beta chain precursor | 399492 | P02675 | 1.96 | 0.43 | 1 |
| Mimecan precursor | 129078 | P20774 | 1.71 | 0.13 | 2 |
| Ferritin light chain | 120523 | P02792 | 1.66 | 0.50 | 2 |
| C. Novel potential biomarkers associated with pediatric brainstem gliomas | |||||
| α1-Collagen type VI | 125987811 | P12109 | 4.56 | 1.90 | 2 |
| Transgelin | 3123283 | Q01995 | 3.42 | 1.40 | 4 |
| Collagen alpha-2(VI) chain precursor | 125987812 | P12110 | 2.92 | 1.40 | 3 |
| Ras-related protein Rap-1A precursor | 51338596 | P62836 | 2.80 | 1.40 | 1 |
| ADAMTS-12 precursor | 17366354 | P58397 | 2.80 | 0.62 | 2 |
| Spectrin beta chain, brain 1 | 116242799 | Q01082 | 2.73 | 0.45 | 2 |
| Keratin, type I cytoskeletal 10 | 547749 | P13645 | 2.49 | 0.55 | 1 |
| Myelin transcription factor 1-like protein | 76789661 | Q9UL68 | 2.46 | 1.20 | 2 |
| Excitatory amino acid transporter 1 | 1169458 | P43003 | 2.35 | 0.45 | 2 |
| ATP synthase subunit beta, mitochondrial | 114549 | P06576 | 2.34 | 0.42 | 5 |
| Creatine kinase B-type | 125294 | P12277 | 2.33 | 1.19 | 4 |
| α3-Collagen type VI precursor | 5921193 | P12111 | 2.30 | 0.65 | 11 |
| Heat shock protein HSP 90-alpha (HSP 86) | 92090606 | P07900 | 2.29 | 0.64 | 1 |
| Collagen alpha-2(I) chain precursor | 124056488 | P08123 | 2.28 | 0.66 | 4 |
| Spectrin alpha chain, brain | 94730425 | Q13813 | 2.23 | 0.30 | 3 |
| Histone H2B type 1-L | 7387743 | Q99880 | 2.21 | 0.69 | 2 |
| Tropomyosin alpha-4 chain | 54039746 | P67937 | 2.18 | 0.90 | 2 |
| Myelin basic protein (MBP) | 17378805 | P02686 | 2.17 | 0.07 | 5 |
| Protein S100-B | 134138 | P04271 | 2.16 | 0.45 | 2 |
| Histone H2A type 2-A (H2A.2) | 74757558 | Q6FI13 | 2.07 | 0.81 | 5 |
| Tubulin beta-4 chain | 75075849 | Q4R4X8 | 2.03 | 0.90 | 6 |
| Tubulin alpha-3/alpha-7 chain | 135418 | P05214 | 1.97 | 0.15 | 2 |
| Transgelin-2 | 586000 | P37802 | 1.97 | 0.34 | 2 |
| Alpha-actinin-1 | 46397817 | P12814 | 1.91 | 0.38 | 2 |
| Actin, alpha skeletal muscle | 55976646 | P68139 | 1.89 | 0.64 | 4 |
| Collagen alpha-1(I) chain precursor | 124056487 | P02452 | 1.85 | 0.60 | 4 |
| 2,3″-cyclic-nucleotide 3″-phosphodiestera″ | 1705945 | P09543 | 1.70 | 0.01 | 2 |
| Ferritin light chain | 120523 | P02792 | 1.66 | 0.51 | 2 |
| Elongation factor 1-alpha 1 | 55584035 | P68104 | 1.64 | 0.20 | 2 |
| Tubulin alpha-6 chain | 20455322 | Q9BQE3 | 1.61 | 0.10 | 4 |
| Plectin-1 (PLTN) | 134044255 | Q15149 | 1.61 | 0.01 | 2 |
| Keratin, type II cytoskeletal 1 | 1346343 | P04264 | 1.60 | 0.14 | 7 |
| Glyceraldehyde-3-phosphate dehydrogenase | 120649 | P04406 | 1.60 | 0.31 | 4 |
4 Discussion
We present the first proteome profile of childhood BSG, thus providing new insight into the molecular pathogenesis of this disease. BSG differentially expressed proteins can be potential biomarkers and molecular signatures of disease progression for possible therapeutic intervention. Importantly, because the etiology of pediatric brainstem glioma has remained unknown, primarily due to the lack of viable tissue for experimental purposes, we describe a technique that may be utilized to study BSG from existing archived specimens, as well as more broadly for all other cancers. Given that FFPE tumor samples are routinely archived and accessible throughout the world, our goal was to develop methodologies to extract and quantify peptides from FFPE samples.
In this study, the largest amount of cells recovered from tumor, were neoplastic astrocytes. However, because of the heterogeneous nature of astrocytomas, other cell types that are an integral part of the tumor, such as the proliferating endothelial cells of vessels and to a much lesser extent, reactive stromal macroglial cells, normal glial cells and neurons, were necessarily present as well. It is important to note that we did not use LCM to recover the cells. Our aim was to recover the most representative area of tumor with the entire intrinsic cellular components in order to obtain the most robust protein signature specific to brainstem astrocytomas given that tumors are molecularly highly heterogeneous and thus random LCM may likely give an erroneous signature based on sampling alone. The same technique was used to recover the adjacent normal area of the brainstem not involved by tumor. The adjacent areas are composed of all the normal elements anatomically present in that specific area of the brain, including neurons, astrocytes, oligodendrocytes and macroglial cells.
Extracting intact proteins from FFPE samples involves removal of the paraffin wax and the reversal of formalin-induced cross-links. Wax removal is accomplished by a combination of heating, xylene and ethanol treatment. In 1991, Shi and colleagues [17] described a method for retrieval of antigens from FEPE tissues through microwave heating in the presence of metal solutions. The molecular mechanism of formalin fixation, wax removal and cross-link reversal of FFPE samples is well studied for antigen retrieval in immunohistochemical assays [16–20]. However, the majority of our BSG FFPE samples are between 10–25 years old. These tissue samples have been processed by different pathologists and possibly by different methods (e.g. fixation period from days to months). To address these issues, we combined and slightly modified existing protocols [16, 17, 20–23, 26, 27] and tested the feasibility of extracting proteolytically digested peptides for analysis and protein identification rather than extracting whole intact proteins. The concept of proteolytic digestion is that the enzyme (trypsin in this case) has access to cleavage sites on cross-linked proteins resulting in the easy release of peptides from the paraffin-embedded tissue. Here, we used a stepwise process in which de-waxed tissues were rehydrated in decreasing ethanol gradient followed by a prolonged tryptic digestion period (Fig. 1).
To ensure the validity of our findings we checked the yield of peptide extraction as well as the efficiency of 18O labeling at each step of our methodology. Since normal and tumor areas were not equal in size, protein measurement for each fraction was required. However, concentration measurements were hindered by the fact that we primarily dealt with peptides and not proteins. Although there are methods by which peptide concentrations can be measured (e.g. modified Bradford assays), in our experience, these methods either lack sensitivity or require a large amount of peptides. To address this issue, we spiked each sample with 2 µg of horse CYC (Swiss-Prot ID P00004), which served as internal standard. We chose horse CYC because it generates several unique tryptic peptides that are different in mass from tryptic peptides of human CYC. Since tryptic digestion of horse CYC was performed simultaneously with tryptic digestion of FFPE tissue, we were able to both test for the efficiency of 18O incorporation and measure the relative peptide concentration in each sample. Furthermore, to strengthen our approach, we performed reverse labeling experiments, in which tryptic peptides of normal brainstem sections were labeled with 18O, and compared to 16O-labeled peptides of tumor regions. Therefore, only proteins for which the expression levels were confirmed in both directions were retained.
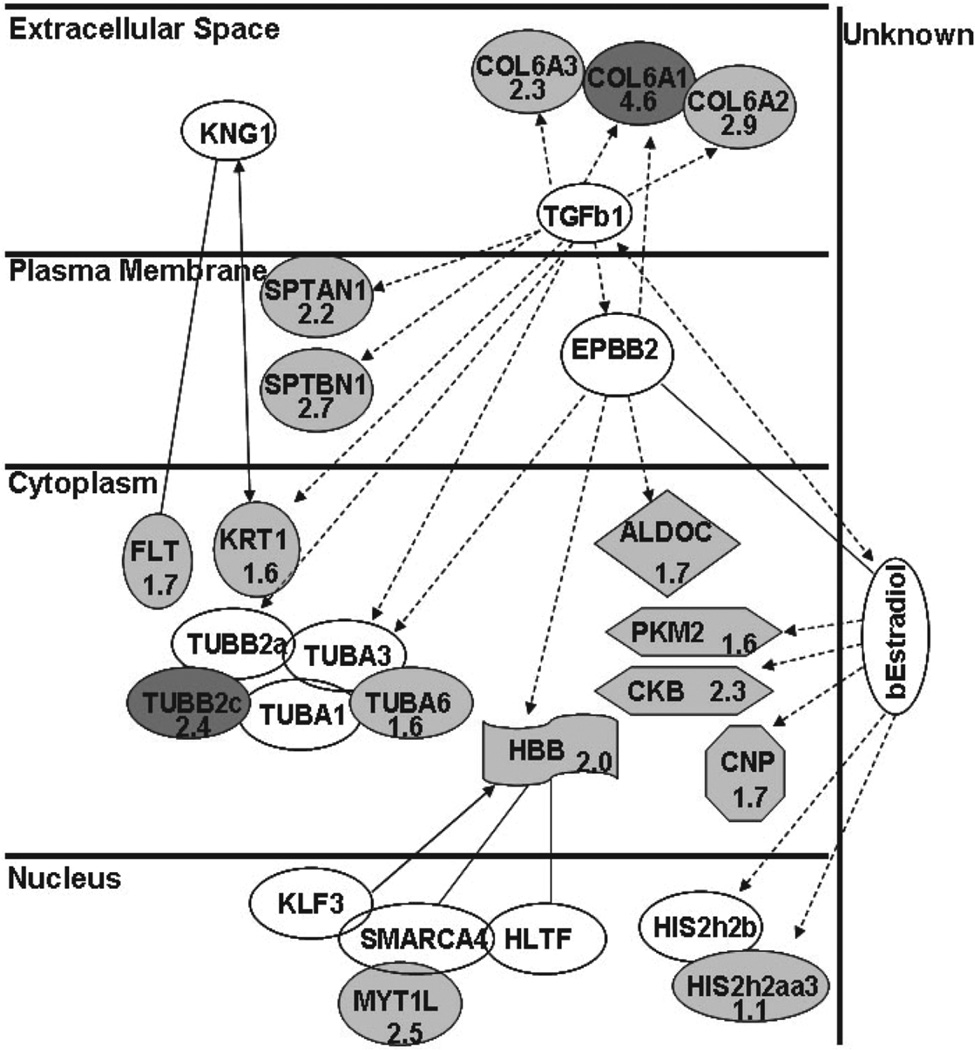
Using this stringent methodology, we identified 54 proteins that were up-regulated in pediatric FFPE BSG when compared to the normal tissue. Our data agree with previous microarray gene expression profiling studies that have used either brain tumor cell lines or tissue from adult supratentorial gliomas [28–30]. We found that 15 out of the 54 differentially expressed proteins have been reported in these previous studies as potential tumor biomarkers. Among these, vimentin, peroxiredoxin-6, SOD1, annexin V, GFAP, and DRP-2 have been shown to be up-regulated in adult glioblastoma multiform (GBM) [28–31] whereas tubulin beta-2C chain, neurofilament light polypeptide, and pyruvate kinase are known to be up-regulated in meduloblastoma cell lines [29, 32]. We also detected a high expression of angiogenesis- related proteins in BSG tumor compared to the normal tissue sections and these included hemoglobin subunits, which are an indication of angiogenesis, as well as periostin and mimecan precursors. Overexpression of periostin is known to enhance invasiveness and angiogenesis in oral cancer cell lines [33]. The remaining 33 proteins that were found to be uniquely up-regulated (≥1.5-fold) in pediatric BSG might be considered as potential novel biomarkers of this disease. Indeed, our analysis of the 54 identified proteins using the ingenuity pathway analysis (IPA) software clustered 16 of these proteins in a single cancer-related pathway. IPA generates pathways based on curated publications taking into account known protein interactions. Perhaps the most intriguing BSG candidates in this pathway are α3-collagen type VI precursor (2.3-fold up-regulated), α1-collagen type VI (4.6-fold up-regulation), and a-spectrin (2.2-fold upregulated), which are excreted or membrane-associated proteins. Furthermore, these mentioned proteins are known to be involved in extracellular matrix remodeling and tumor progression [34, 35] (Fig. 5).
Figure 5.
Pathway generated by the Ingenuity software depicting interaction between proteins, whose expression was altered in BSG versus normal area. Shown is biological pathway generated by Ingenuity software depicting interaction of 16 proteins upregulated in BSG. Protein names are abbreviated and fold changes are shown where applied. Proteins highlighted in dark gray are up-regulated (shown as numerical values) in tumor when compared to the normal brainstem region (score of 45). Protein-protein interactions are shown as follows: indirect ( ), acts on (
), acts on ( ), or (
), or ( ) direct interactions.
) direct interactions.
Our observation that 61% of identified proteins did not overlap with previous studies of gliomas and other brain tumors, implies that pediatric BSG has a distinct pathology compared to gliomas in other locations of the brain. Furthermore, the strengths of our study are in the direct analysis of pediatric BSG tissue, rather than surrogate cell lines or adult glioma tissue, and in our comparison of BSG to the normal brainstem of the same patient, versus a different individual, thereby eliminating two main confounding variables. Further investigation using this methodology with larger sample sizes will be necessary to confirm that these proteins are novel tumor biomarkers and potential therapeutic targets of childhood BSG.
Acknowledgments
The authors would like to thank Dr. Eric P. Hoffman for his support and discussion and Dr. Kristy Brown for assistance with LC-MS/MS analysis via Genetic Medicine Proteomics Core facility. This work was supported by the Isabella Kerr Molina Foundation for Brainstem Glioma Research and Children’s National Medical Center (CNMC) Intellectual and Developmental Disabilities Research Center (IDDRC) grant (P30HD40677).
Abbreviations
BSG
brainstem glioma
CYC
cytochrome C
FFPE
formalin fixed paraffin embedded
Footnotes
The authors have declared no conflict of interest.
References
- 1.Packer RJ. Brain tumors in children. Arch. Neurol. 1999;56:421–425. doi: 10.1001/archneur.56.4.421. [DOI] [PubMed] [Google Scholar]
- 2.Fisher PG, Donaldson SS. Hyperfractionated radiotherapy in the management of diffuse intrinsic brainstem tumors: when is enough enough? Int. J. Radiat. Oncol. Biol. Phys. 1999;43:947–949. doi: 10.1016/s0360-3016(98)00503-3. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg ML, Fisher PG, Freeman C, Korones DN, et al. Etoposide, vincristine, and cyclosporine Awith standard-dose radiation therapy in newly diagnosed diffuse intrinsic brainstem gliomas: a pediatric oncology group phase I study. Pediatr. Blood Cancer. 2005;45:644–648. doi: 10.1002/pbc.20382. [DOI] [PubMed] [Google Scholar]
- 4.Korones DN, Fisher PG, Kretschmar C, Zhou T, et al. Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: A Children’s Oncology Group phase II study. Pediatr. Blood Cancer. 2007 doi: 10.1002/pbc.21154. [DOI] [PubMed] [Google Scholar]
- 5.Fischbein NJ, Prados MD, Wara W, Russo C, et al. Radiologic classification of brain stem tumors: correlation of magnetic resonance imaging appearance with clinical outcome. Pediatr. Neurosurg. 1996;24:9–23. doi: 10.1159/000121010. [DOI] [PubMed] [Google Scholar]
- 6.Packer RJ, Boyett JM, Zimmerman RA, Albright AL, et al. Outcome of children with brain stem gliomas after treatment with 7800 cGy of hyperfractionated radiotherapy. A Childrens Cancer Group Phase I/II Trial. Cancer. 1994;74:1827–1834. doi: 10.1002/1097-0142(19940915)74:6<1827::aid-cncr2820740628>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Jallo G. Brainstem gliomas. Childs Nerv. Syst. 2006;22:1–2. doi: 10.1007/s00381-005-1267-5. [DOI] [PubMed] [Google Scholar]
- 8.Rubin G, Michowitz S, Horev G, Herscovici Z, et al. Pediatric brain stem gliomas: an update. Childs Nerv. Syst. 1998;14:167–173. doi: 10.1007/s003810050205. [DOI] [PubMed] [Google Scholar]
- 9.Fisher PG, Breiter SN, Carson BS, Wharam MD, et al. A clinicopathologic reappraisal of brain stem tumor classification. Identification of pilocystic astrocytoma and fibrillary astrocytoma as distinct entities. Cancer. 2000;89:1569–1576. doi: 10.1002/1097-0142(20001001)89:7<1569::aid-cncr22>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Cartmill M, Punt J. Diffuse brain stem glioma. A review of stereotactic biopsies. Childs Nerv. Syst. 1999;15:235–237. doi: 10.1007/s003810050379. discussion 238. [DOI] [PubMed] [Google Scholar]
- 11.Goyanes VJ, Matsui S, Sandberg AA. The basis of chromatin fiber assembly within chromosomes studied by histone-DNA crosslinking followed by trypsin digestion. Chromosoma. 1980;78:123–135. doi: 10.1007/BF00291911. [DOI] [PubMed] [Google Scholar]
- 12.Jackson V. Studies on histone organization in the nucleosome using formaldehyde as a reversible cross-linking agent. Cell. 1978;15:945–954. doi: 10.1016/0092-8674(78)90278-7. [DOI] [PubMed] [Google Scholar]
- 13.Jackson V. Formaldehyde cross-linking for studying nucleosomal dynamics. Methods. 1999;17:125–139. doi: 10.1006/meth.1998.0724. [DOI] [PubMed] [Google Scholar]
- 14.Dutta R, Gao YG, Priebe W, Wang AH. Binding of the modified daunorubicin WP401 adjacent to a T-G base pair induces the reverse Watson-Crick conformation: crystal structures of the WP401-TGGCCG and WP401-CGG[br5C]CG complexes. Nucleic Acids Res. 1998;26:3001–3005. doi: 10.1093/nar/26.12.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Gao YG, van der Marel GA, van Boom JH, et al. Simultaneous incorporations of two anticancer drugs into DNA. The structures of formaldehyde-cross-linked adducts of daunorubicin-d(CG(araC)GCG) and doxorubicind( CA(araC)GTG) complexes at high resolution. J. Biol. Chem. 1993;268:10095–10101. [PubMed] [Google Scholar]
- 16.Shi SR, Cote C, Kalra KL, Taylor CR, et al. A technique for retrieving antigens in formalin-fixed, routinely acid-decalcified, celloidin-embedded human temporal bone sections for immunohistochemistry. J. Histochem. Cytochem. 1992;40:787–792. doi: 10.1177/40.6.1588025. [DOI] [PubMed] [Google Scholar]
- 17.Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin- fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J. Histochem. Cytochem. 1991;39:741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- 18.Rait VK, O’Leary TJ, Mason JT. Modeling formalin fixation and antigen retrieval with bovine pancreatic ribonuclease A: I-structural and functional alterations. Lab. Invest. 2004;84:292–299. doi: 10.1038/labinvest.3700045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rait VK, Xu L, O’Leary TJ, Mason JT. Modeling formalin fixation and antigen retrieval with bovine pancreatic RNase A II. Interrelationship of cross-linking, immunoreactivity, and heat treatment. Lab. Invest. 2004;84:300–306. doi: 10.1038/labinvest.3700041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Leary TJ, Mason JT. A molecular mechanism of formalin fixation and antigen retrieval. Am. J. Clin. Pathol. 2004;122:154. author reply 154–155. [PubMed] [Google Scholar]
- 21.Hwang SI, Thumar J, Lundgren DH, Rezaul K, et al. Direct cancer tissue proteomics: a method to identify candidate cancer biomarkers from formalin-fixed paraffinembedded archival tissues. Oncogene. 2007;26:65–76. doi: 10.1038/sj.onc.1209755. [DOI] [PubMed] [Google Scholar]
- 22.Hood BL, Conrads TP, Veenstra TD. Mass spectrometric analysis of formalin-fixed paraffin-embedded tissue: unlocking the proteome within. Proteomics. 2006;6:4106–4114. doi: 10.1002/pmic.200600016. [DOI] [PubMed] [Google Scholar]
- 23.Crockett DK, Lin Z, Vaughn CP, Lim MS, et al. Identification of proteins from formalin-fixed paraffin-embedded cells by LC-MS/MS. Lab. Invest. 2005;85:1405–1415. doi: 10.1038/labinvest.3700343. [DOI] [PubMed] [Google Scholar]
- 24.Fenselau C, Yao X. Proteolytic labeling with 18O for comparative proteomics studies: preparation of 18O-labeled peptides and the 18O/16O peptide mixture. Methods Mol. Biol. 2007;359:135–142. doi: 10.1007/978-1-59745-255-7_9. [DOI] [PubMed] [Google Scholar]
- 25.Halligan BD, Slyper RY, Twigger SN, Hicks W, et al. ZoomQuant: an application for the quantitation of stable isotope labeled peptides. J. Am. Soc. Mass Spectrom. 2005;16:302–306. doi: 10.1016/j.jasms.2004.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos-Fernandez A, Lopez-Ferrer D, Vazquez J. Improved method for differential expression proteomics using trypsin-catalyzed 18O labeling with a correction for labeling efficiency. Mol. Cell. Proteomics. 2007;6:1274–1286. doi: 10.1074/mcp.T600029-MCP200. [DOI] [PubMed] [Google Scholar]
- 27.Palmer-Toy DE, Krastins B, Sarracino DA, Nadol JB, Jr, et al. Efficient method for the proteomic analysis of fixed and embedded tissues. J. Proteome Res. 2005;4:2404–2411. doi: 10.1021/pr050208p. [DOI] [PubMed] [Google Scholar]
- 28.Odreman F, Vindigni M, Gonzales ML, Niccolini B, et al. Proteomic studies on low- and high-grade human brain astrocytomas. J. Proteome Res. 2005;4:698–708. doi: 10.1021/pr0498180. [DOI] [PubMed] [Google Scholar]
- 29.Vogel TW, Zhuang Z, Li J, Okamoto H, et al. Proteins and protein pattern differences between glioma cell lines and glioblastoma multiform. Clin. Cancer Res. 2005;11:3624–3632. doi: 10.1158/1078-0432.CCR-04-2115. [DOI] [PubMed] [Google Scholar]
- 30.Iwadate Y, Sakaida T, Hiwasa T, Nagai Y, et al. Molecular classification and survival prediction in human gliomas based on proteome analysis. Cancer Res. 2004;64:2496–2501. doi: 10.1158/0008-5472.can-03-1254. [DOI] [PubMed] [Google Scholar]
- 31.Khalil AA. Biomarker discovery: a proteomic approach for brain cancer profiling. Cancer Sci. 2007;98:201–213. doi: 10.1111/j.1349-7006.2007.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacDonald TJ, Brown KM, LaFleur B, Peterson K, et al. Expression profiling of medulloblastoma: PDGFRA and the RAS/MAPK pathway as therapeutic targets for metastatic disease. Nat. Genet. 2001;29:143–152. doi: 10.1038/ng731. [DOI] [PubMed] [Google Scholar]
- 33.Siriwardena BS, Kudo Y, Ogawa I, Kitagawa M, et al. Periostin is frequently overexpressed and enhances invasion and angiogenesis in oral cancer. Br. J. Cancer. 2006;95:1396–1403. doi: 10.1038/sj.bjc.6603431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shintani Y, Maeda M, Chaika N, Johnson KR, et al. Collagen I promotes EMT in lung cancer cells via TGF-beta3 signaling. Am. J. Respir. Cell. Mol. Biol. 2007 doi: 10.1165/rcmb.2007-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubielecka PM, Jazwiec B, Potoczek S, Wrobel T, et al. Changes in spectrin organisation in leukaemic and lymphoid cells upon chemotherapy. Biochem. Pharmacol. 2005;69:73–85. doi: 10.1016/j.bcp.2004.08.031. [DOI] [PubMed] [Google Scholar]