Early Insulinization to Prevent Diabetes Progression (original) (raw)
This article reviews data collected from clinical studies regarding the place of early insulin treatment in preservation of β-cell function in type 2 diabetic patients. It emphasizes the stepwise progression of the data, starting with small uncontrolled studies and progressing to larger-scale controlled studies. It summarizes current knowledge in the field, emphasizing the additional information gained from the Outcome Reduction with Initial Glargine Intervention (ORIGIN) trial (1).
Effect of glucotoxicity and lipotoxicity on β-cells
Glucotoxicity and lipotoxicity have long been recognized as having a deleterious effect on both β-cell function and insulin action (2–4). Glucolipotoxicity refers to the combined deleterious effects of elevated glucose and free fatty acids on β-cell mass and function (5). Significant progress has been made in recent years toward a better understanding of the cellular and molecular basis of glucolipotoxicity (5–7). Insulin protects the β-cell by inducing rapid reversal of glucolipotoxicity and β-cell rest (8,9). The rapid reversal of glucolipotoxicity by insulin therapy is one of the justifications for early insulin treatment (2–10).
Treat to target or treat to failure?
The importance of avoiding prolonged hyperglycemia in patients with short diabetes duration in order to minimize its negative effect on late microvascular and macrovascular complications has been established (11). Hence, present guidelines (12–16) recommend early initiation of life style changes with or without metformin and subsequent addition of 2nd- and 3rd-line therapy when previous treatments fail to achieve or maintain the goal. The goals in the treatment of hyperglycemia in newly diagnosed type 2 diabetes are to achieve near-normal glucose control as early as possible in order to preserve β-cell function and maintain long-term normoglycemia.
The capacity of antidiabetes medication to maintain prolonged glycemic control (glucose durability) is of great importance. In the ADOPT study, rosiglitazone (17) demonstrated the best “glucose durability” compared with sulfonylurea (SU) and metformin. Glucagon-like peptide (GLP)-1 analogs were shown to have a potential β-cell–protective effect (18,19). In this article, we will focus on the protective effect of insulin on β-cells compared with those of oral antidiabetes drugs (OADs).
Milestones in clinical research of early insulinization for preservation of β-cell function
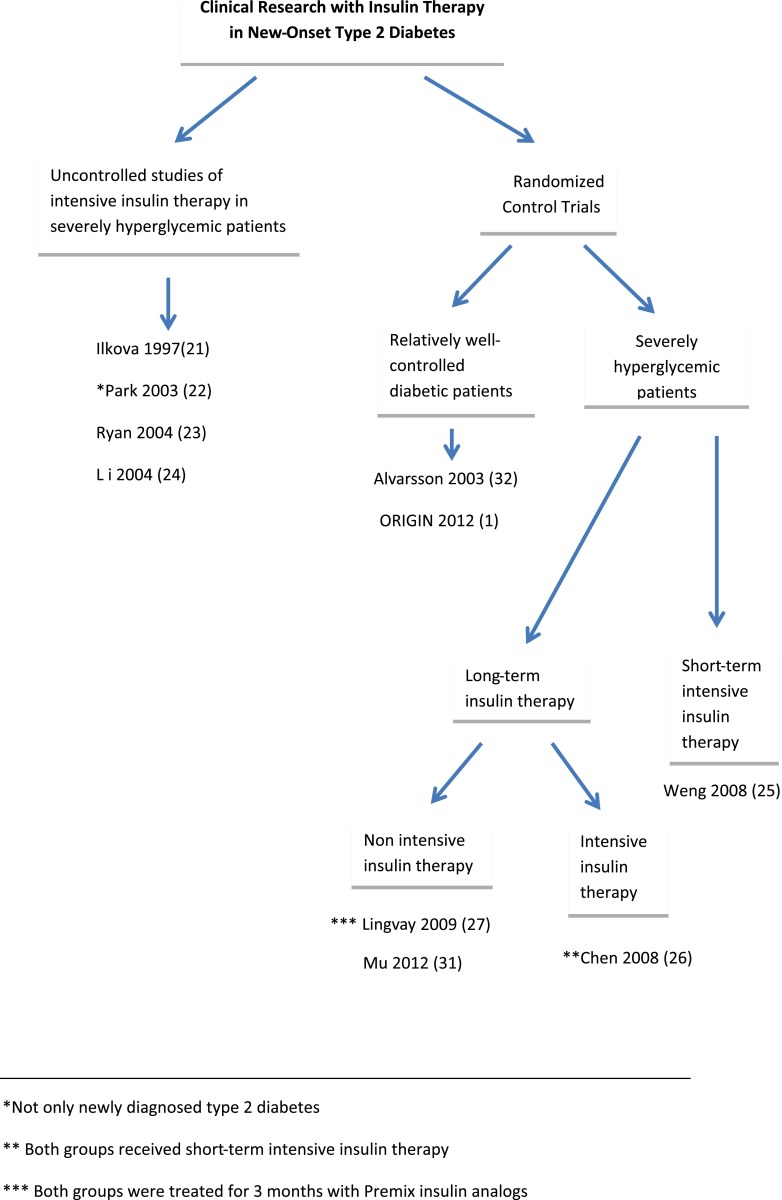
Correction of hyperglycemia with insulin increases peripheral sensitivity and improves residual β-cell function (20). The hypothesis of β-cell protection by early insulin therapy was tested by several clinical studies, beginning with noncontrolled studies in patients with severe hyperglycemia and followed by randomized controlled studies in severely uncontrolled newly diagnosed patients using short-term and longer-term insulin therapy as well as well-controlled type 2 diabetic patients using long-term insulin therapy (Fig. 1 and Table 1).
Figure 1.
Milestones in clinical research of early insulin therapy.
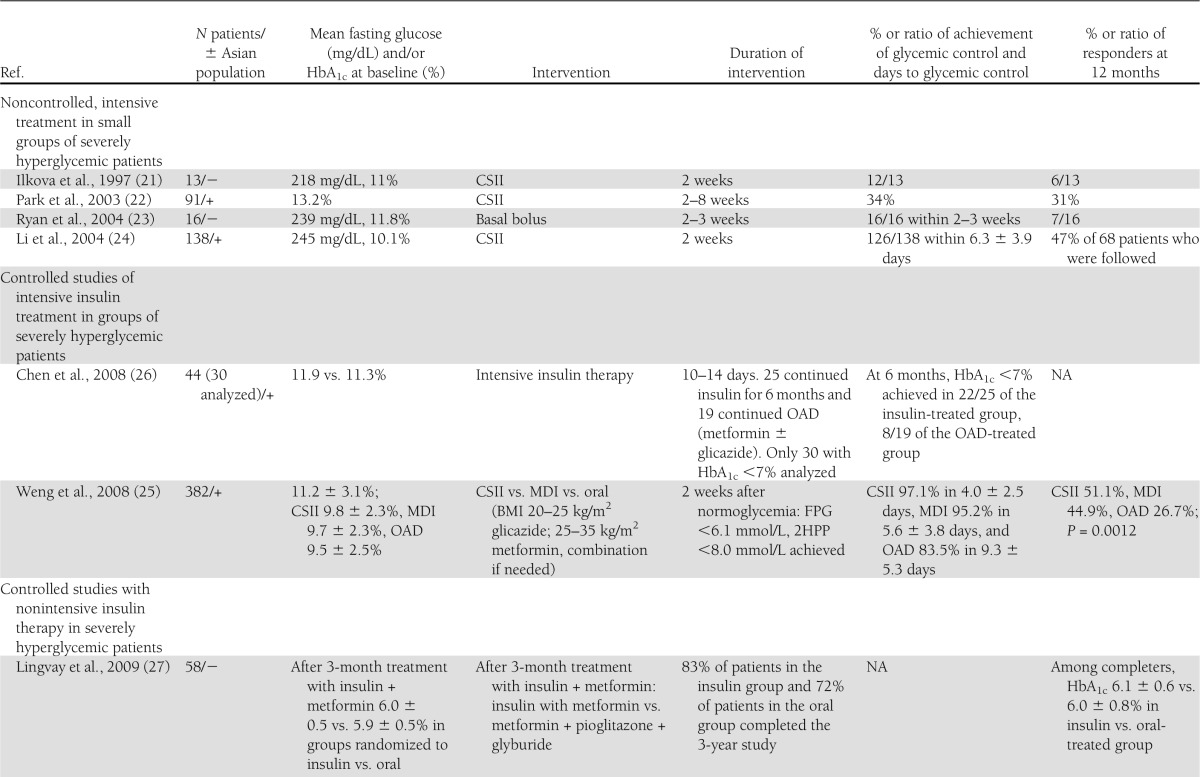
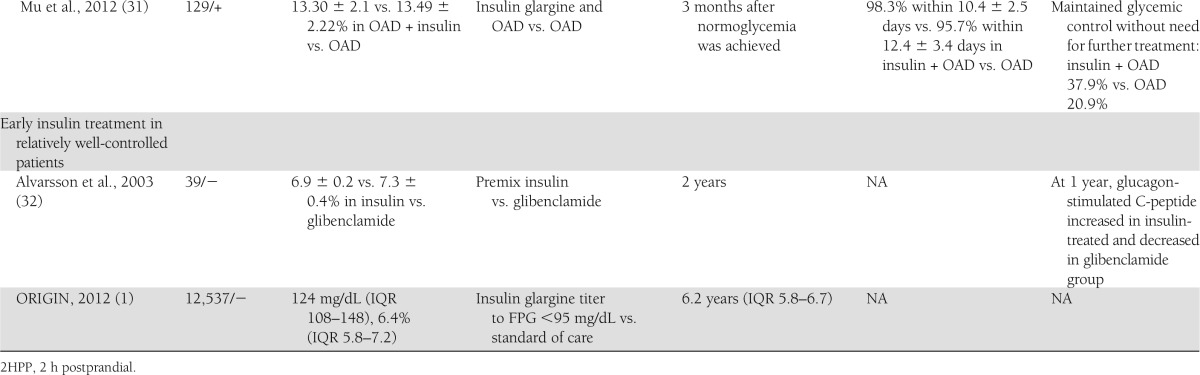
Table 1.
Summary of the baseline characteristics, intervention, and outcomes in the studies presented in the article
Early noncontrolled studies in severe hyperglycemic patients
In 1997, Ilkova et al. (21) published their study of 13 patients with extremely high glucose levels (average HbA1c 11.0%) who were treated with insulin pump–subcutaneous insulin infusion (SCII) for 2 weeks. Twelve of the 13 patients achieved glucose control, and 6 maintained their glucose control for a year without necessitating any further antidiabetes drug (ADD) treatment. This small uncontrolled study may be viewed as a feasibility test for the studies that followed.
The study of Park and Choi (22) included 91 Korean type 2 diabetic patients with average diabetes duration of 7.2 ± 4.9 years. Patients’ diabetes was not well controlled on lifestyle (51.7%), OAD (27.5%), insulin (12.3%), or combination therapy (5.8%), and patients were switched to SCII therapy. Remission rate was higher in patients with short diabetes duration (3.3 ± 2.7 years), lower postprandial glucose levels, and higher BMI and without diabetes complications. This study, however, included patients with long diabetes duration.
Ryan, Imes, and Wallace (23) studied 16 drug naïve, newly diagnosed type 2 diabetic patients with fasting plasma glucose (FPG) levels >11.0 mmol/L in order to define which patients would respond to short (2–3 weeks) intensive insulin therapy. End point was prolonged remission, defined as no need for ADD treatment after 1 year of follow-up. After 1 year, seven patients did not require ADD. These patients required less insulin during the active insulin therapy phase (0.37 ± 0.05 vs. 0.73 ± 0.07 units/kg/day) and had lower FPG at the end of the insulin therapy period (5.9 ± 0.3 vs. 7.7 ± 0.4 mmol/L). This was a noncontrolled study with a small number of patients planned to identify the patients who will most likely respond best to early insulin intervention. Its design and size require repetition for validation of its conclusions.
An important larger uncontrolled study by Li et al. (24) enrolled 138 newly diagnosed type 2 diabetic patients with FPG >11.1 mmol/L. Patients were hospitalized and treated for 2 weeks with continuous subcutaneous insulin infusion (CSII). Optimal glycemic control was achieved in 126 patients within 6.3 ± 3.9 days. Insulin therapy was stopped, and patients were instructed to continue with lifestyle treatment. The percentage of patients who maintained near-normal glucose control defined as FPG <6.1 mmol/L and postprandial glucose <8.0 mmol/L for 3, 6, 12, and 24 months was 72.6, 67.0, 47.1, and 42.3%, respectively. Homeostasis model assessment (HOMA) of β-cell function (HOMA-B) and the area under the curve (AUC) of insulin during intravenous glucose tolerance test were higher in the remission group (145.4 ± 89.6 vs. 78.5 ± 68.5 pmol/L/min, P = 0.002, and 1,423.4 ± 523.2 vs. 1,159.5 ± 476.8 pmol/L/min, P = 0.044). The conclusion of this study was that short-term insulin therapy can induce long-term glycemic control in newly diagnosed type 2 diabetic patients with severe hyperglycemia. The main limitations of this study were the lack of a control group and the exclusion from the analysis of 12 patients who failed to achieve glycemic control after 2 weeks’ CSII treatment.
Randomized controlled studies in new-onset diabetic patients
The results of the previously listed uncontrolled studies were reconfirmed and strengthened by a series of controlled studies. The controlled studies are divided into studies done in severely hyperglycemic patients versus relatively well-controlled patients. The studies done in severely hyperglycemic patients can be further divided into studies with long versus short insulin therapy with intensive insulin treatment in some and less intensive therapy in others.
Randomized controlled studies of short-term insulin intervention in new-onset severely hyperglycemic patients
The first large, multicenter, controlled trial (25) randomized 382 patients from nine different centers in China. The patient population included newly diagnosed type 2 diabetic patients with FPG of 7.0–16.7 mmol/L. The patients were randomly assigned to insulin treatment with multiple daily injections (MDI) or SCII or to treatment with OAD. The type of OAD was given according to BMI: patients with BMI 20–25 kg/m2 were treated with gliclazide, while patients with BMI 25–35 kg/m2 were treated with metformin; combination therapy of these drugs was given if needed. Glycemic control was achieved faster and in a higher percentage of the patients in the insulin-treated arms compared with the OAD group/arm (97.1% of the patients in the CSII group achieved glucose control within 4.0 ± 2.5 days vs. 95.2% within 5.6 ± 3.8 days in the MDI group and 83.5% within 9.3 ± 5.3 days in the OAD group). Two weeks after glycemic control was achieved, antidiabetes treatment was stopped. A year later, the target glycemic control in the insulin-treated groups was maintained in a significantly higher percentage of patients (51.1, 44.9, and 26.7% in the CSII, MDI, and OAD groups, respectively; P = 0.0012). β-Cell function was measured by HOMA-B and acute insulin secretion during the first 10 min after intravenous glucose tolerance test. Both HOMA-B and acute insulin response improved significantly after intensive interventions. The increase in acute insulin response was sustained in the insulin groups but significantly declined in the oral hypoglycemic agents group at 1 year in all patients in the remission group. There are two main limitations to this study: the use of SU in the control group, which limited the ability to separate the protective effect of insulin therapy on β-cell function, from the possible negative effect of SU. This limitation is repeated in other studies that involved treatment with SU in the control group (25–27). The second limitation is its external validity to the non-Asian population. This limitation is also repeated in many other studies (22,24,26).
A recent systematic review and meta-analysis (28) of short-term intensive insulin therapy in type 2 diabetes included the results from seven studies (five of them uncontrolled) (n = 839 participants). The meta-analysis demonstrated an increase in HOMA-B (1.13 [95% CI 1.02–1.25]) and decrease in HOMA of insulin resistance (−0.57 [−0.84 to −0.29]) compared with baseline after short-term intensive insulin therapy. Four of the studies reported glucose remission rates: 66.2, 58.9, 46.3, and 42.1% at 3, 6, 12, and 24 months, respectively. The authors concluded that short-term intensive insulin therapy might modify the natural history of diabetes.
Randomized controlled studies of long-term intensive insulin intervention in new-onset severely hyperglycemic patients
A controlled, single-center study (26) tested the results of prolonged therapy with multiple daily insulin injection (MDII) versus OAD after initial intensive insulin therapy in newly diagnosed diabetic patients. Fifty newly diagnosed type 2 diabetic patients with severe hyperglycemia (defined as FPG >300 mg/dL or random glucose >400 mg/dL) were hospitalized for 10–14 days and treated with MDII. Patients were then randomized to continue insulin therapy (n = 25), or to switch to OAD (metformin or gliclazide) (n = 19). Both groups were followed closely, and their treatment was titrated to preset glycemic goals. The insulin-treated group was better controlled both at 6 months (HbA1c 6.33 ± 0.70 vs. 7.50 ± 1.50%; P = 0.002) and at 1-year follow-up (6.78 ± 1.21 vs. 7.84 ± 1.74%; P = 0.009). Patients who achieved Hba1c <7% were tested for β-cell function using oral glucose tolerance test (OGTT) at baseline and after 6 months: 22 of 25 in the insulin-treated group and 8 of 19 in the OAD-treated group. There was a significant improvement in both groups in glucose AUC during OGTT. HOMA-B and insulin AUC during OGTT were improved significantly only under insulin therapy. The authors concluded that 6 months’ treatment with insulin was better for both glycemic control and preservation of β-cell function in new-onset diabetic patients with severe hyperglycemia. This conclusion, however, is debatable, since it was drawn from a small group that included only the responders and given that the performance of OGTT in the entire study population is missing. Other limitations of this study are its homogenous Asian population, which may decrease external validity to other populations, and the use of SU in the control group. The effect on HOMA-B and on insulin secretion during OGTT might be attributed to the positive effect of insulin on β-cell rest or to the negative effect of SU due to overfunction of β-cell.
Randomized controlled studies of long-term nonintensive insulin intervention in new-onset severely hyperglycemic patients
Lingvay et al. (27) studied the effect of long-term insulin therapy with premix insulin analog twice daily (in combination with metformin) versus a combination of three OADs. Patients were recruited in a single center and were newly diagnosed and drug naïve. They were enrolled in a lead-in period of 3 months during which time they were treated with premix insulin analog (premix insulin aspart 30/70) and metformin and achieved glycemic control, with reduction of HbA1c from 10.8 to 5.9% (29). After completion of the lead-in period, they were randomized to continue insulin-based therapy (29 patients) or to treatment with triple oral therapy: metformin, pioglitazone, and glyburide (29 patients). Eighty-three percent of patients in the insulin group and 72% of patients in the triple oral treatment group completed the 3-year study. Analysis was done on this group and not on the intention-to-treat group. Glycemic control was very well maintained throughout the 3 years in both groups: HbA1c was 6.1 ± 0.6% in the insulin group vs. 6.0 ± 0.8% in the triple oral therapy group (P = 0.26). Weight gain was demonstrated in both groups: 7.2 kg (95% CI 4.2–10.1) and 4.5 kg (0.9–8.0) (P = 0.09) in the oral and insulin-treated groups respectively. The incidences of mild and severe hypoglycemia events were similar in both groups. There were also no differences between the groups regarding compliance or satisfaction with the treatment as well as quality of life as measured by the modified Diabetes Quality of Life Clinical Trial Questionnaire. The conclusion of this study is that prolonged insulin treatment is as effective, safe, and well accepted for new-onset type 2 diabetic patients as triple drug therapy. Another analysis of the same study, which was extended from 36 to 42 months, was published by Harrison et al. (30). β-Cell function was assessed using the results of mixed-meal tolerance test at randomization and at 6, 12, 18, 30, and 42 months. At 3.5 years, both groups had well-preserved β-cell function with no significant change from baseline or within the two groups, as measured by AUC of C-peptide (P = 0.14) or C-peptide to glucose AUC (P = 0.7) during mixed-meal tolerance test. The conclusion of this study was that β-cell function can be preserved in new-onset type 2 diabetic patients for at least 3.5 years by intensive glucose control with either insulin-based therapy or triple drug therapy including peroxisome proliferator–activated receptor-γ.
There are several limitations to this study (27,29,30): dropout rates were high and unequal (17 and 28% in the insulin and triple OAD groups respectively), the initial 3 months’ intensive insulin therapy period might have influenced the results, and the combined triple OAD therapy does not allow distinction among different drugs’ effects on β-cell function. On the other hand, the long-term follow up and the diverse ethnic backgrounds (43% African American, 17% white, and 38% Hispanic) are important strengths of this study.
While earlier studies used intensive insulin treatment with MDII or SCII, a more recent study was conducted in order to determine whether basal insulin can achieve a similar effect. Mu et al. (31) enrolled 129 newly diagnosed type 2 diabetic patients with HbA1c >9% and FPG >9 mmol/L. Patients were randomly divided to receive either OAD alone (glimepiride or metformin) or a combination of OAD and basal insulin (glargine). Treatment was stopped 3 months after normoglycemia was achieved, and patients were followed-up for a year. At 1 year follow-up, a higher percentage of the patients in the insulin plus OAD group maintained target glycemic control without need for further treatment (37.9%) compared with OAD only (20.9%). Both treatment groups had similar improvements in HOMA-IR (P = 0.23) while there was significantly greater improvement of HOMA-B in the insulin plus OAD group (2.17 ± 0.14 vs. 2.11 ± 0.13; P = 0.03). The difference in diabetes remission rates between the insulin plus OAD and the OAD groups in this study was lower than in previous studies (24–26). There are several possible contributing factors for this smaller effect: the use of basal insulin regimen instead of the more complicated MDII or CSII in previous studies, longer time to achieve glucose control in this study, and the use of a different SU (glimepiride instead of gliclazide). More studies in different populations are needed to confirm this finding.
Randomized control study in relatively well-controlled diabetes
The studies described above demonstrated that early insulin therapy in patients with new-onset uncontrolled, severely hyperglycemic type 2 diabetes may restore β-cell function and induce diabetic remission in a large percentage of patients. Can these results be generalized also to patients with relatively well-controlled diabetes?
The first randomized controlled study in relatively well-controlled new-onset diabetic patients was conducted by Alvarsson et al. (32). In this multicenter Swedish study, 39 newly diagnosed (0–2 years) type 2 diabetic patients were randomized to receive either two injections of premix insulin per day or glibenclamide for 2 years. β-Cell function, glucose control, and measurement of quality of life were measured. After 1 year, the glucagon-stimulated C-peptide was increased in the insulin-treated group and decreased in the glibenclamide-treated group (P < 0.02). After 2 years, HbA1c was increased in the glibenclamide group and stable in the insulin-treated group (P < 0.02). The effect on stimulated C-peptide might be attributed to the positive effect of insulin on β-cell rest or to the negative effect of SU due to overfunction of β-cell.
The ORIGIN Trial was planned, designed, and carried out in order to investigate whether early insulin therapy in high–cardiovascular risk patients with diabetes or prediabetes would reduce long-term cardiovascular events (1). This study added important knowledge on the effect of early insulin therapy in diabetic and prediabetic patients. The innovation of the ORIGIN study over previously mentioned studies was its size (12,537 subjects), duration (median follow-up 6.2 years), and inclusion of a diverse population (including prediabetes and well-controlled diabetic patients). Among the 1,456 nondiabetic patients included in the study, in the glargine-treated group compared with the standard-care group there was a 28% reduction in the risk of developing diabetes, as diagnosed by OGTT (odds ratio 0.72 [95% CI 0.58–0.91], P = 0.006) at study end. In a second OGTT done at a median of 100 days (interquartile range [IQR] 95–112) after insulin was discontinued, additional cases of diabetes were detected in both groups. The incidence of diabetes, however, was reduced in the patients previously treated with insulin (i.e., 30 vs. 35%; odds ratio 0.80 [95% CI 0.64–1.0]; P = 0.05). Another important aspect of this study was the high compliance of patients to insulin therapy: after 2 years, 5,398 participants in the insulin glargine group (90%) were adherent to insulin therapy; at 5 years, 4,719 (85%) were adherent. The intervention was insulin glargine titered to reach an FPG level ≤95 mg/dL. After 1 year, 50% of the insulin-glargine group had an FPG level of ≤95 mg/dL and up to 75% had FPG <108 mg/dL. This level of glucose control was maintained over a median follow-up of 6.2 years (IQR 5.8–6.7 years). The control group also had excellent glucose control, and the difference between the two groups was maintained at HbA1c difference of 0.3% throughout the study.
It is interesting to note that most of the patients in the study achieved impressive glucose control even though they were not followed up in diabetology-specialized sites. These findings emphasize the relative ease of glucose control with basal insulin in this group of patients. However, insulin therapy resulted in an increased incidence of hypoglycemic events. The incidence of a first episode of severe hypoglycemia was 1.00/100 person-years in the insulin glargine group and 0.31/100 person-years in the standard care group (P < 0.001). The incidence of any (i.e., confirmed or unconfirmed) nonsevere symptomatic hypoglycemia was 16.72 and 5.16/100 person-years, respectively (P < 0.001). Participants in the insulin glargine group gained a median of 1.6 kg (IQR −2.0 to 5.5), and participants in the standard care group lost a median of 0.5 kg (−4.3 to 3.2) during follow-up.
Identifying patients who are more likely to benefit from early insulin treatment
Defining which patients are more likely to respond to early insulin treatment is a complex but rewarding mission. A recent review by Retnakaran and Zinman (33) divided factors predicting the likelihood of sustaining prolonged euglycemia post–insulin treatment into three categories: factors at baseline, during the insulin treatment, and right after the insulin treatment. At baseline, some of the factors that may predict response are better glycemic control (22,25,28), higher BMI and insulin resistance (22,25,26,28), and shorter diabetes duration (22). Faster achievement of glucose control (25) and requirement of lower exogenous insulin doses during the insulin treatment (23), as well as better glycemic control (23–26) and greater improvement in β-cell function (24–26) immediately after insulin therapy, were all associated with higher remission rates. Patient attitude toward diabetes was another aspect that was found to be related to response rates to early insulin treatment (34). Patients who maintained diabetes remission after short CSII treatment (25) were more likely than those who did not to have higher scores in positive attitude, belief in importance of care, care ability, and self-care compliance scales.
Conclusions
When one considers initiation of insulin therapy in a type 2 diabetic patient with the intention to preserve β-cell function, the level of evidence supporting this decision is relatively high.
For the subgroup of patients with severe symptomatic hyperglycemia, there is strong evidence, in addition to guideline recommendations (American Diabetes Association/ European Association for the Study of Diabetes, International Diabetes Federation, American Association of Clinical Endocrinologists, Canadian, and National Institute for Health and Care Excellence [12–16]), to support initiation of short-term insulin therapy. Insulin therapy is an effective way to reverse short-term glucotoxicity and lipotoxicity and shows evidence of midterm β-cell preservation. Short-term insulin treatment is safe, with low incidence of hypoglycemia (23–25) and less concern for weight gain. However, the best method for insulin treatment in such cases—basal insulin, premix insulin analogs, MDII, or CSII—and the length of insulin therapy should be further studied.
The place of long-term early insulin treatment in well-controlled type 2 diabetic patients is still debatable. Although the ORIGIN study demonstrated that early insulin therapy with insulin glargine is both safe and feasible, there are still the pros and cons of this treatment to consider. The benefits are as follows. 1) Insulin therapy can achieve near-normal FPG. 2) The achievement of such goals, especially in newly-onset diabetes, is relatively easy and can be maintained for many years. 3) Early treatment with insulin is safe with respect to both cardiovascular and tumor genesis. Among the drawbacks to consider regarding early insulin therapy are 1) weight gain (even though weight gain might be mild, its clinical consequences are yet unknown), 2) increased risk of hypoglycemia, and 3) patients’ preference for different treatments.
In light of the above, who will be a good candidate for early insulin therapy? Beyond the consensus concerning severely hyperglycemic patients, other patients who may benefit from early insulin therapy may be those who have a more prominent increase in FPG compared with their increase in postprandial glucose. Another group may be the leaner type 2 diabetic patients, since increased weight is less of a risk, and they are more likely to be insulin deficient. Lastly, we may consider early insulin therapy in obese type 2 diabetes in combination with GLP-1 analogs or in patients treated with GLP-1 analog when FPG still remains high.
Acknowledgments
I.R. is on the advisory boards of Novo Nordisk, AstraZeneca, Bristol-Myers Squibb, Merck Sharp & Dohme, and Eli Lilly; is a consultant for AstraZeneca and Bristol-Myers Squibb, Johnson & Johnson, Eli Lilly, and Israeli firms (Andromeda, HealOr, Insuline, TransPharma, and Teva); and sits on the speakers bureaus of Eli Lilly, Novo Nordisk, AstraZeneca, Roche, and Johnson & Johnson. O.M. sits on the speakers bureaus of Novo Nordisk, Eli Lilly, Sanofi, Novartis, and Merck Sharp & Dohme; sits on the advisory boards of Novo Nordisk, Eli Lilly, Sanofi, and Novartis; and receives grants paid to Hadassah University Hospital as a study physician by AstraZeneca and Bristol-Myers Squibb. No other potential conflicts of interest relevant to this article were reported.
I.R. edited and reviewed the manuscript. O.M. researched, wrote, and revised the manuscript. I.R. and O.M. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This publication is based on the presentations from the 4th World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension (CODHy). The Congress and the publication of this supplement were made possible in part by unrestricted educational grants from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Ethicon Endo-Surgery, Janssen, Medtronic, Novo Nordisk, Sanofi, and Takeda.
References
- 1.Gerstein HC, Bosch J, Dagenais GR, et al. ORIGIN Trial Investigators Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–328 [DOI] [PubMed] [Google Scholar]
- 2.Unger RH, Grundy S. Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia 1985;28:119–121 [DOI] [PubMed] [Google Scholar]
- 3.Poitout V, Robertson RP. Minireview: Secondary beta-cell failure in type 2 diabetes—a convergence of glucotoxicity and lipotoxicity. Endocrinology 2002;143:339–342 [DOI] [PubMed] [Google Scholar]
- 4.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 2008;29:351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poitout V, Amyot J, Semache M, Zarrouki B, Hagman D, Fontés G. Glucolipotoxicity of the pancreatic beta cell. Biochimic Biophys Acta 2010; 1801:289–298 [DOI] [PMC free article] [PubMed]
- 6.Weir GC, Marselli L, Marchetti P, Katsuta H, Jung MH, Bonner-Weir S. Towards better understanding of the contributions of overwork and glucotoxicity to the β-cell inadequacy of type 2 diabetes. Diabetes Obes Metab 2009;11(Suppl. 4):82–90 [DOI] [PubMed] [Google Scholar]
- 7.Cnop M. Fatty acids and glucolipotoxicity in the pathogenesis of Type 2 diabetes. Biochem Soc Trans 2008;36:348–352 [DOI] [PubMed] [Google Scholar]
- 8.Wajchenberg BL. β-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007;28:187–218 [DOI] [PubMed] [Google Scholar]
- 9.Rolla A. The pathophysiological basis for intensive insulin replacement. Int J Obes Relat Metab Disord 2004;28(Suppl. 2):S3–S7 [DOI] [PubMed] [Google Scholar]
- 10.Bonora E. Protection of pancreatic beta-cells: is it feasible? Nutr Metab Cardiovasc Dis 2008;18:74–83 [DOI] [PubMed] [Google Scholar]
- 11.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 12.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–1596 [DOI] [PubMed] [Google Scholar]
- 13.IDF Clinical Guidelines Task Force. Global Guideline for Type 2 Diabetes Brussels, International Diabetes Federation, 2005 [Google Scholar]
- 14.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009;15:540–559 [DOI] [PubMed] [Google Scholar]
- 15.Berard LD, Booth G, Capes S, Quinn K, Woo V. Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Pharmacologic management of type 2 diabetes. Canadian J Diabetes 2008;32:S53–S61 [Google Scholar]
- 16.Home P, Mant J, Diaz J, Turner C, Guideline Development Group Management of type 2 diabetes: summary of updated NICE guidance. BMJ 2008;336:1306–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahn SE, Haffner SM, Heise MA, et al. ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 18.Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol 2003;17:161–171 [DOI] [PubMed] [Google Scholar]
- 19.Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009;32:762–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garvey WT, Olefsky JM, Griffin J, Hamman RF, Kolterman OG. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes 1985;34:222–234 [DOI] [PubMed] [Google Scholar]
- 21.Ilkova H, Glaser B, Tunçkale A, Bagriaçik N, Cerasi E. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients by transient intensive insulin treatment. Diabetes Care 1997;20:1353–1356 [DOI] [PubMed] [Google Scholar]
- 22.Park S, Choi SB. Induction of long-term normoglycemia without medication in Korean type 2 diabetes patients after continuous subcutaneous insulin infusion therapy. Diabetes Metab Res Rev 2003;19:124–130 [DOI] [PubMed] [Google Scholar]
- 23.Ryan EA, Imes S, Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care 2004;27:1028–1032 [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Xu W, Liao Z, et al. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients is associated with improvement of beta-cell function. Diabetes Care 2004;27:2597–2602 [DOI] [PubMed] [Google Scholar]
- 25.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 2008;371:1753–1760 [DOI] [PubMed] [Google Scholar]
- 26.Chen H-S, Wu T-E, Jap T-S, Hsiao L-C, Lee S-H, Lin H-D. Beneficial effects of insulin on glycemic control and β-cell function in newly diagnosed type 2 diabetes with severe hyperglycemia after short-term intensive insulin therapy. Diabetes Care 2008;31:1927–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lingvay I, Legendre JL, Kaloyanova PF, Zhang S, Adams-Huet B, Raskin P. Insulin-based versus triple oral therapy for newly diagnosed type 2 diabetes: which is better? Diabetes Care 2009;32:1789–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kramer CK, Zinman B, Retnakaran R. Short-term intensive insulin therapy in type 2 diabetes mellitus: a systemic review and meta-analysis. Lancet Diabetes & Endocrinology. 30 January 2013 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Lingvay I, Kaloyanova PF, Adams-Huet B, Salinas K, Raskin P. Insulin as initial therapy in type 2 diabetes: effective, safe, and well accepted. J Investig Med 2007;55:62–68 [DOI] [PubMed] [Google Scholar]
- 30.Harrison LB, Adams-Huet B, Raskin P, Lingvay I. β-Cell function preservation after 3.5 years of intensive diabetes therapy. Diabetes Care 2012;35:1406–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mu PW, Chen YM, Lu HY, et al. Effects of a combination of oral anti-diabetes drugs with basal insulin therapy on β-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes. Diabetes Metab Res Rev 2012;28:236–240 [DOI] [PubMed] [Google Scholar]
- 32.Alvarsson M, Sundkvist G, Lager I, et al. Beneficial effects of insulin versus sulphonylurea on insulin secretion and metabolic control in recently diagnosed type 2 diabetic patients. Diabetes Care 2003;26:2231–2237 [DOI] [PubMed] [Google Scholar]
- 33.Retnakaran R, Zinman B. Short-term intensified insulin treatment in type 2 diabetes: long-term effects on β-cell function. Diabetes Obes Metab 2012;14(Suppl. 3):161–166 [DOI] [PubMed] [Google Scholar]
- 34.Chen A, Huang Z, Wan X, et al. Attitudes toward diabetes affect maintenance of drug-free remission in patients with newly diagnosed type 2 diabetes after short-term continuous subcutaneous insulin infusion treatment. Diabetes Care 2012;35:474–481 [DOI] [PMC free article] [PubMed] [Google Scholar]