C2cd3 is critical for centriolar distal appendage assembly and ciliary vesicle docking in mammals (original) (raw)
Significance
The primary cilium is a hair-like cell surface organelle, the loss of which leads to numerous human diseases collectively known as ciliopathies. To better understand the pathology and to develop treatments for these diseases, it is critical to identify important regulators of cilium biogenesis and to reveal their mechanisms of function. We show that C2cd3 is required for the assembly of a structure at the distal end of the mother centriole, which serves as an anchor of the cilium. Without this structure, the mother centriole cannot associate with the membrane, and many other cilium proteins cannot be recruited to the centriole. This work provides a significant insight into one of the earliest steps in cilium formation.
Keywords: centrosome, ciliopathy, Ofd1, Bbs4
Abstract
The primary cilium plays critical roles in vertebrate development and physiology, but the mechanisms underlying its biogenesis remain poorly understood. We investigated the molecular function of C2 calcium-dependent domain containing 3 (C2cd3), an essential regulator of primary cilium biogenesis. We show that C2cd3 is localized to the centriolar satellites in a microtubule- and Pcm1-dependent manner; however, C2cd3 is dispensable for centriolar satellite integrity. C2cd3 is also localized to the distal ends of both mother and daughter centrioles and is required for the recruitment of five centriolar distal appendage proteins: Sclt1, Ccdc41, Cep89, Fbf1, and Cep164. Furthermore, loss of C2cd3 results in failure in the recruitment of Ttbk2 to the ciliary basal body as well as the removal of Cp110 from the ciliary basal body, two critical steps in initiating ciliogenesis. C2cd3 is also required for recruiting the intraflagellar transport proteins Ift88 and Ift52 to the mother centriole. Consistent with a role in distal appendage assembly, C2cd3 is essential for ciliary vesicle docking to the mother centriole. Our results suggest that C2cd3 regulates cilium biogenesis by promoting the assembly of centriolar distal appendages critical for docking ciliary vesicles and recruiting other essential ciliogenic proteins.
The primary cilium is a fundamentally important organelle, the loss of which in humans causes a broad spectrum of genetic disorders known as ciliopathies (1). In addition to sensory and motile functions, the primary cilium plays a central role in the signal transduction of the Hh, PDGF, Wnt, Hippo, and calcium signaling pathways (2–4). Recent genomic and proteomic approaches have identified a ciliome consisting of hundreds of proteins (5, 6). However, the molecular functions of most of these proteins remain elusive.
The primary cilium originates from the basal body, a centriole-derived structure (1). A centriole comprises a core multiprotein complex surrounded by a cylinder of nine microtubule triplets. In addition, the oldest centriole, the mother centriole, possesses distal and subdistal appendages. Centrioles and surrounding pericentriolar material constitute the centrosomes and spindle poles in dividing cells (7). In quiescent cells, Golgi-derived ciliary vesicles dock at the distal end of the mother centriole (8). The mother centriole, now known as the basal body, migrates to the cell surface with the docked ciliary vesicle. The ciliary vesicle fuses with the plasma membrane, allowing the cilium to protrude from the cell surface.
The distal appendages of the mother centriole, also known as transition fibers of the cilium, are protein complexes comprising at least five components (Ccdc41/Cep83, Cep89/Cep123, Sclt1, Fbf1, and Cep164) (9). Distal appendages are critical for the recruitment of Tau tubulin kinase 2 (Ttbk2), which appears to play a critical role in removing centrosomal protein of 110kD (Cp110, also known as Ccp110, Mouse Genome Informatics, www.informatics.jax.org/marker/MGI:2141942), an inhibitor of ciliogenesis, from the distal end of the mother centriole (10). Recent studies have also suggested that components of the distal appendages are essential for docking ciliary vesicles to the mother centriole (9, 11–14).
The axoneme, the microtubule skeleton of the cilium, extends from the distal end of the mother centriole and requires intraflagellar transport (IFT), which was known to mediate cargo movement within the cilium (15). Mutations disrupting the functions of the IFT motors (kinesin II and cytoplasmic dynein) or the IFT cargo adaptor complexes (IFT-A and IFT-B) lead to defects in cilia biogenesis. Recent studies have revealed an essential role for distal appendage proteins in recruiting IFT proteins to the mother centriole, suggesting another mechanism by which centriolar distal appendages promote ciliogenesis (9, 12, 13).
Centriolar satellites are electron-dense particles around the centrosome and basal body (16). Many centriolar satellite components have been identified, including Pcm1, the BBSome, a multiprotein complex comprising seven Bardet–Biedl syndrome-related proteins, as well as Cep290 and Ofd1 (17–20). Interestingly, the functions of various centriolar satellite proteins diverge. Pcm1 and the BBSome promote ciliary membrane biogenesis and the trafficking of ciliary membrane proteins (18, 21). Ofd1, on the other hand, appears to regulate ciliogenesis by recruiting components of the distal appendages and IFT particles, although a recent study appeared to suggest an additional negative role of Ofd1 in ciliogenesis (22, 23).
Through the study of two loss-of-function mouse mutants, we have previously identified a novel C2 domain-containing protein, C2cd3, as an essential regulator of ciliogenesis and mouse embryonic development (24). In the current study, we demonstrate that C2cd3 is localized to centriolar satellites and that its localization is dependent on Pcm1 and dynein-mediated retrograde transport. C2cd3 is also localized to the distal ends of the mother and daughter centrioles and is required for the recruitment of five distal appendage proteins: Ccdc41, Sclt1, Cep89, Fbf1, and Cep164. Moreover, in the absence of C2cd3, Ttbk2 is not recruited to the distal end of the mother centriole, nor is Cp110 removed. In addition, the recruitment of Ift88 and Ift52, two IFT complex-B components, does not occur in C2cd3 mutants. Finally, we observed that the docking of the ciliary vesicles to the mother centriole is dependent on C2cd3. Our results suggest that C2cd3 regulates the initiation of ciliogenesis through centriolar maturation, ciliogenic protein recruitment and ciliary vesicle docking.
Results
C2cd3 Is Associated with Centriolar Satellites.
We previously demonstrated that GFP–C2cd3 was localized to punctae around the ciliary basal body (24). To study the localization of endogenous C2cd3, we generated a polyclonal antibody against the N terminus of C2cd3. Using this antibody, we found that similar to GFP–C2cd3, endogenous C2cd3 was localized to punctae around the centrosome marked with γ-tubulin (Fig. 1_A_ and Fig. S1). We detected no C2cd3 signal in homozygous C2cd3 GT mutant mouse embryonic fibroblasts (MEFs) (24), confirming that this antibody is C2cd3 specific (Fig. 1_A_).
Fig. 1.
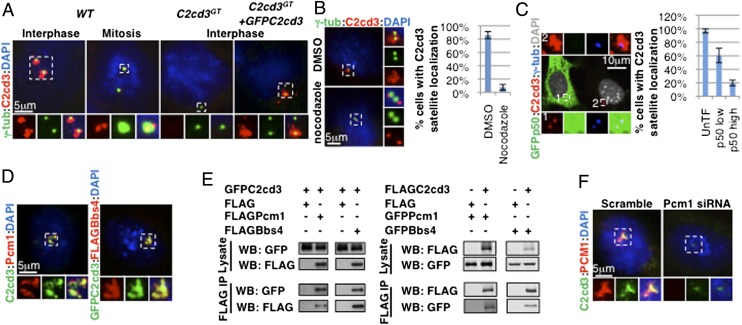
C2cd3 is localized to centriolar satellites. (A) In interphase, C2cd3 is present in punctae around centrosomes, whereas in mitosis, C2cd3 is localized to two punctae at each spindle pole. The lack of staining in C2cd3 GT mutant cells indicates the specificity of the C2cd3 antibody, and GFP–C2cd3 exhibits similar localization to endogenous C2cd3. (B) The centriolar satellite C2cd3 is dispersed upon nocodazole treatment, revealing its centriolar localization. DMSO-treated cells served as a control. (C) The overexpression of p50/Dynamitin disrupts the centriolar satellite localization of C2cd3. (D) C2cd3 is colocalized with Pcm1 and Bbs4. (E) Overexpressed C2cd3 coimmunoprecipitates with Pcm1 and Bbs4. (F) C2cd3 is dispersed from centriolar satellites in cells treated with a Pcm1 siRNA. The lack of Pcm1 staining indicates the effectiveness of the knockdown. Both Pcm1 and C2cd3 are localized to centriolar satellites in the control cells treated with a scramble siRNA. Centrosomes and spindle poles are labeled with γ-tubulin. The nuclei are visualized with DAPI. For quantitative analyses, SD is indicated. n = 3 independent experiments. IP, immunoprecipitation; WB, Western blot.
The punctate localization of C2cd3 around the centrosome was reminiscent of centriolar satellites, dynamic electron-dense granular structures that surround the centrosome and ciliary basal body in interphase but are dispersed in mitosis (16). We observed that in mitosis, C2cd3 was present as two fine dots inside each spindle pole instead of as punctae around it, consistent with the cell cycle-dependent dynamics of centriolar satellites (Fig. 1_A_ and Fig. S1).
Dynein-mediated retrograde transport along microtubules is critical for maintaining centriolar satellites around the centrosomes (17). To confirm the association of C2cd3 with centriolar satellites, we disrupted microtubule polymerization with nocodazole (Fig. S2). As expected, C2cd3 failed to localize around the centrosome in nocodazole-treated cells, suggesting that the centriolar satellite localization of C2cd3 is microtubule-dependent (Fig. 1_B_).
Dynein interacts with some of its cargoes through a dynactin complex (25). Overexpressing the dynactin subunit p50/dynamitin exerts a dominant negative effect on retrograde transport by uncoupling cargo from the motor (26). We observed that high levels of p50 overexpression disrupted C2cd3 centriolar satellite localization, further suggesting that C2cd3 is indeed associated with centriolar satellites (Fig. 1_C_).
Finally, we compared the localization of C2cd3 and two known centriolar satellite components, Pcm1 and Bbs4. We observed that C2cd3 was colocalized with both Pcm1 and Bbs4 (Fig. 1_D_). Moreover, through coimmunoprecipitation analysis between overexpressed proteins, we determined that C2cd3 physically interacted with Pcm1 and Bbs4 (Fig. 1_E_).
Pcm1 is required for the centriolar satellite localization of many other proteins [e.g., the BBS proteins (18), Cep290 (19), etc.]. To test whether the centriolar satellite localization of C2cd3 is also dependent on Pcm1, we depleted Pcm1 in MEFs through RNA interference. Targeting three different parts of the Pcm1 coding sequence with various small interfering RNAs, we observed a consistent reduction in C2cd3 centriolar satellite localization upon Pcm1 depletion, suggesting that the centriolar satellite localization of C2cd3 is dependent on Pcm1 (Fig. 1_F_ and Fig. S3).
In summary, the microtubule-, cell cycle-, and Pcm1-dependent dynamic localization of C2cd3, as well as its colocalization and physical interaction with Pcm1 and Bbs4, provided strong evidence that C2cd3 is associated with centriolar satellites.
C2cd3 Is Dispensable for Centriolar Satellite Integrity.
Despite its centriolar satellite localization, C2cd3 is not required for normal localization of Pcm1, Bbs4, Cep290, and Ofd1 to centriolar satellites, suggesting that C2cd3 is dispensable for centriolar satellite integrity (Fig. S4). Some components of centriolar satellites are involved in activating the small GTPase Rab8, which subsequently enters the cilium (18, 19). To address whether C2cd3 plays a role in Rab8 activation and ciliary localization, we examined the localization of GFP–Rab8a in hypomorphic C2cd3 Hty mutant cells, which produce a small number of cilia (24). We found that ∼30% cilia were Rab8 positive in both wild-type and C2cd3 Hty mutant cells, suggesting that C2cd3 is not required for the activation and ciliary localization of Rab8 (Fig. S5).
C2cd3 Is Localized to the Distal Ends of Centrioles.
In addition to centriolar satellites, C2cd3 is also localized to the centrioles. In mitosis, when centriolar satellite C2cd3 was dispersed, we observed C2cd3 in two fine dots colocalized with γ-tubulin at each spindle pole, suggesting that C2cd3 may associate with both mother and daughter centrioles (Fig. 1_A_). The centriolar localization of C2cd3 in interphase was difficult to observe due to the dense staining of C2cd3 in centriolar satellites. However, when the centriolar satellite C2cd3 was dispersed by nocodazole, p50 overexpression, or Pcm1 depletion, we were able to observe C2cd3 staining partially overlapping with γ-tubulin, suggesting that C2cd3 is localized to centrioles in interphase as well (Fig. 1 B, C, and F and Fig. S3). This result also suggests that distinct from its centriolar satellite localization, the centriolar localization of C2cd3 is independent of microtubules.
To further define the localization of C2cd3 on centrioles, we compared C2cd3 with known centriolar markers. Ninein was localized to the proximal ends of both mother and daughter centrioles as well as to the subdistal appendages of mother centriole (Fig. 2_A_). C2cd3 was more distal to the subdistal appendage staining of ninein in the mother centriole, suggesting a distal localization of C2cd3 on the mother centriole (Fig. 2_A_). Consistent with this, the C2cd3 localization in the daughter centriole was adjacent to, but did not perfectly overlap with the proximal ninein (Fig. 2_A_).
Fig. 2.
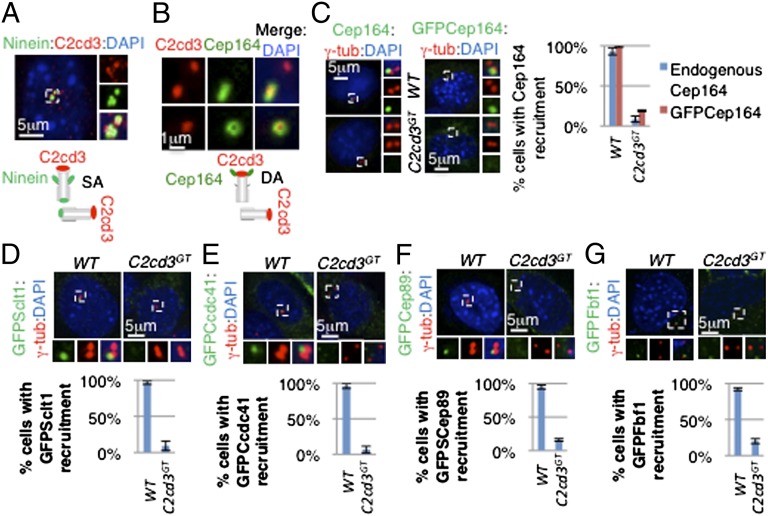
C2cd3 is localized to the distal end of centrioles and is required for distal appendage assembly. (A) C2cd3 is localized distal to ninein on centrioles. On the mother centriole, ninein is localized to both the proximal end and subdistal appendages (SAs), appearing as three dots on a lateral view. Ninein is also localized to the proximal end of the daughter centriole, appearing as a single dot. The illustration schematizes the localization of C2cd3 and ninein on centrioles. (B) C2cd3 is at the same distal level as Cep164 on the centriole. Upper shows a lateral view of the mother centriole in which Cep164 appears as a bar. Lower shows a top view of the mother centriole in which Cep164 appears as a ring. Note that Cep164 is only localized to the distal appendages (DAs) of mother centriole, whereas C2cd3 is localized to both centrioles. The diagram shows the localization of C2cd3 and Cep164 on the centrioles. Endogenous and overexpressed (C) Cep164, (D) GFP–Sclt1, (E) GFP–Ccdc41, (F) GFP–Cep89, and (G) GFP–Fbf1 fail to be recruited to the mother centriole in the absence of C2cd3. The centrosomes are labeled with γ-tubulin, and the nuclei are visualized with DAPI. For quantitative analyses, SD is indicated. n = 3 independent experiments.
Lying more distal to the subdistal appendages are distal appendages, which form the transition fibers that serve as the gateway to the cilium (15). We used Cep164 to label the distal appendages, which looked like a ring in the top view (Fig. 2_B_). We found that C2cd3 was localized in the center of the Cep164 ring-shaped staining (Fig. 2_B_). From the side, Cep164 staining looked like a bar, and C2cd3 was in the center of the bar (Fig. 2_B_). The comparison with ninein and Cep164 suggested that C2cd3 is localized at the distal ends of centrioles.
C2cd3 Is Critical for the Recruitment of Distal Appendage Proteins.
We next investigated whether C2cd3 played a role in the assembly or function of the centrosome. Pericentriolar material protein pericentrin and the subdistal appendage protein ninein were present at the centrioles in C2cd3 GT cells (Figs. S6 and S7). Consistent with the function of subdistal appendages in anchoring microtubules, cytoskeletal microtubule organization and the spindle apparatus were intact in C2cd3 GT cells (Fig. S7).
As C2cd3 was localized to the distal ends of centrioles, we investigated whether the recruitment of the distal appendage protein Cep164 was dependent on C2cd3. Both endogenous Cep164 and overexpressed GFP–Cep164 were localized to the distal end of mother centriole in >90% of wild-type cells (Fig. 2_C_). Interestingly, less than 20% of C2cd3 GT cells exhibited Cep164 centriolar localization, suggesting that C2cd3 is critical for the recruitment of Cep164 to the mother centriole (Fig. 2_C_).
Multiple components of the centriolar distal appendages have been identified recently, including Ccdc41, Cep89, Sclt1, and Fbf1 (9, 11, 12, 14). To study whether C2cd3 regulates the recruitment of additional distal appendage components, we transiently expressed GFP-tagged Ccdc41, Sclt1, Cep89, and Fbf1 in wild-type and C2cd3 GT mutant cells. All four proteins were localized to the distal end of the mother centriole in wild-type cells but not in C2cd3 GT mutant cells (Fig. 2 D_–_G). The failure to recruit multiple components of the distal appendages to the mother centriole indicated that C2cd3 is essential for distal appendage assembly.
C2cd3 Is Required for the Recruitment of Ttbk2 and Removal of Cp110 from the Mother Centriole.
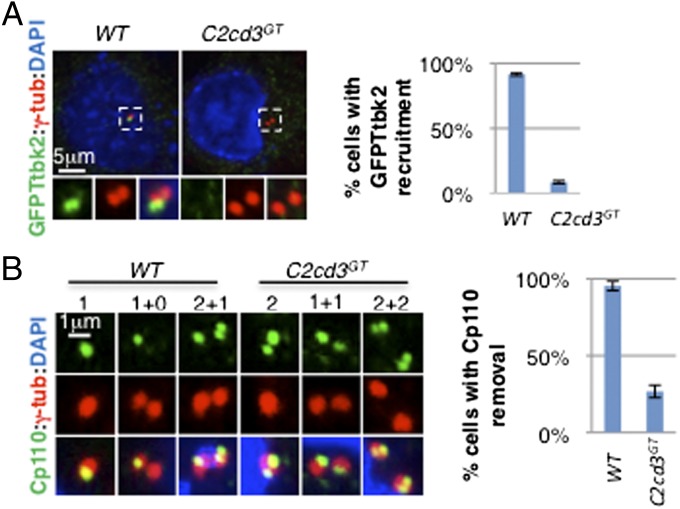
As distal appendages are critical for recruiting Ttbk2, an essential regulator of cilium biogenesis (9), we investigated whether defects in distal appendage assembly affect Ttbk2 recruitment in C2cd3 GT mutant cells. As previously reported, GFP–Ttbk2 was localized to the distal end of the mother centriole in wild-type cells (Fig. 3_A_). Interestingly, the centriolar localization of GFP–Ttbk2 was disrupted in C2cd3 GT mutant cells, suggesting that C2cd3 is essential for recruiting Ttbk2 to the mother centriole (Fig. 3_A_).
Fig. 3.
Ttbk2 recruitment and Cp110 removal are compromised in the absence of C2cd3. (A) GFP–Ttbk2 is localized to the centriole in wild-type cells but not in C2cd3 GT mutant cells. (B) Cp110 is removed from the mother centriole in wild-type cells but not in C2cd3 GT mutant cells. Note that Cp110 staining is dynamic through the cell cycle. Before centrosome duplication, Cp110 was observed as one dot in wild-type cells, representing the daughter centriole (“1”). In contrast, two dots were observed in C2cd3 GT mutant cells, representing both the mother and daughter centrioles (“2”). After centrosome duplication, only one centrosome is positive for Cp110 (“1+0”) in wild-type cells, but both centrosomes are positive for Cp110 (“1+1”) in C2cd3 GT mutant cells. In the G2 phase, the procentrioles are capped with Cp110 such that Cp110 appears as two dots in the centrosome containing the daughter centriole, and one dot representing the procentriole in the centrosome containing the mother centriole (“2+1”) in wild-type cells. In contrast, C2cd3 mutant cells exhibit two Cp110 dots in each centrosome in the G2 phase (“2+2”). The quantitative analysis includes interphase cells. The centrosomes are labeled with γ-tubulin, and the nuclei are visualized with DAPI. For quantitative analyses, SD is indicated. n = 3 independent experiments.
Cp110 inhibits ciliogenesis and must be removed from the distal end of the mother centriole for cilium initiation (27, 28). Recent studies suggested that functional distal appendages and Ttbk2 are both required for the removal of Cp110 (9, 10). To investigate whether defects in distal appendage assembly and Ttbk2 recruitment in C2cd3 GT mutant cells impair the removal of Cp110, we examined the localization of Cp110. In most wild-type cells, Cp110 was localized to the daughter centriole and procentrioles but not to the mother centriole (Fig. 3_B_). In contrast, we observed Cp110 at both the mother and daughter centrioles in C2cd3 GT mutant cells, suggesting that C2cd3 is required for removing Cp110 from the mother centriole (Fig. 3_B_).
C2cd3 Is Required for the Recruitment of IFT Proteins.
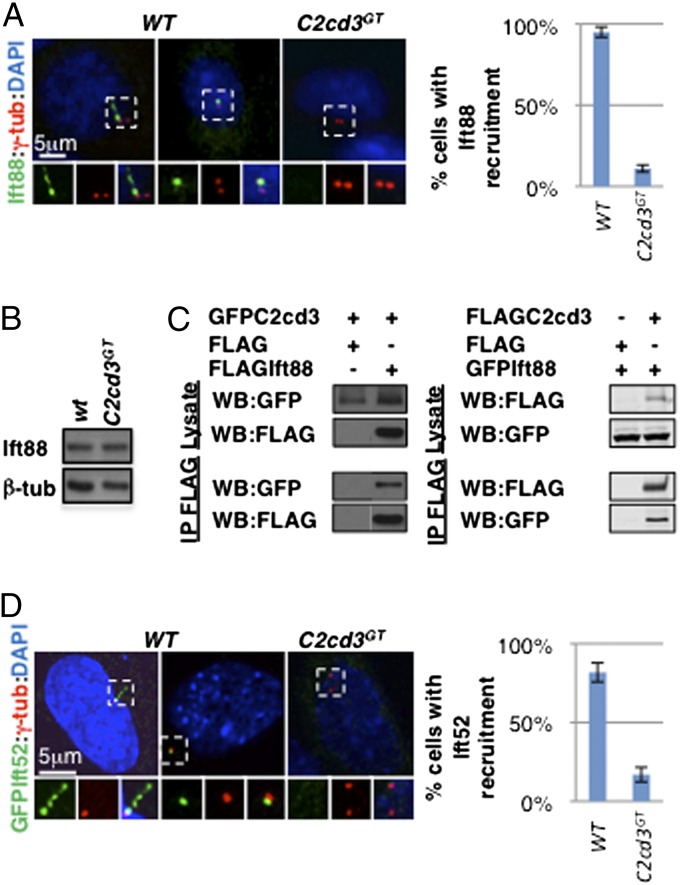
Cilium biogenesis is dependent on IFT, a microtubule-based transport mechanism inside the cilium (15). One core IFT-B complex component, Ift88, is localized to the distal end of the mother centriole and the cilium, and its recruitment depends on some distal appendage proteins (9, 13). As we expected, Ift88 was frequently associated with the mother centriole in both ciliated and nonciliated wild-type cells, but not in C2cd3 GT mutant cells, although the overall protein levels were comparable (Fig. 4 A and B). The failure in Ift88 centriolar recruitment was likely secondary to the defects in distal appendage assembly and Ttbk2 recruitment. However, a more direct role of C2cd3 in Ift88 recruitment could not be ruled out, as coimmunoprecipitation analysis revealed that C2cd3 physically interacts with Ift88 (Fig. 4_C_).
Fig. 4.
C2cd3 is essential for the recruitment of Ift88 and Ift52 to the mother centriole. (A) Ift88 is localized to the basal body and along the cilium in ciliated wild-type cells and to the mother centriole in nonciliated wild-type cells. The centriolar staining of Ift88 is absent in C2cd3 GT mutant cells. (B) Western blot analysis reveals comparable levels of Ift88 protein in C2cd3 GT mutant and wild-type cells. (C) Coimmunoprecipitation analysis reveals the physical interaction between overexpressed C2cd3 and Ift88. (D) GFP–Ift52 is localized to the basal body and along the cilium in ciliated wild-type cells and to the mother centriole in nonciliated wild-type cells. The centriolar staining of GFP–Ift52 is absent in C2cd3 GT mutant cells. The centrosomes are labeled with γ-tubulin, and the nuclei are visualized with DAPI. The quantitative analyses of Ift88 and Ift52 centriolar recruitment only include nonciliated cells. For quantitative analyses, SD is indicated. n = 3 independent experiments.
To test whether the recruitment of other IFT proteins also depends on C2cd3, we examined the localization of GFP–Ift52, another component of the IFT-B complex essential for cilium biogenesis in mice (29). Similar to Ift88, Ift52 was localized to the cilium as well as to the mother centriole in wild-type cells but not in C2cd3 GT mutant cells (Fig. 4_D_).
C2cd3 Is Required to Dock Ciliary Vesicles.
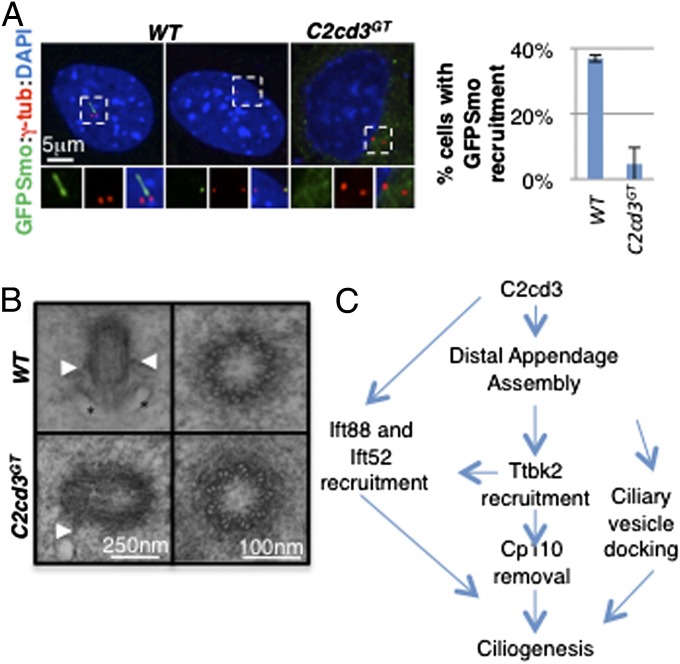
Docking of Golgi-derived ciliary vesicles to the mother centriole is a critical step in cilium biogenesis (8). Recent studies have suggested that centriolar distal appendages are required for docking ciliary vesicles (9, 12, 13). The distal appendage assembly defects in C2cd3 GT mutant cells prompted us to examine whether ciliary vesicle docking to the mother centriole was affected. Using GFP-tagged smoothened (GFP–Smo) as a ciliary vesicle marker (12), we observed that GFP–Smo was localized to the cilia and mother centriole in wild-type cells but not in most C2cd3 GT mutant cells, suggesting that ciliary vesicle docking is disrupted in the absence of C2cd3 (Fig. 5_A_).
Fig. 5.
C2cd3 is important for ciliary vesicle docking during ciliogenesis. (A) GFP–Smo is present in the cilia and ciliary vesicles docked to the mother centriole in wild-type cells, but is not present at the mother centriole in C2cd3 GT mutant cells, suggesting a defect in ciliary vesicle docking. The quantitative analysis only includes nonciliated cells. (B) TEMs of centrioles in E10.5 wild-type and C2cd3 GT mutant mouse embryos. Longitudinal sections (Left) and cross-sections (Right) are presented. Arrowheads indicate subdistal appendages and asterisks indicate docked ciliary vesicles. (C) A model based on our data and published results (9, 10) suggests that C2cd3 is critical for the assembly of centriolar distal appendages, which in turn underlie the recruitment of Ttbk2, Ift88, and Ift52 as well as the removal of Cp110 and the docking of ciliary vesicles. C2cd3 may play a more direct role in recruiting Ift88 to the distal appendages. For quantitative analyses, SD is indicated. n = 3 independent experiments.
In addition, we examined centrioles in wild-type and C2cd3 GT mutant embryos through transmission electron microscopy (TEM). Among the 16 centrioles we imaged in wild-type embryos, 5 were associated with vesicles or the plasma membrane (Fig. 5_B_). Consistent with the lack of GFP–Smo centriolar localization, we did not find any vesicles associated with the 16 centrioles imaged in C2cd3 GT mutant embryos (Fig. 5_B_). These results suggested that the failure in ciliary vesicle docking might, at least partially, underlie the severe ciliogenic defects in C2cd3 GT mutants. Consistent with our immunofluorescent data demonstrating the correct localization of the subdistal appendage marker ninein, we did observe subdistal appendages on some centrioles in C2cd3 GT mutant embryos (Fig. 5_B_). In addition, cross-sections through the centrioles indicated that the ninefold symmetry and microtubule triplet assembly were not affected by the loss of C2cd3 (Fig. 5_B_).
Discussion
In the current study, we demonstrate that C2cd3 is localized to the centriolar satellites in a microtubule- and Pcm1-dependent manner but is not required for centriolar satellite integrity or Rab8 activation. C2cd3 is also localized to the distal ends of centrioles and is required for the recruitment of the distal appendage proteins Sclt1, Ccdc41, Cep89, Fbf1, and Cep164. In addition, in the absence of C2cd3, Ttbk2 is not recruited to the mother centriole, nor is Cp110 removed. Meanwhile, the IFT complex B components Ift88 and Ift52 are not recruited to the mother centriole in the absence of C2cd3. Finally, both GFP–Smo localization and TEM analyses suggest that ciliary vesicle docking to the mother centriole is defective in C2cd3 mutants. Our results indicate that C2cd3 regulates ciliogenesis through the assembly of centriolar distal appendages, which are important for docking the ciliary vesicles and recruiting other important ciliogenic proteins (Fig. 5_C_).
We provide multiple pieces of evidence supporting the association between C2cd3 and centriolar satellites. First, the punctate staining of C2cd3 around the centrosome is cell cycle- and microtubule-dependent, hallmarks of the centriolar satellites. Second, immunofluorescence and coimmunoprecipitation analyses indicate that C2cd3 physically interacts with the two established centriolar satellite proteins Bbs4 and Pcm1. Finally, the loss of C2cd3 centriolar satellite localization in Pcm1-depleted cells further confirms the association between C2cd3 and centriolar satellites.
To our surprise, the localization of multiple centriolar satellite components was not affected in C2cd3 mutant cells, suggesting that C2cd3 is dispensable for centriolar satellite integrity. Consistent with this conclusion, the recruitment of ninein and pericentrin to the centrosome, a known function of centriolar satellites (30), was not disrupted in the absence of C2cd3. Our data using C2cd3 Hty hypomorphic mutant cells also indicated no disruption in Rab8 activation, suggesting that C2cd3 may not be involved in this aspect of centriolar satellite function. Understanding of the function of satellite-localized C2cd3, if any, will require further investigation.
In addition to centriolar satellites, we observed C2cd3 at the distal ends of centrioles. This observation is consistent with a recent report showing the centriolar localization of C2cd3 (31). However, our data more accurately defines the location of C2cd3 at the level of distal appendages, which was not identified in the previous report. More importantly, we demonstrate that C2cd3 plays critical roles in recruiting the distal appendage components and consequently, the recruitment of Ttbk2, Ift88, and Ift52, as well as the removal of Cp110. These important events, along with docking of the ciliary vesicles to the mother centriole, underlie the initiation of the cilium, and defects in these processes may explain the defects in cilium biogenesis in the absence of C2cd3.
We would like to note that although C2cd3 is critical for distal appendage assembly, it is unlikely a structural component of the distal appendages. Distal appendages decorate the periphery of the distal end of the mother centriole; therefore, all known distal appendage proteins exhibit a ring-like pattern at the distal end of the mother centriole in immunofluorecence analysis. In contrast, C2cd3 is localized to the center of the distal ends of both the mother and daughter centrioles (Fig. 2_B_). Therefore, we speculate that C2cd3 may regulate distal appendage assembly by providing docking points for the appendages.
Currently, the only other centriolar protein that specifically regulates distal appendage assembly is Ofd1 (23). Interestingly, Ofd1 is also localized to the centriolar satellites, and a recent study suggested that it might play an additional negative role in ciliogenesis (20, 22). It will be interesting to investigate whether Ofd1 and C2cd3 act in the same pathway leading to the assembly of the distal appendages.
DAZ-interacting zinc finger protein 1 (Dzip1) is also required for centriolar appendage formation (32); however, it regulates the assembly of both distal and subdistal appendages, suggesting that it either acts further upstream or in parallel to C2cd3. It will be interesting to investigate the functional relationship between these two proteins.
In summary, we have identified an essential role for C2cd3 in the assembly of centriolar distal appendages which are critically important for the subsequent recruitment of the ciliogenic proteins Ttbk2, Ift88, and Ift52; the removal of Cp110; and the docking of ciliary vesicles to mother centrioles. Current understanding of this early step of ciliogenesis has been very limited due to a lack of knowledge regarding the factors involved (C2cd3 is only the second protein identified to specifically regulate distal appendage assembly). Therefore, the unveiling of a protein interaction network centered on C2cd3 will be critical to furthering our understanding of the mechanisms underlying the assembly of distal appendages on the mother centriole.
Materials and Methods
Cell Culture, Transfection, and Nocodazole Treatment.
MEFs were established using a previously published protocol (24) and were immortalized by stably expressing SV40 Large T Antigen (a gift from Baolin Wang, Cornell University, New York). Cells were cultured in DMEM supplemented with 10% (vol/vol) FBS, Glutamax (Life Technologies), and antibiotics at 37 °C and 5% (vol/vol) CO2 and were transfected with Lipofectamine 2000 (Life Technologies) or JetPRIME (Polyplus Transfection). For microtubule disassembly, cells were treated with 6 mg/mL nocodazole for 1 h at 37 °C, followed by 0.5 h on ice before immunofluorescence analysis. Control cells were treated with DMSO for 1.5 h at 37 °C.
Immunofluorescence.
For immunofluorescence analyses, cells grown on glass coverslips were fixed in 4% (wt/vol) paraformaldehyde at room temperature or ice-cold methanol for 4 min, permeabilized in PBS containing 0.1% Triton X-100, and incubated in primary antibodies for 2 h at room temperature. Subsequently, the cells were washed three times in PBS and incubated in fluorescently labeled secondary antibodies for 1 h at room temperature. After a final wash with PBS, the coverslips were mounted on microscope slides with VectaShield mounting medium containing DAPI (Vector Laboratories). The cells were imaged with a Nikon E600 epifluorescence microscope or an Olympus FV1000 confocal microscope.
RNA Interference.
A small interfering RNA (siRNA) specific for mouse Pcm1 and a control scramble siRNA were synthesized according to published sequences (30). siRNAs were introduced into MEFs using Lipofectamine RNAiMax (Life Technologies). Forty-eight hours after the first transfection, cells were replated and retransfected with the same siRNA. Forty-eight hours after the second transfection, cells were processed for immunofluorescence.
Additional RNA interference experiments were performed by transfecting MEFs with plasmids expressing small hairpin RNAs (shRNAs; Sigma) against various regions of mouse Pcm1 using JetPRIME (Polyplus Transfection). A plasmid expressing an shRNA against GFP was used as a control. Cells were processed for immunofluoresence 5 d after transfection.
Western Blot and Coimmunoprecipitation.
Western blot analyses were performed as previously described (33). Coimmunoprecipitation between overexpressed GFP- and FLAG-tagged proteins in HEK293T cells was performed using a FLAG immunoprecipitation kit (Sigma) according to the manufacturer’s instructions.
TEM.
Embryonic day 9.5 mouse embryos were dissected in fresh fixative [2 mM CaCl2/2% (wt/vol) paraformaldehyde/2.5% (wt/wt) glutaraldehyde/0.1 M cacodylate buffer, pH 7.4], prefixed in fixative at 4 °C overnight and rinsed with 0.1 M cacodylate buffer. The embryos were then postfixed in 1% OsO4/0.1 M cacodylate buffer for 1 h at 4 °C in the dark and rinsed with 0.1 M cacodylate buffer. Samples were en bloc stained with 2% (wt/vol) aqueous uranyl acetate for 1 h at room temperature in the dark, rinsed with water, dehydrated in ethanol and acetonitrile, infiltrated, and embedded in Eponite 12. Ultrathin sections (80 nm) were cut with a Leica UC6 ultramicrotome (Leica Microsystems), stained with uranyl acetate and lead citrate, and imaged with a JEOL 1200 transmission electron microscope. The use of animals for this study has been approved by the Institutional Animal Care and Use Committee at the Pennsylvania State University.
Supplementary Material
Supporting Information
Acknowledgments
We thank Drs. Charles T. Anderson and Graham Thomas for critically reading the manuscript. We thank Drs. K. V. Anderson, T. Caspary, J. E. Sillibourne, A. Merdes, J. Gleeson, and P. Beachy for providing the reagents. The Pennsylvania State University Nucleic Acid and Microscopy Facilities provided help in DNA sequencing and microscopy services. J.F.R. is supported by National Institutes of Health Grants R01AR054396 and R01GM095941, the Burroughs Wellcome Fund, the Packard Foundation, and the Program for Breakthrough Biomedical Research. A.L. is supported by American Heart Association Scientist Development Grant 0830174N and a laboratory start-up fund from The Pennsylvania State University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137(1):32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerdes JM, Katsanis N. Ciliary function and Wnt signal modulation. Curr Top Dev Biol. 2008;85:175–195. doi: 10.1016/S0070-2153(08)00807-7. [DOI] [PubMed] [Google Scholar]
- 3.Schneider L, et al. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15(20):1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Habbig S, et al. NPHP4, a cilia-associated protein, negatively regulates the Hippo pathway. J Cell Biol. 2011;193(4):633–642. doi: 10.1083/jcb.201009069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inglis PN, Boroevich KA, Leroux MR. Piecing together a ciliome. Trends Genet. 2006;22(9):491–500. doi: 10.1016/j.tig.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Gherman A, Davis EE, Katsanis N. The ciliary proteome database: An integrated community resource for the genetic and functional dissection of cilia. Nat Genet. 2006;38(9):961–962. doi: 10.1038/ng0906-961. [DOI] [PubMed] [Google Scholar]
- 7.Bornens M. The centrosome in cells and organisms. Science. 2012;335(6067):422–426. doi: 10.1126/science.1209037. [DOI] [PubMed] [Google Scholar]
- 8.Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol. 1962;15:363–377. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanos BE, et al. Centriole distal appendages promote membrane docking, leading to cilia initiation. Genes Dev. 2013;27(2):163–168. doi: 10.1101/gad.207043.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goetz SC, Liem KF, Jr, Anderson KV. The spinocerebellar ataxia-associated gene Tau tubulin kinase 2 controls the initiation of ciliogenesis. Cell. 2012;151(4):847–858. doi: 10.1016/j.cell.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei Q, et al. Transition fibre protein FBF1 is required for the ciliary entry of assembled intraflagellar transport complexes. Nat Commun. 2013;4:2750. doi: 10.1038/ncomms3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joo K, et al. CCDC41 is required for ciliary vesicle docking to the mother centriole. Proc Natl Acad Sci USA. 2013;110(15):5987–5992. doi: 10.1073/pnas.1220927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt KN, et al. Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. J Cell Biol. 2012;199(7):1083–1101. doi: 10.1083/jcb.201202126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sillibourne JE, et al. Primary ciliogenesis requires the distal appendage component Cep123. Biol Open. 2013;2(6):535–545. doi: 10.1242/bio.20134457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 16.Bärenz F, Mayilo D, Gruss OJ. Centriolar satellites: Busy orbits around the centrosome. Eur J Cell Biol. 2011;90(12):983–989. doi: 10.1016/j.ejcb.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Kubo A, Sasaki H, Yuba-Kubo A, Tsukita S, Shiina N. Centriolar satellites: Molecular characterization, ATP-dependent movement toward centrioles and possible involvement in ciliogenesis. J Cell Biol. 1999;147(5):969–980. doi: 10.1083/jcb.147.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nachury MV, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129(6):1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Krishnaswami SR, Gleeson JG. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum Mol Genet. 2008;17(23):3796–3805. doi: 10.1093/hmg/ddn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes CA, et al. Centriolar satellites are assembly points for proteins implicated in human ciliopathies, including oral-facial-digital syndrome 1. J Cell Sci. 2011;124(Pt 4):600–612. doi: 10.1242/jcs.077156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin H, et al. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141(7):1208–1219. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Z, et al. Autophagy promotes primary ciliogenesis by removing OFD1 from centriolar satellites. Nature. 2013;502(7470):254–257. doi: 10.1038/nature12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singla V, Romaguera-Ros M, Garcia-Verdugo JM, Reiter JF. Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev Cell. 2010;18(3):410–424. doi: 10.1016/j.devcel.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoover AN, et al. C2cd3 is required for cilia formation and Hedgehog signaling in mouse. Development. 2008;135(24):4049–4058. doi: 10.1242/dev.029835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroer TA. Dynactin. Annu Rev Cell Dev Biol. 2004;20:759–779. doi: 10.1146/annurev.cellbio.20.012103.094623. [DOI] [PubMed] [Google Scholar]
- 26.Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132(4):617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsang WY, et al. CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev Cell. 2008;15(2):187–197. doi: 10.1016/j.devcel.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spektor A, Tsang WY, Khoo D, Dynlacht BD. Cep97 and CP110 suppress a cilia assembly program. Cell. 2007;130(4):678–690. doi: 10.1016/j.cell.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 29.Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132(13):3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- 30.Dammermann A, Merdes A. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J Cell Biol. 2002;159(2):255–266. doi: 10.1083/jcb.200204023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balestra FR, Strnad P, Flückiger I, Gönczy P. Discovering regulators of centriole biogenesis through siRNA-based functional genomics in human cells. Dev Cell. 2013;25(6):555–571. doi: 10.1016/j.devcel.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Low WC, Liu A, Wang B. Centrosomal protein DZIP1 regulates Hedgehog signaling by promoting cytoplasmic retention of transcription factor GLI3 and affecting ciliogenesis. J Biol Chem. 2013;288(41):29518–29529. doi: 10.1074/jbc.M113.492066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jia J, et al. Suppressor of Fused inhibits mammalian Hedgehog signaling in the absence of cilia. Dev Biol. 2009;330(2):452–460. doi: 10.1016/j.ydbio.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information