MicroRNA-708 induces apoptosis and suppresses tumorigenicity in renal cancer cells (original) (raw)
. Author manuscript; available in PMC: 2014 Mar 3.
Abstract
Cancer pathogenesis is restricted by stresses that compromise cell division and survival. In this study we identify miR-708, a little studied member of a set of microRNAs that have been implicated in stress control, as an important tumor suppressor in renal cell carcinoma (RCC). miR-708 expression was attenuated widely in human RCC specimens. Restoration of miR-708 expression in RCC cell lines decreased cell growth, clonability, invasion, migration and elicited a dramatic increase in apoptosis. Moreover, intratumoral delivery of miR-708 was sufficient to trigger in vivo regression of established tumors in a murine xenograft model of human RCC. Investigation of the targets of miR-708 identified the small inhibitor of apoptosis protein survivin as important. siRNA-mediated knockdown of survivin partially phenocopied miR-708 overexpression suggesting that the pro-apoptotic role of miR-708 may be mediated primarily through survivin regulation. Additionally, we identified the E-cadherin regulators ZEB2 and BMI1 as likely miR-708 targets. Taken together, our findings define a major tumor suppressive role for miR-708, which may offer an attractive new target for prognostic and therapeutic intervention in RCC.
Keywords: miR-708, renal cancer, apoptosis, survivin
INTRODUCTION
The incidence and mortality rates of renal cell carcinomas has increased in recent years with approximately 50,000 new cases and 12,000 deaths in 2010. Approximately 30% of localized RCC cases develop metastatic recurrence (1) with very poor prognosis because of the refractory nature of RCC to current treatment regimens. Therefore, there has been much interest in the identification of biomarkers for RCC which could lead to development of better prognostic, diagnostic and therapeutic interventions for the disease.
MicroRNAs (miRNAs) are small noncoding RNAs that negatively regulate expression of multiple genes either by inducing translational silencing or by causing mRNA degradation (2). It has been firmly established that miRNAs control various key cellular processes such as proliferation, apoptosis, differentiation, development (3), and are implicated in human diseases, including cancer (4). miRNAs have been identified that function as classical oncogenes or tumor suppressor genes (4). Genomic deletion or epigenetic silencing of a miRNA that normally represses expression of one or more oncogenes might lead to increased oncogenic expression. Alternatively, amplification, overexpression, or loss of epigenetic silencing of a gene encoding an miRNA that targets one or more tumor suppressor genes could inhibit the activity of an anti-oncogenic pathway (4). Aberrant microRNA profiles have been noted in various cancers (5, 6), including RCC (7–10). Due to their tissue and disease-specific expression patterns and tremendous regulatory potential, miRNAs are being assessed as potential biomarkers for diagnosis and prognosis of human malignancies (11). Recent evidence demonstrates that miRNAs play an important role in the patho-physiology of RCC. Several studies have been conducted to identify the RCC-specific miRNA signature (7–10) though no consensus has been reached on which miRNAs are relevant for development and progression of this malignancy. As the molecular interactions of miRNAs with their cognate target genes in various tumor settings are being explored, the main objective of the present study was to identify novel miRNAs that regulate renal carcinogenesis. In this study, we identified a crucial tumor-suppressive miRNA- miR-708- in renal cancer. Functional studies on miR-708 in RCC indicated that miR-708 is a pro-apoptotic miRNA that regulates renal carcinogenesis. miR-708 is a recently discovered miRNA (12, 13) which has not been extensively studied, though a few reports implicate miR-708 overexpression in lung carcinomas (14, 15) and childhood acute lymphoblastic leukemias (16). This is the first report implicating a tumor suppressor role for this microRNA in renal cancer where we show for the first time an important pro-apoptotic role for miR-708 in renal cancer.
METHODS
Cell lines and cell culture
The non-malignant SV-40 immortalized renal cell line HK2 and human renal cancer cell lines A-498 and Caki2 were obtained from the American Type Culture Collection. The HK-2 cell line was maintained in keratinocyte serum-free medium (GIBCO Laboratories) supplemented with 50 ug/mL bovine pituitary extract, 5% L-glutamine and 5 ng/mL epidermal growth factor (EGF). A498 and Caki2 cells were cultured in Eagle’s minimal essential medium (MEM) and McCoy’s 5A medium, respectively, each supplemented with 10% fetal bovine serum (Atlanta biologicals) and 1% penicillin/streptomycin (UCSF cell culture facility). All cell lines were cultured in a humidified incubator (5% CO2) at 37°C.
miRNA/siRNA transfections
Cells were plated in growth medium without antibiotics ~24hrs before transfections. Transient transfections of miRNA precursor (Ambion)/siRNA (Origene) was carried out by using Lipofectamine 2000 (Invitrogen) according to the manufacturers’s protocol. miR-708 precursor (PM11161) or negative control (miR-CON) (AM17110) (Ambion) was used for assays. All miRNA/siRNA transfections were for 72h.
miRNA microarray
For miRNA microarray, total RNA was extracted from HK2, A498 and Caki2 cells using a miRNeasy mini kit (Qiagen). The miRNA microarray analysis was carried out by a commercial company (Phalanx Biotech) using human v2 miRNA OneArray platform that is designed to contain 100% of miRBase Sequence Database Release 15.0.
Tissue samples
Tissue samples from radical nephrectomy were obtained from the San Francisco Veterans Affairs Medical Center (SFVAMC). Informed consent was obtained from all patients. All slides were reviewed by a board certified pathologist for the identification of tumor foci as well as normal adjacent tissue. For microdissections, 4µm slides were H&E stained, reviewed and marked (tumor and normal areas) by a board certified pathologist at the SFVAMC. After that, 10 µm non-stained sections were microdissected using aforementioned slides as a template.
RNA and miRNA extraction
Total RNA was extracted from microdissected FFPE tissues using a miRNeasy FFPE Kit (Qiagen) and an RNeasy mini kit (Qiagen) was used for RNA extraction from cultured cells following the manufacturer’s instructions.
Quantitative real-time PCR
Mature miRNAs and other mRNAs were assayed using the TaqMan MicroRNA Assays and Gene Expression Assays, respectively, in accordance with the manufacturer's instructions (Applied Biosystems). Samples were normalized to RNU48 or GAPDH (Applied Biosystems), as indicated. The comparative Ct (threshold cycle) method was used to calculate the relative changes in gene expression on the 7500 Fast Real Time PCR System.
Cell viability, clonability, migratory, and invasion assays
Cell viability was determined at 24, 48 and 72 hours by using the CellTiter 96 AQueousOne Solution Cell Proliferation Assay Kit (Promega), according to the manufacturer's protocol. For colony formation assay, cells were counted seeded at low density (1,000 cells/plate or 200 cells/ plate) and allowed to grow until visible colonies appeared. Then, cells were stained with Giemsa, and colonies were counted. Cytoselect Cell migration and invasion assay kit (Cell Biolabs, Inc.) was used for migration and invasion assays, according to the manufacturer's protocol. Briefly, 48 hrs post-transfection, cells were counted and placed on control inserts or Matrigel inserts at 1 x105 cells/ml in serum-free medium and were allowed to migrate for 20 h at 37°C. Cells were removed from the top of the inserts and cells that migrated/invaded though the polycarbonate/basement membrane were fixed, stained and quantified at OD 560nm after extraction.
Apoptosis Assays
Fluorescence-activated cell-sorting (FACS) analysis for apoptosis was done 72 hours posttransfection, using Annexin V-FITC/7-AAD Kit (Beckman Coulter, Inc.) for apoptosis analysis, according to the manufacturer's protocol. Stained cells were immediately analyzed with a flow cytometer (Cell Lab Quanta SC; Beckman Coulter, Inc).
Adhesion assay
Adhesion assays were performed 72 hours posttransfection using a Cytoselect 48-well cell adhesion assay (ECM array) (Cell Biolabs, catalog no. CBA-070) in accordance with the manufacturer's protocol. Briefly, cells were counted and plated at a density of 0.2X106 cells/ml, incubated in a tissue culture incubator for 90 min-5 hours at 37°C. After incubation, adherent cells were stained and quantified at OD 560nm after extraction.
Renal cancer xenografts
We examined the anti-tumor effects of miR-708 by local administration in established tumors as previously described (17, 18). Nude mice (4- 5 week-old, Charles River Laboratories) (n=12) received subcutaneous injections of 3 X 106 A498 cells in the right flank area in a volume of 100 µl. Once palpable tumors developed, caliper measurements were taken twice a week and tumor volume was calculated on the basis of width (x) and length (y): x2: y/2, where x< y. When tumors reached an average volume of 100-150mm36.25 µg of synthetic miRNA (miR-708/miR-CON) complexed with 1.6 µL siPORTamine transfection reagent (Ambion) in 50 µL PBS was delivered intratumorally in 3-day intervals. Synthetic miRNAs are double-stranded, ready-to-use miRNA mimics and were purchased from Ambion, Life Technologies (pre-miR, cat. no. AM17100). Mice were killed 2 days after the last treatment, tumors were collected and total RNA was extracted from tumor tissues for RT-PCR analysis. All animal care was in accordance with the institutional guidelines.
Statistics
All quantified data represents an average of at least triplicate samples or as indicated. Data are represented as mean ± S.E.M. All statistical analyses were performed using StatView (version 5; SAS Institute Inc.). One-way ANOVA or two-tailed Student’s t-test was used for comparisons between groups. The Mann-Whitney U test was used to assess the difference between miRNA expression in tumor/normal adjacent tissue. Chi square test was used for correlation analysis. Results were considered statistically significant at P ≤ 0.05.
Supplemental data
The supplemental data includes supplemental methods, supplemental tables and figures.
RESULTS
miR-708 expression is attenuated in renal cancer
Total RNA was extracted from normal renal (HK2) and primary RCC (A498 and Caki2) cell lines and miRNA expression profiling was performed to identify dysregulated miRNAs in RCC (data not shown). This screening showed that miR-708 is significantly downregulated in RCC cell lines. We validated the microarray data by real time-PCR analysis and confirmed that miR-708 was downregulated in primary RCC cell lines (Fig. 1A). To examine the clinical relevance of this finding, we analysed miR-708 expression in microdissected human renal cancer tissues (n=38) and matched adjacent normal regions by real-time PCR (Fig. 1B). Among these tissues, 28/38 cases (74%) were clear cell carcinomas (ccRCC), 7/38 (18%) were of papillary subtype, 3/38 (8%) represented granular cell carcinoma or oncocytoma. While the expression of miR-708 was unaltered in 8/38 cases (21%) and higher in 10/38 cases (26%), a major fraction of RCC tissue samples (20/38, ~53%) showed lower miR-708 levels (<75%) relative to matched normal tissues. The differences were statistically significant with the Mann-Whitney U test (P-value=0.0179). Patient and tumor characteristics are summarized in Table S1. In both the ccRCC and papillary subtypes, ~57% of the samples had lower miR-708 expression compared to normal adjacent tissues. We further assessed if miR-708 expression in clinical tissues correlated with clinicopathological characteristics such as pathological stage and Fuhrman grade (Fig. 1C). Decreased miR-708 expression was observed in 55% cases of pT1 and 78% cases of higher stage (pT2+pT3) RCC. This data suggests that cases with advanced pathological stage had decreased miR-708 expression compared to adjacent normal tissues (P=0.041). However, no statistically significant correlation was observed between miR-708 expression and Fuhrman grade. We extended our analysis of clinical specimens by assessing miR-708 levels in 20 additional cases of ccRCC and matched normal adjacent tissues by in situ hybridization analysis and again observed attenuated expression of miR-708 in ~60% of RCC tissues compared to normal tissues (Fig. 1D). These results suggest a potential tumor suppressor role for miR-708 in renal carcinoma.
Fig. 1. miR-708 expression is attenuated in renal cancer.
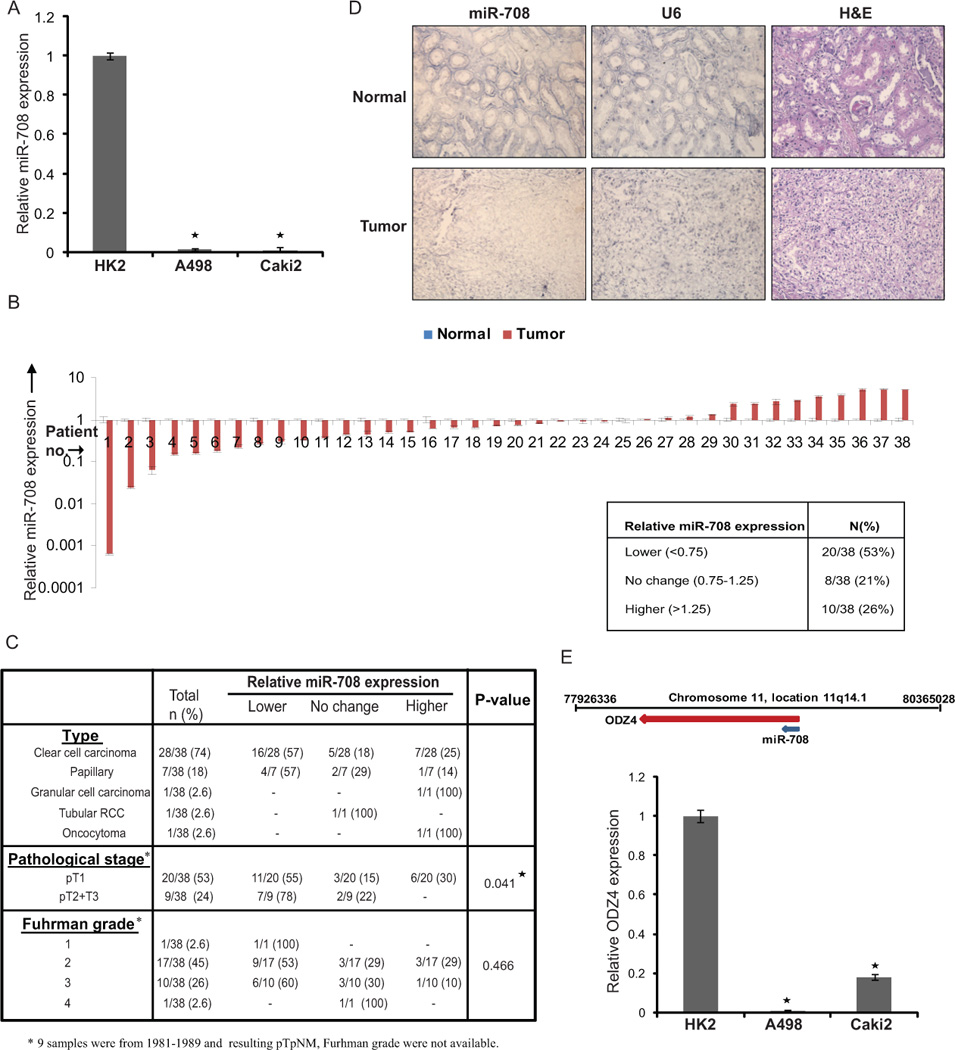
(A) Quantitative RT-PCR analysis of relative miR-708 expression levels in human renal cancer (A498 and Caki2) and normal immortalized renal cell line (HK2). Data were normalized to RNU48 control and are represented as mean ± SEM.
(B) Relative miR-708 expression levels in RCC clinical specimens and patient-matched normal tissues as assessed by real time PCR. Table below summarizes the relative miR-708 expression levels in these specimens.
(C) Correlation of miR-708 expression with subtype, clinicopathological stage and Fuhrman grade of renal cancer tissues used for miR-708 expression analysis in (B).
(D) In-situ hybridization (ISH) analysis of miR-708 expression in human renal cancer tissues and matched cancer adjacent normal tissues showing attenuated miR-708 expression in RCC tumor (lower panels) compared to matched normal adjacent tissue (upper panels). U6 staining confirmed the preservation of intact small RNAs in the same tissues (middle panels); H & E stained sections allowed the identification of tumors (right panels).
(E) Schematic representation of the genomic location of the miR-708 locus in humans at chromosomal position 11q14.1 in ODZ4 gene intron. Relative expression levels of ODZ4 in RCC cell lines as assessed by real time PCR (* P< .05).
Regulation of miR-708 locus
Human miR-708 gene is located at chromosomal position 11q14.1. miR-708 is an intronic miRNA that is located in the first intron of a coding gene, ODZ4 (odz, odd Oz/ten-m homolog 4 (Drosophila) (Fig. 1E), which is transcribed in the same direction as miR-708. ODZ4 (also called Teneurin-4) is a homolog of a Drosophila pair-rule gene that encodes a transmembrane protein involved in intercellular signaling during development (19, 20). To determine if miR-708 is coordinately transcribed with its host gene, we assessed ODZ4 expression in the same RCC cell lines by RT-PCR. We found that ODZ4 expression is similarly downregulated in A498 and Caki2 cell lines as compared to the non-tumorigenic epithelial cell line HK2 indicating that transcription of miR-708 may be coupled with its host gene (Fig. 1E). Further, Trichostatin A, a pharmacological inhibitor of histone deacetylases, increased miR-708 expression alone or in combination with the demethylating agent, 5-aza-2’-deoxycytidine (5–Aza) (Fig. S1) suggesting that this locus is epigenetically regulated primarily by histone modifications in RCC.
miR-708 reexpression suppresses tumorigenicity in vitro
To assess the potential for a tumor suppressive role of miR-708, we reexpressed miR-708 in primary RCC cell lines (A498 and Caki2) followed by functional assays. Transient transfection of miR-708 precursor led to overexpression of miR-708 as determined by real-time PCR (Fig. 2A). Reexpression of miR-708 led to marked morphological changes in both the cell lines (Fig. 2B). Specifically, a pronounced decrease in the fraction of elongated, spindle shaped cells was paralleled by an increase in rounded, apoptotic cells. The morphological alterations observed with miR-708 reexpression suggested a profound increase in apoptotic cells. This was confirmed by staining with Annexin-V FITC followed by fluorescence microscopy (Fig. S2). A significant decrease in cell viability was observed over time in A498/Caki2 cells overexpressing miR-708 (Fig. 2C) as compared to cells expressing control miR (miR-CON). miR-708 reexpression also decreased clonogenicity of A498/Caki2 cells compared to miR-CON (Fig. 2D). Transwell migration and invasion assays showed that miR-708 reintroduction decreased the migration (Fig. 2E) and invasion (Fig. 2F) of both cell lines. These observations suggest that miR-708 reexpression suppresses the tumorigenicity of renal cancer cells in vitro.
Fig. 2. Restoration of miR-708 expression suppresses tumorigenicity in vitro.
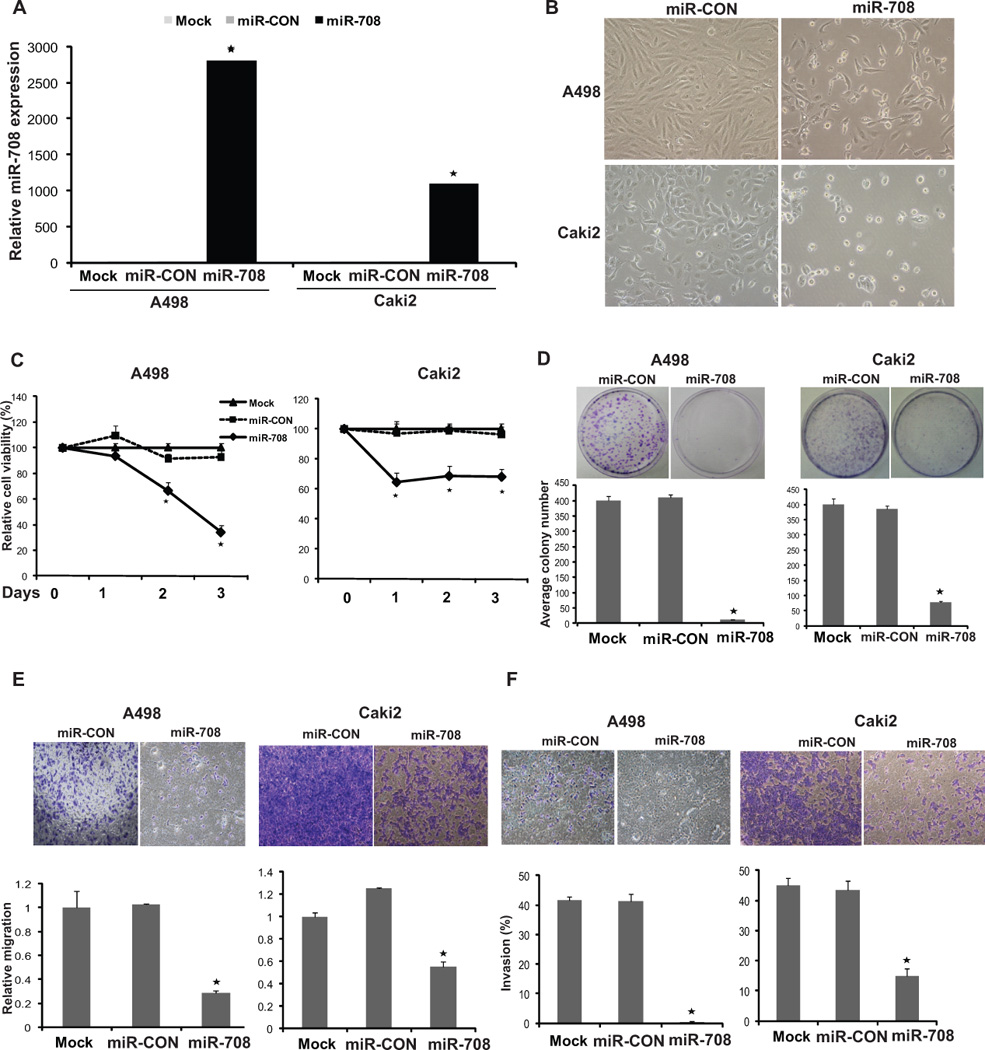
(A) Relative miR-708 expression in A498/Caki2 cells transfected with either control miR /miR-708/ mock transfected cells as assessed by real time PCR.
(B) Morphological alterations in A498/Caki2 cells upon miR-708 restoration assessed by phase-contrast microsopy.
(C) Cellular viability assay, (D) Colony formation assay, (E) Transwell-migration assay and (F) Invasion assay in A498/Caki2 cells mock transfected or transfected with miR-CON or miR-708 (* P< .05).
Reintroduction of miR-708 induces apoptosis in renal cancer cells
We measured apoptosis in control (mock or miR-CON transfected) and miR-708-tranfected cells by flow cytometric analysis of Annexin-V-FITC-7-AAD stained A498 and Caki2 cells (Fig. 3A, B). It was observed in both the cell lines that the average apoptotic cell fractions (Early apoptotic + Apoptotic) were significantly increased upon miR-708 reexpression compared to miR-CON (p<0.001) or mock transfected cells (p<0.001) with a concomitant decrease in the viable cell population. This points to a pro-apoptotic role of miR-708 and suggests that miR-708 affects apoptotic pathways in regulating tumorigenicity. We also examined the expression of various apoptotic components by Western blot analysis and found that miR-708 reexpression leads to induction of TNF-related apoptosis-inducing ligand (TRAIL) (Fig. 3C). Also, cleaved caspases- 3 and -7 and cleaved poly-ADP-ribose polymerase (PARP) were detected by immunoblot analysis (Fig. 3C), further supporting the fact that miR-708 is a pro-apoptotic miRNA.
Fig. 3. miR-708 induces apoptosis and reduces adherence to ECM.
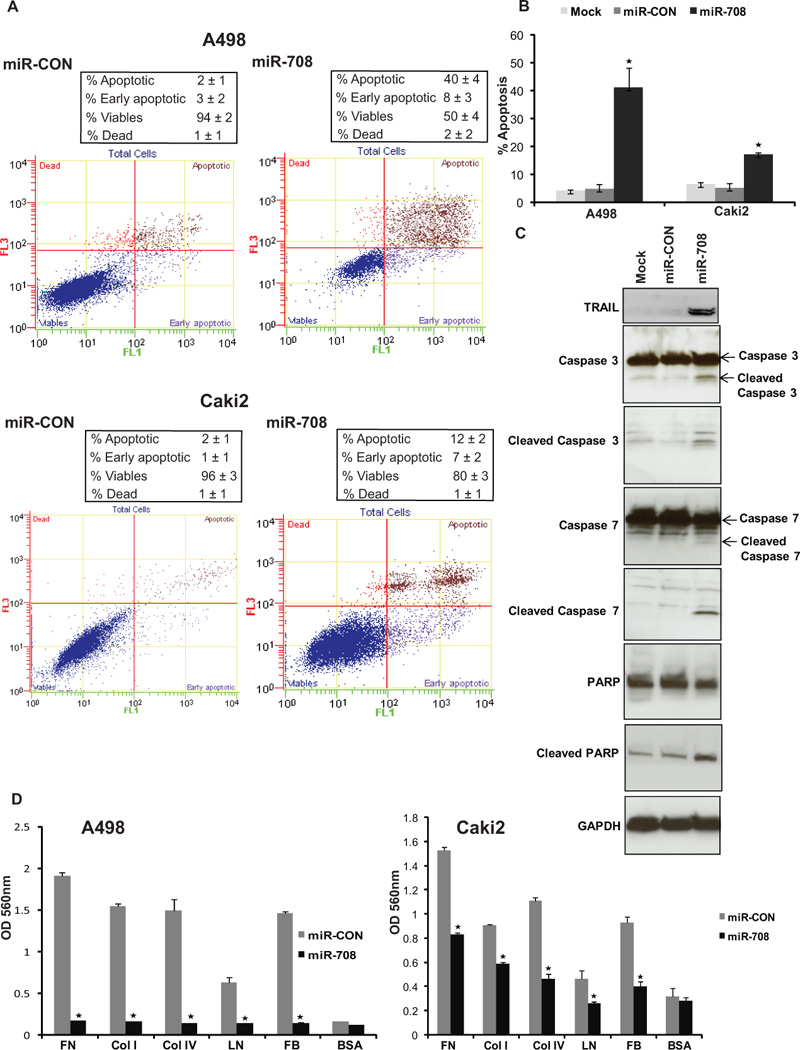
(A) Apoptosis assay in A498/Caki2 cells after miR-CON (left panels)/ miR-708 (right panels) treatments.
(B) Representation of average apoptotic fractions (early + late apoptotic) in each group (* P< .0001).
(C) Immunoblot analysis for apoptotic markers in mock/miR-CON/miR-708 transfected A498 cells. GAPDH was used as a loading control.
(D) Adhesion assay for miR-CON/miR-708 transfected A498 and Caki2 cells to following ECM components: fibronectin (FN), collagens I and IV (COL I + IV), laminin (LN), fibrinogen (FB). BSA was used as negative control (* P< .05).
miR-708 expression reduces adherence of renal cancer cells to extracellular matrix (ECM)
In view of the observed effects of miR-708 reexpression on the morphology of renal cancer cells and its effect on tumorigenicity, we monitored the ability of miR-708 overexpressing cells to attach to the ECM with in vitro adhesion assays. It was observed that miR-708 reintroduction led to decreased ability to attach to a range of ECM components as compared to control cells (Fig. 3D). However, adhesion to the negative control bovine serum albumin (BSA) did not differ between miR-708 overexpressing cells and control cells (Fig. 3C).
Intratumoral delivery of miR-708 leads to regression of tumors in a renal cancer xenograft model
Since the in vitro data showed an anti-tumorigenic role for miR-708 in RCC, we examined the therapeutic potential of synthetic miR-708 mimics in vivo in a mouse renal cancer xenograft model. Nude mice were subcutaneously inoculated with A498 cells and maintained until the tumor cells had formed solid, palpable tumors with an average volume of 100–150mm3. Thirty days following inoculation, miR-708 or a negative control miRNA was repeatedly administered by intratumoral injections every 3 days. All mice were killed on day 58. As shown in Fig. 4A-B, intratumoral delivery of synthetic miR-708 induced a specific inhibitory response and robustly interfered with tumor growth compared to control mice. To correlate the therapeutic response with delivery of miR-708, RNA was extracted from harvested tumors and miR-708 expression was assessed by Q-RT-PCR. Tumors injected with miR-708 mimic contained ~5000-fold more miR-708 than control tumors (Fig. 4C).
Fig. 4. Intratumoral delivery of miR-708 leads to regression of tumors in a renal cancer xenograft mouse model.
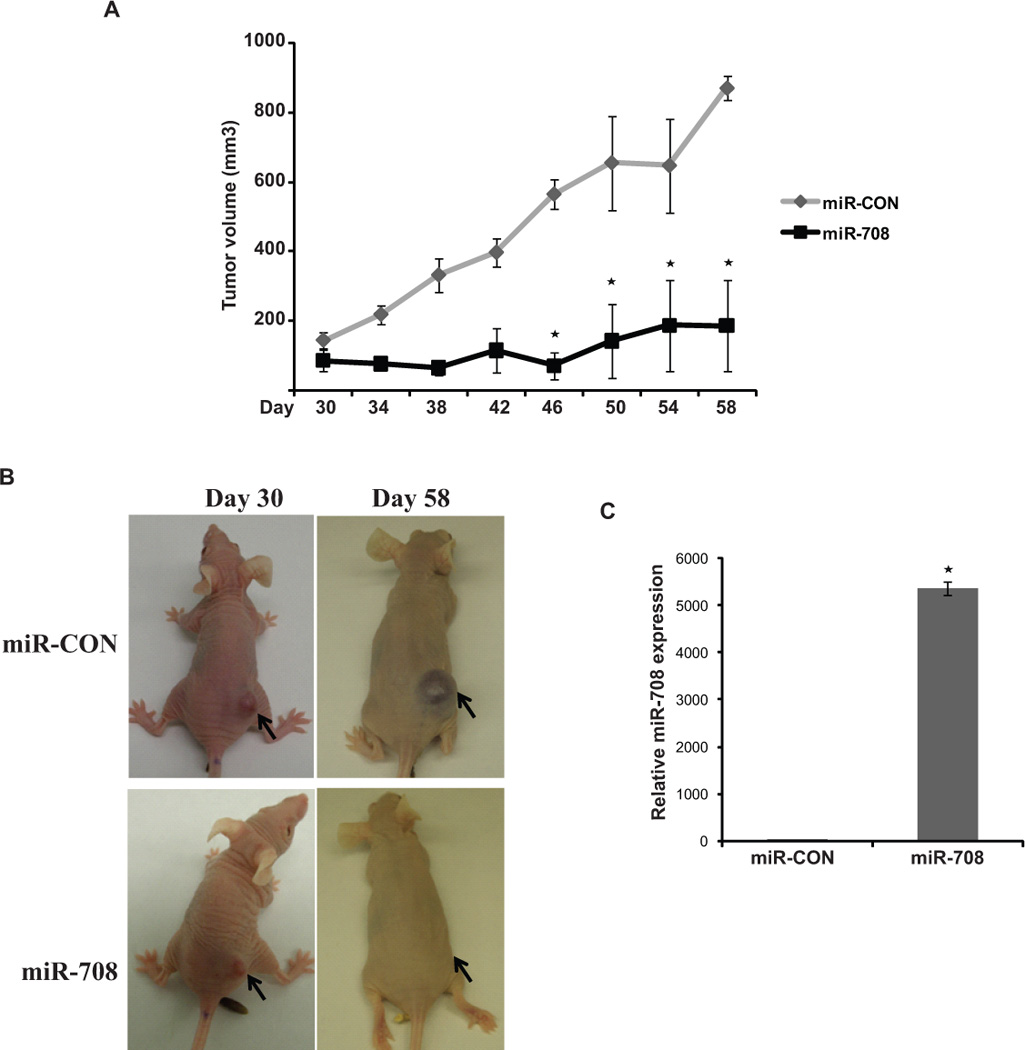
(A) A498 cells were subcutaneously injected into nude mice to form solid, palapable tumors (Day 30), following which synthetic miR-708 or control miR were intratumorally delivered for 4 weeks. Tumor volumes following miR-708 administration were significantly reduced (* P< .05).
(B) Representative images of mice from the two groups before intratumoral injections were started (day 30) and on day 58 are shown. Subcutaneous tumors are indicated by arrows.
(C). Relative miR-708 expression in renal cancer xenografts as assessed by real-time PCR. The average values are represented (* P< .05).
miR-708 targets a cohort of genes in renal cancer cells
To identify effectors of miR-708, we used the miRANDA (21) algorithm that predicts the mRNA targets of a miRNA. Guided by the target prediction algorithms, we performed Western blot analysis for putative miR-708 targets in A498 cells that were either mock tranfected or transfected with miR-708/ miR-CON (Fig. 5A). Consistent with the observed effects of miR-708 on apoptosis and cellular adhesion, we found key molecules of these cellular processes as direct targets of miR-708. miR-708 overexpression led to decreased protein levels of survivin, a small inhibitor of apoptosis protein that is differentially expressed in cancer (22) and plays a pivotal role in renal cancer progression and metastasis (23, 24). Also, miR-708 repressed the protein levels of MCAM (Melanoma Cell Adhesion Molecule) which plays an important role in malignant progression and tumor metastasis (25).
Fig. 5. miR-708 targets a cohort of genes in renal cancer cells.
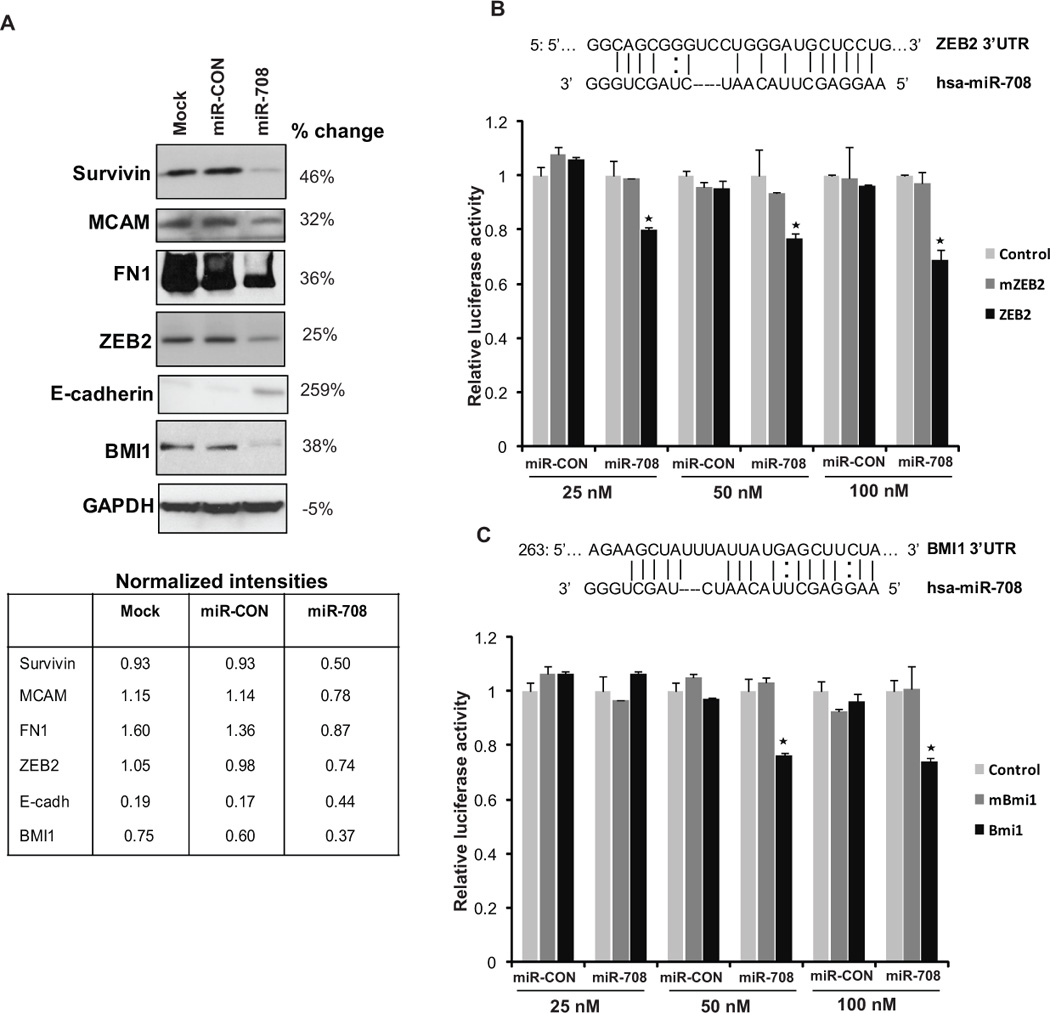
(A) Immunoblots for endogenous survivin, MCAM, FN1, ZEB2, E-cadherin, BMI1 protein in A498 cells transfected as indicated. GAPDH was used a loading control. Quantifications of immunoblots are represented below and % changes in miR-708 transfectants relative to miR-CON are also indicated.
(B) Schematic representation of ZEB2 3’-UTR and (C) BMI1 3’-UTR showing putative miR-708 target sites and luciferase activity assay with respective wt and mutant luciferase constructs and control construct contranfected with increasing concentrations of miR-CON/miR-708. For all luciferase activity assays, firefly luciferase values were normalized to renilla luciferase activity and plotted as relative luciferase activity (* P< .05).
Interestingly, miR-708 repressed the protein levels of transcription factor ZEB2, a transcriptional repressor that regulates the expression of E-cadherin and EMT (26, 27). In keeping with this novel finding, examination of E-cadherin levels in miR-708 transfected cells showed that miR-708 reexpression augments the levels of this epithelial marker and represses fibronectin. Also, miR-708 inhibited the expression of polycomb repressor, BMI1. We investigated whether the 3’UTR of ZEB2 and BMI1 are functional targets of miR-708 in renal cancer. Transient transfection of human A498 cancer cells with the respective 3’UTR plasmids along with different concentrations of miR-708 precursor led to a significant decrease in promoter activity when compared with the control vector (Fig. 5B-C) suggesting that miR-708 directly represses these genes. The luciferase activity of the reporter vectors containing a mutated 3’UTR of the respective genes was unaffected by miR-708. These observations led us to conclude that miR-708 regulates a cohort of genes which play an important role in cellular survival, adhesion, invasion and metastasis.
Survivin is a direct target of miR-708
miR-708 significantly reduced the expression (Fig.5A) of survivin in A498 cells. The 3’-UTR of survivin mRNA has three putative miR-708 binding sites (Fig. 6A). We performed luciferase reporter assays with the survivin construct in miR-203/miR-CON expressing A498 cells (Fig. 6A) and observed a consistent reduction of luciferase activity upon miR-708 transfection suggesting that miR-708 represses survivin directly. We also examined expression of survivin mRNA in 12 pairs of human tissue samples from Fig. 1B to determine if there was an inverse relationship between survivin and miR-708 expression. The relative level of survivin expression in normal versus tumor tissue is shown in Fig. 6B. Survivin expression was inversely correlated with miR-708 expression in these tested samples (p=0.0001), further supporting the idea that miR-708 directly regulates survivin levels in RCC. To further validate survivin as a direct target, we extracted tumor RNA from the renal cancer xenograft mouse model study (Fig. 4), and asked if intratumoral administration of miR-708 alters survivin expression. Analysis of relative survivin expression in these tumors showed that miR-708 injected tumors show a lower average expression of survivin than control tumors (Fig. 6C).
Fig. 6. Survivin is a direct target of miR-708.
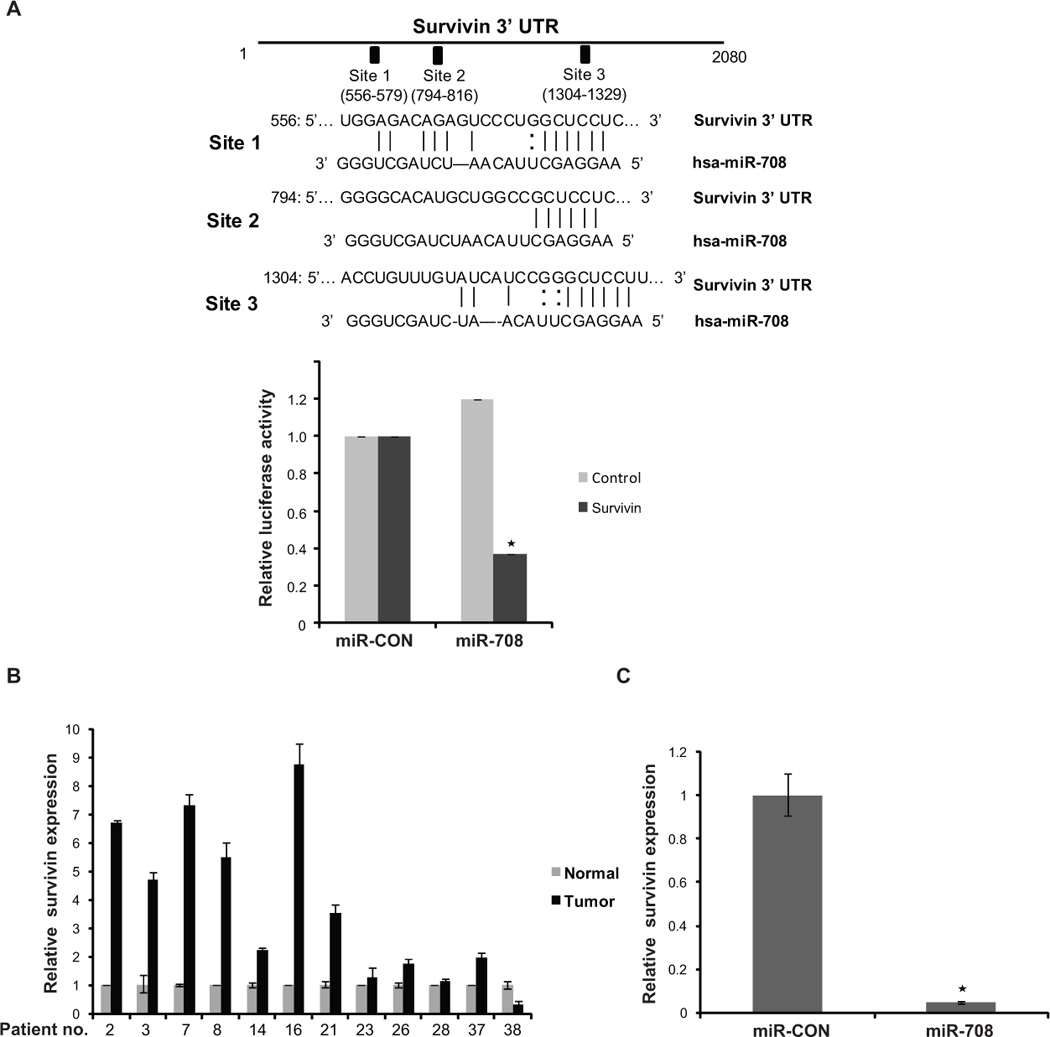
(A) Schematic representation of survivin 3’-UTR showing the relative positions of three putative miR-708 target sites. Survivin 3’ UTR construct encompassing these sites or the control construct was cotransfected into A498 cells alongwith miR-708/ miR-CON and assayed for relative luciferase activity (* P< .05).
(B) Relative survivin expression in human RCC tumor tissues and patient-matched normal tissues as assessed by real time PCR. For this analysis, 12/38 pairs of human tissue samples used in Fig. 1B of this study (indicated by patient no.) were used to determine if there was an inverse relationship between survivin and miR-708 expression.
(C) Relative survivin expression in harvested tumors from mouse renal cancer xenografts intratumorally injected with miR-CON/miR-708. Survivin expression from the two groups was averaged and plotted (* P< .05).
Survivin knockdown partially phenocopies miR-708 reexpression in renal cancer cells
In view of the observed profound effects of miR-708 on apoptosis, we sought to determine if miR-708 mediates its anti-tumorigenic effects primarily through survivin. To address this, we treated A498 cells with survivin siRNA followed by functional assays. RT-PCR and immunoblot analysis confirmed specific knockdown of survivin by siRNA (Fig. 7A). As expected, survivin siRNA-transfected cells assumed a rounded, apoptotic morphology (Fig. 7B) similar to the phenotype observed upon miR-708 overexpression in A498 cells. Apoptosis assay showed that apoptotic cell fractions (Early apoptotic + Apoptotic) were significantly increased upon survivin knockdown compared to control siRNA-treated cells (p=0.018) as observed upon miR-708 reexpression (Fig. 7C). Treatment with survivin siRNA also led to decreased proliferation and clonogenic survival of A498 cells (Fig. S3). These results suggest that the growth-suppressive effects of miR-708 are in part facilitated by survivin downregulation.
Fig. 7. Survivin knockdown partially phenocopies miR-708 reexpression in renal cancer cells.
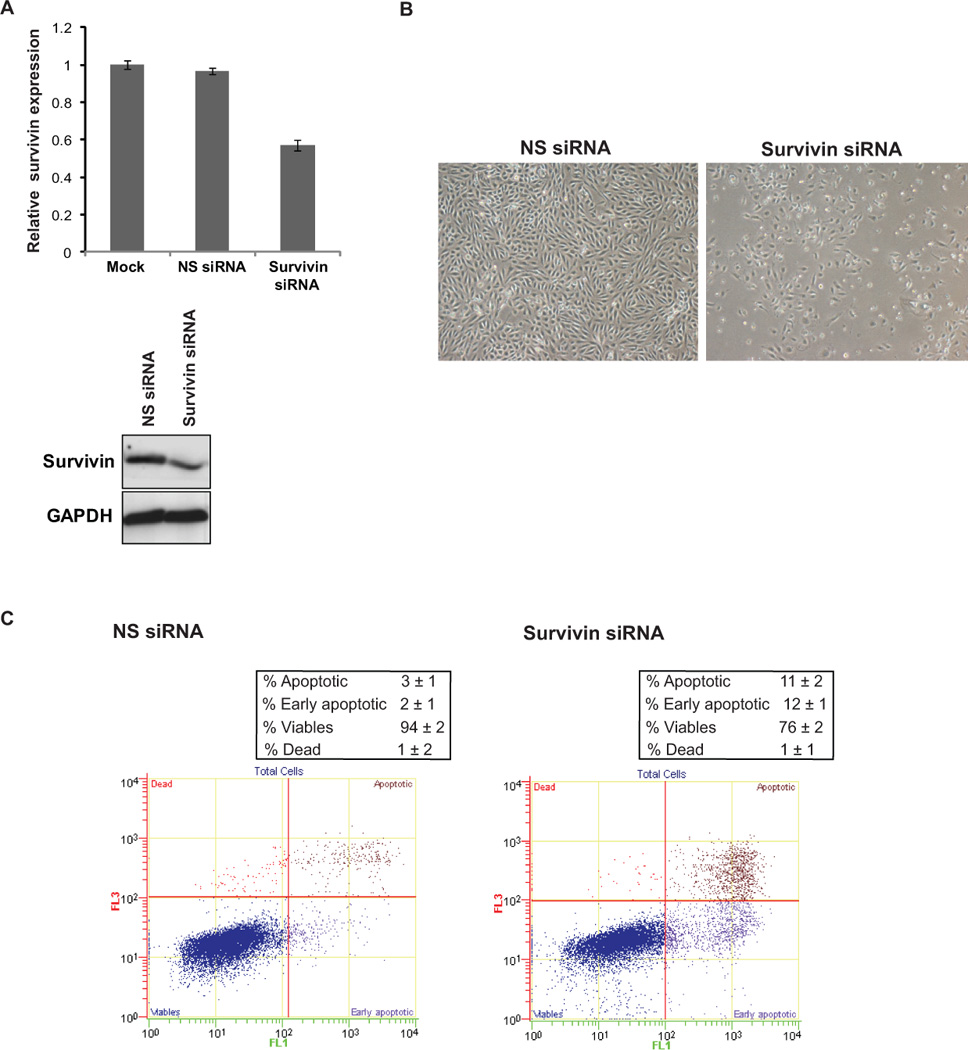
A498 cells were transfected with siRNA specific to survivin and a control nonspecific (NS) siRNA for 72 h followed by various assays.
(A) Relative survivin mRNA expression (upper panel) and protein expression (lower panel) after siRNA transfections as assessed by real time PCR and immunoblotting respectively.
(B) Morphological alterations observed after survivin knockdown in A498 cells as assessed by phase-contrast microsopy.
(C) Apoptosis assay in A498 cells after NS siRNA (left panel) or survivin siRNA (right panel) treatments.
DISCUSSION
In this study we identify a crucial tumor-suppressive miRNA, miR-708, that plays an important role in renal carcinogenesis. Several prior studies have reported on the dysregulation of various miRNAs in RCC (7–10). However, this is the first report that implicates miR-708 in the pathogenesis of RCC. In contrast to non-tumorigenic immortalized renal epithelial cells, tumorigenic cell lines A498 and Caki2 contained low amounts of miR-708, suggesting that miR-708 may have tumor-suppressive activity. Assessment of miR-708 in human kidney tissues also pointed to a statistically significant attenuation of miR-708 expression in ~50-60% of RCC cases. A limitation to our study was the relatively small number of clinical samples at our disposal. Further studies with more clinical samples are warranted.
In addition, our in vitro and in vivo data suggests that reexpression of miR-708 suppresses tumorigenicity confirming the tumor-suppressive role of miR-708 in RCC.
Apoptosis is a well-orchestrated cellular mechanism that balances cell proliferation and cell death. In fact, the ability to evade apoptosis is a hallmark of tumorigenesis. Here we discovered an important pro-apoptotic role of miR-708. miR-708 reexpression led to dramatic induction of apoptosis concomitant with cleavage of caspases-3 and -7 in RCC cell lines. Also, our study suggests that miR-708 is a regulator of death receptor TRAIL that selectively induces apoptosis in tumor-derived cell types through caspase activation and is a promising antitumor agent (28). Recent findings show that miRNAs regulate death receptors and pro- and anti-apoptotic genes involved in programmed cell death pathways (29, 30). In a large scale screening for gene networks regulating TRAIL-induced apoptosis, Ovcharenko et al. transfected ~ 200 synthetic miRNAs in MDA-MB-453 breast cancer cells and identified that miR-28, miR-10a, 196a and miR-337 led to induction of caspase-3 activity (31). In the present study, we found that miR-708 is a novel miRNA that regulates apoptosis in renal cancer cells. In addition, miR-708 impairs the ability of renal cancer cells to attach to ECM components.
Our results show that miR-708 can pleiotropically regulate a cohort of genes that play a role in cellular survival, adhesion, invasion and metastasis. Among the putative targets of miR-708, we have validated that survivin is an important target. Survivin knockdown studies suggest that the pro-apoptotic role of miR-708 may be mediated primarily through regulation of survivin. In addition, lower expression of survivin in miR-708 xenografts provide evidence that the mechanism we identified in cell lines is repeatable in xenograft tumor cells. miR-708 mediated regulation of survivin is a highly significant finding as this nodal protein orchestrates extensive, tumor specific signaling networks and is an attractive drug target (32). Survivin is a regulator of cellular homeostasis, modulating cell death, survival, the cell cycle and microtubule dynamics (22). Survivin expression is an independent predictor of clear cell RCC (ccRCC) progression and death from RCC (33). Survivin expression has been correlated with several adverse pathologic features in ccRCC including tumor size, nuclear grade, TNM classification, presence of metastatic disease, coagulative tumor necrosis, and sarcomatoid differentiation. Thus, survivin has the potential to be important in disease prognosis and to serve as a novel target for the development of new adjuvant therapies (34).
Interestingly, we found that BMI1 and ZEB2 are targets of miR-708. BMI1, a member of the polycomb-repressive complex 1 (35, 36) is frequently overexpressed in cancers (37–39). Overexpression of BMI1 in carcinoma cell lines resulted in the acquisition of Epithelial-to-mesenchymal (EMT) characteristics, induction of stem-cell markers and enhancement of tumor-initiating capability (40). ZEB2, a repressor of E-cadherin (CDH1), coordinates EMT, a key embryonic process (41) that is reactivated during tumorigenesis. Recent evidence indicates that ZEB transcription factors may also regulate apoptosis and senescence apart from regulation of EMT (42, 43). Our results suggest that miR-708 reexpression induces apoptosis and also causes decreased motility and invasiveness. Due to the predominant and dramatic pro-apoptotic effects of miR-708, decreased migratory and invasive properties may be caused partly by effects on apoptosis. However, our present results showing that in addition to targeting survivin, miR-708 also causes reduced expression of EMT regulators ZEB2 and BMI1 concomitant with induction of E-cadherin expression and repression of fibronectin expression suggests that miR-708 may also affect migration and invasion directly. A recent study suggests that miR-200c represses ZEB1 and ZEB2 and also regulates induction of apoptosis through the death receptor CD95 (44).
In conclusion, our study identifies miR-708 as an important miRNA that regulates renal carcinogenesis through a cohort of effectors. This study shows that miR-708 plays an important role as a pro-apoptotic miRNA in renal cancers. In view of our present results showing an attenuation of miR-708 expression in human RCC clinical specimens and the suppression of tumorigenicity upon restoration of miR-708 expression, we hypothesize that miR-708 may be an attractive target for prognostic and therapeutic interventions in RCC. Furthermore, our in vivo data showing growth inhibition of established renal tumor xenografts by intratumoral delivery of synthetic miR-708 oligonucleotides supports the therapeutic potential of this novel miRNA in RCC.
Supplementary Material
1
2
3
4
5
6
ACKNOWLEDGEMENTS
We thank Dr. Roger Erickson for his assistance with the preparation of manuscript. This study was supported by Grants T32DK007790, RO1CA138642, RO1CA130860 (NIH), VA Research Enhancement Award Program and Merit Review grants.
REFERENCES
- 1.Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001;166:1611–1623. [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schickel R, Boyerinas B, Park SM, Peter ME. MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene. 2008;27:5959–5974. doi: 10.1038/onc.2008.274. [DOI] [PubMed] [Google Scholar]
- 4.Ventura A, Jacks T. MicroRNAs and cancer: short RNAs go a long way. Cell. 2009;136:586–591. doi: 10.1016/j.cell.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 6.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 7.Gottardo F, Liu CG, Ferracin M, Calin GA, Fassan M, Bassi P, et al. Micro-RNA profiling in kidney and bladder cancers. Urol Oncol. 2007;25:387–392. doi: 10.1016/j.urolonc.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, Dai Y, Yang J, Chen T, Yin Y, Tang M, et al. Microarray analysis of microRNA expression in renal clear cell carcinoma. Eur J Surg Oncol. 2009;35:1119–1123. doi: 10.1016/j.ejso.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Juan D, Alexe G, Antes T, Liu H, Madabhushi A, Delisi C, et al. Identification of a microRNA panel for clear-cell kidney cancer. Urology. 2010;75:835–841. doi: 10.1016/j.urology.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 10.Jung M, Mollenkopf HJ, Grimm C, Wagner I, Albrecht M, Waller T, et al. MicroRNA profiling of clear cell renal cell cancer identifies a robust signature to define renal malignancy. J Cell Mol Med. 2009;13:3918–3928. doi: 10.1111/j.1582-4934.2009.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartels CL, Tsongalis GJ. MicroRNAs: novel biomarkers for human cancer. Clin Chem. 2009;55:623–631. doi: 10.1373/clinchem.2008.112805. [DOI] [PubMed] [Google Scholar]
- 12.Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lui WO, Pourmand N, Patterson BK, Fire A. Patterns of known and novel small RNAs in human cervical cancer. Cancer Res. 2007;67:6031–6043. doi: 10.1158/0008-5472.CAN-06-0561. [DOI] [PubMed] [Google Scholar]
- 14.Patnaik SK, Kannisto E, Knudsen S, Yendamuri S. Evaluation of microRNA expression profiles that may predict recurrence of localized stage I non-small cell lung cancer after surgical resection. Cancer Res. 2010;70:36–45. doi: 10.1158/0008-5472.CAN-09-3153. [DOI] [PubMed] [Google Scholar]
- 15.Xing L, Todd NW, Yu L, Fang H, Jiang F. Early detection of squamous cell lung cancer in sputum by a panel of microRNA markers. Mod Pathol. 2010;23:1157–1164. doi: 10.1038/modpathol.2010.111. [DOI] [PubMed] [Google Scholar]
- 16.Schotte D, Chau JC, Sylvester G, Liu G, Chen C, van der Velden VH, et al. Identification of new microRNA genes and aberrant microRNA profiles in childhood acute lymphoblastic leukemia. Leukemia. 2009;23:313–322. doi: 10.1038/leu.2008.286. [DOI] [PubMed] [Google Scholar]
- 17.Majid S, Saini S, Dar AA, Hirata H, Shahryari V, Tanaka Y, et al. MicroRNA-205 Inhibits Src-Mediated Oncogenic Pathways in Renal Cancer. Cancer Res. 2011;71:2611–2621. doi: 10.1158/0008-5472.CAN-10-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trang P, Medina PP, Wiggins JF, Ruffino L, Kelnar K, Omotola M, et al. Regression of murine lung tumors by the let-7 microRNA. Oncogene. 2010;29:1580–1587. doi: 10.1038/onc.2009.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tucker RP, Chiquet-Ehrismann R. Teneurins: a conserved family of transmembrane proteins involved in intercellular signaling during development. Dev Biol. 2006;290:237–245. doi: 10.1016/j.ydbio.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 20.Tucker RP, Kenzelmann D, Trzebiatowska A, Chiquet-Ehrismann R. Teneurins: transmembrane proteins with fundamental roles in development. Int J Biochem Cell Biol. 2007;39:292–297. doi: 10.1016/j.biocel.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: targets and expression. Nucleic Acids Res. 2008;36:D149–D153. doi: 10.1093/nar/gkm995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol. 2006;18:609–615. doi: 10.1016/j.ceb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Mehrotra S, Languino LR, Raskett CM, Mercurio AM, Dohi T, Altieri DC. IAP regulation of metastasis. Cancer Cell. 2010;17:53–64. doi: 10.1016/j.ccr.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang M, Coen JJ, Suzuki Y, Siedow MR, Niemierko A, Khor LY, et al. Survivin Is a Potential Mediator of Prostate Cancer Metastasis. Int J Radiat Oncol Biol Phys. 2010;78:1095–1103. doi: 10.1016/j.ijrobp.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson JP, Rummel MM, Rothbacher U, Sers C. MUC18: A cell adhesion molecule with a potential role in tumor growth and tumor cell dissemination. Curr Top Microbiol Immunol. 1996;213:95–105. doi: 10.1007/978-3-642-61107-0_7. [DOI] [PubMed] [Google Scholar]
- 26.Vandewalle C, Comijn J, De Craene B, Vermassen P, Bruyneel E, Andersen H, et al. SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions. Nucleic Acids Res. 2005;33:6566–6578. doi: 10.1093/nar/gki965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, et al. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- 28.Griffith TS, Stokes B, Kucaba TA, Earel JK, Jr, VanOosten RL, Brincks EL, et al. TRAIL gene therapy: from preclinical development to clinical application. Curr Gene Ther. 2009;9:9–19. doi: 10.2174/156652309787354612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subramanian S, Steer CJ. MicroRNAs as gatekeepers of apoptosis. J Cell Physiol. 2010;223:289–298. doi: 10.1002/jcp.22066. [DOI] [PubMed] [Google Scholar]
- 30.Garofalo M, Condorelli GL, Croce CM, Condorelli G. MicroRNAs as regulators of death receptors signaling. Cell Death Differ. 2010;17:200–208. doi: 10.1038/cdd.2009.105. [DOI] [PubMed] [Google Scholar]
- 31.Ovcharenko D, Kelnar K, Johnson C, Leng N, Brown D. Genome-scale microRNA and small interfering RNA screens identify small RNA modulators of TRAIL-induced apoptosis pathway. Cancer Res. 2007;67:10782–10788. doi: 10.1158/0008-5472.CAN-07-1484. [DOI] [PubMed] [Google Scholar]
- 32.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 33.Parker AS, Kosari F, Lohse CM, Houston Thompson R, Kwon ED, Murphy L, et al. High expression levels of survivin protein independently predict a poor outcome for patients who undergo surgery for clear cell renal cell carcinoma. Cancer. 2006;107:37–45. doi: 10.1002/cncr.21952. [DOI] [PubMed] [Google Scholar]
- 34.Lei Y, Geng Z, Guo-Jun W, He W, Jian-Lin Y. Prognostic significance of survivin expression in renal cell cancer and its correlation with radioresistance. Mol Cell Biochem. 2010;344:23–31. doi: 10.1007/s11010-010-0525-3. [DOI] [PubMed] [Google Scholar]
- 35.Valk-Lingbeek ME, Bruggeman SW, van Lohuizen M. Stem cells and cancer; the polycomb connection. Cell. 2004;118:409–418. doi: 10.1016/j.cell.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Widschwendter M, Fiegl H, Egle D, Mueller-Holzner E, Spizzo G, Marth C, et al. Epigenetic stem cell signature in cancer. Nat Genet. 2007;39:157–158. doi: 10.1038/ng1941. [DOI] [PubMed] [Google Scholar]
- 37.Chiba T, Miyagi S, Saraya A, Aoki R, Seki A, Morita Y, et al. The polycomb gene product BMI1 contributes to the maintenance of tumor-initiating side population cells in hepatocellular carcinoma. Cancer Res. 2008;68:7742–7749. doi: 10.1158/0008-5472.CAN-07-5882. [DOI] [PubMed] [Google Scholar]
- 38.Leung C, Lingbeek M, Shakhova O, Liu J, Tanger E, Saremaslani P, et al. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature. 2004;428:337–341. doi: 10.1038/nature02385. [DOI] [PubMed] [Google Scholar]
- 39.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12:982–992. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- 41.Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–923. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- 42.Browne G, Sayan AE, Tulchinsky E. ZEB proteins link cell motility with cell cycle control and cell survival in cancer. Cell Cycle. 2010;9:886–891. doi: 10.4161/cc.9.5.10839. [DOI] [PubMed] [Google Scholar]
- 43.Sayan AE, Griffiths TR, Pal R, Browne GJ, Ruddick A, Yagci T, et al. SIP1 protein protects cells from DNA damage-induced apoptosis and has independent prognostic value in bladder cancer. Proc Natl Acad Sci U S A. 2009;106:14884–14889. doi: 10.1073/pnas.0902042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schickel R, Park SM, Murmann AE, Peter ME. miR-200c regulates induction of apoptosis through CD95 by targeting FAP-1. Mol Cell. 2010;38:908–915. doi: 10.1016/j.molcel.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2
3
4
5
6