Combination Anastrozole and Fulvestrant in Metastatic Breast Cancer (original) (raw)
. Author manuscript; available in PMC: 2014 Mar 12.
Published in final edited form as: N Engl J Med. 2012 Aug 2;367(5):435–444. doi: 10.1056/NEJMoa1201622
Abstract
BACKGROUND
The aromatase inhibitor anastrozole inhibits estrogen synthesis. Fulvestrant binds and accelerates degradation of estrogen receptors. We hypothesized that these two agents in combination might be more effective than anastrozole alone in patients with hormone-receptor (HR)–positive metastatic breast cancer.
METHODS
Postmenopausal women with previously untreated metastatic disease were randomly assigned, in a 1:1 ratio, to receive either 1 mg of anastrozole orally every day (group 1), with crossover to fulvestrant alone strongly encouraged if the disease progressed, or anastrozole and fulvestrant in combination (group 2). Patients were stratified according to prior or no prior receipt of adjuvant tamoxifen therapy. Fulvestrant was administered intramuscularly at a dose of 500 mg on day 1 and 250 mg on days 14 and 28 and monthly thereafter. The primary end point was progressionfree survival, with overall survival designated as a prespecified secondary outcome.
RESULTS
The median progression-free survival was 13.5 months in group 1 and 15.0 months in group 2 (hazard ratio for progression or death with combination therapy, 0.80; 95% confidence interval [CI], 0.68 to 0.94; P = 0.007 by the log-rank test). The combination therapy was generally more effective than anastrozole alone in all subgroups, with no significant interactions. Overall survival was also longer with combination therapy (median, 41.3 months in group 1 and 47.7 months in group 2; hazard ratio for death, 0.81; 95% CI, 0.65 to 1.00; P = 0.05 by the log-rank test), despite the fact that 41% of the patients in group 1 crossed over to fulvestrant after progression. Three deaths that were possibly associated with treatment occurred in group 2. The rates of grade 3 to 5 toxic effects did not differ significantly between the two groups.
CONCLUSIONS
The combination of anastrozole and fulvestrant was superior to anastrozole alone or sequential anastrozole and fulvestrant for the treatment of HR-positive metastatic breast cancer, despite the use of a dose of fulvestrant that was below the current standard.
Endocrine therapy plays a central role in the treatment of hormone-receptor (HR)–positive metastatic breast cancer.1 Selective aromatase inhibitors, such as anastrozole, letrozole, and exemestane, lower the estrogen level and are used as first-line endocrine treatments of HR-positive metastatic disease, owing to their superiority over tamoxifen.1 Fulvestrant (Faslodex, AstraZeneca) is an analogue of estradiol that downregulates the estrogen receptor by disrupting estrogen-receptor dimerization and accelerating degradation of the unstable fulvestrant–estrogenreceptor complex.2 This effect leads to reduced cross-talk between the estrogen receptor and estrogen-independent growth factor signaling, thus delaying resistance to hormone therapy.2 Clinically, fulvestrant at a dose of 250 mg monthly is as active as tamoxifen when used as first-line therapy for metastatic disease3 and as active as anastrozole when administered in patients who have had disease progression after receiving tamoxifen therapy.4,5
In preclinical models, fulvestrant has been shown to have high efficacy in a low-estrogen environment.6 The combination of fulvestrant and an aromatase inhibitor, as compared with either agent alone, delays the development of resistance by down-regulating several signaling molecules involved in the development of resistance.7,8 We therefore conducted a phase 3, randomized trial to determine whether the combination of anastrozole and fulvestrant would be superior to anastrozole alone as first-line therapy for metastatic breast cancer.
METHODS
STUDY DESIGN AND OVERSIGHT
The study was designed and conducted, and the data were analyzed, by the Southwest Oncology Group (SWOG) Cooperative Group, which was funded by the National Cancer Institute (NCI), with review and collaboration by the other participating cooperative groups and the NCI Cancer Therapy Evaluation Program. The first two authors assume full responsibility for the quality and completeness of the data and vouch for the data analysis and for the fidelity of the study to the protocol. All drafts of the manuscript were prepared and approved by all the authors, and members of the SWOG made the decision to submit it for publication. The trial data were reviewed by a data and safety monitoring committee every 6 months. AstraZeneca provided the study medications at no cost to enrolled patients. AstraZeneca provided comments on an early draft of the manuscript but contractually was not allowed to approve or disapprove of the submission of the manuscript for publication. AstraZeneca was not provided with the trial data and did not participate in the statistical analysis. The study protocol, including the statistical analysis plan, is available with the full text of this article at NEJM.org.
ELIGIBILITY
Eligible patients were postmenopausal women with HR-positive metastatic breast cancer (estrogenreceptor–positive, progesterone-receptor–positive, or both), diagnosed according to local institutional standards. Women were eligible if they had had no prior chemotherapy, hormonal therapy, or immunotherapy for metastatic disease. Neoadjuvant or adjuvant chemotherapy had to have been completed more than 12 months before enrollment. In the original protocol, women who had received prior adjuvant therapy with an aromatase inhibitor or fulvestrant were excluded, but those who had received prior adjuvant tamoxifen therapy were eligible. In an early amendment, women who had received prior adjuvant therapy with an aromatase inhibitor were also eligible if the therapy had been completed more than 12 months before enrollment. Patients were not allowed to receive concurrent chemotherapy or other hormonal therapy during the study treatment period (bisphosphonates were allowed). Women with either measurable or nonmeasurable disease were eligible. Other major eligibility criteria included no known metastases in the central nervous system and a Zubrod’s performance score of 0 to 2 (with a score of 0 indicating that the patient is fully active, 1 that the patient is restricted in strenuous activity but is ambulatory, and 2 that the patient is unable to work but is ambulatory and capable of self-care).9 Patients with bleeding diathesis or long-term anticoagulant therapy (except antiplatelet therapy) were ineligible. Patients with other cancers were ineligible unless the cancer had been adequately treated or had been in remission for at least 5 years. All patients provided written informed consent before enrollment.
RANDOMIZATION AND TREATMENT
Randomization was performed at a central location, with stratification according to prior receipt or no prior receipt of adjuvant tamoxifen therapy. Patients were randomly assigned, in a 1:1 ratio, to anastrozole alone (group 1) or to fulvestrant in combination with anastrozole (group 2). Patients in group 1 received 1 mg of anastrozole orally each day. Patients in group 2 received 1 mg of anastrozole orally each day, as well as an initial loading dose (500 mg) of fulvestrant administered intramuscularly on day 1, followed by 250 mg (low-dose fulvestrant) administered intramuscularly on day 14 and day 28 of the first cycle, and thereafter every 28 days. Treatment was continued until disease progression, the development of unacceptable toxic effects, a delay in treatment of 4 weeks or longer, or withdrawal of the patient from the trial. After progression, the treating physician could choose the appropriate therapy, although crossover to low-dose fulvestrant (after initial loading dose) was strongly recommended for patients in group 1 after discontinuation of anastrozole, and fulvestrant was provided free of charge to encourage crossover to that agent. After a higher monthly dose of fulvestrant (500 mg) was shown to be superior to the low dose10 and the Food and Drug Administration approved the higher monthly dose, the protocol was amended (on February 2, 2011) to allow patients in either group to receive the 500-mg dose after progression.
ASSESSMENT OF PROGRESSION AND SURVIVAL
Progression was assessed every 3 months and was defined according to the Response Evaluation Criteria in Solid Tumors (RECIST) in the case of measurable disease11 and according to an assessment of the worsening of symptoms or increasing disease (as determined by the patient’s oncologist) in the case of nonmeasurable disease. After progression, overall survival was assessed every 6 months for the first 2 years from the time of random assignment and then annually for the next 2 years. Follow-up beyond 4 years was not required, although 32 patients without progression at 48 months continued to be followed.
ASSESSMENT OF TOXIC EFFECTS
Toxic effects were measured according to the Common Terminology Criteria for Adverse Events, version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf). Patients with grade 3 or grade 4 toxic effects could have treatment interrupted for up to 4 weeks to allow resolution of the toxic effects to grade 2 or less. The study treatment was withdrawn in the case of grade 3 or 4 toxic effects that did not resolve by 4 weeks.
STATISTICAL ANALYSIS
The primary outcome was progression-free survival, which was defined as the time from random assignment to disease progression or death from any cause. Data from patients who were alive and progression-free at the time of cutoff of the data (September 29, 2011) were censored at the last follow-up visit at which progression had not yet been observed. Overall survival, which was a secondary outcome, was defined as the time from random assignment to death from any cause. We calculated the rates of clinical benefit using the number of patients with a complete or partial response or stable disease as the numerator and the number of all patients (even those in whom a response could not be assessed or for whom response data were missing) as the denominator. The rate of objective response was calculated only for patients with measurable disease, whereas the rate of clinical benefit applied to all patients. Both the primary analysis of progressionfree survival and the analysis of overall survival were specified as log-rank tests stratified according to prior receipt or no prior receipt of adjuvant tamoxifen therapy. Kaplan–Meier methods were used to construct survival plots and to estimate the survival percentages and the median times to progression-free and overall survival. Cox regression was used to estimate hazard ratios and 95% confidence intervals.
Post hoc subgroup analyses were performed on the basis of the stratification variable (status with respect to prior adjuvant tamoxifen therapy); therefore, the results should be interpreted cautiously. A forest plot was used to compare the overall hazard ratio with the hazard ratios obtained in subgroups defined on the basis of several potentially prognostic or predictive factors. P values for interaction were obtained from a Cox regression analysis. Two interim analyses of progression-free survival were performed when 50% and 75% of the expected events had occurred. The final analysis was set at an alpha level of 0.02 (one-sided) so that the one-sided cumulative alpha level was 0.025 or the two-sided alpha level was 0.05. All tests were two-sided, so the P value for the final analysis of the primary outcome (progressionfree survival) had to be 0.04 or less to indicate statistical significance. We estimated that with a sample of 690 patients and an expected median progression-free survival of 10 months in group 1 and 13 months in group 2, the trial would have 90% overall power to show a between-group difference in the primary outcome. The projected medians for overall survival were 36 months and 48 months, respectively.
RESULTS
PATIENTS
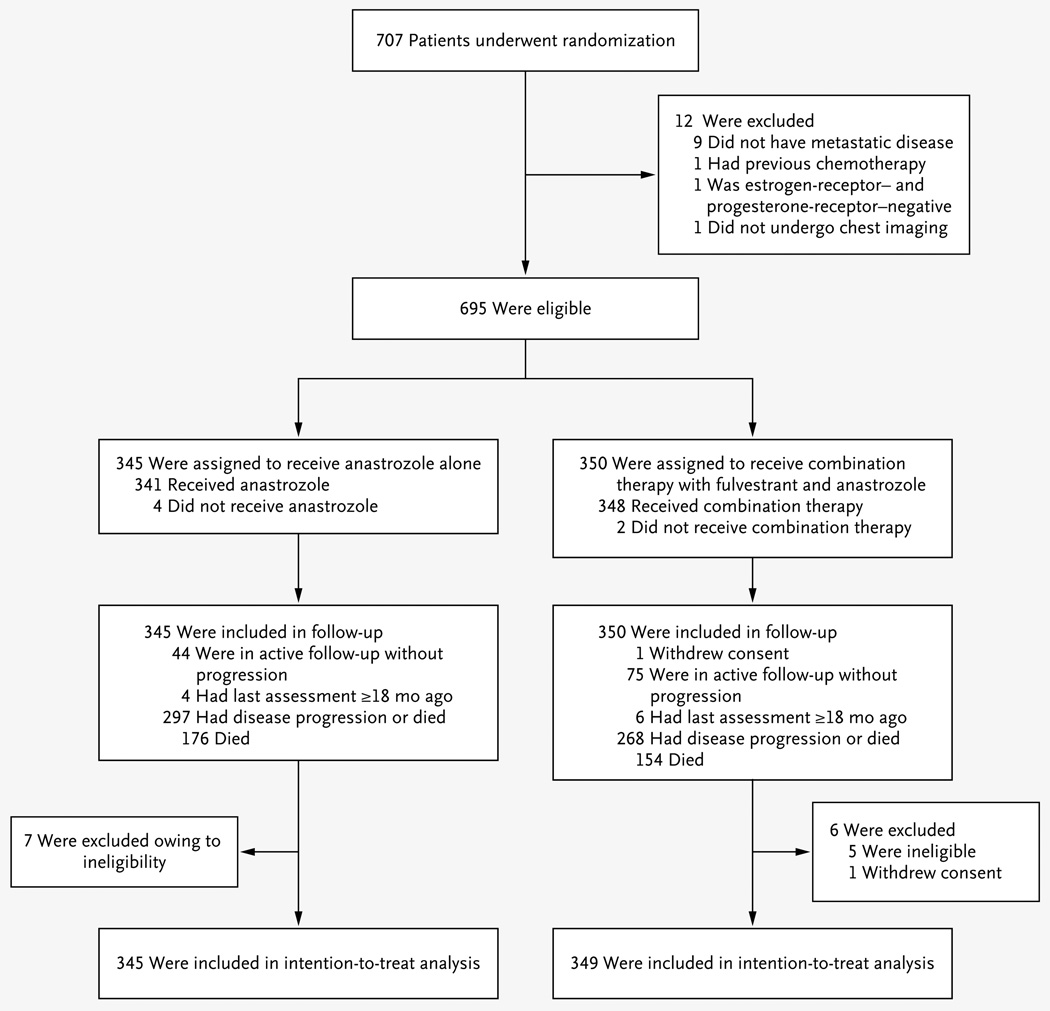
A total of 707 patients underwent randomization during the period from June 1, 2004, through July 1, 2009. Figure 1 shows the enrollment, random assignment, and follow-up of the patients. After randomization, 12 patients were found to be ineligible, in most cases because there was not a definitive diagnosis of metastatic disease; in addition, 1 patient withdrew consent. Therefore, 694 patients were included in the intention-totreat analysis. Table 1 shows the characteristics of the patients, which were well balanced between the two groups.
Figure 1. Enrollment, Randomization, and Follow-up.
Table 1.
Baseline Characteristics of the Patients and the Disease, According to Treatment Group.*
| Characteristic | Anastrozole Alone(N = 345) | Anastrozoleand Fulvestrant(N = 349) | Total(N = 694) |
|---|---|---|---|
| Age — yr | |||
| Median | 65 | 65 | 65 |
| Range | 36–91 | 27–92 | 27–92 |
| Prior adjuvant tamoxifen — no. (%) | |||
| Yes | 139 (40.3) | 141 (40.4) | 280 (40.3) |
| No | 206 (59.7) | 208 (59.6) | 414 (59.7) |
| Prior adjuvant chemotherapy — no. (%) | |||
| Yes | 103 (29.9) | 129 (37.0) | 232 (33.4) |
| No | 242 (70.1) | 220 (63.0) | 462 (66.6) |
| Measurable disease — no. (%) | |||
| Yes | 188 (54.5) | 188 (53.9) | 376 (54.2) |
| No | 157 (45.5) | 161 (46.1) | 318 (45.8) |
| Disease site — no. (%) | |||
| Bone only | 76 (22.0) | 75 (21.5) | 151 (21.8) |
| Visceral | 167 (48.4) | 181 (51.9) | 348 (50.1) |
| Nonvisceral | 102 (29.6) | 93 (26.6) | 195 (28.1) |
| Time between diagnosis of primary and metastatic disease — no./total no. (%) | |||
| None | 141/337 (41.8) | 122/339 (36.0) | 263/676 (38.9) |
| 3 mo to <5 yr | 40/337 (11.9) | 47/339 (13.9) | 87/676 (12.9) |
| 5 to <10 yr | 68/337 (20.2) | 66/339 (19.5) | 134/676 (19.8) |
| ≥10 yr | 88/337 (26.1) | 104/339 (30.7) | 192/676 (28.4) |
| HER2 status — no./total no. (%) | |||
| Positive | 25/295 (8.5) | 31/297 (10.4) | 56/592 (9.5) |
| Negative | 270/295 (91.5) | 266/297 (89.6) | 536/592 (90.5) |
PRIMARY OUTCOME
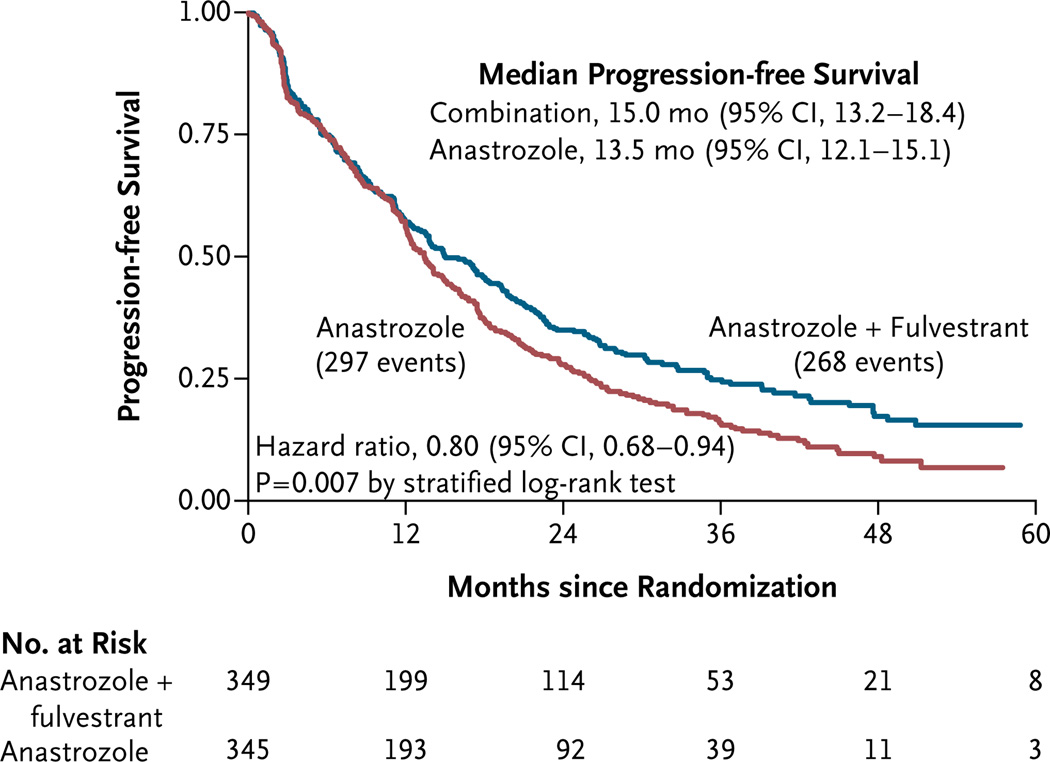
There were 565 events (progression or death) for the primary analysis, with 297 occurring in group 1 and 268 in group 2. The median follow-up time for progression-free survival among the 129 patients without an event was 35 months (range, 3 to 78) as of September 29, 2011. Data for 101 of the 694 patients (14.6%) were censored before the end of the 4-year follow-up period, primarily owing to the fact that, with a 28-month interval between the closure of data accrual and the final analysis, complete follow-up data on patients enrolled at the end of the trial would not have been available for the final analysis. The median progressionfree survival was 13.5 months (95% confidence interval [CI], 12.1 to 15.1) in the group that received anastrozole alone and 15.0 months (95% CI, 13.2 to 18.4) in the group that received the combination therapy (Fig. 2). The study objective, showing a significant improvement in the primary end point of progression-free survival with the combination therapy, was met (P = 0.007 with the use of a two-sided stratified log-rank test). Generally, the superiority of the combination therapy over anastrozole alone with respect to progressionfree survival emerged over time: at 1 year, the rate of progression-free survival was 57% with combination therapy and 56% with anastrozole alone; at 2 years, the corresponding rates were 35% and 28%, and at 3 years, the rates were 25% and 16% (Fig. 2). The hazard ratio for progression or death with the combination therapy was 0.80 (95% CI, 0.68 to 0.94), as calculated by means of a stratified Cox regression analysis.
Figure 2. Kaplan–Meier Curves for Progression-free Survival, According to Treatment Group.
The overall hazard ratio for progression or death with the combination therapy is shown.
Analyses of treatment benefit were performed within the subgroups based on stratification (women who had received prior tamoxifen therapy and those who had not), although these analyses were not prespecified. Among the 414 women (59.7%) who had not received prior tamoxifen therapy, the median progression-free survival was 12.6 months in group 1 and 17.0 months in group 2 (hazard ratio for progression or death with combination therapy, 0.74; 95% CI, 0.59 to 0.92; P = 0.006 with the use of the log-rank test). Among women who had received prior tamoxifen therapy, the estimated median progression-free survival was 14.1 months and 13.5 months, respectively (hazard ratio, 0.89; 95% CI, 0.69 to 1.15; P = 0.37 with the use of the log-rank test). The interaction between treatment and use of prior adjuvant tamoxifen therapy was not significant (P = 0.22).
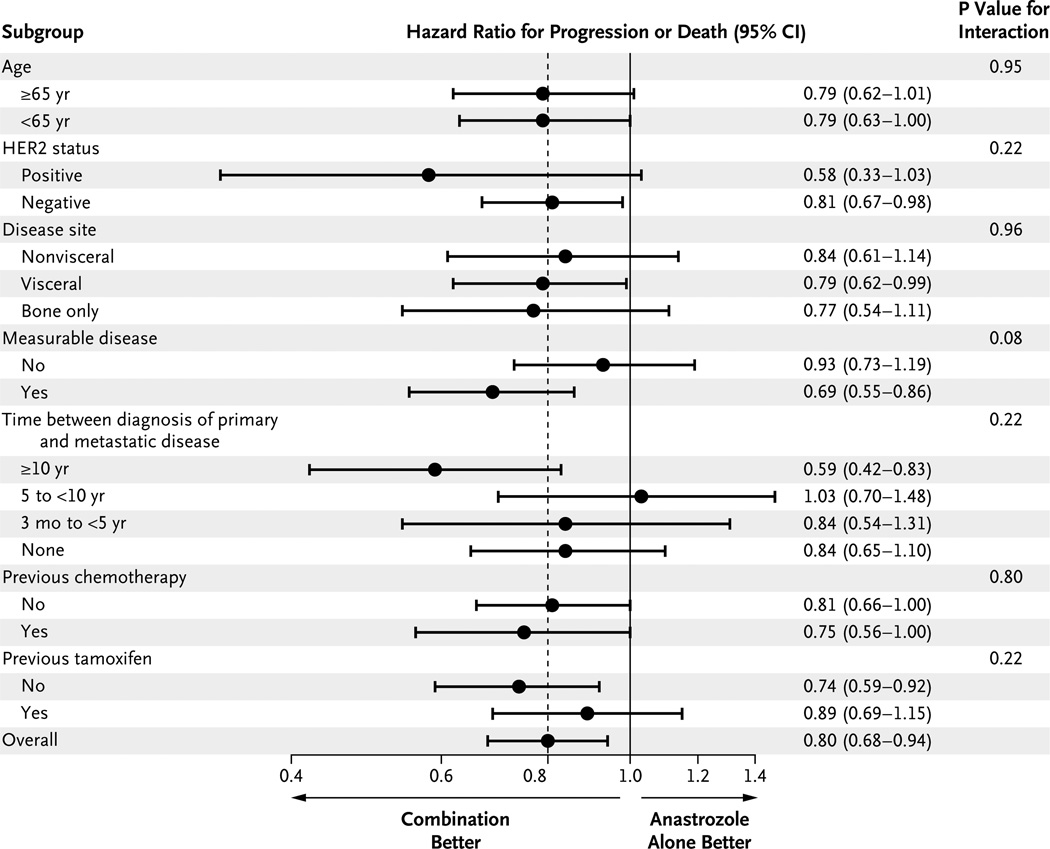
Prior tamoxifen therapy was strongly associated with the length of time between the diagnosis of the primary disease and the diagnosis of metastatic disease. By definition, women with metastatic disease at presentation could not have received prior adjuvant tamoxifen therapy. Among women for whom the interval between the initial diagnosis of disease and the diagnosis of metastatic disease was 3 months to less than 5 years, the rate of prior receipt of tamoxifen therapy was 47%; among women for whom the interval was 5 years to less than 10 years, the rate was 23%; and among women for whom the interval was 10 years or more, the rate was 38%. Figure 3 shows a forest plot of the hazard ratio for progression or death according to subgroups and the P value for interaction. Across all but one subgroup, there was at least a trend toward a benefit with the combination therapy.
Figure 3. Progression-free Survival, According to Subgroups.
The hazard ratio for progression or death with the combination therapy is shown for each indicated subgroup. The dashed line indicates the overall hazard ratio, and the solid vertical line indicates no difference between treatments. The P values shown for the interaction of each factor with treatment are based on a Cox regression model. HER2 denotes human epidermal growth factor receptor type 2.
SECONDARY OUTCOME
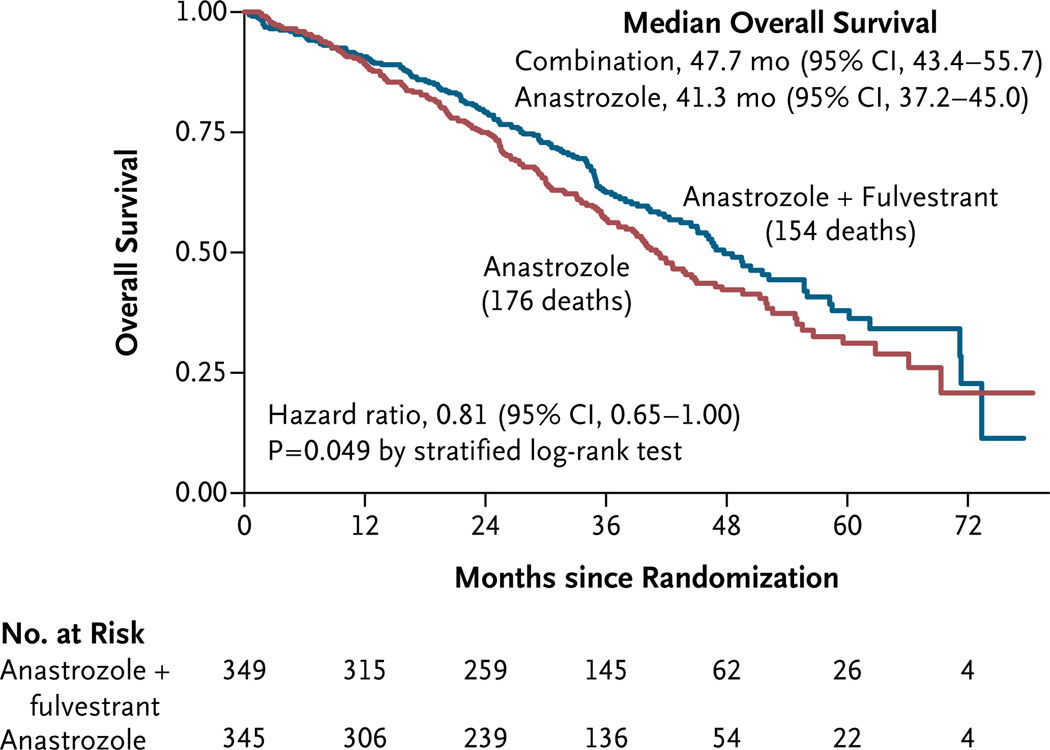
There were 176 deaths (the prespecified secondary outcome) in group 1 and 154 in group 2. The median overall survival was 41.3 months (95% CI, 37.2 to 45.0) with anastrozole alone and 47.7 months (95% CI, 43.4 to 55.7) with the combination therapy (two-sided P = 0.049, with the use of a log-rank test, stratified according to prior or no prior tamoxifen therapy). As was the case with progression-free survival, the magnitude of the difference in overall survival between the group that received anastrozole alone and the group that received the combination therapy increased over time: the rate of overall survival at 1 year was 89% with anastrozole alone and 91% with the combination therapy; the corresponding rate at 2 years was 75% and 79%, and the rate at 3 years was 57% and 62%. The estimated hazard ratio for death with combination therapy was 0.81 (95% CI, 0.65 to 1.00), with the use of a stratified Cox regression analysis (Fig. 4). Overall survival differed significantly between group 1 and group 2 among women who had not received prior tamoxifen therapy (hazard ratio for death with combination therapy, 0.74; 95% CI, 0.56 to 0.98; P = 0.04) but not among women who had received prior tamoxifen therapy (hazard ratio, 0.91; 95% CI, 0.65 to 1.28; P = 0.59), though the interaction was not significant (P = 0.22), and the combination therapy showed a benefit in both groups.
Figure 4. Kaplan–Meier Curves for Overall Survival, According to Treatment Group.
The overall hazard ratio for death with the combination therapy is shown.
A total of 143 patients in group 1 (41.4%) crossed over to fulvestrant therapy, whereas most of the other patients proceeded directly from anastrozole therapy to chemotherapy. The time to death after progression (a post hoc analysis) did not differ according to the randomly assigned treatment (hazard ratio for death with combination therapy, 0.92; 95% CI, 0.72 to 1.15; P = 0.44), suggesting that the benefit provided by the combination therapy was the delay in disease progression.
The rate of clinical benefit (complete or partial response or stable disease) was 73% with the combination therapy and 70% with anastrozole alone (P = 0.39). Among patients with measurable disease, the rate of response (complete or partial) was 27% with the combination therapy and 22% with anastrozole alone (P = 0.26). Stable disease was the most common outcome among all patients.
TOXIC EFFECTS
Information on toxic effects was collected for 678 patients (332 in group 1 and 346 in group 2); information was not collected for the 6 patients who received no study treatment and was incomplete for 10 patients. Only 15 patients discontinued the study treatment early owing to toxic effects (4 in group 1 and 11 in group 2, P = 0.12). In general, the toxic effects were mild and did not differ significantly in grade between the two groups (see Table 1 in the Supplementary Appendix, available at NEJM.org). Toxic effects of grade 3 or higher were observed in 42 patients who received anastrozole alone (12.7%) and in 51 patients who received combination therapy (14.7%) (P = 0.44). The most common grade 3 toxic effects were musculoskeletal pain (2.8%), influenza-like symptoms (2.4%), gastrointestinal disturbances (1.5%), and hematologic effects (1.5%).
Toxic effects of grade 4 or higher were observed in four patients who received anastrozole alone (1.2%) and in five patients who received combination therapy (1.4%) (P = 1.00). The four observed grade 4 toxic effects among patients who received anastrozole alone were thrombosis or embolism, joint pain, thrombocytopenia, and dyspnea. Two patients receiving the combination therapy had grade 4 toxic effects (thrombosis or embolism in one patient and neutropenia or lymphopenia in one patient). In addition, there were three deaths in the group that received combination therapy: one patient, 80 years of age, died from diverticular perforation and heparin-induced thrombocytopenia with thromboembolism; one patient, 83 years of age, died from cerebrovascular ischemia after septic shock associated with worsening disease; and one patient, 73 years of age, who had a Zubrod’s performance score of 2, died from a thromboembolism.
DISCUSSION
This trial tested the hypothesis that the combination of anastrozole and fulvestrant would delay the development of progressive disease in postmenopausal women with HR-positive breast cancer. Progression-free survival was significantly longer with the combination therapy than with anastrozole alone. The hazard ratio of 0.80 for progression or death with combination therapy was notable, especially given the fact that the group receiving anastrozole alone had a higher median progression-free survival than had been projected in the design of the trial. Both regimens were associated with mild-to-moderate toxic effects, and although grade 3 to 5 toxic effects occurred more frequently in the combination group than in the anastrozole-alone group, the between-group difference was not significant.
Combination therapy, as compared with anastrozole alone, resulted in a significant improvement in the secondary end point of overall survival by more than 6 months (hazard ratio for death with combination therapy, 0.81). This finding suggests that the combination therapy is more effective than is the sequential use of anastrozole and fulvestrant. Although a substantial proportion of patients in the anastrozole-alone group (41%) who had disease with a good prognosis crossed over to fulvestrant (albeit low-dose fulvestrant), the overall survival for the combined cohort of patients who received anastrozole alone or anastrozole followed by fulvestrant remained inferior to the overall survival for patients who received the combination therapy. It is unclear whether the former group would have fared better if patients who crossed over to fulvestrant had received high-dose fulvestrant.10, 12,13 The indirect data that speak to this point are inconsistent. On the one hand, high-dose fulvestrant was superior to anastrozole as first-line hormonal therapy in a phase 2 trial.13 On the other hand, in the Comparison of Faslodex in Recurrent or Metastatic Breast Cancer (CONFIRM) trial, high-dose fulvestrant, as compared with low-dose fulvestrant, as second-line endocrine treatment was associated with a nonsignificant median survival benefit of only 2 months.10
Although the benefit with respect to median progression-free survival appears to be small, this outcome was assessed only at the 50th percentile of the survival curves (Fig. 2), which did not reflect the late separation of the curves. The overall effect of the combination therapy across all time points is better summarized by the hazard ratio and the log-rank P value (the primary analysis), which were both highly significant. The median difference in overall survival was larger because the medians were assessed much later than the point of separation of the curves.
Most prior studies have failed to show the superiority of concurrently administered hormonal modulators over single agents,14–21 particularly with respect to overall survival. Indeed, in the adjuvant setting, the combination of anastrozole with tamoxifen is inferior to anastrozole alone.21
Most specifically, our results contrast with the Anastrozole Monotherapy Versus Maximal Oestrogen Blockade with Anastrozole and Fulvestrant Combination Therapy (FACT) trial, in which the same combination of anastrozole and fulvestrant was not superior to anastrozole alone.22 However, the FACT trial included fewer patients (514 patients) than did the current trial, and because that trial included patients with locally recurrent disease, which is associated with fewer failure events, a larger study would have been required to detect a difference between the groups. Moreover, the FACT trial also included patients in whom the disease had progressed while they were receiving adjuvant chemotherapy, and all patients were in relapse after treatment of local disease. In contrast, 39% of the patients enrolled in our study had disease that was metastatic at presentation. Furthermore, in the FACT trial, in the combination-therapy group, 70% of the patients had received prior antiestrogen therapy and 32% were treated during or up to 12 months after stopping adjuvant endocrine therapy.
The improvement in overall survival that was observed in our study has not been seen in other trials of first-line hormonal therapy for HR-positive metastatic breast cancer.23–27 Specifically, in the trials comparing aromatase-inhibitor therapy with tamoxifen therapy, the benefit from aromatase inhibitors with respect to progressionfree survival failed to translate into a benefit with respect to overall survival, a finding that was attributed to the crossover of some patients in the tamoxifen group to an aromatase inhibitor. In contrast, the results of our study are not confounded by crossover to combination therapy, and the benefit with respect to overall survival closely mirrored the benefit with respect to progression-free survival (hazard ratio, 0.81 and 0.80, respectively). A study comparing low-dose fulvestrant with tamoxifen did not show a between-group difference in progression-free survival or overall survival, suggesting that the combination therapy, rather than fulvestrant therapy alone, mediated the improvement in our study.3
Although the percentage of patients who had metastatic disease at presentation in this study may seem high (almost 40%), the population in our study was selected to be at sufficiently low risk to forego chemotherapy. Moreover, a previous study involving patients with metastatic breast cancer showed that although only 18% of the patients had metastatic disease at presentation, this percentage increased with age and hormonereceptor positivity.28
Taken together, the results of our study suggest that trials of adjuvant therapy should be performed in which the combination of an aromatase inhibitor and high-dose fulvestrant is compared with an aromatase inhibitor alone or high-dose fulvestrant alone, in patients with estrogen-receptor–positive tumors for whom chemotherapy is not necessary.
Supplementary Material
Supplement1
Acknowledgments
Supported by grants from the National Cancer Institute (CA32102, CA38926, CA35431, CA35281, CA20319, CA04919, CA67575, CA58861, CA37981, CA46368, CA86780, CA46282, CA63848, CA12644, CA27057, CA95860, CA35119, CA46441, CA11083, CA45450, CA35178, CA13612, CA58882, CA45377, CA45807, CA58723, C14028, CA128567, CA22433, CA52654, CA45560, CA35261, CA073590, CA45808, CA35192, CA42777, CA16385, CA35176, CA63845, CA35128, CA35090, CA63844, CA76447, CA58416, CA63850, CA76462, CA35262, CA77202, and CCSRI155469) and AstraZeneca.
(Funded by the National Cancer Institute and AstraZeneca; SWOG ClinicalTrials.gov number, NCT00075764.)
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Mauri D, Pavlidis N, Polyzos NP, Ioannidis JP. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285–1291. doi: 10.1093/jnci/djj357. [DOI] [PubMed] [Google Scholar]
- 2.Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–3873. [PubMed] [Google Scholar]
- 3.Howell A, Robertson JF, Abram P, et al. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol. 2004;22:1605–1613. doi: 10.1200/JCO.2004.02.112. [DOI] [PubMed] [Google Scholar]
- 4.Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20:3386–3395. doi: 10.1200/JCO.2002.10.058. [DOI] [PubMed] [Google Scholar]
- 5.Howell A, Robertson JF, Quaresma Albano J, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20:3396–3403. doi: 10.1200/JCO.2002.10.057. [DOI] [PubMed] [Google Scholar]
- 6.Osborne CK, Coronado-Heinsohn EB, Hilsenbeck SG, et al. Comparison of effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. J Natl Cancer Inst. 1995;87:746–750. doi: 10.1093/jnci/87.10.746. [DOI] [PubMed] [Google Scholar]
- 7.Macedo LF, Sabnis GJ, Goloubeva OG, Brodie A. Combination of anastrozole with fulvestrant in the intratumoral aromatase xenograft model. Cancer Res. 2008;68:3516–3522. doi: 10.1158/0008-5472.CAN-07-6807. [DOI] [PubMed] [Google Scholar]
- 8.Jelovac D, Macedo L, Goloubeva OG, Handratta V, Brodie AM. Additive antitumor effect of aromatase inhibitor letrozole and antiestrogen fulvestrant in a postmenopausal breast cancer model. Cancer Res. 2005;65:5439–5544. doi: 10.1158/0008-5472.CAN-04-2782. [DOI] [PubMed] [Google Scholar]
- 9.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–755. [PubMed] [Google Scholar]
- 10.Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28:4594–4600. doi: 10.1200/JCO.2010.28.8415. [Erratum, J Clin Oncol 2011;29:2293.] [DOI] [PubMed] [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Robertson JF, Nicholson RI, Bundred NJ, et al. Comparison of the short term biological effects of 7alpha-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)-nonyl]estra-1,3,5(10)triene-3, 17beta-diol (Faslodex) versus tamoxifen in postmenopausal women with breast cancer. Cancer Res. 2001;61:6739–6746. [PubMed] [Google Scholar]
- 13.Robertson JF, Llombart-Cussac A, Rolski J, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol. 2009;27:4530–4540. doi: 10.1200/JCO.2008.21.1136. [DOI] [PubMed] [Google Scholar]
- 14.Rubens RD, Knight RK. The contribution of prednisolone (P) to primary endocrine therapy (PET) in advanced breast cancer. Proc Am Soc Clin Oncol. 1985;4:53. abstract. [Google Scholar]
- 15.Rubens RD, Tinson CL, Coleman RE, et al. Prednisolone improves the response to primary endocrine treatment for advanced breast cancer. Br J Cancer. 1988;58:626–630. doi: 10.1038/bjc.1988.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tormey DC, Lippman ME, Edwards BK, Cassidy JG. Evaluation of tamoxifen doses with and without fluoxymesterone in advanced breast cancer. Ann Intern Med. 1983;98:139–244. doi: 10.7326/0003-4819-98-2-139. [DOI] [PubMed] [Google Scholar]
- 17.Ingle JN, Green SJ, Ahmann DL, et al. Randomized trial of tamoxifen alone or combined with aminoglutethimide and hydrocortisone in women with metastatic breast cancer. J Clin Oncol. 1986;4:958–964. doi: 10.1200/JCO.1986.4.6.958. [DOI] [PubMed] [Google Scholar]
- 18.Ingle JN, Twito DI, Schaid DJ, et al. Combination hormonal therapy with tamoxifen plus fluoxymesterone versus tamoxifen alone in postmenopausal women with metastatic breast cancer: an updated analysis. Cancer. 1991;67:886–991. doi: 10.1002/1097-0142(19910215)67:4<886::aid-cncr2820670405>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Russell CA, Green SJ, O’Sullivan J, et al. Megestrol acetate and aminoglutethimide/hydrocortisone in sequence or in combination as second-line endocrine therapy of estrogen receptor-positive metastatic breast cancer: a Southwest Oncology Group phase III trial. J Clin Oncol. 1997;15:2494–2501. doi: 10.1200/JCO.1997.15.7.2494. [DOI] [PubMed] [Google Scholar]
- 20.Goss P, Bondarenko IN, Manikhas GN, et al. Phase III, double-blind, controlled trial of atamestane plus toremifene compared with letrozole in postmenopausal women with advanced receptor-positive breast cancer. J Clin Oncol. 2007;25:4961–4976. doi: 10.1200/JCO.2006.09.5455. [DOI] [PubMed] [Google Scholar]
- 21.Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomized trial. Lancet. 2002;359:2131–2239. doi: 10.1016/s0140-6736(02)09088-8. [Erratum, Lancet 2002;360: 1520.] [DOI] [PubMed] [Google Scholar]
- 22.Bergh J, Jönsson PE, Lidbrink EK, et al. FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol. 2012;30:1919–1925. doi: 10.1200/JCO.2011.38.1095. [DOI] [PubMed] [Google Scholar]
- 23.Bonneterre J, Thürlimann B, Robertson JF, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol. 2000;18:3748–3757. doi: 10.1200/JCO.2000.18.22.3748. [Erratum, J Clin Oncol 2012;30:343.] [DOI] [PubMed] [Google Scholar]
- 24.Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. J Clin Oncol. 2000;18:3758–3767. doi: 10.1200/JCO.2000.18.22.3758. [DOI] [PubMed] [Google Scholar]
- 25.Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21:2101–2119. doi: 10.1200/JCO.2003.04.194. [DOI] [PubMed] [Google Scholar]
- 26.Bonneterre J, Buzdar A, Nabholtz JM, et al. Anastrozole is superior to tamoxifen as first-line therapy in hormone receptor positive advanced breast carcinoma. Cancer. 2001;92:2247–2258. doi: 10.1002/1097-0142(20011101)92:9<2247::aid-cncr1570>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 27.Paridaens RJ, Dirix LY, Beex LV, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:4883–4990. doi: 10.1200/JCO.2007.14.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 2010;21:2169–2174. doi: 10.1093/annonc/mdq220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement1