Pathophysiology of Portal Hypertension (original) (raw)
. Author manuscript; available in PMC: 2015 May 1.
Published in final edited form as: Clin Liver Dis. 2014 Feb 25;18(2):281–291. doi: 10.1016/j.cld.2013.12.001
Abstract
Portal hypertension is a major complication of liver disease, which results from a variety of pathological conditions that increase the resistance to the portal blood flow into the liver. The primary cause of portal hypertension in cirrhosis is an increase in intrahepatic vascular resistance due to massive structural changes associated with fibrosis and increased vascular tone in the hepatic microcirculation. As portal hypertension develops, the formation of collateral vessels and arterial vasodilation progress, which results in increased blood flow to the portal circulation. Eventually the hyperdynamic circulatory syndrome develops, leading to esophageal varices or ascites. This review article will summarize the factors that increase 1) intrahepatic vascular resistance and 2) the blood flow in the splanchnic and systemic circulations in liver cirrhosis. Finally, the future directions of basic/clinical research in portal hypertension will be discussed.
Keywords: Hyperdynamic circulation, fibrosis, cirrhosis, nitric oxide, lymphatic system, splenomegaly
Introduction
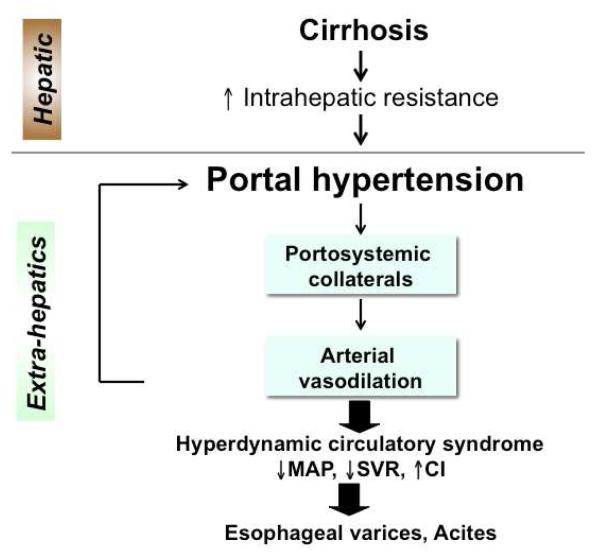
Portal hypertension is a detrimental complication resulting from obstruction of portal blood flow, such as cirrhosis or portal vein thrombosis. 1, 2 In liver cirrhosis, increased intrahepatic vascular resistance to the portal flow elevates portal pressure and leads to portal hypertension (Figure 1). Once portal hypertension develops, it influences extrahepatic vascular beds in the splanchnic and systemic circulations, causing collateral vessel formation and arterial vasodilation. This helps to increase the blood flow into the portal vein, which exacerbates portal hypertension and eventually brings the hyperdynamic circulatory syndrome. 1, 2 Consequently, esophageal varices or ascites develops. This review article will discuss recent advances in understanding of factors that contribute to: 1) an increase in intrahepatic vascular resistance and 2) an increase in blood flow in the splanchnic and systemic circulations, and 3) the future directions of basic/clinical research in portal hypertension.
Figure 1.
Portal hypertension leads to the development of the hyperdynamic circulatory syndrome, characterized by decreased mean arterial pressure (MAP), decreased systemic vascular resistance (SVR) and increased cardiac index (CI).
I. Intrahepatic circulation
An overview
The primary cause of portal hypertension in cirrhosis is an increase in intrahepatic vascular resistance. In cirrhosis, increased intrahepatic vascular resistance is a result of massive structural changes associated with fibrosis/cirrhosis and intrahepatic vasoconstriction2-4. It is reported that intrahepatic vasoconstriction accounts for at least 25% of increased intrahepatic vascular resistance. 5 Phenotypic changes in hepatic cells, such as hepatic stellate cells (HSCs) and liver sinusoidal endothelial cells (LSECs), are known to play pivotal roles in increased intrahepatic vascular resistance and have been studied intensively. This section summarizes important factors that increase intrahepatic vascular resistance in liver fibrosis/cirrhosis.
1. Endothelial cell dysfunction
LSECs are the first line of defense protecting the liver from injury2, and the cells exert diverse effects on liver functions including blood clearance, vascular tone, immunity, hepatocyte growth6, and angiogenesis/sinusoidal remodeling.7, 8 Therefore, LSEC dysfunction could lead to impaired vasomotor control (primarily vasoconstrictive), inflammation, fibrosis, and impaired liver regeneration1, 9, all of which facilitate the development of liver cirrhosis and portal hypertension.
Decreased vasodilators
Nitric oxide (NO) is likely the most potent vasodilator molecule known today. In cirrhotic livers, NO production/bioavailability is significantly diminished, which contributes to increased intrahepatic vascular resistance.2, 9-12 At least two mechanisms explain the decreased NO production. First, the NO synthesizing enzyme endothelial NO synthase (eNOS) is inhibited by negative regulators (such as caveolin-1), which are up-regulated during cirrhosis; as a result, NO production decreases.11 Details regarding eNOS regulation in liver cirrhosis can be found elsewhere.2, 12 Second, oxidative stress is increased in cirrhosis. LSECs receive oxidative stress in response to a wide variety of agents, such as bacterial endotoxins, viruses, drugs, and ethanol.13-15 During cirrhosis, increased superoxide radicals spontaneously react with NO to form peroxynitrite (ONOO-), an endogenous toxicant16, thereby decreasing NOs bioavailability as a vasodilator.13 Antioxidant molecules such as vitamin C14, vitamin E17, superoxide dismutase (SOD)15, 18, and N-acetylcysteine19, have been shown to ameliorate intrahepatic vascular resistance and portal hypertension.
Increased vasoconstrictors
In cirrhosis, not only are vasodilators decreased, but vasoconstrictors, such as thromboxane A2 (TXA2), are also increased. TXA2 is produced by the action of COX-1 in LSECs.20 The activity of COX-1 increases in cirrhotic livers, which results in greater quantities of TXA2 and thereby increased intrahepatic vascular resistance. Inhibition of TXA2 by the prostaglandin H2/TXA2 receptor blocker, SQ-29548, or blocking COX-1 activity by the COX-1 inhibitor, SC-560, attenuates the increased intrahepatic vascular resistance.20, 21 ET-1 is another important vasoconstrictor when it binds to receptors on HSCs.22-24
2. Activated hepatic stellate cells
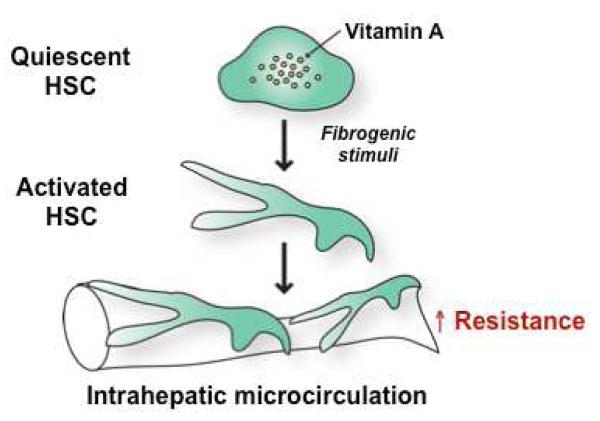
HSCs are perisinusoidal and pericyte-like cells, and reside in the space between LSECs and hepatocytes. In response to liver injury, HSCs are activated and transformed into myofibroblasts, which start to express several pro-inflammatory and fibrotic genes. Importantly, HSCs become contractile in an activated state. 22, 25-27 Increased recruitment of these activated HSCs around newly formed sinusoidal vessels increases intrahepatic vascular resistance in cirrhosis (Figure 2). 8, 28, 29 Therefore, activated HSCs play a crucial role in the development of portal hypertension due to their contractile phenotype.
Figure 2. Activated hepatic stellate cells (HSCs) in liver cirrhosis increase intrahepatic vascular resistance.
Quiescent HSCs are vitamin A storage cells and found in normal livers. In response to fibrogenic stimuli, such as transforming growth factor beta, HSCs are activated to become myofibroblasts, which exhibit a contractile and fibrogenic (collagen-producing) phenotype. These activated HSCs, located underneath liver sinusoidal endothelial cells, exert a contractile effect on the hepatic microcirculation, resulting in an increase in intrahepatic resistance.
Furthermore, activated HSCs display a decreased response to vasodilators, such as NO.30 In addition, ET-1,which is increased in cirrhosis, enhances the contractions of HSCs.25-27 Increased ET-1 production and decreased NO production in cirrhotic livers, therefore, augment intrahepatic resistance to the portal blood flow through activated HSCs, which facilitates the development of portal hypertension. However, the manipulation of ET receptors with ET receptor antagonists is complex due to their differential vasoactive effects based on their cellular locations.
3. Angiogenesis in the liver
In portal hypertension, angiogenesis plays a crucial role in intrahepatic circulation. An increased number of vessels in the fibrotic septa and the surrounding regenerative nodules has been observed in cirrhotic livers.31 Activated HSCs and/or other myofibroblasts such as portal myofibroblasts are thought to promote angiogenesis in liver cirrhosis. In fact, activated HSCs activate LSECs by releasing angiogenic factors, such as angiopoietin8, 32, 33 and vascular endothelial growth factor (VEGF).34
Irregular flow patterns, which are generated as a result of splitting (or intussusceptive) angiogenesis, may contribute to an increase in intrahepatic vascular resistance. In splitting angiogenesis, the two opposing walls of a capillary stretch and connect to each other, forming an intraluminal pillar. The junctions of the opposing endothelial cells are restructured, and the growth of the pillar is promoted. Finally, the capillary splits into two new vessels.35 It has been reported that conditional Notch 1 knockout mice develop splitting angiogenesis, nodular regenerative hyperplasia, and portal hypertension. LSECs from these knockout mice exhibit reduced endothelial fenestrae. These observations indicate that Notch 1 is necessary for LSEC fenestration, and that the absence of Notch 1 leads to pathological angiogenesis, the development of nodular regenerative hyperplasia, and portal hypertension.36
II. Extrahepatic circulation
An overview
Once portal hypertension develops, porto-systemic collateral vessels form. Blood from the digestive organs diverts into these collateral vessels, but portal blood flowing from the splanchnic circulation increases to compensate for the blood escaping into the collateral vessels. Increased portal blood flow exacerbates portal hypertension. Furthermore, arterial vasodilation in the splanchnic and systemic circulations observed in cirrhosis helps to increase the blood flow to the portal vein. Therefore, reducing the collateral vessel formation alone would not ameliorate portal hypertension. Inhibiting arterial vasodilation in the splanchnic circulation to reduce blood flow to portal vein together is important in the treatment of portal hypertension.2 This section discusses the mechanisms of collateral vessel formation and arterial vasodilation in the splanchnic and systemic circulations in cirrhosis with portal hypertension.
1. Collateral vessel formation
Porto-systemic collateral vessels develop in response to an increase in portal pressure. These collateral vessels form through the opening of pre-existing vessels or angiogenesis37, 38, and are known to cause serious complications, including variceal bleeding and hepatic encephalopathy.2, 39, 40 A change in portal pressure is thought to be detected first by the intestinal microcircular vascular bed, followed by arteries of the splanchnic circulation.41 Subsequently, these vascular beds generate various angiogenic factors, such as VEGF42-44 and placental growth factor (PlGF)45, which promote the formation of porto-systemic collaterals.
Studies in experimental models of portal hypertension and cirrhosis have shown that porto-systemic collaterals are reduced by 18 to 78% with treatment by anti-VEGFR246, a combination of anti-VEGF (rapamycin)/anti-PDGF (Gleevec)47, anti-PlGF45, apelin antagonist48, sorafenib49, 50, and a cannabinoid receptor 2 agonist.51 However, the reduction of these collaterals does not necessarily decrease portal pressure because it does not substantially change the blood flow to the portal vein. Therefore, the concomitant mitigation of arterial vasodilation is also needed to reduce portal pressure.
2. Arterial vasodilation in the splanchnic and systemic circulations
Vasodilation
NO is the most important vasodilator molecule that contributes to excessive vasodilation observed in the arterial splanchnic and systemic circulations in portal hypertension. Experimental models of portal hypertension with or without cirrhosis have shown that other vasodilator molecules, such as carbon monoxide (CO), prostacyclin (PGIs), endocannabinoids, and endothelium-derived hyperpolarizing factor (EDHF) are also induced.2, 9, 12 The identity of EDHF is currently unknown, and the candidates include arachidonic acid metabolites [epoxyeicosatrienoic acid (EET)], potassium ions (K+), components of gap junctions, or hydrogen peroxide.2
An increase in portal pressure triggers eNOS activation and subsequent NO overproduction. Changes in portal pressure are detected at different vascular beds depending on the severity of portal hypertension.41 A small increase in portal pressure is sensed first by the intestinal microcirculation and increases VEGF production with a subsequent increase in eNOS levels in the intestinal microcirculation. When portal pressure further increases and reaches a certain level, vasodilation develops in the arterial splanchnic circulation (i.e., the mesenteric arteries). It is postulated that mechanical forces including cyclic strains and shear stress, which are caused by an increased blood flow associated with an increased portal pressure, activate eNOS and lead to NO production.41, 46, 52-54 Subsequently, vasodilation develops in the arterial systemic circulation (i.e., the aorta).
Hypocontractility
Hypocontractility, decreased contractility to vasoconstrictors, is a characteristic of the arterial splanchnic and systemic circulations in portal hypertension. This phenomenon occurs largely due to the presence of excessive vasodilator molecules (i.e., NO) and the resulting excessive arterial vasodilation, but is to some degree attributable to various molecules produced in smooth muscle cells and neurons. Those molecules include endocannabinoids (vasodilators)55, 56, neuropeptide Y57, urotensin II58, 59, angiotensin60, and bradykinin61, 62 (all vasoconstrictors), with the vasodilators increased and the vasoconstrictors decreased.
Neural factors
Neural factors are postulated to be involved in the development of the hyperdynamic circulatory syndrome, especially through the sympathetic system.57, 63, 64 It is reported that sympathetic nerve atrophy/regression observed in the mesenteric arteries of portal hypertensive rats contributes to vasodilation and/or hypocontractility of those arteries.65, 66 The role of neural factors in decreased contractile responses has not yet been fully understood and is an important area to be explored.
Structural changes of arteries
The thinning of arterial walls is observed in the splanchnic and systemic circulations of rats with cirrhotic livers.67, 68 While this arterial thinning results from hemodynamic changes caused by portal hypertension, it may also sustain arterial vasodilation and worsen portal hypertension.2, 24 While NO plays a role at least in part, the molecular mechanisms responsible for arterial thinning remain to be fully elucidated.
III. Future directions
An overview
Four important areas in the study of portal hypertension that have not been sufficiently explored are specified.
1. Microflora/bacterial translocation
In recent years, an accumulating body of evidence suggests the importance of gut microflora and bacterial translocation for the pathogenesis of a variety of diseases. Due to the anatomically-close location and the connection through the vascular system, the liver is continuously exposed to microbial products from the gut.69 It has been known that bacterial translation is closely related to the development of ascites.70 In addition, small changes in portal pressure are first sensed in the intestinal microcirculation. Increased portal pressure caused by portal hypertension may influence the gut–liver axis, further advance the pathology of liver fibrosis/cirrhosis, and exacerbate portal hypertension itself. Therefore, gut microflora may have an important role in a pathological loop that develops and maintains portal hypertension. Additionally, microflora may influence cytokine/chemokine production in the liver, which may also exacerbate portal hypertension.
2. Stem cell therapy
Stem cell therapy has received considerable attention as an alternative to liver transplantation. Indeed, studies have shown that stem cell transplantation improved liver functions in cirrhotic patients71, 72 as well as experimental models of liver cirrhosis.73, 74 While stem cell therapy has shown promising effects on the amelioration of liver fibrosis and portal hypertension, more studies are still needed.
3. The lymphatic system
The lymphatic system plays a central role in ascites and edema formation.75 Further, an association between lymphangiogenesis and portal hypertension has been reported.76 However, the detailed role and mechanisms of the lymphatic system in liver cirrhosis and portal hypertension are largely unknown, and these are important areas to be explored.77, 78
4. Splenomegaly
Spleen stiffness has recently received considerable attention as an indicator of portal hypertension79 because it can be examined by non-invasive imaging systems such as transient elastrography80 and acoustic radiation force impulse imaging.79, 81 Some studies also suggest that spleen stiffness could predict the presence of varices79-81 or ascites.82 An experimental model of cirrhosis with portal hypertension has demonstrated that portal pressure positively correlated with the spleen size.42
In addition, a study using rats with partial portal vein ligation (PVL) showed that fibrosis and angiogenesis in the spleen was accompanied with splenomegaly induced by PVL, and that administration of rapamycin, an immunosuppressive agent, reduced splenomegaly as well as fibrosis and angiogenesis in the spleen.83 Currently, the detailed mechanisms of how portal pressure induces splenomegaly remain to be fully elucidated.
Summary/Conclusion
With our knowledge of vascular biology, our understanding of the pathogenesis of portal hypertension has significantly advanced, revealing how vascular abnormalities both inside and outside the liver contribute to portal hypertension.84 To ameliorate portal hypertension, first and foremost, a decrease in intrahepatic vascular resistance in cirrhotic liver is needed. Therefore, an increased production of vasodilator molecules in LSECs and a decrease in HSC contraction are important. For example, induction of apoptosis of enhanced activated HSCs85, 86, thereby decreasing contractile HSCs, could be a useful therapeutic strategy to decrease portal pressure.
Key Points.
- The primary cause of portal hypertension in liver cirrhosis is increased intrahepatic vascular resistance.
- A reduction of intrahepatic vascular resistance could ameliorate portal hypertension.
- Arterial vasodilation in the splanchnic and systemic circulations worsens portal hypertension.
Acknowledgement
This work was supported by grant R01DK082600 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure The author has nothing to disclose.
References
- 1.Bosch J. Vascular deterioration in cirrhosis: the big picture. J Clin Gastroenterol. 2007;41(Suppl 3):S247–53. doi: 10.1097/MCG.0b013e3181572357. [DOI] [PubMed] [Google Scholar]
- 2.Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43:S121–31. doi: 10.1002/hep.20993. [DOI] [PubMed] [Google Scholar]
- 3.Rockey DC. Cell and molecular mechanisms of increased intrahepatic resistance and hemodynamic correlates. Humana Press Inc; Totowa, NJ: 2005. [Google Scholar]
- 4.Pinzani M, Vizzutti F. Anatomy and vascular biology of the cells in the portal circulation. Humana Press Inc; Totowa, NJ: 2005. [Google Scholar]
- 5.Wiest R, Groszmann RJ. The paradox of nitric oxide in cirrhosis and portal hypertension: too much, not enough. Hepatology. 2002;35:478–91. doi: 10.1053/jhep.2002.31432. [DOI] [PubMed] [Google Scholar]
- 6.Ding BS, Nolan DJ, Butler JM, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–5. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jagavelu K, Routray C, Shergill U, et al. Endothelial cell toll-like receptor 4 regulates fibrosis-associated angiogenesis in the liver. Hepatology. 2010;52:590–601. doi: 10.1002/hep.23739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J Hepatol. 2010;53:976–80. doi: 10.1016/j.jhep.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46:927–34. doi: 10.1016/j.jhep.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Shah V, Haddad FG, Garcia-Cardena G, et al. Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. Journal of Clinical Investigation. 1997;100:2923–30. doi: 10.1172/JCI119842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah V, Toruner M, Haddad F, et al. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology. 1999;117:1222–8. doi: 10.1016/s0016-5085(99)70408-7. [DOI] [PubMed] [Google Scholar]
- 12.Iwakiri Y. The molecules: mechanisms of arterial vasodilatation observed in the splanchnic and systemic circulation in portal hypertension. J Clin Gastroenterol. 2007;41:S288–94. doi: 10.1097/MCG.0b013e3181468b4c. [DOI] [PubMed] [Google Scholar]
- 13.Gracia-Sancho J, Lavina B, Rodriguez-Vilarrupla A, et al. Increased oxidative stress in cirrhotic rat livers: A potential mechanism contributing to reduced nitric oxide bioavailability. Hepatology. 2008;47:1248–56. doi: 10.1002/hep.22166. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez-Guerra M, Garcia-Pagan JC, Turnes J, et al. Ascorbic acid improves the intrahepatic endothelial dysfunction of patients with cirrhosis and portal hypertension. Hepatology. 2006;43:485–91. doi: 10.1002/hep.21080. [DOI] [PubMed] [Google Scholar]
- 15.Lavina B, Gracia-Sancho J, Rodriguez-Vilarrupla A, et al. Superoxide dismutase gene transfer reduces portal pressure in CCl4 cirrhotic rats with portal hypertension. Gut. 2009;58:118–25. doi: 10.1136/gut.2008.149880. [DOI] [PubMed] [Google Scholar]
- 16.Radi R. Peroxynitrite, a stealthy biological oxidant. J Biol Chem. 2013;288:26464–72. doi: 10.1074/jbc.R113.472936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang YY, Lee TY, Huang YT, et al. Asymmetric dimethylarginine (ADMA) determines the improvement of hepatic endothelial dysfunction by vitamin E in cirrhotic rats. Liver Int. 2012;32:48–57. doi: 10.1111/j.1478-3231.2011.02651.x. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Caldero H, Rodriguez-Vilarrupla A, Gracia-Sancho J, et al. Tempol administration, a superoxide dismutase mimetic, reduces hepatic vascular resistance and portal pressure in cirrhotic rats. J Hepatol. 2011;54:660–5. doi: 10.1016/j.jhep.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 19.Yang YY, Lee KC, Huang YT, et al. Effects of N-acetylcysteine administration in hepatic microcirculation of rats with biliary cirrhosis. J Hepatol. 2008;49:25–33. doi: 10.1016/j.jhep.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Gracia-Sancho J, Lavina B, Rodriguez-Vilarrupla A, et al. Enhanced vasoconstrictor prostanoid production by sinusoidal endothelial cells increases portal perfusion pressure in cirrhotic rat livers. J Hepatol. 2007;47:220–7. doi: 10.1016/j.jhep.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Graupera M, March S, Engel P, et al. Sinusoidal endothelial COX-1-derived prostanoids modulate the hepatic vascular tone of cirrhotic rat livers. Am J Physiol Gastrointest Liver Physiol. 2005;288:G763–70. doi: 10.1152/ajpgi.00300.2004. [DOI] [PubMed] [Google Scholar]
- 22.Kawada N, Tran-Thi TA, Klein H, et al. The contraction of hepatic stellate (Ito) cells stimulated with vasoactive substances. Possible involvement of endothelin 1 and nitric oxide in the regulation of the sinusoidal tonus. Eur J Biochem. 1993;213:815–23. doi: 10.1111/j.1432-1033.1993.tb17824.x. [DOI] [PubMed] [Google Scholar]
- 23.Bauer M, Bauer I, Sonin NV, et al. Functional significance of endothelin B receptors in mediating sinusoidal and extrasinusoidal effects of endothelins in the intact rat liver. Hepatology. 2000;31:937–47. doi: 10.1053/he.2000.5922. [DOI] [PubMed] [Google Scholar]
- 24.Iwakiri Y. Endothelial dysfunction in the regulation of cirrhosis and portal hypertension. Liver Int. 2012;32:199–213. doi: 10.1111/j.1478-3231.2011.02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawada N, Seki S, Kuroki T, et al. ROCK inhibitor Y-27632 attenuates stellate cell contraction and portal pressure increase induced by endothelin-1. Biochem Biophys Res Commun. 1999;266:296–300. doi: 10.1006/bbrc.1999.1823. [DOI] [PubMed] [Google Scholar]
- 26.Pinzani M, Milani S, De Franco R, et al. Endothelin 1 is overexpressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology. 1996;110:534–48. doi: 10.1053/gast.1996.v110.pm8566602. [DOI] [PubMed] [Google Scholar]
- 27.Rockey DC, Weisiger RA. Endothelin induced contractility of stellate cells from normal and cirrhotic rat liver: implications for regulation of portal pressure and resistance. Hepatology. 1996;24:233–40. doi: 10.1002/hep.510240137. [DOI] [PubMed] [Google Scholar]
- 28.Medina J, Arroyo AG, Sanchez-Madrid F, et al. Angiogenesis in chronic inflammatory liver disease. Hepatology. 2004;39:1185–95. doi: 10.1002/hep.20193. [DOI] [PubMed] [Google Scholar]
- 29.Kim MY, Baik SK, Lee SS. Hemodynamic alterations in cirrhosis and portal hypertension. Korean J Hepatol. 2010;16:347–52. doi: 10.3350/kjhep.2010.16.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perri RE, Langer DA, Chatterjee S, et al. Defects in cGMP-PKG pathway contribute to impaired NO-dependent responses in hepatic stellate cells upon activation. Am J Physiol Gastrointest Liver Physiol. 2006;290:G535–42. doi: 10.1152/ajpgi.00297.2005. [DOI] [PubMed] [Google Scholar]
- 31.Rappaport AM, MacPhee PJ, Fisher MM, et al. The scarring of the liver acini (Cirrhosis) Tridimensional and microcirculatory considerations. Virchows Arch A Pathol Anat Histopathol. 1983;402:107–37. doi: 10.1007/BF00695054. [DOI] [PubMed] [Google Scholar]
- 32.Taura K, De Minicis S, Seki E, et al. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008;135:1729–38. doi: 10.1053/j.gastro.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 33.Thabut D, Routray C, Lomberk G, et al. Complementary vascular and matrix regulatory pathways underlie the beneficial mechanism of action of sorafenib in liver fibrosis. Hepatology. 2011;54:573–85. doi: 10.1002/hep.24427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novo E, Cannito S, Zamara E, et al. Proangiogenic cytokines as hypoxia-dependent factors stimulating migration of human hepatic stellate cells. Am J Pathol. 2007;170:1942–53. doi: 10.2353/ajpath.2007.060887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagy JA, Morgan ES, Herzberg KT, et al. Pathogenesis of ascites tumor growth: angiogenesis, vascular remodeling, and stroma formation in the peritoneal lining. Cancer Res. 1995;55:376–85. [PubMed] [Google Scholar]
- 36.Dill MT, Rothweiler S, Djonov V, et al. Disruption of Notch1 induces vascular remodeling, intussusceptive angiogenesis, and angiosarcomas in livers of mice. Gastroenterology. 2012;142:967–977. e2. doi: 10.1053/j.gastro.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 37.Sumanovski LT, Battegay E, Stumm M, et al. Increased angiogenesis in portal hypertensive rats: role of nitric oxide. Hepatology. 1999;29:1044–9. doi: 10.1002/hep.510290436. [DOI] [PubMed] [Google Scholar]
- 38.Sieber CC, Sumanovski LT, Stumm M, et al. In vivo angiogenesis in normal and portal hypertensive rats: role of basic fibroblast growth factor and nitric oxide. J Hepatol. 2001;34:644–50. doi: 10.1016/s0168-8278(00)00064-7. [DOI] [PubMed] [Google Scholar]
- 39.Groszmann RJ, Kotelanski B, Cohn JN. Different patterns of porta-systemic shunting in cirrhosis of the liver studied by an indicator dilution technique. Acta Gastroenterol Latinoam. 1971;3:111–6. [PubMed] [Google Scholar]
- 40.Bosch J, Pizcueta P, Feu F, et al. Pathophysiology of portal hypertension. Gastroenterol Clin North Am. 1992;21:1–14. [PubMed] [Google Scholar]
- 41.Abraldes JG, Iwakiri Y, Loureiro-Silva M, et al. Mild increases in portal pressure upregulate vascular endothelial growth factor and endothelial nitric oxide synthase in the intestinal microcirculatory bed, leading to a hyperdynamic state. Am J Physiol Gastrointest Liver Physiol. 2006;290:G980–7. doi: 10.1152/ajpgi.00336.2005. [DOI] [PubMed] [Google Scholar]
- 42.Huang HC, Haq O, Utsumi T, et al. Intestinal and plasma VEGF levels in cirrhosis: the role of portal pressure. J Cell Mol Med. 2012;16:1125–33. doi: 10.1111/j.1582-4934.2011.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandez M, Vizzutti F, Garcia-Pagan JC, et al. Anti-VEGF receptor-2 monoclonal antibody prevents portal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology. 2004;126:886–94. doi: 10.1053/j.gastro.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Geerts AM, De Vriese AS, Vanheule E, et al. Increased angiogenesis and permeability in the mesenteric microvasculature of rats with cirrhosis and portal hypertension: an in vivo study. Liver Int. 2006;26:889–98. doi: 10.1111/j.1478-3231.2006.01308.x. [DOI] [PubMed] [Google Scholar]
- 45.Van Steenkiste C, Geerts A, Vanheule E, et al. Role of placental growth factor in mesenteric neoangiogenesis in a mouse model of portal hypertension. Gastroenterology. 2009;137:2112–24. e1–6. doi: 10.1053/j.gastro.2009.08.068. [DOI] [PubMed] [Google Scholar]
- 46.Fernandez M, Mejias M, Angermayr B, et al. Inhibition of VEGF receptor-2 decreases the development of hyperdynamic splanchnic circulation and portal-systemic collateral vessels in portal hypertensive rats. J Hepatol. 2005;43:98–103. doi: 10.1016/j.jhep.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez M, Mejias M, Garcia-Pras E, et al. Reversal of portal hypertension and hyperdynamic splanchnic circulation by combined vascular endothelial growth factor and platelet-derived growth factor blockade in rats. Hepatology. 2007;46:1208–17. doi: 10.1002/hep.21785. [DOI] [PubMed] [Google Scholar]
- 48.Tiani C, Garcia-Pras E, Mejias M, et al. Apelin signaling modulates splanchnic angiogenesis and portosystemic collateral vessel formation in rats with portal hypertension. J Hepatol. 2009;50:296–305. doi: 10.1016/j.jhep.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 49.Mejias M, Garcia-Pras E, Tiani C, et al. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49:1245–56. doi: 10.1002/hep.22758. [DOI] [PubMed] [Google Scholar]
- 50.Reiberger T, Angermayr B, Schwabl P, et al. Sorafenib attenuates the portal hypertensive syndrome in partial portal vein ligated rats. J Hepatol. 2009;51:865–73. doi: 10.1016/j.jhep.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 51.Huang HC, Wang SS, Hsin IF, et al. Cannabinoid receptor 2 agonist ameliorates mesenteric angiogenesis and portosystemic collaterals in cirrhotic rats. Hepatology. 2012;56:248–58. doi: 10.1002/hep.25625. [DOI] [PubMed] [Google Scholar]
- 52.Iwakiri Y. The Systemic and Splanchnic Circulation. In: Gines P, Kamath PS, Arroyo V, editors. Chronic Liver Failure, Mechanisms and Management. Humana Press; New York, NY: 2011. [Google Scholar]
- 53.Tsai MH, Iwakiri Y, Cadelina G, et al. Mesenteric vasoconstriction triggers nitric oxide overproduction in the superior mesenteric artery of portal hypertensive rats. Gastroenterology. 2003;125:1452–61. doi: 10.1016/j.gastro.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 54.Iwakiri Y, Tsai MH, McCabe TJ, et al. Phosphorylation of eNOS initiates excessive NO production in early phases of portal hypertension. Am J Physiol Heart Circ Physiol. 2002;282:H2084–90. doi: 10.1152/ajpheart.00675.2001. [DOI] [PubMed] [Google Scholar]
- 55.Moezi L, Gaskari SA, Liu H, et al. Anandamide mediates hyperdynamic circulation in cirrhotic rats via CB(1) and VR(1) receptors. Br J Pharmacol. 2006;149:898–908. doi: 10.1038/sj.bjp.0706928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Batkai S, Jarai Z, Wagner JA, et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med. 2001;7:827–32. doi: 10.1038/89953. [DOI] [PubMed] [Google Scholar]
- 57.Moleda L, Trebicka J, Dietrich P, et al. Amelioration of portal hypertension and the hyperdynamic circulatory syndrome in cirrhotic rats by neuropeptide Y via pronounced splanchnic vasoaction. Gut. 2011 doi: 10.1136/gut.2010.226407. [DOI] [PubMed] [Google Scholar]
- 58.Trebicka J, Leifeld L, Hennenberg M, et al. Hemodynamic effects of urotensin II and its specific receptor antagonist palosuran in cirrhotic rats. Hepatology. 2008;47:1264–76. doi: 10.1002/hep.22170. [DOI] [PubMed] [Google Scholar]
- 59.Kemp W, Krum H, Colman J, et al. Urotensin II: a novel vasoactive mediator linked to chronic liver disease and portal hypertension. Liver Int. 2007;27:1232–9. doi: 10.1111/j.1478-3231.2007.01539.x. [DOI] [PubMed] [Google Scholar]
- 60.Hennenberg M, Trebicka J, Kohistani AZ, et al. Vascular hyporesponsiveness to angiotensin II in rats with CCl(4)-induced liver cirrhosis. Eur J Clin Invest. 2009;39:906–13. doi: 10.1111/j.1365-2362.2009.02181.x. [DOI] [PubMed] [Google Scholar]
- 61.Chu CJ, Wu SL, Lee FY, et al. Splanchnic hyposensitivity to glypressin in a haemorrhage/transfused rat model of portal hypertension: role of nitric oxide and bradykinin. Clin Sci (Lond) 2000;99:475–82. [PubMed] [Google Scholar]
- 62.Chen CT, Chu CJ, Lee FY, et al. Splanchnic hyposensitivity to glypressin in a hemorrhage-transfused common bile duct-ligated rat model of portal hypertension: role of nitric oxide and bradykinin. Hepatogastroenterology. 2009;56:1261–7. [PubMed] [Google Scholar]
- 63.Heinemann A, Wachter CH, Fickert P, et al. Vasopressin reverses mesenteric hyperemia and vasoconstrictor hyporesponsiveness in anesthetized portal hypertensive rats. Hepatology. 1998;28:646–54. doi: 10.1002/hep.510280307. [DOI] [PubMed] [Google Scholar]
- 64.Song D, Liu H, Sharkey KA, et al. Hyperdynamic circulation in portal-hypertensive rats is dependent on central c-fos gene expression. Hepatology. 2002;35:159–66. doi: 10.1053/jhep.2002.30417. [DOI] [PubMed] [Google Scholar]
- 65.Coll M, Martell M, Raurell I, et al. Atrophy of mesenteric sympathetic innervation may contribute to splanchnic vasodilation in rat portal hypertension. Liver Int. 2010;30:593–602. doi: 10.1111/j.1478-3231.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- 66.Ezkurdia N, Coll M, Raurell I, et al. Blockage of the afferent sensitive pathway prevents sympathetic atrophy and hemodynamic alterations in rat portal hypertension. Liver Int. 2012;32:1295–305. doi: 10.1111/j.1478-3231.2012.02762.x. [DOI] [PubMed] [Google Scholar]
- 67.Fernandez-Varo G, Ros J, Morales-Ruiz M, et al. Nitric oxide synthase 3-dependent vascular remodeling and circulatory dysfunction in cirrhosis. Am J Pathol. 2003;162:1985–93. doi: 10.1016/S0002-9440(10)64331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fernandez-Varo G, Morales-Ruiz M, Ros J, et al. Impaired extracellular matrix degradation in aortic vessels of cirrhotic rats. J Hepatol. 2007;46:440–6. doi: 10.1016/j.jhep.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 69.Seo YS, Shah VH. The role of gut-liver axis in the pathogenesis of liver cirrhosis and portal hypertension. Clin Mol Hepatol. 2012;18:337–46. doi: 10.3350/cmh.2012.18.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frances R, Chiva M, Sanchez E, et al. Bacterial translocation is downregulated by anti-TNF-alpha monoclonal antibody administration in rats with cirrhosis and ascites. J Hepatol. 2007;46:797–803. doi: 10.1016/j.jhep.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 71.Terai S, Ishikawa T, Omori K, et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292–8. doi: 10.1634/stemcells.2005-0542. [DOI] [PubMed] [Google Scholar]
- 72.Jang YO, Kim YJ, Baik SK, et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int. 2013 doi: 10.1111/liv.12218. [DOI] [PubMed] [Google Scholar]
- 73.Sakamoto M, Nakamura T, Torimura T, et al. Transplantation of endothelial progenitor cells ameliorates vascular dysfunction and portal hypertension in carbon tetrachloride-induced rat liver cirrhotic model. J Gastroenterol Hepatol. 2013;28:168–78. doi: 10.1111/j.1440-1746.2012.07238.x. [DOI] [PubMed] [Google Scholar]
- 74.Sakaida I, Terai S, Yamamoto N, et al. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304–11. doi: 10.1002/hep.20452. [DOI] [PubMed] [Google Scholar]
- 75.Cardenas A, Bataller R, Arroyo V. Mechanisms of ascites formation. Clin Liver Dis. 2000;4:447–65. doi: 10.1016/s1089-3261(05)70118-5. [DOI] [PubMed] [Google Scholar]
- 76.Barrowman JA, Granger DN. Effects of experimental cirrhosis on splanchnic microvascular fluid and solute exchange in the rat. Gastroenterology. 1984;87:165–72. [PubMed] [Google Scholar]
- 77.Chung C, Iwakiri Y. The lymphatic vascular system in liver diseases: its role in ascites formation. Clin Mol Hepatol. 2013;19:99–104. doi: 10.3350/cmh.2013.19.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ribera J, Pauta M, Melgar-Lesmes P, et al. Increased nitric oxide production in lymphatic endothelial cells causes impairment of lymphatic drainage in cirrhotic rats. Gut. 2013;62:138–45. doi: 10.1136/gutjnl-2011-300703. [DOI] [PubMed] [Google Scholar]
- 79.Takuma Y, Nouso K, Morimoto Y, et al. Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology. 2013;144:92–101. e2. doi: 10.1053/j.gastro.2012.09.049. [DOI] [PubMed] [Google Scholar]
- 80.Colecchia A, Montrone L, Scaioli E, et al. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology. 2012;143:646–54. doi: 10.1053/j.gastro.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 81.Berzigotti A, Seijo S, Arena U, et al. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144:102–111. e1. doi: 10.1053/j.gastro.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 82.Mori K, Arai H, Abe T, et al. Spleen stiffness correlates with the presence of ascites but not esophageal varices in chronic hepatitis C patients. Biomed Res Int. 2013;2013:857862. doi: 10.1155/2013/857862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mejias M, Garcia-Pras E, Gallego J, et al. Relevance of the mTOR signaling pathway in the pathophysiology of splenomegaly in rats with chronic portal hypertension. J Hepatol. 2010;52:529–39. doi: 10.1016/j.jhep.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 84.Iwakiri Y, Grisham M, Shah V. Vascular biology and pathobiology of the liver: Report of a single-topic symposium. Hepatology. 2008;47:1754–63. doi: 10.1002/hep.22203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tashiro K, Satoh A, Utsumi T, et al. Absence of Nogo-B (reticulon 4B) facilitates hepatic stellate cell apoptosis and diminishes hepatic fibrosis in mice. Am J Pathol. 2013;182:786–95. doi: 10.1016/j.ajpath.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang D, Utsumi T, Huang HC, et al. Reticulon 4B (Nogo-B) is a novel regulator of hepatic fibrosis. Hepatology. 2011;53:1306–15. doi: 10.1002/hep.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]