Chimpanzees empathize with group mates and humans, but not with baboons or unfamiliar chimpanzees (original) (raw)
Abstract
Human empathy can extend to strangers and even other species, but it is unknown whether non-humans are similarly broad in their empathic responses. We explored the breadth and flexibility of empathy in chimpanzees, a close relative of humans. We used contagious yawning to measure involuntary empathy and showed chimpanzees videos of familiar humans, unfamiliar humans and gelada baboons (an unfamiliar species). We tested whether each class of stimuli elicited contagion by comparing the effect of yawn and control videos. After including previous data on the response to ingroup and outgroup chimpanzees, we found that familiar and unfamiliar humans elicited contagion equal to that of ingroup chimpanzees. Gelada baboons did not elicit contagion, and the response to them was equal to that of outgroup chimpanzees. However, the chimpanzees watched the outgroup chimpanzee videos more than any other. The combination of high interest and low contagion may stem from hostility towards unfamiliar chimpanzees, which may interfere with an empathic response. Overall, chimpanzees showed flexibility in that they formed an empathic connection with a different species, including unknown members of that species. These results imply that human empathic flexibility is shared with related species.
Keywords: chimpanzees, empathy, contagious yawning, empathic flexibility
1. Introduction
The concept of empathy is increasingly applied to explain animal sensitivity to the emotional states of others. Without necessarily implying the cognitively advanced forms found in human adults (e.g. theory of mind), it takes as its basis bodily connections and involuntary mimicry, also known as the perception–action core of empathic processing [1]. There are now studies of empathy in mammals, from mice (Mus musculus) [2], rats (Rattus norvegicus) [3] and dogs (Canis lupus familiaris) [4] to elephants (Loxodonta africana) [5], and also in birds [6,7]. One common behavioural measure is contagious yawning (CY), which appears to fit the empathy framework because of four key findings: (i) human adults high on other measures of empathy show more CY [8]; (ii) humans with developmental and personality disorders in which empathy is impaired show diminished CY [8–11]; (iii) CY is positively biased by familiarity in humans (Homo sapiens) [12], chimpanzees (Pan troglodytes) [13], bonobos (Pan paniscus) [14], gelada baboons (Theropithecus gelada) [15] and dogs [16–18], as is typical of other measures of empathy; and (iv) presented with a variety of body movements apes exclusively increase yawning in response to observed yawning, suggesting CY's high specificity [19,20]. Aiding this specificity, brain areas associated with the human mirror neuron system activate in humans viewing yawns [21–23], with mirror neurons having been implied as a proximate neural mechanism for empathy [24,25]. Thus, CY fits better with an empathy framework than with explanations in terms of imitation or behavioural facilitation.
Human empathic functioning, although biased towards similar and familiar individuals, is flexible enough to include empathy for strangers and even other species [26–28]. Is an empathy response flexible enough to include strangers uniquely human, related to our well-developed capacity to cooperate with outsiders [29]? Empirical studies with non-humans show both the importance of familiarity in forming empathic connections and potential for moving beyond it. Mice showed heightened pain responses after viewing cage-mates in pain, but not after viewing strangers in pain [2]. Chimpanzees made a similar distinction, showing CY in response to familiar individuals but not unfamiliar individuals [13]. Rats, however, would help unfamiliar individuals, but only if the strain of rat was familiar [30]. Domestic dogs show empathy-related responses to unfamiliar humans [4,17,31] (although there are conflicting results with CY [32,33]), but with this species it is unclear whether this ability stems from natural or artificial selection. Young orphaned chimpanzees showed a CY response to an unfamiliar human [20], but positive interactions between the two may have influenced the response. A similar population of chimpanzees also showed helping behaviour towards an unfamiliar human [34]. When combined with anecdotes of inter-species helping behaviour [35], a pattern emerges that non-humans may indeed share some of the human's empathic flexibility.
To explore the origins of flexible empathy in humans, we studied the responses of one of our closest living relatives, the chimpanzee, in which CY is well established [13,19,20,36–38]. Chimpanzees live in fission–fusion communities, which often compete [39,40]. Female migration at sexual maturity is the only movement of individuals between groups. In chimpanzee society, all known individuals are members of the community, and unknown individuals belong per definition to a different community. Using the contagiousness of yawning as a measure of involuntary body synchronization and empathy, we previously showed an ingroup–outgroup bias: chimpanzees were affected by the yawns of known individuals, but not unknown individuals of their species [13]. However, having an existing positive relationship with an individual is not a prerequisite for contagion, as chimpanzees have also shown CY in response to computer-generated animations [37].
Is motor mirroring in chimpanzees flexible enough to induce yawns in response to species different from themselves? And would chimpanzees distinguish between known and unknown individuals from other species? Captive-reared chimpanzees interact daily with humans, so we wanted to know whether chimpanzees would express an empathic connection with humans in CY. However, chimpanzees may respond to known and unknown humans differently. The research and animal care staff at the Yerkes National Primate Research Center Field Station use positive reinforcement when working with the chimpanzees. Hence, the chimpanzees have an established history of positive interactions with these specific individuals. Potentially, known humans may be categorized as something approaching an ingroup and unknown humans as something approaching an outgroup, with the latter potentially limiting the strength of a contagious response.
To control for species familiarity, we also showed chimpanzees yawns from gelada baboons, a species they have never seen before. Videos of gelada baboons yawning were available from a previous study by Palagi et al. [15]. Comparing the response to humans and gelada baboons allowed us to test whether a familiar, meaningful species is necessary for cross-species contagion, or whether cross-species contagion could be elicited via similarities in motor muscle activation alone.
We employed the same experimental methods as in our previous study [13], which allowed us to compare the results directly as a gauge of how chimpanzees view familiar humans, unfamiliar humans and gelada baboons compared with their own species. This way, we could ask chimpanzees about how our species fits into their social world.
2. Material and methods
The subjects were 19 adult chimpanzees (P. troglodytes; age 13–48; 1 male, 18 females) living at the Field Station of the Yerkes National Primate Research Center (Atlanta, GA). The chimpanzees were housed in two separate social groups in large outdoor enclosures (group 1: 711 m2; group 2: 528 m2) with indoor sleeping quarters. The chimpanzees were tested in their sleeping quarters or a testing building (available to group 1 only), and saw only one stimulus per day between 10.30 and 13.30, a time window that appears narrow enough to avoid circadian fluctuations in rates of yawning or contagion [41–43].
The new stimuli consisted of three classes of yawn and control videos: familiar humans, unfamiliar humans and gelada baboons (T. gelada). The familiar humans consisted of researchers or husbandry staff who had worked with the chimpanzees for at least 1 year prior to the start of the experiment. The unfamiliar humans comprised individuals who had never been to the Yerkes Field Station before. Gelada baboons were housed at the NaturZoo (Rheine, Germany; see [15] for husbandry details).
For the human videos, we dressed all individuals in a white shirt and placed them in front of a neutral-coloured wall, to minimize potential distracters. The only difference between each video was the identity of the person. The volunteers acted out several yawns, with several seconds between each one. We recorded yawns from seven individuals in each class (familiar to the chimpanzees and unfamiliar) with a PV-GS500 (Panasonic) digital video camera. For the gelada baboons, we reviewed the videos recorded by Palagi et al. [15] and selected yawns from seven individuals. We edited each yawn clip to 9 s using iMovie (Apple). From the same videos of humans and gelada baboons, we selected 9 s control segments from each of the same individuals at rest, performing no expression. By using the same videos, the control clips are virtually identical to the yawn clips, except for the expression itself. We used this control previously [13], and the effect sizes were even larger than when we used open mouth movements as a control [37]. Massen et al. [38] also used this control and found a significant difference between yawn and control conditions. As no one has explicitly tested the performance of different controls against each other [44], we do not know whether any one control is better at eliciting baseline levels of yawning than any other (see [44] for a more in-depth discussion of controls used for studying CY). We inserted 1 s of green screen between each clip, and assembled them into a yawn video and a control for each class (yielding a total of six videos). Each clip was shown once before repeating the entire set, and the order within a set was randomized except that a clip could not be shown twice in a row.
The procedure was identical to [13]. We used an iPod Touch (Apple; screen: 7.5 × 5 cm) to play the videos. We presented the iPod on its own to a single subject when other individuals could not also see the video. When more than one chimpanzee could see the screen, we placed the iPod at one end of an opaque container with an eyehole at the opposite end. The eyehole allowed only one chimpanzee to look through it at a time. Chimpanzees were tested by themselves, in small groups or with the entire group, based upon their comfort. Some individuals were comfortable being alone and were tested that way. Most individuals were more comfortable in the group, but even then the combination of the iPod and the container with the eyehole ensured individual testing.
Test sessions lasted for 10 min from the time at which the chimpanzee first looked at the video. The chimpanzee could continue to watch or not as it chose; however, the entire 10 min session was after observing the video and thus reflects the influence of the video. Each chimpanzee had one session with each video and saw only one video per day. The order of all six videos was counter-balanced (as closely as possible due to the odd number of subjects), with the chimpanzees watching one video from each class before repeating with the other expression.
The Panasonic PV-GS500 recorded each session. We counted the number of yawns by the subject after the start of the session and coded the time spent watching the video in seconds. Previously, we ran 20 min sessions [13]. To incorporate these results into the analysis, we considered only the first 10 min of each condition. We analysed the results using SPSS Statistics v. 20.0 for Macintosh (IBM) based on our previous rationale [44]. Looking at all of our data on yawn contagion [13,37] (this study), we see an approximately normal distribution and the presence of an outlier. The outlier is a consistently high performer in our studies of CY, so we do not believe that these data are anomalous and need to be discarded. As the rest of the distribution (without this individual) is normal (Shapiro–Wilk, p = 0.128), we used parametric statistics. These data are also located in the electronic supplementary material table. The primary analysis consisted of planned comparisons of theoretical importance between responses to the yawn and control videos (paired-sample _t_-tests, unless indicated). All statistics were two-tailed with an alpha of 0.05.
3. Results
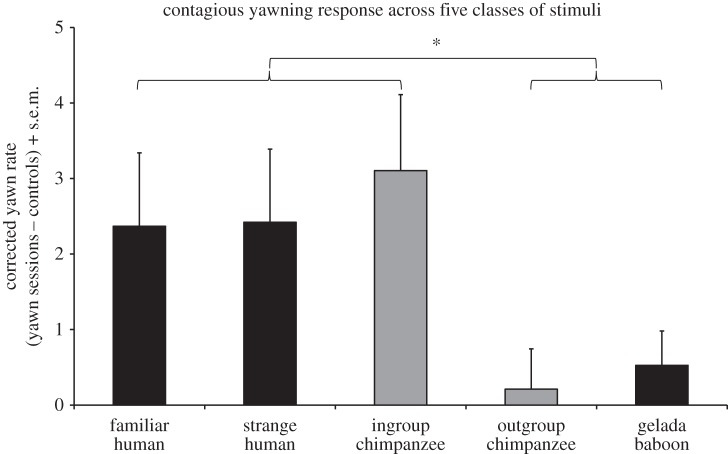
Chimpanzees yawned more while watching familiar human yawn videos than the controls (_t_18 = 2.45, p = 0.025, d = 0.81) and also more while watching the unfamiliar human yawn videos than the controls (_t_18 = 2.50, p = 0.022, d = 0.81). The difference in response to the gelada baboon yawn and control videos was non-significant, however (_t_18 = 1.16, p = 0.26, d = 0.28, figure 1). Incorporating previous data on the response to ingroup versus outgroup chimpanzees [13] allows us to compare yawning rates across the five classes of video. We first calculated a yawning index for each individual within each class by subtracting the rate in the control sessions from the rate in the yawning sessions (figure 2). Thus, the yawning rate in each class is corrected for its control, and an index was calculated for each individual before computing the means. Based on figure 2, we hypothesized that there are two distinct response types: one indicative of contagion (including familiar human, unfamiliar human and ingroup chimpanzee) and one indicative of a lack of contagion (including outgroup chimpanzee and gelada baboon).
Figure 1.
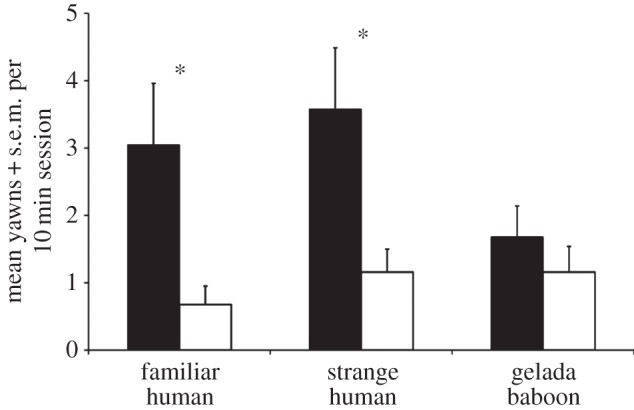
Mean rate of yawning in each session by class and condition. The chimpanzees yawned significantly more when watching the yawn (black bars) than the control (white bars) videos for familiar humans (p = 0.025) and strange humans (p = 0.022), but the difference was non-significant for gelada baboons.
Figure 2.
We calculated a yawning index for each individual by subtracting the number of yawns in the control sessions from the number of yawns in the yawn sessions for each class. The graph presents the mean differences + s.e.m. Data for ingroup and outgroup chimpanzees come from our earlier study [13] (grey bars). Previously, we studied 23 chimpanzees in 20 min sessions [13], and in this study (black bars) we worked with 19 chimpanzees in 10 min sessions. The data for [13] have been sampled and restricted to match the current parameters (the same 19 chimpanzees for 10 min), thus a side-by-side comparison with the graphs from [13] will not match. The response to familiar humans, strange humans and ingroup chimpanzees was significantly greater than the response to outgroup chimpanzees and gelada baboons (p = 0.003).
To test this post hoc hypothesis, we conducted a _k_-means cluster analysis (10 iterations maximum) on all five means in which we selected for two clusters. The analysis returned final cluster centres of 2.63 and 0.37, with no change after the first iteration. The analysis assigned familiar human, unfamiliar human and ingroup chimpanzee to the first cluster (centre = 2.63), and outgroup chimpanzee and gelada baboon to the second cluster (centre = 0.37). We then calculated a mean yawn index response for each individual for cluster 1 (i.e. mean of familiar human, unfamiliar human and ingroup chimpanzee) and cluster 2 (i.e. mean of outgroup chimpanzee and gelada baboon). We compared these means using a paired-sample _t_-test and found a significant difference (_t_18 = 3.41, p = 0.003, d = 0.97).
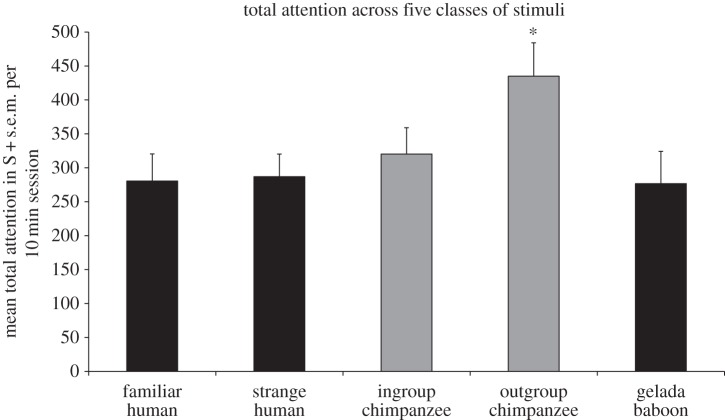
There were no differences in the amount of attention paid to the yawn and control videos within each class (familiar human: _t_18 = 0.23, p = 0.83; unfamiliar human: _t_18 = 1.81, p = 0.087, d = 0.60; gelada baboon: _t_18 = 0.27, p = 0.79). To compare the attention paid to the three classes, we summed the attention to the yawn and control conditions for each class, as these did not differ, and conducted a one-way ANOVA comparing the total attention paid to the familiar humans, unfamiliar humans and geladas (_f_2,54 = 0.81, p = 0.45). We next incorporated our earlier data on the total attention paid to the ingroup chimpanzee and outgroup chimpanzee videos. The one-way ANOVA comparing all five classes showed significant variation (_f_4,90 = 2.49, p = 0.049). Post hoc comparisons using Fisher's LSD showed that our subjects spent significantly more time watching the outgroup chimpanzee video than the familiar human (p = 0.011), unfamiliar human (p = 0.015) or gelada (p = 0.009) videos, and there was a nearly significant trend towards watching the outgroup chimpanzee video more than the ingroup (p = 0.058; figure 3). There were no correlations between the amount of time spent watching any of the yawn videos and the number of yawns observed (familiar human: Pearson's r = −0.10, p = 0.68; unfamiliar human: Pearson's r = 0.43, p = 0.07; gelada baboon: Pearson's r = −0.20, p = 0.40).
Figure 3.
The chimpanzees spent significantly more time watching the videos of outgroup chimpanzees than each of the other classes (_f_4,90 = 2.49, p = 0.049 with Fisher's LSD), but all other comparisons were non-significant. The bars show total attention (yawn + control), as there were no significant differences between these two conditions. The data for ingroup and outgroup chimpanzees come from our earlier study [13] (grey bars). Previously, we studied 23 chimpanzees in 20 min sessions [13], and in this study (black bars) we worked with 19 chimpanzees in 10 min sessions. The data for [13] have been sampled and restricted to match the current parameters (the same 19 chimpanzees for 10 min), thus a side-by-side comparison with the graphs from [13] will not match.
4. Discussion
The chimpanzees yawned significantly more when viewing the familiar human yawn video than the control. This result demonstrates that familiar humans did stimulate CY; however, we did not find that familiarity with the humans in the video was required. Unfamiliar humans stimulated the same yawn contagion, and the yawn rates were not significantly different from those for familiar humans. The third species tested, the gelada baboon, failed to elicit the same yawn contagion, however. Our interpretation is that chimpanzees do not need to know each yawning individual to show contagion, but the individuals do need to belong to a species with which the chimpanzees have a history of positive social interactions.
We found a difference in the yawning rate between stimuli that did and those that did not elicit contagion (figure 2). Among the three stimuli that elicited contagion (i.e. ingroup chimpanzees, familiar humans and unfamiliar humans), the yawning rates were similar and none of these stimuli was more potent than another. Thus, we did not observe different magnitudes of CY, in contrast to human studies in which the degree of contagion follows a continuum based on social closeness [12]. Either humans are more discriminating in their CY responses, or we have not yet designed the experiment in the right way for chimpanzees.
Human stimuli elicited a similar level of contagion as the chimpanzees’ friends and kin, and significantly higher than strange chimpanzees. For our subjects, a different species (but one they have a history of positive experiences with) was more potent at eliciting empathy-based contagion than outsiders of their own species. Many of our chimpanzees have not seen or interacted with strangers of their own species since the groups were assembled decades ago, while others were born into the group and may never even have seen a chimpanzee stranger. While it is possible that the arousal of seeing strange chimpanzees may have suppressed the physiological yawn response [45] irrespective of an empathic connection, increased yawning is also a possible outcome of high arousal [46], including in a CY context [47]. Rather, given the pervasive xenophobia among wild chimpanzees, in which strangers are invariably treated with hostility [40], we think that it is more likely that antagonism inhibited yawn contagion to the unfamiliar chimpanzee stimuli. Subjects may never have reached the positive engagement needed for an empathy-based response. The human stimuli, on the other hand, are not expected to arouse the same hostility as our subjects are used to new people. Students come, complete their studies and leave, and care staff gain and lose members in the normal course of people changing jobs. The chimpanzees may have been conditioned to take a positive view of humans in general, not just the ones that they know. This is not to preclude that the chimpanzees do not make distinctions between familiar and unfamiliar humans, only that this distinction was not detected by our behavioural measure.
How do the chimpanzees view gelada baboons? The rate of yawning was the same as that for outgroup chimpanzees (i.e. an absence of any significant contagion). Does this mean that chimpanzees responded with hostility to gelada baboons as well? This possibility cannot be excluded, yet given chimpanzee natural history it seems unlikely. At Gombe National Park, where chimpanzees interact freely with baboons (Papio anubis), affiliative interactions are common and competition between both species is limited [48]. Our data rather suggest a different possibility. The chimpanzees spent significantly more time looking at the outgroup chimpanzee videos than at gelada baboons or any other stimulus class (figure 3). Our subjects thus seemed far more interested in outgroup chimpanzees than gelada baboons, yet they showed a similar, minimal yawn response to both. Outgroup chimpanzees possibly elicited a hostile response, which interfered with empathy-based engagement [49], whereas the gelada baboons were viewed as a socially meaningless stimulus. If true, we could say that CY with strange chimpanzees was actively thwarted, whereas with geladas it was not there to begin with.
The different responses to the different stimuli further support the idea that CY is socially modulated, and thus serves as a measure of empathic engagement with the stimulus. As outgroup chimpanzees and gelada baboons did not stimulate significant rates of yawning, CY does not seem to be a simple fixed-action pattern for which any yawn may serve as a releaser. As for why humans but not gelada baboons stimulated contagion, physical resemblance or lack thereof probably does not alone account for the differences as outgroup chimpanzees did not stimulate CY. Rather, social experience probably plays a role. Would mere exposure to gelada baboons make them more familiar and lead to contagion? Or is mere exposure not enough and CY requires a history of positive social interactions, such as the chimpanzees have with most humans? Most interestingly, could experience change the way chimpanzees respond to outgroup chimpanzees? These are unanswered questions.
By forming measurable empathy-based contagion with unfamiliar humans, chimpanzees showed that the ability to connect with unfamiliar individuals is not unique to humans. Conditions within the human evolutionary lineage may have altered the expression of this ability, but flexible social engagement was probably already present in the most recent common ancestor with chimpanzees. This flexibility opens a door to examining how we can modify who chimpanzees will form an empathy-based connection with and how strongly. Understanding this flexibility in social engagement may help explain the proximate mechanisms that allow for switching between cooperation and competition within chimpanzee and human societies [50].
Acknowledgements
We are indebted to Elisabetta Palagi for providing the videos of gelada baboons, and we thank Alessia Leone and Giada Mancini for their time in finding clips that met our requirements. We also wish to thank all of our human yawn volunteers, who clearly made an impression on the chimpanzees. We thank the animal care and veterinary staff of the Yerkes National Primate Research Center's Field Station. The comments of two anonymous reviewers greatly improved this article.
The Yerkes NPRC is fully accredited by the American Association for Accreditation for Laboratory Animal Care.
Data accessibility
The data on yawns and attention are included in the electronic supplementary material tables.
Funding statement
This research was supported by the FIRST programme (NIH/NIGMS (USA) IRACDA grant no. K12 GM000680, the base grant to the YNPRC by the National Center for Research Resources P51RR165 (currently supported by the Office of Research Infrastructure Programs/OD P51OD11132) and the Living Links Center.
References
- 1.Preston SD, de Waal FBM. 2002. Empathy: its ultimate and proximate bases. Behav. Brain Sci. 25, 1–72 [DOI] [PubMed] [Google Scholar]
- 2.Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, Chanda ML, Levitin DJ, Mogil JS. 2006. Social modulation of pain as evidence for empathy in mice. Science 312, 1967–1970 (doi:10.1126/science.1128322) [DOI] [PubMed] [Google Scholar]
- 3.Bartal IB-A, Decety J, Mason P. 2011. Empathy and pro-social behavior in rats. Science 334, 1427–1430 (doi:10.1126/science.1210789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Custance D, Mayer J. 2012. Empathic-like responding by domestic dogs (Canis familiaris) to distress in humans: an exploratory study. Anim. Cogn. 15, 851–859 (doi:10.1007/s10071-012-0510-1) [DOI] [PubMed] [Google Scholar]
- 5.Bates LA, Lee PC, Njiraini N, Poole JH, Sayialel K, Sayialel S, Moss CJ, Byrne R. 2008. Do elephants show empathy? J. Conscious. Stud. 15, 204–225 [Google Scholar]
- 6.Edgar JL, Lowe JC, Paul ES, Nicol CJ. 2011. Avian maternal response to chick distress. Proc. R. Soc. B 278, 3129–3134 (doi:10.1098/rspb.2010.2701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wascher CAF, Scheiber IBR, Kotrschal K. 2008. Heart rate modulation in bystanding geese watching social and non-social events. Proc. R. Soc. B 275, 1653–1659 (doi:10.1098/rspb.2008.0146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Platek SM, Critton SR, Myers TE, Gallup GG. 2003. Contagious yawning: the role of self-awareness and mental state attribution. Cogn. Brain Res. 17, 223–227 (doi:10.1016/S0926-6410(03)00109-5) [DOI] [PubMed] [Google Scholar]
- 9.Senju A, Maeda M, Kikuchi Y, Hasegawa T, Tojo Y, Osanai H. 2007. Absence of contagious yawning in children with autism spectrum disorder. Biol. Lett. 3, 706–708 (doi:10.1098/rsbl.2007.0337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giganti F, Esposito Ziello M. 2009. Contagious and spontaneous yawning in autistic and typically developing children. Curr. Psychol. Lett. 25, 1–11 [Google Scholar]
- 11.Helt MS, Eigsti IM, Snyder PJ, Fein DA. 2010. Contagious yawning in autistic and typical development. Child Dev. 81, 1620–1631 (doi:10.1111/j.1467-8624.2010.01495.x) [DOI] [PubMed] [Google Scholar]
- 12.Norscia I, Palagi E. 2011. Yawn contagion and empathy in Homo sapiens. PLoS ONE 6, e28472 (doi:10.1371/journal.pone.0028472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell MW, de Waal FBM. 2011. Ingroup-outgroup bias in contagious yawning by chimpanzees supports link to empathy. PLoS ONE 6, e18283 (doi:10.1371/journal.pone.0018283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demuru E, Palagi E. 2012. In bonobos yawn contagion is higher among kin and friends. PLoS ONE 7, e49613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palagi E, Leone A, Mancini G, Ferrari PF. 2009. Contagious yawning in gelada baboons as a possible expression of empathy. Proc. Natl Acad. Sci. USA 106, 19 262–19 267 (doi:10.1073/pnas.0910891106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva K, Bessa J, de Sousa L. 2012. Auditory contagious yawning in domestic dogs (Canis familiaris): first evidence for social modulation. Anim. Cogn. 15, 721–724 (doi:10.1007/s10071-012-0473-2) [DOI] [PubMed] [Google Scholar]
- 17.Madsen EA, Persson T. 2013. Contagious yawning in domestic dog puppies (Canis lupus familiaris): the effect of ontogeny and emotional closeness on low-level imitation in dogs. Anim. Cogn. 16, 233–240 (doi:10.1007/s10071-012-0568-9) [DOI] [PubMed] [Google Scholar]
- 18.Romero T, Konno A, Hasegawa T. 2013. Familiarity bias and physiological responses in contagious yawning by dogs support link to empathy. PLoS ONE 8, e71365 (doi:10.1371/journal.pone.0071365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amici F, Aureli F, Call J. 2014. Response facilitation in the four great apes: is there a role for empathy? Primates 55, 113–118 (doi:10.1007/s10329-013-0375-1) [DOI] [PubMed] [Google Scholar]
- 20.Madsen EA, Persson T, Sayehli S, Lenninger S, Sonesson G. 2013. Chimpanzees show a developmental increase in susceptibility to contagious yawning: a test of the effect of ontogeny and emotional closeness on yawn contagion. PLoS ONE 8, e76266 (doi:10.1371/journal.pone.0076266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnott SR, Singhal A, Goodale MA. 2009. An investigation of auditory contagious yawning. Cogn. Affect. Behav. Neurosci. 9, 335–342 (doi:10.3758/cabn.9.3.335) [DOI] [PubMed] [Google Scholar]
- 22.Cooper N, Puzzo I, Pawley A, Bowes-Mulligan R, Kirkpatrick E, Antoniou P, Kennett S. 2012. Bridging a yawning chasm: EEG investigations into the debate concerning the role of the human mirror neuron system in contagious yawning. Cogn. Affect. Behav. Neurosci. 12, 393–405 (doi:10.3758/s13415-011-0081-7). [DOI] [PubMed] [Google Scholar]
- 23.Haker H, Kawohl W, Herwig U, Rössler W. 2013. Mirror neuron activity during contagious yawning: an fMRI study. Brain Imaging Behav. 7, 28–34 (doi:10.1007/s11682-012-9189-9) [DOI] [PubMed] [Google Scholar]
- 24.Gallesse V. 2003. The manifold nature of interpersonal relations: the quest for a common mechanism. Phil. Trans. R. Soc. Lond. B 358, 517–528 (doi:10.1098/rstb.2002.1234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Waal FBM, Ferrari PF. 2010. Towards a bottom-up perspective on animal and human cognition. Trends Cogn. Sci. 14, 201–207 (doi:10.1016/j.tics.2010.03.003) [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Zuo X, Wang X, Han S. 2009. Do you feel my pain? Racial group membership modulates empathic neural responses. J. Neurosci. 29, 8525–8529 (doi:10.1523/JNEUROSCI.2418-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avenanti A, Sirigu A, Aglioti SM. 2010. Racial bias reduces empathic sensorimotor resonance with other-race pain. Curr. Biol. 20, 1018–1022 (doi:10.1016/j.cub.2010.03.071) [DOI] [PubMed] [Google Scholar]
- 28.Mathur VA, Harada T, Lipke T, Chiao JY. 2010. Neural basis of extraordinary empathy and altruistic motivation. NeuroImage 51, 1468–1475 (doi:10.1016/j.neuroimage.2010.03.025) [DOI] [PubMed] [Google Scholar]
- 29.Ridley M. 2010. The rational optimist: how prosperity evolves. New York, NY: HarperCollins [Google Scholar]
- 30.Ben-Ami Bartal I, Rodgers DA, Bernardez Sarria MS, Decety J, Mason P, Fernald R. 2014. Pro-social behavior in rats is modulated by social experience. eLife 3, e01385 (doi:10.7554/eLife.01385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joly-Mascheroni RM, Senju A, Shepherd AJ. 2008. Dogs catch human yawns. Biol. Lett. 4, 446–448 (doi:10.1098/rsbl.2008.0333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harr A, Gilbert V, Phillips K. 2009. Do dogs (Canis familiaris) show contagious yawning? Anim. Cogn. 12, 833–837 [DOI] [PubMed] [Google Scholar]
- 33.O'Hara SJ, Reeve AV. 2011. A test of the yawning contagion and emotional connectedness hypothesis in dogs, Canis familiaris. Anim. Behav. 81, 335–340 (doi:10.1016/j.anbehav.2010.11.005) [Google Scholar]
- 34.Warneken F, Hare B, Melis AP, Hanus D, Tomasello M. 2007. Spontaneous altruism by chimpanzees and young children. PLoS Biol. 5, e184 (doi:10.1371/journal.pbio.0050184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Waal FBM. 2009. The age of empathy. Toronto, Canada: McLelland and Stewart [Google Scholar]
- 36.Anderson JR, Myowa-Yamakoshi M, Matsuzawa T. 2004. Contagious yawning in chimpanzees. Proc. R. Soc. Lond. B 271, S468–S470 (doi:10.1098/rsbl.2004.0224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell MW, Carter JD, Proctor D, Eisenberg ML, de Waal FBM. 2009. Computer animations stimulate contagious yawning in chimpanzees. Proc. R. Soc. B 276, 4255–4259 (doi:10.1098/rspb.2009.1087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massen JJM, Vermunt DA, Sterck EHM. 2012. Male yawning is more contagious than female yawning among chimpanzees (Pan troglodytes). PLoS ONE 7, e40697 (doi:10.1371/journal.pone.0040697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishida T, Hiraiwa-Hasegawa M, Hasegawa T, Takahata Y. 1985. Group extinction and female transfer in wild chimpanzees in the Mahale National Park, Tanzania. Zeitschrift für Tierpsychol. 67, 284–301 (doi:10.1111/j.1439-0310.1985.tb01395.x) [Google Scholar]
- 40.Wilson ML, Wrangham RW. 2003. Intergroup relations in chimpanzees. Annu. Rev. Anthropol. 32, 363–392 (doi:10.2307/25064834) [Google Scholar]
- 41.Deputte B. 1994. Ethological study of yawning in primates. I. Quantitative analysis and study of causation in two species of Old World monkeys (Cercocebus albigena and Macaca fascicularis). Ethology 98, 221–245 [Google Scholar]
- 42.Baenninger R, Binkley S, Baenninger M. 1996. Field observations of yawning and activity in humans. Physiol. Behav. 59, 421–425 (doi:10.1016/0031-9384(95)02014-4) [DOI] [PubMed] [Google Scholar]
- 43.Giganti F, Zilli I. 2011. The daily time course of contagious and spontaneous yawning among humans. J. Ethol. 29, 215–219 (doi:10.1007/s10164-010-0242-0) [Google Scholar]
- 44.Campbell MW, de Waal FBM. 2010. Methodolical problems in the study of contagious yawning. In The mystery of yawning in physiology and disease (ed. Walusinski O.), pp. 120–127 Basel, Switzerland: Karger [Google Scholar]
- 45.Walusinski O. 2013. How yawning switches the default-mode network to the attentional network by activating the cerebrospinal fluid flow. Clin. Anat. 27, 201–209 (doi:10.1002/ca.22280) [DOI] [PubMed] [Google Scholar]
- 46.Provine RR. 2005. Yawning. Am. Sci. 93, 532–539 (doi:10.1511/2005.56.980) [Google Scholar]
- 47.Paukner A, Anderson JR. 2006. Video-induced yawning in stumptail macaques (Macaca arctoides). Biol. Lett. 2, 36–38 (doi:10.1098/rsbl.2005.0411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morris K, Goodall J. 1977. Competition for meat between chimpanzees and baboons of the Gombe National Park. Folia Primatol. 28, 109–121 (doi:10.1159/000155801) [DOI] [PubMed] [Google Scholar]
- 49.Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD. 2006. Empathic neural responses are modulated by the perceived fairness of others. Nature 439, 466–469 (doi:10.1038/nature04271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Waal FBM. 1982. Chimpanzee politics. New York, NY: Harper and Row [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data on yawns and attention are included in the electronic supplementary material tables.