Structural and dynamic determinants of type I interferon receptor assembly and their functional interpretation (original) (raw)
. Author manuscript; available in PMC: 2014 Apr 15.
Published in final edited form as: Immunol Rev. 2012 Nov;250(1):317–334. doi: 10.1111/imr.12001
Summary
Type I interferons (IFNs) form a network of homologous cytokines that bind to a shared, heterodimeric cell surface receptor and engage signaling pathways that activate innate and adaptive immune responses. The ability of IFNs to mediate differential responses through the same cell surface receptor has been subject of a controversial debate and has important medical implications. During the past decade, a comprehensive insight into the structure, energetics, and dynamics of IFN recognition by its two-receptor subunits, as well as detailed correlations with their functional properties on the level of signal activation, gene expression, and biological responses were obtained. All type I IFNs bind the two-receptor subunits at the same sites and form structurally very similar ternary complexes. Differential IFN activities were found to be determined by different lifetimes and ligand affinities toward the receptor subunits, which dictate assembly and dynamics of the signaling complex in the plasma membrane. We present a simple model, which explains differential IFN activities based on rapid endocytosis of signaling complexes and negative feedback mechanisms interfering with ternary complex assembly. More insight into signaling pathways as well as endosomal signaling and trafficking will be required for a comprehensive understanding, which will eventually lead to therapeutic applications of IFNs with increased efficacy.
Keywords: interferon, receptors, structure/function
The type I interferon system
The innate immune system is the first line of defense against infection by pathogens and against malignant cells. Jawed vertebrates that evolved an adaptive immune system of T and B cells, also developed the type I interferon (IFN) cytokine family, which are secreted proteins dedicated to signal the presence of intracellular infection to surrounding cells and thus provide defense against viruses and intracellular bacteria (1). Upon binding to their cell surface receptor, these secreted cytokines activate the expression of over 1000 genes involved in a wide variety of activities, which were suggested to be required for targeting different viruses, with each virus targeted by a unique set of activities (2). The initiation of an antiviral state is indeed the most intensively studied outcome of IFN stimulation and is also the eponym of IFNs: these cytokines interfere with viral replication within host cells (3). The family of IFNs comprises sixteen members in humans, including IFNβ, IFNε, IFNκ, IFNω, and 12 subtypes of IFNα. All IFNs bind to a shared cell surface receptor comprising two transmembrane subunits, IFNAR1 and IFNAR2 (4, 5). Following ternary complex assembly (Fig. 1), the Janus family kinases (JAK) tyrosine kinase 2 (Tyk2) and Jak1, which are associated with the membrane-proximal part of the cytoplasmic domains of IFNAR1 and IFNAR2, respectively, are activated by reciprocal transphosphorylation (6). Subsequently, they phosphorylate several tyrosine residues in the membrane-distal, intracellular domains of IFNAR1 and IFNAR2, which, in turn, recruit further effector proteins that propagate downstream signaling. This activates an antiviral response in the host organism, including direct cellular defense mechanisms and activation of further elements of the innate and the adaptive immune response. IFNs evoke a wide range of additional biological activities, both protective and counterprotective (7). For example, IFNβ was shown to be lethal during certain bacterial infections (7), but protective against some protozoa and fungi (8, 9). Comparative studies of wildtype mice with IFN receptor knockout mice have revealed that in addition to its activities in defense against pathogens and viruses, IFNs play an important role in cancer prevention, cell differentiation, and cell-type-specific activities, such as dendritic and natural killer cell activation, T-cell proliferation and survival, B-cell antibody class switching, memory T-cell survival, apoptosis, and angiogenesis (10, 11).
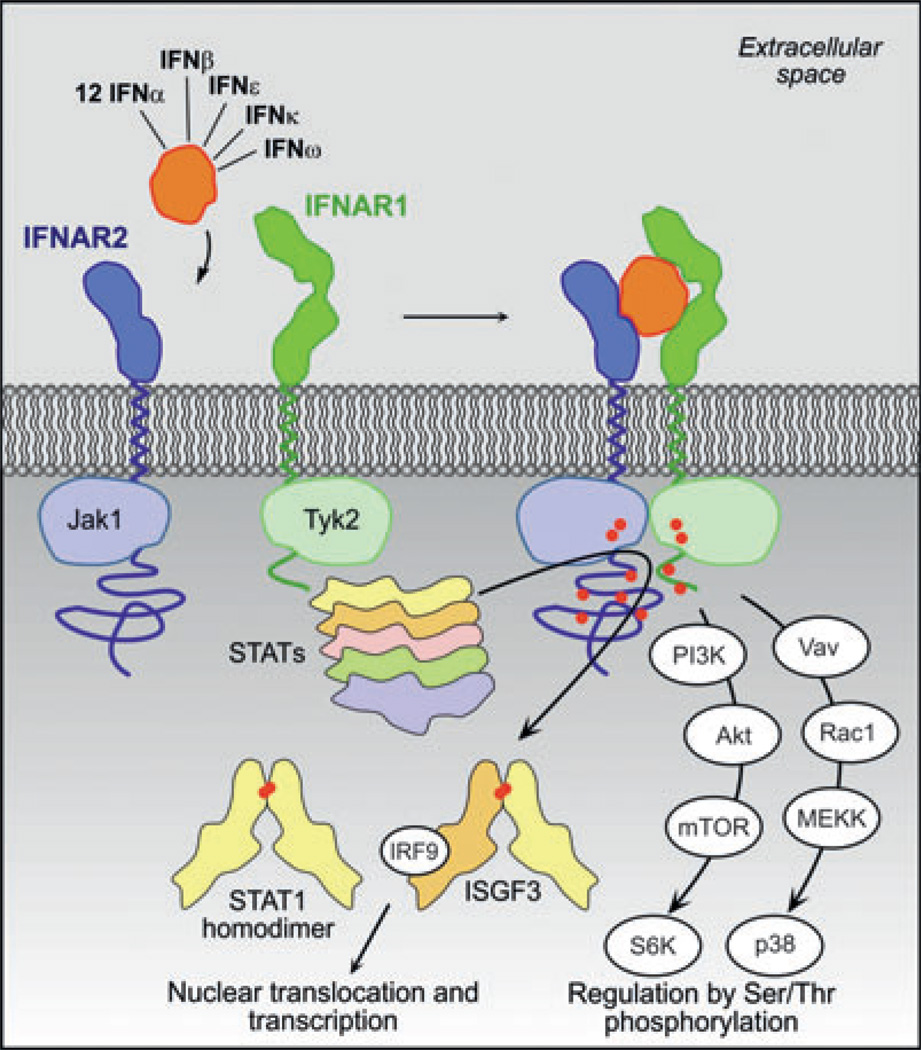
Fig. 1. The type I interferon (IFN) signaling network.
By simultaneous interaction of IFNs with the two-receptor subunits IFNAR1 and IFNAR2, the active signaling complex is formed. Subsequently, the tyrosine kinase 2 (Tyk2) and Janus family kinases (Jak1) associated with IFNAR1 and IFNAR2, respectively, transphosphorylate each other, and phosphorylate-specific tyrosine residues of IFNAR1 and IFNAR2 (indicated as red dots). These serve as docking sites for effector proteins of the signal transducers and activators of transcription (STAT) family. Upon phosphorylation, STAT1 and STAT2 form homo- and heterodimers, which translocate into the nucleus to activate transcription.
A striking feature of IFN signaling is the high redundancy of ligands, which engage the same receptor. Interestingly, differential cellular responses have been observed for different IFNs. Although all IFNs potently activate antiviral responses, more complex cellular responses requiring long-term signaling were reported to be preferentially activated by a subset of IFNs. A number of excellent recent reviews have covered many of the biological aspects of the type I IFN system and their importance in the immune system (12–19). Herein, we focus on the interplay between structure, energetics, and dynamics of IFN–receptor interactions and their role in regulating biological responses. On the basis of this highly quantitative understanding, we propose a model for differential cellular signaling using a single cell surface receptor.
Signaling pathways induced by type I IFNs
Following the formation of the IFN–receptor complex, the signal is propagated to various effector proteins (Fig. 1), many of which belong to the family of signal transducers and activators of transcription (STAT). Upon activation of the receptor-associated kinases Tyk2 and Jak1 by transphosphorylation, they phosphorylate several tyrosine residues on the membrane-distal part of both receptor subunits. These serve as docking sites for STAT proteins (STAT1, STAT2, STAT3, STAT4, and STAT5), which in turn are phosphorylated by the JAKs on a specific tyrosine residue (pSTATs). pSTATs form homo- and heterodimers by respective intermolecular interactions of their Src homology 2 (SH2) domains with the phosphorylated tyrosine residue and subsequently translocate into the nucleus, where they directly regulate gene transcription. The hallmark of IFN signaling is a STAT1/STAT2 heterodimer, which, together with IRF9, forms the transcription factor ISGF3 that binds IFN-stimulated regulatory elements (ISREs) within the promoter of IFN-stimulated genes (ISGs). Other IFN-activated STATs have been described to bind the IFNγ activation sequence (GAS) elements present in the ISG promoters. ISGs encode for a multitude of proteins that are responsible for antiviral, antiproliferative, and immunoregulatory cellular responses, and it is believed that specificity is made possible by the preferential binding of different STAT dimers to specific sequence elements. For antiviral responses, primarily phosphorylation of STAT1 and STAT2 is required (13). In addition to members of the classical JAK–STAT pathway, other signaling factors have a role in IFN activity. These include isoforms of the protein kinase C and themultifunctional adapter protein CrkL, members of the p38 mitogen-activated protein kinase (MAPK) pathway, the phosphoinositol 3-kinase (PI3K) signaling pathway, and the extracellular signal-regulated kinase (ERK) MAPK pathway (12, 13). Notably, although the importance of these pathways in IFN signaling is well established in some systems, it seems that cell-type specificity plays a crucial role in their relevance.
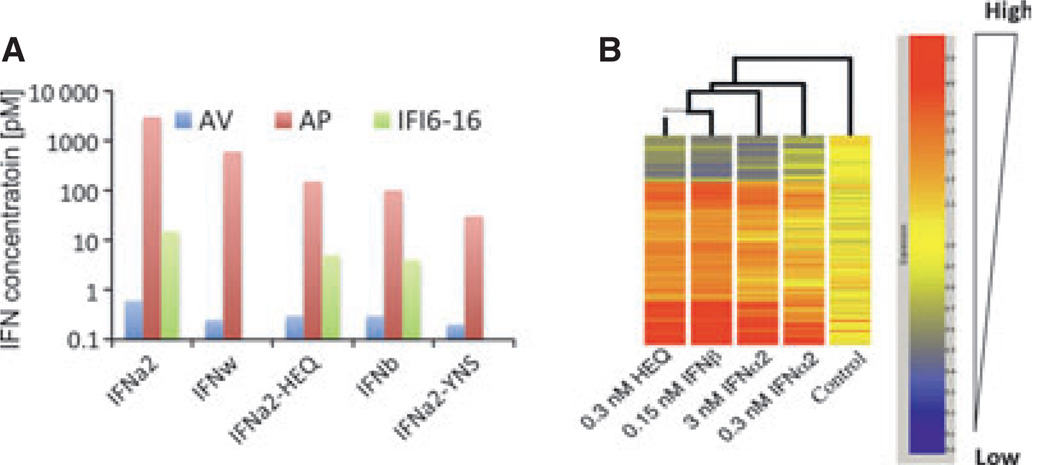
Although all type I IFNs bind to the same cell surface receptor, they are functionally not fully redundant, and many instances of differential activities of IFNs have been reported (20–30). Differential activity refers to the observation that all IFNs hardly differ in their potencies of activating STAT phosphorylation and antiviral responses, but some members show strongly different gene expression patterns and elicit further cellular responses much more potently than others (Fig. 2A). In particular, IFNβ has been shown to be capable to mount cellular responses, such as antiproliferative activity, for which very high concentrations of the α-subtypes are required that are probably above physiological levels (Fig. 2A). Interestingly, those responses specifically mounted by IFNβ require long-term activation of cellular signaling, suggesting that negative feedback mechanisms may play an important role in dictating the differential IFN activities (31).
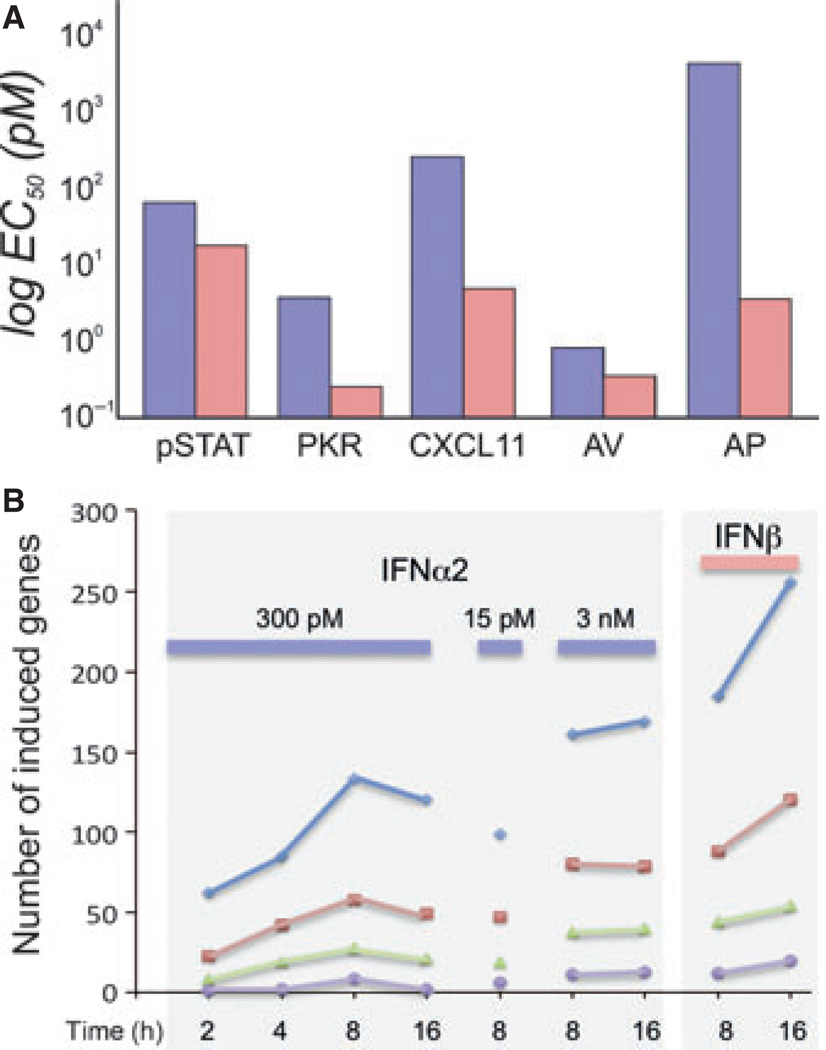
Fig. 2. Differential activities of interferon α 2 (IFNα2) and interferon β (IFNβ) in WISH cells.
(A) Comparison of the potencies of IFNα2 (blue) and IFNβ (pink) in signal transducers and activators of transcription (STAT) phosphorylation (pSTAT), gene expression [protein kinase R (PKR) and CXCL11], as wells as antiviral (AV) and antiproliferative (AP) activity. (B) Gene induction after activation at different concentrations and times (levels of gene induction: blue, >2-fold; red, >3-fold; green, >5-fold; violet, >10-fold).
A major challenge in defining the activities of IFNs is their pleiotropy, i.e., their strongly different behavior in different cell lines. Thus, STAT activation in primary immune cells shows that the potency of IFNs in inducing phosphorylation of STAT1, STAT2, STAT3, STAT4, and STAT5 are similar in CD4, CD8, and monocytes, with up to fourfold variations in EC50 values being observed between the different STATs (32). Contradicting results were shown for the variation in STAT activation in B cells versus CD4+ T cells and monocytes. Whereas only minor differences were observed in one study (32), much lower levels of activation of pSTATs in B cells were recorded in another study (33). All tested IFNs have shown similar potencies with respect to STAT phosphorylation (32) (Fig. 2A). pSTAT activation was found to have a short half-life peaking at about 30 min after induction. pSTAT1 and pSTAT3 decline faster than pSTAT2 (26), reaching non-detectable levels after about 16 h of continuous IFN induction (34). It is still an open question whether also non-phosphorylated STATs are active as transcription factors. It was shown that IFNs stimulate a substantial increase in the concentration of STAT1 that persists for several days. Moreover, increasing concentrations of STAT1 were shown to result in an increased expression of IFN-induced genes (35). Consistent with these findings, STAT1 was present in the nuclei of these cells, suggesting that IFN-induced expression of unphosphorylated STAT1 may act as long-term effector of IFNs, long after the phosphorylation of STATs decays.
Upon IFN treatment, cells undergo a dramatic shift in gene expression, with over 1000 genes being affected, most of them being upregulated (36, 37). Fig. 2B shows a comparison of the number of upregulated genes upon induction with IFNα2 at 15, 300 pm, and 3 nm concentrations versus 150 pm IFNβ (31, 38). The figure shows a number of interesting characteristics of IFN-induced gene expression. At 300 pm IFNα2 (which is a concentration saturating antiviral response, while not significantly activating antiproliferative response in WISH cells), the number of activated genes peaks after 8 h. The number of genes induced is somewhat smaller when 15 pm was applied, and larger with 3 nm. This is true, independent of the fold-change threshold. The number of activated genes at 16 h increased relative to 8 h only when 3 nm IFNα2 or 150 pm IFNβ were used. These concentrations already promote antiproliferative activity in WISH cells (39). However, even at a 20-fold higher concentration of IFNα2 versus IFNβ, a substantially higher number of genes is induced by IFNβ. The EC50 required for activation of individual genes shows that the concentration of IFN required to activate different genes varies (Fig. 2A). In the two examples shown, 100-fold less IFNα2 is required to induce the expression of PKR than CXCL11, whereas much lower concentrations of IFNβ are required for both genes (32, 40). In summary, it appears that IFN-induced genes can be divided into two groups: (i) genes that are highly sensitive and require only pm concentrations for activation, and (ii) genes that require 100-fold higher IFN concentrations. Analysis of gene array results has shown that genes related to the IFN antiviral activity belong to the first group (such as Mx1, PKR, and OAS2), whereas the functions of genes of the second group relate to cell proliferation, chemokine activity, inflammation, and more [e.g. IL6, CXCL11, and Trail (2, 31, 32, 38, 41–44)].
How can such differences in activity of different IFNs be communicated through the same cell surface receptor? Initially, specific, additional components have been suspected to be responsible, but these have never been found. Thus, differential recognition of IFNs by the receptor subunits IFNAR1 and IFNAR2 must be responsible for differential activity, and therefore a comprehensive picture of the structure, the energetics, and the dynamics of the IFN–receptor interactions is required.
Structure of the unbound and bound components of the type I IFN system
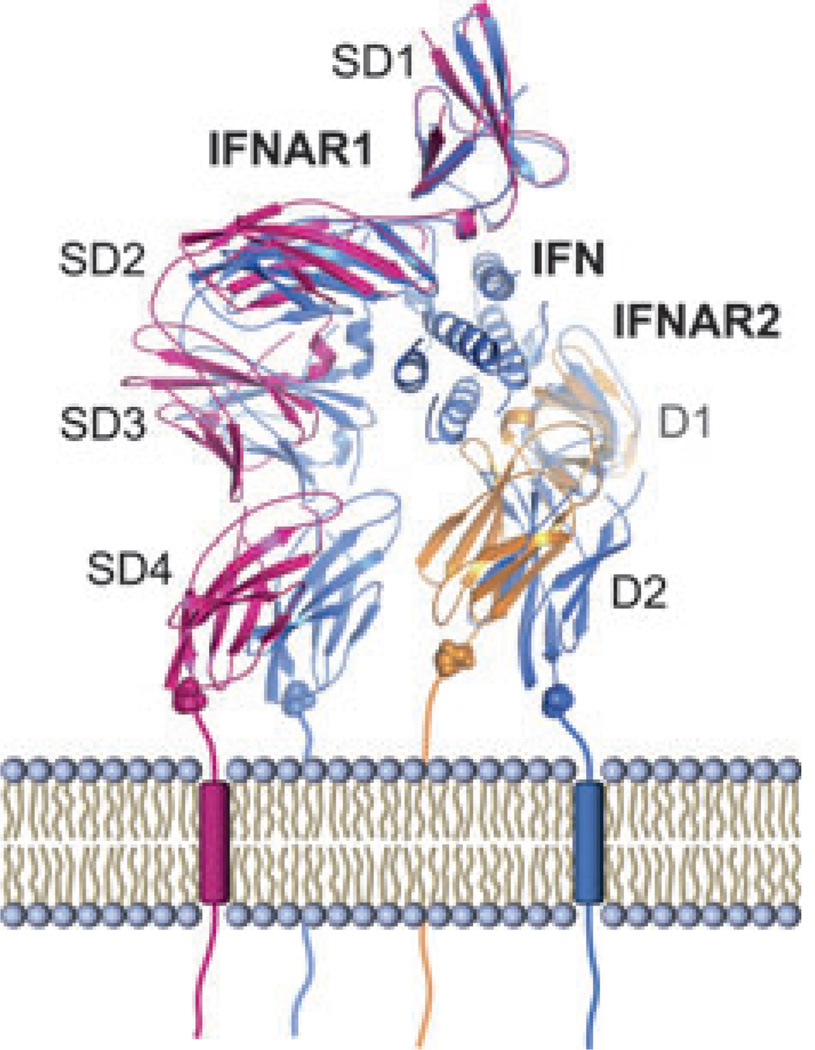
IFNAR1 and IFNAR2 are both transmembrane proteins that belong to the class II helical cytokine receptors (19, 45). Like other receptors in this class, the ectodomain (ECD) of the high-affinity subunit IFNAR2 comprises two fibronectin type III (FNIII)-like subdomains (5) referred to as D1 and D2, respectively. The low-affinity receptor chain IFNAR1, however, consists of four FNIII-like subdomains (SD1–SD4), which most likely emerged from gene duplication (4).
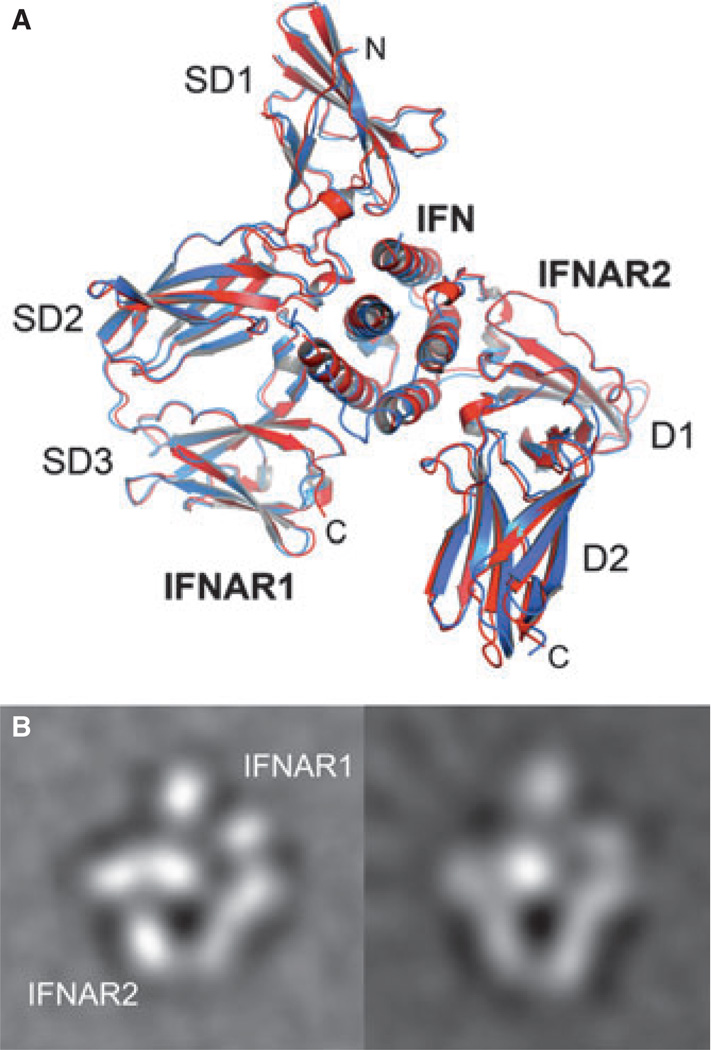
The first structure of a type I IFN (murine IFNβ) was reported in 1992 (46), followed by the X-ray and NMR structures of human IFNα2b (47, 48), as well as the crystal structure of human IFNβ (49). The structure of the ECD of IFNAR2 (IFNAR2-EC) was solved using NMR (49). In that and follow up studies, models of IFNα2–IFNAR2 complexes were build using double-mutant cycle data or/and NMR transfer data as constraints for docking (51, 52). Indeed, the determined structures confirmed that double-mutant cycle and NMR determined distance constraints are valuable inputs to obtain accurate models of complexes. In addition, low-resolution structures of the ternary complexes harboring IFNα2 and IFNβ, respectively, were obtained by single particle electron microscopy. These correctly conceived the general architecture of the ternary complex (53, 54) and showed no difference in binding of these two IFNs (Fig. 3B). More recently, the structures of two heterotrimeric type I IFN receptor–ligand complexes have been determined by X-ray crystallography, in addition to the high-resolution structures of unliganded IFNAR1 and the binary IFNα2–IFNAR2 complex (32) (Fig. 4). Thus, we now know the experimental structures of all the components of IFN–receptor complexes in the bound and unbound state. The recently solved ternary complex structures contain two different ligands with distinct physiological activities, IFNω and a mutant of IFNα2, called YNS, that binds IFNAR1 50-fold tighter than the wildtype protein (see below). These are the first structures of a complete signaling complex of the class II helical cytokine family. Before, only structures of class II helical cytokines, including IFNγ, IL-10, IL-22, and IFNλ, in complex with their high-affinity receptor subunits were known (55–58). Interestingly, despite the different physiological activities of the two ligands, the heterotrimeric receptor–ligand complexes share the same architecture (Fig. 3A). Upon superimposition of the two complexes, the root-mean-square deviation of Cα atoms is 0.9 Å.
Fig. 3. Architectures of the ternary complex observed for different interferons (IFNs).
(A) Overlay of the X-ray structures of IFNα2 (red) and IFNω (blue) in complex with IFNAR1 and IFNAR2. (B) Averaged single particle electron microscopy (EM) images of the ternary complex with IFNα2 (left) and IFNβ (right).
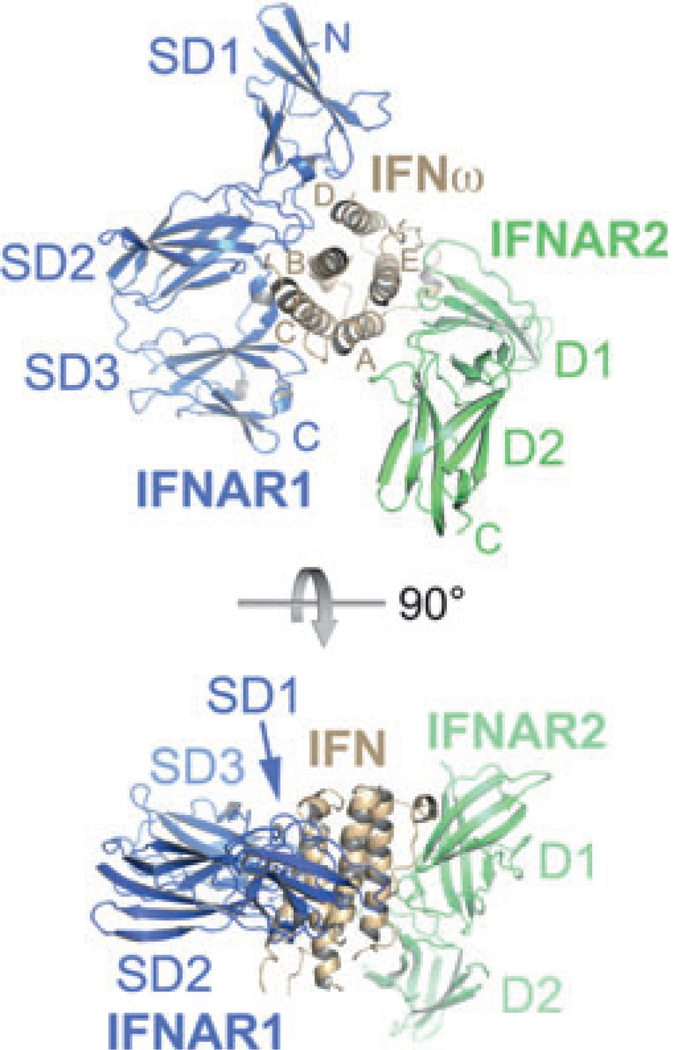
Fig. 4. X-ray structure of IFNω (brown) in complex with IFNAR1-ectodomain (ECD; blue) and IFNAR2-ECD (green).
The helices of IFNω are labeled with the letters (A–E). The FNIII-like domains of IFNAR1 (SD) and IFNAR2 (D) are numbered starting from the N-terminus.
IFNAR1 and IFNAR2 bind on opposing sides of the IFN ligand in an almost orthogonal arrangement that is unique among cytokine–receptor complexes. The interface between IFNAR2 and the ligand buries approximately 1800 Å2 of surface area and is formed between parts of helices A, E, and the A–B loop of IFN and the D1 subdomain of IFNAR2 (Fig. 4). The loop between strands 13 and 14 is the only portion of the IFNAR2-D2 subdomain that is involved in the interface. Thus, unlike most type I and II cytokine–receptor complexes, the IFN ligand does not bind at the apex of the elbow region between the D1 and D2 subdomains of IFNAR2, but its long axis shows almost parallel alignment with the β-strands of D1 (Fig. 4). The overall IFN–IFNAR2 binding mode resembles those observed in the IFNγ–IFNGR1 (58) and IFNλ–IFNLR1 (59) complexes. However, IFNγ and IFNλ bind closer to the apex of the hinge region between the two subdomains of the receptor, and the interdomain angles in IFNGR1 and IFNLR1 are wider than in IFNAR2, allowing larger portions of the C-terminal D2 subdomain to interact with the ligand. The IFNAR2 interface is characterized by the presence of several hot spot residues.
The IFNAR1–IFN interface buries approximately 2200 Å2 of surface area and is formed by contacts between helices B, C, and D of the IFN molecule (52, 60) and subdomains 1–3 of IFNAR1 (32). The membrane-proximal subdomain 4 is not involved in ligand binding (61). There was no electron density for this part of IFNAR1 in the maps of both ternary complexes (32), suggesting that it is flexible. A variable location and orientation of SD4 with respect to the remaining complex has also been suggested by electron-microscopic studies (53, 54). As the SD1–SD2 and SD3–SD4 modules most likely arose by gene duplication and are thought to share a similar architecture and interdomain angle, it was possible to determine the approximate position of SD4 by superimposing SD1 of the SD1–SD2 module onto SD3. In this modeled complex, the C-termini of SD4 of IFNAR1 and D2 of IFNAR2 are 4.5 Å apart (Fig. 5). The binding mode of IFNAR1 is unprecedented among cytokine–receptor interactions: the IFN ligand binds to IFNAR1 at the hinge between subdomains 2 and 3, and the long axis of the helical bundle lies perpendicular to the IFNAR1 receptor chain (Fig. 4). The top of the IFN molecule is capped by SD1. In contrast to type I (e.g. human growth hormone, IL-2, and erythropoietin) and other type II cytokine-receptor systems, where the ligands interact with the loops in the ‘elbow’ region of the receptor, important receptor–ligand interactions are mediated by a region of IFNAR1 that is opposing the hinge between SD2 and SD3 (Fig. 4). The binding mode of the SD1–SD2 module conforms more to the canonical ‘elbow’-mediated cytokine–receptor interaction.
Fig. 5. Ligand-induced conformational changes in IFNAR (based on a comparison of unbound and bound structures).
The bound conformation is in blue. SD4 of IFNAR1 was not visible in the X-ray structures and was modeled for clarity.
The ligand-docking mode seen in the two ternary complexes does not seem to be restricted to IFNα and IFNω, but appears to be shared by all type I IFNs. IFNβ, for example, exhibits only 30% and 33% sequence identity with IFNω and IFNα2, respectively. Mutational studies, single particle electron microscopy (Fig. 3B), and blocking-antibody experiments, however, suggest that IFNβ shares the overall receptor-binding mode with IFNα, except for some differences in details in the interfaces (62, 63). Superimposing human IFNβ [(49) PDB code 1AU1] onto the ligand in the IFNω ternary complex structure leads to only two clashes of side chains (Tyr92 and Tyr155) with the receptors, indicating that the IFNβ ligand could be easily accommodated by the receptors in a position similar to IFNω and IFNα2. Furthermore, the superposition of IFNβonto IFNα2 in the IFNα2–IFNAR2 binary complex shows that Trp22 on IFNβ and Ala19 on IFNα2 overlay onto each other and suggests a direct interaction between Trp22 on IFNβ and Trp100 of IFNAR2. Trp100 of IFNAR2 has been shown to be a specific hot spot residue for IFNβ binding, and double-mutant cycle analysis has demonstrated that Trp100 in IFNAR2 and Trp19 in an IFNα2–A19W mutant interact (64), corroborating the hypothesis that Ala19 of IFNα2 and Trp22 of IFNβ occupy similar positions with respect to Trp100 of IFNAR2 in the ligand–receptor complexes. Taken together, these findings substantiate the notion that all type I IFNs share the same receptor-binding mode and form structurally highly similar ternary signaling complexes.
Conformational dynamics and its role in ligand binding and signaling
Comparison of the unbound receptor subunits with the bound forms revealed a large movement in the receptor orientation and an outward movement of IFN (Fig. 5 and http://proteopedia.org/wiki/index.php/Journal:Cell:1). Structural movements of receptor components upon ligand binding have previously been implicated to have an important role in signal propagation across the membrane. A mechanism that involves the transduction of a conformational change in a preformed extracellular receptor domain toward the intracellular domain and thus inducing signaling was suggested for IFNγ (65), growth hormone (66), erythropoietin (67, 68), and others (69). For the prolactin receptor, ligand-dependent conformational switch that stabilizes the dimeric state was observed, demonstrating a structural link between the WSXWS motif, hormone binding, and receptor dimerization (70).
In the case of IFNAR1, a conformational change was first observed by fluorescence resonance energy transfer (FRET) measurements (54), which showed an increase of approximately 12 Å in the distance between the C- and N-terminal domains upon ligand binding. Moreover, it was established that the ligand-induced conformational changes were propagated to the membrane-proximal Ig domain of IFNAR1, which does not interact with the ligand. This was shown by an electron transfer-sensitive fluorescence dye attached covalently to residue N349C (54, 71). Fluorescence quenching of this dye by a neighboring Trp residue (Trp347) is abrogated upon IFN binding, suggesting that the accessibility of this Trp residue is altered by a conformational change. As SD4 is not required for ligand binding (61), the observed conformational movement in SD4 suggests a transfer of signal from the IFN-binding site to the membrane-proximal domain of IFNAR1. The key role of SD4 in signaling was also shown by the inability of a chimeric IFNAR1 with SD4 being replaced with the corresponding domains of other class II cytokine receptors to form a ternary complex and to activate an IFN response (61). Both the global and the local conformational changes were observed for IFNα2 and IFNβ. Thus, although the conformational change may have important implications for the transmembrane signal activation, it is not likely that they are the basis for differential signal activation.
Energetics and dynamics of IFN recognition by IFNAR1 and IFNAR2
All IFNs bind IFNAR1 with micromolar affinity and the IFNAR2 receptor with nanomolar affinity. This highly asymmetric binding affinity is a common feature in cytokine receptors (72). However, IFN subtypes vary substantially in their respective binding affinities toward IFNAR1 and IFNAR2. A systematic analysis of all IFNα subtypes has shown that they bind IFNAR1 at affinities of 0.5–5 µm, and IFNAR2 at affinities ranging from 0.4 to 5 nm [except for IFNα1 – 220 nm (27, 73)]. The tightest binding IFN is IFNβ, which binds IFNAR1 with 100 nm affinity and IFNAR2 with 0.1 nm affinity (74, 75). The binding affinity of IFNω is close to that of IFNα [0.4 µm toward IFNAR1 and 2 nm toward IFNAR2 (27)]. Comparing the product of the affinities toward both receptor subunits, as measured in vitro using surface plasmon resonance, to the cell surface binding affinity of a range of mutants and IFN subtypes showed a good correlation between the two (38, 73). The product of the receptor binding affinities of IFNα2, 4, 5, 10, 17, and 21 are similar, IFNα7, 8, and 16 have a three- to fourfold higher binding affinity, IFNω has a fivefold higher affinity, and IFNα6 and 14 have an eightfold higher binding affinity compared with IFNα2. Two IFNs are clear outliers, IFNα1 (40-fold lower affinity) and IFNβ (1000-fold higher affinity) than IFNα2.
Likewise, the differences in the kinetics of the interactions of IFNs with the receptor subunits are characteristic. All IFNs rapidly bind to IFNAR2 with association rate constants of 106–107/M/s (27, 63, 73, 75), which have been shown to be enhanced by electrostatic attraction (77). In contrast, the association with IFNAR1 is relatively slow, with association rate constants of approximately 5 × 105/M/s. Differences in binding affinities between different IFNs are mainly manifested as differences in the dissociation rate constants. Thus, the lifetime of IFNα2 in complex with IFNAR1 is approximately 1 s (78), whereas it is approximately 100 s in case of IFNβ. The lifetimes of IFN complexes with IFNAR2 range from approximately 10 s for IFNα1 over approximately 100 s for IFNα2 and IFNω to 1000 s for IFNβ.
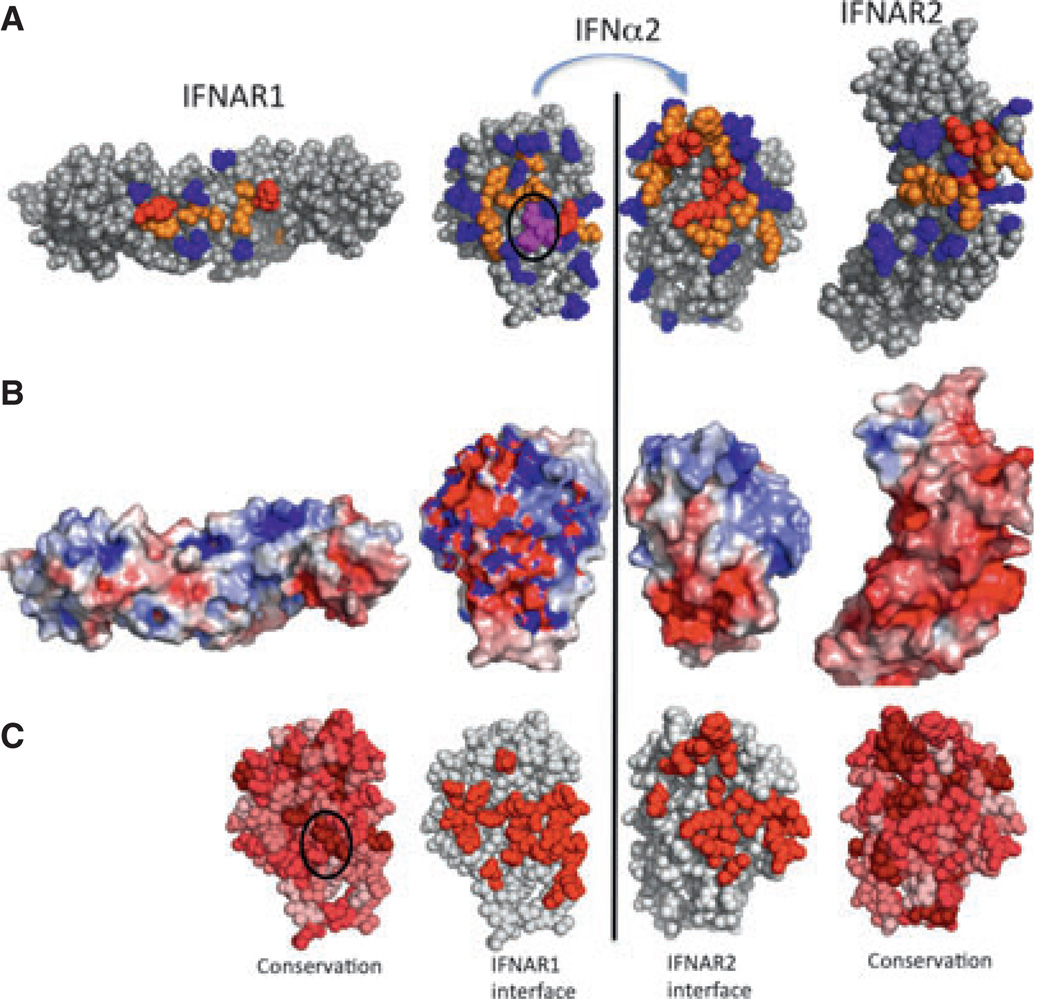
To obtain a comprehensive biophysical understanding of the relations between sequence, three-dimensional structure, energetics, and function, the ligand–receptor interfaces between IFN and IFNAR1 and IFNAR2 were subjected to systematic alanine scanning mutagenesis, and the binding affinities and biological activities of the muteins were determined. The most comprehensive mutational analysis was carried out using IFNα2 as template, but mutational studies were also done with IFNω, IFNβ, and several other IFNα subtypes (32, 62, 79). The energetic contributions of amino acid residues in the binding interfaces of IFNAR1 and IFNAR2 with IFNα2 are summarized in Fig. 6A in an open-book representation of the ligand–receptor complexes. The two IFNα2 representations are rotated by 180° with respect to each other. Side chains are colored according to their contribution to binding. In the high-affinity IFNα2/IFNAR2 interface, hotspot residues are located at the center, surrounded by a ring of residues contributing less, with mutations of the outer-ring residues having only a minor effect on binding. Conversely, on the IFNAR1 binding site of IFNα2, only a single hotspot residue (R120) was found (80). On IFNAR1, two hotspot residues located on SD2 and SD3 were identified (32). This fragmented distribution of binding energies may explain the low affinity of IFNs toward IFNAR1. The IFNAR1 binding site buries 2200 Å2 of surface area, whereas the IFNAR2 binding site buries 1840 Å2, showing again that the size of the interface is not related to its binding affinity.
Fig. 6. Functional characterization of the interferon–receptor complex.
Interferon alpha 2 (IFNα2) is pictured with its IFNAR1 binding site (left) and IFNAR2 binding site (right). (A) Changes in affinity upon mutation: red, >10-fold reduction; orange, 2- to 10-fold reduction; blue, no change; magenta, increase in binding affinity upon mutation. (B) Electrostatic potential of the proteins. (C) Residue conservation between IFNα2, IFNα8, IFNβ, and IFNω and the location of IFN interfaces. The shading from dark to light red marks the degree of conservation, with residues colored dark red being conserved in all four IFNs (note that these are the minority, even in the binding site). The circle marks the location of the HEQ residues [colored in magenta in (A)].
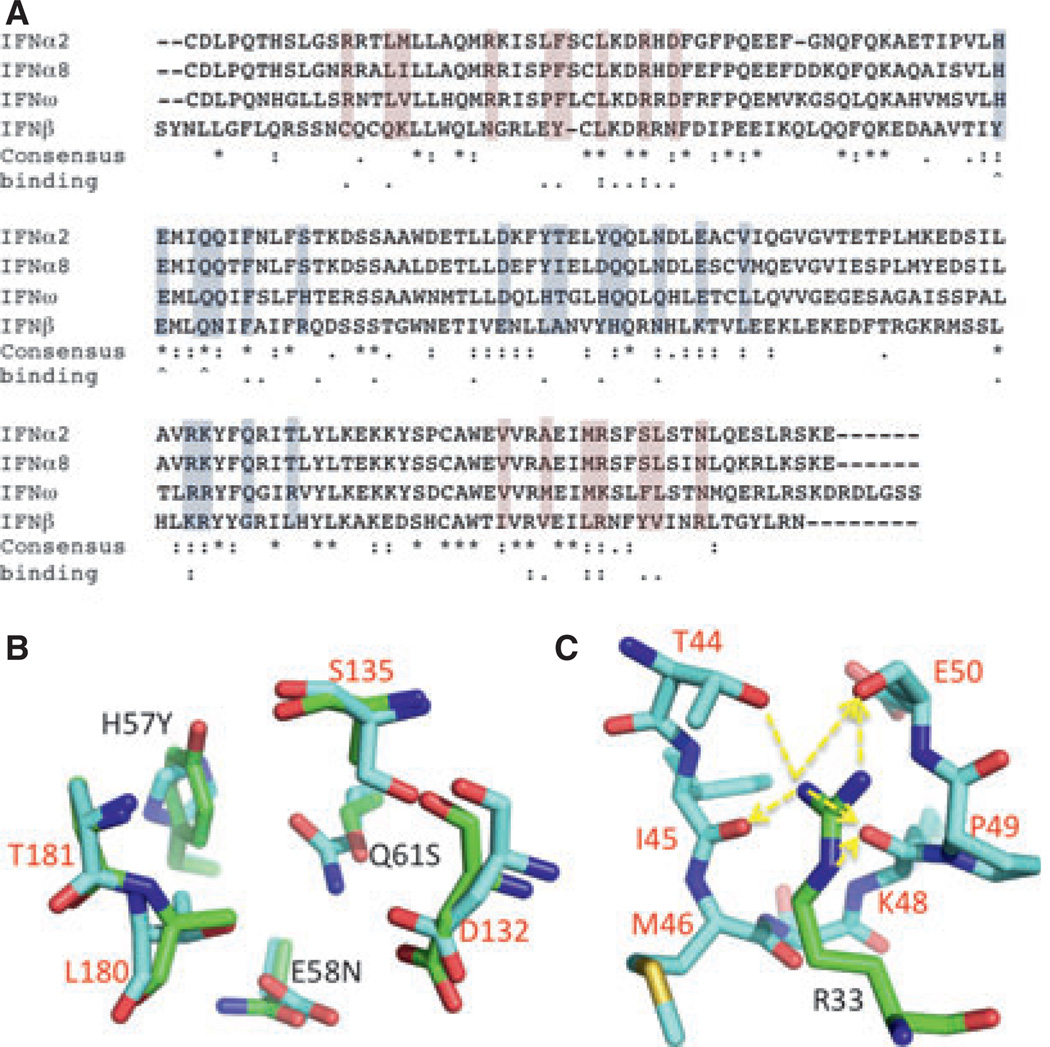
Mutagenesis and structural studies have identified the IFNAR2 binding site of IFNs to be located on helix A, the A–B loop, and helix E, whereas the IFNAR1 binding site involves helices B, C, and D (Fig. 7A). By far the most important hotspot in the IFN–IFNAR2 binding interface is the conserved Arg33IFN. Replacing this residue in IFNα2 by alanine destabilizes binding by over four orders of magnitude, with even the conserved mutation R33K reducing the binding affinity by 1000-fold (39). This is due to five side chains to backbone interactions of Arg33IFN to Ile45, Met46, Lys48, Pro49, and Glu50 on IFNAR2, in addition to the single side-chain–side-chain interaction with Thr44 (Fig. 7C). A second critical residue located on the A–B loop is L30: mutating this residue to Ala reduces binding by over 100-fold (39). Interestingly, the structures of the binary and ternary complexes do not reveal a direct strong contact between Leu30IFN and IFNAR2, suggesting that this residue may stabilize the IFN-binding site rather than directly contributing to binding. Two hydrophobic-interaction clusters are present in the IFNα–IFNAR2 interface, one is formed between Leu15IFN/Met16IFN and Trp100R2/Ile103R2, and the second one comprises Leu26IFN/Phe27IFN/Val142IFN binding Met46R2/Leu52R2/Val80R2/Thr44R2. Mutations of these residues (except Met16IFN) reduce binding by 3- to 50-fold. While none of the mutations in IFN to alanine increases binding, we found that replacing the C-terminal tail of IFNα2 with the strongly positively charged tail of IFNα8 increases binding to IFNAR2 by 20-fold [fourfold slower dissociation and fivefold faster association (64)]. Further mutagenesis work suggested that this is a result of binding to the three negatively charged residues Glu 132–134 on IFNAR2.
Fig. 7. Sequence alignment of several interferons (IFNs).
(A) Colored in gray–blue is the IFNAR1 binding site, and in pink the IFNAR2 binding site. The consensus relates to all IFNs, and not just to the four subtypes displayed. Binding relates to change in binding affinity upon mutation. Two dots are for hotspots (over 10-fold change upon mutation). ^denotes mutations to Ala that result in increase in binding. (B) Overlay of the binding site between IFN (numbers in black) and IFNAR1 (numbers in red) of the site of the YNS tight-binding mutation (green) and the consensus HEQ sequence (cyan). (C) The binding site between R33 of IFNα2 (numbered in black) and IFNAR2 (residues in cyan, numbers in red). Side-chain to main-chain hydrogen bonds are marked by arrows.
Except for Arg120IFN, no hotspot residue was identified on the IFNAR1 binding surface on IFN (60, 80) (Figs 6A and 7A). Surprisingly, three neighboring interface residues on IFNα2 (His57, Glu58, and Gln61, circled in Fig. 6A, C) were identified to increase binding affinity to IFNAR1 upon mutation to alanine (31). Two of the three residues (Glu58 and Gln61) are conserved between all IFNs, whereas His57 is conserved in all but IFNβ (Fig. 7A). Selection using phage display has identified the triple mutant H57Y, E58N, Q61S to increase the binding affinity to IFNAR1 by 50-fold [to 30 nm (81)]. With an affinity threefold higher than that of IFNβ, the IFNα2-YNS mutant is currently the IFN with the highest affinity for IFNAR1. Comparing the ternary complex structure of IFNω (which contains the original HEQ sequence) with that of IFNα2-YNS does not reveal the underlying reason for the 50-fold stabilization of this mutant, as no apparent clashes or stabilizing effects are observed (Fig. 7B). The fact that the IFNα2 affinity toward IFNAR1 was readily increased by individual mutations clearly suggests that weak binding to the IFNAR1 receptor chain is of biological importance, as it is conserved between the different subtypes.
Fig. 7 shows the consensus sequence of IFNs, the location of the binding interfaces, and the energetic consequence of mutations. As can be expected, most (but not all) residues that affect binding affinity are located at the interfaces; however, only five of 34 interface residues are fully conserved. Two of those are His57 and Glu58, which upon mutation into Ala increase binding to IFNAR1. There is also no direct relation between energetically important residues and their conservation. Still, three of six binding hotspots are fully conserved, and the other three are conserved in all IFNs, except for IFNβ or IFNω. The mutation data suggest that there are multiple solutions to obtain a similar binding affinity between IFN and its receptors.
Promiscuity of the receptor interface tolerates binding of the different type I IFNs
A detailed analysis of the residues involved in the IFNα2–IFNAR2 versus IFNω–IFNAR2 interaction highlights the complexity of IFN crossreactivity (32). An example of structural differences that affect the relative binding affinities is Arg149 in IFNα2 and the analogous Lys152 in IFNω and their respective energies of interaction with Glu77R2. Whereas Arg149 is a hotspot in IFNα2, Lys152 is of lesser importance for binding of IFNω (32). Indeed, the binding affinity of IFNω to IFNAR2 is increased by the K152R mutation. Additional examples of IFN sequence differences playing a role in ligand subtype discrimination include Leu26α2 (the L26A mutation causes a fivefold reduction in binding affinity), which corresponds to Pro28 in IFNω. The IFNω mutation P28A had no effect on receptor binding, whereas swapping Pro28ω with Leu26α2 (i.e. P28Lω) reduced binding by sixfold in IFNω. Thus, these residues have evolved distinct energetic contributions by substituting side chains. Two additional residues that differ between IFNα2 and IFNω in the IFNAR2 binding site are Phe152α2 that is a Ser in IFNω, and Ala145α2 that is Met148 in IFNω. The mutation S155Fω reduces binding by 3.5-fold, while F152Aα2 reduces binding by 10-fold (32). Within IFNα subtypes, alanine is the consensus residue in position 145 (148 in IFNω). Still, M148Aω reduces binding by approximately 2.5-fold. The complementary mutation of A145M in IFNα2 reduces binding by approximately sixfold. This shows that Ala in this position is preferred for IFNα subtypes, whereas Met is preferred for IFNω (and Val is preferred for other IFNs). These examples demonstrate again that swapping of these residues does not lead to reconstitution of the donor affinity.
On IFNAR2, the V80A mutation differentially affects IFN binding, with the contribution to IFNω being significantly smaller than to IFNα2 binding. Two other residues in IFNAR2, His76R2 and Met46R2, contribute to ligand discrimination. The double mutation on IFNAR2, H76A, N98A increases the binding affinity to IFNβ by 50-fold, while having no effect on binding to IFNα2 (63). The high affinity toward IFNβ makes the soluble, ECD of IFNAR2 with the 76/98 to Ala mutation an ideal carrier protein or antagonist for IFNβ, while not affecting IFNα binding or activity. Two other mutations on IFNAR2 that differentially affect IFNβ and IFNα2 binding are M46A that results in a 500-fold decrease in IFNα2 binding, but only 10-fold decrease in IFNβ binding, and W100A that reduces binding to IFNα2 by threefold and binding to IFNβ by 100-fold (82).
While mutation coverage of IFNAR1 is less complete than the mutational analysis of IFNAR2, a number of residues were found that upon mutation differently affect binding to IFN subtypes. Most notable is N155T that has no effect on binding of IFNα2 or IFNω, but increases the affinity toward IFNβ by eightfold [resulting in a nanomolar affinity interaction between these two proteins (54)]. Conversely, the IFNAR1 mutant E111A reduces the affinity toward IFNα2 by threefold and to IFNβ by >10-fold. A third residue is N242, which, upon mutation into Ala, reduces the affinity to IFNα2 by twofold, but increases the affinity to IFNβ by 50%. Overall, the mutagenesis data clearly show that in particular ligand binding to IFNAR1 but also to IFNAR2 is not optimized for high affinities. Moreover, specific amino acids on the receptors confer different energetic contributions toward different IFN subtypes. In conclusion, it appears that IFNs and their receptors have evolved to keep a certain range of binding affinities with significant variations in the absolute binding affinity as well as the ratio of binding affinity toward the receptor subunits. The IFNAR1 binding affinity of all IFNs is always much lower than the affinity for IFNAR2. With respect to the integral binding affinity toward the cell surface receptor, the range between the tightest binder IFNβ and the weakest binder IFNα1 covers more than three orders of magnitude. The IFNα subtypes, however, bind within a relatively narrow range of affinities, in between those measured for IFNα1 and IFNβ. These insights substantiate a hypothesis that rather than the structure of the signaling complex, the large differences in binding affinities and complex stabilities of IFNs are responsible for differential activity.
Systematic correlation of binding affinities with IFN activities
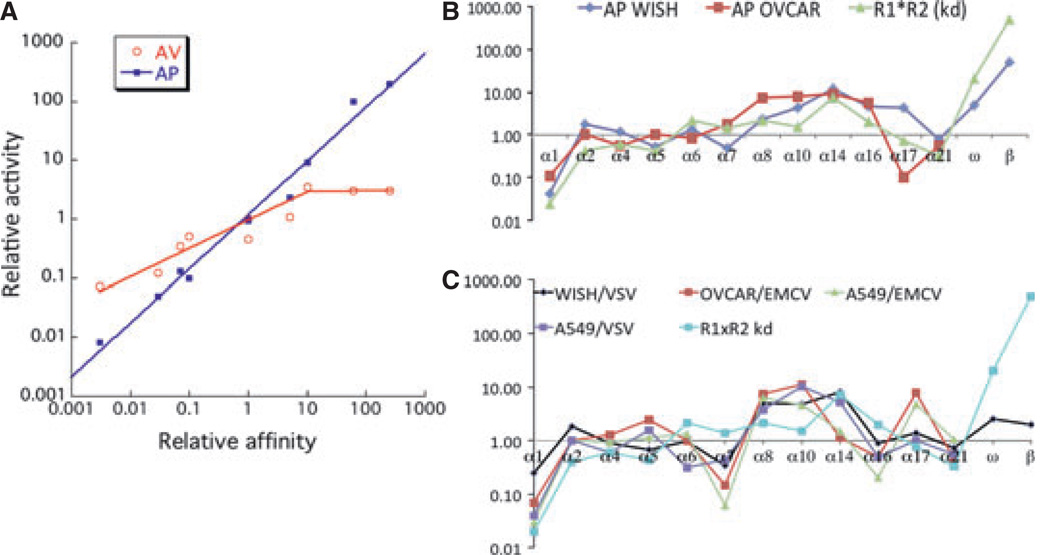
The detailed energetic information on the binding interfaces and the possibility to systematically reduce and increase IFNs affinities toward IFNAR1 and IFNAR2 allowed exploring the role of receptor binding affinity in activating biological activities. Using protein engineering, the complete range of naturally occurring binding affinities of the different IFNs was reconstructed on the template of IFNα2 (38–40, 60, 81). Using these muteins allowed investigating the relations between affinities to either one of the receptors and the seemingly differential biological activities of the different IFNs. To this end, the relative inverse EC50 values of antiviral and antiproliferative dose–response analyses were compared with the product of the binding affinities toward the two-receptor subunits (Fig. 8A). A very good linear relation between relative binding affinity and antiproliferative potency is observed spanning five orders of magnitude (Fig. 8A). Fig. 8B shows that the binding affinities measured for the different IFNα subtypes also correlate with their antiproliferative potencies. This strict correlation clearly demonstrates that antiproliferative activity is foremost determined by ligand affinity. In contrast, the antiviral activity correlates with the affinity only for low binding affinities (Fig. 8A). For IFNs with binding affinities higher than IFNα2, only a marginal increase in the antiviral activity is observed. Moreover, the slope of the linear affinity–activity correlation for mutants with lower binding affinity than wildtype IFNα2 is below 1, suggesting that binding affinity is not the sole factor dictating antiviral potency (39). Thus, rather than the higher antiproliferative activity of IFNβ compared with IFNα2, which can be explained by its higher binding affinity, the very similar antiviral activity of these two IFNs is a puzzling factor responsible for their differential activity.
Fig. 8. Correlation with affinities and activities of interferon (IFN) subtypes and mutants.
(A) Antiviral (AV) and antiproliferative (AP) potencies of IFNα2 mutants as a function of the product of the binding affinities toward IFNAR1 and IFNAR2. All the values are relative to those determined for IFNα2. (B) Antiproliferative activities and (C) antiviral protection of all IFNα subtypes as well as IFNβ and IFNω in different cell lines (WISH, OVCAR, and A549) in comparison to the product of the binding affinity (R1 × R2). The viruses used were vesicular stomatitis virus (VSV) and encephalomyocarditis virus (EMCV).
Consistent with the mutant data, significant variations between receptor binding affinity and antiviral potency were observed also between the different IFNα subtypes and between the different viruses and cell lines used (Fig. 8C). The low activity of IFNα1 is consistent with its low receptor binding affinity, whereas the relative high antiviral activity of IFNα17 is surprising (83), as its binding affinity is similar to IFNα2. Yamamoto et al. (79) selected by phage display IFNα8 variants that differ by 100-fold in their antiviral potency on amnionic FL challenged with Sindbis virus versus LS 174 T cells challenged with VSV. This finding suggests that yet unknown factors tune the specific antiviral potency (in addition to binding affinity). One suggestion was that structural changes in the C-helix of IFNα alter the ability of IFN to limit retroviral activity and reduce toxicity (84). Classifying IFNα proteins by their ability to induce specific genes showed that this is a good marker for their ability to confer cellular protection against pathogens, independent of the target or cellular background. Differences in IFN activity were only observed at subsaturating levels. The divergent potencies of IFNαs and the cell-type-specific regulation of target genes may result in the tuning of the cellular defense (10). Another way to analyze the importance of individual IFN subtypes is by monitoring their divergence in human population. Type II and type III IFNs as well as some type I variants have evolved under strong purifying selection, whereas other type I variants have more relaxed selective constraints, in agreement to the degree of importance in immunity to infection (85).
Systematic modulation of binding affinities toward IFNAR1 and IFNAR2 allowed addressing the question whether higher binding to either of the two-receptor subunits dictates a specific activity (38). Increasing binding to IFNAR1 while decreasing binding to IFNAR2 affected the antiproliferative potency consistent with the change in the integral affinity toward both receptor subunits, independent on the identity of the high-affinity receptor. A similar result was also obtained for the antiviral activity; however, the relation between affinity and activity was much weaker as described above (Fig. 8A). Another critical difference between the activation of an antiviral state and of an antiproliferative response is the duration of IFN induction. To initiate antiviral activity requires a few hours of IFN, whereas antiproliferative activity requires days of constant IFN induction.
The number of surface receptors is another important variable in signaling, which differs between individual cells (76). Manipulating receptor expression by siRNA concentrations reduced the fraction of responsive cells independent of the IFN used. A correlation between receptor numbers, STAT activation, and gene induction was observed. Our data suggest that for a given cell the response is binary (±) and dependent on the stochastic expression level of the receptor subunits on an individual cell. All of our data suggest that the antiviral activity of IFN is a robust feature, requiring very low ligand and receptor concentrations over a short time and is common to all cells. Conversely, antiproliferative activity (and other IFN-related activities requiring signaling over longer time periods) requires high IFN and receptor concentrations as well as tight binding over a prolonged time and thus is a tunable function of IFN that strongly differs between different cells and different IFNs (40). Indeed, it was shown that IFN-receptor expression regulates the antiproliferative effects of IFNs on cancer cells and solid tumors, suggesting that IFN-receptor expression can play a major role in determining the clinical outcome of IFN-based cancer therapeutics (86).
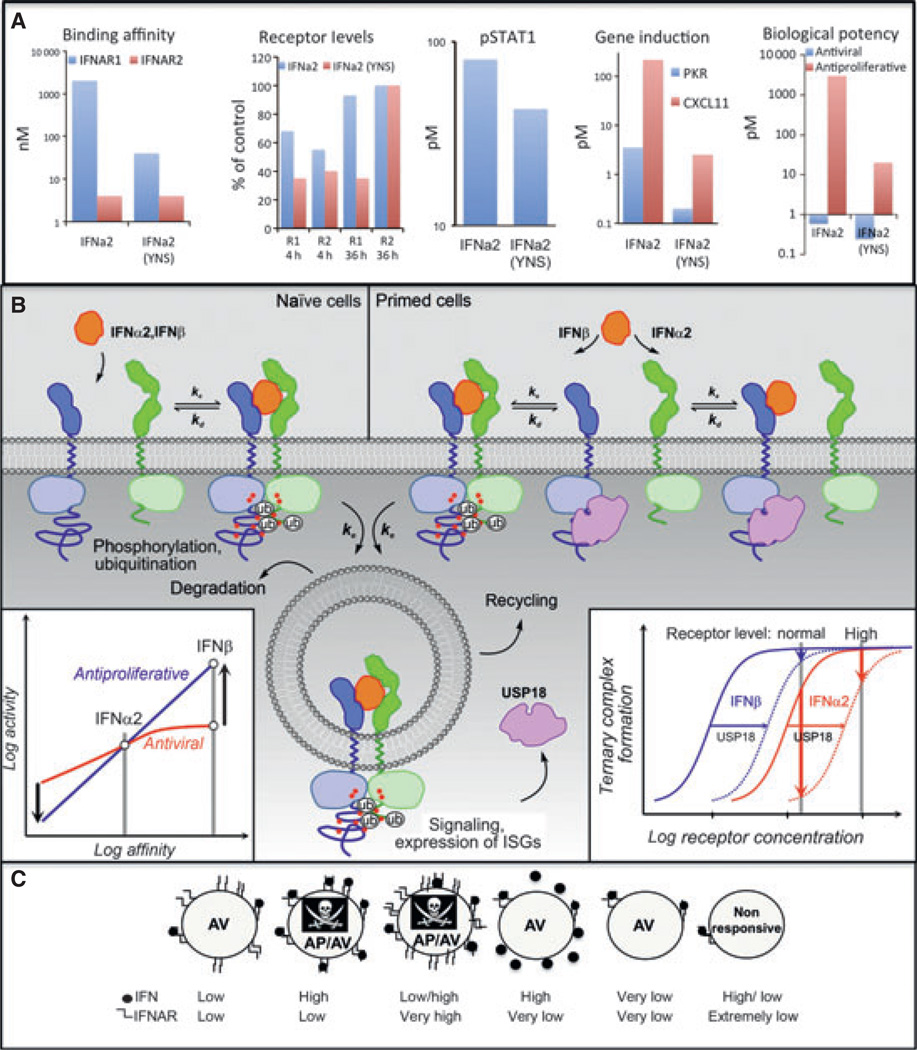
These systematic correlations of binding affinities with functional properties of IFNs in different cellular context clearly established the key role of receptor binding affinity for differential IFN activity. For the proof-of-concept studies, an engineered IFNα2 variant containing the mutations H57A, E58A, and Q61A (IFNα2-HEQ) was used, which binds IFNAR1 with a similar affinity as IFNβ (31). Strikingly, this IFNα2 mutant already very closely mimicked the functional properties of IFNβ (Fig. 9), whereas STAT activation and antiviral activity by IFNα2-HEQ increased only slightly compared with wildtype IFNα2, a dramatic increase in the antiproliferative activity was obtained (Fig. 9A). IFNα2-HEQ also reproduced the gene activation pattern of IFNβ with high fidelity, which was not observed for wildtype IFNα2 even at strongly elevated concentrations (Fig. 9B). By further optimization of the binding affinity toward IFNAR1 (IFNα2-YNS), the differential activity compared with wildtype IFNα2 was even further increased (81).
Fig. 9. Similar and differential activities by type I interferons.
(A) EC50 values of activation of the antiviral and antiproliferative responses and of IFI6–16 gene induction. (B) Gene induction after different treatments with interferons as determined by gene array.
The dynamics of receptor assembly on artificial membranes and in living cells
How can differential binding affinities modulate cellular response patterns? To answer this question, a quantitative mechanistic understanding is required on the role of differential binding affinities and rate constants for the formation and the dynamics of the ternary IFN-receptor signaling complex on the plasma membrane. To this end, extensive studies were performed in vitro using artificial membranes. To mimic membrane anchoring of the receptor subunits, the extracellular domains of IFNAR1 and IFNAR2 were site-specifically tethered onto solid-supported membranes through their C-terminal His-tag (75, 87, 88). For probing IFN binding and ternary complex assembly in a quantitative manner, real-time detection by simultaneous total internal reflection fluorescence spectroscopy and reflectance interference was used (87). By using this approach, the surface concentration of the receptor subunits could be readily controlled and quantified and the kinetics of interactions could be probed in a highly versatile manner (89).
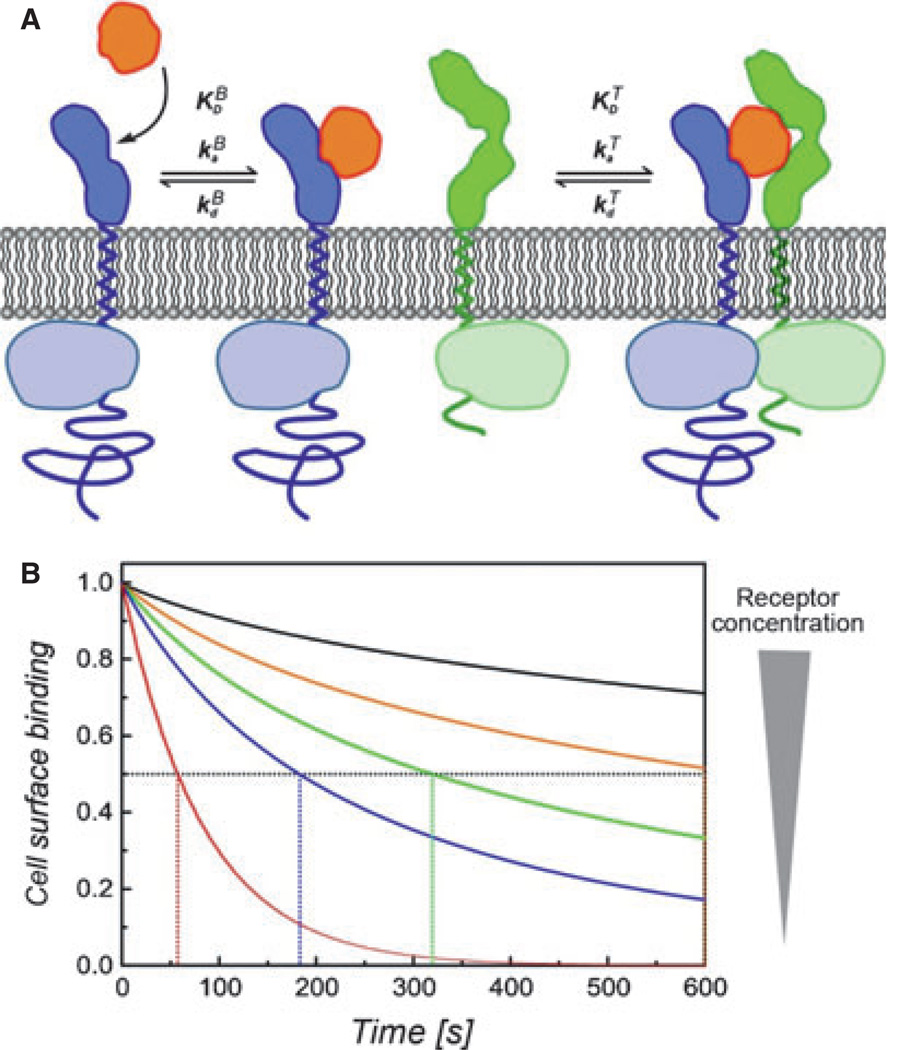
No interaction between the receptor subunits was detectable in the absence of IFN and a two-step assembly of the ternary complex was observed (Fig. 10): after IFN binding to the high-affinity subunit IFNAR2, IFNAR1 is subsequently recruited on the membrane. The sequence of binding events is determined by the substantially faster binding of IFN to IFNAR2 than to IFNAR1 (kaB) rather than the higher equilibrium binding affinity (KDB). This mechanism entails a dynamic equilibrium between binary and ternary complexes on the plasma membrane, which is determined by (i) the affinity of IFN toward IFNAR1 (KDT), and (ii) the concentration of the receptor subunits. Both, the concentrations of the receptor subunits and KDT refer to area (e.g., molecules/µm2) rather than to volume (e.g., µm) concentrations because the receptor subunits are anchored in the membrane. Thus, KDT cannot be readily inferred from the equilibrium constant of the binary complex as determined by standard-binding assays. However, the equilibrium between binary and ternary complex can be assessed by ligand-binding experiments (78). As ligand dissociation from the ternary complex is much slower than from the binary complex, the equilibrium between binary and ternary complexes also affects the total ligand-binding affinity to the cell surface receptor (Fig. 10B). Thus, the decrease in ligand dissociation kinetics compared with the binary IFN–IFNAR2 interaction can be used as a measure for ternary complex formation (87). Based on this approach, KDT was determined for the ligand IFNα2 to be approximately 40 molecules/µm2, which in solution binds IFNAR1 with a KDBof approximately 5 µm. This KDT is surprisingly high compared with the concentration of the IFNAR1 and IFNAR2 in the plasma membrane, for which typically only a few hundred copies per cell are found (i.e., 0.1–1 molecule/µm2). Thus, the formation of ternary complexes by IFNα2 and other IFNα subtypes, which all have very similar binding affinities toward IFNAR1, is probably limited by the physiological cell surface concentrations of the receptor subunits. Under these conditions, two interdependent equilibria have to be considered: (i) IFN in solution and bound to the cell surface receptor, and (ii) binary and ternary complexes. In contrast, the approximately 30-fold higher binding affinity of IFNβ toward IFNAR1 (see above) probably ensures efficient ternary complex formation at physiologically relevant receptor surface concentrations. It is important to note that the limitation of ternary complex formation by KDTcannot be compensated by increasing the IFN concentration, as the cell surface concentration of IFNα2 bound to IFNAR2 is the determining parameter. Thus, differential efficacy in the recruitment of IFNAR1 into the ternary complex achieved by IFNα subtypes and IFNβ would be a good candidate to explain the biophysical basis of differential activities.
Fig. 10. Receptor assembly and dynamics in the plasma membrane.
(A) Two-step assembly of the ternary interferon(IFN)–receptor complex in the plasma membrane (orange, IFN; blue, IFNAR2; green, IFNAR1): rapid and high-affinity binding of IFN to IFNAR2 is followed by recruitment of IFNAR1 into the ternary complex. The dynamic equilibrium between binary and ternary complexes depends on the 2D dissociation constant KDT and 2D concentrations of the receptor subunits. (B) Implications of this mechanism for ligand-binding affinity and kinetics: normalized ligand dissociation kinetics at different cell surface concentrations of the receptor subunits compared with the dissociation from IFNAR2 only (red curve). The half-life of IFN binding to the cell surface receptor (indicated by dotted lines) increases with increasing receptor concentrations. The orange curve approximately corresponds to the affinity observed in cell surface binding experiments.
A dynamic equilibrium between binary and ternary complexes results in a constant exchange of receptor subunits. This may play a critical role for signaling, as the lifetime of individual signaling complexes is given by KdT. Again, the 2D rate constants KdT and KdT cannot be readily inferred from the corresponding 3D rate constants obtained by classic-binding assays, as membrane anchoring also changes the energy landscape and thus the reaction coordinate. By exploiting the experimental flexibility of the model system described above, the rate constant KdT of individual ternary complexes was probed by FRET and ligand chasing experiments (78, 88, 89). These experiments yielded a complex stability that is three to fivefold higher compared with the same interaction measured in 3D. From KdT and KDT, also KaT was estimated. Interestingly, collisions on the membrane are 10- to 100-fold more productive with respect to complex formation when compared with the interaction in solution. At the same time, the number of collisions is lower on the membrane because of the reduced diffusion constant.
These results obtained with artificial, fully homogeneous membranes, which allow free diffusion of the tethered receptor subunits, suggest that the much more spatial organization and diffusion of the receptor subunits in the plasma membrane (90) may play an important role for the dynamics of individual ternary complexes. A detailed, quantitative picture of assembly and dynamics of the IFN signaling complex in the plasma membrane is currently not available, but some important features are emerging. For several homodimeric class I cytokine receptors, pre-association of the receptor subunits has been observed (66–68), and this has also been suggested to hold true for the IFNγ receptor (64), which belongs to the class II cytokine-receptor family. For IFNAR1 and IFNAR2, pre-association of the receptor subunits has not been detected in living cells (6). However, the more than 10-fold increased ligand-binding affinity compared with binding to IFNAR2 only – even at endogenous receptor levels (6) – suggests approximately 100-fold more efficient recruitment of IFNAR1 than predicted by the studies described above (Fig. 10B). As the concentration of the receptor subunits plays a critical role for the equilibrium between binary and ternary complexes, studies with cells overexpressing IFNAR1 and IFNAR2 as required for fluorescence imaging techniques are of limited use. Imaging techniques with single molecule sensitivity are required for resolving receptor assembly at physiological expression levels. Tracking of individual IFNAR1 and IFNAR2 yielded strongly inhomogeneous diffusion behavior with average diffusion constants of 0.05–0.08 µm2/s (6, 91), which can be ascribed to corralling by the cortical actin skeleton (92). Pre-assembly or clustering of IFNAR1 and IFNAR2 was not observed in absence of the ligand (JP, unpublished results). IFN stimulates very rapid formation of ternary complexes, which dynamically form and dissociate as suggested by the artificial membrane system described above (JP, unpublished results). However, not only the efficiency of ternary complex formation but also the lifetime of individual complexes is substantially higher in the plasma membrane of living cells compared with the artificial membrane (JP, unpublished results). These results suggest that further organization principles such as lipid microdomains and/or the membrane-proximal actin corrals may contribute to the receptor assembly on the cell surface (90).
The importance of the lifetime of individual complexes (determined by KdT) for signaling is not clear. Short-term signal activation measured as STAT phosphorylation levels does not significantly differ for stimulation by IFNα2 or IFNβ despite the strong differences in affinity of the interaction with IFNAR1 and the resulting long lifetime of individual ternary complexes. Upon a further decrease in the stability of the interaction of IFNα2 with IFNAR1, a strong loss in STAT phosphorylation is observed (60, 80), but this is probably due to the loss in ternary complex formation. The increased lifetime of ternary complexes formed with IFNβ compared with IFNα2, however, could be responsible for more efficient activation of other, STAT-independent signaling pathways and thus explain differential IFN activities. So far, this has not been experimentally demonstrated.
Receptor endocytosis and negative feedback mechanisms and their possible role for differential signaling
The numbers of IFNAR1 and IFNAR2 on the cell surface are notoriously low, between 100 and a few 1000 copies/cell, dependent on the cell type (76). Cell surface expression and endocytosis of the IFN-receptor subunits is regulated on different levels and endocytic trafficking is not fully resolved. The cytoplasmic domain of IFNAR1 contains an endocytic motif mediating recruitment of the AP2 complex, which is responsible for stimulation-independent endocytosis and degradation of IFNAR1 (93). Interestingly, this motif is masked by the JAK kinase Tyk2, which is constitutively associated with IFNAR1, thus stabilizing cell surface expression of IFNAR1 (93). Upon IFN-induced receptor activation, ubiquitination of IFNAR1 is induced via specific phosphoserine residues on the cytoplasmic domain of IFNAR1 (94). These serve as docking sites for an ubiquitin E3 ligase, which in turn ubiquitinates IFNAR1 on several lysine residues (95). The ligase is recruited to IFNAR1 upon its degron phosphorylation by PKD2, the inhibition of which (and subsequent increase in IFNAR levels) increases the sensitivity of cells to IFN (96). Interestingly, ubiquitination results into a conformational change, by which the constitutive endocytic motif is unmasked (97), leading to endocytosis of the activated signaling complex. Endocytosis of the activated IFN signaling complex was confirmed to follow a classic clathrin –dynamin pathway (98). The non-catalytic role of Tyk2 in sustaining the steady-state IFNAR1 level at the plasma membrane is well documented (99). Recently, it was shown that SOCS1 acts in sequestering the IFN signal by directly interacting with Tyk2. However, the SOCS1 inhibition of Tyk2 does not only inhibit Tyk2 kinase-mediated STAT signaling but also negatively impacts IFNAR1 surface expression, which is stabilized by Tyk2, and thus provides an additional level of regulation (100). Analysis of the endosomal compartment after IFN stimulation revealed ubiquitinated IFNAR1 and a small amount of IFNAR2 as well as tyrosine-phosphorylated Tyk2 and Jak1, suggesting continuous signaling during endocytosis, which may relate to the ability of IFN to induce a multitude of signals (101). Despite the extensive work showing the importance and mechanism of IFNAR1 ubiquitination and its importance in endocytosis and subsequent sorting, no clear evidence exists to show that ubiquitination has a positive role in differential signaling by IFNs (102).
A rapid decrease in both IFNAR1 and IFNAR2 levels on the cell surface is observed after IFN stimulation (31, 32, 95, 103). Whereas transient downregulation was observed also when applying low-IFN concentrations, a tight correlation was observed between the ability of IFNs to continuously induce endocytosis of IFNAR1 and IFNAR2 and their antiproliferative potency (81), with tighter binding IFNs (such as IFNβ or IFNα2-YNS) promoting an increased receptor downregulation (32). However, tight binding to only one of the receptor subunits, without binding to the second receptor subunit, will neither promote antiproliferative activity nor cause receptor downregulation, and thus will act as an antagonist (80). Moreover, irreversible IFN uptake is observed, confirming endocytosis of the entire signaling complex. Interestingly, the K152R mutation on IFNω, which specifically increases binding to IFNAR2 by fivefold, dramatically increases IFNAR2 endocytosis (32). Thus, also the interaction with IFNAR2 seems to play a critical role for regulating endocytosis or endocytic trafficking.
Several implications of IFN-receptor endocytosis for (differential) signaling have been suggested. (i) The resulting decrease in surface concentration of IFNAR1 and IFNAR2 on the cell surface probably shifts the equilibrium from ternary to binary complex (31). Indeed, the concentrations of IFNAR1 and IFNAR2 in the plasma membrane play a critical role for their sensitivity toward IFNs. While in cells with native receptor numbers IFNβ was more potent than IFNα2 in antiproliferative activity, IFNα2 matched IFNβ in cells with highly increased receptor numbers (76). Decreasing the cell surface expression of IFNAR subunits has been shown to affect responsiveness toward IFNα2 stronger than toward IFNβ (40). This can be explained by more efficient recruitment of IFNAR1 by IFNβ due to its higher affinity. These observations could explain why long-term signaling is possible by IFNβ but not IFNα2. (ii) It is very likely that entire signaling complexes are taken up by endocytosis. Signaling may proceed in early endosomes, and notably, even a critical role of endocytosis for signaling has been suggested (98). In any case, endocytosis could explain why the potency of IFNs with respect to very early cellular responses does not increase with affinity above a certain threshold (Fig. 8A): an increase in binding affinity is typically accompanied by an increase in complex stability; if reversible ternary complex formation is followed by irreversible endocytosis, an increase in complex stability pays off only up to a certain threshold, above which complexes are faster endocytozed than they dissociate (Fig. 11).
Fig. 11. A basic model to explain differential activity of interferon (IFN), i.e. diverging affinity–activity relationships for different cellular responses.
(A) The bar-graphs show similarities and differences in response to IFNα2 and the tight binding variant IFNα2-YNS. (B) Shows a model that explains how differential activation (highlighted by the arrows in the bottom-left inset) can be rationalized. In naïve cells (left), reversible IFN binding and ternary complex formation is followed by endocytosis of the signaling complex. If the rate constant of endocytosis _k_e significantly exceeds the rate constant of complex dissociation _k_d, ternary complex formation can be considered irreversible, independent on the IFN binding affinity. While signaling probably proceeds in early endosomes, endocytosis also leads to receptor degradation. Thus, similar activities for IFNα2 and IFNβ are observed in naïve cells. Cells activated with IFNs over extended time periods (primed cells) express the negative feedback regulator USP18, which interferes with ternary complex formation by interacting membrane proximal with the intracellular region of IFNAR. Owing to the lower affinity of IFNα2 toward IFNAR1, a further reduction by USP18 drastically reduces ternary complex formation for this ligand at the typically low receptor surface concentrations (bottom-left inset). Thus, signaling by IFNα2 is abrogated, while this effect is overcome by the much higher affinity of IFNβ toward IFNAR1 and IFNAR2. At increased receptor levels, only minor desensitization is observed. (C) Low occupancy of few receptors is sufficient to initiate an antiviral state, whereas only high occupancy of many receptors will initiate the antiproliferative response. Therefore, cells with reduced receptor numbers will not initiate an antiproliferative response, whereas cells with increased receptor numbers will induce an antiproliferative response even at lower IFN concentration.
Specific functions of IFNβ compared with IFNα subtypes require long-term activation of the signaling complex. Thus, negative feedback mechanisms on the level of gene transcription are likely to play a critical role in regulating differential cellular responses. An important negative feedback mechanism is based on IFN-induced expression of the isopeptidase USP18 (UBP43). USP18 specifically removes the ubiquitin-like protein ISG15 from target proteins (104). Protein ISGylation has complex regulatory functions in IFN signaling (105). Interestingly, USP18-deficient mice are hypersensitive to IFN, suggesting a critical role in negative feedback regulation (106). Rather than by its enzymatic activity, USP18 was shown to interfere with IFN signaling by binding to the membrane-proximal region of the cytoplasmic domain of IFNAR2 (107). Interestingly, USP18 regulates signal activation on the level of the assembly of the signaling complex, as binding and uptake of IFNα2 is strongly reduced in cells expressing USP18 (108). Binding of USP18 to the cytoplasmic domain of IFNAR2 does not affect the binding affinity of the extracellular domain, which binds IFNs independent of the membrane and the transmembrane domain (JP, unpublished results). Rather, it appears that USP18 interferes with the assembly of the ternary complex by increasing KDT substantially above the cell surface concentration of the receptor subunits. This hypothesis is consistent with the observation that expression of USP18 results in a strong loss of sensitivity toward IFNα2 but not toward IFNβ (108). This can be explained by the substantially higher binding affinity of IFNβ toward IFNAR1, corroborating the notion that binding of USP18 to the cytoplasmic domain of IFNAR2 interferes with ternary complex formation. However, the important consequence of differential negative feedback by USP18 is that signaling by IFNα subtypes but not by IFNβ is abrogated by the first wave of cellular responses. This could explain why IFNβ can more efficiently elicit responses, which require maintaining signaling over extended time periods.
Concluding remarks
In this review, we have highlighted the comprehensive insight into the structure, energetics, and dynamics of IFN recognition by its receptor subunits, and how this is translated into gene induction and biological activities (briefly summarized in Fig. 11A). One of the challenges of studying type I IFNs is that they are produced by and act on all nucleated cells and activate a wide range of activities. The common denominator for all type I IFNs is their ability to induce an antiviral state as part of the innate immunity. More cell-type-specific activities include the promotion/inhibition of cell differentiation, antiproliferation (cell cycle arrest and apoptosis), and others. The differential activities of IFNs are manifested as different affinity–activity relationships for these different cellular responses. One cannot expect to obtain a simple yet comprehensive model to explain IFN action in all cell types, particularly as signal transduction cascades may vary in different backgrounds. However, based on the conceptual understanding of receptor assembly and dynamics and its regulation by endocytosis and USP18, we can put forward a basic model, how differential IFN activity can be mediated through a shared receptor (Fig. 11B). In naïve cells, all type I IFNs apparently bind the same two receptor subunits at the same orientation but with varied affinities. Still, at physiological receptor densities, IFN binding to the cell surface receptor rapidly induces formation of the ternary signaling complex with similar efficiency for all IFNs. Binding affinities even of the IFNα subtypes toward IFNAR1 (KDT) and the receptor surface concentrations are probably adjusted to a ratio, which allows efficient recruitment of IFNAR1 into the ternary complex (Fig. 11B, bottom-right insert). After signal activation, signaling complexes are rapidly endocytozed. Thus, the rate of endocytosis and degradation/recycling rather than the ligand dissociation kinetics dictates the decomposition of the signaling complex. This could explain why similar activities can be observed for all IFNs except IFNα1 in the early STAT phosphorylation and the immediate antiviral responses. For IFNα2 mutants with decreased complex lifetimes (as well as for IFNα1), endocytosis competes with ligand dissociation. Thus, the activities of these IFNs decrease with decreasing receptor binding affinity. Differences in potencies of IFNα subtypes may be explained also by differences in endocytic trafficking (i.e. rates of degradation and recycling), an issue requiring further investigation. IFNα2 mutants with lower binding affinity dissociate more rapidly than they are endocytozed and, for this reason, show reduced activity compared with wildtype IFNα2.
After the first wave of gene transcription/translation, negative feedback by USP18 reduces IFN affinity to the cell surface receptor, probably caused by increasing KDT toward IFNAR1 (‘primed cells' in Fig. 11B). As the lifetime of the ternary complex is reduced, ligand dissociation competes with endocytosis and the activity of IFNα2 in primed cells decreases. Owing to its much higher binding affinity, IFNβ (as well as IFNα2 mutants with increased binding affinities) can even in the presence of USP18 form highly stable ternary complexes, which are probably endocytozed prior to dissociation. Thus, the responsiveness of primed cells is substantially more reduced for IFNα2 compared with IFNβ, and cellular responses requiring prolonged signaling can be sustained more efficiently by IFNβ compared with the IFNα subtypes. This model implicates that the low binding affinity of IFNα subtypes toward IFNAR1 relative to the receptor cell surface expression level is evolutionary optimized to a level that allows selective tuning by the negative feedback regulator USP18. For cells with an increased level of cell surface receptor expression, this regulatory mechanism is not viable, which is consistent with the observation that differential activity is observed only for cells with low receptor expression levels. This model explains why receptor numbers affect biological outcome (Fig. 11C). Cells with very high receptor numbers will be able to initiate an antiproliferative response even with lower concentration of IFNα, whereas cells with very low receptor numbers will not be able to initiate such response even with high IFNβ. Conversely, the antiviral response is robust and will be initiated both with high and low receptor numbers.
Our model explains why the low binding affinity of IFNα subtypes appears to be evolutionary conserved, i.e. optimized for ensuring that cellular responsiveness is abrogated by the early cellular response. However, other features of differential IFN signaling remain enigmatic. For example, signaling leading to IFN-induced antiproliferative activity that requires the continuous presence of IFN at concentrations that saturate the surface receptors for a prolonged time cannot be explained by STAT signaling alone. Moreover, pSTATs are not detectable during long-term stimulation that is a requirement for this activity. Also the molecular mechanism supporting induction of transcription of many antiviral genes by picomolar IFN concentrations, whereas other genes require 100-fold higher concentration is not resolved. The linear relation between antiproliferative potency and binding affinity and the higher number of activated surface receptors required for the induction of antiproliferative activity suggest that this activity may be dominated by alternative signaling cascades. It is possible that the higher lifetime of individual ternary complexes formed by IFNβ compared with IFNα allows more efficient activation of alternative signaling pathways that are not related to the antiviral activity. In this context, the role of endosomal signaling, sorting, and trafficking of the IFN–receptor complex remains to be explored in more detail. Answers to these questions may hold the promise to apply natural and engineered IFNs in more efficacious ways to combat diseases, such as cancer, multiple sclerosis, viral infections, and others.
Acknowledgements
We thank Sandra Pellegrini, Gilles Uzé, and Jörg Stelling for the fruitful discussions. Financial support by the European Community’s Seventh Framework Programme (FP7/2007–2013) under grant agreement n° 223608 (IFNaction) is gratefully acknowledged.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Schoggins JW, et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–267. [PubMed] [Google Scholar]
- 4.Uze G, Lutfalla G, Gresser I. Genetic transfer of a functional human interferon alpha receptor into mouse cells: cloning and expression of its cDNA. Cell. 1990;60:225–234. doi: 10.1016/0092-8674(90)90738-z. [DOI] [PubMed] [Google Scholar]
- 5.Novick D, Cohen B, Rubinstein M. The human interferon alpha/beta receptor: characterization and molecular cloning. Cell. 1994;77:391–400. doi: 10.1016/0092-8674(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 6.Cohen B, Novick D, Barak S, Rubinstein M. Ligand-induced association of the type I interferon receptor components. Mol Cell Biol. 1995;15:4208–4214. doi: 10.1128/mcb.15.8.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decker T, Muller M, Stockinger S. The yin and yang of type I interferon activity in bacterial infection. Nat Rev Immunol. 2005;5:675–687. doi: 10.1038/nri1684. [DOI] [PubMed] [Google Scholar]
- 8.Morrell CN, et al. Beta interferon suppresses the development of experimental cerebral malaria. Infect Immun. 2011;79:1750–1758. doi: 10.1128/IAI.00810-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meissner NN, Swain S, Tighe M, Harmsen A, Harmsen A. Role of type I IFNs in pulmonary complications of Pneumocystis murina infection. J Immunol. 2005;174:5462–5471. doi: 10.4049/jimmunol.174.9.5462. [DOI] [PubMed] [Google Scholar]
- 10.Moll HP, Maier T, Zommer A, Lavoie T, Brostjan C. The differential activity of interferon-alpha subtypes is consistent among distinct target genes and cell types. Cytokine. 2011;53:52–59. doi: 10.1016/j.cyto.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berry CM, Hertzog PJ, Mangan NE. Interferons as biomarkers and effectors: lessons learned from animal models. Biomark Med. 2012;6:159–176. doi: 10.2217/bmm.12.10. [DOI] [PubMed] [Google Scholar]
- 12.De Weerd NA, Nguyen T. The interferons and their receptors – distribution and regulation. Immunol Cell Biol. 2012;90:483–491. doi: 10.1038/icb.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 14.Stark GR, Darnell JEJ. The JAK–STAT pathway at twenty. Immunity. 2012;36:503–514. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang BX, Rahbar R, Fish EN. Interferon: current status and future prospects in cancer therapy. J Interferon Cytokine Res. 2011;31:545–552. doi: 10.1089/jir.2010.0158. [DOI] [PubMed] [Google Scholar]
- 16.Croze E. Differential gene expression and translational approaches to identify biomarkers of interferon beta activity in multiple sclerosis. J Interferon Cytokine Res. 2010;30:743–749. doi: 10.1089/jir.2010.0022. [DOI] [PubMed] [Google Scholar]
- 17.Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 18.Pitha PM, Kunzi MS. Type I interferon: the ever unfolding story. Curr Top Microbiol Immunol. 2007;316:41–70. doi: 10.1007/978-3-540-71329-6_4. [DOI] [PubMed] [Google Scholar]
- 19.Uze G, Schreiber G, Piehler J, Pellegrini S. The receptor of the type I interferon family. Curr Top Microbiol Immunol. 2007;316:71–95. doi: 10.1007/978-3-540-71329-6_5. [DOI] [PubMed] [Google Scholar]
- 20.Rani MR, Foster GR, Leung S, Leaman D, Stark GR. Characterization of beta-R1, a gene that is selectively induced by interferon-beta (IFN-beta) compared with IFN-alpha. J Biol Chem. 1996;271:22878–22884. doi: 10.1074/jbc.271.37.22878. [DOI] [PubMed] [Google Scholar]
- 21.Abramovich C, et al. Differential tyrosine phosphorylation of the IFNAR chain of the type I interferon receptor and of an associated surface protein in response to IFN-alpha and IFN-beta. EMBO J. 1994;13:5871–5877. doi: 10.1002/j.1460-2075.1994.tb06932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Platanias LC, Uddin S, Domanski P, Colamonici OR. Differences in interferon alpha and beta signaling. Interferon beta selectively induces the interaction of the alpha and betaL subunits of the type I interferon receptor. J Biol Chem. 1996;271:23630–23633. doi: 10.1074/jbc.271.39.23630. [DOI] [PubMed] [Google Scholar]
- 23.Russell-Harde D, Wagner TC, Perez HD, Croze E. Formation of a uniquely stable type I interferon receptor complex by interferon beta is dependent upon particular interactions between interferon beta and its receptor and independent of tyrosine phosphorylation. Biochem Biophys Res Commun. 1999;255:539–544. doi: 10.1006/bbrc.1998.0105. [DOI] [PubMed] [Google Scholar]
- 24.Foster GR, et al. IFN-alpha subtypes differentially affect human T cell motility. J Immunol. 2004;173:1663–1670. doi: 10.4049/jimmunol.173.3.1663. [DOI] [PubMed] [Google Scholar]
- 25.Kalinke U, Prinz M. Endogenous, or therapeutically induced, type I interferon responses differentially modulate Th1/Th17-mediated autoimmunity in the CNS. Immunol Cell Biol. 2012;90:505–509. doi: 10.1038/icb.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marijanovic Z, Ragimbeau J, Van der Heyden J, Uze G, Pellegrini S. Comparable potency of IFNalpha2 and IFNbeta on immediate JAK/STAT activation but differential down- regulation of IFNAR2. Biochem J. 2007;407:141–151. doi: 10.1042/BJ20070605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaks E, Gavutis M, Uze G, Martal J, Piehler J. Differential receptor subunit affinities of type I interferons govern differential signal activation. J Mol Biol. 2007;366:525–539. doi: 10.1016/j.jmb.2006.11.053. [DOI] [PubMed] [Google Scholar]
- 28.Langer JA. Interferon at 50: new molecules, new potential, new (and old) questions. Sci STKE. 2007;2007:pe53. doi: 10.1126/stke.4052007pe53. [DOI] [PubMed] [Google Scholar]
- 29.Severa M, et al. Differential responsiveness to IFN-alpha and IFN-beta of human mature DC through modulation of IFNAR expression. J Leukoc Biol. 2006;79:1286–1294. doi: 10.1189/jlb.1205742. [DOI] [PubMed] [Google Scholar]
- 30.Brierley MM, Fish EN. Review: IFN-alpha/beta receptor interactions to biologic outcomes: understanding the circuitry. J Interferon Cytokine Res. 2002;22:835–845. doi: 10.1089/107999002760274845. [DOI] [PubMed] [Google Scholar]
- 31.Jaitin DA, et al. Inquiring into the differential action of interferons (IFNs): an IFN-alpha2 mutant with enhanced affinity to IFNAR1 is functionally similar to IFN-beta. Mol Cell Biol. 2006;26:1888–1897. doi: 10.1128/MCB.26.5.1888-1897.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas C, et al. Structural linkage between ligand discrimination and receptor activation by type I interferons. Cell. 2011;146:621–632. doi: 10.1016/j.cell.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Boxel-Dezaire AH, Zula JA, Xu Y, Ransohoff RM, Jacobberger JW, Stark GR. Major differences in the responses of primary human leukocyte subsets to IFN-beta. J Immunol. 2010;185:5888–5899. doi: 10.4049/jimmunol.0902314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaitin DA, Schreiber G. Upregulation of a small subset of genes drives type I interferon-induced antiviral memory. J Interferon Cytokine Res. 2007;27:653–664. doi: 10.1089/jir.2006.0162. [DOI] [PubMed] [Google Scholar]
- 35.Cheon H, Stark GR. Unphosphorylated STAT1 prolongs the expression of interferon-induced immune regulatory genes. Proc Natl Acad Sci USA. 2009;106:9373–9378. doi: 10.1073/pnas.0903487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Der SD, Zhou A, Williams BR, Silverman RH. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci USA. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Veer MJ, et al. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol. 2001;69:912–920. [PubMed] [Google Scholar]
- 38.Kalie E, Jaitin DA, Podoplelova Y, Piehler J, Schreiber G. The stability of the ternary interferon–receptor complex rather than the affinity to the individual subunits dictates differential biological activities. J Biol Chem. 2008;283:32925–32936. doi: 10.1074/jbc.M806019200. [DOI] [PubMed] [Google Scholar]
- 39.Piehler J, Roisman LC, Schreiber G. New structural and functional aspects of the type I interferon–receptor interaction revealed by comprehensive mutational analysis of the binding interface. J Biol Chem. 2000;275:40425–40433. doi: 10.1074/jbc.M006854200. [DOI] [PubMed] [Google Scholar]
- 40.Levin D, Harari D, Schreiber G. Stochastic receptor expression determines cell fate upon interferon treatment. Mol Cell Biol. 2011;31:3252–3266. doi: 10.1128/MCB.05251-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leaman DW, et al. Novel growth and death related interferon-stimulated genes (ISGs) in melanoma: greater potency of IFN-beta compared with IFN-alpha2. J Interferon Cytokine Res. 2003;23:745–756. doi: 10.1089/107999003772084860. [DOI] [PubMed] [Google Scholar]
- 42.Samarajiwa SA, Forster S, Auchettl K, Hertzog PJ. INTERFEROME: the database of interferon regulated genes. Nucleic Acids Res. 2009;37:D852–D857. doi: 10.1093/nar/gkn732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coelho LF, De Freitas Almeida GM, Mennechet FJ, Blangy A, Uze G. Interferon-{alpha} and -{beta} differentially regulate osteoclastogenesis: role of differential induction of chemokine CXCL11 expression. Proc Natl Acad Sci USA. 2005;102:11917–11922. doi: 10.1073/pnas.0502188102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoggins JW, Rice CM. Interferon-stimulated genes and their antiviral effector functions. Curr Opin Virol. 2011;1:519–525. doi: 10.1016/j.coviro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kotenko SV, Langer JA. Full house: 12 receptors for 27 cytokines. Int Immunopharmacol. 2004;4:593–608. doi: 10.1016/j.intimp.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Senda T, et al. Three-dimensional crystal structure of recombinant murine interferon-b. EMBO J. 1992;11:3193. doi: 10.1002/j.1460-2075.1992.tb05396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radhakrishnan R, et al. Zinc mediated dimer of human interferon-alpha 2b revealed by X-ray crystallography. Structure. 1996;4:1453–1463. doi: 10.1016/s0969-2126(96)00152-9. [DOI] [PubMed] [Google Scholar]
- 48.Klaus W, Gsell B, Labhardt AM, Wipf B, Senn H. The three-dimensional high resolution structure of human interferon alpha-2a determined by heteronuclear NMR spectroscopy in solution. J Mol Biol. 1997;274:661–675. doi: 10.1006/jmbi.1997.1396. [DOI] [PubMed] [Google Scholar]
- 49.Karpusas M, Nolte M, Benton CB, Meier W, Lipscomb WN, Goelz S. The crystal structure of human interferon beta at 2.2-A resolution. Proc Natl Acad Sci USA. 1997;94:11813–11818. doi: 10.1073/pnas.94.22.11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chill JH, Quadt SR, Levy R, Schreiber G, Anglister J. The human type I interferon receptor: NMR structure reveals the molecular basis of ligand binding. Structure. 2003;11:791–802. doi: 10.1016/s0969-2126(03)00120-5. [DOI] [PubMed] [Google Scholar]
- 51.Roisman LC, Piehler J, Trosset JY, Scheraga HA, Schreiber G. Structure of the interferon–receptor complex determined by distance constraints from double-mutant cycles and flexible docking. Proc Natl Acad Sci USA. 2001;98:13231–13236. doi: 10.1073/pnas.221290398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akabayov SR, Biron Z, Lamken P, Piehler J, Anglister J. NMR mapping of the IFNAR1-EC binding site on IFNalpha2 reveals allosteric changes in the IFNAR2-EC binding site. Biochemistry. 2010;49:687–695. doi: 10.1021/bi901313x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Z, Strunk JJ, Lamken P, Piehler J, Walz T. The EM structure of a type I interferon–receptor complex reveals a novel mechanism for cytokine signaling. J Mol Biol. 2008;377:715–724. doi: 10.1016/j.jmb.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strunk JJ, et al. Ligand binding induces a conformational change in ifnar1 that is propagated to its membrane-proximal domain. J Mol Biol. 2008;377:725–739. doi: 10.1016/j.jmb.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 55.Bleicher L, et al. Crystal structure of the IL-22/IL-22R1 complex and its implications for the IL-22 signaling mechanism. FEBS Lett. 2008;582:2985–2992. doi: 10.1016/j.febslet.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 56.Jones BC, Logsdon NJ, Walter MR. Structure of IL-22 bound to its high-affinity IL-22R1 chain. Structure. 2008;16:1333–1344. doi: 10.1016/j.str.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Josephson K, Logsdon NJ, Walter MR. Crystal structure of the IL-10/IL-10R1 complex reveals a shared receptor binding site. Immunity. 2001;15:35–46. doi: 10.1016/s1074-7613(01)00169-8. [DOI] [PubMed] [Google Scholar]
- 58.Walter MR, et al. Crystal structure of a complex between interferon-gamma and its soluble high-affinity receptor. Nature. 1995;376:230–235. doi: 10.1038/376230a0. [DOI] [PubMed] [Google Scholar]
- 59.Miknis ZJ, Magracheva E, Li W, Zdanov A, Kotenko SV, Wlodawer A. Crystal structure of human interferon-lambda1 in complex with its high-affinity receptor interferon-lambdaR1. J Mol Biol. 2010;404:650–664. doi: 10.1016/j.jmb.2010.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roisman LC, Jaitin DA, Baker DP, Schreiber G. Mutational analysis of the IFNAR1 binding site on IFNalpha2 reveals the architecture of a weak ligand-receptor binding-site. J Mol Biol. 2005;353:271–281. doi: 10.1016/j.jmb.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 61.Lamken P, Gavutis M, Peters I, Van der Heyden J, Uze G, Piehler J. Functional cartography of the ectodomain of the type I interferon receptor subunit ifnar1. J Mol Biol. 2005;350:476–488. doi: 10.1016/j.jmb.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 62.Runkel L, et al. Systematic mutational mapping of sites on human interferon-b-1a that are important for receptor binding and functional activity. Biochemistry. 2000;39:2538–2551. doi: 10.1021/bi991631c. [DOI] [PubMed] [Google Scholar]
- 63.Peleg-Shulman T, Roisman LC, Zupkovitz G, Schreiber G. Optimizing the binding affinity of a carrier protein: a case study on the interaction between soluble ifnar2 and interferon {beta} J Biol Chem. 2004;279:18046–18053. doi: 10.1074/jbc.M400033200. [DOI] [PubMed] [Google Scholar]
- 64.Slutzki M, Jaitin DA, Yehezkel TB, Schreiber G. Variations in the unstructured C-terminal tail of interferons contribute to differential receptor binding and biological activity. J Mol Biol. 2006;360:1019–1030. doi: 10.1016/j.jmb.2006.05.069. [DOI] [PubMed] [Google Scholar]
- 65.Krause CD, et al. Preassembly and ligand-induced restructuring of the chains of the IFN-gamma receptor complex: the roles of Jak kinases, Stat1 and the receptor chains. Cell Res. 2006;16:55–69. doi: 10.1038/sj.cr.7310008. [DOI] [PubMed] [Google Scholar]
- 66.Brown RJ, et al. Model for growth hormone receptor activation based on subunit rotation within a receptor dimer. Nat Struct Mol Biol. 2005;12:814–821. doi: 10.1038/nsmb977. [DOI] [PubMed] [Google Scholar]
- 67.Kubatzky KF, Liu W, Goldgraben K, Simmerling C, Smith SO, Constantinescu SN. Structural requirements of the extracellular to transmembrane domain junction for erythropoietin receptor function. J Biol Chem. 2005;280:14844–14854. doi: 10.1074/jbc.M411251200. [DOI] [PubMed] [Google Scholar]
- 68.Seubert N, et al. Active and inactive orientations of the transmembrane and cytosolic domains of the erythropoietin receptor dimer. Mol Cell. 2003;12:1239–1250. doi: 10.1016/s1097-2765(03)00389-7. [DOI] [PubMed] [Google Scholar]
- 69.Lopez AF, et al. Molecular basis of cytokine receptor activation. IUBMB Life. 2010;62:509–518. doi: 10.1002/iub.350. [DOI] [PubMed] [Google Scholar]
- 70.Dagil R, et al. The WSXWS motif in cytokine receptors is a molecular switch involved in receptor activation: insight from structures of the prolactin receptor. Structure. 2012;20:270–282. doi: 10.1016/j.str.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 71.Strunk JJ, et al. Probing protein conformations by in situ non-covalent fluorescence labeling. Bioconjug Chem. 2008;20:41–46. doi: 10.1021/bc8002088. [DOI] [PubMed] [Google Scholar]
- 72.Whitty A, et al. Interaction affinity between cytokine receptor components on the cell surface. Proc Natl Acad Sci USA. 1998;95:13165–13170. doi: 10.1073/pnas.95.22.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lavoie TB, et al. Binding and activity of all human alpha interferon subtypes. Cytokine. 2011;56:282–289. doi: 10.1016/j.cyto.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 74.Piehler J, Schreiber G. Fast transient cytokine–receptor interactions monitored in real-time by reflectometric interference spectroscopy. Anal Biochem. 2001;289:173–186. doi: 10.1006/abio.2000.4920. [DOI] [PubMed] [Google Scholar]
- 75.Lamken P, Lata S, Gavutis M, Piehler J. Ligand-induced assembling of the type I interferon receptor on supported lipid bilayers. J Mol Biol. 2004;341:303–318. doi: 10.1016/j.jmb.2004.05.059. [DOI] [PubMed] [Google Scholar]
- 76.Moraga I, Harari D, Schreiber G, Uze G, Pellegrini S. Receptor density is key to the alpha2/beta interferon differential activities. Mol Cell Biol. 2009;29:4778–4787. doi: 10.1128/MCB.01808-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Piehler J, Schreiber G. Biophysical analysis of the interaction of human ifnar2 expressed in E. coli with IFNalpha2. J Mol Biol. 1999;289:57–67. doi: 10.1006/jmbi.1999.2726. [DOI] [PubMed] [Google Scholar]
- 78.Gavutis M, Lata S, Piehler J. Probing 2-dimensional protein–protein interactions on model membranes. Nat Protoc. 2006;1:2091–2103. doi: 10.1038/nprot.2006.270. [DOI] [PubMed] [Google Scholar]
- 79.Yamamoto K, et al. Creation of interferon-alpha8 (IFN-alpha8) mutants with amino acid substitutions against IFN-alpha receptor-2 (IFNAR-2)-binding sites using phage display system and evaluation of their biologic properties. J Interferon Cytokine Res. 2009;29:161–170. doi: 10.1089/jir.2008.0038. [DOI] [PubMed] [Google Scholar]
- 80.Pan M, Kalie E, Scaglione BJ, Raveche ES, Schreiber G, Langer JA. Mutation of the IFNAR-1 receptor binding site of human IFN-alpha2 generates type I IFN competitive antagonists. Biochemistry. 2008;47:12018–12027. doi: 10.1021/bi801588g. [DOI] [PubMed] [Google Scholar]
- 81.Kalie E, Jaitin DA, Abramovich R, Schreiber G. An interferon {alpha}2 mutant optimized by phage display for IFNAR1 binding confers specifically enhanced antitumor activities. J Biol Chem. 2007;282:11602–11611. doi: 10.1074/jbc.M610115200. [DOI] [PubMed] [Google Scholar]
- 82.Piehler J, Schreiber G. Mutational and structural analysis of the binding interface between type I interferons and their receptor ifnar2. J Mol Biol. 1999;294:223–237. doi: 10.1006/jmbi.1999.3230. [DOI] [PubMed] [Google Scholar]
- 83.Dubois A, et al. Enhanced anti-HCV activity of interferon alpha 17 subtype. Virol J. 2009;6:70. doi: 10.1186/1743-422X-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vazquez N, Schmeisser H, Dolan MA, Bekisz J, Zoon KC, Wahl SM. Structural variants of IFNalpha preferentially promote antiviral functions. Blood. 2011;118:2567–2577. doi: 10.1182/blood-2010-12-325027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Manry J, et al. Evolutionary genetic dissection of human interferons. J Exp Med. 2011;208:2747–2759. doi: 10.1084/jem.20111680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wagner TC, et al. Interferon receptor expression regulates the antiproliferative effects of interferons on cancer cells and solid tumors. Int J Cancer. 2004;111:32–42. doi: 10.1002/ijc.20236. [DOI] [PubMed] [Google Scholar]
- 87.Gavutis M, Lata S, Lamken P, Muller P, Piehler J. Lateral ligand–receptor interactions on membranes probed by simultaneous fluorescence-interference detection. Biophys J. 2005;88:4289–4302. doi: 10.1529/biophysj.104.055855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lata S, Gavutis M, Piehler J. Monitoring the dynamics of ligand–receptor complexes on model membranes. J Am Chem Soc. 2006;128:6–7. doi: 10.1021/ja054700l. [DOI] [PubMed] [Google Scholar]
- 89.Gavutis M, Jaks E, Lamken P, Piehler J. Determination of the two-dimensional interaction rate constants of a cytokine receptor complex. Biophys J. 2006;90:3345–3355. doi: 10.1529/biophysj.105.072546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kusumi A, et al. Membrane mechanisms for signal transduction: the coupling of the mesoscale raft domains to membrane-skeleton-induced compartments and dynamic protein complexes. Semin Cell Dev Biol. 2012;23:126–144. doi: 10.1016/j.semcdb.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 91.You C, et al. Self-controlled monofunctionalization of quantum dots for multiplexed protein tracking in live cells. Angew Chem Int Ed Engl. 2010;49:4108–4112. doi: 10.1002/anie.200907032. [DOI] [PubMed] [Google Scholar]
- 92.Kusumi A, et al. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct. 2005;34:351–378. doi: 10.1146/annurev.biophys.34.040204.144637. [DOI] [PubMed] [Google Scholar]
- 93.Kumar KG, et al. Basal ubiquitin-independent internalization of interferon alpha receptor is prevented by Tyk2-mediated masking of a linear endocytic motif. J Biol Chem. 2008;283:18566–18572. doi: 10.1074/jbc.M800991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kumar KG, Krolewski JJ, Fuchs SY. Phosphorylation and specific ubiquitin acceptor sites are required for ubiquitination and degradation of the IFNAR1 subunit of type I interferon receptor. J Biol Chem. 2004;279:46614–46620. doi: 10.1074/jbc.M407082200. [DOI] [PubMed] [Google Scholar]
- 95.Kumar KG, Tang W, Ravindranath AK, Clark WA, Croze E, Fuchs SY. SCF(HOS) ubiquitin ligase mediates the ligand-induced down-regulation of the interferon-alpha receptor. EMBO J. 2003;22:5480–5490. doi: 10.1093/emboj/cdg524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zheng H, Qian J, Varghese B, Baker DP, Fuchs S. Ligand-stimulated downregulation of the alpha interferon receptor: role of protein kinase D2. Mol Cell Biol. 2011;31:710–720. doi: 10.1128/MCB.01154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kumar KG, et al. Site-specific ubiquitination exposes a linear motif to promote interferon-{alpha} receptor endocytosis. J Cell Biol. 2007;179:935–950. doi: 10.1083/jcb.200706034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marchetti M, et al. Stat-mediated signaling induced by type I and type II interferons (IFNs) is differentially controlled through lipid microdomain association and clathrin-dependent endocytosis of IFN receptors. Mol Biol Cell. 2006;17:2896–2909. doi: 10.1091/mbc.E06-01-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marijanovic Z, Ragimbeau J, Kumar KG, Fuchs SY, Pellegrini S. TYK2 activity promotes ligand-induced IFNAR1 proteolysis. Biochem J. 2006;397:31–38. doi: 10.1042/BJ20060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Piganis RA, et al. Suppressor of cytokine signaling (SOCS) 1 inhibits type I interferon (IFN) signaling via the interferon alpha receptor (IFNAR1)-associated tyrosine kinase Tyk2. J Biol Chem. 2011;286:33811–33818. doi: 10.1074/jbc.M111.270207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Payelle-Brogard B, Pellegrini S. Biochemical monitoring of the early endocytic traffic of the type I interferon receptor. J Interferon Cytokine Res. 2010;30:89–98. doi: 10.1089/jir.2009.0044. [DOI] [PubMed] [Google Scholar]
- 102.Fuchs SY. Ubiquitination-mediated regulation of interferon responses. Growth Factors. 2012;30:141–148. doi: 10.3109/08977194.2012.669382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dupont SA, Goelz S, Goyal J, Green M. Mechanisms for regulation of cellular responsiveness to human IFN-beta1a. J Interferon Cytokine Res. 2002;22:491–501. doi: 10.1089/10799900252952280. [DOI] [PubMed] [Google Scholar]
- 104.Malakhov MP, Malakhova OA, Kim KI, Ritchie KJ, Zhang DE. UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J Biol Chem. 2002;277:9976–9981. doi: 10.1074/jbc.M109078200. [DOI] [PubMed] [Google Scholar]
- 105.Zhang D, Zhang DE. Interferon-stimulated gene 15 and the protein ISGylation system. J Interferon Cytokine Res. 2011;31:119–130. doi: 10.1089/jir.2010.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ritchie KJ, et al. Role of ISG15 protease UBP43 (USP18) in innate immunity to viral infection. Nat Med. 2004;10:1374–1378. doi: 10.1038/nm1133. [DOI] [PubMed] [Google Scholar]
- 107.Malakhova OA, et al. UBP43 is a novel regulator of interferon signaling independent of its ISG15 isopeptidase activity. EMBO J. 2006;25:2358–2367. doi: 10.1038/sj.emboj.7601149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Francois-Newton V, et al. USP18-based negative feedback control is induced by type I and type III interferons and specifically inactivates interferon alpha response. PLoS ONE. 2011;6:e22200. doi: 10.1371/journal.pone.0022200. [DOI] [PMC free article] [PubMed] [Google Scholar]