Molecular Pathways: Molecular Basis for Sensitivity and Resistance to JAK Kinase Inhibitors (original) (raw)
. Author manuscript; available in PMC: 2015 Apr 15.
Abstract
Janus kinases (JAK) are the mediators of a variety of cytokine signals via their cognate receptors that result in activation of intracellular signaling pathways. Alterations in JAK1, JAK2, JAK3 and TYK2 signaling contribute to different disease states, and dysregulated JAK-STAT signaling is associated with hematological malignancies, autoimmune disorders and immune-deficient conditions. Genetic alterations of JAK2 occur in the majority of patients with myeloproliferative neoplasms (MPN) and occur in a subset of patients with acute leukemias. JAK-mediated signaling critically relies on STAT transcription factors, and on activation of the MAPK and PI3K/Akt signaling axes. Hyperactive JAK at the apex of these potent oncogenic signaling pathways therefore represents an important target for small molecule kinase inhibitors in different disease states. The JAK1/2 inhibitor ruxolitinib and the JAK3 inhibitor tofacitinib were recently approved for the treatment of myelofibrosis and rheumatoid arthritis, respectively and additional ATP-competitive JAK inhibitors are in clinical development. Although these agents show clinical activity, the ability of these JAK inhibitors to induce clinical/molecular remissions in hematological malignancies appears limited and resistance upon chronic drug exposure is seen. Alternative modes of targeting JAK2 such as allosteric kinase inhibition or HSP-90 inhibition are under evaluation as is the use of histone deacetylase inhibitors. Combination therapy approaches integrating inhibition of STAT, PI3K/Akt and MAPK pathways with JAK kinase inhibitors might be critical to overcome malignancies characterized by dysregulated JAK signaling.
Background
A modular receptor tyrosine kinase
Janus kinases (JAK) are cytoplasmic tyrosine kinases that associate with transmembrane class I/II cytokine receptors. The JAK-cytokine receptor complex equals a functional receptor tyrosine kinase and propagates extracellular cytokine signals across the cell membrane to activate intracellular messenger pathways. JAK kinases mediate a variety of cytokine signals affecting cellular growth, differentiation and survival predominantly in hematopoiesis and immune response(1). Dysregulated JAK activity is involved in hematological malignancies, autoimmune disorders and immunodeficient conditions and has been implicated in the pathogenesis of a subset of solid tumors. Most prominent is the role of activated JAK2 signaling due to the V617F mutation observed in the majority of patients with myeloproliferative neoplasms (MPN)(2–5).
The JAK family
Numerous cytokines signal through the 4 JAK family members. JAK1, JAK2, JAK3 and TYK2 range from 120–140 kDa in size and share 7 JAK homology domains (JH1-7) which include the C-terminal kinase domain, an adjacent pseudokinase domain and the N-terminal Src homology 2 (SH2) and FERM (Band-4.1, ezrin, radixin and moesin)-like domain mediating the association with the cytokine receptor. The kinase domain contains an N- and C-lobe surrounding the ATP binding site and an activation loop with tandem tyrosine residues Y1007/Y1008 which regulate kinase activity through autophosphorylation(6). The pseudokinase domain, which classically has been thought to be deficient of catalytic activity, negatively regulates the kinase domain by phosphorylation of S523 and Y570(7). However, recent studies have suggested the pseudokinase domain might indeed have catalytic activity, which is required for autoinhibition of the JAK kinase domain(7, 8). The crystal structures of complete JAK molecules will be critical to clarify the JAK structure – function relationship in more detail and to reveal specific structural differences between the JAK family members.
JAK1, JAK2, JAK3 and TYK2 associate with different cytokine receptors and activate specific members of the signal transducer and activator of transcription (STAT) family as downstream effectors and are thus critically involved in different aspects of hematopoiesis and immune response. JAK2 is the most extensively investigated of the JAK family of kinases due to its pathogenic role in myeloproliferative neoplasms (MPN) and other malignancies. JAK2 is essential for signaling through hematopoietic cytokine receptors, including type I homodimeric erythropoietin (EpoR) and thrombopoietin receptors (TPOR or MPL) and the heterodimeric GM-CSF (GM-CSFR), IL3 and IL5 receptors. JAK2 also mediates signaling from the prolactin, growth hormone and leptin receptors and is involved in signaling through INFγ and members of the IL10- and IL12-type cytokine receptor family. The critical relation of JAK2 and hematopoietic cytokine signaling is exemplified by its interaction with the EpoR. In the absence of JAK2 expression, EpoR signaling is abolished and the germline JAK2 knockout mouse is embryonically lethal at day 12.5 of embryogenesis due to loss of definitive erythropoiesis(9). Germline activating mutations in JAK2 lead to inherited polycythemia while acquired JAK2 mutations are critical in the pathogenesis of MPN and are also seen in acute leukemia. The transforming capacity of JAK2 in hematopoietic cells is restricted to its EpoR- or MPL-bound form highlighting the functional interdependence of JAK2 and hematopoietic cytokine receptors(10).
JAK1 is critical for interferon γ and interferon α/β signaling, mostly as part of a heterodimer with JAK2 or TYK2, and is involved in IL2 receptor signaling as a heterodimer with JAK3. Somatic gain-of-function mutations in JAK1 have been identified in acute leukemia(11), whereas JAK1 deficiency is perinatally lethal due to impaired lymphopoiesis and CNS development(12).
JAK3 mutations were the first human germline JAK mutations reported and give rise to severe combined immunodeficiency (SCID) with absent T- and NK-cells (13). JAK3 associates with cytokine receptors containing the IL-2 common γ-chain in hematopoietic cells, which includes the IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 receptors.
TYK2 binds a variety of receptors including the interferon, IL-10 and IL-6 family and the IL-12/23/27 group. Germline Tyk2 deficiency in murine models results in defective viral, bacterial and fungal immune defense(14), whereas inherited TYK2 mutations have been described in autosomal recessive hyperimmunoglobulin E syndrome in humans. The current understanding of the different JAK family members implicates JAK1, JAK3 and TYK2 mainly in lymphopoiesis and immune response whereas JAK2 is most critically involved in the development, production and function of the myeloid lineages.
The JAK signaling cascade
The JAK kinases, in association with dimeric cytokine receptors, are activated upon ligand binding, leading to JAK autophosphorylation predominantly at the tandem tyrosines in the activation loop. JAKs then phosphorylate intracellular receptor tyrosines creating binding sites for SH2 domain-containing proteins such as the STATs. In case of JAK2, STAT3 and STAT5 are recruited and directly phosphorylated, while TYK2 mostly acts through STAT4 and STAT1 phosphorylation. STATs then dimerize and translocate to the nucleus to initiate transcription of effector genes involved in cell cycle regulation, apoptosis and proteasomal degradation (Figure 1). JAK2 also activates the PI3K/Akt and the MAPK signaling pathways, which further promote proliferation and survival(5, 10, 15) (Fig.1). Subsequent expression of the serine/threonine kinase Pim favors cell survival by phosphorylation of Bad and increased activity of anti-apoptotic Bcl2 and BclxL, while increased expression of cyclin D1/2 and Cdc25A promotes cell cycle progression from G1 to S phase(16).
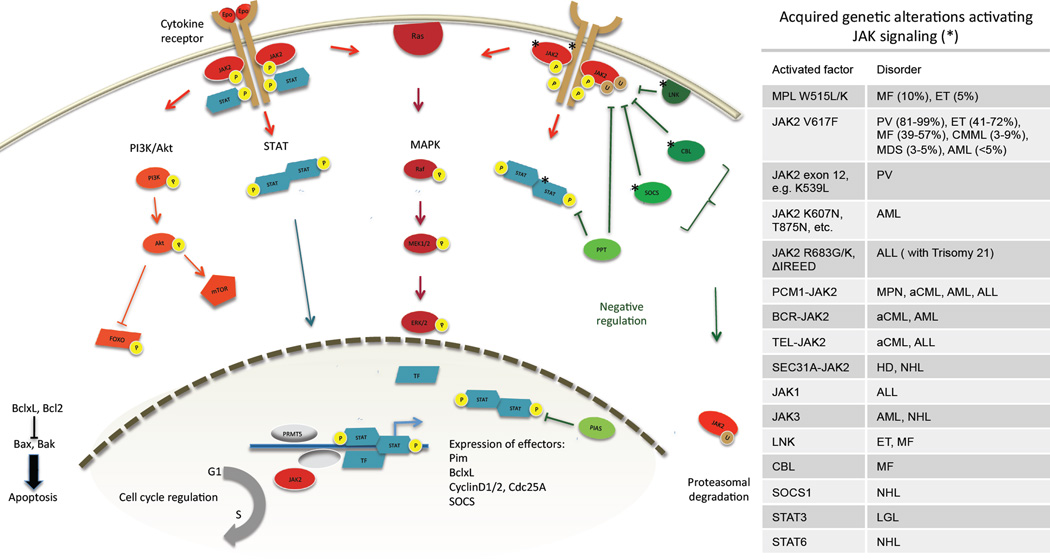
Figure 1. Overview of molecular JAK signaling.
Ligand binding to cell surface cytokine receptors initiates autophosphorylation of JAK2 and phosphorylation of cytoplasmic receptor tyrosines. STATs bind receptor phosphotyrosines via SH2 domains and translocate to the nucleus to induce expression of effector genes such as anti-apoptotic Pim kinase and BclxL, cyclins promoting cell cycle progression and SOCS forming a negative feedback loop. JAK2 also activates the PI3K/Akt and the MAPK pathways promoting proliferation and survival. Nuclear JAK2 is involved in epigenetic modifications. The negative regulators SOCS and CBL mark JAK2 for proteasomal degradation, LNK sequesters JAK2 by direct binding, and protein tyrosine phosphatases (PTP) dephosphorylate cytokine receptors, JAK and STATs. The protein inhibitors of STATs (PIAS) prevent STATs from binding target DNA. Stars indicate signaling components with reported genetic alterations. Transcription factors are shown in blue, negative regulators in green. MF myelofibrosis, ET essential thrombocythemia, PV polycythemia vera, CMML chronic myelomonocytic leukemia, MDS myelodysplastic syndrome, AML acute myeloid leukemia, ALL acute lymphatic leukemia, MPN myeloproliferative neoplasms, aCML atypical chronic myeloid leukemia, HD Hodgkin disease, NHL non hodgkin lymphoma, LGL large granular lymphocyte leukemia.
In contrast, expression of the suppressor of cytokine signaling proteins (SOCS) forms a negative feedback loop. SOCS 1 and 3 compete with STATs for docking at the cytokine receptor, promote proteasomal degradation of JAK2 by ubiquitinylation and interfere with its catalytic function via their kinase inhibitory region (KIR)(17). JAK2 is also negatively regulated by the Casitas B-cell lymphoma (CBL) proteins which act as ubiquitin ligases for numerous tyrosine kinases, and by the adaptor protein LNK (also called SH2B3) which sequesters JAK2(18). Further regulatory control of JAK signaling comes from protein tyrosine phosphatases (PTP) which dephosphorylate receptors, JAKs and STATs, and from protein inhibitors of activated STAT (PIAS), which prevent STATs from binding to target DNA.
Beyond activating cytoplasmic signaling cascades, JAK2 has recently been shown to translocate to the nucleus and have a direct impact on chromatin state. Nuclear JAK2 phosphorylates histone H3 at Y41 leading to displacement of heterochromatin protein 1 (HP1)(19, 20), and PRMT5, a histone arginine methyltransferase(21). JAK2 also phosphorylates p27Kip1, a cyclin-dependent kinase (CDK) inhibitor involved in cell cycle regulation. These nuclear functions of JAK2 warrant further investigation and may represent a novel therapeutic opportunity.
Oncogenic JAK2 signaling in myeloproliferative neoplasms
Dysregulated JAK2 signaling is a hallmark of myeloproliferative neoplasms (MPN), clonal stem cell disorders characterized by excessive proliferation of differentiated hematopoietic cells derived from one or more myeloid lineages. William Dameshek in 1951 recognized polycythemia vera (PV), essential thrombocythemia (ET) und primary myelofibrosis (PMF) as interrelated diseases due to a characteristic “pancytosis“ with proliferation of the erythroid, megakaryocyte and granulocyte lineages(20). PV patients present with erythrocytosis, ET patients with thrombocytosis, and PMF patients with splenomegaly, a leukoerythroblastic blood smear, and bone marrow fibrosis. These myeloproliferative neoplasms share cardinal clinical features, including an increased risk of thrombosis and bleeding, development of bone marrow fibrosis, splenomegaly and a risk of transformation to acute leukemia. While patients with polycythemia vera or essential thrombocythemia may transform to a post-PV/ET myelofibrotic phase similar to primary myelofibrosis, a subset of patients with essential thrombocythemia subsequently develop erythrocytosis and are diagnosed with polycythemia vera. These clinical observations highlight the biological overlap between the different forms of myeloproliferative neoplasms.
On a molecular level, the first insight into the molecular cause of these diseases came in 2005 with the discovery of the somatic V617F mutation in exon 14 of JAK2 in more than half of all MPN patients(2–5). Crystal structures of the wild-type and mutant pseudokinase domain have revealed that the valine to phenylalanine substitution rigidifies JH2 and relieves negative feedback regulation of the tyrosine kinase(8). In addition, JAK2 V617F escapes negative feedback by SOCS3 as, in contrast to the wild-type kinase, phosphorylation of JAK2 V617F is paradoxically increased upon overexpression of SOCS3 in vitro. JAK2 V617F constitutively activates JAK-STAT signaling and, in combination with expression of a hematopoietic cytokine receptor, leads to transformation of hematopoietic stem cells (HSC)(10).
The JAK2 V617F mutation is seen in 81–99% of patients with polycythemia vera, 41–72% of patients with essential thrombocythemia and 39–57% of patients with primary myelofibrosis and has greatly facilitated the diagnostics of myeloproliferative neoplasms. However, it is incompletely understood how one specific mutation contributes to the pathogenesis of three phenotypically distinct disease entities. JAK2 V617F gene dosage may influence the phenotype of myeloproliferative neoplasms, as hematopoietic cells are homozygous for V617F in most patients with polycythemia vera, but rarely in patients with essential thrombocythemia (22). High V617F allele burden associates with an increased risk for thrombotic complications and progression to myelofibrosis, while the impact of allele burden on clinical outcome remains controversial(23). Homozygosity for V617F does not arise from sequential mutation events, but rather as a result of mitotic recombination and resultant uniparental disomy (UPD) of the 9p24 locus(24). Recent studies have identified a specific “46/1” haplotype at the JAK2 locus prone to acquire JAK2 V617F (25). Analysis of familial myeloproliferative neoplasms suggests there are additional, unknown inherited alleles which predispose to familial MPN. Genetic events preceding JAK2 V617F are also suggested by cases of secondary acute myeloid leukemia which are JAK2 V617F negative in contrast to the preceding myeloproliferative neoplasm. In such cases the presence of shared additional cytogenetic and molecular alterations demonstrates the myeloproliferative neoplasm and subsequent acute myeloid leukemia arise from the same clone(26).
In JAK2 V617F negative polycythemia vera, missense mutations in JAK2 exon 12 in the pseudokinase-SH2 linker region are identified in nearly all cases(27). They analogously lead to constitutive kinase activation and dysregulated JAK signaling, but result in isolated erythrocytosis while V617F positive polycythemia vera mostly shows concomitant thrombo- and leukocytosis. JAK2 exon 12 mutations are not found in essential thrombocythemia and primary myelofibrosis.
Essential thrombocythemia is characterized by thrombocytosis due to increased proliferation of the megakaryocytic lineage and by an increased risk for thrombotic and bleeding complications. In V617F negative essential thrombocythemia, 5% of patients carry acquired mutations in exon 10 of the thrombopoietin receptor MPL, most commonly MPL W515L or W515K(28). They result in constitutive receptor activation and downstream JAK2 signaling including the STAT-, PI3K/Akt- and MAPK – axis, and transform hematopoietic cells analogously to JAK2 mutations. In contrast to murine models of JAK2 V617F which largely result in a polycythemia vera-like phenotype, expression of MPL W515L in vivo causes pronounced leukocytosis and thrombocytosis. Another 5% of V617F negative essential thrombocythemia harbor mutations in LNK affecting the negative regulation of JAK2 activity.
Myelofibrosis may develop as a primary manifestation of myeloproliferative neoplasms or develop from preceding polycythemia vera or essential thrombocytosis. Beyond leukocytosis and thrombocytosis, splenomegaly and progressive bone marrow fibrosis leading to extramedullar hematopoiesis and ultimately cytopenias are characteristic of primary myelofibrosis. In addition the levels of circulating inflammatory cytokines such as INFγ, TNFα, IL1, IL6 and IL8 are elevated(29). The MPL W515L mutation is identified in 10% of JAK2 V617F negative myelofibrosis, while mutations in the negative regulators LNK and CBL are found in approximately 5% of myelofibrosis patients. Very recently, two groups independently identified somatic mutations in exon 9 of CALR encoding the endoplasmic reticulum (ER) chaperone calreticulin, in 70–84% of _JAK2_-unmutated myelofibrosis and essential thrombocythemia(30, 31). Several variants due to indels with subsequent frameshift all displayed an altered C-terminus deficient of an ER retention signal. The discovery of calreticulin mutations in _JAK_-unmutated myeloproliferative neoplasms for the first time implicates an ER chaperone in leukemogenesis and will further improve the diagnostics of myeloproliferative neoplasms. While the functional effects of CALR mutations require further investigation, first in vitro studies suggest a critical involvement of STAT5 activation.
The STAT-, PI3K/Akt- and MAPK-pathways represent major routes of oncogenic signaling in many tyrosine kinase-driven systems, including JAK kinase signaling. The essential requirement of the STAT axis downstream of activated JAK2 is highlighted by the critical role of STAT5 for the transforming activity of JAK2 V617F and of TEL-JAK2 in murine bone marrow transplantation models(32, 33). The PI3K/Akt pathway is involved in malignant transformation by JAK2 V617F(34), while the relative significance of the MAPK pathway requires more detailed evaluation.
Beyond myeloproliferative neoplasms: Oncogenic JAK signaling in hematological malignancies
Hyperactive JAK signaling is not exclusive to myeloproliferative neoplasms. JAK2 V617F is found in other myeloid malignancies, including 7.8–13 % of chronic myelomonocytic leukemia (CMML) and 1–4.2 % of myelodysplastic syndrome (MDS)(35). JAK2 V617F positive acute myeloid leukemia (AML) is mostly observed in patients with a previous myeloproliferative neoplasm, but cases of V617F in de novo acute myeloid leukemia have been described. Alternative JAK2 mutations cluster in the pseudokinase domain, including the K607N mutation observed in acute myeloid leukemia(36) and L611S allele observed in acute lymphatic leukemia (ALL)(37). A gain of function mutation in the kinase domain, T875N, was reported in acute megakaryoblastic leukemia(38). JAK2 mutations are relatively rare in lymphoid malignancies except for acute lymphatic leukemia, most commonly in Down syndrome-associated ALL which has a high frequency of mutations at R682 in the pseudokinase domain. JAK1, JAK2, and JAK3 mutations and translocations are observed in high risk BCR-ABL B-cell ALL, and it is important to identify patients with mutations in JAK kinases as these alleles have prognostic and therapeutic relevance(39, 40). JAK2 may alternatively acquire constitutive activation as a fusion partner in oncogenic translocations described in both myeloid and lymphoid neoplasms. A TEL-JAK2 fusion combining the catalytic domain of JAK2 with the oligomerization domain of an ETS family transcription factor was described in childhood T-cell ALL(41) and atypical CML, while the PCM1-JAK2 fusion between JAK2 and the centrosomal factor PCM1 has been identified in a variety of hematological malignancies (42). JAK2 has also been partnered by BCR in atypical CML and AML and by SEC31A in Hodgkin lymphoma. Amplification of the JAK2 locus has been detected in Hodgkin lymphoma and mediastinal B-cell lymphoma(43). The diversity of JAK2 dysregulation highlights its significance as an oncogenic driver and as a prominent therapeutic target in hematological malignancies.
Beyond JAK2, oncogenic mutations occur in other JAK family members and their regulators. Gain-of-function mutations in JAK3 occur in megakaryoblastic leukemia(44) and, NK-T-cell lymphoma(45)and cutaneous T-cell lymphoma. JAK1 mutations are seen in ALL(11), and biallelic inactivating mutations of SOCS1 in mediastinal B-cell lymphoma(46). Several missense mutations in STAT3 and STAT5b have been identified in large granulocytic leukemia and(47) recurrent mutations of the STAT6 DNA binding domain in mediastinal B-cell lymphoma.
JAK2 mutations and translocations are very rare in solid cancers. However, activated JAK signaling in certain solid tumors in the absence of JAK2 mutations fosters interest into JAK2 inhibition as a therapeutic strategy. Ectopic expression of EpoR was shown to desensitize HER2 positive breast cancer to the HER2 inhibitor trastuzumab (48), and is also seen in head and neck squamous cell carcinoma where it correlates with tumor aggressiveness. Reduced levels of microRNA miR-375 which negatively regulates JAK2 expression, represents another mechanism of JAK2 activation seen in gastric cancer (49). In non small cell lung cancer (NSCLC) IL6/JAK2/STAT3 signaling was shown to promote dedifferentiation and to correlate with microvessel density. JAK signaling is increasingly implicated in the pathogenesis of several solid tumors, but its role is incompletely understood and requires detailed investigation to validate JAK2 as a therapeutic target in these malignancies.
Clinical-translational advances
Therapeutic JAK inhibition
The identification of the JAK2 V617F mutation in patients with myeloproliferative neoplasms fueled the development of small molecule JAK inhibitors to specifically target hyperactive JAK signaling. Several compounds differing in structure and JAK selectivity profile are at different stages of clinical development (Table 1) after the previous standard of care in myeloproliferative neoplasms had been restricted to palliation of symptoms and to prevention and treatment of thrombohemorrhagic events by low-dose aspirin, phlebotomy and hydroxyurea, while allogeneic stem cell transplantation represented the only curative approach in these patients. Beyond myeloproliferative neoplasms, there is an increasing interest in JAK inhibitors for targeted therapy of other hematological malignancies, certain solid cancers and, given the role of JAK-mediated cytokine signaling in immune response, in autoimmune disorders. The compounds currently in clinical testing target both wild-type and mutated JAKs in an ATP-competitive manner by occupying the ATP-binding pocket. This type 1 mode of inhibition stabilizes the kinase in its active conformation and results in paradoxically increased phosphorylation of the JAK2 activation loop, a phenomenon whose relevance is incompletely understood(50).
Table 1.
JAK inhibitors in clinical development. Small molecule JAK inhibitors and their stage of clinical development are indicated. * The phase II and III studies of SAR302503 in PV, ET and MF were terminated in November 2013 due to suspected CNS toxicity. PV polycythemia vera, ET essential thrombocythemia, MDS myelodysplastic syndrome, AML acute myeloid leukemia.
| Compound | Previous name | Targets | Disorder | Stage | Generic Name |
|---|---|---|---|---|---|
| Ruxolitinib | INCB18424 | JAK1, JAK2 | Myelofibrosis | FDA approved | Jakafi® |
| PV | Phase III | ||||
| ET | Phase II | ||||
| MDS | Phase II | ||||
| AML | Phase II | ||||
| Lymphoma | Phase II | ||||
| SAR302503 | TG101348 | JAK2, FLT3 | Myelofibrosis | Phase III terminated* | |
| PV and ET | Phase II terminated* | ||||
| Momelotinib | CYT387 | JAK1, JAK2 | Myelofibrosis | Phase II | |
| Pacritinib | SB1518 | JAK2, FLT3 | Myelofibrosis | Phase III | |
| MDS | Phase I/II | ||||
| AML | Phase I/II | ||||
| Lymphoma | Phase I/II | ||||
| AZD1480 | - | JAK1, JAK2, FLT3, Aurora | Myelofibrosis | Phase I/II | |
| BMS911543 | - | JAK2 | Myelofibrosis | Phase I/II | |
| Lestaurtinib | CEP701 | JAK2, FLT3 | Myelofibrosis, PV, ET | Phase I/II | |
| Baricitinib | - | JAK1, JAK2 | Rheumatoid arthritis, Psoriasis | Phase II | |
| Tofacitinib | - | JAK1, JAK3 | Rheumatoid arthritis | FDA approved | Xeljanz® |
| Psoriasis, IBD | Phase II |
Ruxolitinib (JAKAFI®) is the first JAK inhibitor approved by the FDA in 2011 and by the European Medicines Agency in 2012 for treatment of intermediate and high risk myelofibrosis. Two phase III studies demonstrated superiority of ruxolitinib over placebo (COMFORT I(51)) and best available therapy (COMFORT II(52)) in treatment of myelofibrosis. A >35% reduction of spleen size was achieved in 28–41% of patients at 24 weeks of therapy and substantial improvement of constitutional symptoms was observed in 46% of patients treated with ruxolitinib. Leukocytosis and thrombocytosis were decreased and JAK inhibitor therapy resulted in a marked reduction in inflammatory cytokine levels. Ruxolitinib is well tolerated with grade 3 and 4 adverse events due to myelosuppression in <10% of patients. Anemia and thrombocytopenia are most probably due to the essential role of JAK2 in hematopoiesis. Consequently, platelet counts <200 G/l require treatment at a reduced dose while platelets <100 G/l preclude the use of ruxolitinib therapy outside of a clinical trial. Ruxolitinib is currently under evaluation in a phase III trial in polycythemia vera and a phase II study in essential thrombocythemia. As the significance of activated JAK signaling is increasingly recognized across hematological and solid cancers as well as autoimmune disorders, phase II studies of ruxolitinib in acute myeloid leukemia, myelodysplastic syndrome, lymphoma, multiple myeloma, androgen-independent prostate cancer, rheumatoid arthritis and psoriasis are under way.
SAR302503 (former TG101348) selectively inhibits JAK2 at an IC50 value of 3 nM as compared to 105 nM for JAK1 and also shows Flt3 inhibitory activity. A phase 1 trial on 59 myelofibrosis patients showed prompt response of splenomegaly, leukocytosis, thrombocytosis and constitutional symptoms as well as significant reduction of V617F allele burden(53). Inflammatory cytokine levels where not affected which might relate to the more JAK2-specific action. Besides cytopenias, gastrointestinal side effects were most prominent. A phase III study comparing SAR302503 with placebo in myelofibrosis was completed (JAKARTA, NCT01437787), while a phase II study in polycythemia vera and essential thrombocythemia was previously initiated (NCT01420783). However, recent reports have suggested this agent is associated with an increased risk of Wernicke’s encephalopathy, and clinical development is currently on hold pending further evaluation of these CNS toxicities.
Momelotinib (CYT387) is a JAK1/2 inhibitor which reduced splenomegaly, constitutional symptoms and inflammatory cytokines in a single-center phase I/II study in myelofibrosis(54). Of note, 70% of patients with transfusion-dependent anemia at initiation of the study became transfusion-independent for a minimum of 3 months. Results of multi-center follow-up phase II/III studies are awaited. Confirmation of a beneficial anemia response would render momelotinib a favorable treatment in transfusion-dependent myelofibrosis patients.
Pacritinib (SB1518) is a pyrimidine-based inhibitor of JAK2 and FLT3 with efficacy in a phase I/II study of myelofibrosis(55). Its safety profile is favorable particularly regarding hematological toxicity. While type 1 JAK2 inhibitors in current clinical evaluation typically cause anemia and thrombocytopenia, pacritinib shows less myelosuppression with sustained platelet counts. Adverse events are mainly gastrointestinal, but low grade. A phase III trial in myelofibrosis has been initiated (NCT01773187). The compound is also being evaluated in phase I/II studies for myelodysplastic syndrome, acute myeloid leukemia and lymphoma.
Additional JAK1 and JAK2 inhibitors in clinical development. AZD1480 is a JAK1/JAK2 inhibitor with activity also towards FLT3 and Aurora kinases. It has shown inhibition of the TEL-JAK2 fusion in acute myeloid leukemia and a phase I/II study in myelofibrosis is ongoing. AZD1480 is being evaluated as a targeted therapy in Hodgkin lymphoma, multiple myeloma and certain solid tumors. BMS911543 is a JAK2 selective inhibitor which is currently evaluated in myelofibrosis. Baricitinib is a JAK1/JAK2 inhibitor with favorable effects in murine models of arthritis(56) which has entered clinical trials for rheumatoid arthritis and psoriasis.
Tofacitinib (Xeljanz®) is a JAK1/JAK3 inhibitor recently approved for therapy of rheumatoid arthritis(57). JAK3 represents a favorable target for novel immunosuppressive agents due to its expression restricted to the hematopoietic system. Tofacitinib interferes with stimulation of T-lymphoblasts via IL-2/STAT5 and also with expression of inflammatory cytokines via IL-6/STAT3 and STAT1. Clinical phase II and III studies in rheumatoid arthritis, renal transplant rejection, psoriasis and inflammatory bowel disease were favorable and additional trials are under way. Adverse events include increased incidence of viral infections and decreased hemoglobin most probably due to a inhibitory effect on JAK2 at clinically active doses.
Limitations of type 1 JAK inhibition in myeloproliferative neoplasms
The translation of type 1 JAK inhibitors to clinical application in myelofibrosis represents a significant advance for the treatment of patients with myeloproliferative neoplasms who benefit from improved quality of life. Ruxolitinib’s success has led to the rapid development of additional JAK inhibitors which will extend the armamentarium for targeted therapy of MPN and other malignancies driven by JAK signaling. However, the curative potential of type 1 JAK inhibitors in myeloproliferative neoplasms appears limited, as reductions of JAK2 V617F allele burden are modest and effects on disease pathology and survival remain controversial with limited follow-up. Hematologic toxicities due to the essential role of JAK2 in hematopoiesis may contribute by limiting dose-escalation and limiting the extent of target inhibition.
Furthermore, chronic exposure to JAK inhibitors leads to a loss of response in vitro, in animal models as well as in myelofibrosis patients. Acquisition of secondary kinase mutations during tyrosine kinase inhibition is a well-known phenomenon, as illustrated by BCR-ABL TKI resistance mutations in chronic myeloid leukemia. Saturation mutagenesis screens in JAK2 V617F- or R683G-mutated and _TEL-JAK2_-translocated cell lines identified a small set of secondary mutations in the JAK2 kinase domain which inferred resistance to ruxolitinib and caused cross-resistance to other type 1 JAK2 inhibitors(58). So far, none of these mutations has been identified in JAK inhibitor resistant patients suggesting mutation independent mechanisms likely mediate the survival of MPN cells in the setting of chronic JAK kinase inhibition. We recently described functional adaptation and reactivation of JAK-STAT signaling as an escape mechanism from chronic type 1 JAK inhibition. This resulted from heterodimeric activation of JAK2 by other JAK family members such as JAK1 and TYK2 which reactivate downstream signaling in the absence of secondary mutations. The phenomenon is seen in cell lines, murine models and in patient samples and is reversible, as after drug withdrawal sensitivity to type 1 JAK inhibitors is restored(59). A recent study suggested intrinsic resistance to JAK inhibitors in myelofibrosis as an additional mechanism. Primary cells from myelofibrosis patients showed more modest responses, as assessed by the degree of inhibition of STAT phosphorylation to ruxolitinib ex vivo, compared to polycythemia vera or essential thrombocytopenia patient samples. (60).
Novel concepts to target JAK signaling
While type 1 JAK inhibition has led therapy of myeloproliferative neoplasms to a new stage of molecularly targeted cancer treatment, its potential is limited due to insufficient efficacy. Several alternative options to target myeloproliferative neoplams are being explored.
Type 2 JAK inhibition. Novel JAK inhibitors such as BBT594 bind JAK2 in its inactive state at a hydrophobic pocket adjacent to the ATP binding site, that is uncovered by a conformational change of the activation loop with dislocation of a DFG motif. In contrast to type 1 JAK inhibition, inactive JAK2 is stabilized and activation loop phosphorylation is decreased(50). BBT594 was initially designed as an inhibitor of T315I mutated bcr-abl similar to nilotinib which also acts via a type 2 mode of kinase inhibition. BBT594 can overcome persistence to ruxolitinib in JAK2 V617F- and MPL W515L-positive cells and represents an interesting candidate chemical scaffold for further evaluation(59).
HSP90 inhibitors such as PU-H71 and AUY922 attenuate JAK2 expression by interference with the chaperone function of HSP90 and subsequent degradation of JAK2. They efficiently impair JAK-mediated signaling(61) and overcome resistance due to secondary mutations (58) and through JAK heterodimer formation in vitro(59). A phase II study of AUY922 in myelofibrosis has recently been initiated (NCT01668173). HSP90 inhibition as a monotherapy or in combination with a JAK inhibitor will provide a favorable option for treatment of myeloproliferative neoplasms if not compromised by side-effects due to inhibition of other HSP90 client proteins.
HDAC inhibition. HDAC inhibitiors, including panobinostat reduce JAK2 expression most probably due to effects on JAK2 mRNA expression, and through increased proteasomal JAK2 degradation. Phase II studies of givinostat and panobinostat in myelofibrosis showed improvement of splenomegaly and constitutional symptoms(62) and are now followed by a phase I study of panobinostat combined with ruxolitinib (NCT01433445).
Combined pathway inhibition. Dysregulated JAK2 is at the apex of three classical oncogenic signaling cascades including STAT, PI3K/Akt/mTOR and MAPK pathways. Their interrelationship and dependencies downstream of JAK2 in MPN cells is incompletely understood. In vitro studies suggest synergistic effects of PI3K/mTOR inhibitors such as BEZ235(63) or of the MEK1/2 inhibitor Selumetinib with JAK2 inhibitors(64). Synergism of JAK- and PI3K-inhibition was also seen in a murine model of myelofibrosis. A phase I/II trial of the mTOR inhibitor everolimus showed reduction of splenomegaly and constitutional symptoms, but was not as efficient as with JAK inhibition(65). Combination therapy might prove beneficial due to a synergistic impact on functionally redundant oncogenic axes, might allow for lower doses of the different agents with better tolerability, and might avoid or delay the development of resistance.
Perspectives
The ongoing molecular dissection of JAK signaling has enabled the development of specifically targeted therapies for patients with myeloproliferative neoplasms. The interest in applications of clinical JAK inhibition in other hematological malignancies and solid tumors is increasing. The next challenges will be to define the signaling dynamics of JAK-driven malignancies, to test novel JAK inhibitor scaffolds, and to test targeted combination therapies in order to improve outcomes for patients with JAK-dependent malignancies.
Acknowledgments
S.C. Meyer receives funding by the Swiss National Science Foundation and by the Huggenberger-Bischoff Foundation for Cancer Research Switzerland. R.L. Levine receives funding by the LLS Translational Research Program and NIH 1R01CA151949-01
Footnotes
Conflicts of interest: none
References
- 1.Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 2.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 3.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 4.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 5.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 6.Boggon TJ, Li Y, Manley PW, Eck MJ. Crystal structure of the Jak3 kinase domain in complex with a staurosporine analog. Blood. 2005;106:996–1002. doi: 10.1182/blood-2005-02-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ungureanu D, Wu J, Pekkala T, Niranjan Y, Young C, Jensen ON, et al. The pseudokinase domain of JAK2 is a dual-specificity protein kinase that negatively regulates cytokine signaling. Nat Struct Mol Biol. 2011;18:971–976. doi: 10.1038/nsmb.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandaranayake RM, Ungureanu D, Shan Y, Shaw DE, Silvennoinen O, Hubbard SR. Crystal structures of the JAK2 pseudokinase domain and the pathogenic mutant V617F. Nat Struct Mol Biol. 2012;19:754–759. doi: 10.1038/nsmb.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 10.Lu X, Levine R, Tong W, Wernig G, Pikman Y, Zarnegar S, et al. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. Proc Natl Acad Sci U S A. 2005;102:18962–18967. doi: 10.1073/pnas.0509714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flex E, Petrangeli V, Stella L, Chiaretti S, Hornakova T, Knoops L, et al. Somatically acquired JAK1 mutations in adult acute lymphoblastic leukemia. J Exp Med. 2008;205:751–758. doi: 10.1084/jem.20072182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373–383. doi: 10.1016/s0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 13.O'Shea JJ, Husa M, Li D, Hofmann SR, Watford W, Roberts JL, et al. Jak3 and the pathogenesis of severe combined immunodeficiency. Mol Immunol. 2004;41:727–737. doi: 10.1016/j.molimm.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Karaghiosoff M, Neubauer H, Lassnig C, Kovarik P, Schindler H, Pircher H, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549–560. doi: 10.1016/s1074-7613(00)00054-6. [DOI] [PubMed] [Google Scholar]
- 15.Roder S, Steimle C, Meinhardt G, Pahl HL. STAT3 is constitutively active in some patients with Polycythemia rubra vera. Exp Hematol. 2001;29:694–702. doi: 10.1016/s0301-472x(01)00637-3. [DOI] [PubMed] [Google Scholar]
- 16.Gautier EF, Picard M, Laurent C, Marty C, Villeval JL, Demur C, et al. The cell cycle regulator CDC25A is a target for JAK2V617F oncogene. Blood. 2012;119:1190–1199. doi: 10.1182/blood-2011-01-327742. [DOI] [PubMed] [Google Scholar]
- 17.Haan S, Wuller S, Kaczor J, Rolvering C, Nocker T, Behrmann I, et al. SOCS-mediated downregulation of mutant Jak2 (V617F, T875N and K539L) counteracts cytokine-independent signaling. Oncogene. 2009;28:3069–3080. doi: 10.1038/onc.2009.155. [DOI] [PubMed] [Google Scholar]
- 18.Tong W, Zhang J, Lodish HF. Lnk inhibits erythropoiesis and Epo-dependent JAK2 activation and downstream signaling pathways. Blood. 2005;105:4604–4612. doi: 10.1182/blood-2004-10-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawson MA, Bannister AJ, Gottgens B, Foster SD, Bartke T, Green AR, et al. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature. 2009;461:819–822. doi: 10.1038/nature08448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6:372–375. [PubMed] [Google Scholar]
- 21.Liu F, Zhao X, Perna F, Wang L, Koppikar P, Abdel-Wahab O, et al. JAK2V617F-mediated phosphorylation of PRMT5 downregulates its methyltransferase activity and promotes myeloproliferation. Cancer Cell. 2011;19:283–294. doi: 10.1016/j.ccr.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott LM, Scott MA, Campbell PJ, Green AR. Progenitors homozygous for the V617F mutation occur in most patients with polycythemia vera, but not essential thrombocythemia. Blood. 2006;108:2435–2437. doi: 10.1182/blood-2006-04-018259. [DOI] [PubMed] [Google Scholar]
- 23.Guglielmelli P, Barosi G, Specchia G, Rambaldi A, Lo Coco F, Antonioli E, et al. Identification of patients with poorer survival in primary myelofibrosis based on the burden of JAK2V617F mutated allele. Blood. 2009;114:1477–1483. doi: 10.1182/blood-2009-04-216044. [DOI] [PubMed] [Google Scholar]
- 24.Kralovics R, Guan Y, Prchal JT. Acquired uniparental disomy of chromosome 9p is a frequent stem cell defect in polycythemia vera. Exp Hematol. 2002;30:229–236. doi: 10.1016/s0301-472x(01)00789-5. [DOI] [PubMed] [Google Scholar]
- 25.Jones AV, Chase A, Silver RT, Oscier D, Zoi K, Wang YL, et al. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet. 2009;41:446–449. doi: 10.1038/ng.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theocharides A, Boissinot M, Girodon F, Garand R, Teo SS, Lippert E, et al. Leukemic blasts in transformed JAK2-V617F-positive myeloproliferative disorders are frequently negative for the JAK2-V617F mutation. Blood. 2007;110:375–379. doi: 10.1182/blood-2006-12-062125. [DOI] [PubMed] [Google Scholar]
- 27.Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356:459–468. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:e270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29:1356–1363. doi: 10.1200/JCO.2010.32.9490. [DOI] [PubMed] [Google Scholar]
- 30.Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–2390. doi: 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- 31.Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369:2391–2405. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walz C, Ahmed W, Lazarides K, Betancur M, Patel N, Henninghausen L, et al. Essential role for Stat5a/b in myeloproliferative neoplasms induced by BCR-ABL1 and JAK2(V617F) in mice. Blood. 2012;119:3550–3560. doi: 10.1182/blood-2011-12-397554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwaller J, Parganas E, Wang D, Cain D, Aster JC, Williams IR, et al. Stat5 is essential for the myelo- and lymphoproliferative disease induced by TEL/JAK2. Mol Cell. 2000;6:693–704. doi: 10.1016/s1097-2765(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 34.Kamishimoto J, Tago K, Kasahara T, Funakoshi-Tago M. Akt activation through the phosphorylation of erythropoietin receptor at tyrosine 479 is required for myeloproliferative disorder-associated JAK2 V617F mutant-induced cellular transformation. Cell Signal. 2011;23:849–856. doi: 10.1016/j.cellsig.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Levine RL, Loriaux M, Huntly BJ, Loh ML, Beran M, Stoffregen E, et al. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood. 2005;106:3377–3379. doi: 10.1182/blood-2005-05-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sulong S, Case M, Minto L, Wilkins B, Hall A, Irving J. The V617F mutation in Jak2 is not found in childhood acute lymphoblastic leukaemia. Br J Haematol. 2005;130:964–965. doi: 10.1111/j.1365-2141.2005.05697.x. [DOI] [PubMed] [Google Scholar]
- 37.Kratz CP, Boll S, Kontny U, Schrappe M, Niemeyer CM, Stanulla M. Mutational screen reveals a novel JAK2 mutation, L611S, in a child with acute lymphoblastic leukemia. Leukemia. 2006;20:381–383. doi: 10.1038/sj.leu.2404060. [DOI] [PubMed] [Google Scholar]
- 38.Mercher T, Wernig G, Moore SA, Levine RL, Gu TL, Frohling S, et al. JAK2T875N is a novel activating mutation that results in myeloproliferative disease with features of megakaryoblastic leukemia in a murine bone marrow transplantation model. Blood. 2006;108:2770–2779. doi: 10.1182/blood-2006-04-014712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mullighan CG, Zhang J, Harvey RC, Collins-Underwood JR, Schulman BA, Phillips LA, et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2009;106:9414–9418. doi: 10.1073/pnas.0811761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts KG, Morin RD, Zhang J, Hirst M, Zhao Y, Su X, et al. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer Cell. 2012;22:153–166. doi: 10.1016/j.ccr.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lacronique V, Boureux A, Valle VD, Poirel H, Quang CT, Mauchauffe M, et al. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278:1309–1312. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- 42.Reiter A, Walz C, Watmore A, Schoch C, Blau I, Schlegelberger B, et al. The t(8;9)(p22;p24) is a recurrent abnormality in chronic and acute leukemia that fuses PCM1 to JAK2. Cancer Res. 2005;65:2662–2667. doi: 10.1158/0008-5472.CAN-04-4263. [DOI] [PubMed] [Google Scholar]
- 43.Joos S, Kupper M, Ohl S, von Bonin F, Mechtersheimer G, Bentz M, et al. Genomic imbalances including amplification of the tyrosine kinase gene JAK2 in CD30+ Hodgkin cells. Cancer Res. 2000;60:549–552. [PubMed] [Google Scholar]
- 44.Walters DK, Mercher T, Gu TL, O'Hare T, Tyner JW, Loriaux M, et al. Activating alleles of JAK3 in acute megakaryoblastic leukemia. Cancer Cell. 2006;10:65–75. doi: 10.1016/j.ccr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Koo GC, Tan SY, Tang T, Poon SL, Allen GE, Tan L, et al. Janus kinase 3-activating mutations identified in natural killer/T-cell lymphoma. Cancer Discov. 2012;2:591–597. doi: 10.1158/2159-8290.CD-12-0028. [DOI] [PubMed] [Google Scholar]
- 46.Weniger MA, Melzner I, Menz CK, Wegener S, Bucur AJ, Dorsch K, et al. Mutations of the tumor suppressor gene SOCS-1 in classical Hodgkin lymphoma are frequent and associated with nuclear phospho-STAT5 accumulation. Oncogene. 2006;25:2679–2684. doi: 10.1038/sj.onc.1209151. [DOI] [PubMed] [Google Scholar]
- 47.Koskela HL, Eldfors S, Ellonen P, van Adrichem, Kuusanmaki H, Andersson EI, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med. 2012;366:1905–1913. doi: 10.1056/NEJMoa1114885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang K, Esteva FJ, Albarracin C, Stemke-Hale K, Lu Y, Bianchini G, et al. Recombinant human erythropoietin antagonizes trastuzumab treatment of breast cancer cells via Jak2-mediated Src activation and PTEN inactivation. Cancer Cell. 2010;18:423–435. doi: 10.1016/j.ccr.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding L, Xu Y, Zhang W, Deng Y, Si M, Du Y, et al. MiR-375 frequently downregulated in gastric cancer inhibits cell proliferation by targeting JAK2. Cell Res. 2010;20:784–793. doi: 10.1038/cr.2010.79. [DOI] [PubMed] [Google Scholar]
- 50.Andraos R, Qian Z, Bonenfant D, Rubert J, Vangrevelinghe E, Scheufler C, et al. Modulation of activation-loop phosphorylation by JAK inhibitors is binding mode dependent. Cancer Discov. 2012;2:512–523. doi: 10.1158/2159-8290.CD-11-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 53.Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM, et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 2011;29:789–796. doi: 10.1200/JCO.2010.32.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pardanani A, Laborde RR, Lasho TL, Finke C, Begna K, Al-Kali A, et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia. 2013;27:1322–1327. doi: 10.1038/leu.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verstovsek S, Odenike O, Scott B, Estrov Z, Cortes J, Thomas DA, et al. Phase I dose-escalation trial of SB1518, a novel JAK2/FLT3 inhibitor, in acute and chronic myeloid diseases, including primary or post-essential thrombocythemia/polycythemia vera myelofibrosis. Blood. 2009;114:3905. [Google Scholar]
- 56.Fridman JS, Scherle PA, Collins R, Burn TC, Li Y, Li J, et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol. 2010;184:5298–5307. doi: 10.4049/jimmunol.0902819. [DOI] [PubMed] [Google Scholar]
- 57.Ghoreschi K, Jesson MI, Li X, Lee JL, Ghosh S, Alsup JW, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550) J Immunol. 2011;186:4234–4243. doi: 10.4049/jimmunol.1003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weigert O, Lane AA, Bird L, Kopp N, Chapuy B, van Bodegom D, et al. Genetic resistance to JAK2 enzymatic inhibitors is overcome by HSP90 inhibition. J Exp Med. 2012;209:259–273. doi: 10.1084/jem.20111694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koppikar P, Bhagwat N, Kilpivaara O, Manshouri T, Adli M, Hricik T, et al. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature. 2012;489:155–159. doi: 10.1038/nature11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalota A, Jeschke GR, Carroll M, Hexner EO. Intrinsic resistance to JAK2 inhibition in myelofibrosis. Clin Cancer Res. 2013;19:1729–1739. doi: 10.1158/1078-0432.CCR-12-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marubayashi S, Koppikar P, Taldone T, Abdel-Wahab O, West N, Bhagwat N, et al. HSP90 is a therapeutic target in JAK2-dependent myeloproliferative neoplasms in mice and humans. J Clin Invest. 2010;120:3578–3593. doi: 10.1172/JCI42442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DeAngelo DJ, Mesa RA, Fiskus W, Tefferi A, Paley C, Wadleigh M, et al. Phase II trial of panobinostat, an oral pan-deacetylase inhibitor in patients with primary myelofibrosis, post-essential thrombocythaemia, and post-polycythaemia vera myelofibrosis. Br J Haematol. 2013;162:326–335. doi: 10.1111/bjh.12384. [DOI] [PubMed] [Google Scholar]
- 63.Fiskus W, Verstovsek S, Manshouri T, Smith JE, Peth K, Abhyankar S, et al. Dual PI3K/AKT/mTOR inhibitor BEZ235 synergistically enhances the activity of JAK2 inhibitor against cultured and primary human myeloproliferative neoplasm cells. Mol Cancer Ther. 2013;12:577–588. doi: 10.1158/1535-7163.MCT-12-0862. [DOI] [PubMed] [Google Scholar]
- 64.Suryani S, Sia KCS, Bracken L, Carol H, Evans K, Kurmasheva R, et al. Dual inhibition of JAK/STAT and MAPK pathways results in synergistic cell killing of JAK-mutated pediatric acute lymphoblastic leukemia. Blood. 2012;120:3562. [Google Scholar]
- 65.Guglielmelli P, Barosi G, Rambaldi A, Marchioli R, Masciulli A, Biamonte F, et al. Safety and efficacy of everolimus, a mTOR inhibitor, as single agent in a phase 1/2 study in patients with myelofibrosis. Blood. 2011;118:2069–2076. doi: 10.1182/blood-2011-01-330563. [DOI] [PMC free article] [PubMed] [Google Scholar]