Differences in Lactococcal Cell Wall Polysaccharide Structure Are Major Determining Factors in Bacteriophage Sensitivity (original) (raw)
ABSTRACT
Analysis of the genetic locus encompassing a cell wall polysaccharide (CWPS) biosynthesis operon of eight strains of Lactococcus lactis, identified as belonging to the same CWPS type C genotype, revealed the presence of a variable region among the strains examined. The results allowed the identification of five subgroups of the C type named subtypes C1 to C5. This variable region contains genes encoding glycosyltransferases that display low or no sequence homology between the subgroups. In this study, we purified an acidic polysaccharide from the cell wall of L. lactis 3107 (subtype C2) and confirmed that it is structurally different from the previously established CWPS of subtype C1 L. lactis MG1363. The CWPS of L. lactis 3107 is composed of pentasaccharide repeating units linked by phosphodiester bonds with the structure 6-α-Glc-3-β-Gal_f_-3-β-GlcNAc-2-β-Gal_f_-6-α-GlcNAc-1-P. Combinations of genes from the variable region of subtype C2 were introduced into a mutant of subtype C1 L. lactis NZ9000 deficient in CWPS biosynthesis. The resulting recombinant mutant synthesized a polysaccharide with a composition characteristic of that of subtype C2 L. lactis 3107 and not wild-type C1 L. lactis NZ9000. By challenging the recombinant mutant with various lactococcal phages, we demonstrated that CWPS is the host cell surface receptor of tested bacteriophages of both the P335 and 936 groups and that differences between the CWPS structures play a crucial role in determining phage host range_._
IMPORTANCE
Despite the efforts of nearly 80 years of lactococcal phage research, the precise nature of the cell surface receptors of the P335 and 936 phage group receptors has remained elusive. This work demonstrates the molecular nature of a P335 group receptor while bolstering the evidence of its role in host recognition by phages of the 936 group and at least partially explains why such phages have a very narrow host range. The information generated will be instrumental in understanding the molecular mechanisms of how phages recognize specific saccharidic receptors located on the surface of their bacterial host.
INTRODUCTION
Because of their detrimental effect on commercial dairy fermentations, (bacterio)phages infecting lactic acid bacteria (LAB), in particular, Lactococcus lactis, have been the subject of extensive scientific scrutiny (1–3). Lactococcal phages are currently classified into 10 groups on the basis of sequence homology and morphology (4). Of these, three phage groups, namely, 936 (5), P335 (6) and c2 (7), are routinely isolated from dairy processing environments (1, 8). Of the currently recognized lactococcal phage groups, the host-encoded receptor has been conclusively identified only for phages belonging to the c2 group. Phage c2 recognizes a membrane protein, termed PIP (phage infection protein), which exhibits modest similarity to the YueB receptor of Bacillus subtilis phage SPP1 (9–11). It is assumed that most bacteriophages infecting Gram-positive bacteria recognize a carbohydrate moiety on the cell surface (12), such as cell wall polysaccharides (CWPS) (13), wall teichoic acids (11), or lipoteichoic acids (14). The diversity, structural composition, and architecture of CWPS produced by LAB are relatively poorly defined. In contrast, significant research attention has been focused on the nature of exopolysaccharides (EPS), which impart important rheological and organoleptic properties upon fermented milk products (15). Since EPS are loosely associated with the cell wall, produced only by some strains, and often encoded on mobile elements (16), EPS are not thought to be involved in host recognition by lactococcal bacteriophages (17), although their presence has been reported to block adsorption abilities for 936 and c2 group phages 712 and c2, respectively (18, 19).
Two recent studies have investigated the structural nature and biological functions of CWPS in LAB. The CWPS or pellicle on the surface of L. lactis MG1363 was shown to be a polymer composed of repeated hexasaccharide subunits linked by phosphodiester bonds (20). Interestingly, it was found that this cell wall layer provides a protective barrier against host phagocytosis by murine macrophages. Furthermore, three different CWPS structures from various Lactobacillus helveticus strains were recently reported, revealing strain-specific polysaccharides with differing chemical properties that were correlated with different autolytic profiles displayed by individual L. helveticus strains (21).
Evidence that the CWPS of L. lactis is exploited by certain phages for host recognition has been steadily building through structural analyses of phage-encoded receptor-binding proteins (RBPs) and genetic analyses of bacteriophage-insensitive mutants (BIMs). The RBPs of 936 group phages p2 and bIL170 and P335 group phage TP901-1 have been shown to possess carbohydrate-binding properties (22, 23). In addition, the atomic structures of the baseplates of phages p2 and TP901-1 display the same structural architecture but employ different mechanisms of host adsorption (24). BIMs of L. lactis IL1403 generated by means of random insertion mutagenesis showed a sedimenting phenotype in liquid medium and resistance to phage infection by members of the 936 group (3). The ISS1 insertion elements were shown to be located within a gene cluster that is presumed to be responsible for the biosynthesis of a CWPS. Interestingly, a similar sedimenting phenotype was observed in several (but not all) BIMs of L. lactis 3107, which had been isolated as being insensitive to phages TP901-1 and φLC3 (both of which belong to the P335 group) (25). Finally, L. lactis MG1363 mutants deficient in CWPS pellicle biosynthesis were shown to display a sedimenting phenotype and to be insensitive to the 936 group bacteriophage sk1 (20). These previous studies suggest that L. lactis genomes possess a single genetic locus implicated in CWPS biosynthesis and that loss of CWPS production will cause a sedimenting phenotype in addition to insensitivity to infection by certain phages. However, it is not clear if phage insensitivity is due directly to loss of CWPS as a receptor or to the associated sedimenting phenotype.
On the basis of bioinformatic analysis of currently available lactococcal genomes, three major groups of L. lactis strains can be distinguished (types A, B, and C) on the basis of differences in the gene cluster that is presumed to be involved in CWPS biosynthesis (26). The latter study also revealed that correlations exist among the CWPS-based genotype, host range, and phylogeny of the RBPs encoded by sequenced members of the 936 group.
Here, we present a bioinformatic analysis of the genetic region associated with type C CWPS biosynthesis in eight different lactococcal genomes, allowing the identification of various subtypes. We show that such genetic diversity is responsible, at least in the case of one of the subtypes, for the biosynthesis of a CWPS with a distinct chemical structure. Through an approach that combines mutagenesis and genetic complementation, we demonstrate that the CWPS biosynthesis gene cluster is responsible for the production of a receptor used by (tested) lactococcal phages belonging to the P335 and 936 groups.
RESULTS
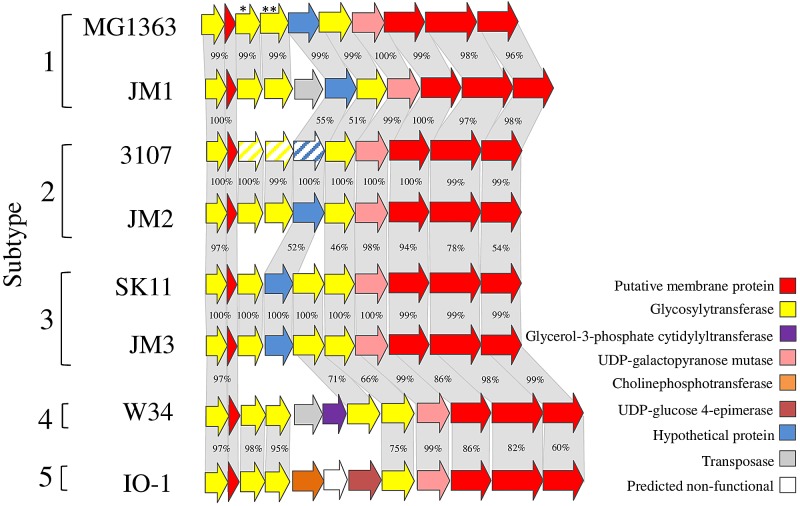
We previously determined the existence of three distinct genetic loci for CWPS biosynthesis in L. lactis, termed the A, B, and C types, which can be linked to the RBP phylogeny of 936 phages (26). Although 936 phage RBP phylogeny groups cluster in one particular CWPS type, the host range of these phages is still limited to a few strains within the particular CWPS type. To determine the degree of genetic diversity within the type C CWPS biosynthesis gene cluster, an analysis of the genetic locus encompassing the presumed CWPS biosynthetic operon of eight type C strains was performed. This analysis consisted of three currently available genomes and the CWPS regions of five strains from our own collection. Results revealed the presence of a variable region among the CWPS type C loci examined (Fig. 1). This allowed the identification of five subtypes among members of the C type (designated subtypes C1 to C5; Fig. 1) on the basis of differences in this variable region within the various type C CWPS biosynthesis loci. This variable region contains genes encoding glycosyltransferases that display low or nonsignificant levels of sequence identity between the subtypes (Fig. 1). Comparative analysis showed that strains belonging to the same subtype display a high level of sequence identity across all of the genes that make up their respective CWPS biosynthesis locus, including the genes located within this variable region (which display 99 to 100% sequence identity). Strains W34 and IO-1, belonging to the C4 and C5 subtypes, respectively, harbor a variable region with genes encoding glycosyltransferases, which display high levels of sequence identity (75 to 98%). Despite this sequence similarity, substantial differences exist between the gene clusters of these subtypes, such as the presence in the C4 subtype of additional genes encoding a predicted glycosyltransferase and a putative glycerol-3-phosphate cytidyltransferase and the presence in the C5 subtype of putative choline phosphotransferase and UDP-glucose 4-epimerase-specifying genes. Furthermore, lower levels of identity (ranging between 100 and 54%) are observed at the 3′ ends of the various C subtype CWPS biosynthesis gene clusters (Fig. 1). The sequence differences between subtypes suggest that subtype C2 to C5 strains produce CWPS structurally different from those of subtype C1 L. lactis MG1363, whose molecular structure has been determined previously (20).
FIG 1 .
Comparison of the variable regions in the type C CWPS biosynthesis cluster of lactococcal strains MG1363, JM1, 3107, JM2, SK11, JM3, W34, and IO-1. Genes with a high level of identity (indicated as percent nucleotide sequence identity) are joined by gray blocks compared to the adjoining strain. Five subtypes (C1 to C5) of the C genotype are highlighted. Genes marked with * and ** are interrupted in MG1363 derivatives NZ9000-GT1 (LLNZ_01145) and NZ9000-GT2 (LLNZ_01150), respectively. Genes with diagonal lines represent strain 3107 subtype C2 genes cloned into pPTPiC2.
Structural analysis of L. lactis 3107 CWPS.
To ascertain if differences in C subtype CWPS biosynthesis gene clusters would lead to differences in the CWPS structure from the previously determined structure of subtype C1 MG1363 CWPS (20), a structural analysis of the CWPS of subtype C2 strain L. lactis 3107 was performed. Fractionation of the trichloroacetic acid (TCA) extract by anion-exchange chromatography afforded the separation of several acidic fractions. The main acidic product was shown to contain Glc, Gal, and GlcNAc, which were shown to be in the d configuration. The detailed chemical structure of this compound was elucidated by nuclear magnetic resonance (NMR) spectroscopy and revealed that it is a phosphorylated oligosaccharide (OS1) (Fig. 2). Sets of two-dimensional NMR spectra (obtained by gradient-selected correlation spectroscopy [COSY], total correlation spectroscopy [TOCSY], nuclear Overhauser effect spectroscopy [NOESY], 1H-13C heteronuclear single-quantum coherence [HSQC] spectroscopy, 1H-13C heteronuclear multiple-bond correlation [HMBC] spectroscopy, and 1H-31P heteronuclear multiple-quantum correlation [HMQC] spectroscopy) were recorded and interpreted (see Table S2 and Fig. S1 in the supplemental material). Monosaccharides in the pyranose form (Glc, GlcNAc) were identified by COSY, TOCSY, and NOESY cross-peak patterns and 13C NMR chemical shifts. Amino group location was concluded from the high field signal position of aminated carbons (CH signals at 45 to 60 ppm). β-Gal_f_ was identified by 13C chemical shifts in comparison with published values (27) on the basis of the known monosaccharide composition.
FIG 2 .
(A) Structure of L. lactis 3107 CWPS and OS1. The structure of the MG1363 CWPS (20) is shown as a comparison. Similar component constituents of L. lactis 3107 and MG1363 CWPS are colored to highlight similarity. Bold letters D, C, F, A, and B represent individual detected monosaccharide residues. (B) Representation of the oligosaccharide fractions (OS2 to OS6) resulting from partial hydrolysis of L. lactis 3107 CWPS. Fractions were purified by RP-HPLC and analyzed by MALDI-TOF MS, confirming the L. lactis 3107 CWPS structure in panel A. The nature of the linkages present in the L. lactis 3107 CWPS, determined by methylation analysis, is displayed adjacent to each fraction.
HMQC spectroscopy of OS1 indicated six anomeric signals (see Fig. S1 in the supplemental material). Complete assignment of 1H and 13C spectra showed one α-Glc residue (D in Fig. 2), two β-Gal_f_ residues (A and C), one β-GlcNAc residue (F), and one GlcNAc residue (B) at the reducing end, which was present in the α and β configurations. The following connections between monosaccharides were determined from transglycosidic NOE and HMBC spectroscopy correlations: A1-B6, C1-F3, D1-C3, and F1-A2. The structure presented in Fig. 2 was assigned to OS1. Another minor acidic fraction with a molecular weight (MW) higher than that of OS1 was also analyzed by NMR. It was identified as a short polysaccharide composed of repeating units with a structure identical to that of OS1, linked by phosphodiester bonds between the α-Glc (D) and the α-GlcNAc (B) (Fig. 2; see Fig. S1 in the supplemental material).
To confirm this structure, the TCA extract of defatted L. lactis cells was treated with hydrofluoric acid (HF) and the fragments generated (OS fraction) were purified by reversed-phase high-performance liquid chromatography (RP-HPLC) and analyzed by matrix-assisted laser desorption ionization–time of flight mass spectroscopy (MALDI-TOF MS). The observed molecular masses correspond to those expected for oligosaccharide fragments derived from the polysaccharide repeating unit shown in Fig. 2, formed by cleavage of phosphodiester bonds and additional cleavage of glycosidic bonds of Gal_f_ residues (Fig. 2; see Table S3 in the supplemental material). These results therefore confirmed the structure of the L. lactis 3107 CWPS shown in Fig. 2.
Part of the OS fraction was reduced with NaBD4, desalted on a Biogel P2 column, and subjected to methylation analysis in order to confirm the nature of the linkages between the monosaccharide units. In agreement with the structure shown in Fig. 2, methylation analysis resulted in the identification of partially methylated alditol acetates from terminal Glc_p_, 2- and 3-substituted Gal_f_, and 3- and 6-substituted HexNAc. Terminal Glc_p_, present in the OS fragments formed by cleavage of glycosidic bonds of Gal_f_ residues (Fig. 2), was also identified.
CWPS of C subtype strains can be swapped.
Because of the high levels (99 to 100%) of DNA sequence identity observed across conserved regions of the CWPS biosynthesis gene clusters found in subtype C1 strain L. lactis MG1363 and subtype C2 strain L. lactis 3107 (Fig. 1), we hypothesized that if the variable genes found in the subtype C2 CWPS biosynthesis locus of L. lactis 3017 were supplied in trans to the L. lactis MG1363 nisin-controlled gene expression system derivative L. lactis NZ9000 (28), this recombinant strain would produce the structural equivalent of L. lactis 3107 CWPS. Combinations of genes from the variable region of the subtype C2 CWPS biosynthesis gene cluster were amplified from L. lactis 3107 and cloned into the low-copy-number, nisin-inducible expression vector pPTPi. The pPTPi derivatives were then introduced into L. lactis NZ9000-GT1, an NZ9000 derivative in which the LLNZ_01145 gene of the native CWPS biosynthesis gene cluster had been mutated by recombineering. This mutation introduces a TGA stop codon into LLNZ_01145. The resulting mutant, NZ9000-GT1, was shown to be resistant to the 936 group phages tested and displayed a sedimenting phenotype (data not shown). The results are consistent with the expected loss of CWPS biosynthesis (3, 20). Cloning and induced expression of the variable region of the subtype C2 CWPS biosynthesis gene cluster from L. lactis 3107 (i.e., genes 3107_003, 3107_004, and 3107_005 located on nisin-inducible plasmid pPTPiC2) in L. lactis NZ9000-GT1 restored wild-type (WT), nonsedimenting cell growth. While pPTPi-based constructs containing genes 3107_003 through 3107_006 also restored WT growth, pPTPi derivatives that contain gene complements less than those present in pPTPiC2 failed to restore WT growth (data not shown). This suggests that NZ9000-GT1 was able to produce a functional CWPS provided that the C2 variable-region genes from 3107 are supplied (and expressed), implying that the CWPS produced was that of subtype C2. Similar results were obtained with a second mutant, NZ9000-GT2, where the second variable subtype C1 variable gene encoding a glycosyltransferase (LLNZ_01150) was interrupted (data not shown).
To investigate if alternative CWPS biosynthesis was indeed occurring in NZ9000-GT1 containing induced pPTPiC2, analysis of the corresponding CWPS content was performed. The CWPS of L. lactis NZ9000 (which is a derivative of MG1363) and 3107 contain the same monosaccharide constituents (i.e., Glc, Gal, and GalNAc), with the exception of Rha, which is present only in NZ9000 CWPS (Fig. 2). Detection of Rha was therefore taken as a distinctive marker for the presence of subtype C1 CWPS.
Extraction of CWPS from the different strains and mutants with or without nisin induction was performed as described in Materials and Methods, after which the CWPS monosaccharide composition was determined (see Fig. S2 in the supplemental material). Results show that CWPS preparations from L. lactis NZ9000-GT1 carrying uninduced pPTPiC2 contain traces of monosaccharides characteristic of NZ9000 CWPS, which suggests that a trace amount of NZ9000 CWPS is present. This may be due to partial readthrough of the introduced stop codon within LLNZ_01145 (29). These results correlate with previously published observations (20), such as the observed phenotypes of cell sedimentation and phage resistance, characteristic of the MG1363 derivatives VES5748 and VES5751, which are CWPS-negative strains (20). The CWPS composition of L. lactis NZ9000-GT1/pPTPiC2 is altered following nisin induction. Results reveal a monosaccharide composition characteristic of the CWPS of L. lactis 3107 rather than that of L. lactis NZ9000, with a very small amount of Rha, in contrast to the other monosaccharides Glc, Gal, and GlcNAc (see Fig. S2).
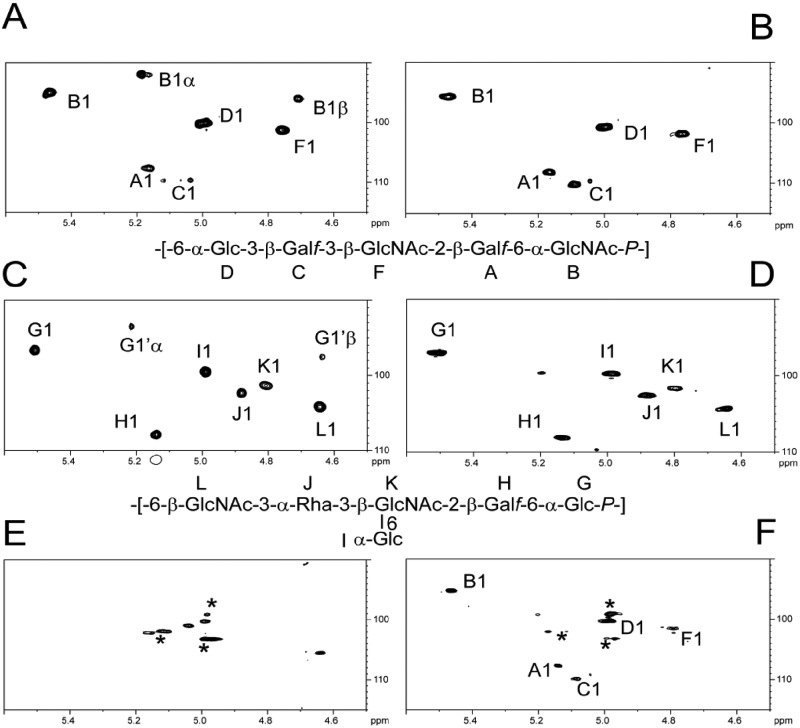
The expression of surface polysaccharides in the different strains was further investigated by high-resolution magic-angle spinning (HR-MAS) NMR. This technique, which enables a molecular examination of surface components on intact cells, was effectively used previously to analyze CWPS of Candida albicans and Bacillus cereus without the need for any purification steps (30, 31). Comparison of the liquid NMR spectra of L. lactis 3107 CWPS and L. lactis NZ9000 CWPS with the HR-MAS NMR spectra of the corresponding intact cells showed that HR-MAS NMR permitted us to unambiguously identify polysaccharides from the L. lactis surface that retain similar 1H-13C NMR parameters irrespective of the method used. As observed from the anomer region of 1H-13C HSQC NMR spectra (Fig. 3A to D), the only difference concerns the presence of reducing ends in liquid NMR spectra, generated by cleavage of the phosphodiester bond linking B and G residues during the CWPS purification procedure, an artifact that is absent from the analysis of intact cells. In contrast to NZ9000, G-L NMR signals associated with NZ9000 CWPS disappeared from the HR-MAS 1H-13C HSQC NMR spectrum of intact, uninduced NZ9000-GT1/pPTPiC2 cells, confirming the absence of this surface polysaccharide (Fig. 3D). Nonetheless, a distinctive set of NMR carbohydrate signals was observed, but none matching known L. lactis polysaccharides was observed, which points at the presence of an as-yet-undefined, surface-associated polysaccharide, which is presumably covered by the NZ9000 CWPS in the WT strain. Finally, an HR-MAS NMR spectrum of intact, nisin-induced NZ9000-GT1/pPTPiC2 cells (Fig. 3E) clearly shows the presence of a surface polysaccharide with NMR parameters that are essentially identical to those of L. lactis 3107 CWPS (Fig. 3B). Altogether, these results support the conclusion that the nisin-induced L. lactis NZ9000-GT1/pPTPiC2 strain produces the subtype C2 CWPS. Nisin induction of pPTPiC2 in L. lactis NZ9000-GT2 similarly leads to production of the CWPS with a composition characteristic of that of L. lactis 3107 (data not shown).
FIG 3 .
NMR analysis of intact cell surface polysaccharides. 1H-13C HSQC NMR liquid spectra of purified polysaccharides (A and C) were compared with HR-MAS spectra of intact cells (B, D to F). Comparison of the NMR spectra of purified L. lactis 3107 CWPS (A) and intact L. lactis 3107 cells (B) confirms that the L. lactis 3107 CWPS is clearly expressed at the cell surface. Intact L. lactis NZ9000 cells clearly express a CWPS at their surface (D) that is, as expected, identical to the previously described (20) purified CWPS of L. lactis MG1363 (C). HR-MAS NMR analysis of uninduced NZ9000-GT1/pPTPiC2 cells (E) does not reveal the presence of the L. lactis NZ9000 CWPS (compare panel E with panels C and D), whereas the analysis of nisin-induced cells shows the presence of L. lactis 3107 CWPS (compare panel F with panels A and B). Asterisks indicate anomer signals associated with an undefined underlying polysaccharide. B1α/β and G1α/β signals originate from free reducing ends of both polysaccharides.
CWPS of L. lactis 3107 is the cell surface receptor of φLC3.
To determine if the C2 subtype CWPS produced in L. lactis NZ9000-GT1/pPTPiC2 functions as a bacteriophage-host cell surface receptor, we challenged the induced strain with various 936 and P335 group phages (Table 1), whose indicator strains are L. lactis 3107 or NZ9000, and monitored phage sensitivity by plaque assay. Of the phages tested, only P335 group phage φLC3, which is unable to form plaques on WT L. lactis NZ9000 or uninduced L. lactis NZ9000-GT1/pPTPiC2, was able to infect and form plaques on induced L. lactis NZ9000-GT1/pPTPiC2 at an efficiency of plating (EOP) of 10−1 (Table 2) and can be propagated to a level of 108 PFU/ml (data not shown). This clearly demonstrates that the CWPS of L. lactis 3107 is the host cell surface receptor of φLC3 and that this CWPS, when produced in NZ9000, is sufficient for this strain to become susceptible to φLC3 infection. Interestingly, another P335 group phage, TP901_erm_, which also uses L. lactis 3107 as a host, was not able to form plaques on induced L. lactis NZ9000-GT1/pPTPiC2. However, the frequency of lysogeny of TP901_erm_ on induced L. lactis NZ9000-GT1/pPTPiC2 is 104-fold that on L. lactis NZ9000-GT1/pPTPi (TP901_erm_ can lysogenize L. lactis NZ9000 at a very low frequency [32]), reaching levels similar to those observed with L. lactis 3107 (Table 2), indicating that CWPS from 3107 is, as expected, the cell surface receptor for TP901_erm_. Additionally, TP901-1_erm_ exhibits an increased frequency of lysogeny on uninduced L. lactis NZ9000-GT1/pPTPiC2 (Table 2), despite exhibiting a sedimentation phenotype and the inability of φLC3 to form plaques on this (uninduced) strain. An increased frequency of lysogeny is not observed in NZ9000-GT1 harboring empty pPTPi (Table 2), suggesting that the observed increase in lysogeny is due to the presence of the pPTPiC2-carrying insert and not a result of the absence of subtype C1 CWPS. This observation may be explained by possible leakage of the nisin promoter in uninduced cells, resulting in the production of a small but undetectable (under the conditions used) amount of subtype C2 CWPS that may be sufficient for phage adsorption yet insufficient to reverse the sedimentation phenotype.
TABLE 1.
Strains, plasmids, and phages used in this study
| Strain, plasmid, or phage | Relevant feature(s) | Reference(s) or source |
|---|---|---|
| Bacterial strains | ||
| L. lactis subsp. cremoris NZ9000 | L. lactis MG1363 derivative containing nisRK, host to sk1 | 28 |
| L. lactis subsp. cremoris 3107 | Host to φLC3, TP901-1_erm_, and _Viridus_JM2 | 50 |
| L. lactis subsp. cremoris NZ9700 | Nisin-producing strain | 28 |
| L. lactis subsp. cremoris NZ9000-GT1 | NZ9000 with GAATTC insert in LLNZ_01145, resulting in in-frame TGA stop codon | This work |
| L. lactis subsp. cremoris NZ9000-GT2 | NZ9000 with GAATTC insert in LLNZ_01150, resulting in in-frame TGA stop codon | This work |
| E. coli One Shot TOP10 | F− mcrA Δ(_mrr_-hsdRMS_-mcrBC) φ80_lac_ZΔM15 Δ_lacX74 recA1 ara_Δ_139 Δ(_ara_-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| Plasmids | ||
| pNZ8048 | Expression vector, P_nisA_, Cmr | 28 |
| pJP005 | pNZ8048 containing recA | 44, 45 |
| pPTPi | E. coli_-L. lactis shuttle vector, P_nisA, Tetr | 43 |
| pPTPiC2 | pPTPi containing genes 3107_003, 3107_4, and 3107_5 | This work |
| Bacteriophages | ||
| φLC3 | P335 species, propagated on 3107 | 51 |
| TP901_erm_ | P335 species, Emr, propagated on 3107 | 52 |
| sk1 | 936 species, propagated on NZ9000 | 5 |
| _Viridus_JM2 | 936 species, propagated on 3107 | 26 |
TABLE 2.
EOP of φLC3 and frequency of lysogeny of TP901-1_erm_ on various strainsa
| Strain and condition | φLC3 EOP | TP901-1_erm_ frequency of lysogeny |
|---|---|---|
| L. lactis NZ9000 | 0 | 4.02 × 10−8 |
| L. lactis 3107 | 1 | 5.40 × 10−4 |
| L. lactis NZ9000-GT1/pPTPi | ||
| Uninduced | 0 | NDb |
| Induced | 0 | ND |
| L. lactis NZ9000-GT1/pPTPiC2 | ||
| Uninduced | 0 | 1.94 × 10−5 |
| Induced | 3.53 × 10−1 | 1.30 × 10−4 |
CWPS is directly involved in 936 group phage interaction.
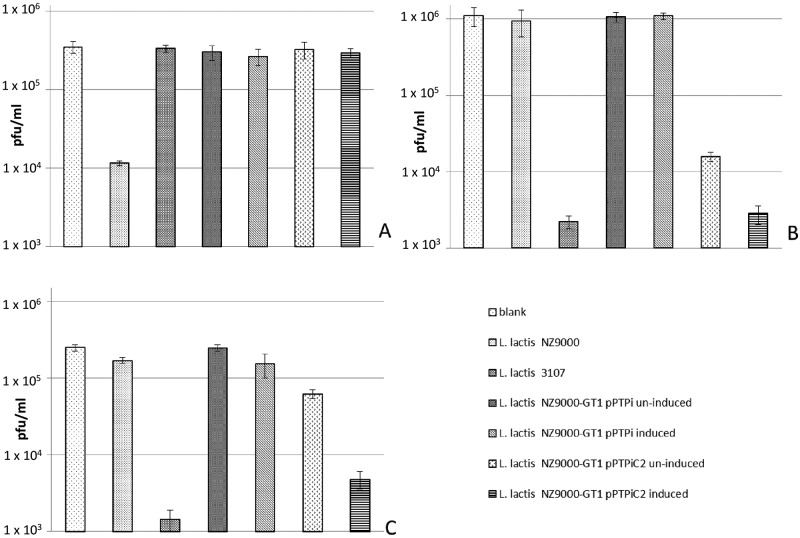
Several 936 group phages, which can form plaques on L. lactis strain 3107, were unable to produce visible plaques on induced L. lactis NZ9000-GT1/pPTPiC2. However, importantly, all of the available 936 group phages, capable of infecting L. lactis NZ9000 (and forming plaques), lost the ability to infect induced L. lactis NZ9000-GT1/pPTPiC2 with restored WT nonsedimenting growth though with an alternative CWPS content. Phage adsorption assays demonstrated that adsorption efficiencies are reversed between the WT indicator strains and induced L. lactis NZ9000-GT1/pPTPiC2 for 936 group phages (Fig. 4). Similar to the frequency of lysogeny experiments, the adsorption efficiency of _Viridus_JM2 on uninduced L. lactis NZ9000-GT1/pPTPiC2 appeared to be greater than that on negative-control strain L. lactis NZ9000-GT1/pPTPi.
FIG 4 .
Irreversible-adsorption assay results displaying various free-phage titers (A, sk1; B, _Viridus_JM2; C, φLC3) postadsorption to various strains. Induction of pPTPi and pPTPiC2 was done with a 1/2,000 dilution of L. lactis NZ9700 cell-free supernatant containing nisin.
DISCUSSION
While the presence of a high diversity of capsular polysaccharides and the importance of CWPS in streptococci have previously been shown (33, 34), the molecular nature and diversity level of CWPS in lactococci remain poorly characterized. In this study, we have characterized the level of diversity of the genetic locus responsible for so-called type C lactococcal CWPS biosynthesis. Variable levels of sequence identity among strains that harbor a type C CWPS biosynthesis gene cluster suggest that there are (subtle) differences in the structure of CWPS produced by five identified C subtype member strains. This notion was substantiated through the structural determination of the subtype C2 CWPS from L. lactis 3107.
The only CWPS of L. lactis described previously is the pellicle polysaccharide from prototypical strain MG1363. As in subtype C1 L. lactis MG1363, the CWPS of L. lactis 3107 is composed of oligosaccharide repeating units linked by phosphodiester bonds and containing a 3-β-GlcNAc-2-β-Gal_f_ sequence. However, the 3107 CWPS repeating unit is composed of pentasaccharide phosphate versus hexasaccharide phosphate in MG1363 CWPS and lacks the Glc-branching at the 3-β-GlcNAc moiety; also, the α-Glc-3-β-Gal_f_-fragment is present instead of β-GlcNAc-3-α-Rha in the repeating units of MG1363 CWPS, thus overall representing a substantially different CWPS structure despite the fact that the corresponding genetic loci responsible for the biosynthesis of their respective CWPS are rather similar (Fig. 1 and 2).
We have also shown that CWPS swapping can be achieved by genetic engineering without any detectable negative impact on growth. As a result of this CWPS swapping experiment and phage challenge assays, we can now conclusively state that the subtype C2 CWPS is the host cell surface receptor of the two P335 group bacteriophages φLC3 and TP901-1, while we also demonstrate that various 936 phages use the CWPS as their receptor to mediate adsorption.
It has previously been shown that certain BIMs of L. lactis 3107, which had been generated by ethyl methanesulfonate exposure, display distinctly different infection profiles with respect to phages φLC3 and TP901-1 (25). Of five TP901-1-resistant BIMs generated, two were shown to be resistant to φLC3. These two BIMs exhibit severely reduced adsorption efficiencies for TP901-1 and φLC3, consistent with the idea that their CWPS production had been affected. Interestingly, three TP901-1-resistant BIMs were still sensitive to φLC3 and exhibited nearly normal adsorption properties. The authors suggested that the two bacteriophages use the same receptor, which is supported by the fact that the C-terminal region of their RBPs are almost identical (35, 36), but possibly utilize different pathways for DNA injection. Our results clearly demonstrate that the receptor used by φLC3 and TP901-1_erm_ (and, by inference, TP901-1) is the CWPS produced by L. lactis 3107. Stockdale et al. (32) have previously demonstrated that TP901-1_erm_ can complete a replication cycle in L. lactis NZ9000 and can produce high numbers (~107 PFU/ml) of infective particles capable of infecting L. lactis 3107 upon induction in liquid growth medium. The failure to observe TP901-1 plaques on lawns of L. lactis NZ9000-GT1 producing the subtype C2 CWPS may therefore be due to a high lysogeny frequency under the plating conditions employed or some other mechanism that prevents plaque formation on this strain. Similarities in the CWPS of L. lactis MG1363 and 3107, specifically, the 3-β-GlcNAc-2-β-Gal_f_-fragment, may explain why TP901-1 can infect both strains, yet with very different efficiencies.
936 bacteriophages constitute a highly conserved group, especially in terms of their structural genes (37, 38), although they typically display narrow host ranges (8). The structural differences between the CWPS of MG1363 and 3107 may be at least partially responsible for the observed narrow host range of the 936 phages. Our data provide conclusive evidence and corroborate previous studies (3, 20, 22, 26, 39) that suggest that lactococcal CWPS is involved in 936 group phage recognition. Although plaque formation on strains expressing alternative CWPS was not observed, the expression of a different CWPS resulted in a changed adsorption profile of the 936 phages tested. The inability to form plaques despite the demonstration of normal adsorption to the CWPS-swapped strain suggests that such 936 phages are blocked in an essential step following adsorption. The nature of this elusive, and apparently essential, step required for successful 936 group phage infection postadsorption may further explain the observed narrow host ranges and justifies further investigation.
The finding that five different subtypes could be distinguished among the eight type C strains examined indicates that further subtypes may be present, and future research will focus on the discovery of additional subtypes among the three main CWPS types so far identified. The possibility of the CWPS subtypes influencing other functions, such as bacteriocin sensitivity or interaction with eukaryotic immune systems (20), also provides further exciting research prospects. In conclusion, this work has significantly advanced our understanding of the molecular identity of the receptor for representatives of two of the three main groups of lactococcal phages. These findings will serve as a springboard for future studies aimed at discovering how phages (and not only lactococcal phages but Gram-positive and perhaps Gram-negative phages as well) can recognize their host via saccharidic cell surface receptors.
MATERIALS AND METHODS
Strains and growth conditions used in this study.
The bacterial strains used in this study are listed in Table 1. All of the L. lactis strains were grown in M17 broth or on M17 agar (Oxoid) supplemented with 5 g/liter glucose and incubated overnight at 30°C. Where necessary, chloramphenicol or tetracycline (Sigma) was added to a particular growth medium at a concentration of 5 µg/ml. To induce the transcription of genes that were placed under the control of a nisin-inducible promoter (see below), the growth medium was supplemented with a 1:2,000 dilution of cell-free supernatant of nisin-producing strain L. lactis NZ9700 (40).
Bacteriophage assays.
The bacteriophages used in this study are listed in Table 1. Bacteriophages were propagated on their respective host strains as previously described (41), and lysates were maintained at 4°C. Spot assays and plaque assays were performed by the overlay method as previously described (42). Adsorption assays were performed as described previously (25). Briefly, cells at an optical density at 600 nm of 0.5 were harvested and resuspended in 0.02 volume of 10 mM MgSO4. A 150-µl volume of this suspension was then added to a 107-PFU/ml bacteriophage lysate containing 10 mM CaCl2 and incubated at 30°C for 5 min. Immediately after incubation, cells were diluted 1:100 in ice-cold quarter-strength Ringer solution containing 1 M NaCl and collected by centrifugation at 14,000 × g for 1 min. Supernatants were then assayed for free phages by standard plaque assays with an appropriate indicator strain. Frequency-of-lysogeny assays using erythromycin-tagged phage TP901_erm_ (a derivative of TP901-1, also designated TP901-BC1034) were performed as previously described (25).
Cloning.
All recombinant plasmids (Table 1) were generated in Escherichia coli TOP10 (Invitrogen). All of the primers, except where stated otherwise, were ordered from Eurofins MWG (Ebersberg, Germany). The variable section (i.e., variable within type C strains) of the CWPS biosynthesis gene cluster of L. lactis 3107, encompassing genes 3107_003 to 3107_005, was amplified with KOD DNA polymerase (Invitrogen) and cloned into the low-copy-number, nisin-inducible vector pPTPi (43). Plasmid constructs were then transformed into the L. lactis MG1363 _nisRK_-containing derivative L. lactis NZ9000 (28), into which plasmid pJP005 (44) had been introduced to allow recombineering and nisin-inducible expression.
Recombineering and oligonucleotides.
Recombineering (45) was performed with L. lactis NZ9000 or derivatives thereof as previously described (32, 44), with associated modifications as optimized for L. lactis and executing a given transformation with 500 µg of a particular oligonucleotide, which in some cases contained phosphorothioate linkages. Recombineering oligonucleotides (see Table S1 in the supplemental material) were ordered from Integrated DNA Technologies (Leuven, Belgium).
Bioinformatic analyses.
For comparative analysis of the CWPS biosynthesis gene clusters that belong to the C type, as identified by multiplex PCR (26), relevant genomic regions encompassing the CWPS biosynthesis gene cluster from lactococcal strains MG1363 (accession number NC_009004.1), SK11 (accession number NC_008527.1), and IO-1 (accession number AP012281) were employed. Full-genome analyses of L. lactis strains 3107, W34, JM1, JM2, and JM3 are currently in progress, and the results will be published elsewhere. The presumed CWPS region of each genome was analyzed and compared in detail by BLASTP (46) and InterPro (47) analyses. The genomic regions responsible for CWPS biosynthesis in L. lactis strains 3107, W34, JM1, JM2, and JM3 were identified on the basis of BLASTN analysis against the reference CWPS biosynthesis gene cluster of L. lactis MG1363. By using the genomic data corresponding to the CWPS biosynthesis region of the above-mentioned strains, conserved and variable regions were identified.
Preparation of carbohydrate material from L. lactis 3107.
L. lactis 3107 cells were harvested after overnight growth. Cells were defatted by boiling in 4% SDS and extracted with TCA as described previously (20). One portion of the TCA extract was fractionated on a HiTrap Q ion-exchange column, and several acidic fractions were collected, desalted on a Sephadex G-50 column, and analyzed further. The main acidic product was a phosphate-containing oligosaccharide (OS1), while another fraction corresponded to short forms of the polysaccharide. Another portion of unfractionated TCA extract (20 mg) was treated with 48% HF (50 µl) for 48 h at 4°C. The HF was allowed to evaporate, and the resulting residue was resuspended in water and applied to a Sephadex G-50 column for separation into two major fractions. The lower-MW fraction contained oligosaccharide fragments of polysaccharide and OS1 (OS preparation) and was used in methylation analysis. The OS preparation was reduced with NaBD4, desalted on a PD cartridge (Pharmacia), and used for methylation analysis. Also, the OS fraction was analyzed by MS as described below.
Chromatographic methods.
Ion-exchange chromatography was performed on a HiTrap Q column (GE Healthcare). The column was washed with water for 10 min and then eluted with a linear gradient of 0 to 1 M NaCl over 60 min at a flow rate of 3 ml min−1 with UV detection at 220 nm. Fractions were desalted by gel chromatography on a Sephadex G-50 column.
Gel filtration chromatography was performed with a Sephadex G-50 column (1.6 by 80 and 1 by 40 cm) and a Bio-Gel P2 column (1.6 by 80 cm) eluted with 0.01% acetic acid. Aliquots of each fraction were assayed for neutral sugars (48) and, if necessary, amino sugars (49).
Gas chromatography (GC)-MS was performed with a Trace GC ULTRA system (Thermo Scientific) equipped with an NMTR-5MS capillary column (30 m by 0.25 mm) with a temperature gradient of 170°C (3 min) to 250°C at 5°C min−1 and with a DSQ II MS detector and with a Varian Saturn 2000 ion trap instrument equipped with a DB-17 capillary column by using a temperature gradient of 160 to 260°C at 4°C min−1.
RP-HPLC was performed with an Agilent UHPLC1290 system equipped with a C18 column (Gemini, 250 by 4.6 mm, 5 µm; Thermo Electron Corporation). Oligosaccharides were separated with a 10-min isocratic step of 0.11% aqueous TFA (buffer A) and then a 20-min linear gradient (0 to 5%) of acetonitrile in buffer A at a flow rate of 0.5 ml min−1. They were detected by UV spectroscopy at 206 nm. Fractions were collected and analyzed by MALDI-TOF MS.
MALDI-TOF MS.
HPLC fractions containing oligosaccharides were analyzed by MALDI-TOF MS with a Voyager-DE STR mass spectrometer (Applied Biosystems) with a 2,5-dihydroxybenzoic acid matrix.
NMR spectroscopy analysis.
NMR experiments were carried out with a Varian INOVA 500-MHz (1H) spectrometer with a 3-mm gradient probe at 25°C with an acetone internal reference (2.225 ppm for 1H and 31.45 ppm for 13C) by using standard pulse sequences for double-quantum filtered COSY, TOCSY (mixing time, 120 ms), rotating-frame NOESY (mixing time, 500 ms), HSQC spectroscopy, and HMBC spectroscopy (100-ms long-range transfer delay). The AQ time was kept at 0.8 to 1 s for H-H correlations and 0.25 s for HSQC spectroscopy, and 256 increments were acquired for t1. Assignment of spectra was performed with the TopSpin 2 (Bruker Biospin) program for spectrum visualization and overlap.
HR-MAS NMR experiments were performed with an 18.8 T Avance III Bruker spectrometer. Results were acquired with a 1H-13C-31P-2H probe with uniaxial gradients. Before analysis, cell pellets were washed twice with deuterium oxide (Eurisotop, Gif-sur-Yvette, France). The 4-mm ZrO2 rotors (CortecNet, Paris, France) were filled with 50 µl of cell pellets, including 0.5 µl of acetone as the internal standard and finally centrifuged at 3,000 rpm. All of the spectra were recorded at 300K, and the rotor spinning rate was 8 kHz. All of the experiments were sourced from the Bruker library pulse program, and delays and powers were optimized for each. For 1H-13C HSQC spectroscopy, the spectral widths were 12,820 Hz (1H) with 1,024 points for free induction decay resolution and 29,994 Hz (13C) during 400 scans, giving 12.5 and 75.0 Hz/point, respectively.
Rapid extraction of CWPS fragments from L. lactis strains for monosaccharide analysis.
A rapid method was developed to release CWPS-associated carbohydrates directly from bacterial cells by treatment with HF. Cells were washed with water and lyophilized. Ten milligrams of dry cells was treated with 48% HF (150 µl) for 48 h at 4°C. HF was evaporated under a stream of nitrogen, the residue was resuspended in water (1 ml), insoluble material was removed by centrifugation, and the clear supernatant was applied to a Sephadex G-50 column. Fractions containing oligosaccharide fragments of CWPS (CWPS OS), identified by colorimetric detection of neutral and amino sugars, were collected. Ninety micrograms of _m_-inositol was added to the CWPS OS fraction prior to lyophilization. The dry residue was hydrolyzed, converted into alditol acetates, and analyzed by GC-MS as described above. The quantity of each individually identified monosaccharide was calculated relative to an _m_-inositol standard reference.
Nucleotide sequence accession numbers.
The nucleotide sequences of the genomic regions responsible for CWPS biosynthesis in L. lactis strains 3107, W34, JM1, JM2, and JM3 have been submitted to GenBank and assigned the following accession numbers: 3107, KF498848; W34, KF498852; JM1, KF498849; JM2, KF498850; JM3, KF498851.
SUPPLEMENTAL MATERIAL
Figure S1
HSQC spectroscopy of OS1 of the CWPS isolated from L. lactis 3107. Download
Figure S2
Quantitative monosaccharide analysis of polysaccharide fragments, released from dry cells by HF treatment (in µg/10 mg of dry cells). Download
Table S1
Recombineering oligonucleotides and screening primers used in this study. Asterisks denote phosphorothioate linkage modifications. Inserted sequences are in uppercase. Target gene in L. lactis NZ9000.
Table S2
NMR data for L. lactis 3107 OS1 (δ, ppm; Varian INOVA 500-MHz spectrometer, 25°C).
Table S3
MALDI mass spectral data ([M+Na]+) of HPLC fractions of OS preparation from L. lactis 3107 CWPS after HF treatment (OS2 preparation). The structures of the different oligosaccharides are shown in Fig. 2
ACKNOWLEDGMENTS
This research was funded by a Science Foundation Ireland (SFI) Principal Investigatorship award (08/IN.1/B1909) to D.V.S. and French ANR Project “Lactophages” (ANR-11-BSV8-004-01) to I.S., P.C., Y.G., T.G., C.C., and M.-P.C.-C. Financial support from the IR-RMN Fr3050 for conducting the research on the 800-MHz NMR spectrometer is gratefully acknowledged.
E. Maes is acknowledged for technical support in NMR spectroscopy.
Footnotes
Citation Ainsworth S, Sadovskaya I, Vinogradov E, Courtin P, Guerardel Y, Mahony J, Grard T, Cambillau C, Chapot-Chartier M-P, van Sinderen D. 2014. Differences in lactococcal cell wall polysaccharide structure are major determining factors in bacteriophage sensitivity. mBio 5(3):e00880-14. doi:10.1128/mBio.00880-14.
REFERENCES
- 1.Garneau JE, Moineau S. 2011. Bacteriophages of lactic acid bacteria and their impact on milk fermentations. Microb. Cell Fact. 10(Suppl 1):S20. 10.1186/1475-2859-10-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahony J, Murphy J, van Sinderen D. 2012. Lactococcal 936-type phages and dairy fermentation problems: from detection to evolution and prevention. Front. Microbiol. 3:335. 10.3389/fmicb.2012.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dupont K, Janzen T, Vogensen FK, Josephsen J, Stuer-Lauridsen B. 2004. Identification of Lactococcus lactis genes required for bacteriophage adsorption. Appl. Environ. Microbiol. 70:5825–5832. 10.1128/AEM.70.10.5825-5832.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deveau H, Labrie SJ, Chopin MC, Moineau S. 2006. Biodiversity and classification of lactococcal phages. Appl. Environ. Microbiol. 72:4338–4346. 10.1128/AEM.02517-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandry PS, Moore SC, Boyce JD, Davidson BE, Hillier AJ. 1997. Analysis of the DNA sequence, gene expression, origin of replication and modular structure of the Lactococcus lactis lytic bacteriophage sk1. Mol. Microbiol. 26:49–64. 10.1046/j.1365-2958.1997.5491926.x [DOI] [PubMed] [Google Scholar]
- 6.Labrie SJ, Josephsen J, Neve H, Vogensen FK, Moineau S. 2008. Morphology, genome sequence, and structural proteome of type phage P335 from Lactococcus lactis. Appl. Environ. Microbiol. 74:4636–4644. 10.1128/AEM.00118-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarvis AW, Lubbers MW, Beresford TP, Ward LJ, Waterfield NR, Collins LJ, Jarvis BD. 1995. Molecular biology of lactococcal bacteriophage c2. Dev. Biol. Stand. 85:561–567 [PubMed] [Google Scholar]
- 8.Kleppen HP, Bang T, Nes IF, Holo H. 2011. Bacteriophages in milk fermentations: diversity fluctuations of normal and failed fermentations. Int. Dairy J. 21:592–600. 10.1016/j.idairyj.2011.02.010 [DOI] [Google Scholar]
- 9.Geller BL, Ivey RG, Trempy JE, Hettinger-Smith B. 1993. Cloning of a chromosomal gene required for phage infection of Lactococcus lactis subsp. lactis C2. J. Bacteriol. 175:5510–5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monteville MR, Ardestani B, Geller BL. 1994. Lactococcal bacteriophages require a host cell wall carbohydrate and a plasma membrane protein for adsorption and ejection of DNA. Appl. Environ. Microbiol. 60:3204–3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baptista C, Santos MA, São-José C. 2008. Phage SPP1 reversible adsorption to Bacillus subtilis cell wall teichoic acids accelerates virus recognition of membrane receptor YueB. J. Bacteriol. 190:4989–4996. 10.1128/JB.00349-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahony J, Ainsworth S, Stockdale S, van Sinderen D. 2012. Phages of lactic acid bacteria: the role of genetics in understanding phage-host interactions and their co-evolutionary processes. Virology 434:143–150. 10.1016/j.virol.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 13.Shibata Y, Yamashita Y, van der Ploeg JR. 2009. The serotype-specific glucose side chain of rhamnose-glucose polysaccharides is essential for adsorption of bacteriophage M102 to Streptococcus mutans. FEMS Microbiol. Lett. 294:68–73. 10.1111/j.1574-6968.2009.01546.x [DOI] [PubMed] [Google Scholar]
- 14.Räisänen L, Draing C, Pfitzenmaier M, Schubert K, Jaakonsaari T, von Aulock S, Hartung T, Alatossava T. 2007. Molecular interaction between lipoteichoic acids and Lactobacillus delbrueckii phages depends on d-alanyl and alpha-glucose substitution of poly(glycerophosphate) backbones. J. Bacteriol. 189:4135–4140. 10.1128/JB.00078-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorska S, Grycko P, Rybka J, Gamian A. 2007. Exopolysaccharides of lactic acid bacteria: structure and biosynthesis. Postepy Hig. Med. Dosw. 61:805–818 (In Polish.)http://www.phmd.pl/abstracted.php?level=5&icid=621720 [PubMed] [Google Scholar]
- 16.van Kranenburg R, Marugg JD, van Swam II, Willem NJ, de Vos WM. 1997. Molecular characterization of the plasmid-encoded eps gene cluster essential for exopolysaccharide biosynthesis in Lactococcus lactis. Mol. Microbiol. 24:387–397. 10.1046/j.1365-2958.1997.3521720.x [DOI] [PubMed] [Google Scholar]
- 17.Deveau H, Van Calsteren MR, Moineau S. 2002. Effect of exopolysaccharides on phage-host interactions in Lactococcus lactis. Appl. Environ. Microbiol. 68:4364–4369. 10.1128/AEM.68.9.4364-4369.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forde A, Fitzgerald GF. 2003. Molecular organization of exopolysaccharide (EPS) encoding genes on the lactococcal bacteriophage adsorption blocking plasmid, pCI658. Plasmid 49:130–142. 10.1016/S0147-619X(02)00156-7 [DOI] [PubMed] [Google Scholar]
- 19.Forde A, Fitzgerald GF. 1999. Analysis of exopolysaccharide (EPS) production mediated by the bacteriophage adsorption blocking plasmid, pCI658, isolated from Lactococcus lactis subsp. cremoris HO2. Int. Dairy J. 9:465–472. 10.1016/S0958-6946(99)00115-6 [DOI] [Google Scholar]
- 20.Chapot-Chartier M-P, Vinogradov E, Sadovskaya I, Andre G, Mistou MY, Trieu-Cuot P, Furlan S, Bidnenko E, Courtin P, Péchoux C, Hols P, Dufrêne YF, Kulakauskas S. 2010. Cell surface of Lactococcus lactis is covered by a protective polysaccharide pellicle. J. Biol. Chem. 285:10464–10471. 10.1074/jbc.M109.082958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinogradov E, Valence F, Maes E, Jebava I, Chuat V, Lortal S, Grard T, Guerardel Y, Sadovskaya I. 2013. Structural studies of the cell wall polysaccharides from three strains of Lactobacillus helveticus with different autolytic properties: DPC4571, BROI, and LH1. Carbohydr. Res. 379:7–12. 10.1016/j.carres.2013.05.020 [DOI] [PubMed] [Google Scholar]
- 22.Tremblay DM, Tegoni M, Spinelli S, Campanacci V, Blangy S, Huyghe C, Desmyter A, Labrie S, Moineau S, Cambillau C. 2006. Receptor-binding protein of Lactococcus lactis phages: identification and characterization of the saccharide receptor-binding site. J. Bacteriol. 188:2400–2410. 10.1128/JB.188.7.2400-2410.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spinelli S, Campanacci V, Blangy S, Moineau S, Tegoni M, Cambillau C. 2006. Modular structure of the receptor binding proteins of Lactococcus lactis phages. The RBP structure of the temperate phage TP901-1. J. Biol. Chem. 281:14256–14262. 10.1074/jbc.M600666200 [DOI] [PubMed] [Google Scholar]
- 24.Veesler D, Spinelli S, Mahony J, Lichière J, Blangy S, Bricogne G, Legrand P, Ortiz-Lombardia M, Campanacci V, van Sinderen D, Cambillau C. 2012. Structure of the phage TP901-1 1.8 MDa baseplate suggests an alternative host adhesion mechanism. Proc. Natl. Acad. Sci. U. S. A. 109:8954–8958. 10.1073/pnas.1200966109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostergaard Breum S, Neve H, Heller KJ, Vogensen FK. 2007. Temperate phages TP901-1 and phiLC3, belonging to the P335 species, apparently use different pathways for DNA injection in Lactococcus lactis subsp. cremoris 3107. FEMS Microbiol. Lett. 276:156–164. 10.1111/j.1574-6968.2007.00928.x [DOI] [PubMed] [Google Scholar]
- 26.Mahony J, Kot W, Murphy J, Ainsworth S, Neve H, Hansen LH, Heller KJ, Sørensen SJ, Hammer K, Cambillau C, Vogensen FK, van Sinderen D. 2013. Investigation of the relationship between lactococcal host cell wall polysaccharide genotype and 936 phage receptor binding protein phylogeny. Appl. Environ. Microbiol. 79:4385–4392. 10.1128/AEM.00653-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bock K, Pedersen C. 1983. Carbon-13 nuclear magnetic resonance spectroscopy of monosaccharides. Adv. Carbohydr. Chem. Biochem. 41:27–66. 10.1016/S0065-2318(08)60055-4 [DOI] [Google Scholar]
- 28.Kuipers OP, de Ruyter PGGA, Kleerebezem M, de Vos WM. 1998. Quorum sensing-controlled gene expression in lactic acid bacteria. J. Biotechnol. 64:15–21. 10.1016/S0168-1656(98)00100-X [DOI] [Google Scholar]
- 29.Poole ES, Brown CM, Tate WP. 1995. The identity of the base following the stop codon determines the efficiency of in vivo translational termination in Escherichia coli. EMBO J. 14:151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maes E, Mille C, Trivelli X, Janbon G, Poulain D, Guérardel Y. 2009. Molecular phenotyping of mannosyltransferases-deficient Candida albicans cells by high-resolution magic angle spinning NMR. J. Biochem. 145:413–419. 10.1093/jb/mvp008 [DOI] [PubMed] [Google Scholar]
- 31.Candela T, Maes E, Garénaux E, Rombouts Y, Krzewinski F, Gohar M, Guérardel Y. 2011. Environmental and biofilm-dependent changes in a Bacillus cereus secondary cell wall polysaccharide. J. Biol. Chem. 286:31250–31262. 10.1074/jbc.M111.249821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stockdale SR, Mahony J, Courtin P, Chapot-Chartier M-P, van Pijkeren JP, Britton RA, Neve H, Heller KJ, Aideh B, Vogensen FK, van Sinderen D. 2013. The lactococcal phages Tuc2009 and TP901-1 incorporate two alternate forms of their tail fiber into their virions for infection specialization. J. Biol. Chem. 288:5581–5590. 10.1074/jbc.M112.444901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bentley SD, Aanensen DM, Mavroidi A, Saunders D, Rabbinowitsch E, Collins M, Donohoe K, Harris D, Murphy L, Quail MA, Samuel G, Skovsted IC, Kaltoft MS, Barrell B, Reeves PR, Parkhill J, Spratt BG. 2006. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2:e31. 10.1371/journal.pgen.0020031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mavroidi A, Aanensen DM, Godoy D, Skovsted IC, Kaltoft MS, Reeves PR, Bentley SD, Spratt BG. 2007. Genetic relatedness of the Streptococcus pneumoniae capsular biosynthetic loci. J. Bacteriol. 189:7841–7855. 10.1128/JB.00836-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brøndsted L, Ostergaard S, Pedersen M, Hammer K, Vogensen FK. 2001. Analysis of the complete DNA sequence of the temperate bacteriophage TP901-1: evolution, structure, and genome organization of lactococcal bacteriophages. Virology 283:93–109. 10.1006/viro.2001.0871 [DOI] [PubMed] [Google Scholar]
- 36.Blatny JM, Godager L, Lunde M, Nes IF. 2004. Complete genome sequence of the Lactococcus lactis temperate phage phiLC3: comparative analysis of phiLC3 and its relatives in lactococci and streptococci. Virology 318:231–244. 10.1016/j.virol.2003.09.019 [DOI] [PubMed] [Google Scholar]
- 37.Castro-Nallar E, Chen H, Gladman S, Moore SC, Seemann T, Powell IB, Hillier A, Crandall KA, Chandry PS. 2012. Population genomics and phylogeography of an Australian dairy factory derived lytic bacteriophage. Genome Biol. Evol. 4:382–393. 10.1093/gbe/evs017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahony J, Deveau H, Mc Grath S, Ventura M, Canchaya C, Moineau S, Fitzgerald GF, van Sinderen D. 2006. Sequence and comparative genomic analysis of lactococcal bacteriophages jj50, 712 and P008: evolutionary insights into the 936 phage species. FEMS Microbiol. Lett. 261:253–261. 10.1111/j.1574-6968.2006.00372.x [DOI] [PubMed] [Google Scholar]
- 39.Bebeacua C, Tremblay D, Farenc C, Chapot-Chartier M-P, Sadovskaya I, van Heel M, Veesler D, Moineau S, Cambillau C. 2013. Structure, adsorption to host, and infection mechanism of virulent lactococcal phage p2. J. Virol. 87:12302–12312. 10.1128/JVI.02033-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuipers OP, Beerthuyzen MM, Siezen RJ, De Vos WM. 1993. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis. Requirement of expression of the nisA and nisI genes for development of immunity. Eur. J. Biochem. 216:281–291. 10.1111/j.1432-1033.1993.tb18143.x [DOI] [PubMed] [Google Scholar]
- 41.Mahony J, McGrath S, Fitzgerald GF, van Sinderen D. 2008. Identification and characterization of lactococcal-prophage-carried superinfection exclusion genes. Appl. Environ. Microbiol. 74:6206–6215. 10.1128/AEM.01053-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lillehaug D. 1997. An improved plaque assay for poor plaque-producing temperate lactococcal bacteriophages. J. Appl. Microbiol. 83:85–90. 10.1046/j.1365-2672.1997.00193.x [DOI] [PubMed] [Google Scholar]
- 43.O’Driscoll J, Glynn F, Cahalane O, O’Connell-Motherway M, Fitzgerald GF, van Sinderen D. 2004. Lactococcal plasmid pNP40 encodes a novel, temperature-sensitive restriction-modification system. Appl. Environ. Microbiol. 70:5546–5556. 10.1128/AEM.70.9.5546-5556.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Pijkeren JP, Neoh KM, Sirias D, Findley AS, Britton RA. 2012. Exploring optimization parameters to increase ssDNA recombineering in Lactococcus lactis and Lactobacillus reuteri. Bioengineered 3:209–217. 10.4161/bioe.21049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Pijkeren JP, Britton RA. 2012. High efficiency recombineering in lactic acid bacteria. Nucleic Acids Res. 40:e76. 10.1093/nar/gks147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and psi-blast: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402. 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Apweiler R, Attwood TK, Bairoch A, Bateman A, Birney E, Biswas M, Bucher P, Cerutti L, Corpet F, Croning MD, Durbin R, Falquet L, Fleischmann W, Gouzy J, Hermjakob H, Hulo N, Jonassen I, Kahn D, Kanapin A, Karavidopoulou Y, Lopez R, Marx B, Mulder NJ, Oinn TM, Pagni M, Servant F, Sigrist CJ, Zdobnov EM. 2001. The InterPro database, an integrated documentation resource for protein families, domains and functional sites. Nucleic Acids Res. 29:37–40. 10.1093/nar/29.1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350–356. 10.1021/ac60111a017 [DOI] [PubMed] [Google Scholar]
- 49.Enghofer E, Kress H. 1979. An evaluation of the Morgan-Elson assay for 2-amino-2-deoxy sugars. Carbohydr. Res. 76:233–238. 10.1016/0008-6215(79)80022-1 [DOI] [PubMed] [Google Scholar]
- 50.Braun V, Jr, Hertwig S, Neve H, Geis A, Teuber M. 1989. Taxonomic differentiation of bacteriophages of Lactococcus lactis by electron-microscopy, DNA-DNA hybridization, and protein profiles. J. Gen. Microbiol. 135:2551–2560 [Google Scholar]
- 51.Lillehaug D, Lindqvist B, Birkeland NK. 1991. Characterization of phiLC3, a Lactococcus lactis subsp. cremoris temperature bacteriophage with cohesive single-stranded DNA ends. Appl. Environ. Microbiol. 57:3206–3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koch B, Christiansen B, Evison T, Vogensen FK, Hammer K. 1997. Construction of specific erythromycin resistance mutations in the temperate lactococcal bacteriophage TP901-1 and their use in studies of phage biology. Appl. Environ. Microbiol. 63:2439–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
HSQC spectroscopy of OS1 of the CWPS isolated from L. lactis 3107. Download
Figure S2
Quantitative monosaccharide analysis of polysaccharide fragments, released from dry cells by HF treatment (in µg/10 mg of dry cells). Download
Table S1
Recombineering oligonucleotides and screening primers used in this study. Asterisks denote phosphorothioate linkage modifications. Inserted sequences are in uppercase. Target gene in L. lactis NZ9000.
Table S2
NMR data for L. lactis 3107 OS1 (δ, ppm; Varian INOVA 500-MHz spectrometer, 25°C).
Table S3
MALDI mass spectral data ([M+Na]+) of HPLC fractions of OS preparation from L. lactis 3107 CWPS after HF treatment (OS2 preparation). The structures of the different oligosaccharides are shown in Fig. 2