A gene horizontally transferred from bacteria protects arthropods from host plant cyanide poisoning (original) (raw)
Abstract
Cyanogenic glucosides are among the most widespread defense chemicals of plants. Upon plant tissue disruption, these glucosides are hydrolyzed to a reactive hydroxynitrile that releases toxic hydrogen cyanide (HCN). Yet many mite and lepidopteran species can thrive on plants defended by cyanogenic glucosides. The nature of the enzyme known to detoxify HCN to β-cyanoalanine in arthropods has remained enigmatic. Here we identify this enzyme by transcriptome analysis and functional expression. Phylogenetic analysis showed that the gene is a member of the cysteine synthase family horizontally transferred from bacteria to phytophagous mites and Lepidoptera. The recombinant mite enzyme had both β-cyanoalanine synthase and cysteine synthase activity but enzyme kinetics showed that cyanide detoxification activity was strongly favored. Our results therefore suggest that an ancient horizontal transfer of a gene originally involved in sulfur amino acid biosynthesis in bacteria was co-opted by herbivorous arthropods to detoxify plant produced cyanide.
DOI: http://dx.doi.org/10.7554/eLife.02365.001
Research organism: other
eLife digest
Hydrogen cyanide is a poison that is deadly for most forms of life. Also known as prussic acid, it has killed countless humans throughout history in accidents and during the Holocaust. Hydrogen cyanide is also used by plants to defend themselves against insects and other herbivorous animals.
Many plants produce chemicals called cyanogenic glycosides that can be converted into hydrogen cyanide when the plant is eaten. This is an ancient and efficient defense against all sorts of herbivores, including humans. For instance, cassava is a key source of food in sub-Saharan Africa and South America, but it contains cyanogenic glucosides and is highly toxic if eaten in unprocessed form. However, some insects and mites can thrive on cyanogenic plants, often to the extent of becoming pests on these plants.
Certain moths, such as burnet moths, have gone further and now depend on cyanogenic glucosides for their own defenses against predators such as birds. How these mites and insects are capable of fending off cyanide toxicity has long remained a mystery.
Now Wybouw et al. have identified a mite enzyme that detoxifies hydrogen cyanide to produce a compound called beta-cyanoalanine. Remarkably, the DNA that encodes this enzyme did not evolve in animals but originally belonged to a bacterium. Wybouw et al. show that the gene was transferred to the genome of the spider mite Tetranychus urticae perhaps a few hundred million years ago. An equivalent gene was also found in moths and butterflies, which explains why these insects can thrive on plants that produce hydrogen cyanide.
This lateral gene transfer from bacteria to animals is a remarkable coalition of two kingdoms against another, and illustrates a new aspect of the chemical warfare between plants and animals. This study also increases our awareness of the importance of laterally transferred genes in the genomes of higher organisms.
DOI: http://dx.doi.org/10.7554/eLife.02365.002
Introduction
Plants have developed a remarkable diversity of chemical defenses to deter herbivores from feeding. Cyanogenesis is one of the most ancient and widespread of these defenses, and more than 2500 plant species are known to synthesize cyanogenic glucosides and cyanolipids as phytoanticipins (constitutive defense compounds present in the plant prior to herbivore attack). Upon tissue disruption by herbivore feeding, cyanogenic glucosides are degraded by the plant β-glucosidases and α-hydroxynitrile lyases, which results in the release of toxic hydrogen cyanide (HCN) and other toxic products such as their aglycones (Gleadow and Woodrow, 2002; Poulton, 1990; Spencer, 1988; Zagrobelny et al., 2004; Figure 1). The released cyanide is a potent inhibitor of the mitochondrial respiratory chain and has a disruptive effect on various metabolic pathways (Solomonson, 1981), thus providing a broad defense against generalist herbivores.
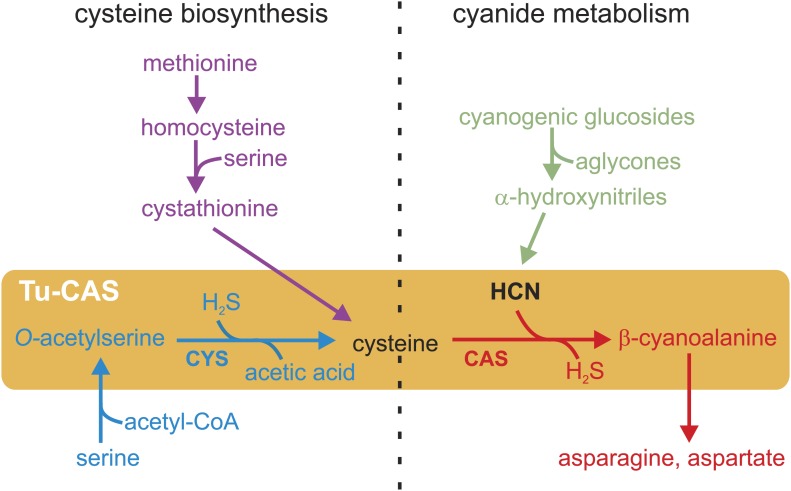
Figure 1. Schematic overview of the cysteine biosynthesis pathway in Metazoa (purple) and Plants/Bacteria (blue), the release of HCN during plant cyanogenesis (green) and the main HCN detoxification pathway in arthropods (red).
The two reactions catalyzed by the gene product of the Tu-CAS gene are marked by an orange background and are indicated by CYS and CAS. CAS detoxifies cyanide by incorporation into cysteine forming β-cyanoalanine, which can be further metabolized by nitrilases. CYS catalyzes the second step of the cysteine synthesis pathway in bacteria and plants, after serine is converted to _O_-acetylserine.
DOI: http://dx.doi.org/10.7554/eLife.02365.003
In their arms race with plants, arthropods have evolved several mechanisms to overcome plant cyanogenesis. A well-documented case of co-evolution is found in insect lepidopteran specialists that sequester the ingested cyanogenic glucosides in their own defense against predators. Remarkably, cyanogenic compounds have become so crucial for some species such as burnet moths (Zygaenidae) that they not only sequester, but also synthesize these compounds de novo by convergent evolution of the biosynthetic pathways (Jensen et al., 2011). Next to sequestration, other mechanisms have evolved to cope with the toxic effects of HCN, such as avoiding the ingestion of cyanogenic compounds or detoxifying HCN upon plant release (Despres et al., 2007). In animals, HCN is thought to be detoxified by two main pathways. The enzyme rhodanese converts cyanide into thiocyanate, but this biochemical reaction is not very common and thought to be inefficient (Beesley et al., 1985; Davis and Nahrstedt, 1985; Long and Brattsten, 1982). Alternatively, the conversion of HCN and cysteine into β-cyanoalanine and sulfide has been suggested as the main detoxification pathway in arthropods (Figure 1). This is supported by several biochemical surveys showing a correlation between β-cyanoalanine synthesis and HCN exposure in lepidopteran species tolerant to HCN (Meyers and Ahmad, 1991; Stauber et al., 2012). However, the enzyme that catalyzes this crucial reaction in arthropods has not been identified to date. The conversion of HCN into β-cyanoalanine by an enzyme called β-cyanoalanine synthase (CAS) has been best studied in bacteria and plants that need to protect themselves from HCN during the synthesis of cyanogenic glucosides or ethylene. The enzymes responsible for CAS activity also have cysteine synthase activity (CYS) and are referred to as CYS or CAS depending on their substrate specificity. CYS catalyzes the conversion of _O_-acetylserine into cysteine, an essential final step in the cysteine biosynthesis pathway unique for plants and bacteria (Bonner et al., 2005). Animals synthesize cysteine by a different pathway and use related enzymes such as cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CGL), which form together with CAS and CYS a group of pyridoxal-5′-phosphate dependent enzymes (Finkelstein et al., 1988; Figure 1). Here we identify the enzyme responsible for detoxification of cyanide to β-cyanoalanine in a spider mite and show that this enzyme is horizontally acquired from bacteria and is also widely distributed in Lepidoptera.
Results
Transcriptional response of the spider mite Tetranychus urticae to a cyanogenic host plant
In order to gain a better insight into arthropod defenses against plant cyanogenesis, we used the two-spotted spider mite T. urticae as a generalist herbivore model in a host plant adaptation experiment. This species is one of the most polyphagous arthropod pests known to date, and feeds on more than 1100 plant species from more than 140 plant families, including many cyanogenic plants (Grbic et al., 2011). We transferred a spider mite strain reared on acyanogenic bean plants (Phaseolus vulgaris) to a cultivar of Phaseolus lunatus containing high levels of well characterized cyanogenic glucosides such as linamarin and lotaustralin (Ballhorn et al., 2006; Jones, 1998; Wybouw et al., 2012) and allowed the strain to adapt to this host plant for more than 30 generations. Gene expression differences between mites feeding on P. vulgaris and on P. lunatus were then determined using genome-wide microarrays (Dermauw et al., 2013).
In contrast to the broad response previously detected after host plant changes from Fabaceae to Solanaceae and Brassicaceae (Grbic et al., 2011; Dermauw et al., 2013), only a limited set of 28 genes (absolute fold change ≥2, Benjamini-Hochberg corrected p-value <0.05) was found differentially expressed between the parental and adapted lines (Supplementary file 1). Within this small set of genes, 18 had an increased expression in the adapted line, while 10 exhibited a lower expression. The most differentially expressed genes after transfer to cyanogenic lima bean encode closely related, small cytoplasmic proteins of unknown function. They were also seen in the transcriptional response after transfer to tomato suggesting that these genes could be part of a broad general stress response of T. urticae (Dermauw et al., 2013). Among the genes with an increased expression were three cytochrome P450 genes, known to respond readily to host plant changes (Grbic et al., 2011; Dermauw et al., 2013). Moreover, we identified a gene (tetur10g01570, Tu-CAS) encoding a predicted cytosolic protein with high similarity to bacterial cysteine synthases (Conserved Domain Database (CDD): COG0031). The reported microarray data have been deposited at the Gene Expression Omnibus (Wybouw et al., 2013).
Phylogeny of Tu-CAS and evidence for a bacterial origin by horizontal transfer
Within chelicerates, we only detected close homologues of Tu-CAS in two closely related tetranychid mites. By sequencing PCR amplicons (see below, this section) and a tBLASTn-search in a published transcriptome (Liu et al., 2011), CAS genes were identified in Tetranychus evansi and Panonychus citri, respectively. A tBLASTn-search in published genomes of mesostigmatid mites and of ticks (Metastigmata) did not reveal homologues (Supplementary file 2). Broadening the search to arthropods by tBLASTn-searches in NCBI databases and additional arthropod genome portals (Supplementary file 2), we only detected close homologues of Tu-CAS in lepidopteran genomes (Bombyx mori, Danaus plexippus, Heliconius melpomene, Manduca sexta and Plutella xylostella) (Figure 2). On average, Tu-CAS showed 75% similarity with lepidopteran protein sequences, and genes encoding these proteins were intronless in both mites and insects. By searching additional NCBI EST-databases and published lepidopteran transcriptomes, a total of 20 (complete and partial) homologous sequences were identified in arthropods.
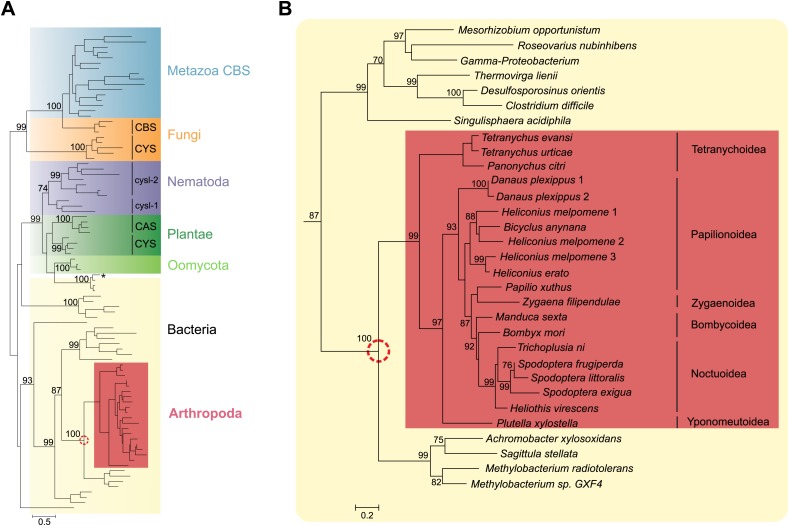
Figure 2. Panel A: Phylogenetic analysis of β-substituted alanine synthases, showing arthropod sequences nested within bacterial cysteine synthases.
The fungal CYS, metazoan and fungal CBS as well as the plant, oomycete and nematode CYS and CAS sequences are marked with a different color. The two branches of nematode sequences, marked as cysl-1 and cysl-2, include the sequences coded by the two genes previously characterized in C. elegans (Budde and Roth, 2011). The CYS and CAS groups within Plantae represent plant protein sequences with CYS and CAS activity, respectively (Yamaguchi et al., 2000). The asterisk represents a CYS sequence of the mealybug P. citri acquired by horizontal gene transfer from its endosymbiont (Husnik et al., 2013). Panel B: Detailed view of the bacterial CYS sequences showing the embedded sequences of tetranychid mites and Lepidoptera. In both panels support values of only important nodes are shown. The scale bar represents 0.5 and 0.2 substitutions per site in panel A and panel B, respectively.
DOI: http://dx.doi.org/10.7554/eLife.02365.004
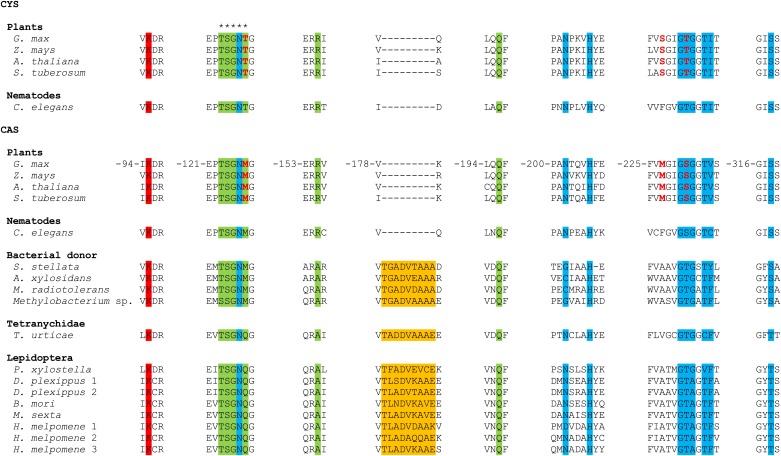
Figure 2—figure supplement 1. MUSCLE alignment of cysteine synthases and β-cyanoalanine synthases discussed in this study.
Residue numbering is shown for the G. max CAS sequence (Yi et al., 2012). The five residues forming an active site loop are marked by asterisks. The amino acid and pyridoxal-5′-phosphate binding sites are indicated by a green and a blue background respectively, while the Schiff base Lys is indicated by a red background (Bonner et al., 2005; Yi et al., 2012). The unique 9 amino acid insertion seen in arthropod enzymes and their closest bacterial homologues is highlighted in orange. The three residues that convert G. max CYS into CAS by creating a triple mutant (Yi et al., 2012) are shown in red.
A phylogenetic reconstruction of these arthropod proteins with CYS, CAS and CBS enzymes of plants, fungi, oomycetes, bacteria and Metazoa indicated that these homologous arthropod sequences might be monophyletic. They were embedded with high node support within bacterial cysteine synthase sequences, indicative of a horizontal gene transfer (Figure 2). The most closely related sequences are from bacteria that belong to the α- and β-Proteobacteria, two of which are Methylobacterium species, free-living epiphytic bacteria known to establish endophytic colonies. These species are reported to be transferred from the plant to phytophagous insects and to survive inside arthropod hosts (Kutschera, 2007; Rampelotti-Ferreira et al., 2010), facilitating a potential horizontal gene transfer. Alternatively, Proteobacteria are known endosymbionts of arthropods and often reside in the reproductive organs for vertical transmission to following generations (Wernegreen, 2002). Because of this intimate relationship, successful horizontal gene transfer is more likely to occur (Hotopp, 2011). The mite and insect sequences formed a branch in bacterial cysteine synthase enzymes, some of which have documented dual CYS and CAS activities (Omura et al., 2003) (Figure 2). The arthropod protein sequences and their closest bacterial homologues shared a unique 9 amino acid insertion not present in cysteine synthases of other organisms, but the residues known to be crucial for substrate and cofactor binding in plants and bacteria showed conservation (Bonner et al., 2005; Yi et al., 2012). The lysine residue (Lys95, Glycine max CAS numbering, Yi et al., 2012) that forms a Schiff base linkage to the cofactor pyridoxal-5′-phosphate was also conserved in arthropods (Figure 2—figure supplement 1).
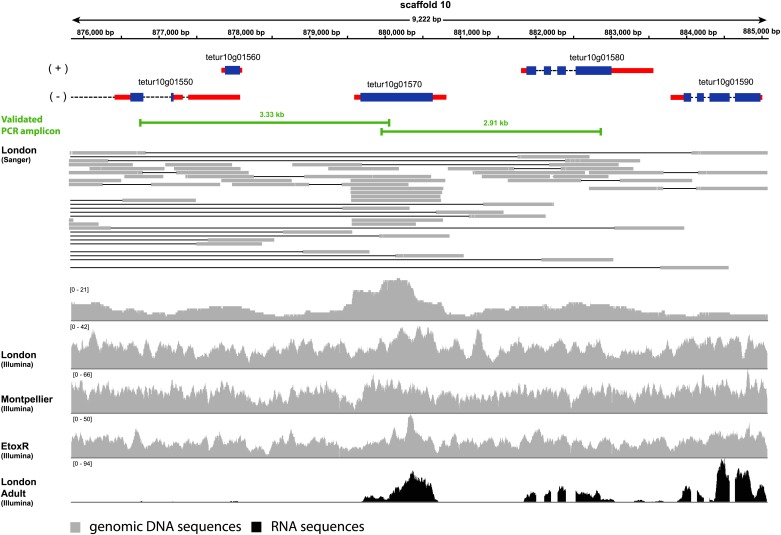
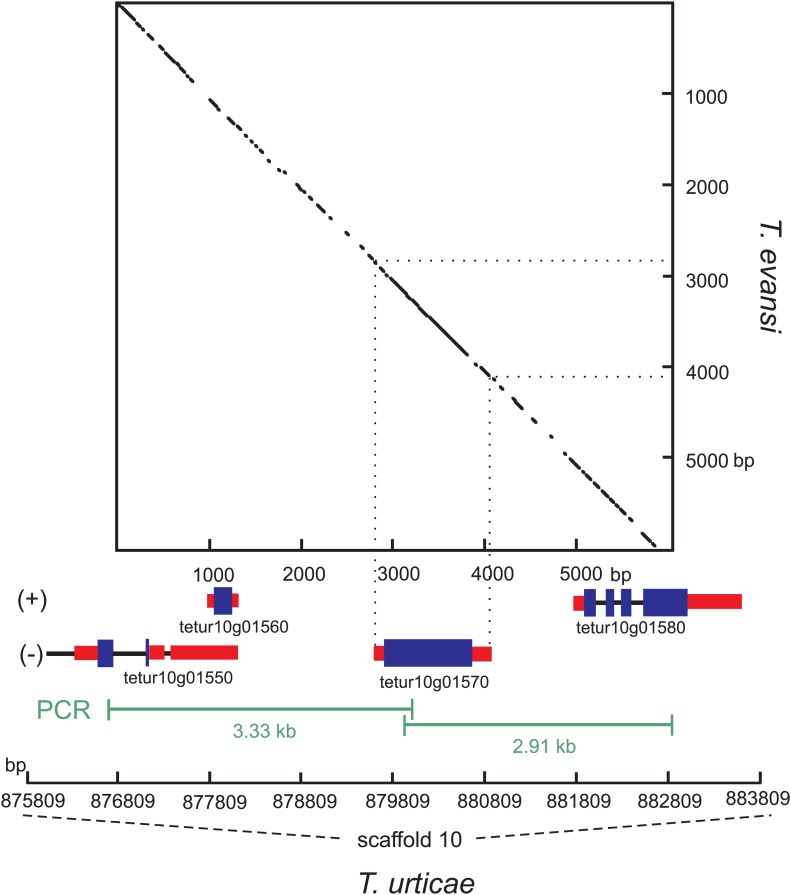
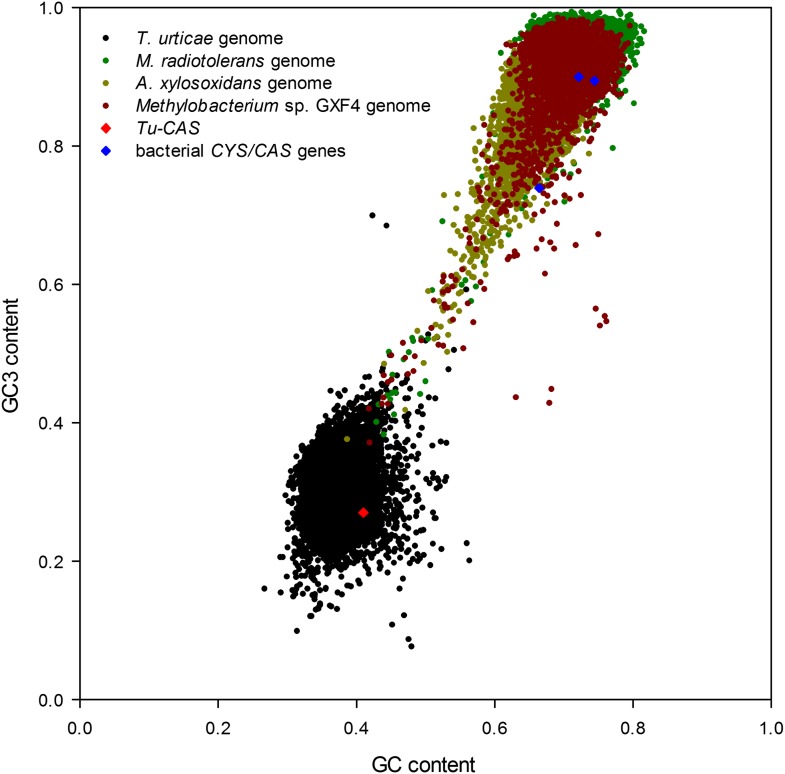
To exclude the possibility that the Tu-CAS sequence was derived from contaminating bacterial DNA, we examined its position in the T. urticae genome into more detail. Tu-CAS is located on a 3 Mb large scaffold (scaffold 10) and is flanked by typical eukaryotic genes, tetur10g01550 and tetur10g01580. Both genes contain introns with splice sites confirmed by EST or RNA-seq data (Grbic et al., 2011, GenBank: LIBEST_025606, Figure 3). Tetur10g01580 encodes a nudix hydrolase (CDD: cl00447) highly similar to other arthropod nudix hydrolases (BLASTp hits with E-value <1e−45). To rule out that the linkage of Tu-CAS to these spliced genes is a genome assembly artefact, we took several independent genomic approaches. First, we remapped the T. urticae Sanger reads used for the genome assembly of the London strain of T. urticae (Grbic et al., 2011) and examined the Illumina read coverage of the Tu-CAS region in two re-sequenced strains (EtoxR and Montpellier) that are geographically distinct from the London strain (Grbic et al., 2011; Van Leeuwen et al., 2012). Neither Sanger nor Illumina-reads revealed any inconsistencies in the Tu-CAS region in any of these strains (Figure 3). Second, using a PCR approach, we amplified a 6 kb genomic region bracketing Tu-CAS with the surrounding spliced genes (tetur10g01550 and tetur10g01580, Figure 4, Figure 4—figure supplement 1). Using a similar strategy we also amplified a 6 kb region in the closely related spider mite T. evansi (Figure 4, Figure 4—figure supplement 1) and a nucleotide dot plot between the amplified region of these two species showed clear synteny and the absence of discontinuity around the CAS gene (Figure 4). This would not be expected by bacterial contamination of either genome. Last, gene compositions of T. urticae and bacteria were analyzed by determining both the GC-content at the synonymous third codon position (GC3) and the overall GC-content (GC) of the genes to look if amelioration occurred. Amelioration is the process by which the DNA composition of the newly acquired gene becomes homogenized to match the composition of the recipient genome (all genes in the recipient genome are subject to the same mutational processes) (Lawrence and Ochman, 1997). Indeed, the GC/GC3 content of Tu-CAS was most similar to the GC/GC3 content of genes from the T. urticae genome, and quite distinct from the GC/GC3 content of genes (including the CYS/CAS genes) from the three annotated bacterial genomes in the closest sister clade to the apparent monophyletic arthropod clade (Figure 5). Taken together, these data provide strong evidence that Tu-CAS is a sequence integrated in the genome of T. urticae and does not represent bacterial contamination. Sequence data is available at Genbank (accession numbers: KF981736 and KF981737).
Figure 3. Coverage plot of Tu-CAS (tetur10g01570) and its surrounding region in the genome of T. urticae.
Gene models of Tu-CAS and its neighboring genes are depicted as follows: blue and red rectangles represent coding sequences and untranslated regions, respectively, while introns are shown as dashed lines. (+) and (−) represent the forward and reverse strand, respectively. Underneath the gene models, indicated in green, are the length and position of amplicons obtained by PCR (Figure 4). Next, an alignment of paired-end Sanger reads (and corresponding coverage plot) with the T. urticae genome of the London strain is displayed. Paired-end Sanger reads for which both reads are mapped in or extend nearby the indicated region are denoted by thin lines to show pair connections (shown are all paired-end Sanger reads that were produced from 2.5, 8.5, and 35.5 kb libraries used for assembly of the T. urticae genome [Grbic et al., 2011]). The Sanger reads coverage plot is followed by coverage plots of Illumina-reads from genomic DNA sequencing of the Montpellier and EtoxR strain of T. urticae (Grbic et al., 2011; Van Leeuwen et al., 2012). The coverage plot at the bottom shows Illumina RNA-seq read coverage produced from adult T. urticae polyA selected RNA (Grbic et al., 2011). Numbers between brackets represent the sequence depth.
DOI: http://dx.doi.org/10.7554/eLife.02365.006
Figure 4. Nucleotide dot plot of the PCR amplified genomic region bracketing T. urticae and T. evansi CAS with adjacent intron-containing eukaryotic genes.
The dot-plot was constructed with 95% identity in a 21 bp window, with the T. evansi and the T. urticae amplified region on the y- and x-axis, respectively. From the T. urticae region, the gene models and their genomic positions on the 10th scaffold are specified below the x-axis. The (+) and (−) signs represent the forward and reverse strand, respectively. Blue and black bars indicate exons and introns respectively, while the untranslated regions are depicted as red bars.
DOI: http://dx.doi.org/10.7554/eLife.02365.007
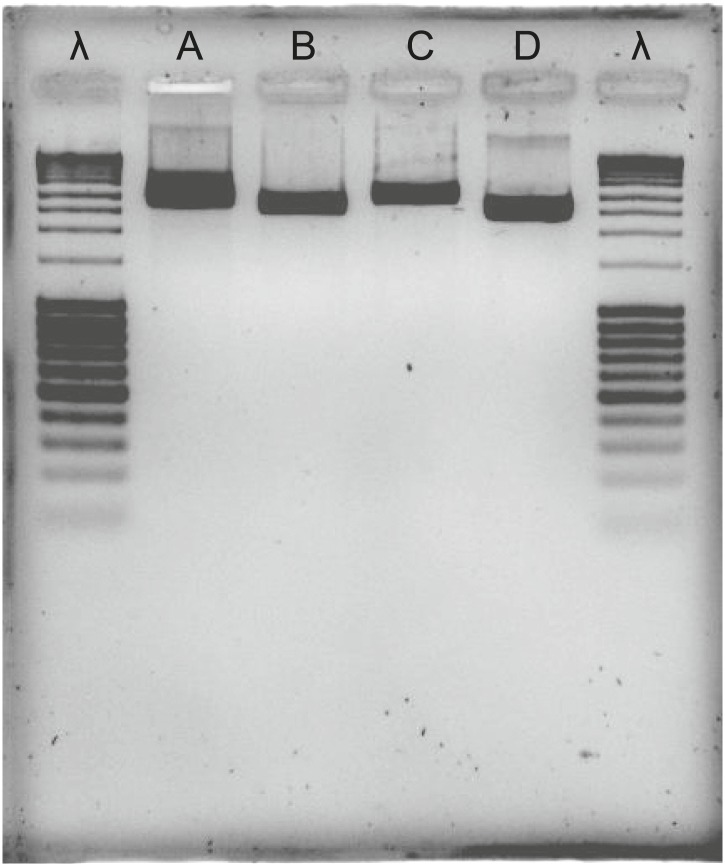
Figure 4—figure supplement 1. Agarose gel of PCR products, bracketing CAS with adjacent eukaryotic genes in T. urticae and T. evansi.
λ: lambda DNA, digested with Pstl; A: tetur10g01550_—_tetur10g01570 fragment in T. urticae; B: tetur10g01570_—_tetur10g01580 fragment in T. urticae; C; tetur10g01550_—_tetur10g01570 fragment in T. evansi; D: tetur10g01570_—_tetur10g01580 fragment in T. evansi. Primers used for the amplification of the fragments are listed in Supplementary file 4.
Figure 5. Graph showing the GC/GC3 gene contents of T. urticae and putative bacterial donor species.
The GC/GC3 contents of the T. urticae genome and the three annotated bacterial genomes of bacteria residing in the sister clade closest to the apparent monophyletic arthropod clade (Figure 2) are shown. The GC/GC3 content of the specific CYS/CAS gene of each organism (T. urticae in red, bacteria in blue) is highlighted.
DOI: http://dx.doi.org/10.7554/eLife.02365.009
Biochemical characterization of Tu-CAS
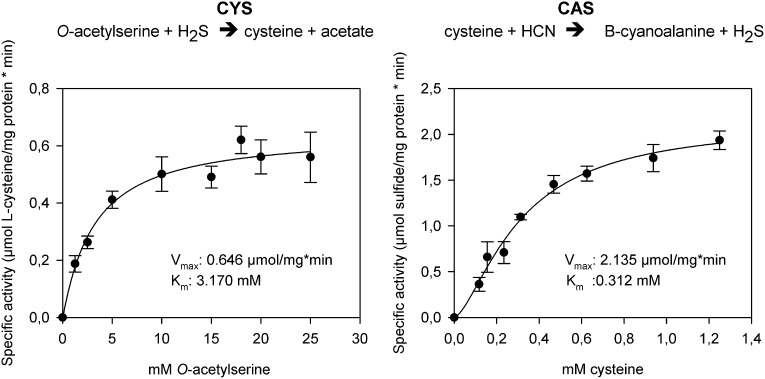
In order to functionally test whether arthropod enzymes are active and still able to catalyze both reactions after horizontal gene transfer, we recombinantly expressed Tu-CAS in Escherichia coli and obtained mg quantities after affinity purification. Subsequent biochemical assays confirmed that Tu-CAS catalyzes both reactions (Figure 6). In order to determine which of the two reactions (cysteine synthesis, CYS and β-cyanoalanine synthesis, CAS) was favored by the Tu-CAS enzyme, we calculated the ratio of the specificity constants for each reaction. The specificity constant kcat/Km defines, at any concentration, the specificity of an enzyme for a particular substrate. The CAS/CYS ratio of the specificity constants was 33.7, showing that CAS activity was strongly favored over CYS activity (Table 1). (The ratio was calculated from the respective values of Vmax/Km, as kcat = Vmax/[E] and as the reactions were measured with the same enzyme preparation.) This preferred CAS activity is typical of known plant CAS enzymes and clearly different from CYS enzymes (Table 1; Yamaguchi et al., 2000; Wada et al., 2004; Bogicevic et al., 2012; Yi et al., 2012). Enzymatic activity was dependent on pyridoxal-5′-phosphate as a cofactor, and the substrate-dependent formation of β-cyanoalanine was further confirmed by thin layer chromatography (TLC) and LC-MS (Figure 7).
Figure 6. The two reactions catalyzed by recombinant Tu-CAS (cysteine synthase, CYS and β-cyanoalanine synthase, CAS), showing the kinetic plots and calculated Vmax and Km values.
DOI: http://dx.doi.org/10.7554/eLife.02365.010
Table 1.
| CAS reaction | CYS reaction | CAS/CYS | |||||
|---|---|---|---|---|---|---|---|
| Activity (s−1) | Km (mM) | Specificity constant (mM−1. s−1) | Activity (s−1) | Km (mM) | Specificity constant (mM−1. s−1) | Ratio of specificity constants | |
| Tetranychus urticae CAS | 2.135* | 0.312 | 6.84† | 0.646* | 3.17 | 0.203† | 33.7 |
| Arabidopsis thaliana CAS | 2.66 | 0.14 | 19 | 2.0 | 8.03 | 0.250 | 76 |
| Glycine max CAS | 38.9 | 0.81 | 48 | 1.82 | 8.87 | 0.205 | 234 |
| Glycine max CYS | 0.21 | 0.30 | 0.7 | 57.5 | 3.6 | 15.97 | 0.044 |
| Corynebacterium glutamaticum CYS | n.d | n.d | – | 435* | 7 | 62† | – |
| Lactobacillus casei CYS | n.d | n.d | – | 89* | 0.6 | 148† | – |
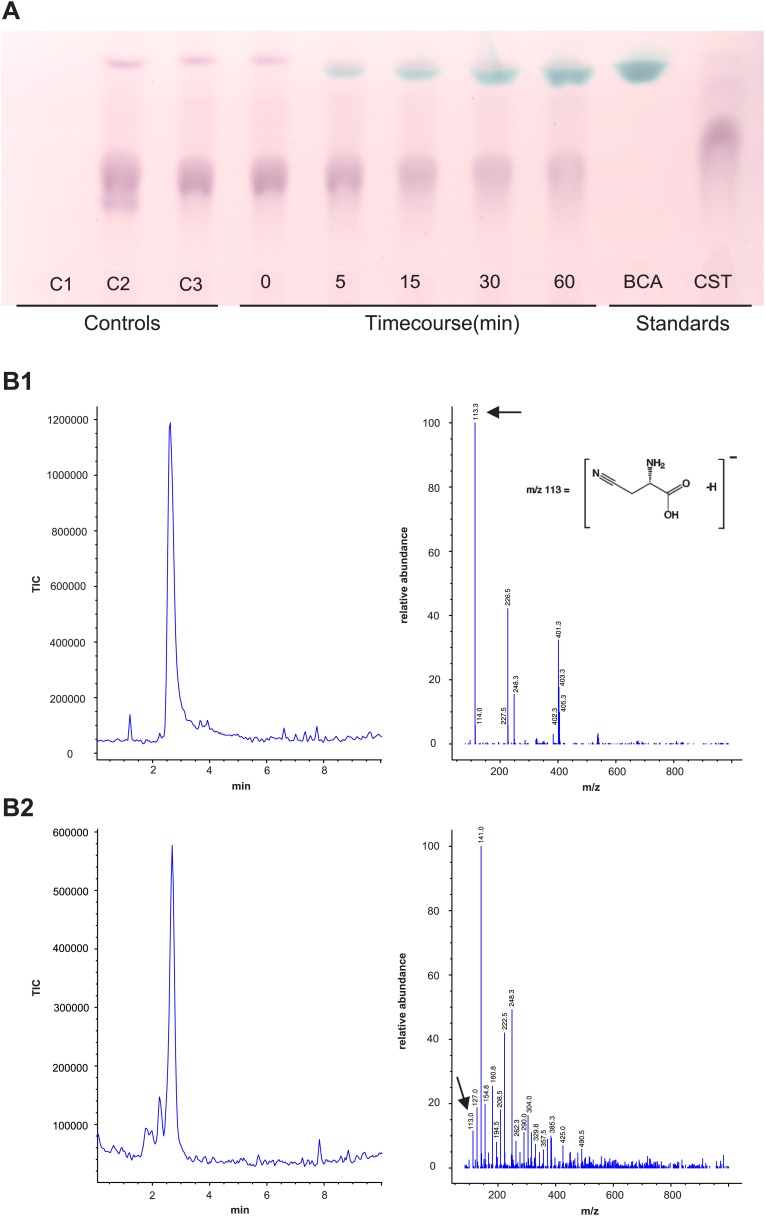
Figure 7. Panel A: Formation and accumulation of β-cyanoalanine by recombinant Tu-CAS as visualized by TLC analysis.
Controls; C1: no cysteine control, C2: no cyanide control, C3: no enzyme control. Time course 0–60 min after adding 3.75 µg of recombinant Tu-CAS. Standards; BCA: 5 µg β-cyanoalanine, CST: 5 µg cysteine. Panel B: LC-MS identification of β-cyanoalanine as a reaction product of Tu-CAS. The enzymatically produced β-cyanoalanine was scraped from silica plates after TLC separation of reaction mixtures, and was analyzed by LC-MS. β-cyanoalanine was identified in the reaction mixture on the basis of a similar elution time on LC and the characteristic ion of m/z = 113 which is [M-H]- as compared to the standard. (The base peak in panel B2 at m/z = 141 is a contaminant from the silica gel [2SiO +2H2O + OH−].) B1: total ion current (TIC) chromatogram and mass spectrum of the β-cyanoalanine standard, B2: TIC chromatogram and mass spectrum of Tu-CAS reaction mixture after separation on TLC.
DOI: http://dx.doi.org/10.7554/eLife.02365.012
The very high sequence similarity between Tu-CAS and the lepidopteran proteins strongly suggests that the lepidopteran proteins can catalyze the same two reactions (cyanide detoxification and cysteine synthesis) and that these proteins are responsible for the known wide occurrence of CAS activity in lepidopteran species (Witthohn and Naumann, 1987; Meyers and Ahmad, 1991; Stauber et al., 2012).
Discussion
In arthropods, the ability to detoxify HCN plays a crucial ecological role and is thought to have allowed the exploitation of cyanogenic plants by circumventing the toxic effects of HCN. We have shown here that the two-spotted spider mite increases transcript levels of a horizontally transferred β-cyanoalanine synthase upon adaptation on cyanogenic bean. Phylogenetic evidence alone does not constitute strong evidence for horizontal gene transfer (HGT), because in the absence of introns in the sequence, a contamination of the mite genome sequence with a sequence from a bacterial symbiont or commensal cannot be excluded. However, the results of mite genomic analysis, codon amelioration, synteny and genomic PCR, combined with the phylogenetic evidence, unambiguously prove that T. urticae CAS has a bacterial origin and was laterally transferred and incorporated into the mite genome prior to the divergence in the Tetranychidae. A homologous lateral gene transfer has also occurred in Lepidoptera (Figure 2) raising the question of the time and number of HGT events needed to explain the phylogenetic pattern of distribution of the CYS/CAS genes. Several hypotheses can be discussed. (a) A single HGT to a common ancestor of mites and insects, followed by selective losses resulting in the present phylogenetic distribution. The broad sampling of arthropod species that revealed the absence of any _CYS/CAS_-like sequence (even as a distant recognizable trace) argues against the origin of the gene in the common ancestor of tetranychid mites and Lepidoptera, followed by multiple independent losses. These losses would have to be numerous: seven in the hexapods (Trichoptera, Diptera, Hymenoptera, Coleoptera/Strepsiptera, Hemiptera/Heteroptera/Thysanoptera/Phthiraptera, Orthoptera, Odonata/Ephemeroptera) and six further in other arthropods (Copepoda/Branchiopoda, Myriapoda, Aranea, Scorpiones, Metastigmata and Mesostigmata). This figure of 13 losses is an absolute minimum that implies that the loss occurred each time at the origin of the lineage, that is before any speciation beyond the point of coalescence. A loss later in the history of each lineage would rapidly increase the total number of losses. We strongly believe that this hypothesis is not parsimonious, and therefore that there was no CYS/CAS gene in the common ancestor of mites and Lepidoptera. (b) Alternatively, two HGT events might have occurred (one to a mite ancestor, one to a lepidopteran ancestor) or (c) a single HGT from a donor bacterial species, followed by a transfer between a mite and a lepidopteran. These two hypotheses are impossible to distinguish based on the topology of the tree (Figure 2). The sampling of bacterial species phylogenetically close to the presumed donor species is too shallow at present. The apparent monophyly of the arthropod CAS gene may be due to the fact that both the mite and the insect gene came from very closely related bacteria, but these bacteria are not represented in the tree. A future survey of Proteobacteria likely to be associated with arthropods (directly or through a plant host) may resolve this question by finding several potential bacterial donor species that would invalidate the apparent monophyly (i.e., split the tree at the point of arthropod coalescence). The transfer from a lepidopteran to mite is improbable, because the mite sequence would branch with the closest relative to the lepidopteran donor, rather than being basal. Conversely, we cannot exclude the transfer from a mite to a lepidopteran, as sampling within the Prostigmata is extremely limited. We therefore favor hypothesis (b), with a very old transfer to an ancestral lepidopteran, and a second transfer to an ancestral mite. Current sampling of mite species is insufficient to give a good estimate of time, but we note the absence of a CAS enzyme in Mesostigmata (typically parasitic and predatory mites) and Metastigmata (ticks), and transfer is likely to have occurred after the split of Prostigmata in the Lower Devonian about 400 MYA (Dabert et al., 2010). The hypothesis of multiple transfers is also compatible with the presence of a _CYS_-like sequence in the mealybug Planococcus citri, apparently derived from its endosymbiont (Husnik et al., 2013). In that case, the phylogenetic tree clearly distinguishes the HGT event from that under study here.
In Lepidoptera, the CAS gene was subsequently duplicated in H. melpomene and D. plexippus that have 3 and 2 copies of the gene, respectively (Sun et al., 2013). The importance of the laterally acquired gene for HCN detoxification in those species was not previously apparent. These species of Papilionoidea, like burnet moths, not only thrive on cyanogenic plants, but have themselves evolved the ability to synthesize cyanogenic compounds de novo (Jensen et al., 2011) that now have crucial functions in their life history. Cyanogenesis serves in predator defense by releasing HCN, but also stores reduced nitrogen that can be mobilized for chitin synthesis, and plays a role in mate choice by determining the attractiveness of nuptial gifts from male to female partners (Zagrobelny et al., 2007). Our results support the early idea that CAS activity is needed for the exploitation of cyanogenic glucosides in insects (Meyers and Ahmad, 1991; Witthohn and Naumann, 1987; Zagrobelny et al., 2008) whether they are sequestered from the plant, or synthesized de novo. Higher β-cyanoalanine synthase activity in Spodoptera eridania than in Trichoplusia ni is related to higher cyanide tolerance (Meyers and Ahmad, 1991), and this enzyme activity is widespread in Lepidoptera (Witthohn and Naumann, 1987).
Moreover, it was recently shown that specialist pierid butterflies that feed on Brassicales, release equimolar concentrations of HCN upon metabolism of benzylglucosinolates, turning the ‘mustard oil bomb’ into a ‘cyanide bomb’ (Stauber et al., 2012). When Pieris rapae feeds on a cyanogenic (dhurrin-containing) plant that this species does not normally consume, an increased production of β-cyanoalanine and thiocyanate is observed, thus implicating both a CAS and a rhodanese activity (Stauber et al., 2012). It was therefore proposed that the ability of P. rapae to metabolize HCN allowed the primary host transfer from Fabales to Brassicales (Stauber et al., 2012). The gene for either CAS or rhodanese has not been identified in arthropods before, and their respective role in detoxification of HCN is not formally demonstrated. There is no close homologue of the known rhodanese (thiosulfate sulfurtransferase) gene in T. urticae or in Lepidoptera. However, we identified Tu-CAS and functionally demonstrated that the enzyme it encodes converts HCN to β-cyanoalanine in vitro. Such evidence is difficult to obtain in vivo with mites, as it would be difficult to exclude the possibility that a plant or a bacterial enzyme rather than the mite enzyme catalyzes the reaction in vivo. We argue that the presence of the same gene in lepidopteran species that display this activity in vivo (Meyers and Ahmad, 1991; Stauber et al., 2012) is strong evidence for the function of the laterally transferred CAS gene. It will be of great interest to confirm that the homologous CAS genes of Lepidoptera that we identified indeed encode a β-cyanoalanine synthase, and to provide evidence of its protective role against HCN poisoning.
Next to the detoxification function, the CYS activity acquired after horizontal gene transfer may also have enhanced the sulfur amino acid economy of mites and lepidopterans (Figure 1). To date, nematodes were the only animal species thought to synthesize cysteine independently from methionine by CYS activity (Budde and Roth, 2011). However, nematode CYS sequences grouped with plants and oomycetes, clearly outside the arthropod-bacterial clade, suggestive of a different origin of CYS between metazoan subgroups (Figure 2). Duplications of cys genes were observed in nematodes, and indeed genetic evidence suggests that in Caenorhabditis elegans cyanide resistance is conferred by the cysl-2 gene, probably encoding an enzyme with CAS activity while cysl-1 is a classical cysteine synthase gene (Budde and Roth, 2011). The acquisition of an alternative cysteine biosynthesis route fits into previously documented horizontal gene transfers in T. urticae that include a cobalamin-independent methionine synthase gene, genes for carotenoid biosynthesis, as well as laterally acquired genes that likewise respond to host plant change such as intradiol ring-cleavage dioxygenases and a cyanase gene (Grbic et al., 2011; Wybouw et al., 2012; Dermauw et al., 2013). The latter encodes an enzyme that decomposes cyanate (CNO−), a bacterial or photochemical decomposition product of cyanide, to carbon dioxide and ammonia (Wybouw et al., 2012). This enzyme may serve as a second line of spider mite defense against cyanogenic plants, or alternatively may have a regulatory function in the amino acid and pyrimidine metabolism as previously suggested (Wybouw et al., 2012). For a polyphagous herbivore, horizontal gene transfer might play an important role in gaining independence from the varying plant nutrients and defense compounds. It remains unclear which reaction (CAS/CYS) of Tu-CAS provides the strongest adaptive advantage, but the CYS activity might be one of the reasons why these horizontally transferred genes have been retained in organisms that are at present not living on cyanogenic plants.
In conclusion, a horizontal gene transfer from a bacterial ancestor underlies the exploitation of cyanogenic host plants in some arthropod lineages and made the subsequent evolution of a convergent pathway for synthesis of cyanogenic glucosides possible, as shown in burnet moths (Jensen et al., 2011).
Material and methods
Mite strains and host plants
The London strain of T. urticae (Grbic et al., 2011) was maintained on acyanogenic P. vulgaris L. cv ‘Prelude’. The strain London-CYANO originated from this population and was transferred to cyanogenic P. lunatus as previously described (Wybouw et al., 2012). Before the start of experiments the cyanogenic potential of both plant species was determined, confirming the acyanogenic nature of P. vulgaris and revealing high levels of cyanogenic precursors produced in the P. lunatus cultivar (Wybouw et al., 2012). For this study, young adult female mites were collected for gene-expression analysis 35 generations after the initial host shift. The T. evansi strain was maintained in the laboratory on Solanum lycopersicum L. cv ‘Moneymaker’ as previously described (Wybouw et al., 2012). All strains were maintained in climatically controlled rooms at 26°C, 60% RH and 16:8 hr light:dark photoperiod.
Transcriptional response of T. urticae to a cyanogenic host plant
Total RNA samples were isolated with the RNeasy minikit (Qiagen, Belgium) and were subsequently treated with DNase (Turbo DNA-free kit, Ambion, Belgium). RNA was extracted from 100–120 young adult female mites in four replicates. Cy5- or Cy3-labeled cRNA was produced using the Low Input Quick Amp Labeling Kit (Agilent Technologies, Belgium) as previously described (Dermauw et al., 2013). Microarray hybridization and scanning procedures were performed as previously described (Dermauw et al., 2013), using the GE2_107_Sep09 protocol. The data was transferred to GeneSpring GX 11.0 software (Agilent Technologies) for statistical analyis. Probes were flag filtered (only probes that had flag-value ‘present’ in 50% of all replicates were retained) and linked to T. urticae genes using the ‘Create New Gene-Level Experiment’ option. Differentially expressed genes were identified by a Student's t test with the cutoff for Fold Change (FC) and corrected p-value (Benjamini-Hochberg correction) set at 2 and 0.05, respectively. The array design is accessible under the GEO-platform format GPL16890 (Wybouw et al., 2013).
Phylogenetic reconstruction
A full-length Tu-CAS (tetur10g01570) homologue was retrieved in T. evansi by sequencing PCR products bracketing the CAS gene with neighboring genes (Supplementary file 4). Additional Tu-CAS homologues were identified by conducting BLASTp and/or tBLASTn searches in NCBI, UniProt, P. citri transcriptome (Liu et al., 2011) and diverse arthropod genome portals (Supplementary file 2) using Tu-CAS as query. As several best BLAST-hits (E-value ≤1e−90) included members of the order Lepidoptera, transcriptome databases from Lepidoptera not included in the NCBI database were also mined for Tu-CAS homologues (Supplementary file 3). This approach yielded 35 arthropod and bacterial protein sequences, which according to the Conserved Domain Database all contained a motif typical for cysteine synthases (COG0031) (Marchler-Bauer et al., 2011). This dataset was further complemented with cysteine synthase M (cysM) protein sequences from bacteria and cysteine synthase protein sequences from fungi, Chromalveolata, plants, nematodes, and Planococcus citri and its three best BLASTp hits, harboring a cysteine synthase CDD motif (COG0031 or PLN2565). Finally, a diverse set of cystathionine-β-synthase protein sequences, related to cysteine synthases and also belonging to the group of pyrodixal-5′-phosphate dependent β-substituted alanine synthases were added as an outgoup.
The final dataset contained 90 protein sequences. Accession numbers of protein sequences, their trivial name, CDD classification (Marchler-Bauer et al., 2011) and cellular localization (Horton et al., 2007) are listed in Supplementary file 3. Protein sequences were aligned with MUSCLE (Edgar, 2004) using default settings. Model selection was done with ProtTest 2.4 and according to the Akaike information criterion the model LG+I+G was optimal for phylogenetic analysis (Abascal et al., 2005). A maximum-likelihood analysis was performed using Treefinder (Jobb et al., 2004) with edge-support calculated by 1000 pseudoreplicates (LR-ELW). Resulting trees were midpoint rooted prior to further analysis (Hess and De Moraes Russo, 2007). Phylogenetic trees were visualized and edited using MEGA5 (Tamura et al., 2011) and CorelDraw X6 (Corel inc., UK), respectively.
Incorporation of Tu-CAS in the spider mite genome
Paired-end T. urticae Sanger reads (available in the Trace Archive at the NCBI website, http://www.ncbi.nlm.nih.gov/Traces/home/) were remapped to the T. urticae genome (Grbic et al., 2011) using Bowtie 2.1.0 (Langmead et al., 2009) and the preset parameter option ‘–very-sensitive’. Resulting SAM files were converted into BAM files using SAMtools (Li et al., 2009). Illumina-reads from genomic DNA sequencing of the London, Montpellier and EtoxR strains of T. urticae and Illumina RNA-seq reads from adult T. urticae polyA selected RNA were mapped as previously described (Grbic et al., 2011; Van Leeuwen et al., 2012). Read alignments and coverage were visualized with IGV 2.3 (Thorvaldsdottir et al., 2013) using the most recent T. urticae genome annotation (‘Tetur_gff3_20130708’, accessible at http://bioinformatics.psb.ugent.be/orcae/-overview/Tetur); for display, Sanger reads were arranged in Adobe Illustrator CS5 while maintaining alignment coordinates.
Genomic DNA was collected from T. evansi and T. urticae by phenol-chloroform extraction (Van Leeuwen et al., 2008). Primer pairs were designed to amplify a genomic region of Tu-CAS and adjacent genes on either the 5′ or the 3′ end on the 10th scaffold of the T. urticae genome (Supplementary file 4). The Expand Long Range PCR kit (Roche, Belgium) was used to conduct PCR, and fragments were sequenced with primers listed in Supplementary file 4. Some primer pairs designed on the T. urticae genome sequence also successfully amplified genomic fragments of T. evansi (Supplementary file 4). The resulting fragments were sequenced by primer walking (Supplementary file 4). A nucleotide dot-blot between the two spider mite species was constructed using the MEGALIGN program of DNASTAR software, allowing 5% mismatch in a 21 bp window. We analyzed overall GC contents and at the third codon position (GC3) of whole coding nucleotide sequences using UGENE (Okonechnikov et al., 2012) of all coding sequences of T. urticae and Achromobacter xylosoxidans, Methylobacterium radiotolerans and Methylobacterium sp. GXF4. These three bacterial genomes were selected based on our phylogenetic analysis as the closest fully annotated bacterial genomes to the arthropod clade (Figure 2).
Recombinant expression of Tu-CAS and enzyme activity assays
Recombinant Tu-CAS was produced by the GenScript Corporation (Piscataway, NJ, USA). After codon optimization of the Tu-CAS coding sequence (Supplementary file 5), an E3 expression vector was used to transform E. coli cells. The transformed cells were cultured in 3 l LB. Using a Ni2+-column, the N-His-tagged Tu-CAS protein was purified from the supernatant. After sample sterilization via a 0.22 µm filter, the recombinant protein was stored in a buffer containing: 50 mM Tris, 150 mM NaCl, 2 mM DTT, 10% glycerol at a pH of 8.0 and finally kept at −80°C. The concentration and purity of the recombinant protein sample was determined respectively by a Bradford protein assay (Bradford, 1976) and a densitometric analysis of a Coomassie Blue-stained SDS-PAGE gel.
Chemicals for the activity assays were purchased from Sigma–Aldrich (Belgium), except β-cyanoalanine, which was acquired from VWR, Cayman Chemical. All reactions were carried out in gastight 7 ml vials with a screw cap having a PTFE/rubber septum (Supelco–Sigma–Aldrich, Belgium). Reagents were added to the reaction volumes using gastight syringes (Hamilton, series 1700, Gastight, 1750RN, VWR Belgium). Prior to measuring enzyme activity, recombinant Tu-CAS was incubated at 30°C for 10 min in the appropriate reaction buffer containing 500 µM pyridoxal-5′-phosphate. For measuring CAS activity, reactions were executed in a 0.2 M Tris buffer at pH 8.5. The CAS activity assay was a modification of the method of Hendrickson (Hendrickson and Conn, 1969). The standard reaction was started with 0.5 ml of 0.01 M cysteine, 0.5 ml of 0.01 M sodium cyanide and 1.5 µg recombinant Tu-CAS. All CAS assays were performed at 37°C on a mechanical shaker. CAS activity was quantified by spectrophotometrically measuring the H2S formed at 650 nm (PowerWavex340, BioTek Instruments Inc., Winooski, VT, USA) by the method of Siegel (Siegel, 1965). The CYS activity assay was based on Lunn et al., 1990 using 0.5 µg recombinant Tu-CAS per reaction. Standard substrate concentrations were 10 mM and 2 mM for _O_-acetylserine and sodium sulfide, respectively. The reaction product cysteine was quantified by measuring the absorbance at 560 nm by the method of Gaitonde (Gaitonde, 1967). For both activity assays, the spontaneous formation of the measured reaction product and its potential presence in protein preparations was corrected by respectively non-enzyme controls and zero time point controls. Each experimental assay condition was analyzed using three independent and three technical replicates. Kinetic data was fitted to the Hill equation, from which the Km and Vmax values for _O_-acetylserine and cysteine were calculated for respectively the CYS and CAS reaction.
Identification of β-cyanoalanine by TLC and LC-MS
Recombinant Tu-CAS was incubated at 30°C for 10 min in a 1 mM phosphate buffer containing 500 µM pyridoxal-5′-phosphate. The standard reaction was executed in a 320 µl reaction volume at 37°C on a mechanical shaker in 1 mM phosphate buffer pH 8.5, with 80 µl of 7.5 mM cysteine and 80 µl of 15 mM sodium cyanide. Each reaction was started by adding 3.75 µg of recombinant protein and was terminated by snap freezing at different time points. No-substrate and no-enzyme controls were included in the analysis. Reaction mixtures were defrosted at 4°C and a 20 µl aliquot was spotted on a thin-layer chromatograph (HPTLC Silica gel 60 F254, Merck, Darmstadt Germany) and run with a mobile phase of (ethanol/28% ammonium hydroxide/water) with a (18/1/4) ratio. Five µg of β-cyanoalanine and cysteine were spotted as standards. After drying, the TLC plate was treated with a ninhydrin solution (20 g ninhydrin in 600 ml ethanol) for amino-acid visualization. In this validated TLC set-up (Yoshikawa et al., 2000), cysteine and β-cyanoalanine can be identified both by color and relative mobility (Rf-value). β-cyanoalanine consistently displayed a blue-green color with a Rf value of around 0.8. In contrast, cysteine colored red and exhibited a consistent different Rf value.
The identification of β-cyanoalanine as a blue spot at 0.8 Rf was further confirmed by LC-MS analysis. After TLC separation, the zone around 0.8 Rf was scrapped from the TLC plate. After scrapping, the plate was colored as described above to confirm that the correct blue/green zone was collected. The collected silica was mixed with 200 µl ddH2O, vortexed and centrifuged at 21000×g for 5 min. The supernatant was collected and directly analyzed using an Agilent 1100 Series LC-MSD with a diode array detector operating at 220 nm. The column oven was programmed at 35°C using a Phenomenex Luna 5u column (particle size) C18(2), 100A (pore size) with a column size of 250 × 3.0 mm. A gradient elution program driven by a quarternary pump was used at a flow rate of 0.5 ml/min (injection volume: 20 µl). The acetonitrile/water gradient used was 0–2 min (5% acetonitrile); 2–17 min (5–100% acetonitrile); 17–22 min (100% acetonitrile); 22–24.5 min (100–5% acetonitrile); 24.5–27 min (5% acetonitrile). The mass spectrometer was operated using the SCAN mode in the electrospray ionization mode. The analyzing sector contained a quadrupole analyzer and an electron multiplier detector.
Acknowledgements
We thank S Bajda and P Zwaenepoel for performing PCR and LC-MS respectively and RM Clark, EJ Osborne and RT Greenhalgh for help and advice in constructing coverage plots.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Funding Information
This paper was supported by the following grants:
- Institute for the promotion of innovation by Science and Technology in Flanders (IWT) SB/101451 to Nicky Wybouw.
- Fund for Scientific Research in Flanders (FWO) to Wannes Dermauw, Thomas Van Leeuwen.
- Fund for Scientific Research in Flanders (FWO) 3G061011 to Luc Tirry, Thomas Van Leeuwen.
- Ghent Special Research Fund 01J13711 to Luc Tirry, Thomas Van Leeuwen.
- This work was partially supported by the government of Canada through Genome Canada and the Ontario Genomics Institute OGI-046 to Miodrag Grbić.
- Fund for Scientific Research in Flanders (FWO) 3G009312 to Thomas Van Leeuwen.
Additional information
Competing interests
The authors declare that no competing interests exist.
Author contributions
NW, Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting and revising the article.
WD, Acquisition of data, Analysis and interpretation of data, Revising the article.
LT, Revising the article, Contributed unpublished essential data or reagents.
MG, Revising the article, Contributed unpublished essential data or reagents.
CS, Analysis and iRnterpretation of data, Contributed unpublished essential data or reagents.
RF, Analysis and interpretation of data, Drafting and revising the article.
TVL, Conception and design, Analysis and interpretation of data, Drafting and revising the article.
Additional files
Major dataset
The following dataset was generated:
N Wybouw, W Dermauw, T Van Leeuwen, 2013, Genome wide gene-expression analysis of the spider mite Tetranychus urticae after long term host transfer from acyanogenic Phaseolus vulgaris cv. ‘Prelude’ bean plants to cyanogenic Phaseolus lunatus cv. ‘8078’ bean plants, http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE50162, Publicly available at NCBI GEO.
References
- Abascal F, Zardoya R, Posada D. 2005. ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21:2104–2105. doi: 10.1093/bioinformatics/bti263 [DOI] [PubMed] [Google Scholar]
- Ballhorn DJ, Heil M, Lieberei R. 2006. Phenotypic plasticity of cyanogenesis in lima bean Phaseolus lunatus - activity and activation of beta-glucosidase. Journal of Chemical Ecology 32:261–275. doi: 10.1007/s10886-005-9001-z [DOI] [PubMed] [Google Scholar]
- Beesley SG, Compton SG, Jones DA. 1985. Rhodanese in insects. Journal of Chemical Ecology 11:45–50. doi: 10.1007/BF00987603 [DOI] [PubMed] [Google Scholar]
- Bogicevic B, Berthoud H, Portmann R, Meile L, Irmler S. 2012. CysK from Lactobacillus casei encodes a protein with _O_-acetylserine sulfhydrylase and cysteine desulfurization activity. Applied Microbiology and Biotechnology 94:1209–1220. doi: 10.1007/s00253-011-3677-5 [DOI] [PubMed] [Google Scholar]
- Bonner ER, Cahoon RE, Knapke SM, Jez JM. 2005. Molecular basis of cysteine biosynthesis in plants - Structural and functional analysis of _O_-acetylserine sulfhydrylase from Arabidopsis thaliana. Journal of Biological Chemistry 280:38803–38813. doi: 10.1074/jbc.M505313200 [DOI] [PubMed] [Google Scholar]
- Bradford MM. 1976. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Analytical Biochemistry 72:248–254. doi: 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Budde MW, Roth MB. 2011. The response of Caenorhabditis elegans to hydrogen sulfide and hydrogen cyanide. Genetics 189:521–532. doi: 10.1534/genetics.111.129841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabert M, Witalinski W, Kazmierski A, Olszanowski Z, Dabert J. 2010. Molecular phylogeny of acariform mites (Acari, Arachnida): Strong conflict between phylogenetic signal and long-branch attraction artifacts. Molecular Phylogenetics and Evolution 56:222–241. doi: 10.1016/j.ympev.2009.12.020 [DOI] [PubMed] [Google Scholar]
- Davis RH, Nahrstedt A. 1985. Cyanogenesis in insects. Comprehensive Insect Physiology, Biochemistry and Pharmacology 11:635–654 [Google Scholar]
- Dermauw W, Wybouw N, Rombauts S, Menten B, Vontas J, Grbic M, Clark RM, Feyereisen R, Van Leeuwen T. 2013. A link between host plant adaptation and pesticide resistance in the polyphagous spider mite Tetranychus urticae. Proceedings of the National Academy of Sciences of the United States of America 110:E113–E122. doi: 10.1073/pnas.1213214110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres L, David JP, Gallet C. 2007. The evolutionary ecology of insect resistance to plant chemicals. Trends in Ecology & Evolution 22:298–307 [DOI] [PubMed] [Google Scholar]
- Edgar RC. 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5:1–19. doi: 10.1186/1471-2105-5-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein JD, Martin JJ, Harris BJ. 1988. Methionine metabolism in mammals - the methionine-sparing effect of cystine. Journal of Biological Chemistry 263:11750–11754 [PubMed] [Google Scholar]
- Gaitonde MK. 1967. A spectrophotometric method for direct determination of cysteine in presence of other naturally occurring amino acids. Biochemical Journal 104:627–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleadow RM, Woodrow IE. 2002. Constraints on effectiveness of cyanogenic glycosides in herbivore defense. Journal of Chemical Ecology 28:1301–1313. doi: 10.1023/A:1016298100201 [DOI] [PubMed] [Google Scholar]
- Grbic M, Van Leeuwen T, Clark RM, Rombauts S, Rouzé P, Grbić V, Osborne EJ, Dermauw W, Ngoc PC, Ortego F, Hernández-Crespo P, Diaz I, Martinez M, Navajas M, Sucena É Magalhães S, Nagy L, Pace RM, Djuranović S, Smagghe G, Iga M, Christiaens O, Veenstra JA, Ewer J, Villalobos RM, Hutter JL, Hudson SD, Velez M, Yi SV, Zeng J, Pires-daSilva A, Roch F, Cazaux M, Navarro M, Zhurov V, Acevedo G, Bjelica A, Fawcett JA, Bonnet E, Martens C, Baele G, Wissler L, Sanchez-Rodriguez A, Tirry L, Blais C, Demeestere K, Henz SR, Gregory TR, Mathieu J, Verdon L, Farinelli L, Schmutz J, Lindquist E, Feyereisen R, Van de Peer Y. 2011. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 479:487–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson HR, Conn EE. 1969. Cyanide metabolism in higher plants .4. Purification and properties of the beta-cyanoalanine synthase of blue lupine. Journal of Biological Chemistry 244:2632–2640 [PubMed] [Google Scholar]
- Hess PN, De Moraes Russo CA. 2007. An empirical test of the midpoint rooting method. Biological Journal of the Linnean Society 92:669–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P, Park K-J, Obayashi T, Fujita N, Harada H, Adams-Collier C, Nakai K. 2007. WoLF PSORT: protein localization predictor. Nucleic acids research 35:W585–W587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotopp JCD. 2011. Horizontal gene transfer between bacteria and animals. Trends in Genetics 27:157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husnik F, Nikoh N, Koga R, Ross L, Duncan RP, Fujie M, Tanaka M, Satoh N, Bachtrog D, Wilson AC, von Dohlen CD, Fukatsu T, McCutcheon JP. 2013. Horizontal gene transfer from diverse bacteria to an insect genome enables a tripartite nested mealybug symbiosis. Cell 153:1567–1578. doi: 10.1016/j.cell.2013.05.040 [DOI] [PubMed] [Google Scholar]
- Jensen NB, Zagrobelny M, Hjernø K, Olsen CE, Houghton-Larsen J, Borch J, Møller BL, Bak S. 2011. Convergent evolution in biosynthesis of cyanogenic defence compounds in plants and insects. Nature Communications 2:273. doi: 10.1038/ncomms1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobb G, von Haeseler A, Strimmer K. 2004. TREEFINDER: a powerful graphical analysis environment for molecular phylogenetics. BMC Evolutionary Biology 4:18. doi: 10.1186/1471-2148-4-18 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jones DA. 1998. Why are so many food plants cyanogenic? Phytochemistry 47:155–162. doi: 10.1016/S0031-9422(97)00425-1 [DOI] [PubMed] [Google Scholar]
- Kutschera U. 2007. Plant-associated methylobacteria as co-evolved phytosymbionts: a hypothesis. Plant Signaling & Behavior 2:74–78. doi: 10.4161/psb.2.2.4073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biology 10:R25. doi: 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JG, Ochman H. 1997. Amelioration of bacterial genomes: rates of change and exchange. Journal of Molecular Evolution 44:383–397. doi: 10.1007/PL00006158 [DOI] [PubMed] [Google Scholar]
- Li H Handsaker B Wysoker A Fennell T Ruan J Homer N Marth G Abecasis G Durbin R, 1000 Genome Project Data Processing Subgroup . 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Jiang G, Zhang Y, Li J, Li X, Yue J, Chen F, Liu H, Li H, Zhu S, Wang J, Ran C. 2011. Analysis of transcriptome differences between resistant and susceptible strains of the citrus red mite Panonychus citri (Acari: tetranychidae). PLOS ONE 6:e28516. doi: 10.1371/journal.pone.0028516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long KY, Brattsten LB. 1982. Is rhodanese important in the detoxification of dietary cyanide in southern armyworm (Spodoptera eridania cramer) larvae. Insect Biochemistry 12:367–375. doi: 10.1016/0020-1790(82)90033-6 [DOI] [Google Scholar]
- Lunn JE, Droux M, Martin J, Douce R. 1990. Localization of ATP Sulfurylase and O-Acetylserine(thiol)lyase in Spinach Leaves. Plant Physiology 94:1345–1352. doi: 10.1104/pp.94.3.1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Lu F, Marchler GH, Mullokandov M, Omelchenko MV, Robertson CL, Song JS, Thanki N, Yamashita RA, Zhang D, Zhang N, Zheng C, Bryant SH. 2011. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Research 39:D225–D229. doi: 10.1093/nar/gkq1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers DM, Ahmad S. 1991. Link between L-3-cyanoalanine synthase activity and differential cyanide sensitivity of insects. Biochimica et Biophysica Acta 1075:195–197. doi: 10.1016/0304-4165(91)90252-C [DOI] [PubMed] [Google Scholar]
- Okonechnikov K Golosova O Fursov M, UGENE team . 2012. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics 28:1166–1167. doi: 10.1093/bioinformatics/bts091 [DOI] [PubMed] [Google Scholar]
- Omura H, Kuroda M, Kobayashi M, Shimizu S, Yoshida T, Nagasawa T. 2003. Purification, characterization and gene cloning of thermostable _O_-acetyl-L-serine sulfhydrylase forming _beta_-cyano-L-alanine. Journal of Bioscience and Bioengineering 95:470–475 [PubMed] [Google Scholar]
- Poulton JE. 1990. Cyanogenesis in plants. Plant Physiology 94:401–405. doi: 10.1104/pp.94.2.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampelotti-Ferreira FT, Ferreira A, Vendramim JD, Lacava PT, Azevedo JL, Araújo WL. 2010. Colonization of rice and Spodoptera frugiperda JE Smith (Lepidoptera: Noctuidae) larvae by genetically modified endophytic Methylobacterium mesophilicum. Neotropical Entomology 39:308–310 [DOI] [PubMed] [Google Scholar]
- Siegel LM. 1965. A direct microdetermination for sulfide. Analytical Biochemistry 11:126–132. doi: 10.1016/0003-2697(65)90051-5 [DOI] [PubMed] [Google Scholar]
- Solomonson LP. 1981. Cyanide as a metabolic inhibitor. In: Vennesland B. editor Cyanide in Biology. London: Academic Press; p11–28 [Google Scholar]
- Spencer KC. 1988. Glycosides - the interface between plant secondary and insect primary metabolism. Acs Symposium Series 380:403–416 [Google Scholar]
- Stauber EJ, Kuczka P, van Ohlen M, Vogt B, Janowitz T, Piotrowski M, Beuerle T, Wittstock U. 2012. Turning the 'mustard oil bomb' into a 'cyanide bomb': aromatic glucosinolate metabolism in a specialist insect herbivore. PLOS ONE 7:e35545. doi: 10.1371/journal.pone.0035545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun BF, Xiao JH, He SM, Liu L, Murphy RW, Huang DW. 2013. Multiple ancient horizontal gene transfers and duplications in lepidopteran species. Insect molecular biology 22:72–87. doi: 10.1111/imb.12004 [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28:2731–2739. doi: 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorvaldsdottir H, Robinson JT, Mesirov JP. 2013. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Briefings in Bioinformatics 14:178–192. doi: 10.1093/bib/bbs017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leeuwen T, Demaeght P, Osborne EJ, Dermauw W, Gohlke S, Nauen R, Grbic M, Tirry L, Merzendorfer H, Clark RM. 2012. Population bulk segregant mapping uncovers resistance mutations and the mode of action of a chitin synthesis inhibitor in arthropods. Proceedings of the National Academy of Sciences of the United States of America 109:4407–4412. doi: 10.1073/pnas.1200068109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leeuwen T, Vanholme B, Van Pottelberge S, Van Nieuwenhuyse P, Nauen R, Tirry L, Denholm I. 2008. Mitochondrial heteroplasmy and the evolution of insecticide resistance: non-Mendelian inheritance in action. Proceedings of the National Academy of Sciences of the United States of America 105:5980–5985. doi: 10.1073/pnas.0802224105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M, Awano N, Yamazawa H, Takagi H, Nakamori S. 2004. Purification and characterization of O-acetylserine sulfhydrylase of Corynebacterium glutamicum. Bioscience Biotechnology and Biochemistry 68:1581–1583 [DOI] [PubMed] [Google Scholar]
- Wernegreen JJ. 2002. Genome evolution in bacterial endosymbionts of insects. Nature Reviews Genetics 3:850–861. doi: 10.1038/nrg931 [DOI] [PubMed] [Google Scholar]
- Witthohn K, Naumann CM. 1987. Cyanogenesis - a general phenomenon in the Lepidoptera? Journal of Chemical Ecology 13:1789–1809. doi: 10.1007/BF01013229 [DOI] [PubMed] [Google Scholar]
- Wybouw N, Balabanidou V, Ballhorn DJ, Dermauw W, Grbić M, Vontas J, Van Leeuwen T. 2012. A horizontally transferred cyanase gene in the spider mite Tetranychus urticae is involved in cyanate metabolism and is differentially expressed upon host plant change. Insect Biochemistry and Molecular Biology 42:881–889. doi: 10.1016/j.ibmb.2012.08.002 [DOI] [PubMed] [Google Scholar]
- Wybouw N, Dermauw W, Van Leeuwen T. 2013. Genome wide gene-expression analysis of the spider mite Tetranychus urticae after long term host transfer from acyanogenic Phaseolus vulgaris cv. ‘Prelude’ bean plants to cyanogenic Phaseolus lunatus cv. ‘8078’ bean plants. NCBI Gene Expression Omnibus. http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE50162 [Google Scholar]
- Yamaguchi Y, Nakamura T, Kusano T, Sano H. 2000. Three Arabidopsis genes encoding proteins with differential activities for cysteine synthase and beta-cyanoalanine synthase. Plant & Cell Physiology 41:465–476. doi: 10.1093/pcp/41.4.465 [DOI] [PubMed] [Google Scholar]
- Yi H, Juergens M, Jez JM. 2012. Structure of Soybean beta-cyanoalanine synthase and the molecular basis for cyanide detoxification in plants. The Plant Cell 24:2696–2706. doi: 10.1105/tpc.112.098954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa K, Adachi K, Nishijima M, Takadera T, Tamaki S, Harada K, Mochida K, Sano H. 2000. beta-cyanoalanine production by marine bacteria on cyanide-free medium and its specific inhibitory activity toward cyanobacteria. Applied and Environmental Microbiology 66:718–722. doi: 10.1128/AEM.66.2.718-722.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagrobelny M, Bak S, Møller BL. 2008. Cyanogenesis in plants and arthropods. Phytochemistry 69:1457–1468. doi: 10.1016/j.phytochem.2008.02.019 [DOI] [PubMed] [Google Scholar]
- Zagrobelny M, Bak S, Olsen CE, Møller BL. 2007. Intimate roles for cyanogenic glucosides in the life cycle of Zygaena filipendulae (Lepidoptera, Zygaenidae). Insect Biochemistry and Molecular Biology 37:1189–1197. doi: 10.1016/j.ibmb.2007.07.008 [DOI] [PubMed] [Google Scholar]
- Zagrobelny M, Bak S, Rasmussen AV, Jørgensen B, Naumann CM, Møller BL. 2004. Cyanogenic glucosides and plant-insect interactions. Phytochemistry 65:293–306. doi: 10.1016/j.phytochem.2003.10.016 [DOI] [PubMed] [Google Scholar]
eLife posts the editorial decision letter and author response on a selection of the published articles (subject to the approval of the authors). An edited version of the letter sent to the authors after peer review is shown, indicating the substantive concerns or comments; minor concerns are not usually shown. Reviewers have the opportunity to discuss the decision before the letter is sent (see review process). Similarly, the author response typically shows only responses to the major concerns raised by the reviewers.
Thank you for sending your work entitled “A gene horizontally transferred from bacteria protects arthropods from host plant cyanide poisoning” for consideration at eLife. Your article has been favorably evaluated by a Senior editor and 3 reviewers, one of whom is a member of our Board of Reviewing Editors, and one of whom, Søren Bak, has agreed to reveal his identity.
The Reviewing editor and the other reviewers discussed their comments before reaching this decision, and the Reviewing editor has assembled the following comments to help you prepare a revised submission:
Your paper describes new evidence that supports a scenario of horizontal transfer of a gene encoding CAS/CYS activity from bacteria to T. urticae. The horizontally transferred gene has a proposed role in T. uritcae's detoxification of cyanide exposure from host plants. In general the external reviewers and the Reviewing editor found much that they liked about this work and recognized its novelty and relevance for a broad scientific audience. The paper appears to be important for our general understanding of the evolution of certain arthropods to colonize host plants that contain cyanogenic glucosides, and might lead to new genomics-enabled discoveries in other systems of plant-arthropod interactions. The paper is well written and easy to follow. The reviewers and the Reviewing editor found the paper convincing in its documentation of a CAS/CYS gene of likely bacterial origin integrated into the T. urticae genome. The biochemical characterization of the encoded T. urticae CAS/CYS enzyme also appears to be solid, although some additional data are requested to substantiate conclusions of product formation and enzyme kinetic properties (please see below). The reviewers and the Reviewing editor commented on a lack of discussion (or lack of data) to explain the phylogenetic pattern of a limited appearance of the CYS/CAS gene in T. urticae and only a relatively small number of Lepidoptera as presented in Figure 2. At the least, the Discussion of the paper needs to be substantially revised with a plausible explanation for this observation (please see below). However, if this important question regarding the phylogeny cannot be resolved with a new Discussion, additional data mining or generation of additional supporting data would be required to fill this critical gap in an otherwise interesting story.
Major issues to address:
To allow the reader to assess enzyme kinetic properties, the KM and kcat values must be shown in Table 1. Vmax values shown in one of the figures are not very informative.
The text says that products of CAS activity, beta-cyanoalanine, were identified by TLC and LC-MS. The authors refer to Figure 9 for these results. However, Figure 9 only shows TLC data, which may not be sufficiently informative. To support statements of product identification, the LC-MS results must be shown for enzyme products and the authentic standard.
While the results appear to be generally sound, the reviewers and the Reviewing editor did not find a convincing explanation of the phylogenetic distribution of the described horizontally transferred CAS/CYS gene in T. urticae and only a few Lepidoptera. This point requires a better discussion and potentially additional data to support or explain the phylogenic pattern of distribution of the CAS/CYS gene.
The phylogenies in Figure 2 suggest that the horizontally transferred gene in the mite and the insects identified share a common ancestor. This is not discussed sufficiently in the text. The fact that this gene is apparently only found in 18 arthropods (of all of those with substantial sequences in public databases) would suggest either a loss of such a gene in most arthropods lineages or more than one independent horizontal gene transfer event in T. urticae and the Lepidoptera.
Although the authors indicate they looked at several insect species for this gene, they do not describe the extent of their search. Did they examine the genomic data available outside of Lepidoptera (e.g., in Coleoptera, Hymenoptera, and Diptera)? Between insects and arachnids, a common ancestor to this gene might suggest that it was introduced prior to the divergence of insects and arachnids, and therefore may be present (or vestiges thereof) in other insect species. Are there any? If not, does this suggest two separate horizontal gene transfer events? This in silico analysis, and an analysis of the predicted transfer event date based on divergence of the orthologous sequences, would add additional insight into this manuscript.
After addressing the above questions, the authors should discuss the alternative scenarios of a single vs. multiple horizontal gene transfer event(s) to explain the phylogeny described in Figure 2 and make a well-supported conclusion to this point.
1) To allow the reader to assess enzyme kinetic properties, the K M and K cat values must be shown in Table 1. V max values shown in one of the figures are not very informative.
The main objective of the enzyme kinetic data was to determine which of the two reactions (cysteine synthesis – CYS and β-cyanoalanine synthesis – CAS) was favored by the Tu-CAS enzyme. For this, a comparison of the specificity constants (kcat/KM) (a.k.a. second order rate constant) for each reaction was needed. The ratio of specificity constants (kcat/KM of CAS activity over kcat/KM of CYS activity) strongly favored CAS activity over CYS activity. As kcat is directly proportional to Vmax (kcat = Vmax/[E]), the ratio is the same whether Vmax or kcat is used. The values given for the ratio of specificity constants are therefore identical and correct whether they are calculated from Vmax/KM or kcat/KM. As the enzyme purity is not known precisely and this would introduce a systematic error, we calculated the Vmax/KM ratio for the mite enzyme, as it cancels out the enzyme concentration in the equation. We have updated Table 1, so it now also includes the KM and activity values.
The paragraph describing the kinetic data has now been amended in the Results section “Biochemical characterization of Tu-CAS”.
2) The text says that products of CAS activity, beta-cyanoalanine, were identified by TLC and LC-MS. The authors refer to Figure 9 for these results. However, Figure 9 only shows TLC data, which may not be sufficiently informative. To support statements of product identification, the LC-MS results must be shown for enzyme products and the authentic standard.
We have measured CAS activity using robust and well validated methods previously used for the bacterial and plant enzymes (Hendrickson and Conn 1969; Warrilow and Hawkesford 2000; Yamaguchi et al. 2000; Hatzfeld et al. 2000). These indirect methods are based on the colorimetric determination of sulfide (Siegel 1965). More directly, we have also shown the enzyme- and time-dependent formation of β-cyanoalanine after separation of reaction mixtures on TLC. This is especially powerful, as it allows the identification of β-cyanoalanine both by Rf value, and by a specific and strong color shift after treatment with a ninhydrin solution. The cyano group of β-cyanoalanine is responsible for a unique deep blue color (as also clear from the standard, see Figure 7, panel A, BCA standard). Finally, we scrapped the enzymatically produced β-cyanoalanine after separation of reaction mixtures from silica plates, and analyzed it by LC-MS. We showed that the elution time on LC and the characteristic ion of m/z = 113 which is [M-H]- of the β-cyanoalanine standard are also found in the enzymatic product. We have added figure panels to illustrate the LC-MS data as requested (Figure 7 panel B1 and B2).
3) While the results appear to be generally sound, the reviewers and the Reviewing editor did not find a convincing explanation of the phylogenetic distribution of the described horizontally transferred CAS/CYS gene in T. urticae and only a few Lepidoptera. This point requires a better discussion and potentially additional data to support or explain the phylogenic pattern of distribution of the CAS/CYS gene.
The phylogenies in Figure 2 suggest that the horizontally transferred gene in the mite and the insects identified share a common ancestor. This is not discussed sufficiently in the text. The fact that this gene is apparently only found in 18 arthropods (of all of those with substantial sequences in public databases) would suggest either a loss of such a gene in most arthropods lineages or more than one independent horizontal gene transfer event in T. urticae and the Lepidoptera.
Although the authors indicate they looked at several insect species for this gene, they do not describe the extent of their search. Did they examine the genomic data available outside of Lepidoptera (e.g., in Coleoptera, Hymenoptera, and Diptera)? Between insects and arachnids, a common ancestor to this gene might suggest that it was introduced prior to the divergence of insects and arachnids, and therefore may be present (or vestiges thereof) in other insect species. Are there any? If not, does this suggest two separate horizontal gene transfer events? This in silico analysis, and an analysis of the predicted transfer event date based on divergence of the orthologous sequences, would add additional insight into this manuscript.
After addressing the above questions, the authors should discuss the alternative scenarios of a single vs. multiple horizontal gene transfer event(s) to explain the phylogeny described in Figure 2 and make a well-supported conclusion to this point.
By a first series of BLAST-searches in NCBI databases (nr/nt), Tu-CAS homologues could not be found outside Lepidoptera even though the genomic data of many other arthropods are present in NCBI. Nevertheless, we have meticulously screened a large number of genome portals directly, and an exhaustive list of databases searched is now included as Supplemental file 2, without revealing any sequence that was not previously detected.
In order to better describe the extent of our search, we altered the first paragraph of the Results section “Phylogeny of Tu-CAS and evidence for a bacterial origin by horizontal transfer”.
We thank the referees and editors for pointing out that the phylogeny should be better discussed in the manuscript. We have now gone as far as the data allow without gratuitous speculation, and we have added the section beginning “A homologous lateral gene transfer has also occurred in Lepidoptera” to the first paragraph of the Discussion.
We also updated Figure 2 now showing also the phylogenetic position of the CAS enzymes of related tetranychid mite species Tetranychus evansi and Panonychus citri.