The R882H DNMT3A Mutation Associated with AML Dominantly Inhibits WT DNMT3A by Blocking its Ability to Form Active Tetramers (original) (raw)
. Author manuscript; available in PMC: 2015 Apr 14.
Published in final edited form as: Cancer Cell. 2014 Mar 20;25(4):442–454. doi: 10.1016/j.ccr.2014.02.010
Summary
Somatic mutations in DNMT3A, which encodes a de novo DNA methyltransferase, are found in ~30% of normal karyotype acute myeloid leukemia (AML) cases. Most mutations are heterozygous and alter R882 within the catalytic domain (most commonly R882H), suggesting the possibility of dominant negative consequences. The methyltransferase activity of R882H DNMT3A is reduced by ~80% compared to the WT enzyme. In vitro mixing of WT and R882H DNMT3A does not affect the WT activity but co-expression of the two proteins in cells profoundly inhibits the WT enzyme by disrupting its ability to homotetramerize. AML cells with the R882H mutation have severely reduced de novo methyltransferase activity and focal hypomethylation at specific CpGs throughout AML cell genomes.
Introduction
Acute myeloid leukemia (AML) is a malignancy of hematopoietic stem/progenitor cells (HSPCs) with considerable clonal and genetic heterogeneity (Welch et al., 2012; Ding et al., 2012; Ley et al., 2013). Some AML patients have recurrent translocations that cause gene fusions (i.e. PML-RARA, RUNX1-RUNX1T1, CBFB-MYH11, MLL fusions, etc.), which are associated with canonical alterations in gene expression and DNA methylation patterns (Figueroa et al., 2010; Ley et al., 2013). Recent studies have revealed that patients with normal karyotype (NK) AML exhibit a high frequency of mutations in genes involved with DNA methylation, including DNMT3A (~30-35%), IDH1 or IDH2 (~15-20%), and TET2 (~25-30%), suggesting that alterations in DNA methylation may play an important role in early disease pathogenesis for this group of AML patients (Delhommeau et al., 2009; Mardis et al., 2009; Ley et al., 2010; Marcucci et al., 2012; Ley et al., 2013).
DNMT3A mutations are generally present in the founding clones of AML samples, suggesting that they may initiate leukemia (Ding et al., 2012; Ley et al., 2013; Miller et al., 2013). However, the mechanisms by which they contribute to leukemogenesis are not yet clear. Previous studies of Dnmt3a function using mouse models have shown that haploinsufficiency for Dnmt3a results in no obvious alterations in hematopoiesis, while HSPCs completely lacking DNMT3A protein exhibit increased self-renewal and decreased differentiation upon serial transplantation (Tadokoro et al., 2007; Challen et al., 2011). However, more than half of DNMT3A mutations in AML samples are heterozygous missense alterations within the catalytic domain of the enzyme at residue R882, most commonly resulting in an arginine to histidine change (Ley et al., 2010; Shen et al., 2011; Thol et al., 2011; Yan et al., 2011; Marcucci et al., 2012; Ribeiro et al., 2012). The high frequency of mutations at this specific site raises the possibility that this amino acid change creates a gain-of-function activity, and/or produces a protein with a dominant negative effect on the residual wild-type (WT) protein.
Previous studies of R882 mutations in recombinant DNMT3A produced in several systems have demonstrated that these mutations confer reduced de novo methyltransferase activity in vitro. Prior to the discovery of this mutation in AML, the homologous R878 residue in murine DNMT3A was mutated in a screen of the C-terminal catalytic methyltransferase domain of DNMT3A purified from E. coli (Gowher et al., 2006); this mutation reduced its methyltransferase activity, and also its DNA and S-adenosylmethionine (SAM, or AdoMet) binding capacity. Full-length human DNMT3A with the R882H mutation purified from Sf9 insect cells confirmed that this mutation has reduced activity in an in vitro methylation assay (Yamashita et al., 2010). Additional studies of recombinant DNMT3A have examined its interactions with DNMT3L, a related (but catalytically inactive) protein that contributes to the regulation of DNMT3A oligomerization and enhances its methyltransferase activity (Jia et al., 2007, Holz-Schietinger et al., 2010). Complexes of full-length DNMT3A and DNMT3L co-purified from E. coli demonstrated the hypomorphic activity of R882H DNMT3A relative to WT DNMT3A (Yan et al., 2011). More detailed analysis of the properties of the catalytic domain of DNMT3A (also purified from E. coli) revealed that the R882H mutation disrupted its ability to form tetramers (Holz-Schietinger et al., 2012). This mutation reduced the processive methylation of consecutive CpG dinucleotides in vitro, but this effect was significantly rescued by the addition of DNMT3L. Importantly, the dominant negative potential of the R882 mutations was highlighted by a recent study where murine DNMT3A R878H (equivalent to R882H in human DNMT3A) was shown to inhibit de novo DNA methylation by WT DNMT3A in murine ES cells (Kim et al., 2013).
In this study, we explored potential mechanisms that could explain how a heterozygous, hypomorphic R882H allele could affect de novo methylation beyond causing simple haploinsufficiency.
Results
Characterizing the DNA Methylation Potential of NK-AML Cells
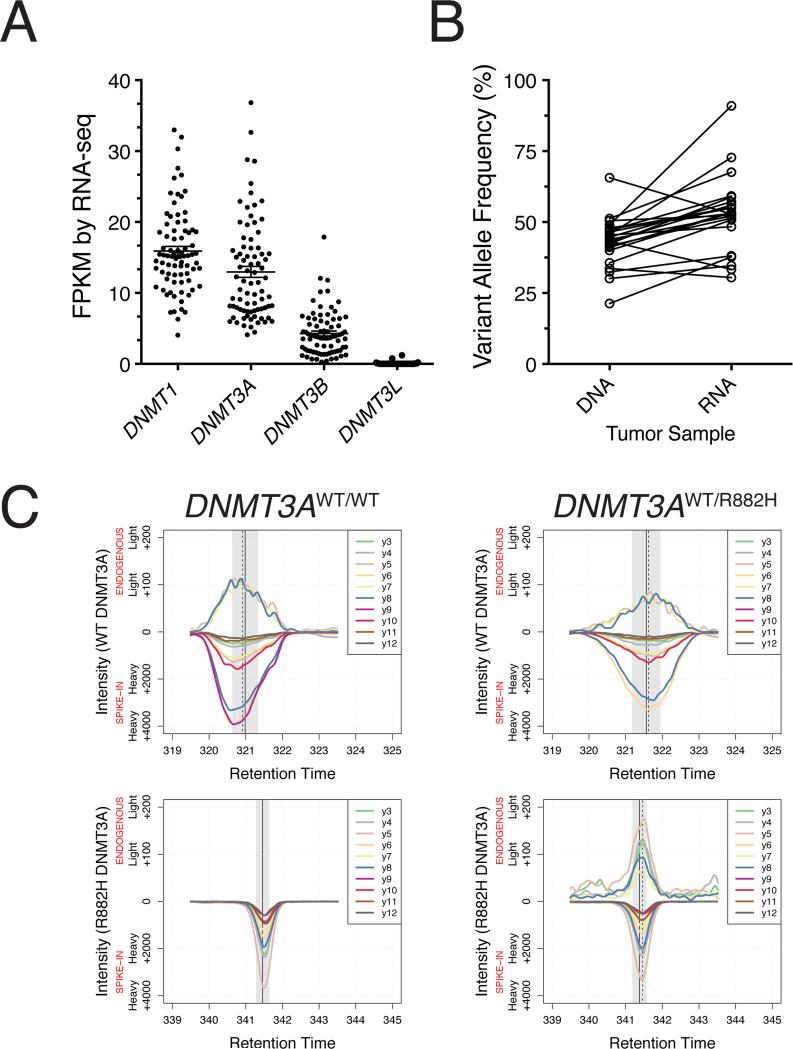
To explore the functional consequences of the R882H DNMT3A mutation in AML, we first characterized patterns of expression of the DNA methyltransferases in a set of 80 primary NK-AML samples from the TCGA AML cohort (Ley et al., 2013). RNA-sequencing data confirmed that DNMT1, the maintenance DNA methyltransferase, was the most highly expressed DNMT gene (mean FPKM = 15.91, standard deviation = 5.82; comparison of DNMT1 relative to DNMT3A p = 0.004; Figure 1A). DNMT3A is known to be dynamically regulated during normal and malignant hematopoiesis (Mizuno et al., 2008; Challen et al., 2011); we observed substantial DNMT3A expression in all NK-AML samples (mean FPKM = 12.97, standard deviation = 6.95, Figure 1A). Further, DNMT3A was expressed on average 2.3-fold higher than DNMT3B (mean FPKM = 4.27, standard deviation = 3.10). Importantly, 95% (76/80) of NK-AML patients predominantly expressed inactive splice variants of DNMT3B, irrespective of FAB or DNMT3A mutation status (median ratio of inactive to active DNMT3B transcripts was 3:1; Figure S1). DNMT3L expression was not detected in the vast majority of NK-AML cases, although very minimal expression was present in 13 cases (mean FPKM = 0.04, standard deviation = 0.16, Figure 1A).
Figure 1. Expression of DNA Methyltransferase Genes in Normal Karyotype (NK)-AML Samples.
(A) FPKM expression of DNA methyltransferase family genes in NK-AML by RNA-seq (n = 80).
(B) Variant allele frequency (%) of R882 mutant DNMT3A in NK-AML tumor DNA and RNA. Each set of connected points corresponds to a single patient (n = 23).
(C) TTOF mass spectrometry identification of WT and R882H DNMT3A in primary NKAML cell lysates. X-axis reflects peptide retention time (minutes), which distinguishes between the two peptides that define WT vs. R882H DNMT3A proteins. Y-axis reflects signal intensity for heavy peptides ([13C6][15N4]-labeled internal standard synthetic peptides corresponding to WT or R882H DNMT3A; negative on axis) or light peptides (endogenous, native WT and R882H DNMT3A; positive on axis), based on specific y-series ion transitions (curves y3-y12). All endogenous DNMT3A signals exhibiting mean retention times (dashed vertical lines) within the 95% CI of the heavy internal standard mean retention time (vertical gray shading and solid vertical lines) are shown.
See also Figure S1.
The variant allele frequency (VAF) of somatic mutations at R882 in DNMT3A was about 50% in most samples, indicating that R882 mutations are almost always heterozygous and are present in nearly all cells in most AML samples (i.e. they are nearly always in founding clones); based on the similar VAFs of the R882 mutations in RNA-seq data, we conclude that these mutations do not alter the expression or stability of DNMT3A mRNA (Figure 1B).
The existence of R882H DNMT3A protein in AML cells has not yet been reported, and it is possible that the R882H mutation produces an unstable protein that causes functional haploinsufficiency for DNMT3A at the protein level. We therefore developed a selected reaction monitoring mass spectrometric assay to specifically quantify the relative abundance of WT and R882H DNMT3A proteins in NK-AML whole cell lysates (one was WT/WT for DNMT3A [TCGA#3008], and the other had a heterozygous R882H DNMT3A mutation [TCGA#2896] with a VAF of 43.1%). We exploited the alteration of a tryptic digest site caused by the R882H mutation (WT peptide = VFGFPVHYTDVSNMSR; R882H peptide = VFGFPVHYTDVSNMSHLAR), and used heavy ([13C6][15N4]-labeled) synthetic peptides containing these sequences as internal standards to identify and quantify endogenous WT and R882H DNMT3A (Figure 1C). We demonstrated that the WT- and R882H-specific peptides from the endogenous proteins co-eluted with isotopically labeled synthetic peptides of the same amino acid sequence; we detected only the WT DNMT3A peptide in the _DNMT3A_WT/WT lysate, and found both the WT and R882H DNMT3A peptides in the mutant lysate. Positive identification was achieved by matching the ion chromatograms from the respective peptide fragmentation spectra (isotopically labeled internal standard and peptides from endogenous proteins). We compared the relative signal intensities of the endogenous WT and R882H peptides to the respective heavy internal standards, and found approximately equal amounts of WT and R882H DNMT3A in the _DNMT3A_WT/R882H sample (WT:R882 = 5.14:6.77 fmol). Together, these data suggest that the de novo DNA methylation potential of NK-AML cells is almost exclusively provided by DNMT3A (since functional DNMT3B and DNMT3L are generally not present in these cells). Further, it suggests that the R882H mutation could produce a maximal 50% reduction in the net de novo DNA methylation potential in these cells – unless the mutant protein has a dominant negative activity.
Hypomethylation in NK-AML Genomes with DNMT3A Mutations at R882
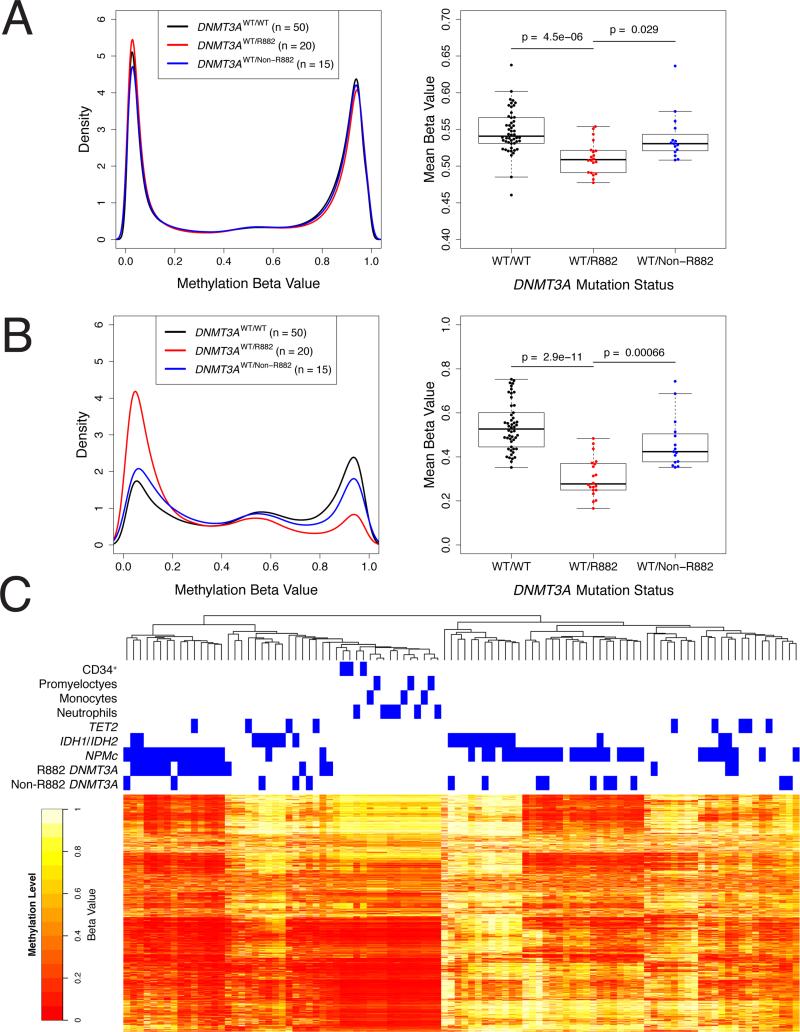
While recent work in murine ES cells has shown that the mouse equivalent of the R882H DNMT3A mutation can lead to DNA hypomethylation in a dominant manner, it is important to note that ES cells and AML cells possess substantially different DNA methylation environments (Ziller et al., 2013) – notably, Dnmt3l and active Dnmt3b are expressed in murine ES cells (Chen et al., 2003), but not in NK-AML cells (Figure 1A). To define the consequences of DNMT3A mutations on DNA methylation in primary human AML cells, we analyzed Illumina Infinium HumanMethylation450 BeadChip (“450K array”) data from 85 cases of NK-AML in the TCGA AML cohort (Ley et al., 2013). Although previous studies of global DNA methylation using liquid chromatography-tandem mass spectrometry showed that DNMT3A mutations had no measureable effects on the total (i.e. ‘bulk’) 5-methylcytosine content in AML samples (Ley et al., 2010), the 450K array platform allowed us to conduct a high resolution analysis of DNA methylation in DNMT3A mutant (‘R882’ or ‘non-R882’) and WT NKAML samples at 464,198 CpGs found throughout the genome (see Supplemental Experimental Procedures). We first compared the mean methylation value across all array CpGs, and found a small but statistically significant difference between DNMT3A WT (n = 50) and R882 mutant samples (n = 20), with R882 mutant samples having a lower mean methylation value (WT = 0.547 vs R882 = 0.511, p = 4.5 × 10-6; Figure 2A). In contrast, samples with non-R882 mutations (n = 15) did not demonstrate a significant difference in mean methylation relative to WT samples. However, R882 and non-R882 mutant samples showed a statistically significant difference in mean methylation that was similar to the difference between R882 mutant and WT samples (non-R882 = 0.539 vs R882 = 0.511, p = 0.029). Levels of DNA methylation in NK-AML were not influenced by DNMT3B expression or active/inactive DNMT3B splice variant ratio (Figure S2A), highlighting the influence of DNMT3A on shaping the methylome of NK-AML cells.
Figure 2. DNA Methylation Profiling of NK-AML Samples Identifies a Focal Hypomethylation Phenotype Associated with DNMT3A R882 Mutations.
(A) Aggregate density distribution of methylation beta values for all CpGs for all patients based on DNMT3A mutation status (black = _DNMT3A_WT/WT [n = 50], red = heterozygous DNMT3A mutation at R882 [n = 20], blue = non-R882 mutation in DNMT3A [n = 15]). Mean methylation beta values are shown for all CpGs for each patient based on the DNMT3A mutation status. P-values were calculated by t-tests corrected for multiple testing.
(B) Aggregate density distribution of methylation beta values for the 5,000 most variably methylated CpGs for all samples (categorized by DNMT3A mutation status: black = _DNMT3A_WT/WT [n = 50], red = heterozygous DNMT3A mutation at R882 [n = 20], blue = non-R882 mutation in DNMT3A [n = 15]). Mean methylation beta values are shown for each patient (categorized by DNMT3A mutation status) for the 5,000 most variably methylated CpGs. P-values were calculated by t-tests corrected for multiple testing.
(C) Heatmap representation of unsupervised hierarchical clustering of 85 NK-AML cases and 15 normal human bone marrow-derived samples (enriched CD34+ cells, promyelocytes, neutrophils, or monocytes), based on methylation beta values for the 5,000 most variably methylated CpGs in the sample set. The methylation beta value for each CpG is represented by a color scale (red = less methylated, yellow/white = more methylated). CpG probes were ordered by similarity, as assessed by hierarchical clustering analysis. The mutation status of relevant, recurrently mutated genes in these NK-AML samples is indicated above the heatmap. See also Figure S2 and Table S1.
We next asked whether CpGs associated with particular genomic features accounted for the hypomethylation phenotype of samples with R882 mutations. To assess this, we compared mean methylation levels of subsets of CpGs based on their relationships to gene loci (promoter, gene body, 3’ UTR, or intergenic) as well as CpG islands (islands, shores, shelves, or open sea) between WT, R882, and non-R882 mutant NK-AMLs. All eight subsets demonstrated consistent hypomethylation in NK-AMLs with R882 mutations (Figure S2B), and, similarly, hypomethylation was observed when examining each chromosome independently (Figure S2C), demonstrating the genome-wide nature of this phenotype.
To determine whether R882 mutations might have a more striking effect on the methylation of a smaller subset of CpGs, we identified the 5,000 most variably methylated CpGs according to their methylation value standard deviations across all 85 NK-AML samples. This set was comprised of genic (n = 3,466) and non-genic (n = 1,534), as well as CGI (n = 1,775) and non-CGI (n = 3,225) CpGs (Table S1). Hypomethylation was observed in R882 mutant samples relative to those with WT DNMT3A in 73.8% (3690/5000) of the CpGs (mean methylation value difference between genotypes > 0.15). Additionally, samples with R882 mutations exhibited lower mean methylation values across these 5,000 CpGs than WT samples (WT = 0.539 vs R882 = 0.306, p = 2.2 × 10-16; Figures 2B, S2D), a much greater difference than that detected across all CpGs on the 450K array. This effect was also observed when these 5,000 CpGs were organized by gene and CGI relationships (Figure S2E). Hierarchical clustering of all 85 NK-AML samples (as well as 15 normal human control samples derived from purified cells from human bone marrow samples: three CD34-enriched, three promyelocyte-enriched, three monocyte-enriched, and six neutrophil-enriched samples) using these 5,000 CpGs resulted in a distinct cluster of fourteen cases with R882 mutations (and two cases with non-R882 mutations) exhibiting very low levels of DNA methylation at these 5,000 CpGs (Figure 2C). As expected, a separate cluster of cases with IDH1 or IDH2 mutations was detected, which was associated with higher levels of DNA methylation at specific locations.
The most hypomethylated cluster of NK-AML cases was also highly enriched for NPM1 mutations (p = 3.6 × 10-4). We therefore calculated mean methylation values across these 5,000 CpGs for all 85 NK-AML cases, and compared the values for DNMT3A WT and R882 mutant samples without NPM1 mutations. All comparisons (the entire 5,000 CpG set, as well as the genic/non-genic/CGI/non-CGI subsets of these 5,000 CpGs) revealed significant hypomethylation in R882 mutant samples relative to WT samples (Figure S2F), demonstrating that the hypomethylation observed in the cases with R882 mutations is independent of NPM1 mutations (although it is exaggerated with NPM1 mutations).
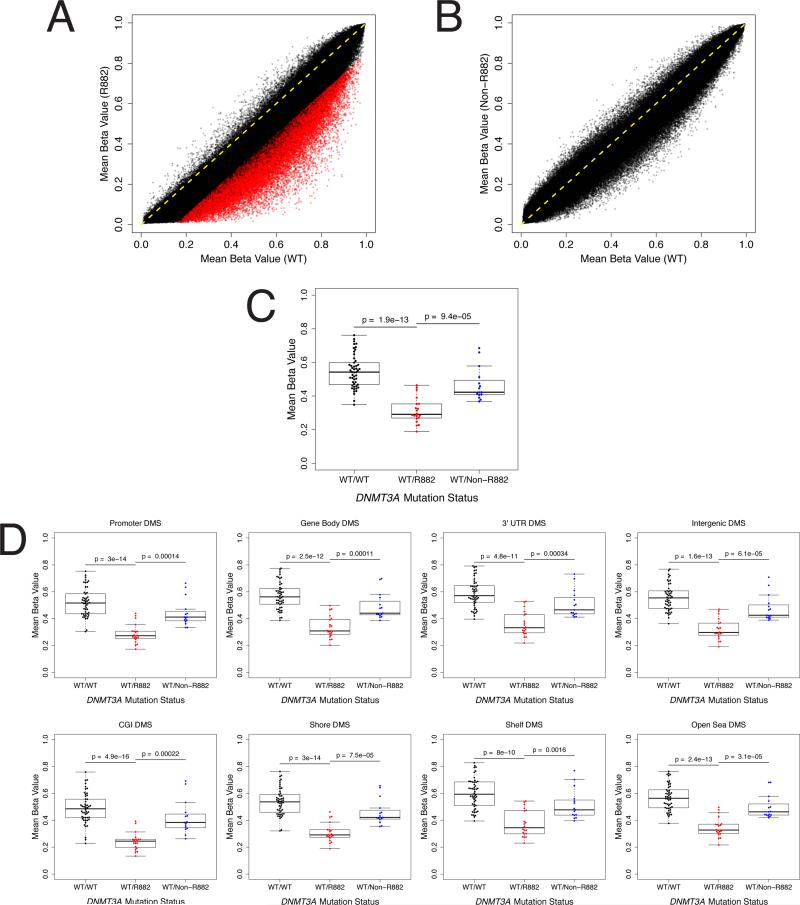
To determine whether the hypomethylation associated with R882 mutations in DNMT3A occurs consistently at specific CpGs, we performed a supervised differential methylation analysis to compare NK-AML genomes based on DNMT3A mutation status (WT vs R882). Of the 464,198 CpGs included in our analysis, 29,660 (6.4% of assessed loci) exhibited significant differential methylation between WT and R882 mutant samples (FDR-adjusted p-value < 0.01 and mean methylation value difference between genotypes > 0.15; Table S2), of which 29,658 (99.9%) were hypomethylated in R882 samples (Figure 3A). We performed targeted bisulfite sequencing and validated a specific, differentially methylated CpG detected by the 450K array platform just upstream from the p15/CDKN2B gene (Figure S3A). Importantly, no CpGs were differentially methylated between WT and non-R882 mutant samples (Figure 3B). Genome-wide DNA methylation studies have recently shown that most tissue-specific differential DNA methylation occurs outside CGIs in the neighboring 2 kb shores (Irizarry et al., 2009). Accordingly, we found that the 29,658 hypomethylated CpGs specific to R882 mutant samples were significantly enriched for CpG island shore loci (p = 3.14 × 10-223 by Chi-squared; Figure S3B). Further, in this NK-AML cohort, the R882 mutant alleles consisted of both R882H (15/20) and R882C (5/20). We confirmed that the hypomethylation phenotype was nearly identical for the R882H and R882C mutant samples (no CpGs were found to be significantly differentially methylated between R882H and R882C samples by supervised analysis, and no significant differences between R882H and R882C samples were found when comparing mean methylation values across the entire genome or within subsets of CpGs based on their relation to gene loci or CGIs; Figures S3C, S3D). This suggests that the dominant negative activity of the R882H mutation in DNMT3A can likely be extrapolated to other R882 mutations. Together, these findings demonstrate that NK-AML genomes with DNMT3A mutations specifically at R882 exhibit focal hypomethylation at specific CpG residues, which are found throughout the genome (Figure S3E). Discrete, large-scale regional changes in methylation (Berman et al., 2011) are not observed in these samples, however (Figures S3F, S3G).
Figure 3. Differential Methylation in NK-AML Samples with R882 Mutant DNMT3A.
(A) Scatter plot comparing mean methylation beta values at individual CpGs across all NK-AML cases categorized by DNMT3A mutation status (WT vs. R882 mutant). Individual points represent single CpGs (x-axis = mean methylation beta value for all WT samples; y-axis = mean methylation beta value for all R882 mutant samples). CpGs with equal mean methylation beta values between WT and R882 samples appear along the line Y=X, indicated in yellow. CpGs hypomethylated in R882 samples appear below the line Y=X. CpGs that were differentially methylated between the two sample sets (FDR < 0.01 and absolute value of mean methylation difference > 0.15) are indicated in red.
(B) Scatter plot comparing mean methylation beta values at individual CpGs across all NK-AML cases categorized by DNMT3A mutation status (WT vs. non-R882 mutant). Individual points represent single CpGs (x-axis = mean methylation beta value for all WT samples; y-axis = mean methylation beta value for all non-R882 mutant samples). CpGs with equal mean methylation beta values between WT and non-R882 samples appear along the line Y=X, indicated in yellow. CpGs hypomethylated in non-R882 samples appear below the line Y=X. CpGs that were differentially methylated between the two sample sets (FDR < 0.01 and absolute value of mean methylation difference > 0.15) are indicated in red.
(C) Mean methylation beta values are shown for each patient (categorized by DNMT3A mutation status) for the 29,660 differentially methylated CpGs (by supervised analysis between WT and R882 mutant samples). P-values were calculated by t-tests corrected for multiple testing.
(D) Mean methylation beta values are shown for each patient (categorized by DNMT3A mutation status) for subsets (based on CpG relationship to gene loci or CGIs) of the 29,660 differentially methylated CpGs (by supervised analysis between WT and R882 mutant samples). P-values were calculated by t-tests corrected for multiple testing.
See also Figure S3 and Table S2.
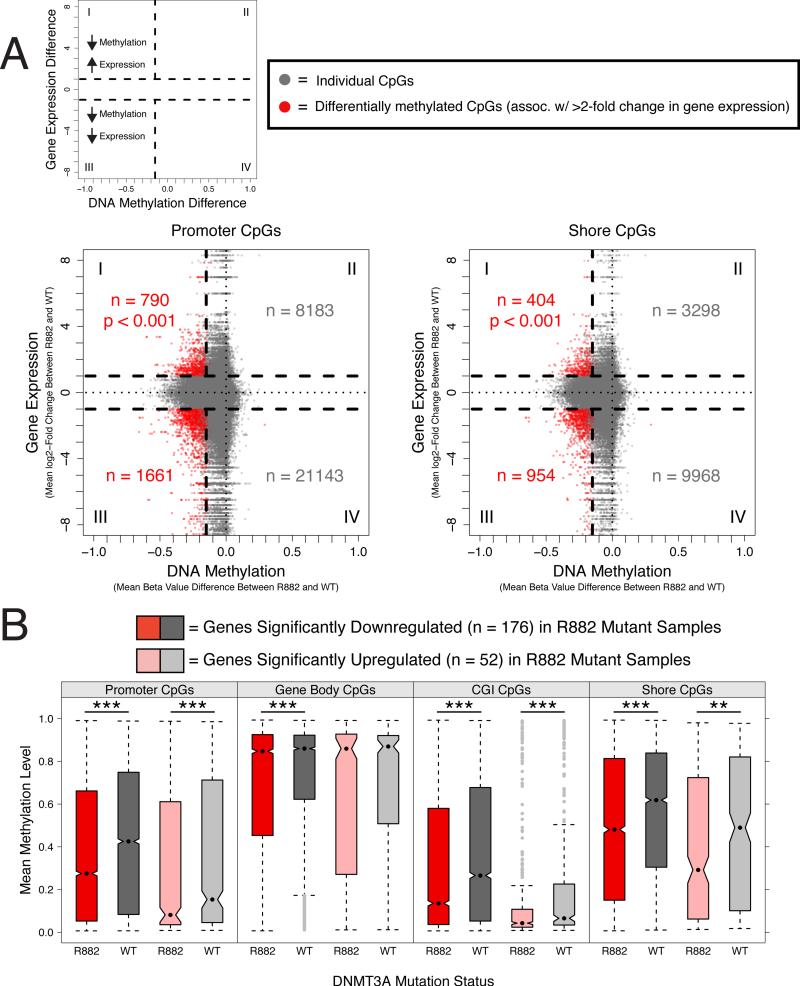
We also assessed the relationship between gene expression and DNA methylation in the 74 NK-AML cases with both RNA-seq and 450K methylation array data. All samples exhibited canonical relationships between levels of DNA methylation and gene expression – increased promoter, CGI, and CpG island shore methylation was associated with significantly decreased gene expression, whereas increased gene body methylation was associated with increased gene expression (Figures S4A, S4B). However, hypomethylated CpGs in R882 mutant samples identified by the supervised differential methylation analysis were associated with variable expression changes (Figures 4A, S4C). Genes associated with significantly hypomethylated promoter or shore CpGs in R882 samples were statistically overrepresented among upregulated genes (>2-fold mean change) relative to the overall gene expression changes between WT and R882 mutant samples (p < 0.001 for both promoter and shore CpGs, by Chi-squared with Yates’ correction). In contrast, genes associated with hypomethylated gene bodies or CGI CpGs showed no enrichment in either upregulated or downregulated genes (Figure S4C).
Figure 4. Effects of DNMT3A Mutations on the Relationship Between DNA Methylation and Gene Expression in NK-AML.
(A) Variable expression changes are observed in genes associated with differentially methylated promoter or shore CpGs in R882 mutant DNMT3A NK-AML samples. Each data point in the starburst plots represents the mean DNA methylation (x-axis) and mean gene expression (y-axis, log2-fold change) difference at an individual CpG comparing WT and R882 mutant DNMT3A samples. Data points in red are CpGs exhibiting significant differential methylation (by supervised analysis between WT and R882 mutant samples) and also >2-fold changes in expression of their associated genes. Genes associated with significantly hypomethylated promoter or shore CpGs were statistically enriched for upregulated genes relative to the overall gene expression changes between WT and R882 mutant samples (p < 0.001 for both promoter and shore CpGs by Chi-squared with Yates’ correction; contingency table = quadrants I/III vs. II/IV). Gene body and CGI CpGs are shown in Figure S4C.
(B) Differentially expressed genes in R882 mutant NK-AML consistently exhibit hypomethylation. Box-and-whisker plots compare R882 mutant and WT samples by mean methylation levels of individual promoter, gene body, CGI, or shore CpGs associated with either significantly downregulated (n = 176) or upregulated genes (n = 52) in R882 mutant NK-AML. p < 0.05 denoted by ‘*’, p < 0.005 denoted by ‘**’, and p < 0.0005 denoted by ‘***’ by Wilcoxon signed-rank test corrected for multiple testing. Note that both upregulated and downregulated differentially expressed genes are hypomethylated in R882 mutant samples.
See also Figure S4 and Tables S3 and S4.
We next conducted a supervised differential gene expression analysis (Anders et al., 2010), which identified only 228 differentially expressed genes between WT and R882 mutant NK-AMLs (52 upregulated and 176 downregulated in the R882 samples; Benjamini-Holm adjusted p-value < 0.05 and absolute log2-fold-change > 1; Tables S3 and S4; Figure S4D). Hierarchical clustering of all NK-AML samples using only these 228 differentially expressed genes revealed a high degree of intra-genotype variance (Figures S4D), reiterated by the failure of the first two principal components based on gene expression to significantly distinguish R882 mutant from WT NK-AML samples (Figure S4E). This is in contrast to the extent to which the first two principal components based on global DNA methylation successfully distinguish the majority of WT and R882 mutant samples (Figure S4F), emphasizing the greater similarity of R882 mutant NKAML samples by DNA methylation than by gene expression patterns. The 52 genes that were significantly upregulated in R882 mutant samples exhibited the predicted canonical hypomethylation of their promoters, CGIs, and CpG island shores (Figures 4B). However, the 176 genes that were significantly downregulated in R882 mutant samples were also hypomethylated compared to WT samples (Figures 4B) – these genes may be regulated by mechanisms other than DNA methylation. Overall, these findings highlight the uniformity of the hypomethylation signature found in R882 mutant samples, but illustrate the lack of a universal correlation between changes in the methylation state of individual CpGs and corresponding changes in the expression of linked genes. Clearly, further studies will be needed to better understand these findings.
Structural and Functional Consequences of the R882H Mutation in DNMT3A
Several previous studies have demonstrated that the R882H mutation reduces (but does not eliminate) the de novo DNA methyltransferase activity of DNMT3A in vitro (Yamashita et al., 2010; Yan et al., 2011; Holz-Schietinger et al., 2012). However, it is not yet clear why mutations at R882 are over-represented among the spectrum of DNMT3A mutations if their major effect is a simple reduction in methyltransferase activity. We therefore explored the possibility that R882 mutations may also possess novel gain-of-function activities, such as altered cellular localization and/or modified CpG substrate specificity.
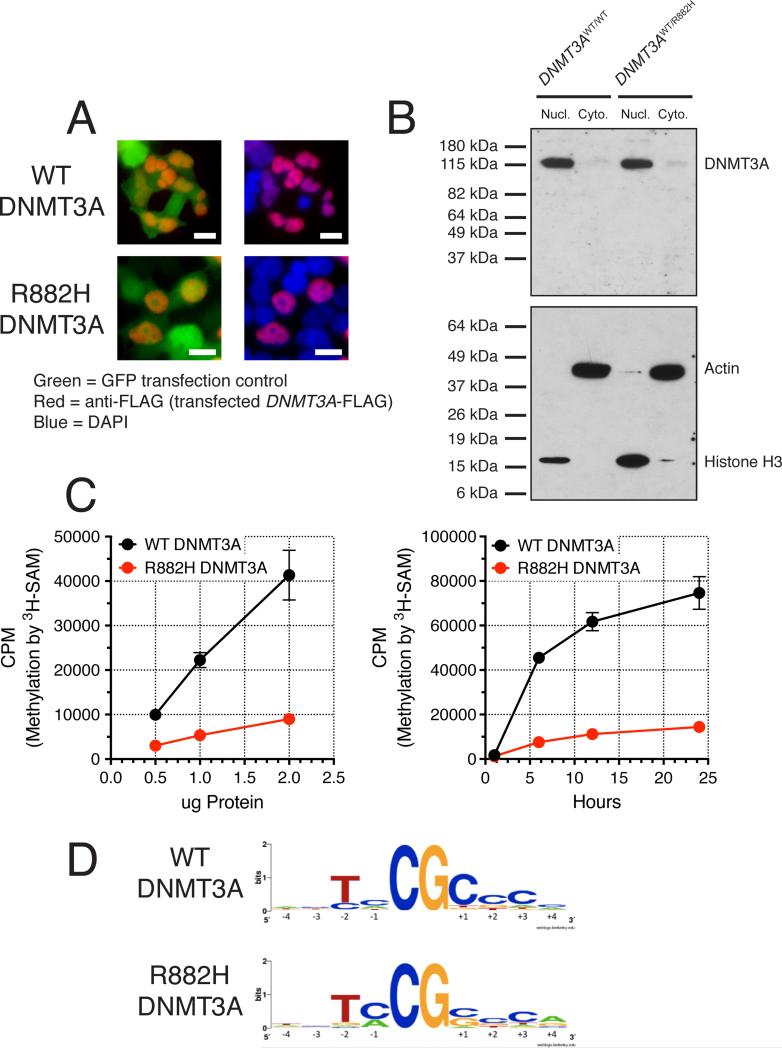
To determine whether R882 mutations cause improper subcellular targeting of DNMT3A, we expressed FLAG-tagged WT and R882H DNMT3A (as well as GFP for a transfection and cytoplasmic-distribution control) in HEK293T cells, and used immunofluorescence to assess subcellular localization. This revealed appropriate nuclear localization of both WT and R882H DNMT3A (Figure 5A). To verify this finding in primary NK-AML cells, we performed nuclear-cytoplasmic separations after hypotonic lysis of the leukemic cells, followed by Western blotting for DNMT3A (since the abundance of this protein is too low to be detected by immunofluorescence). Since WT and R882H DNMT3A protein are present in equal abundance in primary AML cells (Figure 1C), cytoplasmic mislocalization of mutant DNMT3A protein should be easily detected by this method. All measurable DNMT3A protein was in the nuclear fraction regardless of the R882 mutation status (Figure 5B).
Figure 5. Cellular Localization and Function of R882H DNMT3A Protein.
(A) Immunofluorescence imaging of FLAG-tagged WT and R882H DNMT3A. Note that WT and R882H DNMT3A proteins have the same nuclear distribution. The scale bar indicates 10 μm.
(B) Nuclear/cytoplasmic fractionation of primary NK-AML cells (WT/WT and WT/R882H for DNMT3A) to assess DNMT3A nuclear localization. Top panel = western blot with anti-DNMT3A antibody. Bottom panel = western blot with anti-histone-H3 (nuclear) and anti-actin (cytoplasmic) antibodies. Lanes were loaded with identical cell equivalents of lysate volumes.
(C) In vitro methylation of a linearized plasmid DNA substrate (pcDNA3.1) by recombinant full-length human WT DNMT3A or R882H DNMT3A: dose-response assay with 6-hour incubation, and time-course assay using 1 μg total protein per reaction (250 nM). Data are means +/- SEM of three independent experiments, each performed in triplicate.
(D) LOGOS motifs demonstrating preferentially methylated CpG sequences of WT and R882H DNMT3A based on bisulfite sequencing of in vitro methylated DNA templates.
See also Figure S5.
To further evaluate the consequences of the R882H mutation on DNMT3A functions, we purified full-length, human WT and R882H DNMT3A using a mammalian tissue culture system (Figures S5A-C). We first used the recombinant proteins to confirm that the R882H mutation reduces the de novo methylation activity of DNMT3A. We measured the transfer of tritiated methyl groups from 3H-SAM to substrate DNA (linearized DNA from the pcDNA3.1 plasmid, which contains 334 CpG residues), and found that R882H DNMT3A had only 21.7% (+/- SD of 4.4%) of the methyltransferase activity of WT DNMT3A (Figure 5C).
Previous work demonstrated that DNMT3A has a preference for specific DNA sequences flanking target CpGs (Wienholz et al., 2010). To assess whether the R882H mutation alters the CpG-flanking sequence preference, we performed in vitro methylation on linearized pcDNA3.1 plasmid DNA with recombinant WT or R882H DNMT3A. We then performed bisulfite sequencing of the methylated plasmid DNA from these reactions, with 13 amplicons covering 114 unique CpG-containing 6-mers. Lists of -10 to +10 sequence contexts of all target CpGs were generated from the bisulfite sequencing results of these in vitro methylation reactions. Sequences were then assigned weights corresponding to their measured methylation beta values, and these weighted sequence motifs were used to quantify preferential enrichment of bases upstream or downstream of target CpGs using WebLogo (Crooks et al., 2004). These data revealed identical CpG-flanking sequence preferences for WT and R882H DNMT3A (Figure 5D; Spearman correlation of rank ordered CpGs by methylation beta value between WT DNMT3A and R882H DNMT3A = 0.99), consistent with the expected “TNCGCY” motif previously described (Wienholz et al., 2010). Interestingly, the differentially methylated CpGs found in our primary AML cohort (Figure 3A; Table S2) were not enriched for the intrinsic DNMT3A -2 to +2 flanking sequence preference.
R882H DNMT3A Exhibits Dominant Negative Inhibition of WT DNMT3A Activity
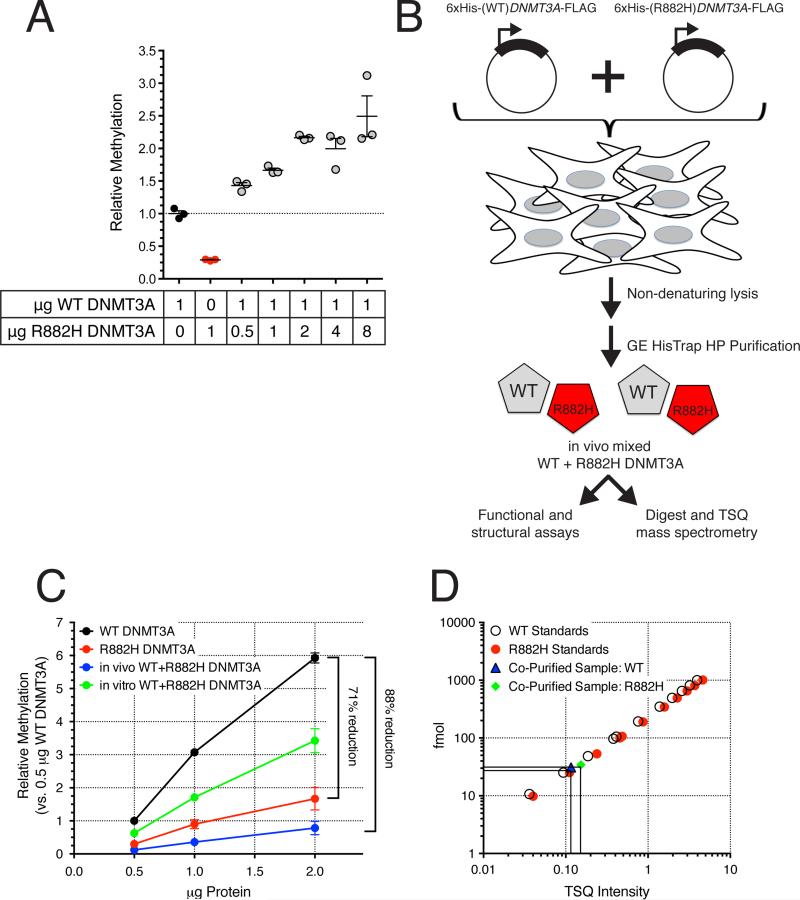
To determine whether recombinant R882H DNMT3A can inhibit the activity of the WT enzyme, we measured the methylation activity of increasing amounts of R882H DNMT3A mixed with a fixed amount of WT DNMT3A protein. Surprisingly, we observed a linear increase in the net enzymatic activity, reflecting the summed activity of the two forms of DNMT3A in these 4-hour in vitro reactions (Figure 6A). Therefore, using this assay, no dominant negative activity of the R882H mutant protein was detected.
Figure 6. Dominant Negative Effects of Recombinant R882H DNMT3A Protein are Found Only After “In Vivo” Mixing.
(A) In vitro methylation assay of linearized pcDNA3.1 using recombinant full-length human WT, R882H, or in vitro mixed WT and R882H DNMT3A.
(B) Schematic of co-transfection/co-purification of WT and R882H DNMT3A for structure/function analysis and TSQ mass spectrometry quantification of DNMT3A proteins.
(C) In vitro methylation assay of linearized pcDNA3.1 using WT, R882H, in vitro mixed WT and R882H DNMT3A, vs. co-transfected/co-purified “in vivo_”_ mixed WT and R882H DNMT3A. Data are means +/- SEM of four independent experiments, each performed in triplicate.
(D) Example of WT:R882H DNMT3A ratio quantification by TSQ mass spectrometry in “in vivo” mixed samples. Open circles = WT DNMT3A standards; red closed circles = R882H DNMT3A standards; blue triangle = WT DNMT3A peptide from co-purified WT+R882H sample; green diamond = R882H DNMT3A peptide from co-purified WT+R882H sample.
However, we suspected that in vitro mixing of WT and R882H DNMT3A proteins might not recapitulate their interactions in primary AML cells, where both proteins would be produced and folded in the same compartment at the same time. We therefore cotransfected HEK293T cells with equal amounts of 6xHis-WT and 6xHis-R882H DNMT3A expression vectors, and then co-purified both proteins (Figure 6B). In contrast to the in vitro mixing experiment, the co-expressed, co-purified, “in vivo” mixed WT and R882H DNMT3A proteins displayed a >80% reduction in methyltransferase activity relative to WT DNMT3A (Figure 6C). To further test whether this represented a dominant negative activity of the R882H protein, we measured the relative abundance of both WT and R882H proteins in each of our “in vivo” mixed samples generated by co-expression/co-purification. We used a triple stage quadrupole (TSQ) mass spectrometric approach that exploited the altered tryptic cleavage site in DNMT3A produced by the R882H mutation, as described above. This revealed similar amounts of each protein in the co-purified enzyme preparations, with WT:R882H ratios ranging from 0.79 to 1.60 in four separate purifications (mean = 1.05; Figure 6D). The R882H mutant protein therefore must act in a dominant fashion to inhibit the methyltransferase function of the WT protein, but only when the two proteins are co-produced in the same cell.
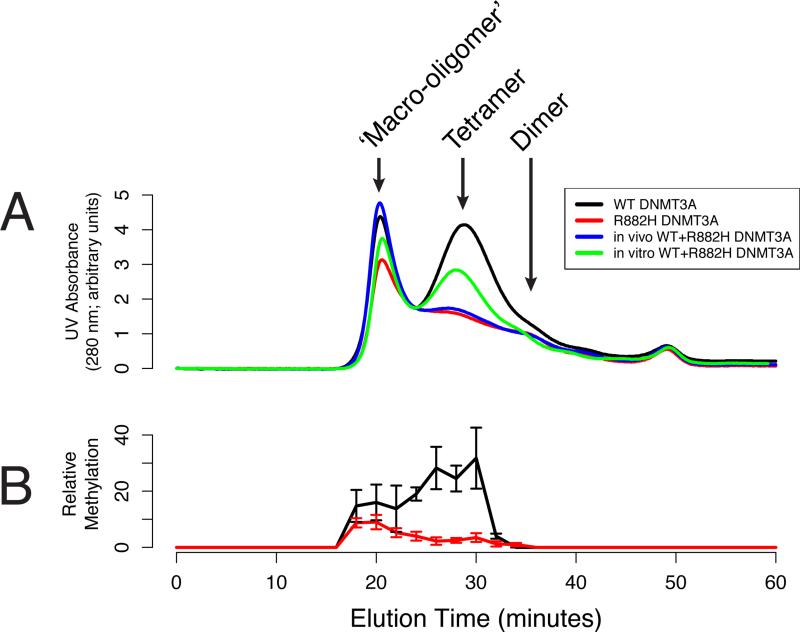
Given the location of the R882H mutation in the ‘RD’ self-interacting domain of DNMT3A, and the knowledge that the catalytic domain of WT DNMT3A preferentially forms tetramers, we postulated that the dominant negative effect of R882H may be due to an altered and/or disrupted oligomerization potential of DNMT3A when WT and R882H proteins form complexes. Using a Superose 6 size exclusion column, we identified two populations of full-length WT DNMT3A oligomers: a ‘macro-oligomer’ peak with a mean molecular weight of ~800 kDa (i.e. 8 or more DNMT3A molecules), and a tetramer peak of DNMT3A molecules with a mean molecular weight of ~450 kDa (Figure 7A). A significant deficit in the tetramer complex was observed for R882H DNMT3A, and a second, minor peak at a later elution time (consistent with dimers) was also detected. “In vivo” mixed (co-purified) WT and R882H DNMT3A complexes exhibited a pattern of oligomerization identical to R882H DNMT3A alone. In contrast, in vitro mixed WT and R882H DNMT3A exhibited a distribution of oligomers corresponding to the expected average of the WT and R882H curves (Figure 7A). All species (WT, R882H, as well as “in vivo” and in vitro mixed WT and R882H DNMT3A) were able to form ‘macro-oligomers’. Previous studies, however, have suggested that tetramers of the catalytic domain represent the most active form of the methyltransferase (Kareta et al., 2006; Jia et al., 2007, Holz-Schietinger et al., 2011).
Figure 7. R882H DNMT3A Fails to Form Homotetramers, and Blocks WT Homotetramer Formation in a Dominant Negative Fashion.
(A) Size exclusion chromatography tracings of DNMT3A complexes (WT = black; R882H = red; co-transfected/co-purified “in vivo” mixed WT and R882H = blue; in vitro WT and R882H DNMT3A = green). X-axis = Superose 6 column elution time (run at 0.5 mL/min; 20 minutes = ~800 kDa estimated molecular weight, 30 minutes = ~450 kDa estimated molecular weight). Y-axis = arbitrary units of UV280 nm absorption of column eluates.
(B) Methyltransferase activity of WT DNMT3A or R882H DNMT3A complexes (purified with size exclusion chromatography) assessed by bisulfite pyrosequencing of column fractions after in vitro methylation reactions. X-axis = Superose 6 column elution time (run at 0.5 mL/min; 20 minutes = ~800 kDa estimated molecular weight, 30 minutes = ~450 kDa estimated molecular weight). Y-axis = relative methyltransferase activity, calculated as the sum of methylation beta values across all 14 CpGs within the pyrosequencing amplicon. Data are means +/- SEM of three independent experiments.
To assess the relative methyltransferase activity of the different oligomeric complexes, we used Superose 6 fractions of WT DNMT3A in in vitro methylation assays that were assessed by pyrosequencing (Figure 7B). Of the eight fractions with detectable levels of methyltransferase activity, the three most active fractions were found in the tetramer elution peak. In contrast, the three DNMT3A fractions associated with the ‘macro-oligomer’ peak contained only 53% of the total methyltransferase activity of the three tetramer-associated DNMT3A fractions. We additionally verified the absence of catalytic activity from the corresponding tetramer fraction for R882H DNMT3A (Figure 7B). Together, these findings provide further evidence that the homotetramer is the most active multimeric species of WT DNMT3A, and suggests that the R882H protein must interact with the WT protein intracellularly to form stable heterodimers that are virtually inactive, and that inhibit the formation of the active WT homotetramers.
Discussion
In this study, we explored the consequences of the R882H mutation on DNMT3A function, and identified the mechanism of its dominant negative effect on WT DNMT3A in mammalian cells. Further, we identified a focal hypomethylation phenotype in NKAML cases possessing heterozygous DNMT3A mutations at R882, which demonstrates the profound loss of de novo methyltransferase activity resulting from the dominant negative consequences of these heterozygous alterations.
We and others have shown that recombinant DNMT3A with the R882H mutation has a reduced de novo DNA methyltransferase activity (~20% of WT levels), which therefore would predict only a ~40% reduction of the total de novo DNA methyltransferase activity of an AML cell with a heterozygous mutation – unless the mutant protein acts as a dominant negative against the WT protein. Surprisingly, mixing of purified recombinant WT and R882H DNMT3A demonstrated additive methylation capacity in an in vitromethylation assay, reflecting the summed activities of the two enzymes. This suggests that WT and R882H DNMT3A do not functionally interact with each other when purified separately and mixed in solution.
DNMT3A possesses two protein-protein interacting faces within the C-terminal catalytic domain. One is a hydrophobic ‘FF interface’, which mediates both homo-oligomerization and the DNMT3A-DNMT3L interaction via stacking of four phenylalanine residues (two from each subunit). The other is a polar ‘RD interface’ for DNMT3A-DNMT3A self-interaction, which occurs through a hydrogen-bonding network between arginine and aspartate residues (Jurkowska et al., 2011). This ‘RD interface’, in which the R882H mutation is found, forms the DNA binding site, while both interfaces are essential for cofactor S-adenosylmethionine (SAM) binding and overall catalytic activity of the enzyme (Holz-Schietinger et al., 2011). In vitro, DNMT3A-DNMT3A self-interactions are stable even in the presence of 2 M NaCl and 0.1% Triton X-100 (Purdy et al., 2010), suggesting that these complexes may have a very slow dissociation rate.
We suspected that in vitro mixing of WT and R882H DNMT3A might not recapitulate the DNMT3A complexes that would form within a NK-AML cell that express both WT and R882H DNMT3A. In vitro mixing may simply combine two pools of highly stable DNMT3A multimers (one exclusively WT, the other exclusively R882H) that do not form mixed multimers of WT and R882H enzymes during incubations lasting several hours. We therefore performed an “in vivo_”_ mixing experiment by co-transfecting and then co-purifying WT and R882H DNMT3A from the same mammalian cells. Co-purified enzymes (which were shown to possess WT and R882H DNMT3A in equal abundance by mass spectrometry) indeed demonstrated that the R882H mutant form of DNMT3A possesses a potent dominant negative activity, essentially eliminating the methyltransferase activity of the WT enzyme. Size exclusion chromatography analysis demonstrated that the mutant enzyme inhibits the ability of the WT enzyme to form functional tetramers. Earlier work suggested that tetrameric WT DNMT3A catalytic domains formed the most active methyltransferase (Kareta et al., 2006; Purdy et al., 2010; Jurkowska et al., 2011); we have shown here that tetramers of full-length WT DNMT3A protein are required for maximal de novo methyltransferase activity. Together, these findings define the mechanism through which R882H DNMT3A is able to function as a dominant negative inhibitor of de novo DNA methylation.
We also examined the 85 NK-AML cases from the TCGA AML cohort (Ley et al., 2013), identifying a small (but statistically significant) overall reduction in CpG methylation in the genomes of AML cases with DNMT3A mutations at R882, which was not found in cases with non-R882 mutations. Many common cancer-associated DNA methylation phenotypes involve changes at specific functional or structural elements of the genome, such as the CpG-Island Methylator Phenotype (CIMP) in glioblastoma (Noushmehr et al., 2010) and the long-range hypomethylation of nuclear lamina-associated domains in colon cancer (Berman et al., 2011). In contrast, the focal hypomethylation associated with R882 mutations occurred at specific CpGs throughout the genome, instead of in specific blocks.
The hypomethylated loci in R882 mutant NK-AML samples were enriched for CpG island shores, which others have shown to be regions of enrichment for tissue-specific differential methylation (Irizarry et al., 2009). Nonetheless, the profound R882 mutant hypomethylation phenotype entailed significant reductions in methylation in all subsets of CpGs based on their relationships to annotated genetic loci (promoter, gene body, 3’ UTR, or intergenic) or CGIs (CpG island, shore, shelf, or open sea). Both WT and R882 mutant cases exhibited canonical relationships between global levels of DNA methylation and gene expression; however, significantly hypomethylated CpGs in R882 mutant samples were associated with variable changes in the expression of linked genes. Although many of the affected genes have been shown to play important roles in AML, additional studies will be required to fully understand how these changes in gene expression may contribute to leukemogenesis.
In sum, we have elucidated the protein-intrinsic mechanism that explains the dominant negative potential of the R882H alteration in DNMT3A, and have demonstrated that mutations at this residue contribute to a focal hypomethylation phenotype in NK-AML samples. These genetic and molecular data suggest that there are two distinct classes of DNMT3A mutations that contribute to leukemogenesis in different ways. The first entails the dominant negative R882 mutations, which are expected to cause a near complete loss of de novo methyltransferase activity in an affected AML cell, which may directly cause the focal hypomethylation phenotype. The second class includes the other ~40% of DNMT3A mutations, which are equally distributed between frameshift/nonsense/splice-site mutations and other missense alleles (the most common of which, S714C, has only been reported in 11 samples to date, compared to >1000 R882 mutations [Forbes et al., 2011]). Many cases with non-R882 mutations are predicted to cause haploinsufficiency for DNMT3A (e.g. deletions, frameshifts, splice-site, and nonsense mutations); these cases do not exhibit significant changes in DNA methylation. Additional missense mutations in DNMT3A that are not at position R882 may contribute to AML pathogenesis by alternative mechanisms that do not directly involve altered de novo methyltransferase activity or DNA methylation patterns. Since DNMT3A interacts with many other proteins, one or more alternative mechanisms may be relevant for AML associated with the wide variety of mutations that are ‘non-R882’. Regardless, these data suggest that R882 and non-R882 DNMT3A mutations in AML patients may in fact be different entities that will ultimately require different therapeutic approaches.
Experimental Procedures
Mass Spectrometry, Immunofluorescence, Western Blot, Cell Culture, Bisulfite Pyrosequencing, Bisulfite Sequencing, and Oligonucleotide Sequences
These are described in the Supplemental Experimental Procedures.
Primary AML Samples
All cryopreserved primary AML samples were collected as part of a study approved by the Human Research Protection Office at Washington University School of Medicine after patients provided informed consent in accordance with the Declaration of Helsinki.
DNA Methylation and RNA-seq Analysis
Illumina Infinium HumanMethylation450 BeadChip and RNA-seq data from NK-AML patients (and normal human control samples derived from purified cells from bone marrow) were obtained from the TCGA-AML dataset (Ley et al., 2013). All DNA methylation and RNA-seq analyses were performed using the “R” software environment. The methylation data were normalized for background correction using the BioConductor MethyLumi package. Probes were removed from the dataset that were non-unique, within 10 bases of a SNP found in dbSNP, and/or on the X- or Y-chromosomes (yielding 464,198 probes for downstream analysis). Supervised differential methylation analysis was performed using CpGassoc with logit-transformed methylation beta values. Gene and CGI annotations were based on annotation provided by Illumina. For the purposes of this study, CpGs from the four categories TSS1500, TSS200, 5’-UTR, and 1st Exon were grouped into one “promoter” annotation. Hierarchical clustering analysis was performed with the heatmap.2 function (gplots package) using the 5,000 CpGs with the greatest standard deviation across all NK-AML samples. Supervised differential expression analysis was performed using DESeq2.
Statistical Analysis
Statistical analyses were performed using chi-squared tests for categorical variables and t-tests for continuous variables. All reported significance metrics are corrected for multiple testing by Benjamini-Holm (FDR) or Bonferonni methods (p-values) unless otherwise noted.
Protein Purification
N-terminal 6xHis-tags and C-terminal FLAG-tags were cloned into a full-length DNMT3A (NM_175629.1) expression vector using the pCMV6 (Origene) backbone. Five million HEK293T cells were plated per 15-cm plate and transfected after 24 hours by standard calcium-phosphate transfection protocols with 25 μg WT, 25 μg R882H, or 12.5:12.5 μg WT:R882H 6xHis-DNMT3A-FLAG expression vectors. Twenty-four hours after transfection, the media on the cells was replaced, and at 48 hours, cells were harvested in PBS by trituration. Cells were then centrifuged and resuspended at 5 million cells/mL in 20 mM sodium phosphate, 250 mM NaCl, 30 mM imidazole pH 7.4 plus a protease inhibitor cocktail (Sigma P8465). Cells were lysed by repeated (>3x) snap-freezing and thawing, then the lysates were clarified by centrifugation at 10,000 g for 20 minutes. The supernatants were filtered through a Whatman 25 mm GD/X PES 0.45 μm pore filter and then loaded onto a 2-mL GE HisTrap HP column and washed with 20 mL of 20 mM sodium phosphate, 500 mM NaCl, 30 mM imidazole pH 7.4. The protein was eluted in 20 mM sodium phosphate, 250 mM NaCl, 400 mM imidazole pH 7.4 and then dialyzed into 25 mM Tris-HCl, 150 mM NaCl, 0.5 mM DTT, 0.5 mM EDTA pH 7.2 (with 5% v/v glycerol for making -80°C frozen stocks). Protein concentrations were determined by Pierce BCA Kit (ThermoScientific), and then purity was confirmed using the Pierce Silver Stain Kit (ThermoScientific).
In vitro Methylation
In vitro methylation reactions were performed on linearized pcDNA3.1 (Invitrogen) or linearized pcDNA3.1 containing a 144-bp fragment of the GSTP1 promoter (see Supplemental Experimental Procedures) cloned into the EcoRI restriction digest site. Reactions were carried out at 37°C in a buffer of 20 mM HEPES, 30 mM NaCl, 0.5 mM DTT, 1 mM EDTA pH 7.2, plus 0.2 mg/mL BSA and 5 μM 3H-labelled SAM (Perkin-Elmer). Reactions were quenched by 100-fold dilution in ice-cold 10% TCA with 35 μg tRNA carrier (Sigma). Samples were spotted onto Whatman GF/C 25 mm filters and washed with 5 mL of ice-cold 10% TCA twice, then 5 mL of 95% ethanol once, dried, and measured by a scintillation counter.
Size-exclusion Chromatography
Purified DNMT3A proteins (100 μL of 0.5 mg/mL protein) were loaded onto a Superose 6 HR 10/30 column controlled by an AKTApurifier with UNICORN software (GE v5.11). The flow rate was kept constant at 0.5 mL/min using a running buffer of 25 mM Tris-HCl, 150 mM NaCl, 0.5 mM DTT, 0.5 mM EDTA pH 7.2. To generate a standard molecular weight curve, 100 μg of each of five proteins were used (urease, BSA, chicken egg albumin, carbonic anhydrase, and α-lactalbumin; Sigma MWND500). Molecular weights of DNMT3A complexes were calculated by non-linear regression based on Kav values determined from elution values, the column void volume, and the total column volume (GraphPad Prism 6.0).
Supplementary Material
01
02
03
04
05
Highlights.
- AML cases with DNMT3A mutations at R882 exhibit focal hypomethylation
- R882H DNMT3A is a dominant negative inhibitor of WT DNMT3A
- WT DNMT3A forms stable, active homotetramers
- R882H DNMT3A dominantly disrupts DNMT3A tetramerization
Significance.
Mutations in epigenetic modifier genes have been identified in several cancers, including DNMT3A, IDH1, IDH2, TET2, EZH2, KDM6A, ASXL1, MLL, and others in AML. The mechanisms by which mutations in DNMT3A contribute to AML pathogenesis are not entirely clear. Here we demonstrate that the common R882H mutation creates an altered protein with dominant negative activity against WT DNMT3A, disrupting the ability of the WT enzyme to form active homotetramers. As a consequence, AML samples with DNMT3A mutations at R882 are associated with focal hypomethylation throughout their genomes. This suggests that two classes of DNMT3A mutations exist: R882 mutations cause a striking reduction of de novo DNA methyltransferase activity, whereas non-R882 mutations may contribute to AML pathogenesis via different mechanisms.
Acknowledgements
The authors thank Daniel George and Anne Kettler for technical assistance. This work was funded by the NIH (T32-HL007088 (supporting D.A.R.-G.), and CA162086, CA101937, to T.J.L.), and the Barnes Jewish Hospital Foundation (T.J.L.). The Washington University Proteomics Core is supported by grants from the National Institute of General Medical Sciences (8 P41 GM103422-35), the National Cancer Institute (P30 CA091842) and the National Center for Advancing Translational Sciences (UL1 TR000448).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman BP, Weisenberger DJ, Aman JF, Hinoue T, Ramjan Z, Liu Y, Noushmehr H, Lange CPE, van Dijk CM, Tollenaar RAEM, et al. Regions of focal DNA hypermethylation and long-range hypomethylation in colorectal cancer coincide with nuclear lamina–associated domains. Nat Genet. 2011;44:40–46. doi: 10.1038/ng.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS, Bock C, Vasanthakumar A, Gu H, Xi Y, et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat. Genet. 2011;44:23–31. doi: 10.1038/ng.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Ueda Y, Dodge JE, Wang Z, Li E. Establishment and Maintenance of Genomic Methylation Patterns in Mouse Embryonic Stem Cells by Dnmt3a and Dnmt3b. Mol Cell Biol. 2003;23:5594–5605. doi: 10.1128/MCB.23.16.5594-5605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia J-M, Brenner SE. WebLogo: a sequence logo generator. Genome Research. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhommeau F, Dupont S, Valle Della, V., James C, Trannoy S, Massé A, Kosmider O, Le Couedic J-P, Robert F, Alberdi A, et al. Mutation in TET2 in myeloid cancers. N. Engl. J. Med. 2009;360:2289–2301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, Ritchey JK, Young MA, Lamprecht T, McLellan MD, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481:506–510. doi: 10.1038/nature10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa ME, Lugthart S, Li Y, Erpelinck-Verschueren C, Deng X, Christos PJ, Schifano E, Booth J, van Putten W, Skrabanek L, et al. DNA Methylation Signatures Identify Biologically Distinct Subtypes in Acute Myeloid Leukemia. Cancer Cell. 2010a;17:13–27. doi: 10.1016/j.ccr.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, Jia M, Shepherd R, Leung K, Menzies A, et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011;39:945–950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowher H, Loutchanwoot P, Vorobjeva O, Handa V, Jurkowska RZ, Jurkowski TP, Jeltsch A. Mutational Analysis of the Catalytic Domain of the Murine Dnmt3a DNA-(cytosine C5)-methyltransferase. J. of Mol. Biol. 2006;357:928–941. doi: 10.1016/j.jmb.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Holz-Schietinger C, Reich NO. The inherent processivity of the human de novo methyltransferase 3A (DNMT3A) is enhanced by DNMT3L. J. of Biol. Chem. 2010;285:29091–29100. doi: 10.1074/jbc.M110.142513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz-Schietinger C, Matje DM, Harrison MF, Reich NO. Oligomerization of DNMT3A Controls the Mechanism of de Novo DNA Methylation. J. of Biol. Chem. 2011;286:41479–41488. doi: 10.1074/jbc.M111.284687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz-Schietinger C, Matje DM, Reich NO. Mutations in DNA methyltransferase (DNMT3A) observed in acute myeloid leukemia patients disrupt processive methylation. J. of Biol. Chem. 2012;287:30941–30951. doi: 10.1074/jbc.M112.366625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, Onyango P, Cui H, Gabo K, Rongione M, Webster M, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet. 2009;41:178–186. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia D, Jurkowska RZ, Zhang X, Jeltsch A, Cheng X. Structure of Dnmt3a bound to Dnmt3L suggests a model for de novo DNA methylation. Nature. 2007;449:248–251. doi: 10.1038/nature06146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkowska RZ, Rajavelu A, Anspach N, Urbanke C, Jankevicius G, Ragozin S, Nellen W, Jeltsch A. Oligomerization and binding of the DNMT3A DNA methyltransferase to parallel DNA molecules, heterochromatic localization and role of DNMT3L. J. of Biol. Chem. 2011;286:24200–24207. doi: 10.1074/jbc.M111.254987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareta MS, Botello ZM, Ennis JJ, Chou C, Chédin F. Reconstitution and mechanism of the stimulation of de novo methylation by human DNMT3L. J. of Biol. Chem. 2006;281:25893–25902. doi: 10.1074/jbc.M603140200. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Zhao H, Hardikar S, Singh AK, Goodell MA, Chen T. A DNMT3A mutation common in AML exhibits dominant-negative effects in murine ES cells. Blood. 2013;122:4086–4089. doi: 10.1182/blood-2013-02-483487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley TJ, Mardis ER, Ding L, Fulton B, McLellan MD, Chen K, Dooling D, Dunford-Shore BH, McGrath S, Hickenbotham M, et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature. 2008;456:66–72. doi: 10.1038/nature07485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, Kandoth C, Payton JE, Baty J, Welch J, et al. DNMT3A Mutations in Acute Myeloid Leukemia. N. Engl. J. Med. 2010;363:2424–2433. doi: 10.1056/NEJMoa1005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson AG, Hoadley K, Triche TJ, Jr., Laird PW, Baty JD, et al. Genomic and Epigenomic Landscapes of Adult De Novo Acute Myeloid Leukemia. N. Engl. J. Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcucci G, Metzeler KH, Schwind S, Becker H, Maharry K, Mrozek K, Radmacher MD, Kohlschmidt J, Nicolet D, Whitman SP, et al. Age-Related Prognostic Impact of Different Types of DNMT3A Mutations in Adults With Primary Cytogenetically Normal Acute Myeloid Leukemia. J. of Clin. Onc. 2012;30:742–750. doi: 10.1200/JCO.2011.39.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, Koboldt DC, Fulton RS, Delehaunty KD, McGrath SD, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Wilson RK, Ley TJ. Reply to Correspondence: Genomic Landscapes and Clonality of De Novo AML. N. Engl. J. Med. 2013;369:1472–1473. doi: 10.1056/NEJMc1308782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno SI, Chijwa T, Okamura T, Akashi K, Fukumaki Y, Niho Y, Sasaki H. Expression of DNA methyltransferases DNMT1, 3A, and 3B in normal hematopoiesis and in acute and chronic myelogenous leukemia. Blood. 2001;97:1172–1179. doi: 10.1182/blood.v97.5.1172. [DOI] [PubMed] [Google Scholar]
- Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP, Pan F, Pelloski CE, Sulman EP, Bhat KP, et al. Identification of a CpG Island Methylator Phenotype that Defines a Distinct Subgroup of Glioma. Cancer Cell. 2010;17:510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy MM, Holz-Schietinger C, Reich NO. Identification of a second DNA binding site in human DNA methyltransferase 3A by substrate inhibition and domain deletion. Arch. of Bioch. and Biophys. 2010;498:13–22. doi: 10.1016/j.abb.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Renneville A, Boissel N, Nibourel O, Berthon C, Helevaut N, Gardin C, Cayuela J-M, Hayette S, Reman O, Contentin N, et al. Prognostic significance of DNA methyltransferase 3A mutations in cytogenetically normal acute myeloid leukemia: a study by the Acute Leukemia French Association. Leukemia. 2012;26:1247–1254. doi: 10.1038/leu.2011.382. [DOI] [PubMed] [Google Scholar]
- Ribeiro AFT, Pratcorona M, Erpelinck-Verschueren C, Rockova V, Sanders M, Abbas S, Figueroa ME, Zeilemaker A, Melnick A, Lowenberg B, et al. Mutant DNMT3A: a new marker of poor prognosis in acute myeloid leukemia. Blood. 2012;119:5824–5831. doi: 10.1182/blood-2011-07-367961. [DOI] [PubMed] [Google Scholar]
- Tadokoro Y, Ema H, Okano M, Li E, Nakauchi H. De novo DNA methyltransferase is essential for self-renewal, but not for differentiation, in hematopoietic stem cells. J. of Exp. Med. 2007;204:715–722. doi: 10.1084/jem.20060750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thol F, Damm F, Ludeking A, Winschel C, Wagner K, Morgan M, Yun H, Gohring G, Schlegelberger B, Hoelzer D, et al. Incidence and Prognostic Influence of DNMT3A Mutations in Acute Myeloid Leukemia. J. of Clin. Onc. 2011;29:2889–2896. doi: 10.1200/JCO.2011.35.4894. [DOI] [PubMed] [Google Scholar]
- Wienholz BL, Kareta MS, Moarefi AH, Gordon CA, Ginno PA, Chédin F. DNMT3L Modulates Significant and Distinct Flanking Sequence Preference for DNA Methylation by DNMT3A and DNMT3B In Vivo. PLoS Genet. 2010;6:e1001106. doi: 10.1371/journal.pgen.1001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, Wartman LD, Lamprecht TL, Liu F, Xia J, et al. The Origin and Evolution of Mutations in Acute Myeloid Leukemia. Cell. 2012;150:264–278. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y, Yuan J, Suetake I, Suzuki H, Ishikawa Y, Choi YL, Ueno T, Soda M, Hamada T, Haruta H, et al. Array-based genomic resequencing of human leukemia. Oncogene. 2010;29:3723–3731. doi: 10.1038/onc.2010.117. [DOI] [PubMed] [Google Scholar]
- Yan XJ, Xu J, Gu ZH, Pan CM, Lu G, Shen Y, Shi JY, Zhu YM, Tang L, Zhang XW, et al. Exome sequencing identifies somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nat. Genet. 2011;43:309–315. doi: 10.1038/ng.788. [DOI] [PubMed] [Google Scholar]
- Ziller MJ, Gu H, Müller F, Donaghey J, Tsai LT-Y, Kohlbacher O, De Jager PL, Rosen ED, Bennett DA, Bernstein BE, et al. Charting a dynamic DNA methylation landscape of the human genome. Nature. 2013;500:477–481. doi: 10.1038/nature12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01
02
03
04
05