Targeting the Myofibroblast Genetic Switch: Inhibitors of Myocardin-Related Transcription Factor/Serum Response Factor–Regulated Gene Transcription Prevent Fibrosis in a Murine Model of Skin Injury (original) (raw)
Abstract
Systemic sclerosis (SSc), or scleroderma, similar to many fibrotic disorders, lacks effective therapies. Current trials focus on anti-inflammatory drugs or targeted approaches aimed at one of the many receptor mechanisms initiating fibrosis. In light of evidence that a myocardin-related transcription factor (MRTF)–and serum response factor (SRF)–regulated gene transcriptional program induced by Rho GTPases is essential for myofibroblast activation, we explored the hypothesis that inhibitors of this pathway may represent novel antifibrotics. MRTF/SRF-regulated genes show spontaneously increased expression in primary dermal fibroblasts from patients with diffuse cutaneous SSc. A novel small-molecule inhibitor of MRTF/SRF-regulated transcription (CCG-203971) inhibits expression of connective tissue growth factor (CTGF), _α_-smooth muscle actin (α_-SMA), and collagen 1 (COL1A2) in both SSc fibroblasts and in lysophosphatidic acid (LPA)–and transforming growth factor β (TGF_β)–stimulated fibroblasts. In vivo treatment with CCG-203971 also prevented bleomycin-induced skin thickening and collagen deposition. Thus, targeting the MRTF/SRF gene transcription pathway could provide an efficacious new approach to therapy for SSc and other fibrotic disorders.
Introduction
Systemic sclerosis (scleroderma, SSc) is a multisystem autoimmune disorder that can cause fibrosis of the skin and internal organ systems (lungs, heart, kidneys, and gastrointestinal system). It has the highest case fatality of any rheumatic disease. SSc predominately affects women (4–8:1), and the incidence increases with age. The precise pathogenesis of SSc is yet to be defined, but the major clinical features of SSc—collagen production, vascular damage, and inflammation/autoimmunity—require environmental triggers and genetic effects that interact with the three cardinal features of the disease at several points (Charles et al., 2006). In general, there is initial inflammation but fibrosis persists even after the inflammation has resolved or has been suppressed by medications (Beyer et al., 2012; Wynn and Ramalingam, 2012). This has led to the concept that understanding and targeting the fibrosis mechanism per se will be critical to successful therapies (Beyer et al., 2012; Wynn and Ramalingam, 2012; Gilbane et al., 2013).
A central feature of virtually all diseases of fibrosis is the activation of fibroblasts and their transition into myofibroblasts (Sappino et al., 1990; Boukhalfa et al., 1996; Zhang et al., 1996; Beyer et al., 2010; Hinz et al., 2012; Wynn and Ramalingam, 2012; Gilbane et al., 2013; Hu and Phan, 2013). The expression of _α_-smooth muscle actin (α_-SMA) is a widely recognized marker for this transition but it also contributes to the maintenance of fibrosis (Sappino et al., 1990; Boukhalfa et al., 1996; Zhang et al., 1996; Tomasek et al., 2002; Gilbane et al., 2013; Hu and Phan, 2013). There are multiple signaling pathways that induce myofibroblast transition. Transforming growth factor β (TGF_β) is a critical mediator, but lysophosphatidic acid (LPA), endothelin, thrombin, angiotensin, and connective tissue growth factor (CTGF) have all been implicated (Beyer et al., 2012; Wynn and Ramalingam, 2012; Gilbane et al., 2013). Furthermore, tissue stiffness has been identified as a positive feedback mechanism that leads to further myofibroblast activation—probably through integrins and focal adhesion kinase (FAK) mechanisms (Tomasek et al., 2002, 2008; Wynn and Ramalingam, 2012).
Emerging evidence implicates gene transcription induced by serum response factor (SRF) as a critical driver of myofibroblast activation by nearly all of these mechanisms (Small et al., 2010; Sandbo et al., 2011; Small, 2012). Indeed, key genes involved in fibrosis are direct SRF targets, including CTGF, collagen 1 (COL1A2), and even ACTA2, the gene for α_-SMA itself. The concept of a central role for SRF helps rationalize the complex signaling mechanisms that have been implicated in fibrosis. SRF-regulated gene expression is dependent on Rho-GTPase–stimulated nuclear localization of its transcriptional coactivator myocardin-related transcription factor (MRTF). RhoA appears to be a convergent downstream mediator activated by virtually all of the signal pathways controlling myofibroblast transition (Tomasek et al., 2008; Sandbo et al., 2009; Small et al., 2010; Small, 2012). G protein–coupled receptors for LPA, endothelin, thrombin, angiotensin, and even chemokines activate RhoA (Kranenburg et al., 1999; Seasholtz et al., 1999). Other factors important in fibrosis, including TGF_β and focal adhesion kinase, also modulate MRTF/SRF activity through activation of Rho signaling and actin dynamics (Crider et al., 2011; Huang et al., 2012; Sakai et al., 2013), which in turn drives expression of CTGF (CCN2), which synergizes with TGF_β_ in its profibrotic actions (Chaqour and Goppelt-Struebe, 2006; Liu et al., 2013; Serrati et al., 2013). Indeed, recent evidence suggests that CTGF release from SSc endothelial cells can enhance fibrosis, providing a connection between the vascular and mesenchymal attributes of SSc (Serrati et al., 2013).
Disruption of the Rho pathway with Rho kinase (ROCK) inhibitors has reversed myofibroblast differentiation in vitro and fibrosis in several animal models (Buhl et al., 1995; Masszi et al., 2003; Zhao et al., 2007; Akhmetshina et al., 2008; Sandbo et al., 2011; Small, 2012; Zhou et al., 2013). We recently identified in high-throughput screens, a compound, CCG-1423, that blocks MRTF nuclear localization by interfering with the regulation of intranuclear actin polymerization mediated by microtubule-associated mono-oxygenase, calponin and LIM domain–containing 2 (MICAL-2) (Evelyn et al., 2007; Lundquist et al., 2014). CCG-1423 is more effective than ROCK inhibitors in reducing SRF-mediated transcription (Evelyn et al., 2007). Several groups have used this compound to interdict myofibroblast formation (Sandbo et al., 2009; Zhou et al., 2013), and it was recently shown to have in vivo activity in a chlorhexidine gluconate model of peritoneal fibrosis (Sakai et al., 2013). We have now optimized this chemical series to reduce off-target toxicity (Evelyn et al., 2010; Bell et al., 2013).
In the present study, we demonstrated in human SSc dermal fibroblasts that there is spontaneous activation of an MRTF-regulated gene transcription program. CCG-203971, a new MRTF/SRF-gene transcription inhibitor, reverses the myofibroblast phenotype of both TGF_β_-stimulated normal dermal fibroblasts as well as the spontaneous activation of SSc-derived fibroblasts in vitro. Furthermore, it prevents the development of fibrosis in a mouse bleomycin skin injury model. These results suggest that targeting the MRTF/SRF-gene transcription mechanism may provide a novel and particularly effective approach to antifibrotic therapy.
Materials and Methods
Patient Sample and Animal Use.
All patients with SSc fulfilled the American College of Rheumatology criteria for classification of SSc (LeRoy et al., 1988). Two punch biopsies (4 mm) were taken from the forearm of mixed sex, SSc patients with the diffuse cutaneous variant. Normal skin tissue was obtained similarly from mixed sex, healthy volunteers. Written informed consent was obtained for all subjects and the study was approved by the University of Michigan Institutional Review Board. All experiments performed with animals were done with the approval from the University of Michigan Committee on Use and Care of Animals. C57BL/6 mice were used for the bleomycin prevention model.
Primary Cell Culture.
Both normal and SSc dermal fibroblasts were isolated from human skin (ages 53.4 ± 13.1 years for SSc; 47.5 ± 18.4 years for normal). The tissue was digested using enzyme digestion solution containing 2.4 U/ml dispase, 650 U/ml type II collagenase, and 10,000 Dornase U/ml DNase. Dermal fibroblasts were maintained in RPMI 1640 medium with 10% fetal bovine serum (FBS), penicillin, and streptomycin. Cells of passages between four and six were used.
Quantitative Polymerase Chain Reaction.
Dermal fibroblasts (1.0 × 105) were plated into six-well plates (Falcon; BD Biosciences Discovery Labware, Bedford, MA) and starved for 24 hours in Dulbecco’s modified Eagle’s medium (DMEM) containing 0.5% FBS with the concentrations of CCG-203971 indicated in the figure legends, 0.1% dimethyl sulfoxide (DMSO) and stimulated with 10 ng/ml TGF_β_1 (R&D Systems, Minneapolis, MN). Cells were lysed and RNA was isolated using the RNeasy kit (Qiagen, Valencia, CA) following the manufacturer’s directions. DNase-treated RNA was quantified using a NanoDrop spectrophotometer, and 1 _μ_g was used as a template for synthesizing cDNA using the TaqMan Reverse-Transcription Reagents kit (Invitrogen, Carlsbad, CA). SYBR Green quantitative polymerase chain reaction (qPCR) (SABiosciences/Qiagen) was performed using a Stratagene Mx3000P (Agilent Technologies, Santa Clara, CA). Ct values were determined and mRNA expression calculated relative to that of GAPDH. Primer sequences were: GAPDH, 5′-GGAAGGGCTCATGACCACAG-3′, 3′-ACAGTCTTCTGGGTGGCAGTG-5′; CTGF, 5′-CAGAGTGGAGCGCCTGTT-5′, 3′-CTGCAGGAGGCGTTGTCA-5′; ACTA2, 5′-AATGCAGAAGGAGATCACGC-3′, 3′-TCCTGTTTGCTGATCCACATC-5′; and COL1A2-hn, 5′-CTTGCAGTAACCTTATGCCTAGCA-3′, 3′-CCCATCTAACCTCTCTACCCAGTCT-5′. All mRNA values were normalized to a control (either normal fibroblasts or a vehicle control) run the same day.
Proliferation.
Human dermal fibroblasts (2.0 × 104) were plated into a 96-well plate and grown overnight in DMEM containing 10% FBS. Media were removed and replaced with DMEM containing 2% FBS and 30 _μ_M CCG-203971 or 0.1% DMSO control. After 72 hours WST-1 dye (Roche, Indianapolis, IN) was added to each well according to the manufacturer, and after 60 minutes absorbance at 490 nm was read using a Wallac Victor2 plate reader (PerkinElmer Life and Analytical Sciences, Waltham, MA).
Immunocytochemistry.
Dermal fibroblasts (3.0 × 104) from normal individuals or from patients with diffuse SSc were plated on 20-mm glass cover slips in DMEM containing 10% FBS and allowed to attach overnight. Medium was changed to low-serum medium (DMEM with 0.5% FBS) for 72 hours with the indicated concentration of CCG-203971 (and 0.1% DMSO control) with or without stimulation by 10 ng/ml TGF_β_1 (R&D Systems, Minneapolis, MN). The 3-day time point was chosen because in initial experiments TGF_β_ did not induce significant myofibroblast transition at 24 hours (data not shown). Cells were then fixed in 3.7% formaldehyde for 10 minutes at room temperature and permeabilized with 0.25% Triton X-100 for 10 minutes at room temperature. Primary antibody for _α_-SMA (ab5694; Abcam, Cambridge, MA) was diluted 1:300 and incubated for 2 hours at room temperature. Fluorophore-conjugated secondary antibody (Alexa Fluor 594 goat anti-rabbit IgG; Invitrogen, Carlsbad, CA) was diluted 1:1000 and added for 1 hour at room temperature. Coverslips were mounted (Prolong Gold antifade reagent with DAPI; Invitrogen) and imaged on an upright fluorescence microscope (Nikon E-800) at 40× magnification. For quantification, cells from three random nonoverlapping fields of view were scored as _α_-SMA positive or negative by an observer blinded to the treatment.
Bleomycin-Induced Skin Fibrosis Model.
Skin fibrosis was induced in C57BL/6 mice (female, 8 weeks old) by local intracutaneous injection of 100 _μ_l of bleomycin (1 mg/ml) in phosphate-buffered saline (PBS), every day for 2 weeks in a defined area (∼1 cm2) on the upper back. Intracutaneous injection of 100 _μ_l of PBS was used as a control. Three groups of mice with a total of 21 mice were used. One group received injections of PBS and the other two were challenged with bleomycin. Twice-a-day intraperitoneal administration of CCG-203971 (100 mg/kg in 50 _μ_l of DMSO) was initiated together with the first challenge of bleomycin and continued for 2 weeks. DMSO was used as the vehicle control. The three groups of animals were: (1) PBS/DMSO; (2) bleomycin/DMSO; and (3) bleomycin/CCG-203971. After treatment, animals were humanely killed by cervical dislocation, and tissue was collected.
Histology Analysis.
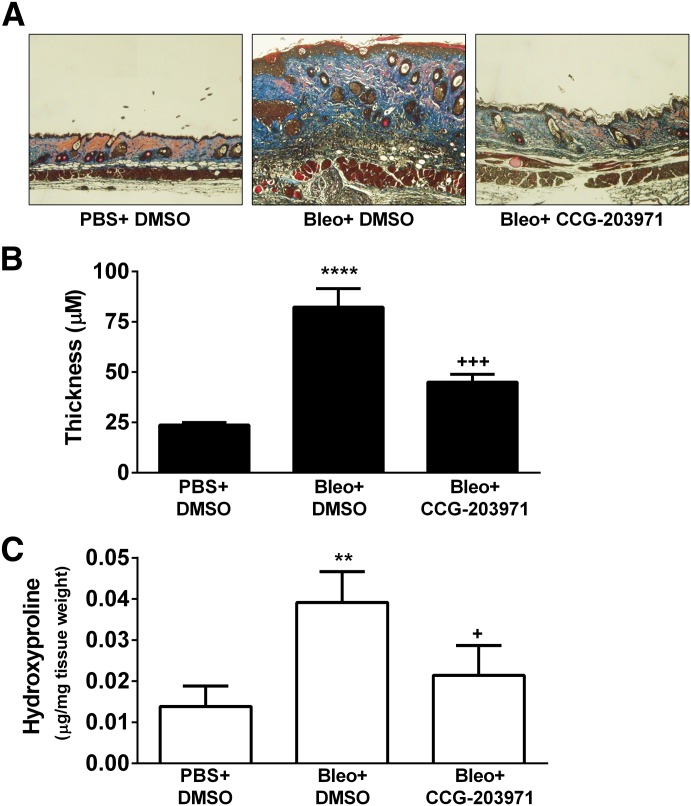
Skin obtained from the upper back at the site of the bleomycin or PBS injections was fixed and embedded in paraffin at the University of Michigan Comprehensive Cancer Center Histology Core. Skin sections were stained with Masson’s trichrome. Dermal thickness was determined by measuring the maximal distance between the epidermal-dermal junction and the dermal-subcutaneous fat junction. Three measurements were averaged from each skin section. The measurement was performed using the analysis tool in Photoshop.
Hydroxyproline Assay.
The collagen content from lesional skin samples was quantified using a hydroxyproline assay kit from Sigma-Aldrich (St. Louis, MO) and normalized with tissue weight.
Statistics.
Statistical analysis for two-group comparisons used a two-tailed, unpaired t test. For three or more groups analysis was performed by one-way analysis of variance (ANOVA) in GraphPad Prism (La Jolla, CA) followed by a Bonferroni post-test comparing all pairs in the dataset. Statistical significance was defined as P < 0.05.
Results
LPA Induces a Rho/MRTF/SRF Pathway–Dependent Fibrotic Gene Program in NIH-3T3 Fibroblasts.
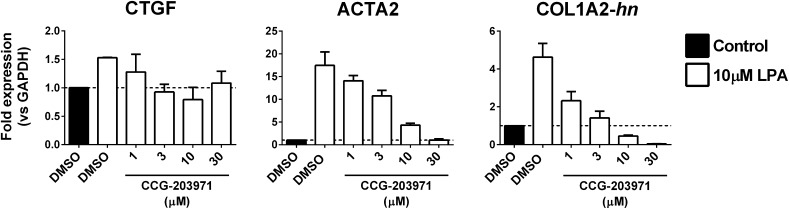
LPA treatment of NIH-3T3 fibroblasts (10 _μ_M, 60 minutes) stimulates expression of several Rho-regulated, fibrosis-associated genes (Fig. 1). Levels of mRNA for CTGF and ACTA2, both known MRTF/SRF targets, were induced 1.5- and 17.5-fold, respectively. Likewise, the heterogeneous nuclear precursor RNA for COL1A2 (COL1A2-hn), another MRTF/SRF target gene, was also increased by LPA (4.6-fold). Induction of all three genes was blocked by our recently described MRTF/SRF transcription pathway inhibitor CCG-203971 (Bell et al., 2013) in a concentration-dependent manner. The IC50 for these effects is ∼1–3 _μ_M.
Fig. 1.
LPA activates fibrotic gene expression in 3T3 fibroblasts in a Rho/MRTF-dependent manner. NIH-3T3 cells were treated with the indicated concentration of CCG-203971 or DMSO for 23 hours. One hour prior to RNA isolation, cells were stimulated with 10 _μ_M LPA. Expression of MRTF target genes CTGF, ACTA2, and COL1A2 was assessed by qPCR. For COL1A2, primers were designed to amplify newly synthesized heterogeneous nuclear RNA (hnRNA). Expression levels were quantified relative to GAPDH. Data are mean ± S.D. of two independent experiments.
SSc Dermal Fibroblasts Overexpress MRTF/SRF Target Genes.
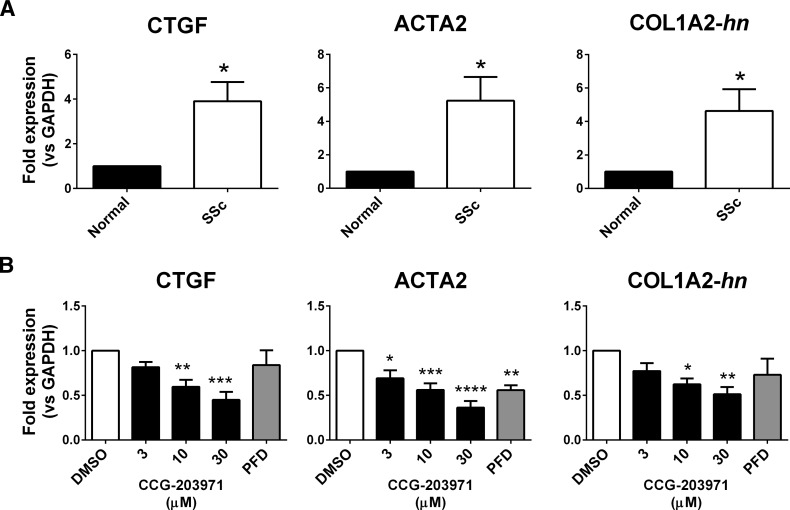
Multiple MRTF/SRF target genes are both markers and drivers of dermal fibrosis. CTGF and ACTA2 are overexpressed in the SSc dermal fibroblasts compared with the normal donor samples (Fig. 2A), as is COL1A2-hn. We also show that TGF_β_ stimulation of normal fibroblasts promotes RNA expression of CTGF, ACTA2, and COL1A2-hn (Supplemental Fig. 1). To further assess the involvement of the MRTF/SRF pathway in profibrotic gene expression, we treated the SSc cells with increasing concentrations of our MRTF/SRF pathway inhibitor, CCG-203971, which reduced expression of CTGF, ACTA2, and COL1A2 (Fig. 2B). In light of the long half-life of mRNA for COL1A2, the primers were designed to amplify the 1st exon/intron border of the gene (COL1A2-hn), as previously described (Luchsinger et al., 2011).
Fig. 2.
SSc-patient dermal fibroblasts show increased expression of fibrosis markers/MRTF target genes, which are inhibited by CCG-203971. (A) mRNA expression for fibrotic markers connective tissue growth factor (CTGF), alpha smooth muscle actin (ACTA2), and collagen (COL1A2) were quantified by qPCR. Primary human dermal fibroblasts isolated from normal donors or patients with SSc were grown in culture for no more than five passages prior to mRNA isolation. Data are mean ± S.E.M. of samples from three individuals. (B) CCG-203971 treatment reduces expression of CTGF, ACTA2, and COL1A2. Prior to mRNA isolation, SSc dermal fibroblasts were treated for 24 hours in the presence of the indicated concentration (_μ_M) of CCG-203971 or 300 _μ_M PFD. Data are mean ± S.E.M. of samples from at least four individuals. (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus DMSO control.)
Approximately 50% inhibition of these MRTF/SRF target genes was seen with 10 _μ_M CCG-203971. Pirfenidone (PFD), which is the only approved therapy directly targeting fibrosis, significantly inhibited ACTA2 gene expression but only showed a trend toward inhibition of CTGF and COL1A2-hn. The PFD effect, however, required a concentration of 300 _μ_M, which is 30–100 times greater than the effective concentrations of CCG-203971.
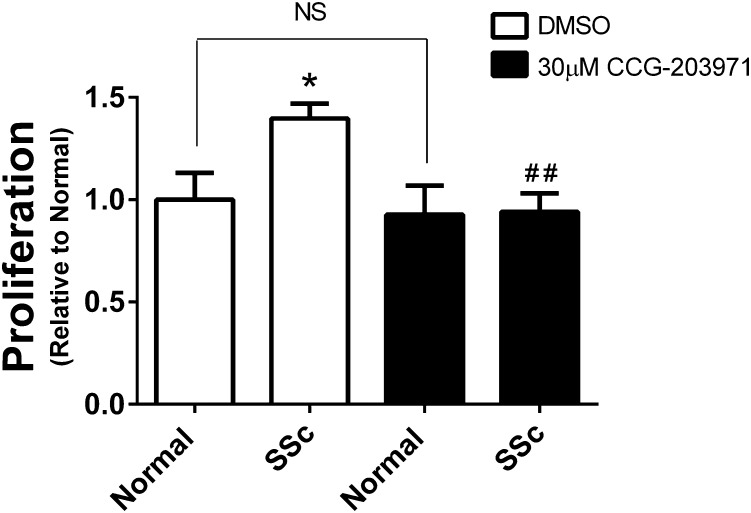
Scleroderma Fibroblasts Proliferate Faster Than Normal Cells and CCG-203971 Selectively Blocks Their Growth.
Autocrine secretion of LPA and overexpression of mitogenic cytokines such as CTGF contributes to cell proliferation in fibrotic tissues (Badri and Lama, 2012; Sakai et al., 2013). SSc cells proliferated faster than normal dermal fibroblasts in vitro, and this increase in growth was selectively blocked by CCG-203971 (Fig. 3). There was no effect on proliferation of the fibroblasts derived from normal individuals, even at 30 _μ_M compound. Combined with the effect on gene expression, these data suggest that the MRTF/SRF pathway is important for the transition of normal dermal fibroblasts into the faster proliferating myofibroblast-like SSc cells.
Fig. 3.
Scleroderma dermal fibroblasts proliferate faster than normal cells, and this is inhibited by CCG-203971. Cells were plated onto 96-well plates and allowed to grow for 3 days in the presence of 30 _μ_M CCG-203971 or DMSO vehicle. Viable cell density was assessed through enzymatic reduction of the water-soluble tetrazolium dye WST-1. Data are mean ± S.E.M. of samples from three individuals. (*P < 0.05 versus DMSO-treated Normal, ##P < 0.01 versus DMSO-treated SSc.)
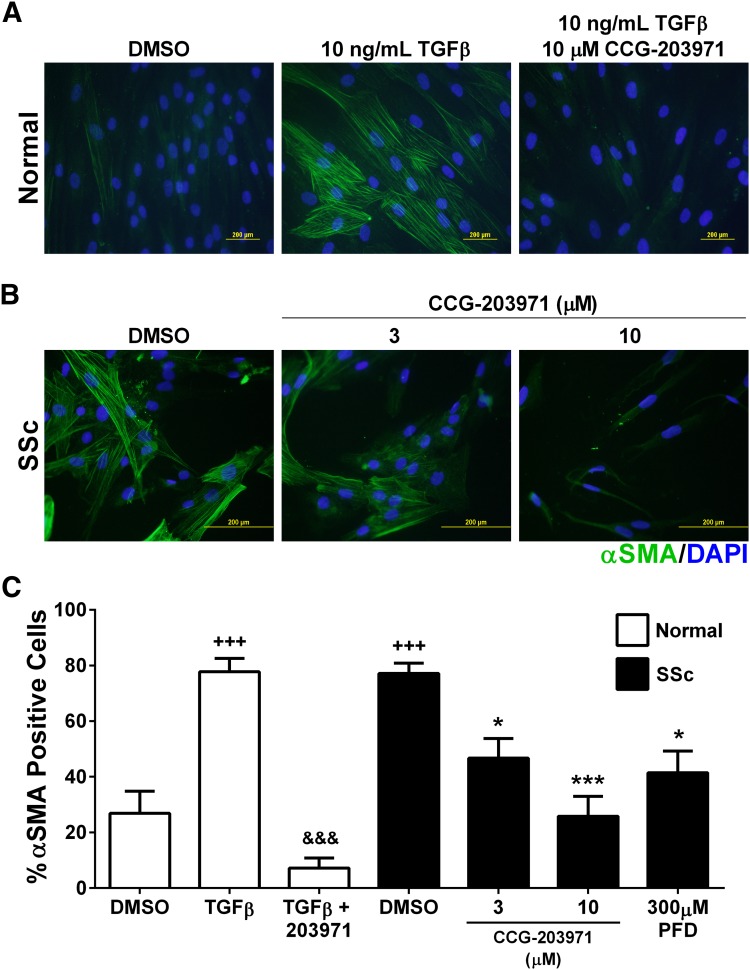
Inhibition of the MRTF/SRF Pathway Modulates Myofibroblast Transition of Dermal Fibroblasts.
_α_-SMA is involved in cellular motility and the contractile apparatus. It is also a well recognized protein marker for myofibroblasts (Tomasek et al., 2002; Masszi et al., 2003; Tomasek et al., 2008). Dermal fibroblasts from normal donors exhibit TGF_β_-stimulated _α_-SMA expression, which is blocked by CCG-203971 (Fig. 4, A and C). In these experiments, at 72 hours 10 _μ_M completely blocks the myofibroblast transition of normal fibroblasts. Consistent with the mRNA expression data above, SSc patient–derived fibroblasts display spontaneously high levels of _α_-SMA protein, which can be reversed by treatment with CCG-203971 (Fig. 4, B and C). In addition, PFD reversed the _α_-SMA expression but with a 50% reduction occurring at 300 _μ_M PFD; it is 100 times less potent than CCG-203971, which shows an IC50 of ∼3 _μ_M in this assay (Fig. 4C).
Fig. 4.
CCG-203971 modulates myofibroblast transition of dermal fibroblasts. (A) Primary human dermal fibroblasts from normal donors were plated onto cover slips and treated with or without 10 ng/ml TGF_β_ for 3 days to induce a myofibroblast transition; during stimulation cells were also treated with 10 _μ_M CCG-203971 or DMSO. Cells were then fixed, and _α_-SMA was visualized using immunocytochemistry along with nuclear DAPI staining. Shown are two representative individual samples. (B) Human dermal fibroblasts from patients with diffuse SSc were plated onto coverslips and treated with the indicated concentration of CCG-203971. Cells were then fixed and visualized using immunocytochemistry along with nuclear DAPI staining. Shown are two representative individual samples. (C) The fraction of cells positive for α_-SMA was scored by an observer blinded to the sample identification. Data are mean ± S.E.M. of samples from at least four individuals. (*P < 0.05, ***P < 0.001 versus DMSO Scleroderma, +++P < 0.001 versus Normal DMSO, &&&P < 0.001 versus Normal TGF_β.)
CCG-203971 Blocks Dermal Fibrosis in Bleomycin-Induced Injury Model.
To determine whether these effects would translate in vivo, we tested CCG-203971 in a bleomycin skin injury model. Because of its modest solubility, bleomycin was administered in 50 _μ_l of DMSO intraperitoneally. Preliminary studies showed that the compound administered in this manner was well tolerated at 100 mg/kg twice a day. Intradermal bleomycin for 2 weeks along with the DMSO control (50 _μ_l i.p.) resulted in marked dermal thickening (P < 0.0001) compared with the PBS+DMSO group, which did not receive bleomycin (Fig. 5, A–B). CCG-203971 treatment strongly and significantly (P < 0.001) suppressed the bleomycin-induced skin thickening in this model (Fig. 5, A–B). Skin collagen amounts, assessed by measurement of hydroxyproline content, showed similar results. Bleomycin injections promoted collagen deposition (P < 0.01) and CCG-203971 was able to block this effect (P < 0.05, Fig. 5C).
Fig. 5.
CCG-203971 prevents bleomycin-induced fibrosis in vivo. Bleomycin (0.1 mg) or vehicle (PBS) was injected intradermally in three groups of seven C57BL/6J mice for 2 weeks. Mice were also treated with twice daily injections of either CCG-203971 (100 mg/kg i.p.) or vehicle control (50 _μ_l i.p. of DMSO). At the end of the treatment period, skin samples were collected and either stained with Masson’s trichrome (A and B) or analyzed for hydroxyl-proline content (C), as described in Materials and Methods. Differences in skin thickness (triplicate measures from each mouse) and hydroxyproline content were assessed by one-way analysis of variance with the Bonferroni post-test to correct for multiple comparisons. (**P < 0.01, ****P < 0.0001, Bleo versus PBS control; +P < 0.05, +++P < 0.001, Bleo and CCG-203971 versus Bleo and DMSO.)
Discussion
There are no effective therapeutics for SSc or other diseases with fibrosis as a primary feature. Most current approaches target the inflammation that initiates fibrosis but do not directly address the process of fibrosis per se. In light of recent evidence for the central role of gene transcription regulated by the MRTF/SRF complex, we have shown that an inhibitor of this mechanism can reverse myofibroblast differentiation and reduce the fibrosis response in a bleomycin skin injury model (Tomasek et al., 2008; Sandbo et al., 2009; Small et al., 2010; Sakai et al., 2013).
In recent years, important signaling pathways of fibrosis have been elucidated (Beyer et al., 2012; Wynn and Ramalingam, 2012; Gilbane et al., 2013). Some key receptors that drive fibrosis include those for TGF_β_, LPA1, endothelin, angiotensin (AT1), chemokines (CXCR4), and serotonin. In addition, tissue stiffness itself can activate integrins and focal adhesion kinase, which sets up a vicious cycle that maintains fibrosis after the initial inflammatory stimulus is resolved (Guiducci et al., 2009; Huang et al., 2012). Many of these mechanisms are currently under consideration as antifibrotic targets (Beyer et al., 2012; Leask, 2012; Wynn and Ramalingam, 2012; Gilbane et al., 2013). However, blocking each individual signal may not be effective. Indeed, despite good preclinical results (Varga and Abraham, 2007), TGF_β_-1-neutralizing antibodies failed to show efficacy in SSc (Denton et al., 2007).
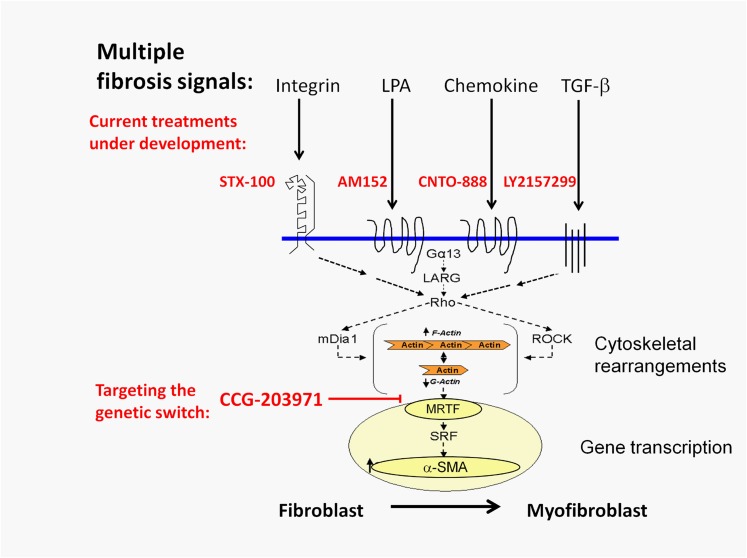
Targeting downstream mechanisms that are engaged by multiple fibrotic inputs may be more effective (see Fig. 6). Epigenetic mechanisms provide one promising avenue of this type, with histone deacetylase and DNA methyltransferase inhibitors (Huber et al., 2007; Beyer et al., 2012). Likewise, kinase inhibitors such as imatinib have been considered, but recent clinical trials showed conflicting results (Khanna et al., 2011; Spiera et al., 2011; Prey et al., 2012). Rho kinase inhibitors have also been used to reduce fibrosis (Zhou et al., 2013). Our compounds, however, are more effective in reducing MRTF/SRF-regulated gene transcriptional signaling than is the ROCK inhibitor Y-27632 (Evelyn et al., 2007).
Fig. 6.
Schematic model of multiple profibrotic stimuli that all use the MRTF/SRF-regulated gene transcription mechanism. Several mechanisms being targeted in fibrosis in SSc and other primary fibrotic diseases are illustrated. All appear to activate Rho GTPase and the downstream MRTF/SRF gene transcription mechanism. By blocking MRTF nuclear localization, our compound CCG-203971 may prove more effective than disrupting each individual profibrotic input.
Here, we propose that the Rho/MTRF/SRF transcription mechanism may represent an important downstream target for antifibrotic therapy. Our results with CCG-203971 in vitro and in vivo are promising. The strong effect to inhibit CTGF expression and collagen synthesis and the reduction in myofibroblast differentiation induced by both TGF_β_ and LPA suggests that we are engaging a key step in the overall fibrosis gene program. Since this family of compounds blocks nuclear localization of MRTF-A and SRF-regulated gene expression regardless of the activating stimulus (Evelyn et al., 2007), it should have actions against many profibrotic ligands. CCG-203971 was tolerated at the relatively high doses used in our study. The potency of this compound in vitro is only modest (IC50 1–3 _μ_M for most effects) but compared with the only approved antifibrotic drug, PFD, it is nearly 100 times more potent (see Fig. 4C). Unlike PFD, the direct molecular target of CCG-1423, to which CCG-203971 is related, was recently identified (Lundquist et al., 2014). This should facilitate the development of new, more potent analogs.
RNAi-mediated MRTF knockdown produces strong antifibrotic effects in several models (Sandbo et al., 2009; Small et al., 2010; Small, 2012; Zhou et al., 2013), which provides important target validation for this pathway. Perhaps the greatest limitation currently is the very short in vivo half-life of CCG-203971 (_T_1/2 ∼25 minutes after intraperitoneal administration, data not shown). Improvements to its pharmacokinetic properties will clearly facilitate clinical translation.
The Rho/MRTF/SRF-regulated transcription pathway plays a critical role in fibrosis by switching on the myofibroblast state. Many different fibrosis signals that are targets of current therapeutic development feed into this pathway (Fig. 6). This mechanism is spontaneously active in human dermal fibroblasts from patients with diffuse SSc. We show that a novel small molecule inhibitor of MRTF/SRF-regulated gene transcription, CCG-203971, reverses myofibroblast activation and collagen synthesis by human SSc dermal fibroblasts and by LPA- and TGF_β_-stimulated fibroblasts. It also prevents skin thickening and collagen deposition in a bleomycin skin injury model. As a consequence, MRTF/SRF transcription pathway inhibitors may represent an efficacious new approach to SSc and other diseases of fibrosis.
Supplementary Material
Data Supplement
Acknowledgments
The authors thank Raelene Van Noord for assistance with the animal studies.
Abbreviations
COL1A2
collagen 1
CTGF
connective tissue growth factor
CCG-1423
2-({[3,5-bis(trifluoromethyl)phenyl]formamido}oxy)-_N_-(4-chlorophenyl)propanamide
CCG-203971
-_N_-(4-chlorophenyl)-1-{[3-{furan-2-yl)phenyl]carbonyl}piperidine-3-carboxamide
DMEM
Dulbecco’s modified Eagle’s medium
DMSO
dimethyl sulfoxide
FBS
fetal bovine serum
LPA
lysophosphatidic acid
MRTF
myocardin-related transcription factor
PBS
phosphate-buffered saline
PFD
pirfenidone
ROCK
Rho kinase
_α_-SMA
_α_-smooth muscle actin
SRF
serum response factor
SSc
systemic sclerosis
TGF_β_
transforming growth factor β
Y-27632
4-[(1_R_)-1-aminoethyl]-_N_-(pyridin-4-yl)cyclohexane-1-carboxamide
Authorship Contributions
Participated in research design: Haak, Tsou, Amin, Ruth, Campbell, Fox, Khanna, Larsen, Neubig.
Conducted experiments: Haak, Tsou, Amin, Ruth, Campbell.
Contributed new reagents or analytical tools: Larsen.
Performed data analysis: Haak, Tsou.
Wrote or contributed to the writing of the manuscript: Haak, Tsou, Fox, Khanna, Neubig.
Footnotes
This work was supported in part by a generous gift from Jon and Lisa Rye; National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases [Grant K24 AR063120-02] (to D.K.); and the Arthritis Foundation (to P.-S.T.).
References
- Akhmetshina A, Dees C, Pileckyte M, Szucs G, Spriewald BM, Zwerina J, Distler O, Schett G, Distler JH. (2008) Rho-associated kinases are crucial for myofibroblast differentiation and production of extracellular matrix in scleroderma fibroblasts. Arthritis Rheum 58:2553–2564 [DOI] [PubMed] [Google Scholar]
- Badri L, Lama VN. (2012) Lysophosphatidic acid induces migration of human lung-resident mesenchymal stem cells through the β-catenin pathway. Stem Cells 30:2010–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JL, Haak AJ, Wade SM, Kirchhoff PD, Neubig RR, Larsen SD. (2013) Optimization of novel nipecotic bis(amide) inhibitors of the Rho/MKL1/SRF transcriptional pathway as potential anti-metastasis agents. Bioorg Med Chem Lett 23:3826–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer C, Schett G, Distler O, Distler JH. (2010) Animal models of systemic sclerosis: prospects and limitations. Arthritis Rheum 62:2831–2844 [DOI] [PubMed] [Google Scholar]
- Beyer C, Distler O, Distler JH. (2012) Innovative antifibrotic therapies in systemic sclerosis. Curr Opin Rheumatol 24:274–280 [DOI] [PubMed] [Google Scholar]
- Boukhalfa G, Desmoulière A, Rondeau E, Gabbiani G, Sraer JD. (1996) Relationship between alpha-smooth muscle actin expression and fibrotic changes in human kidney. Exp Nephrol 4:241–247 [PubMed] [Google Scholar]
- Buhl AM, Johnson NL, Dhanasekaran N, Johnson GL. (1995) G alpha 12 and G alpha 13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J Biol Chem 270:24631–24634 [DOI] [PubMed] [Google Scholar]
- Chaqour B, Goppelt-Struebe M. (2006) Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins. FEBS J 273:3639–3649 [DOI] [PubMed] [Google Scholar]
- Charles C, Clements P, Furst DE. (2006) Systemic sclerosis: hypothesis-driven treatment strategies. Lancet 367:1683–1691 [DOI] [PubMed] [Google Scholar]
- Crider BJ, Risinger GM, Jr, Haaksma CJ, Howard EW, Tomasek JJ. (2011) Myocardin-related transcription factors A and B are key regulators of TGF-β1-induced fibroblast to myofibroblast differentiation. J Invest Dermatol 131:2378–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton CP, Merkel PA, Furst DE, Khanna D, Emery P, Hsu VM, Silliman N, Streisand J, Powell J, Akesson A, et al. Cat-192 Study Group. Scleroderma Clinical Trials Consortium (2007) Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum 56:323–333 [DOI] [PubMed] [Google Scholar]
- Evelyn CR, Wade SM, Wang Q, Wu M, Iñiguez-Lluhí JA, Merajver SD, Neubig RR. (2007) CCG-1423: a small-molecule inhibitor of RhoA transcriptional signaling. Mol Cancer Ther 6:2249–2260 [DOI] [PubMed] [Google Scholar]
- Evelyn CR, Bell JL, Ryu JG, Wade SM, Kocab A, Harzdorf NL, Hollis Showalter HD, Neubig RR, Larsen SD. (2010) Design, synthesis and prostate cancer cell-based studies of analogs of the Rho/MKL1 transcriptional pathway inhibitor, CCG-1423. Bioorg Med Chem Lett 20:665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbane AJ, Denton CP, Holmes AM. (2013) Scleroderma pathogenesis: a pivotal role for fibroblasts as effector cells. Arthritis Res Ther 15:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiducci S, Distler JH, Milia AF, Miniati I, Rogai V, Manetti M, Falcini F, Ibba-Manneschi L, Gay S, Distler O, et al. (2009) Stiff skin syndrome: evidence for an inflammation-independent fibrosis? Rheumatology (Oxford) 48:849–852 [DOI] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, De Wever O, Mareel M, Gabbiani G. (2012) Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol 180:1340–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Phan SH. (2013) Myofibroblasts. Curr Opin Rheumatol 25:71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. (2012) Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol 47:340–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber LC, Distler JH, Moritz F, Hemmatazad H, Hauser T, Michel BA, Gay RE, Matucci-Cerinic M, Gay S, Distler O, et al. (2007) Trichostatin A prevents the accumulation of extracellular matrix in a mouse model of bleomycin-induced skin fibrosis. Arthritis Rheum 56:2755–2764 [DOI] [PubMed] [Google Scholar]
- Khanna D, Saggar R, Mayes MD, Abtin F, Clements PJ, Maranian P, Assassi S, Saggar R, Singh RR, Furst DE. (2011) A one-year, phase I/IIa, open-label pilot trial of imatinib mesylate in the treatment of systemic sclerosis-associated active interstitial lung disease. Arthritis Rheum 63:3540–3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranenburg O, Poland M, van Horck FP, Drechsel D, Hall A, Moolenaar WH. (1999) Activation of RhoA by lysophosphatidic acid and Galpha12/13 subunits in neuronal cells: induction of neurite retraction. Mol Biol Cell 10:1851–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A. (2012) Emerging targets for the treatment of scleroderma. Expert Opin Emerg Drugs 17:173–179 [DOI] [PubMed] [Google Scholar]
- LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr, Rowell N, Wollheim F. (1988) Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 15:202–205 [PubMed] [Google Scholar]
- Liu S, Parapuram SK, and Leask A (2013) Fibrosis caused by loss of PTEN expression by fibroblasts is crucially dependent on CCN2. Arthritis Rheum 65:2940–2944. [DOI] [PubMed]
- Luchsinger LL, Patenaude CA, Smith BD, Layne MD. (2011) Myocardin-related transcription factor-A complexes activate type 1 collagen expression in lung fibroblasts. J Biol Chem 286:44116–44125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist MR, Storaska AJ, Liu TC, Larsen SD, Evans T, Neubig RR, and Jaffrey SR (2014) Redox modification of nuclear actin by MICAL-2 regulates SRF signaling. Cell 156:563–576. [DOI] [PMC free article] [PubMed]
- Masszi A, Di Ciano C, Sirokmány G, Arthur WT, Rotstein OD, Wang J, McCulloch CA, Rosivall L, Mucsi I, Kapus A. (2003) Central role for Rho in TGF-beta1-induced alpha-smooth muscle actin expression during epithelial-mesenchymal transition. Am J Physiol Renal Physiol 284:F911–F924 [DOI] [PubMed] [Google Scholar]
- Prey S, Ezzedine K, Doussau A, Grandoulier AS, Barcat D, Chatelus E, Diot E, Durant C, Hachulla E, de Korwin-Krokowski JD, et al. (2012) Imatinib mesylate in scleroderma-associated diffuse skin fibrosis: a phase II multicentre randomized double-blinded controlled trial. Br J Dermatol 167:1138–1144 [DOI] [PubMed] [Google Scholar]
- Sakai N, Chun J, Duffield JS, Wada T, Luster AD, Tager AM. (2013) LPA1-induced cytoskeleton reorganization drives fibrosis through CTGF-dependent fibroblast proliferation. FASEB J 27:1830–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbo N, Kregel S, Taurin S, Bhorade S, Dulin NO. (2009) Critical role of serum response factor in pulmonary myofibroblast differentiation induced by TGF-beta. Am J Respir Cell Mol Biol 41:332–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbo N, Lau A, Kach J, Ngam C, Yau D, Dulin NO. (2011) Delayed stress fiber formation mediates pulmonary myofibroblast differentiation in response to TGF-β. Am J Physiol Lung Cell Mol Physiol 301:L656–L666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappino AP, Masouyé I, Saurat JH, Gabbiani G. (1990) Smooth muscle differentiation in scleroderma fibroblastic cells. Am J Pathol 137:585–591 [PMC free article] [PubMed] [Google Scholar]
- Seasholtz TM, Majumdar M, Brown JH. (1999) Rho as a mediator of G protein-coupled receptor signaling. Mol Pharmacol 55:949–956 [DOI] [PubMed] [Google Scholar]
- Serratì S, Chillà A, Laurenzana A, Margheri F, Giannoni E, Magnelli L, Chiarugi P, Dotor J, Feijoo E, Bazzichi L, et al. (2013) Systemic sclerosis endothelial cells recruit and activate dermal fibroblasts by induction of a connective tissue growth factor (CCN2)/transforming growth factor β-dependent mesenchymal-to-mesenchymal transition. Arthritis Rheum 65:258–269 [DOI] [PubMed] [Google Scholar]
- Small EM, Thatcher JE, Sutherland LB, Kinoshita H, Gerard RD, Richardson JA, Dimaio JM, Sadek H, Kuwahara K, Olson EN. (2010) Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ Res 107:294–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small EM. (2012) The actin-MRTF-SRF gene regulatory axis and myofibroblast differentiation. J Cardiovasc Transl Res 5:794–804 [DOI] [PubMed] [Google Scholar]
- Spiera RF, Gordon JK, Mersten JN, Magro CM, Mehta M, Wildman HF, Kloiber S, Kirou KA, Lyman S, Crow MK. (2011) Imatinib mesylate (Gleevec) in the treatment of diffuse cutaneous systemic sclerosis: results of a 1-year, phase IIa, single-arm, open-label clinical trial. Ann Rheum Dis 70:1003–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3:349–363 [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Haaksma CJ, McRae JK, Risinger GM, Howard EW. (2008) Mechanoregulated expression of smooth muscle alpha-actin in myofibroblasts is mediated by MRTF-A localization and actin dynamics. FASEB J 22 (Suppl):S21.7 [Google Scholar]
- Varga J, Abraham D. (2007) Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest 117:557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Ramalingam TR. (2012) Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18:1028–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Gharaee-Kermani M, Zhang K, Karmiol S, Phan SH. (1996) Lung fibroblast alpha-smooth muscle actin expression and contractile phenotype in bleomycin-induced pulmonary fibrosis. Am J Pathol 148:527–537 [PMC free article] [PubMed] [Google Scholar]
- Zhao XH, Laschinger C, Arora P, Szászi K, Kapus A, McCulloch CA. (2007) Force activates smooth muscle alpha-actin promoter activity through the Rho signaling pathway. J Cell Sci 120:1801–1809 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ. (2013) Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest 123:1096–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Supplement