Relationship of Hearing loss and Dementia: a Prospective, Population-based Study (original) (raw)
. Author manuscript; available in PMC: 2015 Jun 1.
Abstract
Objective
To determine whether baseline hearing loss increases cognitive decline and risk for all-cause dementia in a population of elderly individuals.
Study design
Longitudinal cohort study
Setting
Community-based, outpatient
Patients
Men and women aged 65 years or older without dementia at baseline
Intervention(s)
All subjects completed the Modified Mini-Mental Status Exam (3MS-R) at baseline and over 3 triennial follow-up visits. Hearing loss (HL) at baseline was based on observation of hearing difficulties during testing or interview. Incident dementia was determined by clinical assessment and expert consensus.
Main outcome measure(s)
Dementia and 3MS-R score.
Results
At baseline 4,463 subjects were without dementia, 836 of whom had HL. Of those with HL, 16.3% developed dementia, compared to 12.1% of those without HL (p<0.001). Mean time to dementia was 10.3 years in the HL group vs. 11.9 years for non-HL (Log Rank test p<0.001). In Cox regression analyses controlling for gender, presence of APOE- ε4 allele, education, and baseline age, and cardiovascular risk factors, HL was an independent predictor of developing dementia (Hazard ratio = 1.27, p=0.026 (95% CI = 1.03, 1.56). Linear mixed models controlling for similar covariates showed HL was associated with faster decline on the 3MS-R, at a rate of 0.26 points/year worse than those without HL.
Conclusions
Elderly individuals with HL have an increased rate of developing dementia and more rapid decline on 3MS-R scores than their non-hearing impaired counterparts. These findings suggest that hearing impairment may be a marker for cognitive dysfunction in adults age 65 and older.
Introduction
Cognitive impairment and hearing loss (HL) are two distinct neurologic conditions that are associated with aging. Cognitive impairment generically refers to a spectrum of conditions ranging from mild cognitive impairment to full dementia. It has been estimated that in 2010, there were 4.7 million individuals aged 65 years or older with Alzheimer disease (AD) dementia.(1) Using 2010 United States (US) census data, it is projected that this number will increase to 13.8 million by the year 2050.(1)
As the US and global populations age, the incidence of age-related hearing loss (ARHL) is also rising. The prevalence of significant hearing loss is 40–60% for those over age 60.(2) According to the National Health and Nutritional Examination Survey (NHANES), more than 80% of persons older than 85 years have ARHL.(3) In addition to the loss of peripheral (cochlear) hearing function with age, there is also a decrease of central auditory function, or the way the brain processes auditory information, in aged individuals.(4) It has been shown that there is likely overlap between peripheral auditory, central auditory, and cognitive function.(2,5) Decline in one of these domains could potentially influence the others.
Is there a relationship between HL and cognitive function or dementia? Could functional loss in sensorineural hearing be a harbinger of loss in another domain such as cognition? In multiple epidemiological studies, ARHL has been shown to be independently associated with decreased cognitive function and increased incident dementia.(6-12)
This study evaluates the relationship between dementia and HL based on data from the Cache County Study, a prospective cohort of individuals age 65 years and older who were followed for the development of dementia. We also compare HL to other known risk factors of dementia including presence of the APOE- ε4 allele, education, gender, and age.
Materials and Methods
Study population and Cognitive Assessments
The Cache County Study on Memory, Health, and Aging is a longitudinal inquiry into the prevalence and incidence of dementia in relation to genetic and environmental risk factors that has been conducted on residents of Cache County, Utah.(13) Beginning enrollment in 1995, the study has followed residents of the county age 65 years or older.
Institutional review boards of Utah State University, Duke University and the Johns Hopkins University approved this study and written informed consent was obtained at each interview with participants. All permanent residents of Cache County, UT, age 65 years and older on January 1, 1995 were recruited to this study. A total of 5,092 individuals (90% of eligible seniors in the population) were enrolled and evaluated for cognitive status using a multi-stage dementia ascertainment protocol described previously.(14) A subsample of 359 cases of prevalent dementia and another 188 with incomplete cognitive evaluations were removed from the sample.
Dementia ascertainment
To determine dementia status, we relied on a multi-stage dementia screening and assessment protocol completed in three triennial waves. The first stage of screening consisted of administration of the Modified Mini-Mental State Exam-Revised (3MS-R), a 100-point adaptation of the Mini-Mental State Examination that extends both the floor and ceiling of the instrument.(15,16). Screen positive individuals and a randomly selected 19% designated subsample were invited to complete subsequent stages of evaluation consisting of an informant interview and the next stage, a clinical assessment including neuropsychological testing. The clinical assessment results were reviewed by a geropsychiatrist and neuropsychologist and preliminary diagnoses of dementia or other cognitive disorders were assigned. Those carrying a diagnosis of dementia or its prodrome were invited to complete standard laboratory tests for dementia, an MRI scan, and a geropsychiatrist examination. Final dementia status was determined by an expert panel of clinicians including study geropsychiatrists, neuropsychologists, a neurologist and cognitive neuroscientist. Diagnoses of dementia were based on DSM-III-R criteria as described previously(14) and specific dementia illnesses according to standard criteria. For example, diagnosis of AD followed National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria.(17) Study subjects were identified as those who were diagnosed with no dementia (per clinical assessment) or whose results were negative at each preceding screening stage. Persons with incomplete screening results (i.e., those who were screen positive at one stage, but did not complete the subsequent stage) were excluded from the analyses. All cause dementia is inclusive of AD, Parkinison's dementia, vascular dementia, fronto-temporal dementia, and other less common forms of dementia. The majority of participants in the Cache County Cohort have AD.(14)
Cognitive and functional assessments were made via multiple sources of data, not all of which required auditory participation from the subject.(14) For example, a knowledgeable informant completed a semi-structured interview of the cognitive and functional status of the subject. This minimized diagnostic errors of labeling subjects with cognitive impairment when their poor testing performance was actually due to hearing impairment.
Cognitive assessments
Cognition was assessed using the 3MS-R. However, as reported previously (14), total scores were adjusted in which performance on items confounded by sensory or motor problems were discarded from the total possible points. The total points earned on remaining items were summed and rescaled to a 100-point scale.
Hearing assessments
As part of the 3MS-R administration protocol during an in-person, in-home assessment, when subjects appeared to experience difficulty with any testing item and the examiner detected the presence of any sensory or motor impairment responsible for the subject having missed an item, or if the subjects reported hearing loss, such impairments were noted and coded as: auditory, visual, manual (sensory) or “other.” Interviewers were trained to recognize difficulties in comprehension due to hearing loss and often-repeated questions, speaking more slowly, and articulating more clearly. Interviewers were equipped with an audio amplifier that was used for subjects with any perceived hearing difficulty. Subjects who required an audio amplifier during testing were noted as having hearing loss. Subjects with at least one testing item coded with auditory impairment were then coded in a dichotomous indicator as hearing loss (HL) present or absent. If hearing aids were noted, subjects were marked as having hearing loss, though hearing aid use data were not routinely recorded. Hearing loss was also noted during cognitive testing when there was any indication that hearing problems had affected performance on an item. In other studies, subjective hearing assessments have been shown to be valid and reliable when compared to standard audiometry. (18-23)
Other Covariates
Information on educational level, age, and gender was also collected. Approximately 97% (4,962) of participants provided buccal DNA for APOE-ε4 genotyping, which has been associated with AD.(24) For analytic purposes, we collapsed across genotypes to dichotomize into those with or without one or more ε4 alleles (i.e. ε4 positive or negative).
We included cardiovascular risk factors – diabetes, smoking, high cholesterol, and hypertension - in our multivariate analysis since these risk factors have been associated with hearing loss, dementia, or both.(25-29) The cardiovascular risk assessment was done during the first visit of wave one screening. The methods by which these risk factors were identified in the Cache County Cohort Study have been previously described.(27,28) Briefly, vascular risk factors were determined by a combination of self-reported medical history and questionnaires specifically identifying the diagnoses of diabetes, hypertension, and high cholesterol. Smoking assessment included self-report of ever using at least 100 cigarettes or weekly use of pipes or cigars. Subjects were excluded from multivariate analysis if cardiovascular risk factor data were missing (n=20 for diabetes, 24 for smoking, 53 for cholesterol, and 26 for hypertension).
Statistical Analysis
In exploratory analyses, we examined differences in subject characteristics for those with HL vs. those without HL, using chi-square (χ2) tests for independence for categorical variables and t-tests for means of continuous variables. We used traditional survival analyses to examine whether baseline HL increased the hazard for all-cause dementia. Similar to other population cohort studies, the time on study from baseline to dementia or censoring (loss of follow-up due to refusal, death, or end of observation) was calculated in years.(30) Cox regression analyses were conducted, including the covariates listed above. Exploratory analyses were completed with Kaplan-Meier plots to check the assumption of proportional hazards modeling.
To examine whether HL affected the rate of cognitive decline, we ran linear mixed effects models including a random effect for intercept and time. This approach allowed us to account for the dependence between within-subject repeated measurements and for nonlinear change with respect to time by testing for time-squared effects. To examine the association between predictor variables and change in 3MS-R, we built upon a base mixed model by adding each predictor followed by its interaction with time. A predictor was retained in the model if the individual term had an associated Wald statistic with p < 0.05 or if the likelihood ratio (LR) χ2 test of nested models including vs. excluding the terms yielding a p < 0.05. Education level was kept in the models because it is a known risk factor for dementia.(31) All statistical analyses were completed with SPSS version 21 (IBM, Armonk, NY, USA).
Results
There were 4,545 subjects eligible for this study without baseline dementia (Table 1). Initial screening data were missing for 82 participants. Of the 4,463 participants evaluated, 836 had baseline HL. The mean age of participants was 75.4 years old (range 65.3-102.4 year old, SD 6.85 years). Of those with HL, 16.3% developed all-cause dementia, compared to 12.1% of those without HL (p<0.001). Mean time to all-cause dementia was 10.3 years in the HL group vs. 11.9 years for non-HL (p<0.0001). For patients with HL, 9.9% developed AD compared to 8.0% without HL with a mean time to developing AD at 11.4 years for HL participants and 12.6 years for those without HL, but this difference did not reach statistical significance. In Cox regression analyses of our entire study population, controlling for gender, presence of APOE-ε4 allele, education, and baseline age, HL was an independent predictor of developing dementia, hazard ratio = 1.28, p<0.018 (95% CI = 1.04-1.57) (Figure 1). A multivariate analysis including all four cardiovascular risk factors showed that hearing loss remained an independent risk factor with a hazard ratio of 1.27, p=0.027 (95% CI = 1.03, 1.56). A multivariate analysis including only the two risk factors that were statistically more prevalent among the HL cohort – diabetes and smoking – showed that hearing loss remained an independent risk factor with a hazard ratio of 1.27, p=0.026 (95% CI = 1.03, 1.56).
Table 1. Demographics of the Study Population.
This table shows pertinent demographic variables of the study population. HL = hearing loss; 3MS-R = Modified Mini-Mental State (3MS-R) examination; SD = standard deviation
| Have baseline HL | No baseline HL | Significance | |
|---|---|---|---|
| N = 836 | N=3,627 | N = 4,463 | |
| Categorical Measures | N (%) | N (%) | χ2 test for independence |
| Female gender | 373 (44.6) | 2,170 (59.8) | p<0.001 |
| APOE-ε4 carrier | 213 (25.9) | 1,127 (31.4) | p=0.002 |
| All-cause dementia | 136 (16.3) | 438 (12.1) | p=0.001 |
| Diabetes | 146 (17.5) | 437 (12.1) | p<0.001 |
| Smoking History | 198 (23.7) | 685 (19.0) | p=0.002 |
| High Cholesterol | 215 (26.1) | 1268 (35.4) | p<0.001 |
| Hypertension | 403 (48.4) | 1620 (44.9) | p=0.07 |
| Continuous Measures | Mean(SD) | Mean(SD) | T-test for mean difference |
| Baseline age | 79.45 (7.5) | 74.53 (6.3) | p<0.001 |
| Education, years | 12.83 (3.0) | 13.35 (2.8) | p<0.001 |
| Baseline 3MS-R | 87.59 (7.9) | 91.50 (6.6) | p<0.001 |
| # of visits (1-4) | 2.22 (1.1) | 2.71 (1.1) | p<0.001 |
| Yrs. of Follow-up | 4.32 (4.1) | 6.08 (4.1) | p<0.001 |
Figure 1.
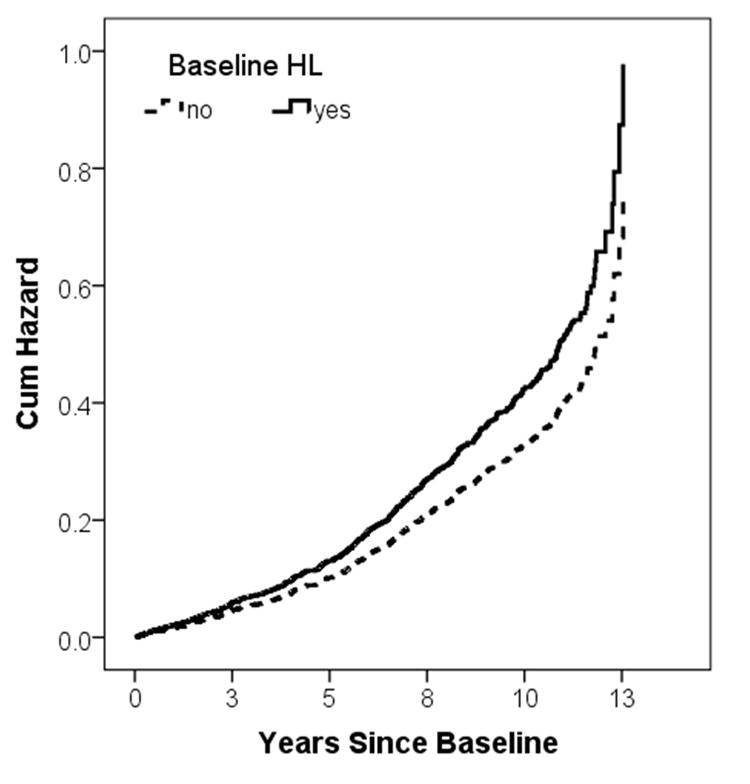
A hazard plot for all-cause dementia based on hearing loss controlled for the four main covariables: gender, presence of APOE-ε4 allele, education, and baseline age. The cumulative hazard is shown on the y-axis with years since baseline assessment on the x-axis. The broken line represents those subjects without baseline hearing loss (HL) and the solid line represents those with HL. Cum Hazard = cumulative hazard, HL = hearing loss
There were 303 of the 4,463 (6.8%) subjects with initial 3MS-R <80, suggestive of mild cognitive impairment (Table 2). Baseline hearing loss was noted in 120 (39.6%) of these vs. 18.7% in the full sample of 4463 (p<0.001). Despite the lower 3MS-R scores, none of these subjects were determined to have dementia at the time of enrollment. Only 64 (1.4%) qualified by comprehensive assessment as having cognitive impairment, 32 of whom went on to develop dementia. Another 40 were determined to be cognitively normal at baseline, but went on to develop dementia at a later date. Compared to subjects with 3MS-R ≥80, those with 3MS-R <80 were more likely to have baseline hearing loss (39.6% vs. 17.2% [p<0.001]) and more likely to develop dementia (25.2% vs. 11.9% [p<0.001]).
We evaluated the subgroup of patients with a 3MS-R score ≥80 (i.e. those with the highest baseline cognitive testing), and found that hearing loss was associated with incident dementia in this subgroup (p<0.001, see Figure 2). When considered with other risk factors such as baseline age, female gender, APOE-ε4, and education level in a Cox regression analysis, however, hearing loss was not statistically significant as an independent risk factor for developing dementia (p=0.09).
Figure 2.
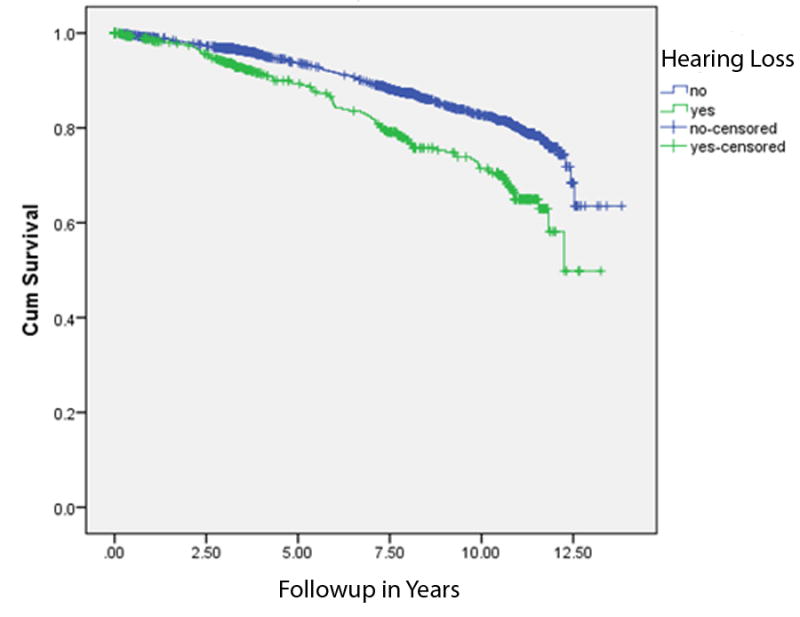
This Kaplan-Meier plot shows time to dementia in years of follow up (“cumulative survival”) based on baseline hearing status. Those with hearing loss depicted on the lower line had a higher rate of incident dementia (p<0.001).
In linear mixed models examining 3MS-R score over time, we observed a significant non-linear, quadratic time effect. Inspection of the plot revealed a slight curvilinear change in 3MS-R score over time that did not appear to differ appreciably by HL group. We ran analyses two ways, modeling time linearly in one model and modeling time with an additional quadratic term in a second model. As the results were consistent, we report the results of the simpler linear time model to facilitate interpretation (Figure 3). The linear mixed model controlled for age, gender, education and presence of APOE-ε4 allele. At baseline, mean 3MS-R for those with hearing loss is 1.27 points lower than those without hearing loss (fixed effect of hearing loss [HL]). Over time, those who had hearing loss at baseline decline 54% faster, an average of 0.71 points/year, as opposed to 0.46 points/year among those without (time effect of HL).
Figure 3.
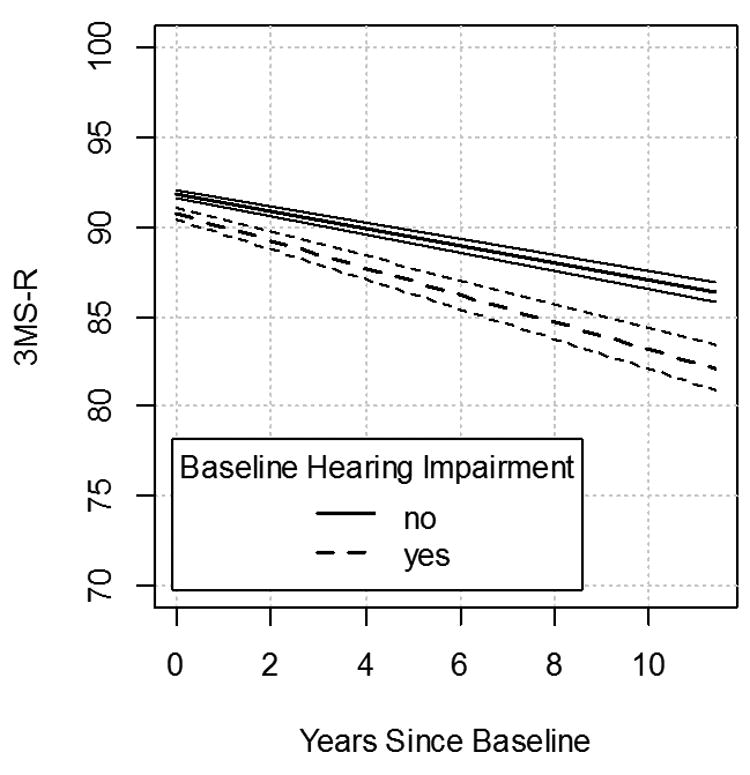
This graph shows a linear mixed effects model controlling for age, gender, education and presence of APOE-ε4 allele. At baseline, mean 3MS-R for those with hearing loss is 1.27 points lower than those without hearing loss (fixed effect of hearing loss [HL]). Over time, those who had hearing loss at baseline decline 54% faster, an average of 0.71 points/year, as opposed to 0.46 points/year among those without (time effect of HL). The parallel lines above the broken and solid line represent 95% confidence interval bars, which are non-overlapping. 3MS-R = Modified Mini-Mental State (3MS-R) examination.
Discussion
In this study, we have shown that participants with baseline HL develop dementia at a higher incidence, displaying a faster rate of developing dementia, than those without HL. While the baseline demographics of the study populations were different (Table 1), these differences were statistically accounted for in our regression analysis and hearing loss was found to be an independent variable associated with dementia (Figure 1). One of the differences highlighted in the Table 1 is follow-up time. Those participants with HL had a mean follow up of 4.32 years while those with no HL followed up for 6.08 years. Some of this difference may be accounted for by the fact that patients were not followed after dementia occurred. Since those with HL developed dementia more rapidly, their follow-up was shorter.
Similar findings correlating hearing loss and dementia have been reported in other population-based epidemiologic studies. From the Baltimore Longitudinal Study of Aging, Lin et al. reported that the risk of incident all-cause dementia increased log linearly with the severity of baseline hearing loss (1.27 per 10 dB loss, 95% CI: 1.06 – 1.50) and that hearing loss is independently associated with lower scores on tests of memory and executive function.(6,7) Data derived from the NHANES population-based study showed that greater HL was significantly associated with lower scores on the Digit Symbol Substitution Test (DSST), a nonverbal test assessing executive function and psychomotor processing (DSST score difference of −1.5 [95% confidence interval: −2.9 to −0.23] per 10 dB of hearing loss).(3) Gallacher et al. reported that in older men followed over a 17-year period in the Caerphilly cohort, worsening auditory threshold was associated with incident dementia and cognitive decline. An additional effect of change in auditory threshold over 8 years was found for nonvascular dementia.(10) An Australian study on aging also showed that cognitive impairment can hasten the degree of sensorineural hearing loss.(11)
In the study population, those with HL had a faster rate of cognitive decline than those without HL at a rate of 0.26 3MS-R points/year worse than those without HL, although this result was confounded with age. Unfortunately, it is not possible to distinguish the effects of aging vs. HL on 3MS-R change over time in this sample due to the correlated nature of HL, age and declines in 3MS-R scores associated with both factors. Further studies are required to evaluate the effect of hearing loss on rate of decline on the 3MS-R.
It should be noted that by design, the Cache County Study followed participants only up to the point of dementia. Thus, individuals who developed dementia in any of the incident waves were not retested at subsequent time points, hence their test scores only contributed up to the wave in which dementia was detected. It is not clear if the results would differ had these subjects received continued follow-ups with the 3MS-R.
Accelerated rate of cognitive decline with hearing loss has been reported by the Health, Aging, and Body Composition Study. Lin et al. found that individuals having HL demonstrating a 30% to 40% accelerated rate of cognitive decline and a 24% increased risk for incident cognitive impairment during a 6-year period compared with individuals having normal hearing.(9) In models adjusting only for main effects of demographics, this study also found that individuals with HL would require 7.7 years to decline by 5 points on the 3MS-R vs. 10.9 years in individuals with normal hearing.
Hearing loss in patients with cognitive decline may be peripheral (cochlear), as is typically seen in presbycusis, but may also be the result of central auditory processing dysfunction or how the brain processes auditory signals. Central auditory testing assessing complex hearing tasks can evaluate central auditory processing. Examples include understanding competing speech constructs or hearing in noise since filtering background noise is thought to be done by central processing.(32) Central auditory processing appears to be affected in dementing disease. It has been shown that people with early Alzheimer's disease fail these tests at the 80% correct level.(33) Gates et al. also reported that central auditory dysfunction is found in patients with mild memory impairment and is a precursor to AD.(34-36) Gates has suggested that all senior citizens with hearing difficulty, specifically with hearing in noise, should be evaluated for central auditory dysfunction.(35)
One of the major limitations of this study is the lack of objective hearing assessment by standard audiometry. As this cohort of patients was enrolled into an epidemiologic study not primarily focused on hearing, only qualitative measurements of hearing were obtained. The measures used, however, were consistently applied across the population of study participants. Moreover, subjective screening tools and reports from patients, family, or caregivers can provide an accurate assessment of hearing status.(37) Qualitative assessments of hearing have been validated for accuracy against the standard of formal audiometry.(38) In multiple studies, the single question, ‘Do you feel you have hearing loss?’ has been shown to have high sensitivity and specificity for hearing impairment when using pure tone audiometry as the standard reference.(18-23) Nondahl et al. showed that hearing loss estimates from subjective assessments were within 3.2 percent of measures derived audiometrically.(18) While not as optimal a formal audiometry, the hearing assessments as reported in this study were not made arbitrarily. The dichotomous hearing designation came from patient-reported and interviewers assessment of hearing status, which are both reliable methods for determining hearing loss.
A qualitative assessment of hearing could arguably be influenced by a study participant's cognition. For example, individuals with MCI will have more difficulty hearing in background noise or with unfamiliar speakers because of impaired central auditory processing rather than peripheral hearing loss. This issue was addressed in part, however, by the fact that only 6.3% of subjects had a 3MS score <80 at the time of enrollment, none of whom met the diagnosis of dementia. Moreover, only 64 of those 303 (1.4% of total study participants) qualified as having mild cognitive impairment at baseline using the full battery of initial diagnostic testing. While this subset of patients did have a higher degree of hearing loss and incident dementia, when evaluated with multivariate analysis, hearing loss did not reach statistical significance (p=0.09) as an independent risk factor for their incident dementia. This finding is likely due to a lack of statistical power in the relatively smaller subgroup analysis and because those participants who were excluded were older and had higher baseline rates of both HL and incident dementia. This finding could potentially confound the result that HL is an independent risk factor because those with lower 3MS-R scores may have been incorrectly labeled with HL due to cognitive factors (i.e. not responding appropriately) rather than true auditory dysfunction. This group did not, however, meet criteria for dementia and the hearing assessment should have remained reliable in their responses. Despite the lack of statistical significance in this subgroup analysis, the relationship of HL as an independent risk factor remained robust with hazard ration changing from 1.28 in the whole population to 1.22 in this subpopulation.
Another limitation of this and other epidemiological studies is that while our results show a compelling association between HL and dementia, the mechanistic nature of that association has yet to be elucidated. We have yet to determine whether these findings reveal an underlying neurobiological causation as opposed to a qualitative correlation. For example, is sensorineural HL an early biologic marker for more extensive future central neurologic dysfunction or rather, do patients with HL become socially withdrawn, lead less enriching lives, and therefore decline more rapidly in their cognitive ability?
There are several lines of evidence that suggest HL may reflect neuronal failure predisposing to dementing illness. Studies have shown a genetic and molecular association between certain types of dementia and HL such as hereditary sensory autonomic neuropathy with dementia and hearing loss (HSAN1E).(39,40) Similar molecular mechanisms may underlie other forms of associated dementia and HL. Alzheimer's disease is the most common cause of late life dementia in the developed countries and in the Cache County Study.(1,14) We therefore expect the observations in our study are driven primarily from Alzheimer's disease rather than less common causes of dementia.
Sinha et al. reported a highly topographically specific and consistent pattern of degeneration in the auditory system of AD patients.(41) Senile plaques and neurofibrillary tangles, histologic hallmarks of AD, were distributed throughout the central auditory pathways in the ventral nucleus of the medial geniculate body and the central nucleus of the inferior colliculus. It is also possible that the effects of hearing loss represent a less specific risk factor increasing the risk of clinical expression of all dementing diseases through a mechanism of reducing cognitive reserve. In this scenario, hearing loss would be an independent co-morbidity functioning similar to stroke, delirium and head injury to increase the expression of dementing disease without altering neuropathology. Without systematic neuropathological data, this hypothesis can't be tested in our population.
An alternative hypothesis to the neurobiological etiology of HL and dementia is that those with ARHL typically have a poorer quality of life and often become more socially withdrawn.(42-45) As a result, their cognitive function may suffer as an indirect result of their HL. There is also the hypothesis that implicates HL to detrimentally impact cognitive load required to accomplish a particular cognitive task: performance is degraded because greater effort is needed in a person with HL. It has been shown that under conditions where auditory perception is difficult (i.e., hearing loss), greater cognitive resources are dedicated to auditory perceptual processing to the detriment of other cognitive processes such as working memory.(6,46-48)
The increased cognitive load, social isolation, and neurobiology of HL may not be mutually exclusive as each may contribute in some way to cognitive impairment.(49) Future research to investigate the relationship and underlying neurobiology of HL and dementia are needed. A fundamental reason to pursue such research would be to know whether intervention via aural rehabilitation could alter the course of cognitive decline or whether detection of cognitive impairment via hearing assessments could lead to early intervention with improved outcomes for those affected by cognitive impairment.
This study has implications for the clinical care of elderly individuals with HL. Memory complaints should be carefully evaluated in patients with HL and not simply assumed to be due to impaired auditory function. Cognitive testing should be administered with extra effort to account for auditory impairments. Health care providers should be more vigilant in longitudinally assessing cognition in elderly individuals with HL because of their increased risk of developing dementia. A clear understanding of the extent of hearing loss and effectively treating it may have more extensive benefits than previously realized.
Conclusion
Subjects age 65 or older with HL have an increased rate of developing dementia and more rapid decline on 3MS-R scoring than their non-hearing-impaired counterparts. After controlling for gender, presence of APOE-ε4 allele, education, baseline age, and cardiovascular risk factors, HL was shown to be an independent predictor of developing dementia. These findings suggest that hearing impairment may be a marker for cognitive dysfunction in adults age 65 and older.
Acknowledgments
Source of funding: NIH Numbers: R01AG11380, R01AG21136
Footnotes
The authors have no relevant financial disclosures or conflict of interest disclosures to make.
This paper was presented at the American Otological Society 146th annual spring meeting, Orlando, FL, U.S.A. April 18th, 2013
References
- 1.Hebert LE, Weuve J, Scherr PA, et al. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013 doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humes LE, Dubno JR, Gordon-Salant S, et al. Central presbycusis: a review and evaluation of the evidence. J Am Acad Audiol. 2012;23:635–66. doi: 10.3766/jaaa.23.8.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin FR. Hearing loss and cognition among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66:1131–6. doi: 10.1093/gerona/glr115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gates GA, Feeney MP, Mills D. Cross-sectional age-changes of hearing in the elderly. Ear and hearing. 2008;29:865–74. doi: 10.1097/aud.0b013e318181adb5. [DOI] [PubMed] [Google Scholar]
- 5.Humes LE. Speech understanding in the elderly. J Am Acad Audiol. 1996;7:161–7. [PubMed] [Google Scholar]
- 6.Lin FR, Ferrucci L, Metter EJ, et al. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011;25:763–70. doi: 10.1037/a0024238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin FR, Metter EJ, O'Brien RJ, et al. Hearing loss and incident dementia. Arch Neurol. 2011;68:214–20. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uhlmann RF, Larson EB, Rees TS, et al. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA. 1989;261:1916–9. [PubMed] [Google Scholar]
- 9.Lin FR, Yaffe K, Xia J, et al. Hearing Loss and Cognitive Decline in Older Adults. JAMA internal medicine. 2013:1–7. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallacher J, Ilubaera V, Ben-Shlomo Y, et al. Auditory threshold, phonologic demand, and incident dementia. Neurology. 2012;79:1583–90. doi: 10.1212/WNL.0b013e31826e263d. [DOI] [PubMed] [Google Scholar]
- 11.Kiely KM, Gopinath B, Mitchell P, et al. Cognitive, health, and sociodemographic predictors of longitudinal decline in hearing acuity among older adults. J Gerontol A Biol Sci Med Sci. 2012;67:997–1003. doi: 10.1093/gerona/gls066. [DOI] [PubMed] [Google Scholar]
- 12.Lin MY, Gutierrez PR, Stone KL, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. Journal of the American Geriatrics Society. 2004;52:1996–2002. doi: 10.1111/j.1532-5415.2004.52554.x. [DOI] [PubMed] [Google Scholar]
- 13.Tschanz JT, Welsh-Bohmer KA, Lyketsos CG, et al. Conversion to dementia from mild cognitive disorder: the Cache County Study. Neurology. 2006;67:229–34. doi: 10.1212/01.wnl.0000224748.48011.84. [DOI] [PubMed] [Google Scholar]
- 14.Breitner JC, Wyse BW, Anthony JC, et al. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53:321–31. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- 15.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 16.Tschanz JT, Welsh-Bohmer KA, Plassman BL, et al. An adaptation of the modified mini-mental state examination: analysis of demographic influences and normative data: the cache county study. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15:28–38. [PubMed] [Google Scholar]
- 17.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 18.Nondahl DM, Cruickshanks KJ, Wiley TL, et al. Accuracy of self-reported hearing loss. Audiology. 1998;37:295–301. doi: 10.3109/00206099809072983. [DOI] [PubMed] [Google Scholar]
- 19.Sindhusake D, Mitchell P, Smith W, et al. Validation of self-reported hearing loss. The Blue Mountains Hearing Study. International journal of epidemiology. 2001;30:1371–8. doi: 10.1093/ije/30.6.1371. [DOI] [PubMed] [Google Scholar]
- 20.Clark K, Sowers M, Wallace RB, et al. The accuracy of self-reported hearing loss in women aged 60-85 years. Am J Epidemiol. 1991;134:704–8. doi: 10.1093/oxfordjournals.aje.a116147. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto M, Nakanishi N, Tatara K. Self-reported hearing difficulty and hearing impairment in Japanese people living in a community. International journal of audiology. 2004;43:54–9. doi: 10.1080/14992020400050008. [DOI] [PubMed] [Google Scholar]
- 22.Voeks SK, Gallagher CM, Langer EH, et al. Self-reported hearing difficulty and audiometric thresholds in nursing home residents. The Journal of family practice. 1993;36:54–8. [PubMed] [Google Scholar]
- 23.Wu HY, Chin JJ, Tong HM. Screening for hearing impairment in a cohort of elderly patients attending a hospital geriatric medicine service. Singapore medical journal. 2004;45:79–84. [PubMed] [Google Scholar]
- 24.Hayden KM, Zandi PP, West NA, et al. Effects of family history and apolipoprotein E epsilon4 status on cognitive decline in the absence of Alzheimer dementia: the Cache County Study. Arch Neurol. 2009;66:1378–83. doi: 10.1001/archneurol.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruickshanks KJ, Klein R, Klein BE, et al. Cigarette smoking and hearing loss: the epidemiology of hearing loss study. JAMA. 1998;279:1715–9. doi: 10.1001/jama.279.21.1715. [DOI] [PubMed] [Google Scholar]
- 26.Gates GA, Cobb JL, D'Agostino RB, et al. The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Arch Otolaryngol Head Neck Surg. 1993;119:156–61. doi: 10.1001/archotol.1993.01880140038006. [DOI] [PubMed] [Google Scholar]
- 27.Hayden KM, Zandi PP, Lyketsos CG, et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer disease and associated disorders. 2006;20:93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- 28.Treiber KA, Lyketsos CG, Corcoran C, et al. Vascular factors and risk for neuropsychiatric symptoms in Alzheimer's disease: the Cache County Study. International psychogeriatrics / IPA. 2008;20:538–53. doi: 10.1017/S1041610208006704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–42. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 30.Morrell CH, Brant LJ, Ferrucci L. Model choice can obscure results in longitudinal studies. J Gerontol A Biol Sci Med Sci. 2009;64:215–22. doi: 10.1093/gerona/gln024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet neurology. 2012;11:1006–12. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gates GA. Central presbycusis: an emerging view. Otolaryngol Head Neck Surg. 2012;147:1–2. doi: 10.1177/0194599812446282. [DOI] [PubMed] [Google Scholar]
- 33.Gates GA, Karzon RK, Garcia P, et al. Auditory dysfunction in aging and senile dementia of the Alzheimer's type. Arch Neurol. 1995;52:626–34. doi: 10.1001/archneur.1995.00540300108020. [DOI] [PubMed] [Google Scholar]
- 34.Gates GA, Anderson ML, Feeney MP, et al. Central auditory dysfunction in older persons with memory impairment or Alzheimer dementia. Arch Otolaryngol Head Neck Surg. 2008;134:771–7. doi: 10.1001/archotol.134.7.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gates GA, Anderson ML, McCurry SM, et al. Central auditory dysfunction as a harbinger of Alzheimer dementia. Arch Otolaryngol Head Neck Surg. 2011;137:390–5. doi: 10.1001/archoto.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gates GA, Cobb JL, Linn RT, et al. Central auditory dysfunction, cognitive dysfunction, and dementia in older people. Arch Otolaryngol Head Neck Surg. 1996;122:161–7. doi: 10.1001/archotol.1996.01890140047010. [DOI] [PubMed] [Google Scholar]
- 37.Pacala JT, Yueh B. Hearing deficits in the older patient: “I didn't notice anything”. JAMA. 2012;307:1185–94. doi: 10.1001/jama.2012.305. [DOI] [PubMed] [Google Scholar]
- 38.Bagai A, Thavendiranathan P, Detsky AS. Does this patient have hearing impairment? JAMA. 2006;295:416–28. doi: 10.1001/jama.295.4.416. [DOI] [PubMed] [Google Scholar]
- 39.Klein CJ, Bird T, Ertekin-Taner N, et al. DNMT1 mutation hot spot causes varied phenotypes of HSAN1 with dementia and hearing loss. Neurology. 2013 doi: 10.1212/WNL.0b013e318284076d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein CJ, Botuyan MV, Wu Y, et al. Mutations in DNMT1 cause hereditary sensory neuropathy with dementia and hearing loss. Nature genetics. 2011;43:595–600. doi: 10.1038/ng.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinha UK, Hollen KM, Rodriguez R, et al. Auditory system degeneration in Alzheimer's disease. Neurology. 1993;43:779–85. doi: 10.1212/wnl.43.4.779. [DOI] [PubMed] [Google Scholar]
- 42.Thomas A, Herbst KG. Social and psychological implications of acquired deafness for adults of employment age. Br J Audiol. 1980;14:76–85. doi: 10.3109/03005368009078906. [DOI] [PubMed] [Google Scholar]
- 43.Kricos PB, Erdman S, Bratt GW, et al. Psychosocial correlates of hearing aid adjustment. J Am Acad Audiol. 2007;18:304–22. doi: 10.3766/jaaa.18.4.5. [DOI] [PubMed] [Google Scholar]
- 44.Strawbridge WJ, Wallhagen MI, Shema SJ, et al. Negative consequences of hearing impairment in old age: a longitudinal analysis. The Gerontologist. 2000;40:320–6. doi: 10.1093/geront/40.3.320. [DOI] [PubMed] [Google Scholar]
- 45.Weinstein BE, Ventry IM. Hearing impairment and social isolation in the elderly. J Speech Hear Res. 1982;25:593–9. doi: 10.1044/jshr.2504.593. [DOI] [PubMed] [Google Scholar]
- 46.Pichora-Fuller MK, Schneider BA, Daneman M. How young and old adults listen to and remember speech in noise. J Acoust Soc Am. 1995;97:593–608. doi: 10.1121/1.412282. [DOI] [PubMed] [Google Scholar]
- 47.Rabbitt PM. Channel-capacity, intelligibility and immediate memory. Q J Exp Psychol. 1968;20:241–8. doi: 10.1080/14640746808400158. [DOI] [PubMed] [Google Scholar]
- 48.Tun PA, McCoy S, Wingfield A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychology and aging. 2009;24:761–6. doi: 10.1037/a0014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parham K, Lin FR, Coelho DH, et al. Comprehensive Management of Presbycusis: Central and Peripheral. Otolaryngol Head Neck Surg. 2013 doi: 10.1177/0194599813477596. [DOI] [PMC free article] [PubMed] [Google Scholar]