Prospective Study of Sequential Reduction in Immunosuppression, Interferon Alpha-2B, and Chemotherapy for Posttransplantation Lymphoproliferative Disorder (original) (raw)
. Author manuscript; available in PMC: 2014 May 21.
Published in final edited form as: Transplantation. 2008 Jul 27;86(2):215–222. doi: 10.1097/TP.0b013e3181761659
Abstract
Background
Several interventions can cure posttransplant lymphoproliferative disease (PTLD); a sequential approach is usual, starting with reduction in immunosuppressives (RI). The efficacy of RI remains poorly defined, particularly in adults. We assessed an algorithm starting with a defined course of RI in all patients, escalating to interferon (IFN) alpha2b, and finally to chemotherapy, in a prospective multicenter phase II study of adult solid organ transplant recipients. The design predated rituximab.
Methods
Reduction in immunosuppressives: cyclosporine or tacrolimus reduction by 50% for 2 weeks; a further 50% reduction for 1 week if not in complete remission (CR). Intravenous acyclovir was given for the duration of all RI. Patients with less than CR, or any rejection, resumed immunosuppressives and proceeded to IFN 3 MIU/m2/day for up to 3 months; if less than CR, ProMACE-CytaBOM chemotherapy.
Results
Twenty patients were registered over 60 months; 16 patients with biopsy-proven PTLD were eligible (13 heart, 3 kidney recipients). Median age was 47 (24–75) years. Reduction in immunosuppressives resulted in only 1 of 16 partial responses (12.5%), no CR. Progressive disease occurred in 8 of 16 (50%) and 6 of 16 (38%) experienced rejection. Only 1 of 13 (7%) patients achieved durable CR with IFN. Seven eligible patients received ProMACE-CytaBOM chemotherapy, five of seven (67%) achieving CR, four of five durable beyond 2 years.
Conclusions
Reduction in immunosuppressives produced no CR, progressive disease and rejection were frequent; response to IFN was rare. A strong case can be made for adding rituximab to RI as initial therapy. Chemotherapy resulted in 57% durable CR, data that are relevant for the up to two thirds of PTLD patients who are refractory to rituximab.
Keywords: PTLD, Lymphoma, Immunosuppressive reduction, S9239
Posttransplant lymphoproliferative disorder (PTLD) represents a significant problem for organ transplant recipients. Although several treatments have been shown to result in durable regression of the disease, it has been difficult to formulate a uniform approach to treatment. This is in part the result of the marked clinical heterogeneity of the disease, and of the heterogeneous clinical situations that pertain to the recipients of different organ transplants. The problem is further compounded by significant differences between adult and pediatric organ transplant recipients resulting from differences in Epstein-Barr virus (EBV) infection patterns between those two groups. The disease can be rapidly progressive and continues to carry a high mortality, with only 30% to 50% of patients surviving long term in the larger published series.
Complete surgical resection, limited field irradiation, reduction or withdrawal in immunosuppressives, acyclovir, interferon (IFN) alpha, and cytotoxic chemotherapy had all been reported as inducing durable remission in small series of patients at the time the current study was designed, before the availability of monoclonal anti B-cell therapy. Rituximab has resulted in complete remission (CR) rates of 30% to 60% with minimal toxicity and has changed the approach to treatment of this disease (1–3). Previous experience had led to the adoption of a stepwise approach to treatment, as reduced immunosuppression could be very effective in some cases, and cytotoxic chemotherapy had been found to carry major risks in this patient population. Experience with cytotoxics had been so poor that such therapy was viewed as virtually contraindicated at some transplant centers.
Reduced immunosuppression had been empiric, with little standardization of either the reductions to be made or endpoints to the intervention. Response rates to reduced immunosuppression were poorly defined, but seemed to be highest in pediatric patients; for PTLD presenting at less than a year after transplantation; and in renal recipients, where immunosuppressives could be discontinued. Essentially all reported clinical data regarding the efficacy of immunosuppressive reduction are retrospective in nature, did not involve a standardized regimen or defined endpoints, and were derived from a mixture of adult and pediatric transplant recipients. The current study is the first prospective clinical trial to examine the efficacy of immunosuppressive reduction in adults with PTLD, and was conducted by the Southwest Oncology Group and Eastern Cooperative Oncology Group, both large national oncology clinical trials groups (protocol S9239). The immunosuppressive reduction algorithm developed for the current protocol was based on common clinical practices; set clear endpoints to the intervention, in terms of response and rejection; and avoided complete discontinuation of immunosuppressives, as that had resulted in a high incidence of both severe rejection and progressive disease (PD) in one series of adult vital organ recipients (4). The use of high-dose acylovir has been associated with regression of lymphoproliferations in a small number of cases, although its activity against EBV is limited to the lytic phase of viral replication (5, 6). A course of full-dose intravenous acyclovir was viewed as relatively nontoxic, and has often been given simultaneously with reduction in immunosuppressives as initial management of PTLD, making it difficult to separate the individual effects. As such, acyclovir was incorporated in the protocol, given in the same way as had originally been reported (5).
Interferon was viewed as an attractive next step in patients not responding to reduced immunossuppressives, because it might obviate the need for cytotoxics. Efficacy had been shown only in a few small series, and neither the response rate nor the durability of response was well defined (7–9). Chemotherapy for PTLD had frequently been cyclophosphamide-doxorubicin-vincristine-prednisone (CHOP) or a reduced-intensity regimen. Efficacy was poor and toxicity appreciable, with mortality ranging from 33% to more than 70% (6, 10–14). On the basis of prior favorable experience with ProMACE-CytaBOM in cardiac recipients, this regimen was selected as the chemotherapy to use in patients not achieving remission with IFN (4). PTLD is frequently a rapidly progressive disease, and performance status is a major determinant of outcome (15, 16). Disease progression over the course of unsuccessful treatment attempts is therefore a concern. The treatment algorithm was structured to move patients to the next treatment if CR was not achieved by a specified time point.
The original intent and statistical design was to assess the efficacy of the treatment algorithm as a whole. The study was closed before full enrollment because of slow accrual and the advent of rituximab as a new treatment option. The study is the only prospective trial of reduced immunosuppression, and provides some data on the efficacy of both subsequent IFN and aggressive chemotherapy in the setting of rigorous cooperative group eligibility, response, and follow-up criteria. Although rituximab clearly represents a valuable treatment advance, half or more of patients with PTLD fail to achieve durable remission and require chemotherapy.
Patients and Methods
Eligibility Criteria
Patients over the age of 15 with biopsy-proven PTLD after any organ allograft (excluding lung transplantation) were eligible for entry onto this trial if they had bidimensionally measurable disease, any stage by Ann Arbor staging criteria. Needle biopsies were considered insufficient for study entry. Patients were excluded if they had received prior chemotherapy or IFN for PTLD; had AIDS or HIV antibody positivity; had PTLD confined to the central nervous system; had prior malignancies, excluding adequately treated basal cell or squamous cell skin cancer, in situ cervical cancer, or any other malignancy for which the patient had been disease-free for 5 years. Relapse of prior PTLD completely removed by biopsy or surgery was allowed. Pregnant or lactating patients were not eligible. All patients were informed of the investigational nature of this study and were required to sign a written informed consent approved in accordance with institutional and federal guidelines.
Staging Studies
Patients were staged by Ann Arbor staging criteria. Studies performed before treatment started included history and physical examination, CT or MRI of the chest, abdomen and pelvis, bone marrow biopsy, lactate dehydrogenase estimation, complete blood count, chemistries, serum protein electrophoresis, electocardiogram, cardiac-gated blood pool scan, or echocardiogram. Restaging studies were performed at each formal disease reassessment point, or at any point if there was clinical suspicion of disease progression.
Study Design
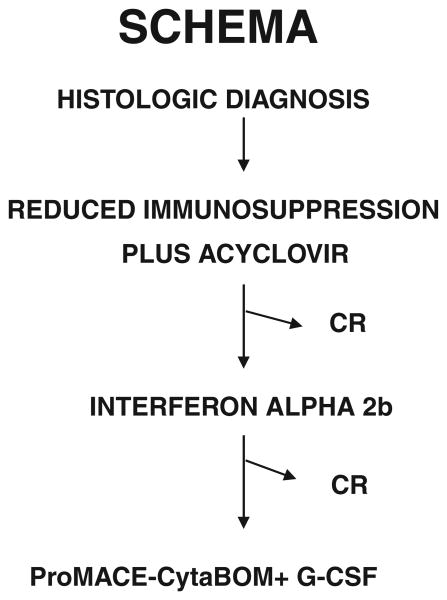
Patients were managed according to a treatment algorithm requiring stepwise escalation in therapy if complete response was not achieved by a predetermined time point (Fig. 1).
Figure 1.
Treatment algorithm. Patients progressed to the next treatment unless complete remission (CR) was achieved.
Reduction in Immunosuppression
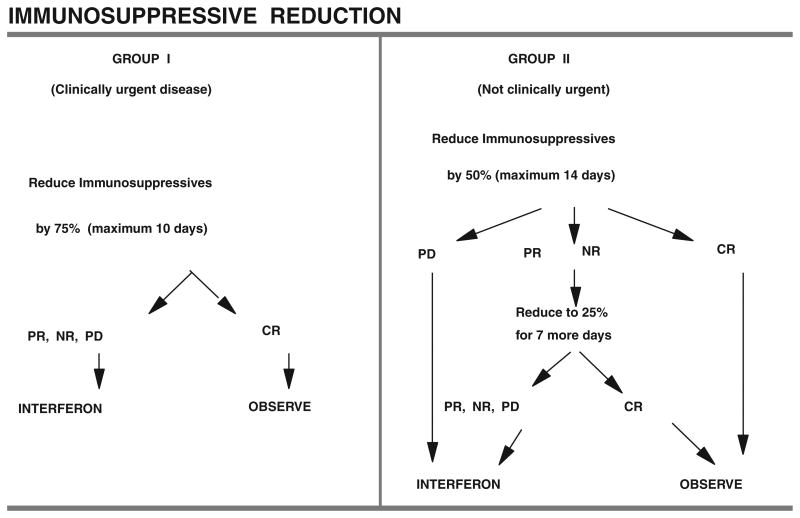
All patients were to receive initial management with immunosuppressive reduction and acyclovir. The immunosuppressive reduction regimen is detailed in Figure 2.
Figure 2.
Schema for immunosuppressive reduction. CR, complete response; PR, partial response; NR, no response; PD, progressive disease.
Patients were grouped into one of two categories, and the initial approach to reduction in immunosuppression was determined by that grouping. Patients were considered to fall in group I, clinically urgent disease, if any of the following applied: (a) histologically proven involvement of the allograft or the bone marrow; (b) liver involvement causing hepatic insufficiency (insufficiency defined as serum bilirubin more than the institutional upper limit of normal, or serum glutamic oxaloacetic transaminase or serum glutamic pyruvic transaminase more than or equal to 2× the institutional upper limit of normal, or prothrombin time (PT) more than the institutional upper limit of normal, or clinical hepatic encephalopathy); (c) lactate dehydrogenase more than or equal to 3× the institutional upper limit of normal; (d) systemic sepsis; (e) locally urgent lesions such as tonsillar enlargement sufficient to threaten airway patency, superior vena cava syndrome, bilateral hydronephrosis, or postobstructive pneumonia; (f) any clinical situation deemed urgent by the treating physician, after discussion with the study coordinator; (g) small noncleaved lymphocytic lymphoma histology (i.e., Burkitt's lymphoma morphology). Gastrointestinal obstruction, perforation, or hemorrhage requiring surgical correction were not assigned to group I for that reason alone. Group II then consisted of all patients who did not have a finding to warrant inclusion in group I.
Management of Immunosuppressives in Group I Patients
Azathioprine (or methotrexate or cyclophosphamide if those drugs were being used) was discontinued, cyclosporine or tacrolimus dose was reduced to 25% of the dose being given at the time of diagnosis, and glucocorticoids were reduced to a physiologic maintenance dose of prednisone 7.5 mg/day. Immunosuppressives were maintained at this level for a maximum of 10 days, with close clinical monitoring for rejection; assessment of tumor response occurred on day 10.
Management of Immunosuppressives in Group II Patients
Azathioprine (or methotrexate or cyclophosphamide if those drugs were being used) was discontinued, cyclosporine or tacrolimus dose was reduced to 50% of the dose being given at the time of diagnosis, and glucocorticoids were reduced by 50% with a lower limit of prednisone of 7.5 mg/day. Immunosuppressives were maintained at this level for a maximum of 14 days, with close clinical monitoring for rejection; assessment of tumor response occurred on day 14. Unless CR or PD were identified, immunsuppressives were reduced by 50% again (i.e., 25% of the dose being given at the time of diagnosis) for a maximum of 7 days, with a lower limit of prednisone of 7.5 mg/day. Reassessment of tumor response occurred on the 7th day. Both groups I and II patients received acyclovir 10 mg/kg intravenously (IV) over 1 hr q 8 hourly until the end of the immunosuppressive reduction therapy (maximum day 10 for group I, day 21 for group II). Patients assessed as having PD at any point, or patients failing to achieve CR on the final disease reassessment, or patients experiencing biopsy-proven allograft rejection of any grade during reduced immunosuppression, had immunosuppressives reinstituted at 80% of the dose in use at the time of diagnosis (except for azathioprine, methotrexate, or cyclophosphamide), and proceeded to treatment with IFN alpha. Patients assessed as having CR at any of the planned disease assessment points also had immunosuppressives reinstituted at 80% of the dose in use at the time of diagnosis (except for azathioprine, methotrexate, or cyclophosphamide), proceeded to observation and started oral acyclovir at 1 g four times per day, to be continued for 3 months, then 600 mg four times per day for 6 months, then 400 mg four times per day for 3 months, provided the patient remained in CR. If relapse occurred, acyclovir was discontinued and the patient proceeded to IFN therapy.
Interferon
Interferon therapy was given as IFN alpha 2b, 3 million IU/m2 or intramuscularly (SC) daily on days 1 to 28, for three cycles. One cycle was 28 days. Immunosuppressives were continued during IFN therapy; treatment of rejection episodes, and adjustment of immunosuppressive doses were carried out according to institutional practice. Disease reassessment occurred at the end of each cycle. A total of three cycles of daily IFN were given, unless disease reassessment showed PD. Patients with PD at any point, and patients failing to achieve CR by the end of the third cycle, proceeded to chemotherapy. Patients who had achieved CR by the end of the third cycle of daily IFN therapy received maintenance IFN, given as IFN alpha 2b, 3 million IU/m2 sq or IM 3 days per week for six cycles. One cycle was 28 days. Disease reassessment was performed at the end of cycles 2, 4, and 6. Patients remaining in CR discontinued IFN after six maintenance cycles and proceeded to observation. Patients with disease relapse at any point during maintenance IFN, discontinued IFN and proceeded to chemotherapy.
Chemotherapy
Chemotherapy was given with the ProMACE-CytaBOM regimen as follows: cyclophosphamide 650 mg/m2 IV on day 1, doxorubicin 25 mg/m2 IV on day 1, etoposide 120 mg/m2 infused IV over 60 min on day 1, prednisone 60 mg/m2 orally (PO) on days 1 to 14, cytosine arabinoside 300 mg/m2 IV on day 8, bleomycin 5 mg/m2 IV on day 8, vincristine 1.4 mg/m2 IV on day 8, methotrexate 120 mg/m2 IV on day 8, and leucovorin 25 mg/m2 PO or IV every 6 hr for four doses beginning 24 hr after methotrexate. The cycle of chemotherapy was repeated every 21 days, for a total of six cycles. Supportive care consisted of filgrastim 5 μg/kg/day, SC, days 2 to 14 or until recovery of WBC more than or equal to 10,000 for 2 consecutive days, trimethoprim-sulfamethoxazole, one double strength tablet PO three times per week for the duration of chemotherapy and for 6 months after completion of the final cycle. From days 15 to 21 prednisone was given at 15 mg/day. No immunosuppressives were given from day 1 of the first cycle of chemotherapy until day 21 of the final cycle. On day 21 of cycle 6 all immunosuppressives were resumed at 80% of the prestudy dose except azathioprine that was started at 50% of the prior dose and subsequently titrated up to 80% according to WBC. Disease reassessment was performed after cycles 2, 4, and 6.
Response Assessment
Complete response was defined as complete disappearance of all measurable and evaluable disease. No new lesions. All measurable, evaluable, and nonevaluable lesions and sites must be assessed. Partial response was defined as more than or equal to 50% decrease under baseline in the sum of the products of perpendicular diameters of all measurable lesions. No progression of evaluable disease and no new lesions. All measurable and evaluable lesions and sites must be assessed. Stable disease was defined as not qualifying for complete response, partial response, or progression. All measurable and evaluable sites and lesions must be assessed. Progression or relapse was defined as more than or equal to 25% increase or an increase of 10 cm2 (whichever was smaller) in the sum of products of measurable lesions over smallest sum observed (over baseline if no decrease), or reappearance of any lesion that had disappeared, or clear worsening of any evaluable disease, or appearance of any new lesion/site, or failure to return for evaluation because of deteriorating condition (unless deterioration was clearly unrelated to this cancer).
Statistical Considerations
The primary objective of the study was to assess prospectively the efficacy, in terms of response rate and survival, of a defined, incremental treatment approach to lymphoproliferations after organ transplantation. The accrual goal was 50 eligible patients, allowing the overall response rate to be estimated to within ± 15% (95% CI). This accrual goal could not be met, and the study was closed to new registrations after accruing 20 patients.
Results
Patient Characteristics
Twenty patients were registered from seven institutions over 60 months. Four patients were ineligible based on central pathology review. Of the 16 eligible patients, 13 were heart recipients, and three were kidney recipients. The median age was 47 years (range, 24–75). Thirteen patients (81%) were male and three (19%) were female. Fourteen were white, one black, and one Native American. Fourteen had a performance status of 0 to 2, two had a performance status of 3 to 4. Data regarding the interval between transplant and diagnosis of PTLD were collected as less than or equal to 6 months, less than or equal to 1 year, or more than 1 year. No patients presented with PTLD at less than 6 months posttransplant, four patients (25%) presented between 6 and 12 months post-transplant, and 12 patients (75%) presented at more than 12 months posttransplant (Table 1). Tumor morphology was classified according to the Working Formulation and the Rappaport classification in the design of this study. The pathologic interpretation was diffuse large B-cell lymphoma in 14 patients, and large cell lymphoma of T-cell origin in two patients. The study design predates the WHO classification of PTLD; by that classification four patients had polymorphic B-cell lymphoma, 10 patients had monomorphic lymphoma of B-cell origin, and two patients had peripheral T-cell lymphoma. Tissue analysis for EBV association was not required by the protocol, and many patients predate the routine use of CD20 determination, so that incomplete data exist for those parameters. Tumor-associated EBV was demonstrated to be present in six cases, absent in three cases (both T-cell lymphomas and one B-cell lymphoma), and not determined in seven cases. Five tumors were CD20 positive, nine were not determined, and the two T-cell tumors were CD20 negative.
Table 1. Patient characteristics (n= 16).
| Age | Median 47 yr. (range 24–75) |
|---|---|
| Sex | |
| Male | 13 (81) |
| Female | 3 (19) |
| Organ allograft | |
| Heart | 13 (81) |
| Kidney | 3 (19) |
| Race | |
| White | 14 (88) |
| Black | 1 (6) |
| Native American | 1 (6) |
| Interval from transplant | |
| >6 mo ≤ 1 yr | 4 (25) |
| >1 yr | 12 (75) |
| Stage | |
| II | 5 (31) |
| III | 4 (25) |
| IV | 7 (44) |
| CNS involvement | 1 (6) |
| Extranodal disease | 9 (56) |
| LDH elevated | 11 (69) |
| Performance status | |
| 0–2 | 14 (87) |
| 3–4 | 2 (13) |
| Disease at presentation | |
| Clinically urgent (group 1) | 3 (19) |
| Not clinically urgent (group 2) | 13 (81) |
| Pathology | |
| B-cell | 14 (87) |
| T-cell | 2 (13) |
Response to Treatment
Reduced Immunosuppression
Sixteen eligible patients started treatment with reduced immunosuppression. The response rate to reduced immunosuppression was 0/16 CR; 1/16 PR (6%). Six of the 16 patients (38%) had documented rejection during the period of reduced immunosuppression. Eight of the 16 patients had documented PD during the period of reduced immunosuppression. There was one early death, which could not be documented as PD, but most likely was because of that. Three patients did not proceed from reduced immunosuppression to IFN: one because of early death and another because of fatal PD during reduced immunosuppression, and a third because of protocol violation. None of the three renal recipients responded to reduced immunosuppression.
Interferon Therapy
Thirteen patients underwent treatment with IFN alpha. The response rate was 2/13 (15%) CR; 2/13 (15%) PR. All of the three renal recipients responded to IFN, two achieving CR and one PR. One of the two patients achieving CR relapsed after 20 months and proceeded to chemotherapy. There were four deaths during IFN therapy (one sepsis, one acute rejection, one acute myocardial infarction, one motor vehicle accident). One patient was withdrawn from protocol therapy for unspecified reasons after IFN therapy, leaving seven patients who proceeded to chemotherapy and one in durable CR after IFN.
ProMACE-CytaBOM Chemotherapy
Seven eligible patients proceeded to ProMACE-CytaBOM chemotherapy. The response rate to chemotherapy was 5/7 (67%) CR. One patient was not assessable, because of nonneutropenic septic death. Four of the five CRs were durable, with remission duration of more than 2 years.
Treatment Algorithm as a Whole
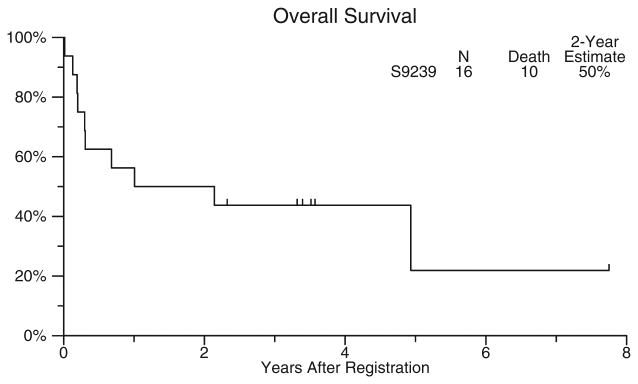
The median survival for the treatment cohort as a whole was 19 months, with a range of 5 days to 60+ months. Overall survival was 50% at 2 years, 44% at 4 years, and 24% at 8 years (Fig. 3). The median follow-up time for those patients still alive was 5.5 years. Two of the three renal transplant recipients were long-term survivors. Patients had been stratified according to the interval between transplantation and the diagnosis of PTLD. Patients presenting at less than or equal to 1 year posttransplant (n=5) had a 60% 4 year survival, whereas patients presenting at more than 1 year post-transplant had a 38% 4 year survival.
Figure 3.
Overall survival for the entire cohort of eligible patients, from the time of study registration.
Toxicity
Reduced Immunosuppression
Grade 3/4 toxicities are detailed in Table 2. The main toxicity of immunosuppressive reduction was rejection. Six of 16 patients experienced rejection, none fatal. No other clearly treatment-related toxicities were evident. One patient experienced grade 3/4 neutropenia and thrombocytopenia, with neutropenic fever, more likely related to the underlying disease than to immunosuppressive reduction.
Table 2. Grade 3 to 5 toxicities by treatment phase.
| Toxicity | Reduced immunosuppression(n=16) | Interferon(n=13) | Chemotherapy(n=7) |
|---|---|---|---|
| Hepatic | 3 | 3 | |
| Renal | 1 | 5 | |
| Cardiac | 2 | ||
| Fatigue | 4 | 2 | |
| Hypotension | 1 | 2 | |
| Infection without neutropenia | 1 | ||
| Infection with neutropenia | 1 | 2 | |
| Anemia | 1 | 1 | 4 |
| Neutropenia | 1 | 4 | 6 |
| Thrombocytopenia | 1 | 1 | |
| Pulmonary | 1 | 1 | 2 |
| Seizures | 1 | ||
| Weakness | 3 |
Interferon
There were four deaths during IFN therapy. One because of non-neutropenic sepsis (nocardia); one because of acute myocardial infarction and allograft vasculopathy; and one because of acute rejection, all possibly associated with IFN therapy. There was one death resulting from a motor vehicle accident. Toxicites that were likely associated with IFN therapy were grade 3/4 increase in creatinine in 4/13 (31%) patients; neutropenia in 4/13 (31%) patients; grade 3/4 fatigue in 4/13 (31%) patients; weakness in 3/13 (23%) patients; hypotension in 2/13 (15%); seizures in 1/13 (8%) patients; and somnolence in 1/13 (8%) patients. Most of these toxicities were manageable with treatment interruptions and dose reductions as specified in the protocol. Two patients experienced a rejection episode during IFN therapy, one was fatal.
Chemotherapy
The principal toxicities were hematologic. One patient died of infection during cycle 6 of chemotherapy, and no patients developed congestive heart failure. Left ventricular ejection fraction was very closely monitored for the cardiac recipients during doxorubicin therapy. Only one patient sustained a cardiac toxicity, which was grade 1. No rejection episodes were documented during chemotherapy with ProMACE-CytaBOM.
Discussion
Southwest Oncology Group protocol S9239 was an attempt at conducting a multicenter cooperative group study for PTLD. The treatment plan applied general principles of management in an area where much has been anecdotal or poorly defined. Accrual was very slow, even after the study was modified to include kidney and liver transplant recipients and was expanded through the participation of the Eastern Cooperative Oncology Group. There were several reasons for this. Organ transplant recipients are a heterogeneous group of patients, multiple subspecialties are involved in their management, clinical events surrounding a PTLD diagnosis are often urgent, many patients were not accrued because immunosuppressive reduction had been started before diagnosis, and the maintenance of low-volume protocols had become problematic at many institutions. This experience may apply more generally to the study of rare or complex diseases through the cooperative group mechanism. The high rate of ineligible patients and protocol deviations was likely also attributable to some of those factors. The accrual goal was not met, and the planned statistical analysis was not possible. Nonetheless, this patient cohort represents the only prospective study of reduced immunosuppression, and is one of only very few prospective studies of PTLD, particularly in adults.
A number of conclusions can be drawn from the results of this study. The lack of efficacy of reduced immunosuppression is striking, despite the fact that more than one third of patients experienced rejection during that maneuver. The efficacy of reduced immunosuppression has varied according to the composition of the patient cohort, being highest among pediatric organ recipients. Posttransplant lymphoproliferative disease within the first year of transplantation may also be more amenable to this therapy, although conflicting results have been published (12, 17, 18). Response rates as high as 50% have been reported in a retrospective study (19), whereas others have indicated extremely low response rates to this maneuver (4, 20). The current study was performed in adult patients, mainly cardiac rather than renal recipients, and 75% presented at more than a year after transplantation, none in the first 6 months. Although it may be reasonable to continue to reduce immunosuppressives for adults with PTLD in the future, a strong argument can be made for the simultaneous initiation of rituximab therapy in all newly diagnosed patients. This is particularly true for a disease where performance status has been identified as one of the major prognostic factors determining outcome, and where rapid disease progression during ineffective therapy is well recognized (14, 15, 17). A sequential approach to therapy was attractive primarily to avoid the significant risks of chemotherapy in this patient population. Those concerns do not apply to rituximab.
The experience gained with IFN alpha on this protocol in 13 patients refractory to a uniform immunosuppressive reduction regimen generates little enthusiasm for a larger role for this drug in PTLD. Toxicity was furthermore not insignificant, primarily fatigue, renal dysfunction, and cytopenias, and two patients experienced rejection despite having resumed immunosuppressive therapy.
When used for PTLD, chemotherapy with the regimen most commonly used for aggressive lymphomas in the immunocompetent population, CHOP, has produced generally poor results (13). A retrospective multicenter study of CHOP for PTLD in 25 patients showed a CR rate of 48%, with half of patients achieving a CR subsequently relapsing. Time to progression was less than a year, median survival was 1 year, and there was a 36% treatment-related mortality (10). The results achieved with ProMACE-CytaBOM in our prospective study are in keeping with prior observations of the feasibility and efficacy of this regimen in PTLD (4), and with very similar results obtained for etoposide-prednisonevincristine-cyclophosphamide-doxorubicin (EPOCH) (21). It is worth noting that ProMACE-CytaBOM contains half the doxorubicin dose of CHOP, which may be relevant in cardiac recipients, (4), and permits discontinuation of all immunosuppressives for the duration of chemotherapy. The latter may reduce the infectious complications otherwise typically seen in organ recipients during chemotherapy, a problem not encountered to a significant extent during chemotherapy on this study. Low-dose chemotherapy has been explored in PTLD, with very favorable results. A 77% CR rate, with most being durable, was achieved with a modest dose of cyclophosphamide and prednisone. However, the patients were all pediatric organ transplant recipients, with a median age of 4.9 years, and a median interval from transplant of 5.3 months (18). In most of these patients, PTLD likely represented primary EBV infection in the setting of immunodeficiency, and therapy modulated the lymphoproliferation while a primary immune response was established. Those results are not likely to be applicable to the majority of adult patients with PTLD. Although aggressive chemotherapy is effective for that situation, it is nonetheless desirable to avoid it, if possible. On the other hand, disease progression, deterioration in performance status, or rejection of a vital organ during ineffective therapy are also to be avoided.
Monoclonal antibody therapy, most recently with rituximab, has shown significant efficacy against PTLD, with minimal toxicity. A retrospective study of rituximab in 26 organ transplant recipients with PTLD showed a 57% CR rate. It should be noted that the median interval between transplant and PTLD diagnosis was 5 months in this series (22). A prospective phase 2 study, by the same group, of 43 organ transplant recipients with a median interval from transplant of 2.4 years, showed a 28% durable CR rate and 1 year survival of 56% (23). Interestingly, a much earlier study of monoclonal antibody therapy for PTLD, using a mixture of anti-CD21 and anti-CD24 antibodies, reported very durable remissions on long-term follow-up (24). The complete response rate among 58 patients was 61%, but only 22% of patients presenting at more than 1 year posttransplant responded (25). The efficacy and durability of rituximab in different PTLD subsets remains to be fully defined, but it is clear that a significant number of patients will require therapy other than rituximab. Despite the valid concerns regarding toxicity, chemotherapy can produce durable remissions in PTLD refractory to other measures. The results of the current study would strongly support, at least in adults, rather the addition of rituximab to the initial management of PTLD, than relying on immunosuppressive reduction alone. Chemotherapy remains an effective treatment option, to be applied as soon as it is clear that initial management has been ineffective.
Acknowledgments
This work (S9239) was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA38926, CA32102, CA46282, CA76132, CA35431, CA04919, CA35178, CA46441, CA21115 CA17145.
References
- 1.Milpied N, Vasseur B, Parquet N, et al. Humanized anti-CD20 monoclonal antibody (Rituximab) in post transplant B-lymphoproliferative disorder: A retrospective analysis on 32 patients. Ann Oncol. 2000;1(11 suppl):113. [PubMed] [Google Scholar]
- 2.Choquet S, Leblond V, Herbrecht R, et al. Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: Results of a prospective multicenter phase 2 study. Blood. 2006;107:3053. doi: 10.1182/blood-2005-01-0377. [DOI] [PubMed] [Google Scholar]
- 3.Oertel SH, Verschuuren E, Reinke P, et al. Effect of anti-CD 20 antibody rituximab in patients with post-transplant lymphoproliferative disorder (PTLD) Am J Transplant. 2005;5:2901. doi: 10.1111/j.1600-6143.2005.01098.x. [DOI] [PubMed] [Google Scholar]
- 4.Swinnen LJ, Mullen GM, Carr TJ, et al. Aggressive treatment for post-cardiac transplant lymphoproliferation. Blood. 1995;86:3333. [PubMed] [Google Scholar]
- 5.Hanto DW, Frizzera G, Gajl-Peczalska KJ, et al. Epstein-Barr virus-induced B-cell lymphoma after renal transplantation: Acyclovir therapy and transition from polyclonal to monoclonal B-cell proliferation. N Engl J Med. 1982;306:913. doi: 10.1056/NEJM198204153061506. [DOI] [PubMed] [Google Scholar]
- 6.Morrison VA, Dunn DL, Manivel JC, et al. Clinical characteristics of post-transplant lymphoproliferative disorders. Am J Med. 1994;97:14. doi: 10.1016/0002-9343(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro RS, Chauvenet A, McGuire W, et al. Treatment of B-cell lymphoproliferative disorders with interferon alfa and intravenous gamma globulin [letter] N Engl J Med. 1988;318:1334. doi: 10.1056/NEJM198805193182013. [DOI] [PubMed] [Google Scholar]
- 8.Liebowitz D, Anastasi J, Hagos F, et al. Post-transplant lymphoproliferative disorders (PTLD): Clinicopathologic characterization and response to immunomodulatory therapy with interferon-alpha. Ann Oncol. 1996;7:28. Abstract. [Google Scholar]
- 9.Davis CL, Wood BL, Sabath DE, et al. Interferon-alpha treatment of posttransplant lymphoproliferative disorder in recipients of solid organ transplants. Transplantation. 1998;66:1770. doi: 10.1097/00007890-199812270-00035. [DOI] [PubMed] [Google Scholar]
- 10.Choquet S, Leblond V, Jager U, et al. Efficacy of CHOP regimen as first line therapy in posttransplantation lymphoproliferative disorders (PTLD) Blood. 2003;102:49b. Abstract 3897. [Google Scholar]
- 11.Nalesnik MA, Makowka L, Starzl TE. The diagnosis and treatment of posttransplant lymphoproliferative disorders. Curr Probl Surg. 1988;25:367. doi: 10.1016/0011-3840(88)90011-1. [DOI] [PubMed] [Google Scholar]
- 12.Armitage JM, Kormos RL, Stuart RS, et al. Posttransplant lymphoproliferative disease in thoracic organ transplant patients: Ten years of cyclosporine-based immunosuppression. J Heart Lung Transplant. 1991;10:877. discussion 886. [PubMed] [Google Scholar]
- 13.Leblond V, Sutton L, Dorent R, et al. Lymphoproliferative disorders after organ transplantation: A report of 24 cases observed in a single center. J Clin Oncol. 1995;13:961. doi: 10.1200/JCO.1995.13.4.961. [DOI] [PubMed] [Google Scholar]
- 14.Leblond V, Dhedin N, Mamzer Bruneel MF, et al. Identification of prognostic factors in 61 patients with posttransplantation lymphoproliferative disorders. J Clin Oncol. 2001;19:772. doi: 10.1200/JCO.2001.19.3.772. [DOI] [PubMed] [Google Scholar]
- 15.Leblond V, Dhedin N, Mamzer Bruneel MF, et al. Identification of prognostic factors in 61 patients with posttransplantation lymphoproliferative disorders. J Clin Oncol. 2001;19:772. doi: 10.1200/JCO.2001.19.3.772. [DOI] [PubMed] [Google Scholar]
- 16.Ghobrial IM, Habermann TM, Maurer MJ, et al. Proposed prognostic model for survival in solid organ transplant recipients with post transplant lymphoproliferative disorders (PTLD) Blood. 2003;102:392a. Abstract 1423. [Google Scholar]
- 17.Tsai DE, Hardy CL, Tomaszewski JE, et al. Reduction in immunosuppression as initial therapy for posttransplant lymphoproliferative disorder: Analysis of prognostic variables and long-term follow-up of 42 adult patients. Transplantation. 2001;71:1076. doi: 10.1097/00007890-200104270-00012. [DOI] [PubMed] [Google Scholar]
- 18.Ghobrial IM, Habermann TM, Macon WR, et al. Differences between early and late posttransplant lymphoproliferative disorders in solid organ transplant patients: Are they two different diseases? Transplantation. 2005;79:244. doi: 10.1097/01.tp.0000144335.39913.5c. [DOI] [PubMed] [Google Scholar]
- 19.Tsai DE, Hardy CL, Tomaszewski JE, et al. Reduction in immunosuppression as initial therapy for posttransplant lymphoproliferative disorder: Analysis of prognostic variables and long-term follow-up of 42 adult patients. Transplantation. 2001;71:1076. doi: 10.1097/00007890-200104270-00012. [DOI] [PubMed] [Google Scholar]
- 20.Leblond V, Dhedin N, Mamzer Bruneel MF, et al. Identification of prognostic factors in 61 patients with posttransplantation lymphoproliferative disorders. J Clin Oncol. 2001;19:772. doi: 10.1200/JCO.2001.19.3.772. [DOI] [PubMed] [Google Scholar]
- 21.Davis CL, Wood BL, Sabath DE, et al. Interferon-alpha treatment of posttransplant lymphoproliferative disorder in recipients of solid organ transplants. Transplantation. 1998;66:1770. doi: 10.1097/00007890-199812270-00035. [DOI] [PubMed] [Google Scholar]
- 22.Milpied N, Antoine C, Garnier JL, et al. Humanized anti-CD20 monoclonal antibody (rituximab) in B post-transplant lymphoproliferative disorders (B PTLDs): A retrospective analysis of 32 patients. Ann Oncol. 1999;10:5. Abstract. [PubMed] [Google Scholar]
- 23.Choquet S, Leblond V, Herbrecht R, et al. Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: Results of a prospective multicenter phase 2 study. Blood. 2006;107:3053. doi: 10.1182/blood-2005-01-0377. [DOI] [PubMed] [Google Scholar]
- 24.Fischer A, Blanche S, Le Bidois J, et al. Anti-B-cell monoclonal antibodies in the treatment of severe B-cell lymphoproliferative syndrome following bone marrow and organ transplantation. N Engl J Med. 1991;324:1451. doi: 10.1056/NEJM199105233242102. [DOI] [PubMed] [Google Scholar]
- 25.Benkerrou M, Jais JP, Leblond V, et al. Anti-B-cell monoclonal antibody treatment of severe posttransplant B-lymphoproliferative disorder: Prognostic factors and long-term outcome. Blood. 1998;92:3137. [PubMed] [Google Scholar]