Rapamycin Reduces Disease Activity and Normalizes T Cell Activation–Induced Calcium Fluxing in Patients With Systemic Lupus Erythematosus (original) (raw)
. Author manuscript; available in PMC: 2014 May 27.
Published in final edited form as: Arthritis Rheum. 2006 Sep;54(9):2983–2988. doi: 10.1002/art.22085
Abstract
Objective
Systemic lupus erythematosus (SLE) is an autoimmune disease of unknown origin. Current treatment options are often ineffective or poorly tolerated. Recent observations have revealed mitochondrial hyperpolarization and enhanced Ca2+ fluxing in T cells from SLE patients. Rapamycin, a lipophilic macrolide antibiotic that regulates mitochondrial transmembrane potential and Ca2+ fluxing, has been used safely and effectively to treat renal transplant rejection since 1999. In addition, rapamycin has been shown to ameliorate T cell function and to prolong survival in lupus-prone MRL/lpr mice. We therefore undertook the present study to investigate whether rapamycin is beneficial in patients with SLE.
Methods
Nine patients with clinically active SLE that had been treated unsuccessfully with other immunosuppressive medications began therapy with rapamycin, 2 mg/day orally. Disease activity was assessed with the British Isles Lupus Assessment Group (BILAG) score, SLE Disease Activity Index (SLEDAI), and requirement for prednisone therapy. Mitochondrial transmembrane potential and Ca2+ fluxing were assessed by flow cytometry.
Results
In patients treated with rapamycin, the BILAG score was reduced by a mean ± SEM of 1.93 ± 0.9 (P = 0.0218), the SLEDAI by 5.3 ± 0.8 (P = 0.00002), and concurrent prednisone use by 26.4 ± 6.7 mg/day (P = 0.0062) compared with pre–rapamycin treatment. While mitochondrial hyperpolarization persisted, pretreatment cytosolic and mitochondrial Ca2+ levels and T cell activation–induced rapid Ca2+ fluxing were normalized in rapamycin-treated patients.
Conclusion
Rapamycin appears to be a safe and effective therapy for SLE that has been refractory to traditional medications. Mitochondrial dysfunction and Ca2+ fluxing could serve as biomarkers to guide decisions regarding future therapeutic interventions in SLE.
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease of unknown origin. It involves multiple organs including the joints, skin, kidneys, and central nervous system and is characterized by a waxing and waning course. We have previously discovered that mitochondrial dysfunction, characterized by elevation of the mitochondrial transmembrane potential and depletion of ATP, plays key roles in aberrant apoptosis and predisposition to necrosis, and likely significantly contributes to the inflammatory process in SLE (1). Recent observations have indicated that mitochondrial dysfunction is associated with enhanced Ca2+ fluxing in T lymphocytes from patients with SLE (2, 3), providing potential new therapeutic targets (4–6).
Rapamycin (sirolimus) is a lipophilic macrolide isolated from a strain of Streptomyces hygroscopius. The intracellular rapamycin receptor is a small, 12-kd FK-506 binding protein (FKBP12). The rapamycin–FKBP12 complex interacts with mammalian target of rapamycin (mTOR), a protein kinase that controls T cell activation (7), by influencing gene transcription and multiple metabolic pathways, including Ca2+ fluxing (8) and maintenance of the mitochondrial transmembrane potential (9). In lupus-prone MRL/lpr mice, rapamycin has been shown to normalize T cell mitogen-stimulated splenocyte proliferation and interleukin-2 production, prevent the typical rise in anti–double-stranded DNA antibody and urinary albumin levels and glomerulonephritis, and prolong survival (10). Rapamycin has been used safely and effectively to treat renal transplant rejection since 1999 (11). We have prescribed rapamycin to treat 9 patients with clinically active SLE who were unable to tolerate or did not respond to other immunosuppressive medications. The results of this treatment after 6–48 months of followup are described herein.
PATIENTS AND METHODS
Patients
Sixteen female patients with SLE fulfilling the American College of Rheumatology criteria (12) were studied. Nine of the patients received treatment with rapamycin, 2 mg/day orally. The other 7 were included as disease controls. As controls for in vitro studies, 7 age-matched healthy female subjects were also examined in parallel. Disease activity was assessed by the British Isles Lupus Assessment Group (BILAG) instrument (13) and the SLE Disease Activity Index (SLEDAI) (14).
Cell culture and T cell activation
Peripheral blood mononuclear cells were isolated from heparinized venous blood on Ficoll-Hypaque gradient. Peripheral blood lymphocytes (PBLs) were separated from monocytes by adherence to autologous serum–coated petri dishes (15). PBLs were resuspended at 106 cells/ml in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 m_M_ l-glutamine, 100 IU/ml penicillin, 100 µg/ml streptomycin, and 0.25 µg/ml amphotericin B, in 12-well plates at 37°C in a humidified atmosphere with 5% CO2. Crosslinking of the CD3 antigen was performed by precoating with 20 µg/ml goat anti-mouse IgG (Sigma, St. Louis, MO) for 2 hours at 37°C, then washing twice with phosphate buffered saline and coating with 1 µg/ml/well monoclonal antibody OKT3 (CRL 8001; American Type Culture Collection, Rockville, MD) for 1 hour at 37°C, followed by addition of PBLs. CD28 costimulation was performed by addition of 500 ng/ml monoclonal antibody CD28.2 (PharMingen, San Diego, CA). Rapid Ca2+ signaling was investigated after direct addition of OKT3 and CD28.2 to PBLs preloaded with Fluo-3 (see below).
Flow cytometric analysis of mitochondrial transmembrane potential
Mitochondrial transmembrane potential (Δψm) was estimated by staining with 1 µ_M_ tetramethylrhodamine for 15 minutes at 37°C in the dark before flow cytometry (excitation 543 nm, emission 567 nm; recorded in fluorescence channel 2 [FL-2]) (3); it was also assessed by staining with 4 nm 3,3′-dihexyloxacarbocyanine iodide (DiOC6), a cationic lipophilic dye (15), for 15 minutes at 37°C in the dark before flow cytometry (excitation 488 nm, emission 525 nm; recorded in FL-1). Cotreatment with a protonophore, 5 µ_M_ carbonyl cyanide m-chlorophenylhydrazone (Sigma), for 15 minutes at 37°C resulted in decreased tetramethylrhodamine and DiOC6 (16,17). All fluorescent probes were obtained from Molecular Probes (Eugene, OR).
Measurement of cytoplasmic and mitochondrial calcium levels
Cytoplasmic calcium ([Ca2+]c) levels were assessed by loading the cells with 1 µ_M_ Fluo-3/acetoxymethyl ester (AM) (excitation 506 nm, emission 526 nm; recorded in FL-1). After entering the cell, AM hydrolysis occurs, and thereafter, the dye is trapped in the cytosol. Fluo-3 elicits a large increase in fluorescence intensity upon binding of calcium. To investigate early Ca2+ fluxing, freshly isolated cells were prelabeled with Fluo-3 and stimulated with OKT3 and anti-CD28 monoclonal antibodies while Fluo-3 fluorescence of annexin V− cells was continuously recorded by flow cytometry. Ca2+ levels 24 hours after CD3/CD28 costimulation were measured by staining with Fluo-3 at the termination of incubation, in parallel with staining with dyes monitoring Δψmphosphatidylserine (PS) externalization, and T cell antigen expression. Mitochondrial calcium levels ([Ca2+]m) were estimated by loading the cells with 1 µ_M_ Rhod-2/AM, which is compartmentalized into the mitochondria (18). Samples were analyzed using an LSRII flow cytometer (Becton Dickinson, Mountain View, CA) equipped with 20 mW argon (emission 488 nm) and 16 mW helium-neon lasers (emission 634 nm). Using 4-color immunofluorescence, cytosolic and mitochondrial Ca2+ levels, Δψmand PS externalization within T cell subsets were concurrently analyzed by parallel staining with 1) Fluo-3 or DiOC6 (FL-1), 2) Rhod-2 or tetramethylrhodamine (FL-2), 3) annexin V–Alexa Fluor 647 (FL-5), and 4) allophycocyanin-Cy7–conjugated monoclonal antibody UCHT1 recognizing the CD3ε chain (PharMingen) (FL-6). Dead cells and debris were excluded from the analysis by electronic gating of forward and side scatter measurements on 10,000 cells. In each experiment, SLE patient and control cells were studied in parallel.
Statistical analysis
Results were analyzed by Student’s _t_-test. P values less than 0.05 were considered significant.
RESULTS
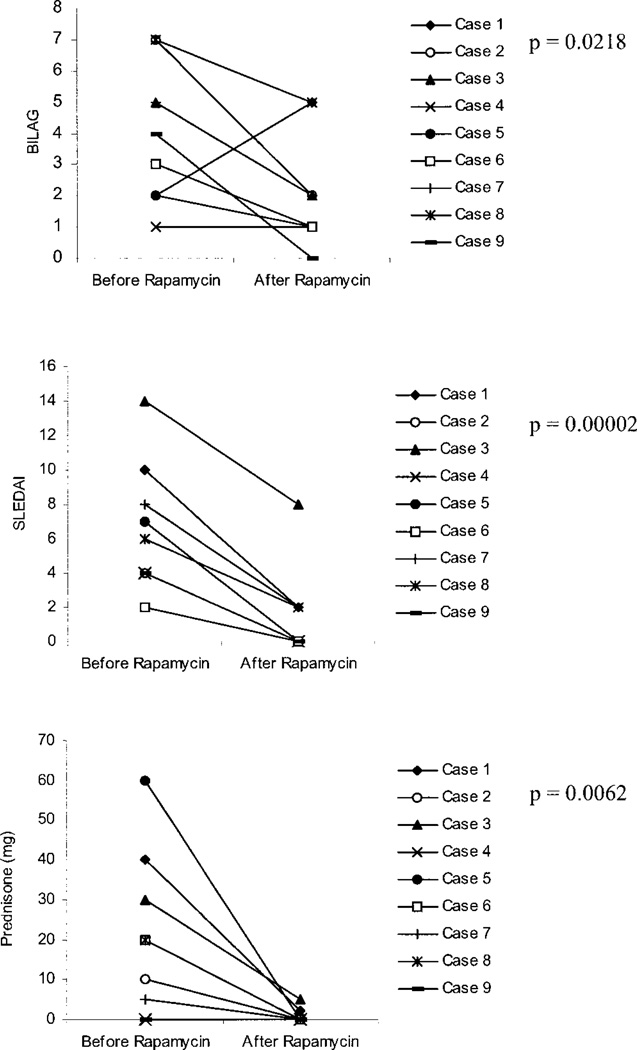
Clinical features of the 9 SLE patients at the time of initiation of rapamycin treatment are shown in Table 1. Rapamycin was well tolerated and proved effective for control of disease activity in all 9 patients. Disease activity (by BILAG score and SLEDAI) and concurrent prednisone dosage at the time of rapamycin initiation and the last followup visit (after 6–48 months of treatment) are shown in Figure 1. The BILAG disease flare index was reduced in 7 patients, unchanged in 1 patient, and increased in 1 patient. In patient 1, the increase in the BILAG score (which is considered to be a highly sensitive instrument for detecting disease flares) was due to transient arthralgias during the last observation period, which did not require adjustment of the prednisone dosage. At the last followup, the mean ± SEM reduction in the BILAG score in the 9 patients compared with pretreatment was 1.93 ± 0.9 (P = 0.0218). The SLEDAI was reduced by 5.3 ± 0.8 (range 2–8) (P = 0.00002). Three of the patients had cyclophosphamide-treated lupus nephritis. In all 3, the nephritis remained in remission throughout the period of rapamycin treatment, with normal serum creatinine levels and urinary protein levels of <300 mg/24 hours.
Table 1.
Clinical characteristics of the 9 female SLE patients treated with rapamycin*
| Patient/age/race | Durationof SLE,years | Clinicalmanifestations | Serologic features | Prednisone dosage,mg/day | Previous medications | Duration of rapamycintreatment, months | BILAG score/SLEDAIscore |
|---|---|---|---|---|---|---|---|
| 1/43/W | 15 | Arthritis, RP, serositis, nephritis, rash, AVN | Anti-DNA, anti-RNP, aCL | 40 | HCQ, MTX, AZA, CYC, MMF | 48 (HCQ, MMF) | 5/2 |
| 2/52/W | 5 | Arthritis, leukopenia, AVN | Anti-DNA, aCL, low C3 | 20 | HCQ, MTX, MMF | 25 | 2/0 |
| 3/26/W | 7 | Arthritis, oral ulcers, serositis, nephritis, thrombocytopenia AVN | Anti-DNA, aCL, low C3/C4 | 30 | HCQ, CYC, AZA, MMF, MTX | 44 (HCQ, MMF) | 5/8 |
| 4/54/W | 5 | Arthritis, leukopenia | Anti-DNA, low C3/C4 | 0 | HCQ, MMF | 16 | 1/0 |
| 5/18/W | 2 | Arthritis, pulmonary embolism, nephritis, serositis, rash, AVN | Anti-DNA, low C3/C4 | 60 | HCQ, CYC, MMF | 11 | 1/0 |
| 6/21/W | 6 | Arthritis, oral ulcers, rash, pleuritis | Anti-DNA, low C3/C4 | 20 | HCQ, MMF | 10 | 1/0 |
| 7/37/B | 4 | Arthritis, oral ulcers, hemolytic anemia, AVN | Low C3/C4 | 5 | HCQ, MMF | 10 (MMF) | 2/2 |
| 8/43/W | 4 | Arthritis, oral ulcers, rash, RP | – | 20 | HCQ, MMF | 6 | 5/2 |
| 9/43/W | 12 | Arthritis, anemia, thyroiditis | Anti-DNA | 0 | HCQ, MMF | 7 | 0/0 |
Figure 1.
Effect of rapamycin on British Isles Lupus Assessment Group (BILAG) and Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) scores and daily prednisone dosage in 9 patients with systemic lupus erythematosus. “After rapamycin” values correspond to the treatment durations shown in Table 1.
At the time of rapamycin initiation, anti-DNA antibody titers and complement levels were elevated in 2 patients (patients 3 and 9). At followup, complement levels were normalized in both patients, and anti-DNA had become negative in patient 3. After 7 months of rapamycin administration, arthritis and fatigue were suppressed and the anti-DNA titer reduced from 1,111 IU/ml to 273 IU/ml (normal ≤99) in patient 9.
Prior to initiation of rapamycin therapy, prednisone treatment was required in 7 of the 9 patients (Figure 1). After clinical control was established and disease activity was reduced as determined by the BILAG and SLEDAI, prednisone at >10 mg/day was tapered by decrements of ≤5 mg in a 2-week period, prednisone at 5–10 mg/day was tapered by decrements of 1 mg in a 2-week period, and prednisone at <5 mg/day was tapered by decrements of 1 mg in a 4-week period. After treatment with rapamycin, the mean ± SEM reduction in the daily dosage of prednisone in the 7 patients was 26.4 ± 6.7 mg (P = 0.0062).
Clinical features of the 7 control SLE patients not treated with rapamycin are shown in Table 2. The disease activity scores of these patients were higher than those of patients who had received 6–48 months of rapamycin treatment (mean BILAG score 5.00 versus 2.11 [P = 0.02]; mean SLEDAI score 3.14 versus 1.55 [P = 0.11]). These observations are also consistent with the notion that rapamycin treatment is beneficial in SLE.
Table 2.
Clinical characteristics of the 7 female SLE patients not treated with rapamycin, at the time of assessment of mitochondrial function and Ca2+ signaling*
| Patient/age/race | Durationof SLE,years | Clinicalmanifestations | Serologic features | Prednisonedosage,mg/day | Othermedications | BILAG score/SLEDAIscore |
|---|---|---|---|---|---|---|
| 1/50/W | 6 | APS, arthritis, oral ulcers, RP | LAC | 0 | HCQ, MTX | 5/2 |
| 2/58/W | 10 | Oral ulcers, APS, arthralgias | Low C3/C4 | 0 | HCQ, dapsone | 5/6 |
| 3/66/B | 25 | alopecia, thrombocytopenia, RP | Anti-SSA | 0 | AZA | 2/2 |
| 4/20/W | 1 | APS, nephritis, myocarditis, thrombocytopenia | Low C3/C4, aCL, LAC, anti-DNA, antihistone | 20 | MMF, rituximab | 13/6 |
| 5/48/W | 12 | Arthralgias, APS, malar rash, oral ulcers, RP, thrombocytopenia | Low C3 | 0 | HCQ, MMF | 4/2 |
| 6/63/W | 10 | Arthritis, oral ulcers | – | 0 | HCQ | 4/4 |
| 7/66/B | 1 | Alopecia, pericarditis, oral ulcers | Anti-DNA | 0 | HCQ | 2/0 |
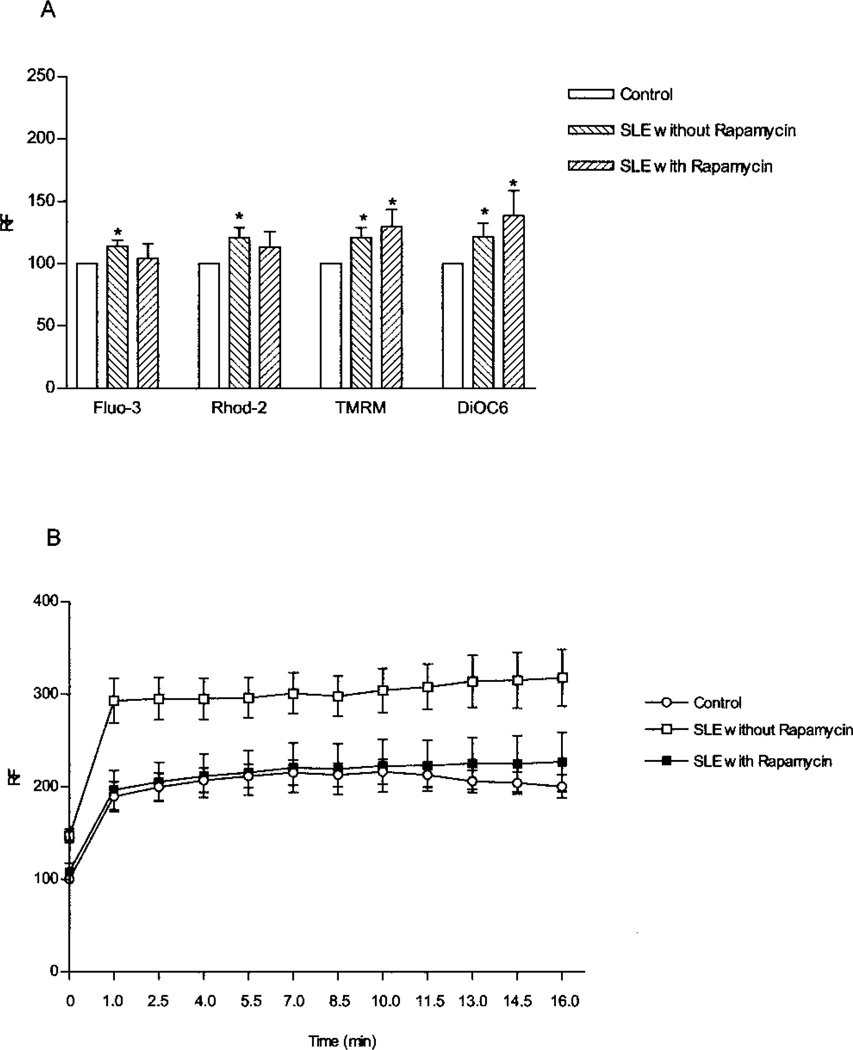
T cells from 7 healthy controls, 7 SLE controls, and 6 rapamycin-treated SLE patients were used for studies of Ca2+ signaling and mitochondrial transmembrane potential. While mitochondrial hyperpolarization (MHP) persisted (Figure 2A), baseline [Ca2+]c and [Ca2+]m and T cell activation–induced rapid Ca2+ fluxing were normalized in rapamycin-treated patients (Figure 2B). T cells from SLE patients not receiving rapamycin showed significantly elevated Ca2+ levels at each time point, with P values versus levels in healthy controls ranging between 0.0008 at time 0 to 0.023 at 16 minutes. In contrast, the level of CD3/CD28-induced Ca2+ fluxing in T cells from rapamycin-treated SLE patients was not significantly different from that in cells from healthy donors.
Figure 2.
A, Levels of cytoplasmic calcium (Fluo-3), mitochondrial calcium (Rhod-2), and mitochondrial transmembrane potential (tetra-methylrhodamine [TMRM] and DiOC6 fluorescence) in 7 healthy controls, 7 systemic lupus erythematosus (SLE) patients not treated with rapamycin, and 6 SLE patients treated with rapamycin. Values are the mean and SEM. * = P < 0.05 versus healthy controls. B, Rapid Ca2+ fluxing in CD3/CD28-costimulated peripheral blood lymphocytes (PBLs) from 7 healthy controls, 7 SLE patients not treated with rapamycin, and 6 SLE patients treated with rapamycin. PBLs were preloaded with Fluo-3 and stimulated with OKT3 and anti-CD28 monoclonal antibodies while Fluo-3 fluorescence of annexin V− T cells was continuously recorded by flow cytometry. Values are the mean ± SEM relative fluorescence (RF) values relative to those in unstimulated cells (set at 100).
DISCUSSION
Findings in this study of 9 rapamycin-treated patients indicate that rapamycin can effectively control disease activity in SLE. Arthritis improved in all 9 patients, and cyclophosphamide-treated nephritis in 3 patients remained in remission during rapamycin treatment. The single daily oral administration and small size of the pill were liked and well-tolerated by all patients. None of the patients discontinued the drug due to lack of efficacy or adverse effects.
Lupus T cells exhibit mitochondrial dysfunction, resulting in altered Ca2+ fluxing and predisposition to a necrotic, rather than apoptotic, form of cell death (1). Increased necrosis may initiate a proinflammatory state and production of interferon in SLE (19). Compared with cells from rheumatoid arthritis patients and healthy controls, T cells from patients with SLE exhibit elevated Δψm or MHP (1, 20) and enhanced Ca2+ fluxing upon CD3/CD28 costimulation (2, 21). MHP is associated with increased mitochondrial mass in lupus T cells (21). Mitochondria can take up, store, and release Ca2+ and play a substantial role in shaping Ca2+ signals in many cell types (18, 22), including human T lymphocytes (3). Due to its effects on Δψm (9) and Ca2+ fluxing (8), rapamycin may be particularly effective for the treatment of SLE. Therefore, we examined Δψm[Ca2+]c[Ca2+]mand CD3/CD28-induced Ca2+ fluxing in T cells from 6 rapamycin-treated patients with SLE. The remarkable impact of rapamycin on T cell activation– induced rapid Ca2+ fluxing is consistent with recent observations on the role of mTOR in regulating inositol triphosphate receptor–induced Ca2+ release from the endoplasmic reticulum (23). Such Ca2+ release is necessary for the operation of Ca2+ release–activated Ca2+ channels in the plasma membrane (24). These findings suggest that the therapeutic effect of rapamycin may be related to its selective effect on Ca2+ fluxing without influencing MHP of lupus T cells.
In summary, rapamycin has shown positive effects in murine lupus, and, although the investigation included only a very small number of patients, the patients in the present study, whose SLE had been refractory to most traditional treatments, benefited from rapamycin therapy. T cell activation–induced rapid Ca2+ fluxing was normalized and could serve as a biomarker of response in rapamycin-treated patients. While additional research is clearly needed to explain its mechanism of action, prospective double-blind and placebo-controlled clinical studies for establishing a role of rapamycin in treatment of SLE seem warranted.
Acknowledgment
Supported in part by NIH grants AI-061066 and AI-48079.
REFERENCES
- 1.Gergely P, Jr, Grossman C, Niland B, Puskas F, Neupane H, Allam F, et al. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:175–190. doi: 10.1002/1529-0131(200201)46:1<175::AID-ART10015>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vassilopoulos D, Kovacs B, Tsokos GC. TCR/CD3 complex-mediated signal transduction pathway in T cells and T cell lines from patients with systemic lupus erythematosus. J Immunol. 1995;155:2269–2281. [PubMed] [Google Scholar]
- 3.Nagy G, Koncz A, Perl A. T cell activation-induced mitochondrial hyperpolarization is mediated by Ca2+- and redox-dependent production of nitric oxide. J Immunol. 2003;171:5188–5197. doi: 10.4049/jimmunol.171.10.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perl A, Gergely P, Jr, Nagy G, Koncz A, Banki K. Mitochondrial hyperpolarization: a checkpoint of T cell life, death, and autoimmunity. Trends Immunol. 2004;25:360–367. doi: 10.1016/j.it.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyttaris VC, Tsokos GC. T lymphocytes in systemic lupus erythematosus: an update [review] Curr Opin Rheumatol. 2004;16:548–552. doi: 10.1097/01.bor.0000132646.55056.e0. [DOI] [PubMed] [Google Scholar]
- 6.Nagy G, Koncz A, Perl A. T- and B-cell abnormalities in systemic lupus erythematosus [review] Crit Rev Immunol. 2005;25:123–140. doi: 10.1615/critrevimmunol.v25.i2.30. [DOI] [PubMed] [Google Scholar]
- 7.Rovira P, Mascarell L, Truffa-Bachi P. The impact of immunosuppressive drugs on the analysis of T cell activation [review] Curr Med Chem. 2000;7:673–692. doi: 10.2174/0929867003374778. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Fang Y. A novel pathway regulating the mammalian target of rapamycin (mTOR) signaling [review] Biochem Pharmacol. 2002;64:1071–1077. doi: 10.1016/s0006-2952(02)01263-7. [DOI] [PubMed] [Google Scholar]
- 9.Paglin S, Lee NY, Nakar C, Fitzgerald M, Plotkin J, Deuel B, et al. Rapamycin-sensitive pathway regulates mitochondrial membrane potential, autophagy, and survival in irradiated MCF-7 cells. Cancer Res. 2005;65:11061–11070. doi: 10.1158/0008-5472.CAN-05-1083. [DOI] [PubMed] [Google Scholar]
- 10.Warner LM, Adams LM, Sehgal SN. Rapamycin prolongs survival and arrests pathophysiologic changes in murine systemic lupus erythematosus. Arthritis Rheum. 1994;37:289–297. doi: 10.1002/art.1780370219. [DOI] [PubMed] [Google Scholar]
- 11.Food and Drug Administration. FDA approves Rapamune to prevent organ rejection. URL: http://www.fda.gov/bbs/topics/ ANSWERS/ANS00974.html.
- 12.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–127. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 13.Symmons DP, Coppock JS, Bacon PA, Bresnihan B, Isenberg DA, Maddison P, et al. Members of the British Isles Lupus Assessment Group (BILAG) Development and assessment of a computerized index of clinical disease activity in systemic lupus erythematosus. QJM. 1988;69:927–937. [PubMed] [Google Scholar]
- 14.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH the Committee on Prognosis Studies in SLE. Derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis Rheum. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 15.Perl A, Gonzalez-Cabello R, Lang I, Gergely P. Effector activity of OKT4+ and OKT8+ T-cell subsets in lectin-dependent cell-mediated cytotoxicity against adherent HEp-2 cells. Cell Immunol. 1984;84:185–193. doi: 10.1016/0008-8749(84)90089-3. [DOI] [PubMed] [Google Scholar]
- 16.Banki K, Hutter E, Gonchoroff N, Perl A. Elevation of mitochondrial transmembrane potential and reactive oxygen intermediate levels are early events and occur independently from activation of caspases in Fas signaling. J Immunol. 1999;162:1466–1479. [PMC free article] [PubMed] [Google Scholar]
- 17.Perl A, Nagy G, Gergely P, Jr, Puskas F, Qian Y, Banki K. Apoptosis and mitochondrial dysfunction in lymphocytes of patients with systemic lupus erythematosus. In: Perl A, editor. Autoimmunity: methods and protocols. Totowa (NJ): Humana; 2004. pp. 87–114. [DOI] [PubMed] [Google Scholar]
- 18.Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 19.Crow MK, Kirou KA. Interferon-α in systemic lupus erythematosus [review] Curr Opin Rheumatol. 2004;16:541–547. doi: 10.1097/01.bor.0000135453.70424.1b. [DOI] [PubMed] [Google Scholar]
- 20.Gergely P, Jr, Niland B, Gonchoroff N, Pullmann R, Jr, Phillips PE, Perl A. Persistent mitochondrial hyperpolarization, increased reactive oxygen intermediate production, and cytoplasmic alkalinization characterize altered IL-10 signaling in patients with systemic lupus erythematosus. J Immunol. 2002;169:1092–1101. doi: 10.4049/jimmunol.169.2.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagy G, Barcza M, Gonchoroff N, Phillips PE, Perl A. Nitric oxide-dependent mitochondrial biogenesis generates Ca2+signaling profile of lupus T cells. J Immunol. 2004;173:3676–3683. doi: 10.4049/jimmunol.173.6.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duchen MR. Mitochondria and calcium: from cell signalling to cell death [review] J Physiol. 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacMillan D, Currie S, Bradley KN, Muir TC, McCarron JG. In smooth muscle, FK506-binding protein modulates IP3 receptor-evoked Ca2+ release by mTOR and calcineurin. J Cell Sci. 2005;118:5443–5451. doi: 10.1242/jcs.02657. [DOI] [PubMed] [Google Scholar]
- 24.Gallo EM, Cante-Barrett K, Crabtree GR. Lymphocyte calcium signaling from membrane to nucleus [review] Nature Immunol. 2006;7:25–32. doi: 10.1038/ni1295. [DOI] [PubMed] [Google Scholar]