The Potential Biomarker Panels for Identification of Major Depressive Disorder (MDD) Patients with and without Early Life Stress (ELS) by Metabonomic Analysis (original) (raw)
Abstract
Objective
The lack of the disease biomarker to support objective laboratory tests still constitutes a bottleneck in the clinical diagnosis and evaluation of major depressive disorder (MDD) and its subtypes. We used metabonomic techniques to screen the diagnostic biomarker panels from the plasma of MDD patients with and without early life stress (ELS) experience.
Methods
Plasma samples were collected from 25 healthy adults and 46 patients with MDD, including 23 patients with ELS and 23 patients without ELS. Furthermore, gas chromatography/mass spectrometry (GC/MS) coupled with multivariate statistical analysis was used to identify the differences in global plasma metabolites among the 3 groups.
Results
The distinctive metabolic profiles exist either between healthy subjects and MDD patients or between the MDD patients with ELS experience (ELS/MDD patients) and the MDD patients without it (non-ELS/MDD patients), and some diagnostic panels of feature metabolites' combination have higher predictive potential than the diagnostic panels of differential metabolites.
Conclusions
These findings in this study have high potential of being used as novel laboratory diagnostic tool for MDD patients and it with ELS or not in clinical application.
Introduction
Major depressive disorder (MDD) is a serious psychiatric mood disorder, resulting in several detrimental socioeconomic effects, including increased healthy care expenditures and suicide rates [1]. And as a complex affective syndrome, the understanding of this disease is insufficient. Several well-established risk factors have been reported to increase an individual's likelihood of developing depression, including family history for depression, past personal history of depression, and early life stress (ELS) [2], [3]. There is study reported that the responsive of depressive disorder developed in relation to early life stress (ELS/MDD) to the treatment of depression is different [4]. So, the early life stress seems to be special in those risk factors. The epidemiological studies have provided strong evidence that the ELS, such as abuse, neglect or loss, is associated with dramatic increases in the risk to develop depression [5]–[7]. The studies in rodents and non-human primates reported that ELS induce persistent structural, functional, and epigenomic changes in some neural circuits. These changes converged in increased endocrine and autonomic reactivity to stress, anxiety-like behavior, anhedonia, cognitive impairment, pain sensitivity, and altered sleep [8]–[10]. Many of the neurobiological and behavioral effects of ELS in animal models closely parallel signs and symptoms of MDD. In addition, a group has conducted a series of clinical studies concerned whether early life adverse experience in humans is associated with neurobiological changes and whether the changes are related to depression. These studies focused on studying alterations of the HPA axis in subjects with histories of ELS and the results suggested that the ELS contributes to the neuroendocrine features of depression [4]. Because not all forms of depression are associated with ELS, the group reported the existence of biologically distinguishable subtypes of depression as a function of ELS [4]. Since the ELS/MDD may be a biologically distinguishable subtype of depression, one major question for clinical research concerned is whether there are characteristic alterations in human blood which can generate a detectable molecular phenotype for diagnoses.
Currently the firm diagnosis of the depressive disorder relies solely on the clinician's subjective identification of symptomatic clusters and scales which has the shortage of subjectivity [11]. Moreover, in routine practice, clinicians are typically challenged by fitting their patients' presentations, which lie along a continuous scale of depression severity, into strict DSM-IV based diagnostic categories, so over one-third of diagnosed depressed patients are not appropriately diagnosed [12]. An earlier clinical meta-analysis of 50371 depressed patients from 41 studies found the accuracy of symptom-based diagnosis of MDD to be a mere 47% [13]. In light of these factors, the development of empirical laboratory-based diagnostic approaches for MDD and its subtypes is required. Plasma is always chosen to be testing sample in the empirical laboratory-based diagnostic method for it can be collected at minimal risk and cost to the patients. And peripheral metabolic disturbances have been increasingly implicated in psychiatric mood disorder, including MDD [14]–[16]; it is therefore conceivable that introduction of metabonomic screen may generate a detectable molecular phenotype for diagnosis MDD and its subgroup (ELS/MDD and non-ELS/MDD) in plasma.
With the development of analytical technologies and methods, metabonomics approaches, which enables simultaneous quantitative measurement of numerous small molecules within a particular sample, are widely applied in the investigation of disease classification, potential biomarker discovery and molecular mechanism of diseases [17]–[19]. The metabolic profiling techniques, such as integrated gas chromatography/mass spectrometry (GC/MS) coupled with multivariate statistical analysis, are being widely used in metabonomics approaches [20]–[22]. Early studies employed the metabonomics approach have identified the panels of metabolites associated with depression-like behavior in animal models [23]–[25] and the metabolic perturbation in diagnosis of MDD patients [26], [27]. In this study, the central hypothesis was that there is characteristic metabolic alteration associated with the pathophysiologic mechanisms of the ELS/MDD in the blood which may generate a detectable molecular phenotype for diagnosis. Therefore, GC/MS coupled with multivariate statistical analyses was used to compare the metabolite profiles of plasma samples from ELS/MDD patients, non-ELS/MDD patients and healthy subjects. Furthermore, Tclass system [28]–[30], a machine learning method combining Fisher's linear discriminant analysis and feature selection based on a stepwise optimization process for classification and feature selection, was applied to overcome the positively biased cross-validation estimate induced by the diagnostic panels which was constituted by the pre-selecting differential metabolites [31] and to improve the predictive power of diagnostic metabolites' panels. The introduction of metabonomic screening and the Tclass system analysis may provide a novel empirical laboratory-based test for diagnosing MDD and its subtypes (ELS/MDD and non-ELS/MDD).
Methods
Subjects and sample collection
Plasma samples were collected from 25 healthy adults, 46 patients with chronic form of MDD, including 23 patients with previous ELS and 23 patients without previous ELS. The age ranges for above three groups were 27±5, 29±8, 30±6 years, respectively. All patients were diagnosed at the Second Xiangya Hospital of Central South University (Changsha, China). All subjects enrolled in this study volunteered to participate in this study. This study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University, China. A complete description of the study was provided to every subject and his or her legal guardians, and all participants had the capacity to consent. Written informed consent was obtained from each subject. All 71 subjects were examined for MDD according to the criteria of Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [32] and the 46 subjects were diagnosed as MDD patients. Severity of depression was measured with Self-Rating Depression Scale (SDS). The SDS was designed to assess the level of depression for patients diagnosed with MDD [33]. The Self-Rating Anxiety Scales (SAS) was used to monitor the anxiety mood in MDD patients. The SAS was only designed to measure the level of anxiety mood [34], [35]. The score on a rating scale, like SDS or SAS, is insufficient for diagnosing, and it just provides an indication of the severity of this symptom for a time period [36]. And then, the MDD patients were examined for childhood trauma. For assignment to the major depressive disorder patients with early life stress experience (ELS/MDD) group, the MDD patients must have had experienced at least one form of sexual or physical abuse before the age of 13 years. In our study, sexual abuse was defined as having been forced to touch another person's intimate parts, having been touched in intimate parts, attempted or completed intercourse. Physical abuse was defined as having been spanked, kicked or choked in a way that left bruises or injuries, having been attacked with a weapon or tied up or locked in a room or a closet. For assignment to the major depressive disorder patients without early life stress experience (non-ELS/MDD) group, the MDD patients could not have had experienced any traumatic or major stressful life event before the age of 13 years. The severity of the ELS was assessed by the Early Trauma Inventory (ETI). The ETI is a structured interview that assesses the number, frequency, and duration of early trauma types, resulting in a score for each trauma type and a total score [37], [38]. Blood samples were collected before breakfast on the second day after hospitalization with the EDTA-anticoagulant tube. After the centrifugation (3000×g) for 10 min at 4°C, the plasma samples were collected and stored at −80°C until analysis.
Sample preparation
The pretreatment of plasma samples and GC/MS analysis were performed as previously described [39]–[41]. 500 µL of methanol (100%) and 20 µL of ribitol stock solution (0.2 mg/mL in deionized water) were added to 100 µL aliquots of thawed plasma samples. The mixture was shaken (100 rpm) at 70°C for 15 min and then centrifuged at 13000×g for 10 min. The supernatant was collected and mixed with chloroform (270 µL) and deionized water (450 µL). The mixture was shaken (80 rpm) at 37°C for 5 min and centrifuged at 4000×g for 10 min. The polar phase was separated and evaporated under a stream of N2 gas to dryness about 90 min. The dried residue was dissolved in 40 µL methoxamine hydrochloride (20 mg/mL pyridine) and incubated at 30°C for 90 min with continuous shaking. Then 40 µL of N-methyl-N-trimethylsilyl-trifluoroacetamide (MSTFA) containing 1% trimethylchlorosilane (TMCS) was added at 37°C for 30 min. The derivative samples were stored at room temperature for 120 min before injection. All chemicals were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MI).
GC/MS
0.3 µL aliquot of sample solution was injected at a split ratio of 25:1 into a GC/MS system consisting of a HP 6890 gas chromatograph and a time-of-flight mass spectrometer (Waters Co., Milford, MA). Chromatography was performed on a DB-5 MS capillary column (30 m×0.25 mm i.d., 0.25 mm thickness). Helium carrier gas was set to a constant flow rate of 1 mL/min. The temperatures of injection, interface, and ion source were adjusted at 230°C, 290°C, and 220°C, respectively, with an electron energy of 70 eV and a trap current of 70 µA. The GC oven temperature was first held at 70°C for 5 min solvent delay and then ramped at 5°C/min to a final temperature of 310°C, and this was followed by a 1 min isocratic, cool-down to 70°C, and an additional 5 min delay. The mass spectra over the m/z range 50–800 were acquired at a scan rate of 0.5 s per scan and an inter scan delay of 0.1 s in centroid mode. The GC/MS system was operated at a multichannel plate voltage of 2800 V, a pushout voltage of 980 V, and a pusher interval of 40 µs.
Data processing and pattern recognition
Total ion current chromatograms (TICs) were obtained by using the MassLynx software (Waters Co.). Peaks with intensity higher than 10-fold of the signal-to-noise (S/N) ratio were recorded and integrated. The electron impact (EI) GC/MS data were converted into CDF files for peak extraction by Automated Mass Spectral Deconvolution and Identification System (AMDIS). The compounds in all recorded peaks in TICs were identified by using National Institute of Standards and Technology (NIST 02) library with EI spectra and then be validated using reference standards [42]. In addition, the GC/MS data were also processed using the MarkerLynx Applications Manager software (Waters Co.). A peak deconvolution package was incorporated in the software, which allowed the alignment of detection and retention times for peaks in each data file across the whole data set. MarkerLynx extracted components and generated a matrix of detected peaks that are represented by their m/z and retention time pairs along with their associated intensities. And intensities of the peaks of the validated compounds were normalized as relative peak area (RPA) to the ribitol's peak intensity which was defined as the internal standard, and the ribitol's peak intensity (internal standard intensity) was arbitrarily set to 1. The RPA was evaluated by multivariate data analysis (PCA and PLS-DA) to reduce the complexity of plasma GC data and facilitate analysis. The RPA data were also subjected to Tclass system for analysis.
Discriminant analysis
To find out the biomarkers to discriminate patients from healthy subjects, the multivariate statistical analysis and the Tclass classification system were used in this study. For multivariate statistical analysis, PCA and PLS-DA were carried out for group discrimination. Based on the variable importance on a projection (VIP) with a threshold of 1.0 from the PLS-DA model, a number of metabolites variables were obtained to be responsible for the difference in the metabolic profiles between different groups, which were defined as the differential metabolites. And then, logistic regression was fit to find the diagnostic panel of differential metabolites between these groups.
In addition, Tclass classification system was applied to find out the diagnostic panel constituted by feature metabolites' combination [28]. At first, a feature forward selection procedure with the leave-one-out cross validation (LOOCV) as the object function was firstly applied to search for the optimal diagnostic panels, and then the stability index analysis was used to get an optimal biased assessment about how well the prediction model constructed by diagnostic panels will fit an independent data set. Through randomly dividing the sample into two parts with the partition ration 85% for 1000 times, the major part was used as the training set and the minor part was taken as the independent test set for each partition. The average of 1000 predictive accuracies from the test sets was defined as the stability index of the diagnostic panels which was suitable value for evaluating the performance of cross-validation estimates and the performance of predictive potential of the diagnostic panels in practice. Finally, the feature metabolites set with the highest stability indexes was found, and the related model that is composed of 1000 classifiers was constructed. Additionally, the ratio of the number of classifiers correctly predicting a sample and 1000 was taken as the probability (P) to predict MDD. Therefore, if P value is more than 0.5, the sample will be predicted to be MDD sample. The subgroups of MDD (ELS/MDD and non-ELS/MDD) were processed by the same analysis procedure.
Areas under the receiver operating characteristic curve (AUC) of the ROC analysis were calculated to evaluate the performance of these diagnostic panels. And these diagnostic biomarker panels were also validated by the Tclass system with the stability analysis.
Results
Demographic and clinical data
The demographic and clinical data were summarized in Table 1. There were no differences in age and racial distribution between different groups. Patients with MDD had significantly higher SDS score than that in healthy subjects [F (2,71) = 64.7, P<0.001]. And according to SAS score, the MDD patients had been observed existing anxiety mood [F (2,71) = 23.4, P = 0.004]. The 1990-92 National Comorbidity Survey (US) reported that 51% of those with MDD also suffer from lifetime anxiety [43]. And a study reported that the anxiety symptoms can have a major impact on the course of a depressive illness, with delayed recovery, increased risk of relapse, greater disability and increased risk of relapse, greater disability and increased suicide attempts [44]. Therefore, the anxiety mood can be often observed in the MDD patients. The MDD patients with a history of ELS had higher mean ETI score than those without ELS [F (1, 46) = 6.42, P<0.001]. There was no difference in current episode duration between two MDD groups (ELS/MDD group and non-ELS/MDD group). There was no difference in objective support between the two subgroups of MDD. Patients with MDD reported less subjective support and utilization of support than healthy subjects [F (2,71) = 3.71, P = 0.03] and [F (2,71) = 7.54, P = 0.001], respectively.
Table 1. Demographic and clinical features of healthy subjects, non-ELS/MDD and ELS/MDD.
| healthy subjects (n = 25) | non-ELS/MDD (n = 23) | ELS/MDD (n = 23) | Statistic | |
|---|---|---|---|---|
| Age (mean, SD)a | 27.5 (4.4) | 29.8 (6.0) | 29.2 (8.3) | F(2,71) = .80,ns |
| Race (n, %) | χ2(2) = 4.36, ns | |||
| the Han nationality | 23 (92) | 23 (100) | 19 (82) | |
| other | 2 (8) | 0 (0) | 4 (17) | |
| Education (mean, SD)b | 17.3 (3.2) | 11.4 (4.0) | 13.8 (2.9) | F(2,71) = 17.6, P<0.001 |
| Married or partnered (n, %) | 8 (32) | 13 (56) | 11 (48) | χ2(2) = 3.09, ns |
| Employed (n, %) | 25 (0) | 16 (70) | 18 (78) | χ2(2) = 7.24, P = 0.027 |
| Length of current depression (mean, SD)c | 21.7 (29.5) | 27.1 (27.4) | F(1,46) = .00, ns | |
| Family history of mental disorder (n, %) | 0 (0) | 2 (9.0) | 3 (39) | χ2(2) = 3.14, ns |
| ETI Total (mean, SD) | 33.3 (8.2) | 49.2 (11.9) | F(1,46) = 6.42, P<0.001 | |
| Emotional abuse | 6.6 (1.8) | 9.7 (3.2) | F(1,46) = 8.38, P<0.001 | |
| Physical abuse | 5.9 (1.7) | 8 (3.1) | F(1,46) = 6.70, P = 0.007 | |
| Sexual abuse | 5.4 (0.7) | 6 (1.1) | F(1,46) = 3.1, P = 0.03 | |
| Emotional neglect | 9.7 (1.9) | 14.4 (4.6) | F(1,46) = 19.5, P<0.001 | |
| Physical neglect | 7.2 (1.3) | 11.1 (3.7) | F(1,46) = 17.5, P<0.001 | |
| SDS (mean, SD) | 28.3 (5.3) | 51.5 (11.0) | 54.4 (8.3) | F(2,71) = 64.7, P<0.001 |
| SAS (mean, SD) | 26.6 (4.6) | 42.3 (11.0) | 42.7 (10.3) | F(2,71) = 23.4, P = 0.004 |
| Social support (mean, SD) | 37 (5.8) | 32.2 (7.4) | 29.5 (8.4) | F(2,71) = 6.10, P = 0.004 |
| Objective support | 8.4 (3.0) | 7.4 (2.0) | 7.1 (3.0) | F(2,71) = 1.44, ns |
| Subjective support | 20.0 (3.8) | 18.0 (5.1) | 16.0 (5.6) | F(2,71) = 3.71, P = 0.03 |
| Utilization of support | 8.6 (1.6) | 6.8 (2.1) | 6.9 (1.6) | F(2,71) = 7.54, P = 0.001 |
Metabolic profiles and differential metabolites of each group
Typical GC/MS TICs of plasma samples from three groups were obtained. Thirty-five peaks of compounds were identified to be amino acids, fatty acids, carbohydrates, organic acids and mineral acid (Table 2). The results of the multivariate statistic analysis towards metabonomic data showed the distinct cluster between each group, indicating that the metabonomic data in each group have distinct metabolic profiles (Figure 1 and Figure S1). Through the PLS-DA loading plot (data not shown), many identified metabolites contributed strongly to the separation of groups were obtained. 15 metabolites stood out the VIP threshold (VIP>1), which were annotated to be differential metabolites between the healthy control and MDD (Table 2). In the 15 differential metabolites, 3 long-chain fatty acid (linoleic acid, oleic acid, heptadecylic acid) were found decreased in MDD patients; as for carbohydrate, the galactose and sorbitol were elevated while the myoinositol and mannose were decreased in MDD compared with healthy control; 4 amino acids (glycine, alanine, proline, serine) were found elevated while only leucine was decreased in MDD compared with healthy control. Erythronic acid was found decreased while butanedioic acid was found increased in MDD. Cholesterol was significantly decreased in MDD patients compared with healthy subjects. Through the same analysis procedure, 16 differential metabolites were annotated between the healthy control and ELS/MDD, including 6 amino acids (aspartic acid, glycine, alanine, threonine, serine, leucine); 5 carbohydrate (sorbitol, myoinositol, mannose, 6-deoxy-mannopyrannose, galactose); 3 long-chain fatty acid (linoleic acid, oleic acid, heptadecylic acid); 1 organic acid (erythronic acid) and cholesterol (Table 2). And then the 12 annotated differential metabolites, which were cholesterol, linoleic acid, glycine, alanine, butanedioic acid, lactic acid, glucose, oleic acid, glucopyranose, sorbitol, proline and stearic acid, were explored by PLS-DA model classifying the non-ELS/MDD and healthy control (Table 2). Moreover, the levels of cholesterol, glucopyranose, linoleic acid, glyceric acid, alanine, butanedioic acid, phosphoric acid, galactose, lactic acid, glycine, glucose, proline and stearic acid were identified relevant to the differentiation between the ELS/MDD and non-ELS/MDD (Table 2). These results suggested that some carbohydrates, amino acids and fatty acids contributed to the discrimination of ELS/MDD from healthy control or non-ELS/MDD.
Table 2. RPA of metabolites detected by GC/MS in human plasmaA.
| No. | Retention time (min) | Identified metabolites | Healthy subjects | MDD patients | ELS/MDD patients | non-ELS/MDD patients | VIPB |
|---|---|---|---|---|---|---|---|
| 1 | 6.116 | Lactic acid | 1.6345±0.41723 | 1.9009±0.47168 | 1.7224±0.48594 | 2.0795±0.39025 | VIPc = 1.23 VIPd = 1.17 |
| 2 | 7.033 | Alanine | 0.4348±0.07446 | 0.5018±0.13743 | 0.4365±0.09907 | 0.5672±0.14104 | VIPa = 1.31 VIPb = 1.25 VIPc = 1.33 VIPd = 1.45 |
| 3 | 7.933 | Oxalic acid | 0.608±0.12699 | 0.6842±0.21615 | 0.6589±0.08991 | 0.7096±0.29350 | |
| 4 | 8.399 | Hydroxybutyric acid | 0.1686±0.16895 | 0.1484±0.168 | 0.1417±0.014071 | 0.1570±0.20161 | |
| 5 | 9.75 | Valine | 0.4496±0.06243 | 0.4517±0.1094 | 0.4410±0.06865 | 0.4624±0.13973 | |
| 6 | 10.866 | Urea | 3.1162±0.96887 | 3.0487±0.90941 | 2.9548±0.66486 | 3.1425±1.10959 | |
| 7 | 11.316 | Phosphoric acid | 3.4182±0.8181 | 3.6007±0.98405 | 3.1813±0.27462 | 4.0201±1.23997 | VIPd = 1.3 |
| 8 | 11.75 | Leucine | 0.17±0.04885 | 0.1661±0.0631 | 0.1535±0.04123 | 0.1805±0.08014 | VIPa = 1.03 VIPb = 1.01 |
| 9 | 11.82 | Proline | 0.1108±0.07821 | 0.1788±0.1161 | 0.1359±0.07335 | 0.2162±0.1339 | VIPa = 1.05 VIPc = 1.1 VIPd = 1.05 |
| 10 | 12.05 | Glycine | 0.3351±0.07559 | 0.4490±0.1344 | 0.3990±0.07520 | 0.4990±0.16146 | VIPa = 1.38 VIPb = 1.55 VIPc = 1.39 VIPd = 1.16 |
| 11 | 12.333 | Butanedioic acid | 0.0239±0.00882 | 0.0434±0.04028 | 0.0269±0.00782 | 0.0625±0.05293 | VIPa = 1.03 VIPc = 1.24 VIPd = 1.36 |
| 12 | 12.377 | Glyceric acid | 0.0204±0.01170 | 0.0214±0.01203 | 0.0162±0.00702 | 0.0285±0.01394 | VIPd = 1.5 |
| 13 | 13.5 | Serine | 0.1421±0.09798 | 0.2032±0.10309 | 0.1944±0.07278 | 0.2128±0.12977 | VIPa = 1.01 VIPb = 1.13 |
| 14 | 14.15 | Threonine | 0.1622±0.10725 | 0.2205±0.11248 | 0.2144±0.08284 | 0.2269±0.13870 | VIPb = 1.15 |
| 15 | 16.233+17.767 | Aspartic acid | 0.9201±0.32851 | 1.1121±0.36793 | 1.1571±0.19671 | 1.0567±0.50531 | VIPb = 1.56 |
| 16 | 17.434 | Pyroglutamate | 0.1631±0.09368 | 0.1895±0.09589 | 0.1762±0.07038 | 0.2064±0.12116 | |
| 17 | 18.55 | Erythronic acid | 0.0260±0.02118 | 0.0168±0.00821 | 0.0169±0.00920 | 0.0167±0.007 | VIPa = 1.09 VIPb = 1.05 |
| 18 | 23.951 | Mannose | 0.0482±0.02763 | 0.0355±0.02175 | 0.0307±0.02069 | 0.0414±0.2214 | VIPa = 1.02 VIPb = 1.37 |
| 19 | 24.234 | Isocitric acid | 0.3288±0.13325 | 0.3388±0.10913 | 0.3373±0.05798 | 0.3410±0.15705 | |
| 20 | 24.867 | Arabopyranose | 0.8943±0.15508 | 0.9838±0.24707 | 0.9478±0.20382 | 1.0197±0.28393 | |
| 21 | 25.734 | Unknown carbohydrate | 1.6946±0.63606 | 1.8667±0.58277 | 1.7689±0.32809 | 2.0119±0.73246 | |
| 22 | 25.851 | Glucose | 3.7220±0.64182 | 4.2806±0.97574 | 3.9382±0.85764 | 4.6231±0.98319 | VIPc = 1.21 VIPd = 1.08 |
| 23 | 26.201 | Galactose | 1.5240±0.17550 | 1.7945±0.34136 | 1.6611±0.32737 | 1.9278±0.30658 | VIPa = 1.32 VIPb = 1 VIPd = 1.19 |
| 24 | 26.434 | Sorbitol | 0.0175±0.0335 | 0.3028±0.63857 | 0.4385±0.819 | 0.1254±0.1759 | VIPa = 1.09 VIPb = 1.44 VIPc = 1.1 |
| 25 | 27.001 | Gluconate | 0.0805±0.04228 | 0.072±0.03247 | 0.0757±0.03107 | 0.0673±0.03449 | |
| 26 | 27.567 | Glucopyranose | 2.5494±0.42851 | 2.7061±0.59253 | 2.3710±0.40559 | 3.0413±0.56461 | VIPc = 1.16 VIPd = 1.76 |
| 27 | 28.468 | 6-deoxy-mannopyrannose | 0.1005±0.05662 | 0.1401±0.08907 | 0.1382±0.05129 | 0.1421±0.11898 | VIPb = 1.31 |
| 28 | 28.784 | Palmitic acid | 0.7542±0.19656 | 0.6426±0.19054 | 0.6827±0.17838 | 0.6024±0.19766 | |
| 29 | 29.534 | Myoinositol | 0.1330±0.05742 | 0.1041±0.05806 | 0.0989±0.02892 | 0.1098±0.07914 | VIPa = 1.1 VIPb = 1.4 |
| 30 | 30.601 | Heptadecylic acid | 0.0169±0.00972 | 0.012±0.00565 | 0.0123±0.00544 | 0.0115±0.00641 | VIPa = 1.21 VIPb = 1.15 |
| 31 | 31.484 | Tryptophane | 0.02±0.01 | 0.0232±0.01423 | 0.02±0.012 | 0.03±0.018 | |
| 32 | 31.751 | Linoleic acid | 0.1394±0.06166 | 0.0689±0.05385 | 0.0914±0.05650 | 0.0344±0.02334 | VIPa = 1.63 VIPb = 1.49 VIPc = 1.75 VIPd = 1.53 |
| 33 | 31.868 | Oleic acid | 0.1874±0.09155 | 0.1102±0.0869 | 0.1292±0.07510 | 0.0836±0.09759 | VIPa = 1.3 VIPb = 1.3 VIPc = 1.21 |
| 34 | 32.384 | Stearic acid | 0.8864±0.18828 | 0.7775±0.2 | 0.8424±0.15551 | 0.7096±0.22150 | VIPc = 1.01 VIPd = 1.01 |
| 35 | 45.652 | Cholesterol | 2.0814±0.4245 | 1.1613±0.91029 | 1.7824±0.66708 | 0.4711±0.58983 | VIPa = 1.6 VIPb = 1.03 VIPc = 2.18 VIPd = 2.23 |
Figure 1. Score plots for PLS-DA of GC/TOF-MS data from healthy subjects, MDD patients and its subgroup (ELS/MDD patients and non-ELS/MDD patients).
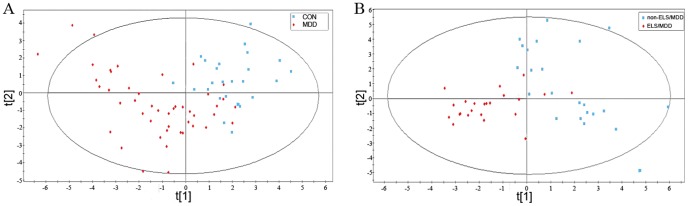
(A) The clustering analysis of metabonomic profiles from healthy subjects and MDD patients; (B) The clustering analysis of metabonomic profiles from ELS/MDD and non-ELS/MDD patients. MDD indicates depressed patients, ELS/MDD indicates depressed patients with early life stress experience and non-ELS/MDD indicates depressed patients without early life stress experience.
Diagnostic biomarker panels for identification of MDD and its subgroups (ELS/MDD and non-ELS/MDD)
Based on quantification of fewer metabolites, diagnosis will be more convenient and economical if the metabolites can provide sufficient information [31]. To explore the simplified and the optimal prediction diagnostic panels for MDD and even its subgroups (ELS/MDD and non-ELS/MDD), the ROC analysis and Tclass system were all applied.
At first, the differential metabolites in the plasma were used as biomarkers candidates. And then, the ROC analysis was carried out to find out the diagnostic panel from these biomarkers candidates. We sorted the VIP for each differential metabolite in a descending order. Logistic regression was then fit from 1 to 9 differential metabolites. According to the AUC of the ROC analysis, the logistic regression with 9 metabolites had the highest predictive potential because the AUC of this differential metabolites panel was 1 between the MDD and healthy control (Figure S2-A). According to the traditional academic scoring system, the AUC of 1 represents a perfect prediction test [31]. These 9 differential metabolites were linoleic acid, cholesterol, glycine, galactose, alanine, oleic acid, heptadecylic acid, myoinositol and sorbitol. Through the same analysis procedure, a logistic regression model with 9 differential metabolites was obtained, which had the highest predictive potential between ELS/MDD and healthy control. The AUC of this panel was 1 (Figure S2-B). These 9 differential metabolites were aspartic acid, glycine, linoleic acid, sorbitol, myoinositol, mannose, 6-deoxy-mannopyrannose, oleic acid and alanine. Between non-ELS/MDD and healthy control, the logistic regression with 3 metabolites was confirmed to have highest predictive potential, and the AUC of this panel was 1 (Figure S2-C). These 3 differential metabolites were cholesterol, linoleic acid and glycine. At last, a logistic regression model with 4 differential metabolites was found that have highest prediction potential between the ELS/MDD group and non-ELS/MDD group (Figure S2-D). These 4 differential metabolites were cholesterol, glucopyranose, linoleic acid and glyceric acid. The AUC of this panel was 1. However, Yang et al. reported that the pre-selecting differential metabolites may result in positively biased cross-validation estimates which will influence the prediction potential of the metabolic biomarkers panel [31]. Cross-validation estimate is an estimate value for assessing how accurately a prediction model will perform in practice. The traditional metabnomics studies have still used the pre-selecting differential metabolites to constitute the diagnostic panel for predicting diseases which will influence the predictive power of metabonomics approach. To overcome positively biased corss-validation estimates of the diagnostic panels, the Tclass system was applied which uses feature selection procedure such as stepwise optimization of all possible feature combinations and does not need the pre-selecting differential metabolites. And stability analysis was carried out to evaluate the performance of cross-validation estimates and the performance of predictive accuracy of each diagnostic panels obtained by Tclass system and the ROC analysis.
First of all, a model was generated by the Tclass system for classification between the healthy subjects and all MDD patients. The highest prediction power was reached through Naïve Bayes method with 9 metabolites combination, including valine, leucine, proline, glyceic acid, pyroglutamate, galactose, glucopyranose, palmitic acid and heptadecylic acid. The AUC of the feature metabolites' combination was 1 (Figure S2-E). Secondly, a model generated by Tclass system with 8 metabolites was obtained which had highest prediction power between the healthy control and ELS/MDD. And the AUC of these feature metabolites' combination including lactic acid, proline, glyceric acid, mannose, gluconate, tryptophane, stearic acid and cholesterol was 1 (Figure S2-F). Thirdly, a model with 3 metabolites (6-deoxidation mannopyrannose, palmitic acid and heptadecylic acid) was obtained which had the highest prediction power between the healthy control and non-ELS/MDD. The AUC of the feature metabolites' combination was also 1 (Figure S2-G). Finally, the optimal model generated by Tclass system with 3 metabolites (oxalic acid, heptadecylic acid and stearic acid) had the highest predictive power and the AUC of the feature metabolites' combination was 1 between the ELS/MDD and non-ELS/MDD (Figure S2-H).
The cross-validation estimates evaluation of the diagnostic biomarker panels
To evaluate the cross-validation estimates and the prediction potential of each biomarker panels, the stability analysis was carried out. Through randomly dividing the sample into two parts with the partition ration 85% for 1000 times, the major part was used as the training set and the minor part was taken as the independent test set for each partition. The average of 1000 predictive accuracies from the test sets was defined as the stability index of the diagnostic panels which was suitable value for evaluating the performance of cross-validation estimates and the performance of predictive potential of the diagnostic panels in practice [28].
When the diagnostic panel of feature metabolites' combination obtained by Tclass system and the diagnostic panel of differential metabolites established by logistic regression of ROC analysis were both used to classify the healthy control and MDD, the stability index of the diagnostic panel constituted by differential metabolites was 0.7546 and the stability index of the diagnostic panel of feature metabolites' combination was 0.9438. When the stability index analysis analyzed the diagnostic panels between the healthy control and ELS/MDD, the stability index of the differential metabolites panel was 0.7872 and the stability index of the feature metabolites' set was 0.997. The stability index of the differential metabolites panel was 0.904 and the stability index of the feature metabolites' combination was 1 between the healthy control and non-ELS/MDD. At last, the stability index of differential metabolites panel was 0.9098 and the feature metabolites' combination was 1 between the ELS/MDD group and non-ELS/MDD group (Figure 2).
Figure 2. The stability index of each diagnostic panel.
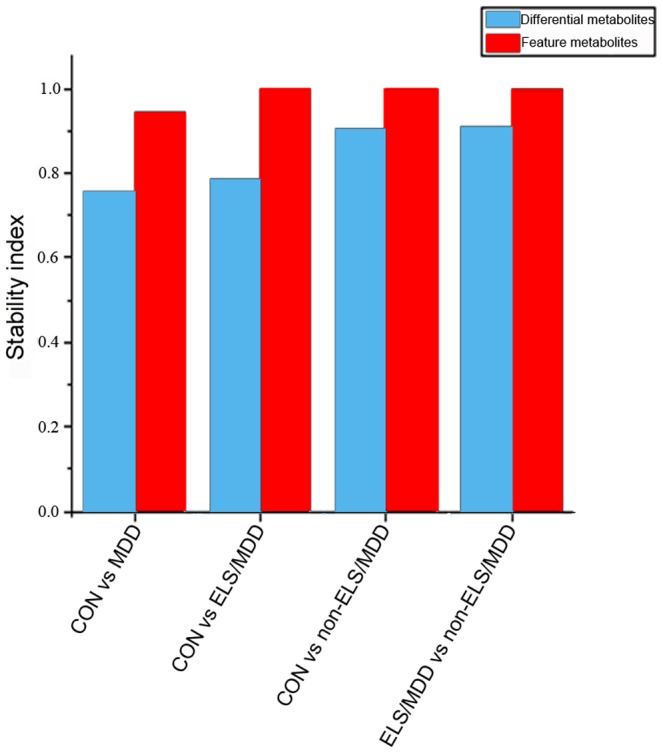
The diagnostic panels of feature metabolites' combination established by Tclass had the higher value than the corresponding diagnostic panels of differential metabolites, indicating the diagnostic panel of feature metabolites had higher predictive potential and more objective cross-validation estimate than the diagnostic panel of differential metabolites.
The ensemble classifier model for identification MDD and its subgroups (ELS/MDD and non-ELS/MDD)
Here, the models generated by Tclass system were used for prediction disease. The relationship between the stability index and the number of feature metabolites for classification between the healthy subjects and all MDD patients was provided in Figure S3-A. The highest predictive power (prediction accuracy and stability index) was reached using Naïve Bayes method and the prediction accuracy through Tclass system was 97.436% at a sensitivity of 95.238% and a specificity of 100%. The final model constructed by the Tclass system was an ensemble classifier consisting of 1000 classifiers. The results were shown in Table S1. Here is the example of the one of the classifier during the ensemble classifier, which can objectively help for MDD identification.
C1 = -193.22739-348.65282X1+637.70420X2+37.96944X3+771.28313X4-179.95208X5-21.87057 X6+82.37111 X7+281.58456 X8+3245.21105X9
MDD1 = -143.65828-251.96156X1+453.25011X2+31.32127X3+475.62189X4-117.87757X5-10.38482X6+68.85657 X7+224.79745X8+2492.61184X9
The X1 (valine), X2 (leucine), X3 (proline), X4 (glyceic acid), X5 (pyroglutamate), X6 (galactose), X7 (glucopyranose), X8 (palmitic acid) and X9 (heptadecylic acid) stand for the RPA level of metabolites in the diagnostic panel. For one plasma sample, the RPA values for these metabolites were applied to the 1000 classifiers like this and the sample will be predicted to be the sample of MDD patients if there were more than 500 classifiers in which the value in the equation of “MDD” is bigger than the value in the equation of “C”. The relationship between the predictive power and the number of feature metabolites for classification between the healthy subjects group and ELS/MDD patients group was displayed in Figure S3-B. The prediction accuracy through Tclass system was 100% (Sensitivity is 100%; Specificity is 100%), when combining eight metabolites. 1000 related classifiers were taken as the final classification profile and the results were shown in Table S1. The relationship between the predictive power and the number of feature metabolites for classification between the healthy subjects and non-ELS/MDD patients was provided in Figure S3-C. The optimal prediction result was obtained from the combination of 3 metabolites. The prediction accuracy was as high as 100% (Sensitivity is 100%; Specificity is 100%). The related classifiers were displayed in Table S1. Finally, the Tclass system was applied to generate a model for classification between the ELS/MDD and non-ELS/MDD groups. The relationship between the predictive performance and the number of metabolites was provided in Figure S3-D. The optimal prediction result was obtained from the combination of 3 metabolites and their prediction accuracy was 100% (Sensitivity is 100%; Specificity is 100%). The related classifiers were shown in Table S1. These models established by the Tclass system could be used to identify MDD and its subgroups.
Discussion
MDD is a serious psychiatric mood disorder and it is also a complex affective syndrome. Numerous epidemiologic and clinical studies have provided compelling evidence for a strong association between various forms of early life stress and depressive symptoms or disorders [5], [45], [46]. Recently, several studies reported that the depression developed in relation to early life stress experiences (ELS/MDD) have a characteristic neuroendocrine alterations associated with the pathophysiologic mechanisms of ELS/MDD [4]. To explore the characteristic metabolic alterations of ELS/MDD, we developed a metabonomics approach that uses GC/MS coupled with multivariate statistical analysis for identifying the differences in the global plasma metabolites and generating mathematic models for diagnosis. Our approach included 3 steps: 1) the distinct metabolic profile of the MDD and its subgroups (ELS/MDD and non-ELS/MDD) exploration; 2) diagnostic biomarker panels for prediction of MDD patients and its subgroups (ELS/MDD and non-ELS/MDD) investigation; 3) the diagnostic models for prediction of MDD patients and its subgroups (ELS/MDD and non-ELS/MDD) construction.
Limitations
Through the multivariate statistical analysis, we found that it is possible to separate the MDD and its subgroup (ELS/MDD and non-ELS/MDD) with the plasma metabonomic data. The results of the analysis indicated that each group has distinct metabolic profiles in the blood (Figure 1 and Figure S1). So the aim of this study was to examine the feasibility of an empirical laboratory-based method to diagnose MDD and even its subgroup (ELS/MDD and non-ELS/MDD). In this study, plasma samples were collected from 25 healthy subjects, 46 patients with MDD, including 23 patients with ELS and 23 patients without ELS. We made appropriate refinement towards the traditional metabonomic data analysis approach to obtain the mathematic model with optimal predictive power from the relatively small sample size. In the most of the metabonomics studies, the diagnostic biomarkers panels for prediction diseases were constituted by the pre-selecting differential metabolites. However, the pre-selecting differential metabolites may result in positively biased cross-validation estimates which will influence the predictive power of the metabonomics approach [31]. In this metabonomics study, the Tclass system was applied to search the optimal feature combinations of metabolites for diagnosis and prognosis. Take the advantages of selecting biomarker panel without needing pre-selecting differential metabolites, the Tclass system can overcome the positively biased cross-validation estimates and improve the predictive power of the metabonomics method [28]. Through application of Tclass system, the GC/MS based plasma metabonomics approach identified MDD patients at a sensitivity of 95.2381% and a specificity of 100%; and identified its subgroups, ELS/MDD patients, at a sensitivity of 100% and specificity of 100%. The small size of the training set for the prediction model required achieving greater than 90% sensitivity and specificity highlights the power of this approach [47]. Our analysis is preliminary, and substantially larger sample size obtained through application of this refined metabonomics approach to clinical practice may further improve the diagnostic sensitivity and specificity of this novel metabonomics approach.
Novel metabonomic insights and diagnostic method about ELS/MDD
The results of multivariate statistic analysis suggested that the ELS/MDD have characteristic metabolic alterations associated with the pathophysiologic mechanisms of ELS/MDD in the blood. The PLS-DA model implicated 16 metabolites responsible for discrimination ELS/MDD and healthy control, and 13 metabolites responsible for discrimination ELS/MDD and non-ELS/MDD. The overlapping metabolites from these two metabolites sets may play a role in ELS/MDD pathophysiologic mechanism and in discriminating ELS/MDD from MDD. These metabolites included amino acids (alanine, glycine), carbohydrate (galactose), fatty acids (linoleic acid), and cholesterol.
The altered amino acid profile was noteworthy in light of previous studies that suggested the synthesis of brain neuro-transmitters related to ELS/MDD pathophysiologic mechanism can be influenced by circulating amino acids levels [27], [48], [49].The previous study reported that the higher plasma levels of alanine, glutamine, glycine, and taurine are existed in MDD patients [50] and our study also found the higher plasma levels of alanine and glycine in MDD including ELS/MDD and non-ELS/MDD. In this study, the plasma levels of alanine and glycine were decreased in the ELS/MDD when they were compared to non-ELS/MDD. These findings implicated that the decreased plasma levels of alanine and glycine may be associated with the ELS/MDD mechanism and provide an ELS-associated metabolic alteration in discriminating ELS/MDD from non-ELS/MDD. The plasma level of galactose was found elevated in MDD compared with healthy control. In MDD, the level of galactose in ELS/MDD was lower than non-ELS/MDD. Galactose is an important metabolites involved in the formation of glycans featuring galactose and its derivatives which were essentially bound by galectins [51], [52]. Converging evidence suggested that galectins play an important role in neuroinflammation and brain development and function [53]–[55], and Kraneveld et al. reported that dietary or pharmacological modulation with small molecules targeting the galectin response in neurodevelopment disorders such as MDD could be a future therapeutic approach [56]. Although galactose's role in MDD is not clear, the foregoing reports implied that galactose may be involved in galectin-glycan interactions associated with the neuro-immune axis in mental disorders such as MDD and ELS/MDD. Linoleic acid level in the plasma was found decreased in MDD (including ELS/MDD and non-ELS/MDD) compared to healthy control. Inside the MDD group, the plasma level of linoleic acid in ELS/MDD patients was higher than the MDD patients without ELS. Consistent with these findings, a previous study reported that the plasma level of linoleic acid in MDD patients was significantly lower than the level of healthy subjects [57]. And many studies reported that ELS altered the metabolic profile of plasma polyunsaturated fatty acids in adulthood [58], [59]. Even a study reported that dietary n-3 polyunsaturated fatty acid, such as linoleic acid, deprivation together with early maternal separation increased anxiety and vulnerability to stress in adult rats [60]. These findings indicated that higher plasma level of the linoleic acid in ELS/MDD compared with non-ELS/MDD may be an ELS-associated metabolic alteration in depression patients. And this study also found the lower plasma level of cholesterol in MDD (including ELS/MDD and non-ELS/MDD) and this finding was consistent with the several previous reports' findings [61], [62]. The plasma level of cholesterol in ELS/MDD was significantly higher than the level of non-ELS/MDD. A previous study also reported that the early-life maltreatment may induce high level of cholesterol in the adulthood of non-human primate [63]. Those ELS-associated metabolic alterations indicated the potential of plasma metabonomics method in discriminating ELS/MDD from MDD and provided a novel plasma metabolic insight about the ELS/MDD.
Due to the lack of empirical laboratory-based tests, the diagnosis of MDD relies solely on the clinician's subjective identification of symptomatic clusters and scales which has the shortage of subjectivity [11]. The lack of the disease molecular markers to support objective laboratory tests constitutes a bottleneck for the research on MDD. In light of this shortage, this study applied ROC analysis and Tclass system to obtain the metabolic biomarker panels for predicting MDD and even its subgroup (ELS/MDD and non-ELS/MDD). Through the ROC analysis, the diagnostic panels with pre-selecting differential metabolites were obtained. The AUC of these obtained diagnostic panels all attained 1. To overcome the positively biased cross-validation estimate of the differential metabolites' panel, the Tclass system was applied which does not need the pre-selecting differential metabolites to constitute the diagnostic panels and construct models [28], [31]. The diagnostic panels with feature metabolites' combination were obtained by Tcalss system analysis. The AUC values of these diagnostic panels all attained 1, as well. To evaluate the cross-validation estimates of these diagnostic panels obtained by Tclass system analysis or ROC analysis, the stability analysis was carried out. The stability index of stability analysis was a suitable cross-validation value for evaluating the performance of cross-validation estimates and the performance of predictive potential of the diagnostic panels in practice. Our preliminary results showed that the stability index of the feature metabolites' combination was generally higher than the differential metabolites panel (Figure 2). When using the diagnostic panels, for instance, classify the healthy control and MDD, the stability index of the diagnostic panel constituted by differential metabolites was 0.7546 and the stability index of the diagnostic panel constituted by feature metabolites' combination was 0.9438. Therefore, the feature metabolites' combination obtained by Tclass system had the optimum biased cross-validation estimate and had more accurate predictive potential compared with the diagnostic panels of differential metabolites obtained by ROC analysis.
Accordingly, 4 mathematical models (Table S1) were generated by Tclass system and these models can be used for prediction MDD and its subgroup (ELS/MDD and non-ELS/MDD). And on the basis of these mathematical models, the 3 prediction tools were designed including the prediction tool for MDD, ELS/MDD, and non-ELS/MDD (Table S1). At first, we should use the prediction tool for MDD to identify the sample from MDD or not. The ensemble classifier model for healthy control and MDD is applied. And if the sample is predicted as MDD, the sample is from a MDD patient. Next, we will use the prediction tool for ELS/MDD to identify the sample from ELS/MDD or not. When using the prediction tool for diagnosing ELS/MDD, we actually apply 2 ensemble classifier models (healthy control vs. ELS/MDD and ELS/MDD vs. non-ELS/MDD). Only when the patient's plasma sample has been both predicted as ELS/MDD by the 2 ensemble classifier model (healthy control vs. ELS/MDD and ELS/MDD vs. non-ELS/MDD), the patient will be predicted as ELS/MDD. In consistent with it, only when the plasma sample has been both predicted as non-ELS/MDD by both 2 models (healthy control vs. non-ELS/MDD and ELS/MDD vs. non-ELS/MDD), the plasma will be identified from non-ELS/MDD. These tools were shown in Table S1. In these prediction tools, what the user needs to do is to extract the RPA of the feature metabolites that constitute the diagnostic panel with highest classification accuracy. Then, paste the above RPA of feature metabolites into the metabolites window. The related discrimination result will be displayed in the discrimination window.
In summary, this study used metabonomics approach based on GC/MS coupled with multivariate statistic analysis to characterize the metabolic profiles of plasma from MDD and its subgroups (ELS/MDD and non-ELS/MDD). The results showed that the subgroup of MDD patients with ELS have the distinct metabolic profiles when compared to non-ELS/MDD patients and healthy subjects. And the diagnostic panels of feature metabolites' combination and the ensemble classifiers provide a novel metabonomics approach for diagnosis and prognosis of MDD even its subgroups (ELS/MDD and non-ELS/MDD) which can improve the predictive power of the biomarker panels obtained by the current metabonomic data analysis approach. Although the introduction of metabonomic screening and the Tclass system analysis can help to simply and objectively diagnose MDD and even its subgroups (ELS/MDD and non-ELS/MDD); the limitations in this study indicated that the further studies with large sample size are required to replicate and validate this novel metabonomic analysis approach.
Supporting Information
Figure S1
Score plots for PLS-DA of GC/TOF-MS data from healthy subjects, ELS/MDD patients and non-ELS/MDD patients. The clustering analysis of metabonomic profiles from healthy subjects and ELS/MDD patients (A); the clustering analysis of metabonomic profiles from healthy subjects and non-ELS/MDD patients (B). MDD indicates depressed patients, ELS/MDD indicates depressed patients with early life stress experience and non-ELS/MDD indicates depressed patients without early life stress experience.
(DOCX)
Figure S2
Scatter plots of the values of area under the receiver operating characteristic curve (AUC) of ROC analyses. The Scatter plots of the values of AUC were drawn for evaluating the diagnostic panels of differential metabolites between the healthy subjects and MDD patients (A), healthy subjects and ELS/MDD patients (B), healthy subjects and non-ELS/MDD patients (C), ELS/MDD patients and non-ELS/MDD patients (D); and the results of ROC analyses for evaluating the diagnostic panels of feature metabolites' combination between healthy subjects and MDD patients (E), healthy subjects and ELS/MDD patients (F), healthy subject and non-ELS/MDD patients (G), ELS/MDD patients and non-ELS/MDD patients (H). The relationship between the number of metabolites and the diagnostic performance was shown by the AUC values which were based on the receiver operating characteristic (ROC) analysis and logistic regression model analysis.
(DOCX)
Figure S3
The results of Tclass discriminant analyses. Results of Tclass discriminant analyses between the healthy subjects and MDD patients (A), healthy subjects and ELS/MDD patients (B), healthy subjects and non-ELS/MDD patients (C), ELS/MDD patients and non-ELS/MDD patients (D). The relationship between the number of metabolites and classification accuracy was shown by Fisher's test and Naïve Bayes discriminant analysis. Both methods were based on the feature forward selection procedure and classification accuracy from leave-one-out cross-validation (LOOCV).
(DOCX)
Table S1
The ensemble classifier models and the prediction tools for identification MDD and its subgroups (ELS/MDD and non-ELS/MDD). This is an excel file with 7 worksheets. “Ensemble classifier model 1” worksheet describes 1000 classifiers which consist of the ensemble classifier model for discrimination healthy control and MDD; “Ensemble classifier model 2” worksheet describes the ensemble classifier model for discrimination healthy control and ELS/MDD; “Ensemble classifier model 3” worksheet describes the ensemble classifier model for discrimination healthy control and non-ELS/MDD; “Ensemble classifier model 4” worksheet describes the ensemble classifier model for discrimination non-ELS/MDD and ELS/MDD. And then, “Prediction tool for MDD” worksheet describes a prediction tool to identify the sample from MDD or not; “Prediction tool for ELSMDD” worksheet describes the prediction tool to identify the sample from ELS/MDD or not; “Prediction tool for non ELSMDD” worksheet describes the prediction tool to identify the sample from non-ELS/MDD or not. In these prediction tools, what the user needs to do is to paste the RPA of the feature metabolites into the metabolites window, and then the related discrimination result will be displayed in the discrimination window.
(XLSX)
Acknowledgments
The authors would like to thank all participants who took part in this study, and the experts at the National Center of Biomedical Analysis for providing technical assistant.
Funding Statement
This work was supported by the Chinese National Key Project of Basic Research (2009CB918301 and 2009CB918303) and the Chinese National Natural Science Foundation (81370051). The funders had no role in study design, data collection and analysis, decision to publish, or perparation of the manuscript.
References
- 1.Simon GE (2003) Social and economic burden of mood disorders. Biol Psychiatry 54: 208–215. [DOI] [PubMed] [Google Scholar]
- 2.Kendler KS, Kessler RC, Walters EE, MacLean C, Neale MC, et al. (1995) Stressful life events, genetic liability, and onset of an episode of major depression in women. Am J Psychiatry 152: 833–842. [DOI] [PubMed] [Google Scholar]
- 3.Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, et al. (2001) Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the Adverse Childhood Experiences Study. JAMA 286: 3089–3096. [DOI] [PubMed] [Google Scholar]
- 4.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB (2008) The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 33: 693–710. [DOI] [PubMed] [Google Scholar]
- 5.Edwards VJ, Holden GW, Felitti VJ, Anda RF (2003) Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry 160: 1453–1460. [DOI] [PubMed] [Google Scholar]
- 6.Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, et al. (2004) Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord 82: 217–225. [DOI] [PubMed] [Google Scholar]
- 7.MacMillan HL, Fleming JE, Streiner DL, Lin E, Boyle MH, et al. (2001) Childhood abuse and lifetime psychopathology in a community sample. Am J Psychiatry 158: 1878–1883. [DOI] [PubMed] [Google Scholar]
- 8.Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, et al. (2000) Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res 122: 81–103. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez MM, Ladd CO, Plotsky PM (2001) Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol 13: 419–449. [DOI] [PubMed] [Google Scholar]
- 10.Meaney MJ, Szyf M (2005) Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci 7: 103–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Eaton WW, Gallo JJ, Nestadt G (2000) Understanding the heterogeneity of depression through the triad of symptoms, course and risk factors: a longitudinal, population-based study. J Affect Disord 59: 1–11. [DOI] [PubMed] [Google Scholar]
- 12.Wittchen HU, Hofler M, Meister W (2001) Prevalence and recognition of depressive syndromes in German primary care settings: poorly recognized and treated? Int Clin Psychopharmacol 16: 121–135. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell AJ, Vaze A, Rao S (2009) Clinical diagnosis of depression in primary care: a meta-analysis. Lancet 374: 609–619. [DOI] [PubMed] [Google Scholar]
- 14.Laugeray A, Launay JM, Callebert J, Surget A, Belzung C, et al. (2010) Peripheral and cerebral metabolic abnormalities of the tryptophan-kynurenine pathway in a murine model of major depression. Behav Brain Res 210: 84–91. [DOI] [PubMed] [Google Scholar]
- 15.Horrobin DF, Bennett CN (1999) Depression and bipolar disorder: relationships to impaired fatty acid and phospholipid metabolism and to diabetes, cardiovascular disease, immunological abnormalities, cancer, ageing and osteoporosis. Possible candidate genes. Prostaglandins Leukot Essent Fatty Acids 60: 217–234. [DOI] [PubMed] [Google Scholar]
- 16.Ashcroft GW, Crawford TB, Eccleston D, Sharman DF, MacDougall EJ, et al. (1966) 5-hydroxyindole compounds in the cerebrospinal fluid of patients with psychiatric or neurological diseases. Lancet 2: 1049–1052. [DOI] [PubMed] [Google Scholar]
- 17.Fernie AR, Trethewey RN, Krotzky AJ, Willmitzer L (2004) Metabolite profiling: from diagnostics to systems biology. Nat Rev Mol Cell Biol 5: 763–769. [DOI] [PubMed] [Google Scholar]
- 18.Bjerrum JT, Nielsen OH, Hao F, Tang H, Nicholson JK, et al. (2010) Metabonomics in ulcerative colitis: diagnostics, biomarker identification, and insight into the pathophysiology. J Proteome Res 9: 954–962. [DOI] [PubMed] [Google Scholar]
- 19.Bertram HC, Duus JO, Petersen BO, Hoppe C, Larnkjaer A, et al. (2009) Nuclear magnetic resonance-based metabonomics reveals strong sex effect on plasma metabolism in 17-year-old Scandinavians and correlation to retrospective infant plasma parameters. Metabolism 58: 1039–1045. [DOI] [PubMed] [Google Scholar]
- 20.Pasikanti KK, Ho PC, Chan EC (2008) Gas chromatography/mass spectrometry in metabolic profiling of biological fluids. J Chromatogr B Analyt Technol Biomed Life Sci 871: 202–211. [DOI] [PubMed] [Google Scholar]
- 21.Koh Y, Pasikanti KK, Yap CW, Chan EC (2010) Comparative evaluation of software for retention time alignment of gas chromatography/time-of-flight mass spectrometry-based metabonomic data. J Chromatogr A 1217: 8308–8316. [DOI] [PubMed] [Google Scholar]
- 22.Krall L, Huege J, Catchpole G, Steinhauser D, Willmitzer L (2009) Assessment of sampling strategies for gas chromatography-mass spectrometry (GC-MS) based metabolomics of cyanobacteria. J Chromatogr B Analyt Technol Biomed Life Sci 877: 2952–2960. [DOI] [PubMed] [Google Scholar]
- 23.Zhang F, Jia Z, Gao P, Kong H, Li X, et al. (2010) Metabonomics study of urine and plasma in depression and excess fatigue rats by ultra fast liquid chromatography coupled with ion trap-time of flight mass spectrometry. Mol Biosyst 6: 852–861. [DOI] [PubMed] [Google Scholar]
- 24.Ni Y, Su M, Lin J, Wang X, Qiu Y, et al. (2008) Metabolic profiling reveals disorder of amino acid metabolism in four brain regions from a rat model of chronic unpredictable mild stress. FEBS Lett 582: 2627–2636. [DOI] [PubMed] [Google Scholar]
- 25.Zheng S, Yu M, Lu X, Huo T, Ge L, et al. (2010) Urinary metabonomic study on biochemical changes in chronic unpredictable mild stress model of depression. Clin Chim Acta 411: 204–209. [DOI] [PubMed] [Google Scholar]
- 26.Paige LA, Mitchell MW, Krishnan KR, Kaddurah-Daouk R, Steffens DC (2007) A preliminary metabolomic analysis of older adults with and without depression. Int J Geriatr Psychiatry 22: 418–423. [DOI] [PubMed] [Google Scholar]
- 27.Zheng P, Gao HC, Li Q, Shao WH, Zhang ML, et al. (2012) Plasma metabonomics as a novel diagnostic approach for major depressive disorder. J Proteome Res 11: 1741–1748. [DOI] [PubMed] [Google Scholar]
- 28.Wuju L, Momiao X (2002) Tclass: tumor classification system based on gene expression profile. Bioinformatics 18: 325–326. [DOI] [PubMed] [Google Scholar]
- 29.Wu B, Cha L, Du Z, Ying X, Li H, et al. (2007) Construction of mathematical model for high-level expression of foreign genes in pPIC9 vector and its verification. Biochem Biophys Res Commun 354: 498–504. [DOI] [PubMed] [Google Scholar]
- 30.Xiao T, Ying W, Li L, Hu Z, Ma Y, et al. (2005) An approach to studying lung cancer-related proteins in human blood. Mol Cell Proteomics 4: 1480–1486. [DOI] [PubMed] [Google Scholar]
- 31.Yang J, Chen T, Sun L, Zhao Z, Qi X, et al. (2013) Potential metabolite markers of schizophrenia. Mol Psychiatry 18: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.First MB, Donovan S, Frances A (1996) Nosology of chronic mood disorders. Psychiatr Clin North Am 19: 29–39. [DOI] [PubMed] [Google Scholar]
- 33.Zung WW (1965) A Self-Rating Depression Scale. Arch Gen Psychiatry 12: 63–70. [DOI] [PubMed] [Google Scholar]
- 34.Zung WW (1971) A rating instrument for anxiety disorders. Psychosomatics 12: 371–379. [DOI] [PubMed] [Google Scholar]
- 35.Zung WW (1974) The measurement of affects: depression and anxiety. Mod Probl Pharmacopsychiatry 7: 170–188. [DOI] [PubMed] [Google Scholar]
- 36.Sharp LK, Lipsky MS (2002) Screening for depression across the lifespan: a review of measures for use in primary care settings. Am Fam Physician 66: 1001–1008. [PubMed] [Google Scholar]
- 37.Bremner JD, Vermetten E, Mazure CM (2000) Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the Early Trauma Inventory. Depress Anxiety 12: 1–12. [DOI] [PubMed] [Google Scholar]
- 38.Fink LA, Bernstein D, Handelsman L, Foote J, Lovejoy M (1995) Initial reliability and validity of the childhood trauma interview: a new multidimensional measure of childhood interpersonal trauma. Am J Psychiatry 152: 1329–1335. [DOI] [PubMed] [Google Scholar]
- 39.Feng B, Wu S, Lv S, Liu F, Chen H, et al. (2007) Metabolic profiling analysis of a D-galactosamine/lipopolysaccharide-induced mouse model of fulminant hepatic failure. J Proteome Res 6: 2161–2167. [DOI] [PubMed] [Google Scholar]
- 40.Arvelo MB, Cooper JT, Longo C, Daniel S, Grey ST, et al. (2002) A20 protects mice from D-galactosamine/lipopolysaccharide acute toxic lethal hepatitis. Hepatology 35: 535–543. [DOI] [PubMed] [Google Scholar]
- 41.Fiehn O, Kopka J, Dormann P, Altmann T, Trethewey RN, et al. (2000) Metabolite profiling for plant functional genomics. Nat Biotechnol 18: 1157–1161. [DOI] [PubMed] [Google Scholar]
- 42.Sun B, Wu S, Li L, Li H, Zhang Q, et al. (2009) A metabolomic analysis of the toxicity of Aconitum sp. alkaloids in rats using gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom 23: 1221–1228. [DOI] [PubMed] [Google Scholar]
- 43.Kessler RC, Nelson CB, McGonagle KA, Liu J, Swartz M, et al.. (1996) Comorbidity of DSM-III-R major depressive disorder in the general population: results from the US National Comorbidity Survey. Br J Psychiatry Suppl: 17–30. [PubMed]
- 44.Hirschfeld RM (2001) The Comorbidity of Major Depression and Anxiety Disorders: Recognition and Management in Primary Care. Prim Care Companion J Clin Psychiatry 3: 244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, et al. (1997) Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA 277: 1362–1368. [PubMed] [Google Scholar]
- 46.Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, et al. (1999) Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry 4: 163–172. [DOI] [PubMed] [Google Scholar]
- 47.Brindle JT, Antti H, Holmes E, Tranter G, Nicholson JK, et al. (2002) Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat Med 8: 1439–1444. [DOI] [PubMed] [Google Scholar]
- 48.Wurtman RJ, Hefti F, Melamed E (1980) Precursor control of neurotransmitter synthesis. Pharmacol Rev 32: 315–335. [PubMed] [Google Scholar]
- 49.Wang L, Maher TJ, Wurtman RJ (2007) Oral L-glutamine increases GABA levels in striatal tissue and extracellular fluid. FASEB J 21: 1227–1232. [DOI] [PubMed] [Google Scholar]
- 50.Mitani H, Shirayama Y, Yamada T, Maeda K, Ashby CR Jr, et al. (2006) Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatry 30: 1155–1158. [DOI] [PubMed] [Google Scholar]
- 51.de Kivit S, Kraneveld AD, Garssen J, Willemsen LE (2011) Glycan recognition at the interface of the intestinal immune system: target for immune modulation via dietary components. Eur J Pharmacol 668 Suppl 1S124–132. [DOI] [PubMed] [Google Scholar]
- 52.Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, et al. (2002) Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim Biophys Acta 1572: 232–254. [DOI] [PubMed] [Google Scholar]
- 53.Shin T (2013) The pleiotropic effects of galectin-3 in neuroinflammation: a review. Acta Histochem 115: 407–411. [DOI] [PubMed] [Google Scholar]
- 54.Starossom SC, Mascanfroni ID, Imitola J, Cao L, Raddassi K, et al. (2012) Galectin-1 deactivates classically activated microglia and protects from inflammation-induced neurodegeneration. Immunity 37: 249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakaguchi M, Okano H (2012) Neural stem cells, adult neurogenesis, and galectin-1: from bench to bedside. Dev Neurobiol 72: 1059–1067. [DOI] [PubMed] [Google Scholar]
- 56.Kraneveld AD, de Theije CG, van Heesch F, Borre Y, de Kivit S, et al. (2014) The neuro-immune axis: prospect for novel treatments for mental disorders. Basic Clin Pharmacol Toxicol 114: 128–136. [DOI] [PubMed] [Google Scholar]
- 57.McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, et al. (2010) Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord 126: 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Das UN (2004) Perinatal supplementation of long-chain polyunsaturated fatty acids, immune response and adult diseases. Med Sci Monit 10: HY19–25. [PubMed] [Google Scholar]
- 59.Logue JA, Howell BR, Bell JG, Cossins AR (2000) Dietary n-3 long-chain polyunsaturated fatty acid deprivation, tissue lipid composition, ex vivo prostaglandin production, and stress tolerance in juvenile Dover sole (Solea solea L.). Lipids 35: 745–755. [DOI] [PubMed] [Google Scholar]
- 60.Mathieu G, Oualian C, Denis I, Lavialle M, Gisquet-Verrier P, et al. (2011) Dietary n-3 polyunsaturated fatty acid deprivation together with early maternal separation increases anxiety and vulnerability to stress in adult rats. Prostaglandins Leukot Essent Fatty Acids 85: 129–136. [DOI] [PubMed] [Google Scholar]
- 61.Jow GM, Yang TT, Chen CL (2006) Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. J Affect Disord 90: 21–27. [DOI] [PubMed] [Google Scholar]
- 62.Olusi SO, Fido AA (1996) Serum lipid concentrations in patients with major depressive disorder. Biol Psychiatry 40: 1128–1131. [DOI] [PubMed] [Google Scholar]
- 63.Kaufman D, Banerji MA, Shorman I, Smith EL, Coplan JD, et al. (2007) Early-life stress and the development of obesity and insulin resistance in juvenile bonnet macaques. Diabetes 56: 1382–1386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Score plots for PLS-DA of GC/TOF-MS data from healthy subjects, ELS/MDD patients and non-ELS/MDD patients. The clustering analysis of metabonomic profiles from healthy subjects and ELS/MDD patients (A); the clustering analysis of metabonomic profiles from healthy subjects and non-ELS/MDD patients (B). MDD indicates depressed patients, ELS/MDD indicates depressed patients with early life stress experience and non-ELS/MDD indicates depressed patients without early life stress experience.
(DOCX)
Figure S2
Scatter plots of the values of area under the receiver operating characteristic curve (AUC) of ROC analyses. The Scatter plots of the values of AUC were drawn for evaluating the diagnostic panels of differential metabolites between the healthy subjects and MDD patients (A), healthy subjects and ELS/MDD patients (B), healthy subjects and non-ELS/MDD patients (C), ELS/MDD patients and non-ELS/MDD patients (D); and the results of ROC analyses for evaluating the diagnostic panels of feature metabolites' combination between healthy subjects and MDD patients (E), healthy subjects and ELS/MDD patients (F), healthy subject and non-ELS/MDD patients (G), ELS/MDD patients and non-ELS/MDD patients (H). The relationship between the number of metabolites and the diagnostic performance was shown by the AUC values which were based on the receiver operating characteristic (ROC) analysis and logistic regression model analysis.
(DOCX)
Figure S3
The results of Tclass discriminant analyses. Results of Tclass discriminant analyses between the healthy subjects and MDD patients (A), healthy subjects and ELS/MDD patients (B), healthy subjects and non-ELS/MDD patients (C), ELS/MDD patients and non-ELS/MDD patients (D). The relationship between the number of metabolites and classification accuracy was shown by Fisher's test and Naïve Bayes discriminant analysis. Both methods were based on the feature forward selection procedure and classification accuracy from leave-one-out cross-validation (LOOCV).
(DOCX)
Table S1
The ensemble classifier models and the prediction tools for identification MDD and its subgroups (ELS/MDD and non-ELS/MDD). This is an excel file with 7 worksheets. “Ensemble classifier model 1” worksheet describes 1000 classifiers which consist of the ensemble classifier model for discrimination healthy control and MDD; “Ensemble classifier model 2” worksheet describes the ensemble classifier model for discrimination healthy control and ELS/MDD; “Ensemble classifier model 3” worksheet describes the ensemble classifier model for discrimination healthy control and non-ELS/MDD; “Ensemble classifier model 4” worksheet describes the ensemble classifier model for discrimination non-ELS/MDD and ELS/MDD. And then, “Prediction tool for MDD” worksheet describes a prediction tool to identify the sample from MDD or not; “Prediction tool for ELSMDD” worksheet describes the prediction tool to identify the sample from ELS/MDD or not; “Prediction tool for non ELSMDD” worksheet describes the prediction tool to identify the sample from non-ELS/MDD or not. In these prediction tools, what the user needs to do is to paste the RPA of the feature metabolites into the metabolites window, and then the related discrimination result will be displayed in the discrimination window.
(XLSX)