Versican and the Control of Inflammation (original) (raw)
. Author manuscript; available in PMC: 2015 Apr 1.
Abstract
Versican is an extracellular matrix (ECM) proteoglycan that interacts with cells by binding to non-integrin and integrin receptors and to other ECM components that associate with the cell surface. Recent studies have shown also that versican interacts with myeloid and lymphoid cells promoting their adhesion and production of inflammatory cytokines. Versican is produced by stromal cells, as well as leukocytes, and is markedly increased in inflammation. Inflammatory agonists, such as double-stranded RNA mimetics (e.g., poly I:C), stimulate stromal cells, such as smooth muscle cells and fibroblasts, to produce fibrillar ECMs enriched in versican and hyaluronan (HA) that interact with leukocytes promoting their adhesion. Interference with the incorporation of versican into this ECM blocks monocyte adhesion and dampens the inflammatory response. Tumor cells also express elevated levels of versican which interact with myeloid cells to promote an inflammatory response, through stimulating cytokine release, and metastasis. In addition, myeloid cells, such as macrophages in tumors, synthesize versican which affects tumor cell phenotypes, inflammation, and subsequent metastasis. Versican, by binding to hyaluronan, influences T lymphocyte phenotypes and in part controls the ability of these cells to synthesize and secrete cytokines that influence the immune response. Collectively, these studies indicate that versican as an ECM molecule plays a central role in inflammation and as a result it is emerging as a potential target promising wide therapeutic benefits.
Keywords: Versican, Hyaluronan, Immunity, Inflammation, Macrophages, T lymphocytes
Introduction
Versican--A Proteoglycan with Multiple Binding Sites for Inflammatory Ligands
Versican is a large chondroitin sulfate proteoglycan (CSPG) that is found in the extracellular matrix (ECM) of most soft tissues (see reviews) (Wight, 2002; Zimmermann, 2000). Normally, it is present in low amounts, but increases dramatically when tissues become inflamed. Versican was first isolated from the medium of cultured fibroblasts (Coster et al., 1979) and subsequently cloned from placental fibroblasts and named versican in recognition of its domain structure and versatility as a highly interactive molecule (Zimmermann and Ruoslahti, 1989). Versican is also known as PG-M (Coster et al., 1979; Kimata et al., 1986) and CSPG-2 (Naso et al., 1994). In humans, versican is encoded from a single gene locus on chromosome 5q14.3 (Iozzo and McPherson, 1992) and is 86% identical between mouse and human (Naso et al., 1994), indicating the importance and highly conserved nature of this proteoglycan.
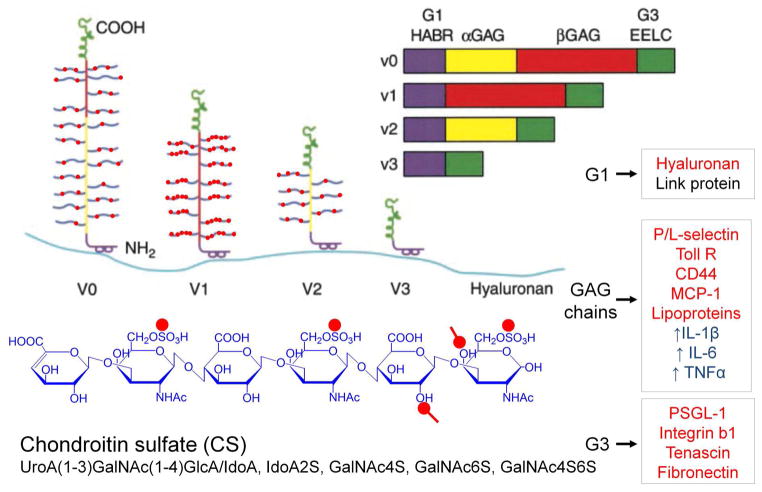
Versican exists in at least four different isoforms due to the alternative splicing of the major exons that code for the attachment regions for the glycosaminoglycan (GAG) in the core protein (Figure 1). Three of these isoforms contain chondroitin sulfate (CS) GAGs, while one of the isoforms, V3, contains no GAGs due to the splicing together of the N- and C- terminal regions (Zako et al., 1995).
Figure 1.
A model of the different isoforms generated by alternative splicing of the mRNA transcript for versican. All isoforms interact with hyaluronan and thus are capable of forming different sized versican-HA aggregates. Different colors denote specific domains in the gene and in the protein product. Purple = hyaluronan binding region (HABR); yellow = α GAG exon and protein product; red = β GAG exon and protein product; green = two epidermal growth factor repeats (EE), a lectin binding domain (L) and a complement regulatory region. Inflammatory molecules that bind to different domains of versican are shown in the boxes to the right, marked in red. Those marked in blue indicate macrophage responses to treatment with versican. Structure of the CS GAG is shown at the bottom in blue with red dots denoting negatively charged residues. Reprinted (with modifications) from Current Opin Cell Biol, 14(5), Thomas N. Wight, Versican: a versatile extracellular matrix proteoglycan in cell biology, pp. 617–23, Copyright (2002), with permission from Elsevier.
Versican is highly interactive due to the charged nature of the GAG chains and the domain structure of the core protein (Wu et al., 2005). Recently, a new versican isoform, V4, consisting of the G1 domain, the first 398 amino acids of the β-GAG region and the G3 domain has been found to be upregulated in human breast cancer lesions (Kischel et al., 2010). Other isoforms potentially may exist, such as a V5 isoform, consisting of essentially only the G1 domain, found by new gene discovery techniques and listed as a reference sequence for mouse versican in Entrez Gene. Interestingly, several molecules with which versican interacts have a role in the inflammatory process and versican is known to stimulate inflammatory cytokine release by immune and inflammatory cells (see below) (Figure 1).
1. Versican: A Component of the Inflammatory Response
Inflammatory responses require the emigration of leukocytes from the vasculature into damaged underlying tissue areas as part of the innate immune response. Upon extravasation into the subendothelial compartment, leukocytes encounter the ECM which functions as a scaffold for leukocytes, influencing their adhesion, migration, activation, and retention (Figure 2A, B) (Gill et al., 2010; Parish, 2006; Sorokin, 2010; Vaday et al., 2001; Vaday and Lider, 2000). Versican interacts with receptors on the surface of immune cells, such as CD44, PSGL-1, Toll-like receptor 2 (TLR2), and P- or L-selectins (Hirose et al., 2001; Kawashima et al., 2002; Kawashima et al., 2000; Kim et al., 2009; Taylor and Gallo, 2006; Wang et al., 2009; Wu et al., 2005; Zheng et al., 2004), and provides intrinsic signals that influence immune and inflammatory cell phenotype (Figure 2B). Versican interacts also with a number of other ECM components that are important in inflammation, such as hyaluronan (HA), TSG-6, inter-alpha-trypsin inhibitor (IαI), fibronectin, and tenascin-R and -C, (Day and de la Motte, 2005; Hascall et al., 2004; LeBaron et al., 1992; Lundell et al., 2004). High resolution confocal microscopy shows that HA, versican, TSG-6, and IαI can be organized into discrete ECM filaments or cables that emanate from the surface of a variety of cells and form a matrix that binds leukocytes (de la Motte et al., 2002; de la Motte, 2011; de la Motte et al., 1999; de la Motte et al., 2003; Evanko et al., 2012; Evanko et al., 2009; Lauer et al., 2008; Lauer et al., 2009a; Lauer et al., 2009b; Majors et al., 2003; Potter-Perigo et al., 2010; Selbi et al., 2006b; Wang et al., 2011; Wang and Hascall, 2004) (Day and de la Motte, 2005) (Figure 3 A–D). These structures can also be generated by epithelial cells in response to inflammatory cytokines, promoting leukocyte adhesion (Lauer et al., 2008; Selbi et al., 2006a; Selbi et al., 2004; Selbi et al., 2006b). Versican-enriched cable-like structures have been found also in tissues exhibiting leukocyte enrichment, such as in lesions of atherosclerosis (Figure 3D) and in inflammatory bowel disease (IBD) (de la Motte et al., 2003). Such interactions between multiple ECM components create highly ordered ECM structures that selectively impact leukocyte/ECM interactions (Day and de la Motte, 2005; Sorokin, 2010). Versican, which binds to HA, can also bind to CD44 (Kawashima et al., 2000) via CS GAGs, suggesting that both versican and HA may stabilize CD44-dependent interactions and subsequent CD44-dependent signaling in inflammatory cells. On the other hand, versican binding to HA may interfere with the binding of HA to CD44 on immune cells, such as T lymphocytes, as recently demonstrated (Evanko et al., 2012) and dampen the immune response. CS chains on versican can interact also with chemokines, growth factors, and proteases to influence chemokine availability, activity and immune cell phenotype (see reviews)(Gill et al., 2010; Wu et al., 2005). Thus, individual components, including versican, in the mixture of several different ECM components may interact specifically with key molecules involved in either promoting or inhibiting inflammation.
Figure 2.
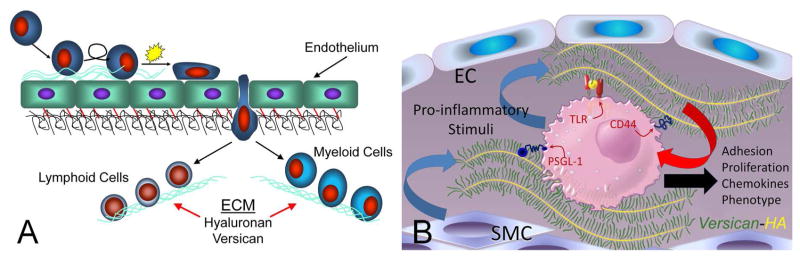
A. Extravasation of myeloid and lymphoid cells across the endothelium into the underlying tissue where they interact with the ECM enriched in versican and HA. (B. ECM and immune cell regulation. Leukocytes come into contact with the ECM as they invade tissue as part of the inflammatory phase of tissue repair. Certain types of ECMs, including those that contain versican and hyaluronan, interact with myeloid and lymphoid cells through specific cell surface receptors such as, PSGL-1, TLR2, and CD44 to promote their adhesion, accumulation, and activation. Such matrices may exhibit either pro- or anti-inflammatory properties. These versican-enriched ECMs may be produced by either stromal cells or the myeloid and lymphoid cells themselves. Image shown in B kindly provided by Dr. Charles W. Frevert, University of Washington, Seattle, WA. Panel A is from Wight TN, “The biomatrix of the vascular system and the control of cell phenotype,” Hyaluronan: From Basic Science to Clinical Applications, vol 5, Structure and Function of Biomatrix: Control of Cell Behavior and Gene Expression, Balazs, EA, Editor. Matrix Biology Institute, Edgewater, NJ, 2012, pp. 315–340. Reprinted with permission of the Matrix Biology Institute.
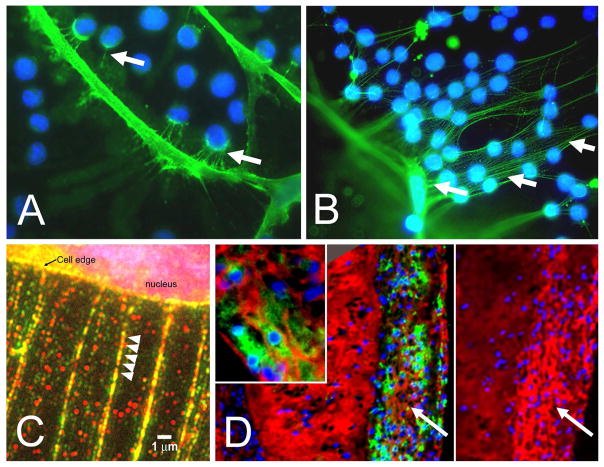
Figure 3.
A. Cell processes of a cultured human lung fibroblast stained for HA (green) with U937 monocytes added (blue nuclei) showing interaction of the monocytes with the fibroblast surface by HA-positive fibrils. B. Increased adhesion of monocytes to HA-positive fibrils of lung fibroblasts treated with poly I:C. C. Poly I:C-treated fibrobasts stained for HA (red) and versican (green) showing that the strands emanating from the cell surface contain both HA and versican (A–C, from Evanko et al., 2009). D. A section from an atherosclerotic lesion in a Watanabe rabbit immunostained for macrophages (green) and affinity-stained for HA (red). Inset of the area indicated by the arrow shows that the HA is organized into cables. Panel to the right is the adjacent section immunostained for versican. These images show that macrophages accumulate in regions of the lesion that contain both hyaluronan and versican, but not in regions devoid of versican (Sakr and Wight, unpublished observation).
Once bound to the versican-containing ECM, leukocytes degrade the ECM to generate pro-inflammatory fragments that further drive the inflammatory response (Adair-Kirk and Senior, 2008; Arroyo and Iruela-Arispe, 2010; Schor et al., 2000; Vaday and Lider, 2000). For example, the G3 domain of versican interacts with P-selectin glycoprotein-1 (PSGL-1) on the surface of macrophages and causes macrophage aggregation (Zheng et al., 2004). Numerous matrix metalloproteinases (MMPs), including MMP-1, -2, -3, -7, and -9, as well as the serine protease plasmin and several members of the ADAMTS family (A Disintegrin And Metalloproteinase with Thrombospondin Motifs), degrade versican (see reviews) (Apte, 2009; Kenagy et al., 2006). The versican cleavage site for some of the ADAMTS enzymes is in the βGAG domain of versican at the Glu441-Ala422 bond which generates a 70 kDa fragment that can be recognized by an antibody against the neoepitope sequence DPEAAE (Sandy et al., 2001; Somerville et al., 2003). This fragment has been shown to be enriched in regions of blood vessels undergoing atrophy and cell death (Kenagy et al., 2005; Sandy et al., 2001). Recent findings by Apte’s group indicate that the ADAMTS-generated G1 fragment of versican regulates apoptosis during mammalian inter-digital web regression (McCulloch et al., 2009). It is of interest that expression of the V1 isoform in NIH3T3 cells is associated with apoptotic resistance, but these cells also exhibit increased sensitivity to cell death induced by cytotoxic agents used in the treatment of tumor cells (LaPierre et al., 2007). Whether intact or fragmented versican can cause apoptosis as part of the immune and inflammatory response awaits further experimentation. Versican degradation also takes place as new blood vessels are generated as part of inflammatory events associated with tissue repair. Injection of an adenoviral vector expressing VEGF164 into the skin of a mouse induces a florid angiogenic response characterized by an increase in ADAMTS-1 and the versican fragment DPEAAE (Fu et al., 2011). These changes are accompanied by loss of versican as angiogenesis proceeds, although it remains to be shown whether versican degradation exhibits direct control over the angiogenic response. Thus, either intact versican or fragmented versican could be a “first step” in the amplification of the inflammatory response in many different diseases (Said et al., 2012; Said and Theodorescu, 2012; Zhang et al., 2012).
2. Versican: Role in Leukocyte Adhesion and Activation
To explore the interaction of inflammatory and immune cells with ECMs enriched in versican, in vitro models have been developed, pioneered by Carol de la Motte and her colleagues at the Cleveland Clinic, in which leukocytes are seeded onto ECM generated by stromal cells stimulated with agents such as viruses and/or endoplasmic reticulum (ER) stress agonists and evaluated for adhesion and accumulation (de la Motte, 2011; de la Motte and Drazba, 2011; de la Motte et al., 1999; Hascall et al., 2004; Lauer et al., 2008; Lauer et al., 2009a; Lauer et al., 2009b; Majors et al., 2003; Wang et al., 2011) (Figure 3A, B). A number of different conditions cause ER stress and it is not clear yet how this pathway promotes the generation of an ECM that binds leukocytes. These pioneering studies showed that monocytes adhere to HA generated by stromal cells including arterial smooth muscle cells (ASMCs) and fibroblasts. We have focused on the role of versican, a proteoglycan that binds to HA (LeBaron et al., 1992) in this process. For example, treatment of human lung fibroblasts with the viral mimetic, poly I:C, causes an enrichment of HA and versican in the ECM deposited by the cultured fibroblasts (Evanko et al., 2009; Potter-Perigo et al., 2010). This enrichment does not appear to be due to increased HA or versican synthesis, but rather to decreases in the degradation of both HA and versican (Potter-Perigo et al., 2010). The HA- and versican-enriched ECMs supported monocyte adhesion, while those generated by cytokine stimulation alone in the absence of poly I:C did not. Interestingly, the ECMs that did not support monocyte adhesion were deficient in versican, but enriched in HA (Potter-Perigo et al., 2010). To further implicate versican as a key component in monocyte adhesion to the ECM, we treated the poly I:C-generated ECM with an antibody to the N-terminal region of versican before adding the monocytes and blocked monocyte adhesion to this ECM. Such results indicate that versican is an important player in forming an ECM that binds monocytes, at least in in vitro experiments.
Another area where versican can influence inflammation is by affecting inflammatory cytokine release by myeloid and lymphoid cells. This appears to be prominent in some types of cancers. For example, versican derived from Lewis lung carcinoma cells appears to induce the secretion of inflammatory cytokines, such as tumor necrosis factor-α (TNFα) and IL-6 from macrophages through interaction with TLR2 and its co-receptors TLR6 and CD14. This interaction promotes metastasis in a mouse model (Kim et al., 2009; Wang et al., 2009; Zhang et al., 2012). It is of interest that highly sulfated CS GAG chains on versican (Kawashima et al., 2002) may be critical to promote inflammatory cytokine release and activity in this model (Kawashima et al., 2002; Li et al., 2008). Intriguingly, CSPGs have been shown to interact with P-selectin (Kawashima et al., 2002), which may be of importance for recruiting inflammatory cells to the tumor. In addition, other cancer cells, such as those from bladder (Said et al., 2012; Said and Theodorescu, 2012) and colon (Bogels et al., 2013) secrete versican, which in turn, induces myeloid inflammatory cytokine release, promoting inflammation and metastasis. Inhibiting versican production in these cancer cells blocks the inflammation associated with these tumors. Most recently, Li and colleagues showed in co-culture experiments of macrophages and ovarian cancer cells that versican produced by the tumor cells promoted the production of an antimicrobial protein by macrophages through TLR2 activation that in turn stimulated ovarian tumor cell proliferation and invasion, providing yet another example of the pro-proliferative nature of versican (Li et al., 2013). Whether similar versican activity exists during inflammation in non-cancerous tissue awaits further investigation. It may be that ligation of immune receptors such as TLR2 by versican is responsible for the activation of multiple cell types and the induction of inflammatory cytokine secretion in many disease situations (Wang et al., 2009; Zhang et al., 2012).
3. Versican: Expression by Myeloid Cells and the Impact on Inflammation
While accumulation of versican during the inflammatory response can originate in the stromal connective tissue, a number of past as well as more recent studies have identified versican as a gene which is upregulated in monocytes in a number of pro-inflammatory states, such as myocardial infarction (Toeda et al., 2005), coronary stenosis (Wingrove et al., 2008), autoimmunity (Masuda et al., 2013; Olsen et al., 2004; Shou et al., 2006), and in response to pro-inflammatory stimulants such as lipopolysaccharide (LPS) (Lang et al., 2002) and hypoxia (Asplund et al., 2011; Asplund et al., 2009). In addition, the versican gene is differentially expressed in M1 macrophages, as opposed to M2 macrophages, as they differentiate from monocytes (Asplund et al., 2011; Martinez et al., 2006). Recent studies by our group have shown that versican mRNA, along with mRNA from other HA binding proteins such as TSG-6 and IαI, is upregulated as monocytes differentiate into macrophages (Chang et al., 2012). Of particular interest, versican produced by macrophages can form complexes with MMPs, such as MMP-9 (Malla et al., 2013), suggesting possible roles for versican in controlling the activity of matrix degrading enzymes. Such changes raise the possibility that the myeloid cells themselves are creating their own microenvironment to support the myeloid phenotype as part of the inflammatory response. For example, versican expression is elevated in CD14 positive monocytes taken from patients with systemic sclerosis (Masuda et al., 2013) and this elevated expression is accompanied by elevated expression of CCL2 (Monocyte Chemoattractant Protein 1). Furthermore, versican protects CCL2 from degradation and this interaction promotes monocyte migration (Masuda et al., 2013). Such results confirm earlier studies that show CCL2 binds to versican and impacts inflammation in a model of neuronal inflammation hyperalgesia (Bogen et al., 2009). In addition, versican expression by macrophages is prominent in tumor-associated macrophages (TAMs) and is thought to be critical for promoting metastasis (Solinas et al., 2009). Using a mouse model of spontaneous breast cancer, Gao and his colleagues demonstrated that bone marrow-derived myeloid progenitor cells in the premetastatic lung secrete versican which promotes a mesenchymal to epithelial transition of tumor cells and subsequent metastasis (Gao et al., 2012a; Gao et al., 2012b). Versican derived from myeloid cells in this model appears to promote tumor growth by enhancing tumor cell proliferation, possibly by blocking the TGF-β-smad2/3 pathway. Previous studies by Yang and colleagues have shown similar activity when V1 versican was expressed in NIH3T3 cells (Sheng et al., 2006). More work is needed to elucidate the role that versican plays in the monocyte/macrophage involvement in the inflammatory response.
4. Versican: Targeting Versican and the Control of Inflammation
Attempts to understand functional aspects of versican’s control over inflammation by gene knockout experiments have been frustrated by the early lethality of versican null mice (Mjaatvedt et al., 1998). However, some recent success has been achieved by obtaining partial knockout of the versican gene and expression of versican lacking the A subdomain of G1, resulting in significantly decreased versican accumulation, but there have been no studies directed at versican’s role in inflammation in this mouse model (Hatano et al.; Suwan et al., 2009).
While a number of studies have implicated versican in the regulation of leukocyte adhesion, it is not clear whether the GAG chains attached to the core protein, or the core protein of versican itself contribute to this activity. One way to address this is to remove the GAG chains from versican and test whether the ECM has a diminished capacity to bind leukocytes (Kang and Wight, 2013, unpublished observations). Indeed, removing the GAG chains from versican by treating the ECM with chondroitin A/C lyase reduces binding of the monocytes in a dose-dependent manner. We also wondered if forced expression of the versican isoform that does not contain GAG chains (V3) could generate an ECM deficient in versican GAGs, but enriched in V3 core protein. Our earlier work indicated that growth factors, such as platelet-derived growth factor (PDGF), dramatically increase versican expression and the proliferation of ASMCs through specific signaling pathways that influence core protein synthesis and GAG synthesis in different ways (Cardoso et al., 2010; Schönherr et al., 1991; Schönherr et al., 1997). We also found that blocking the interaction of HA and versican with the cell surface inhibits the ability of these cells to proliferate and migrate in response to PDGF (Evanko et al., 1999; Evanko et al., 2001). At about the same time, Burton Yang’s group in Toronto was expressing minigenes encoding different domains of versican and finding dramatic effects on the phenotype of cells, such as their ability to proliferate, migrate, and bind monocytes (Sheng et al., 2005; Wu et al., 2001; Yang et al., 1999; Zhang et al., 1999). Following their lead, we chose to overexpress V3 in ASMCs, reasoning that the GAG chains may be critical for creating a microenvironment permissive for cell proliferation and migration (Wight, 2002). Indeed, ASMCs overexpressing the V3 isoform of versican became more adherent and grew and migrated more slowly in response to PDGF than vector-only controls (Lemire et al., 2002). A similar observation has been made in melanoma. Expression of V0/V1 isoforms of versican is increased in malignant melanoma and contributes to the increased proliferation rate and decreased adhesion of the tumor cells (Touab et al., 2003; Touab et al., 2002). Overexpression of V3 by melanoma tumor cells reverses this phenotype by decreasing proliferation and increasing adhesion (Serra et al., 2005). The decrease in proliferation was accompanied by a decrease in the activation of ERK1/2 in response to epidermal growth factor (EGF) (Miquel-Serra et al., 2006). Recent studies by Anna Bassols’ group now show that V3 interferes with the CD44-EGFR/ErbB2 pathway in the regulation of cell proliferation and migration (Hernandez et al., 2011a; Hernandez et al., 2011b).
In addition to the effects on the proliferative and migratory phenotype of ASMCs, we found that forced expression of V3 results in a striking increase in elastic fibers in the ECM deposited in culture dishes (Figure 4A) (Merrilees et al., 2002). Furthermore, injecting these V3-expressing rabbit ASMCs into rabbit carotid arteries that had been injured by a balloon catheter created a remodeled blood vessel enriched with elastic fibers (Merrilees et al., 2011; Merrilees et al., 2002) (Figure 4B). The relationship of versican to elastic fiber assembly is interesting and unusual. It is known that CS inhibits the formation of elastic fibers, through a mechanism involving interference with the binding of the elastin receptor on the surface of stromal cells (Hinek et al., 1991). In Costello Syndrome, in which there is a marked accumulation of CS in the skin of these patients, elastic fiber formation is defective. Transducing skin fibroblasts from Costello Syndrome patients with V3 corrected the inability of these cells to produce elastic fibers (Hinek et al., 2004). Interestingly, in this study, expressing the V3 isoform reduced the accumulation of the CS-containing form of versican (Hinek et al., 2004). Furthermore, we subsequently showed that inhibiting versican synthesis in ASMCs by antisense promoted the synthesis and assembly of elastic fibers both in vitro and in vivo (Huang et al., 2006). These results support the possibility that CS-containing isoforms of versican inhibit elastogenesis, while the non CS-containing isoform of versican, i.e., V3, promotes elastogenesis.
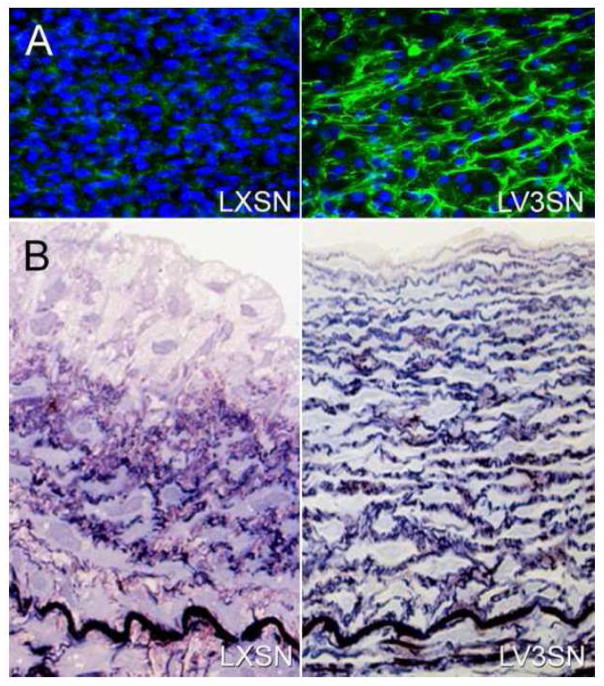
Figure 4.
A. Cultures of ASMCs transduced by an empty retroviral vector (LXSN) or one that contains the V3 gene (LV3SN) and immunostained for tropoelastin (green). Figure kindly supplied by Michel Gooden and Robert Vernon. B. A section from a balloon-injured carotid artery seeded with ASMCs transduced with an empty retroviral vector (LXSN; left panel) or seeded with ASMCs transduced with a retroviral vector containing the V3 gene (LV3SN; right panel). Multiple laminae of elastic fibers are seen in the vessel seeded with the V3-transduced cells. (From Merrilees M.J. et al, 2011).
While we did not completely understand the mechanism(s) responsible for this elastogenic effect, we wondered if the elastin-enriched ECM created by expression of V3 influenced the trafficking and retention of immune and inflammatory cells. In the vessel wall, intact elastic fibers are considered to reduce ongoing invasion of monocytes that normally respond chemotactically to elastin degradation products resulting from elastolysis derived from macrophage-derived MMPs (Senior et al., 1980). Further, decellularized arterial elastic lamellae have been shown to be non-adherent for monocytes and to prevent transmigration through activation of signal regulatory protein α and Src homology 2 domain containing protein-tyrosine phophatase-1 (Liu et al., 2005). To address this question, we turned to our in vitro model and transduced rabbit ASMCs with V3 and subjected those cells to agonists that cause ER stress, including oxidized lipoprotein, which we and others have shown is a stimulant for expression of an ECM that attracts and binds monocytes (Viola et al., 2013) (Figure 5A). Quite remarkably, adhesion of monocytes to the ECM generated by V3-expressing ASMCs treated with oxidized LDL or ECM formed by ASMCs treated with versican antisense was reduced (Figure 5B) (Merrilees et al., 2011). Next, we injected the rabbit V3-transduced ASMCs into injured rabbit carotid arteries and subsequently placed the animals on a lipid-rich diet to determine if remodeling of the ECM would affect lipid build-up and macrophage accumulation. Indeed, V3 expression prevented lipid build-up and monocyte accumulation over an 8-week period (Figure 6) (Merrilees et al., 2011). Evaluation of the V3-expressing neointima revealed an enrichment of elastic fibers when compared to vector-only transduced cells. Collectively, these studies suggest that V3 may be an effective anti-inflammatory agent in the prevention of cardiovascular disease. Certainly, what these studies do show is that vascular tissue enriched in versican, but poor in elastic fibers is more likely to exhibit pro-inflammatory properties and tissue destruction, while vascular tissue that is poor in versican, but rich in elastic fibers is more resistant to inflammation and tissue destruction (Figure 7).
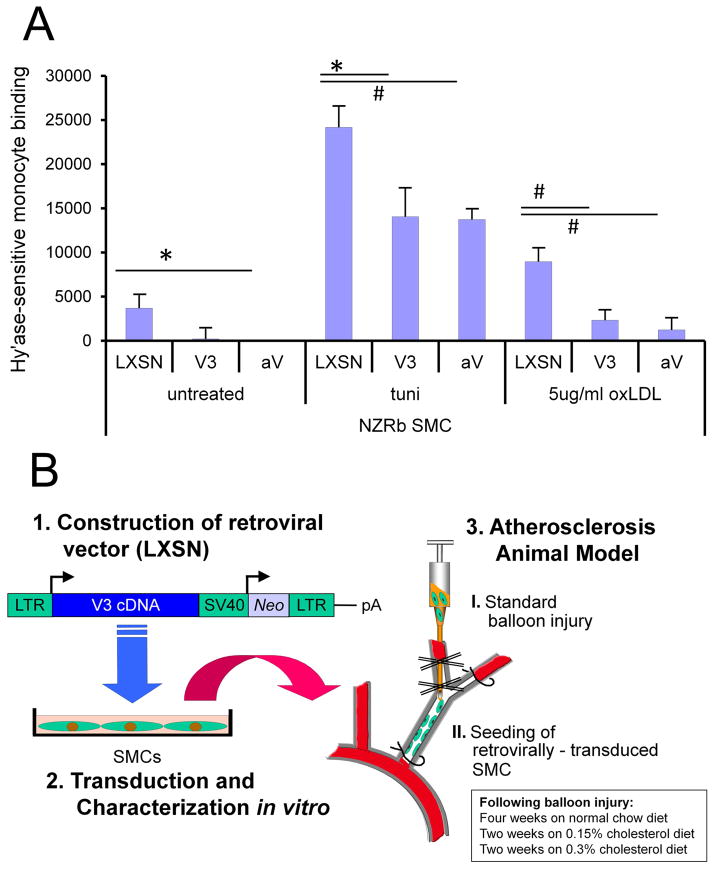
Figure 5.
A. Monocyte adherence in vitro. Hyaluronidase sensitive (U937) monocyte binding to 21-day cultures of empty vector control (LXSN) and V3 ASMC in untreated cultures, and cultures stimulated with 5 μg/mL tunicamycin (tuni) or 5 μg/mL oxLDL for 20 hours, showing reduced binding to matrix generated by V3-expressing ASMC. Degree of binding of monocytes in the V3-expressiong cultures was similar that when versican expression was blocked by versican antisense (AV). B. Experimental protocol to test impact of V3 expression on lipid and macrophages in an animal model of atherosclerosis. Rabbit ASMCs were transduced with a retroviral vector containing the V3 gene and then seeded into balloon-injured rabbit carotid artery. The animals were then placed on a normal chow diet for 4 weeks and then a cholesterol-enriched diet for an additional 4 weeks. The carotid arteries were then processed for histological analysis using histomorphometric measures for evidence of lipid and macrophage accumulation. (From Merrilees M.J. et al, 2011).
Figure 6.
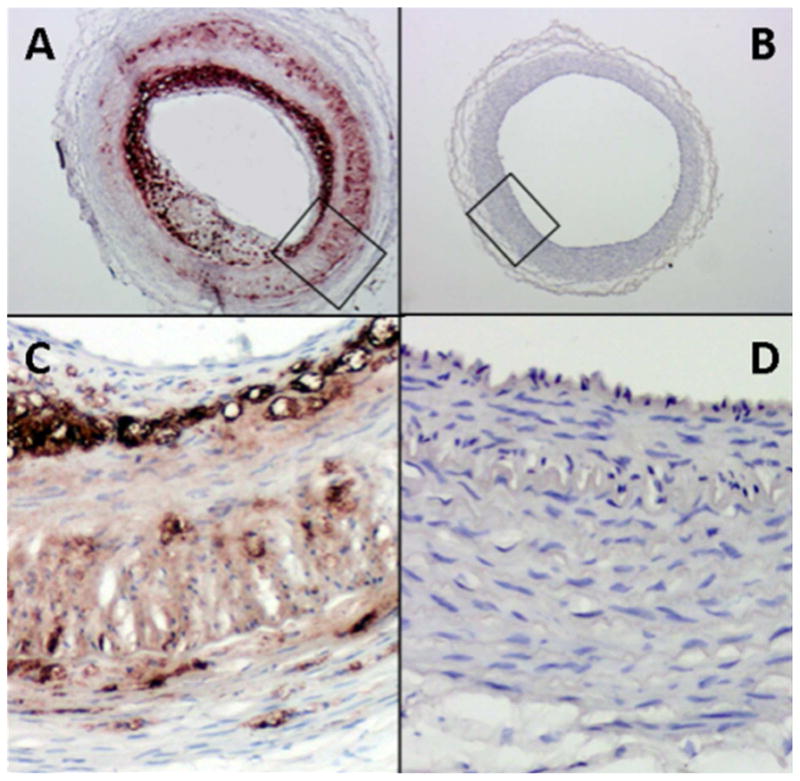
Cross section of vector control (A and C) and V3-seeded (B and D) carotid arteries of rabbits fed a high fat diet after balloon-injury and cell seeding. All sections have been immunostained for macrophages (brown). Control vessels (A & C) shows extensive macrophage accumulation, while V3 cell-seeded vessel is devoid of macrophages. (From Merrilees M.J. et al, 2011).
Figure 7.
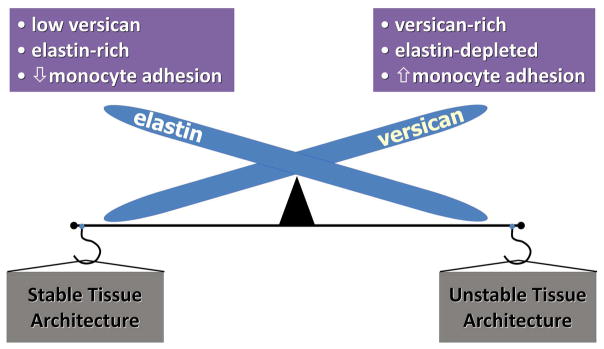
Reciprocal relationship of versican, elastin, and inflammation. Organs such as skin, lung, and blood vessels that are enriched in elastic fibers are low in versican and resistant to monocyte accumulation, leading to a stable tissue architecture with anti-inflammatory properties. When tissues from these organs are enriched in versican, as occurs in disease, they are low in elastic fibers, and show increased macrophage accumulation and inflammation, leading to unstable tissue architecture.
5. Versican: A Role in T Lymphocyte Adhesion and Activation
While it is clear that myeloid cells bind to both HA and versican and use these ECM components to interact, less is known about lymphoid cells and the importance of the ECM and their interactions. The ability of T cells to adhere and migrate through connective tissue ECM is vital to efficient immune responses (Evanko et al., 2012; Sorokin, 2010). Regulatory T cells are a specialized subpopulation of CD4+ T cells that maintain immune homeostasis and immune tolerance (Sakaguchi et al., 2006), and T-cell invasion is critical to the early immune response and is especially prominent in autoimmune diseases. Recently, we showed that activated human CD4+ T cells adhere to ECM generated by human synoviocytes and human lung fibroblasts treated with poly I:C, but not to ECM generated from stromal cells not treated with poly I:C (Evanko et al., 2012). Interestingly, the poly I:C-induced ECM impeded T-cell spreading and migration. Adding the versican monoclonal antibody (12C5) made against the N-terminal of versican during ECM formation reversed the inhibition of migration caused by the poly I:C. These data suggest that versican-rich ECM binds and constrains T cells. Furthermore, blocking HA binding to the surface of T cells by versican negatively impacted the secretion of IL-10 by T cells, thereby reducing the capacity of HA to be immunosuppressive (Evanko et al., 2012). We also examined the contribution of the ECM components on T-cell migration in a three-dimensional collagen gel system to which versican had been added. Addition of versican to the collagen gel impeded T-cell penetration and migration, further indicating that the complex interactions of ECM components can have a significant impact on whether the ECM promotes or inhibits activities associated with inflammation. These results are interesting since we and others have reported that HA, when in high molecular weight (HMW) form, promotes the adhesion, function, and stability of T-regulatory cells (Bollyky et al., 2007; Firan et al., 2006), also see review (Bollyky et al., 2012). Thus, the impact of any one ECM component on immune and inflammatory responses can be clearly influenced by those other ECM components with which it interacts.
Conclusions
The causal role of versican regulating events that drive immunity and inflammation is still somewhat circumstantial. There is no doubt that versican, as an ECM participant, has a role, but whether it acts alone or in combination with other components during inflammatory events is still not fully understood. There are published examples showing versican having both pro- or anti-inflammatory activities. These activities seem to depend on the context in which versican is presented to cells and the temporal and spatial availability of the versican molecule during tissue remodeling and inflammation. The importance of versican in inflammation lies in its versatility and ability to bind to a wide variety of receptors and other inflammatory components to regulate their availability and activity. What is needed are experimental investigations to define the precise mechanisms responsible for these activities, possibly by developing targeted reagents that can interfere with versican processing and accumulation as part of the early inflammatory response. Collectively, however, it is now clear that versican, either intact or fragmented, possesses both structural and functional capacities to influence inflammation and as a result is emerging as a potential target promising wide therapeutic benefits.
Acknowledgments
The authors wish to thank Dr. Virginia M. Green for editing, careful reading, and preparation of the manuscript. The authors are also indebted to all past and present trainees, collaborators, and technicians, who have worked tirelessly to define the importance of this ECM macromolecule in the pathogenesis of disease. The authors also apologize to those authors whose original contributions were not cited in lieu of reviews to fulfill space requirements.
This study was supported by grants from the National Institutes of Health P01 HL030086, P01 HL18645, and U01 AI101984 (T.N.W); the American Heart Association Postdoctoral Fellowship 09 POST 20500065 (I.K.); and from the Health Research Council of New Zealand and the National Heart Foundation of New Zealand (M.J.M.).
Abbreviations
CSPG
chondroitin sulfate proteoglycan
ECM
extracellular matrix
GAG
glycosaminoglycan
CS
chondroitin sulfate
TLR2
Toll-like receptor 2
HA
hyaluronan
IαI
inter-alpha-trypsin inhibitor
IBD
inflammatory bowel disease
PSGL-1
P-selectin glycoprotein-1
MMPs
matrix metalloproteinases
ADAMTS
A Disintegrin And Metalloproteinase with Thrombospondin Motifs
ER
endoplasmic reticulum
ASMCs
arterial smooth muscle cells
TNFα
tumor necrosis factor-α
LPS
lipopolysaccharide
CCL2
Monocyte Chemoattractant Protein 1
TAMs
tumor-associated macrophages
PDGF
platelet-derived growth factor
EGF
epidermal growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40:1101–1110. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009;284:31493–31497. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo AG, Iruela-Arispe ML. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc Res. 2010;86:226–235. doi: 10.1093/cvr/cvq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asplund A, Friden V, Stillemark-Billton P, Camejo G, Bondjers G. Macrophages exposed to hypoxia secrete proteoglycans for which LDL has higher affinity. Atherosclerosis. 2011;215:77–81. doi: 10.1016/j.atherosclerosis.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Asplund A, Stillemark-Billton P, Larsson E, Rydberg EK, Moses J, Hulten LM, Fagerberg B, Camejo G, Bondjers G. Hypoxic regulation of secreted proteoglycans in macrophages. Glycobiology. 2009;20:33–40. doi: 10.1093/glycob/cwp139. [DOI] [PubMed] [Google Scholar]
- Bogels M, Braster R, Nijland PG, Gul N, van de Luijtgaarden W, Fijneman RJ, Meijer GA, Jimenez CR, Beelen RH, van Egmond M. Carcinoma origin dictates differential skewing of monocyte function. Oncoimmunology. 2013;1:798–809. doi: 10.4161/onci.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen O, Dina OA, Gear RW, Levine JD. Dependence of monocyte chemoattractant protein 1 induced hyperalgesia on the isolectin B4-binding protein versican. Neuroscience. 2009;159:780–786. doi: 10.1016/j.neuroscience.2008.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollyky PL, Bogdani M, Bollyky JB, Hull RL, Wight TN. The role of hyaluronan and the extracellular matrix in islet inflammation and immune regulation. Curr Diab Rep. 2012;12:471–480. doi: 10.1007/s11892-012-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollyky PL, Lord JD, Masewicz SA, Evanko SP, Buckner JH, Wight TN, Nepom GT. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:744–747. doi: 10.4049/jimmunol.179.2.744. [DOI] [PubMed] [Google Scholar]
- Cardoso LE, Little PJ, Ballinger ML, Chan CK, Braun KR, Potter-Perigo S, Bornfeldt KE, Kinsella MG, Wight TN. Platelet-derived growth factor differentially regulates the expression and post-translational modification of versican by arterial smooth muscle cells through distinct protein kinase C and extracellular signal-regulated kinase pathways. J Biol Chem. 2010;285:6987–6995. doi: 10.1074/jbc.M109.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MY, Chan CK, Braun KR, Green PS, O’Brien KD, Chait A, Day AJ, Wight TN. Monocyte-to-macrophage differentiation: synthesis and secretion of a complex extracellular matrix. J Biol Chem. 2012;287:14122–14135. doi: 10.1074/jbc.M111.324988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coster L, Carlstedt I, Malmstrom A. Isolation of 35S- and 3H-labelled proteoglycans from cultures of human embryonic skin fibroblasts. Biochem J. 1979;183:669–681. doi: 10.1042/bj1830669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day AJ, de la Motte CA. Hyaluronan cross-linking: a protective mechanism in inflammation? Trends Immunol. 2005;26:637–643. doi: 10.1016/j.it.2005.09.009. [DOI] [PubMed] [Google Scholar]
- de la Motte C, Hascall V, Drazba J, Strong S. Poly I:C induces mononuclear leukocyte-adhesive hyaluronan structures on colon smooth muscle cells: I alpha I and versican facilitate adhesion. In: Kennedy JF, GP, Williams PA, Hascall VC, editors. Hyaluronan, Volume 1 Chemical, Biochemical and Biological Aspects. Woodhead Publishing Limited; Cambridge, England: 2002. pp. 381–388. [Google Scholar]
- de la Motte CA. Hyaluronan in intestinal homeostasis and inflammation: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2011;301:G945–949. doi: 10.1152/ajpgi.00063.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Motte CA, Drazba JA. Viewing hyaluronan: imaging contributes to imagining new roles for this amazing matrix polymer. J Histochem Cytochem. 2011;59:252–257. doi: 10.1369/0022155410397760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Motte CA, Hascall VC, Calabro A, Yen-Lieberman B, Strong SA. Mononuclear leukocytes preferentially bind via CD44 to hyaluronan on human intestinal mucosal smooth muscle cells after virus infection or treatment with poly(I.C) J Biol Chem. 1999;274:30747–30755. doi: 10.1074/jbc.274.43.30747. [DOI] [PubMed] [Google Scholar]
- de la Motte CA, Hascall VC, Drazba J, Bandyopadhyay SK, Strong SA. Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: inter-a-trypsin inhibitor is crucial to structure and function. Am J Pathol. 2003;163:121–133. doi: 10.1016/s0002-9440(10)63636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1004–1013. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- Evanko SP, Johnson PY, Braun KR, Underhill CB, Dudhia J, Wight TN. Platelet-derived growth factor stimulates the formation of versican-hyaluronan aggregates and pericellular matrix expansion in arterial smooth muscle cells. Arch Biochem Biophys. 2001;394:29–38. doi: 10.1006/abbi.2001.2507. [DOI] [PubMed] [Google Scholar]
- Evanko SP, Potter-Perigo S, Bollyky PL, Nepom GT, Wight TN. Hyaluronan and versican in the control of human T-lymphocyte adhesion and migration. Matrix Biol. 2012;31:90–100. doi: 10.1016/j.matbio.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanko SP, Potter-Perigo S, Johnson PY, Wight TN. Organization of hyaluronan and versican in the extracellular matrix of human fibroblasts treated with the viral mimetic poly I:C. J Histochem Cytochem. 2009;57:1041–1060. doi: 10.1369/jhc.2009.953802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firan M, Dhillon S, Estess P, Siegelman MH. Suppressor activity and potency among regulatory T cells is discriminated by functionally active CD44. Blood. 2006;107:619–627. doi: 10.1182/blood-2005-06-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Nagy JA, Brown LF, Shih SC, Johnson PY, Chan CK, Dvorak HF, Wight TN. Proteolytic cleavage of versican and involvement of ADAMTS-1 in VEGF-A/VPF-induced pathological angiogenesis. J Histochem Cytochem. 2011;59:463–473. doi: 10.1369/0022155411401748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Joshi N, Choi H, Ryu S, Hahn M, Catena R, Sadik H, Argani P, Wagner P, Vahdat LT, Port JL, Stiles B, Sukumar S, Altorki NK, Rafii S, Mittal V. Myeloid progenitor cells in the premetastatic lung promote metastases by Inducing mesenchymal to epithelial transition. Cancer Res. 2012a;72:1384–1394. doi: 10.1158/0008-5472.CAN-11-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Vahdat LT, Wong S, Chang JC, Mittal V. Microenvironmental regulation of epithelial-mesenchymal transitions in cancer. Cancer Res. 2012b;72:4883–4889. doi: 10.1158/0008-5472.CAN-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Wight TN, Frevert CW. Proteoglycans: key regulators of pulmonary inflammation and the innate immune response to lung infection. Anat Rec (Hoboken) 2010;293:968–981. doi: 10.1002/ar.21094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall VC, Majors AK, De La Motte CA, Evanko SP, Wang A, Drazba JA, Strong SA, Wight TN. Intracellular hyaluronan: a new frontier for inflammation? Biochim Biophys Acta. 2004;1673:3–12. doi: 10.1016/j.bbagen.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Hatano S, Kimata K, Hiraiwa N, Kusakabe M, Isogai Z, Adachi E, Shinomura T, Watanabe H. Versican/PG-M is essential for ventricular septal formation subsequent to cardiac atrioventricular cushion development. Glycobiology. 22:1268–1277. doi: 10.1093/glycob/cws095. [DOI] [PubMed] [Google Scholar]
- Hernandez D, Miquel-Serra L, Docampo MJ, Marco-Ramell A, Bassols A. Role of versican V0/V1 and CD44 in the regulation of human melanoma cell behavior. Int J Mol Med. 2011a;27:269–275. doi: 10.3892/ijmm.2010.577. [DOI] [PubMed] [Google Scholar]
- Hernandez D, Miquel-Serra L, Docampo MJ, Marco-Ramell A, Cabrera J, Fabra A, Bassols A. V3 versican isoform alters the behavior of human melanoma cells by interfering with CD44/ErbB-dependent signaling. J Biol Chem. 2011b;286:1475–1485. doi: 10.1074/jbc.M110.127522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A, Braun KR, Liu K, Wang Y, Wight TN. Retrovirally mediated overexpression of versican v3 reverses impaired elastogenesis and heightened proliferation exhibited by fibroblasts from Costello syndrome and Hurler disease patients. Am J Pathol. 2004;164:119–131. doi: 10.1016/S0002-9440(10)63103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A, Mecham RP, Keeley F, Rabinovitch M. Impaired elastin fiber assembly related to reduced 67-kD elastin-binding protein in fetal lamb ductus arteriosus and in cultured aortic smooth muscle cells treated with chondroitin sulfate. J Clin Invest. 1991;88:2083–2094. doi: 10.1172/JCI115538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose J, Kawashima H, Yoshie O, Tashiro K, Miyasaka M. Versican interacts with chemokines and modulates cellular responses. J Biol Chem. 2001;276:5228–5234. doi: 10.1074/jbc.M007542200. [DOI] [PubMed] [Google Scholar]
- Huang R, Merrilees MJ, Braun K, Beaumont B, Lemire J, Clowes AW, Hinek A, Wight TN. Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury. Circ Res. 2006;98:370–377. doi: 10.1161/01.RES.0000202051.28319.c8. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Naso MF, Cannizzaro LA, Wasmuth JJ, McPherson JD. Mapping of the versican proteoglycan gene (CSPG2) to the long arm of human chromosome 5 (5q12–5q14) Genomics. 1992;14:845–851. doi: 10.1016/s0888-7543(05)80103-x. [DOI] [PubMed] [Google Scholar]
- Kawashima H, Atarashi K, Hirose M, Hirose J, Yamada S, Sugahara K, Miyasaka M. Oversulfated chondroitin/dermatan sulfates containing GlcAbeta1/IdoAalpha1-3GalNAc(4,6-O-disulfate) interact with L- and P-selectin and chemokines. J Biol Chem. 2002;277:12921–12930. doi: 10.1074/jbc.M200396200. [DOI] [PubMed] [Google Scholar]
- Kawashima H, Hirose M, Hirose J, Nagakubo D, Plaas AH, Miyasaka M. Binding of a large chondroitin sulfate/dermatan sulfate proteoglycan, versican, to L-selectin, P-selectin, and CD44. J Biol Chem. 2000;275:35448–35456. doi: 10.1074/jbc.M003387200. [DOI] [PubMed] [Google Scholar]
- Kenagy RD, Fischer JW, Lara S, Sandy JD, Clowes AW, Wight TN. Accumulation and loss of extracellular matrix during shear stress-mediated intimal growth and regression in baboon vascular grafts. J Histochem Cytochem. 2005;53:131–140. doi: 10.1369/jhc.4A6493.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenagy RD, Plaas AH, Wight TN. Versican degradation and vascular disease. Trends Cardiovasc Med. 2006;16:209–215. doi: 10.1016/j.tcm.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata K, Oike Y, Tani K, Shinomura T, Yamagata M, Uritani M, Suzuki S. A large chondroitin sulfate proteoglycan (PG-M) synthesized before chondrogenesis in the limb bud of chick embryo. J Biol Chem. 1986;261:13517–13525. [PubMed] [Google Scholar]
- Kischel P, Waltregny D, Dumont B, Turtoi A, Greffe Y, Kirsch S, De Pauw E, Castronovo V. Versican overexpression in human breast cancer lesions: known and new isoforms for stromal tumor targeting. Int J Cancer. 2010;126:640–650. doi: 10.1002/ijc.24812. [DOI] [PubMed] [Google Scholar]
- Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169:2253–2263. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- LaPierre DP, Lee DY, Li SZ, Xie YZ, Zhong L, Sheng W, Deng Z, Yang BB. The ability of versican to simultaneously cause apoptotic resistance and sensitivity. Cancer Res. 2007;67:4742–4750. doi: 10.1158/0008-5472.CAN-06-3610. [DOI] [PubMed] [Google Scholar]
- Lauer ME, Erzurum SC, Mukhopadhyay D, Vasanji A, Drazba J, Wang A, Fulop C, Hascall VC. Differentiated murine airway epithelial cells synthesize a leukocyte-adhesive hyaluronan matrix in response to endoplasmic reticulum stress. J Biol Chem. 2008;283:26283–26296. doi: 10.1074/jbc.M803350200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer ME, Fulop C, Mukhopadhyay D, Comhair S, Erzurum SC, Hascall VC. Airway smooth muscle cells synthesize hyaluronan cable structures independent of inter-alpha-inhibitor heavy chain attachment. J Biol Chem. 2009a;284:5313–5323. doi: 10.1074/jbc.M807979200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer ME, Mukhopadhyay D, Fulop C, de la Motte CA, Majors AK, Hascall VC. Primary murine airway smooth muscle cells exposed to poly(I,C) or tunicamycin synthesize a leukocyte-adhesive hyaluronan matrix. J Biol Chem. 2009b;284:5299–5312. doi: 10.1074/jbc.M807965200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBaron RG, Zimmermann DR, Ruoslahti E. Hyaluronate binding properties of versican. J Biol Chem. 1992;267:10003–10010. [PubMed] [Google Scholar]
- Lemire JM, Merrilees MJ, Braun KR, Wight TN. Overexpression of the V3 variant of versican alters arterial smooth muscle cell adhesion, migration, and proliferation in vitro. J Cell Physiol. 2002;190:38–45. doi: 10.1002/jcp.10043. [DOI] [PubMed] [Google Scholar]
- Li D, Wang X, Wu JL, Quan WQ, Ma L, Yang F, Wu KY, Wan HY. Tumor-produced versican V1 enhances hCAP18/LL-37 expression in macrophages through activation of TLR2 and vitamin D3 signaling to promote ovarian cancer progression in vitro. PLoS One. 2013;8:e56616. doi: 10.1371/journal.pone.0056616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Ten Dam GB, Murugan S, Yamada S, Hashiguchi T, Mizumoto S, Oguri K, Okayama M, van Kuppevelt TH, Sugahara K. Involvement of highly sulfated chondroitin sulfate in the metastasis of the lewis lung carcinoma cells. J Biol Chem. 2008;283:34294–34304. doi: 10.1074/jbc.M806015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SQ, Alkema PK, Tieche C, Tefft BJ, Liu DZ, Li YC, Sumpio BE, Caprini JA, Paniagua M. Negative regulation of monocyte adhesion to arterial elastic laminae by signal regulatory protein alpha and Src homology 2 domain-containing protein-tyrosine phosphatase-1. J Biol Chem. 2005;280:39294–39301. doi: 10.1074/jbc.M503866200. [DOI] [PubMed] [Google Scholar]
- Lundell A, Olin AI, Morgelin M, al-Karadaghi S, Aspberg A, Logan DT. Structural basis for interactions between tenascins and lectican C-type lectin domains: evidence for a crosslinking role for tenascins. Structure (Camb) 2004;12:1495–1506. doi: 10.1016/j.str.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Majors AK, Austin RC, de la Motte CA, Pyeritz RE, Hascall VC, Kessler SP, Sen G, Strong SA. Endoplasmic reticulum stress induces hyaluronan deposition and leukocyte adhesion. J Biol Chem. 2003;278:47223–47231. doi: 10.1074/jbc.M304871200. [DOI] [PubMed] [Google Scholar]
- Malla N, Berg E, Theocharis AD, Svineng G, Uhlin-Hansen L, Winberg JO. In vitro reconstitution of complexes between pro-matrix metalloproteinase-9 and the proteoglycans serglycin and versican. FEBS J. 2013;280:2870–2887. doi: 10.1111/febs.12291. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- Masuda A, Yasuoka H, Satoh T, Okazaki Y, Yamaguchi Y, Kuwana M. Versican is upregulated in circulating monocytes in patients with systemic sclerosis and amplifies a CCL2-mediated pathogenic loop. Arthritis Res Ther. 2013;15:R74. doi: 10.1186/ar4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch DR, Nelson CM, Dixon LJ, Silver DL, Wylie JD, Lindner V, Sasaki T, Cooley MA, Argraves WS, Apte SS. ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev Cell. 2009;17:687–698. doi: 10.1016/j.devcel.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrilees MJ, Beaumont BW, Braun KR, Thomas AC, Kang I, Hinek A, Passi A, Wight TN. Neointima formed by arterial smooth muscle cells expressing versican variant v3 is resistant to lipid and macrophage accumulation. Arterioscler Thromb Vasc Biol. 2011;31:1309–1316. doi: 10.1161/ATVBAHA.111.225573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrilees MJ, Lemire JM, Fischer JW, Kinsella MG, Braun KR, Clowes AW, Wight TN. Retrovirally mediated overexpression of versican v3 by arterial smooth muscle cells induces tropoelastin synthesis and elastic fiber formation in vitro and in neointima after vascular injury. Circ Res. 2002;90:481–487. doi: 10.1161/hh0402.105791. [DOI] [PubMed] [Google Scholar]
- Miquel-Serra L, Serra M, Hernández D, Domenzain C, Docampo MJ, Rabanal R, de Torres I, Wight TN, Fabra A, Bassols A. V3 versican isoform expression has a dual role in human melanoma tumor growth and metastasis. Lab Invest. 2006;86:889–901. doi: 10.1038/labinvest.3700449. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol. 1998;202:56–66. doi: 10.1006/dbio.1998.9001. [DOI] [PubMed] [Google Scholar]
- Naso MF, Zimmermann DR, Iozzo RV. Characterization of the complete genomic structure of the human versican gene and functional analysis of its promoter. J Biol Chem. 1994;269:32999–33008. [PubMed] [Google Scholar]
- Olsen NJ, Moore JH, Aune TM. Gene expression signatures for autoimmune disease in peripheral blood mononuclear cells. Arthritis Res Ther. 2004;6:120–128. doi: 10.1186/ar1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish CR. The role of heparan sulphate in inflammation. Nat Rev Immunol. 2006;6:633–643. doi: 10.1038/nri1918. [DOI] [PubMed] [Google Scholar]
- Potter-Perigo S, Johnson PY, Evanko SP, Chan CK, Braun KR, Wilkinson TS, Altman LC, Wight TN. Polyinosine-polycytidylic acid stimulates versican accumulation in the extracellular matrix promoting monocyte adhesion. Am J Respir Cell Mol Biol. 2010;43:109–120. doi: 10.1165/rcmb.2009-0081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said N, Sanchez-Carbayo M, Smith SC, Theodorescu D. RhoGDI2 suppresses lung metastasis in mice by reducing tumor versican expression and macrophage infiltration. J Clin Invest. 2012;122:1503–1518. doi: 10.1172/JCI61392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said N, Theodorescu D. RhoGDI2 suppresses bladder cancer metastasis via reduction of inflammation in the tumor microenvironment. Oncoimmunology. 2012;1:1175–1177. doi: 10.4161/onci.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Setoguchi R, Yagi H, Nomura T. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in self-tolerance and autoimmune disease. Curr Top Microbiol Immunol. 2006;305:51–66. doi: 10.1007/3-540-29714-6_3. [DOI] [PubMed] [Google Scholar]
- Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, Zimmermann DR, Lemire JM, Fischer JW, Wight TN, Clowes AW. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem. 2001;276:13372–13378. doi: 10.1074/jbc.M009737200. [DOI] [PubMed] [Google Scholar]
- Schönherr E, Järveläinen HT, Sandell LJ, Wight TN. Effects of platelet-derived growth factor and transforming growth factor-β 1 on the synthesis of a large versican-like chondroitin sulfate proteoglycan by arterial smooth muscle cells. J Biol Chem. 1991;266:17640–17647. [PubMed] [Google Scholar]
- Schönherr E, Kinsella MG, Wight TN. Genistein selectively inhibits platelet-derived growth factor stimulated versican biosynthesis in monkey arterial smooth muscle cells. Arch Biochem Biophys. 1997;339:353–361. doi: 10.1006/abbi.1996.9854. [DOI] [PubMed] [Google Scholar]
- Schor H, Vaday GG, Lider O. Modulation of leukocyte behavior by an inflamed extracellular matrix. Dev Immunol. 2000;7:227–238. doi: 10.1155/2000/51902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbi W, Day AJ, Rugg MS, Fulop C, de la Motte CA, Bowen T, Hascall VC, Phillips AO. Overexpression of hyaluronan synthase 2 alters hyaluronan distribution and function in proximal tubular epithelial cells. J Am Soc Nephrol. 2006a;17:1553–1567. doi: 10.1681/ASN.2005080879. [DOI] [PubMed] [Google Scholar]
- Selbi W, de la Motte C, Hascall V, Phillips A. BMP-7 modulates hyaluronan-mediated proximal tubular cell-monocyte interaction. J Am Soc Nephrol. 2004;15:1199–1211. doi: 10.1097/01.asn.0000125619.27422.8e. [DOI] [PubMed] [Google Scholar]
- Selbi W, de la Motte CA, Hascall VC, Day AJ, Bowen T, Phillips AO. Characterization of hyaluronan cable structure and function in renal proximal tubular epithelial cells. Kidney Int. 2006b;70:1287–1295. doi: 10.1038/sj.ki.5001760. [DOI] [PubMed] [Google Scholar]
- Senior RM, Griffin GL, Mecham RP. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980;66:859–862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M, Miquel L, Domenzain C, Docampo MJ, Fabra A, Wight TN, Bassols A. V3 versican isoform expression alters the phenotype of melanoma cells and their tumorigenic potential. Int J Cancer. 2005;114:879–886. doi: 10.1002/ijc.20813. [DOI] [PubMed] [Google Scholar]
- Sheng W, Wang G, La Pierre DP, Wen J, Deng Z, Wong CK, Lee DY, Yang BB. Versican mediates mesenchymal-epithelial transition. Mol Biol Cell. 2006;17:2009–2020. doi: 10.1091/mbc.E05-10-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng W, Wang G, Wang Y, Liang J, Wen J, Zheng PS, Wu Y, Lee V, Slingerland J, Dumont D, Yang BB. The roles of versican V1 and V2 isoforms in cell proliferation and apoptosis. Mol Biol Cell. 2005;16:1330–1340. doi: 10.1091/mbc.E04-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou J, Bull CM, Li L, Qian HR, Wei T, Luo S, Perkins D, Solenberg PJ, Tan SL, Chen XY, Roehm NW, Wolos JA, Onyia JE. Identification of blood biomarkers of rheumatoid arthritis by transcript profiling of peripheral blood mononuclear cells from the rat collagen-induced arthritis model. Arthritis Res Ther. 2006;8:R28. doi: 10.1186/ar1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- Somerville RP, Longpre JM, Jungers KA, Engle JM, Ross M, Evanko S, Wight TN, Leduc R, Apte SS. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem. 2003;278:9503–9513. doi: 10.1074/jbc.M211009200. [DOI] [PubMed] [Google Scholar]
- Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10:712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- Suwan K, Choocheep K, Hatano S, Kongtawelert P, Kimata K, Watanabe H. Versican/PG-M Assembles Hyaluronan into Extracellular Matrix and Inhibits CD44-mediated Signaling toward Premature Senescence in Embryonic Fibroblasts. J Biol Chem. 2009;284:8596–8604. doi: 10.1074/jbc.M806927200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. Faseb J. 2006;20:9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- Toeda K, Nakamura K, Hirohata S, Hatipoglu OF, Demircan K, Yamawaki H, Ogawa H, Kusachi S, Shiratori Y, Ninomiya Y. Versican is induced in infiltrating monocytes in myocardial infarction. Mol Cell Biochem. 2005;280:47–56. doi: 10.1007/s11010-005-8051-4. [DOI] [PubMed] [Google Scholar]
- Touab M, Arumi-Uria M, Barranco C, Bassols A. Expression of the proteoglycans versican and mel-CSPG in dysplastic nevi. Am J Clin Pathol. 2003;119:587–593. doi: 10.1309/ME25-J1G5-ENE5-7LM3. [DOI] [PubMed] [Google Scholar]
- Touab M, Villena J, Barranco C, Arumi-Uria M, Bassols A. Versican is differentially expressed in human melanoma and may play a role in tumor development. Am J Pathol. 2002;160:549–557. doi: 10.1016/S0002-9440(10)64874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaday GG, Franitza S, Schor H, Hecht I, Brill A, Cahalon L, Hershkoviz R, Lider O. Combinatorial signals by inflammatory cytokines and chemokines mediate leukocyte interactions with extracellular matrix. J Leukoc Biol. 2001;69:885–892. [PubMed] [Google Scholar]
- Vaday GG, Lider O. Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. J Leukoc Biol. 2000;67:149–159. doi: 10.1002/jlb.67.2.149. [DOI] [PubMed] [Google Scholar]
- Viola M, Bartolini B, Vigetti D, Karousou E, Moretto P, Deleonibus S, Sawamura T, Wight TN, Hascall VC, De Luca G, Passi A. Oxidized low density lipoprotein (LDL) affects hyaluronan synthesis in human aortic smooth muscle cells. J Biol Chem. 2013;288:29595–29603. doi: 10.1074/jbc.M113.508341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, de la Motte C, Lauer M, Hascall V. Hyaluronan matrices in pathobiological processes. FEBS J. 2011;278:1412–1418. doi: 10.1111/j.1742-4658.2011.08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Hascall VC. Hyaluronan structures synthesized by rat mesangial cells in response to hyperglycemia induce monocyte adhesion. J Biol Chem. 2004;279:10279–10285. doi: 10.1074/jbc.M312045200. [DOI] [PubMed] [Google Scholar]
- Wang W, Xu GL, Jia WD, Ma JL, Li JS, Ge YS, Ren WH, Yu JH, Liu WB. Ligation of TLR2 by versican: a link between inflammation and metastasis. Arch Med Res. 2009;40:321–323. doi: 10.1016/j.arcmed.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- Wingrove JA, Daniels SE, Sehnert AJ, Tingley W, Elashoff MR, Rosenberg S, Buellesfeld L, Grube E, Newby LK, Ginsburg GS, Kraus WE. Correlation of peripheral-blood gene expression with the extent of coronary artery stenosis. Circ Cardiovasc Genet. 2008;1:31–38. doi: 10.1161/CIRCGENETICS.108.782730. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhang Y, Cao L, Chen L, Lee V, Zheng PS, Kiani C, Adams ME, Ang LC, Paiwand F, Yang BB. Identification of the motif in versican G3 domain that plays a dominant-negative effect on astrocytoma cell proliferation through inhibiting versican secretion and binding. J Biol Chem. 2001;276:14178–14186. doi: 10.1074/jbc.M100618200. [DOI] [PubMed] [Google Scholar]
- Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- Yang BL, Zhang Y, Cao L, Yang BB. Cell adhesion and proliferation mediated through the G1 domain of versican. J Cell Biochem. 1999;72:210–220. doi: 10.1002/(sici)1097-4644(19990201)72:2<210::aid-jcb5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Zako M, Shinomura T, Ujita M, Ito K, Kimata K. Expression of PG-M (V3), an alternatively spliced form of PG-M without a chondroitin sulfate attachment region in mouse and human tissues. J Biol Chem. 1995;270:3914–3918. doi: 10.1074/jbc.270.8.3914. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cao L, Kiani C, Yang BL, Hu W, Yang BB. Promotion of chondrocyte proliferation by versican mediated by G1 domain and EGF-like motifs. J Cell Biochem. 1999;73:445–457. [PubMed] [Google Scholar]
- Zhang Z, Miao L, Wang L. Inflammation amplification by versican: the first mediator. Int J Mol Sci. 2012;13:6873–6882. doi: 10.3390/ijms13066873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng PS, Vais D, Lapierre D, Liang YY, Lee V, Yang BL, Yang BB. PG-M/versican binds to P-selectin glycoprotein ligand-1 and mediates leukocyte aggregation. J Cell Sci. 2004;117:5887–5895. doi: 10.1242/jcs.01516. [DOI] [PubMed] [Google Scholar]
- Zimmermann D. Versican. In: Iozzo R, editor. Proteoglycans: Structure, Biology and Molecular Interactions. Marcel Dekker, Inc; New York: 2000. pp. 327–341. [Google Scholar]
- Zimmermann DR, Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989;8:2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]