Salmonellae PhoPQ regulation of the outer membrane to resist innate immunity (original) (raw)
. Author manuscript; available in PMC: 2015 Feb 14.
Published in final edited form as: Curr Opin Microbiol. 2014 Feb 14;17:106–113. doi: 10.1016/j.mib.2013.12.005
Abstract
Salmonellae sense host cues to regulate properties important for bacterial survival and replication within host tissues. The PhoPQ two-component regulatory system senses phagosome acidification and cationic antimicrobial peptides to regulate the protein and lipid contents of the bacterial envelope. PhoPQ regulated lipid components of the outer membrane include lipopolysaccharide and glycerolphospholipids. Envelope proteins regulated by PhoPQ, include: components of virulence associated secretion systems, the flagellar apparatus, membrane transport systems, and proteins that are likely structural components of the outer membrane. PhoPQ alteration of the bacterial surface results in increased bacterial resistance to cationic antimicrobial peptides (CAMP) and decreased detection by the innate immune system. This review highlights the complexity of the outer membrane as an environmentally regulated organelle.
Keywords: lipid A, lipopolysaccharide, PagP, PhoPQ, cationic antimicrobial peptide, aminoarabinose, glycerophospholipid, phosphatidylglycerol, cardiolipin, TLR4/MD2/CD14, Gram-negative bacteria, membrane lipids, Salmonellae, phagosome, molecular pathogenesis
Introduction
During infection, Salmonellae encounter a wide-range of host environments including those found in the lumen of the small intestine and phagosomal vacuoles of epithelial cells and macrophages [1]. Dramatic shifts in nutrient abundance [2–5], pH [6,7], as well as the presence of reactive oxygen and nitrogen species [8,9], membrane damaging cationic antimicrobial peptides (CAMP) [10–12] and bile acids [13–15], are among the environmental hazards that bacteria encounter during infection. Here, we review mechanisms of Salmonellae PhoPQ40-regulated envelope remodeling and its role in bacterial resistance to innate immune killing.
PhoPQ is activated when Salmonellae are within acidifying phagosome vacuoles to promote virulence for animals and humans
The PhoPQ virulence regulators are activated within systemic host environments including the acidified phagolysosome of macrophages [6]. Both activating and null mutations in phoPQ severely attenuate S. Typhimurium for virulence in murine models of infection indicating regulation mediated by PhoPQ is critical for disease [16,17]. In broth culture, the PhoPQ system can be activated when Salmonellae are grown in media of acidic pH [6,18], containing subinhibitory concentrations of CAMP [19], or severely limited in divalent cations [20]. Environmental activation of the inner membrane (IM) PhoQ sensor-kinase increases phosphorylation of PhoP, its cytoplasmic response regulator. Phosphorylated PhoP activates genes encoding OM proteins [21–23], regulators [24,25], components of the intracellular type III secretion system[26], IM transporters that buffer cytosolic pH [27], and enzymes that covalently modify OM barrier components [28,29], while repressing genes encoding the flagellar and invasion associated type III secretion systems [30]
PhoPQ regulates the outer membrane barrier
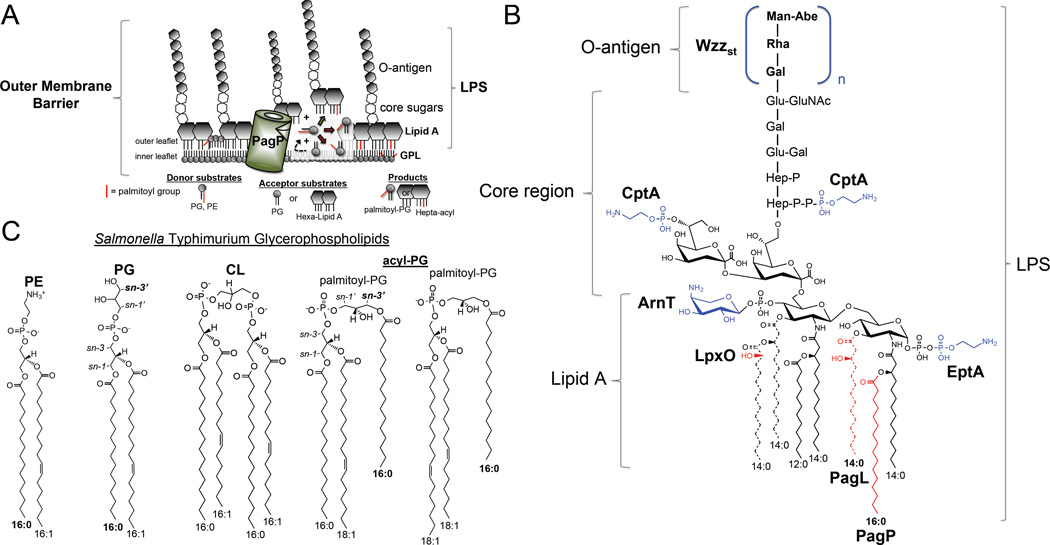
The outer membrane of Gram-negative bacteria is a complex organelle that provides a barrier protecting bacteria from hazards in their environment (Fig. 1A). The outer leaflet of the OM consists of predominantly lipid A (Fig. 1B), the bioactive component of LPS detected by the TLR4/MD2/CD14 innate immune receptor complex [30]. Lipid A anchors LPS to inner leaflet glycerophospholipids (GPL) through hydrophobic interactions between fatty acyl side chains (Fig. 1C). LPS core oligosaccharide and hypervariable repeated O-antigen extend from the diglucosamine polar head group of the lipid A amphiphile to complete what is often referred to as the OM barrier (Fig. 1A). Salmonellae synthesize GPL and lipid A on the inner leaflet of the IM by defined biosynthetic machinery [31]. However, unlike lipid A, which is specifically transported to the OM by an inner and outer membrane spanning protein complex [32] the mechanism of GPL transport to the OM is undefined.
Figure 1. PhoPQ-regulated remodeling of outer membrane structures.
(A) Structural organization of the S. Typhimurium outer membrane (OM). PagP hydrolyzes palmitoyl groups of carbon length sixteen (C16:0) from glycerophospholipid (GPL) donor substrates and transfers them to lipid A molecules with six acyl substituents of carbon length twelve (C12:0) and fourteen (C14:0), or to the polar head group of phosphatidylglycerol (PG) molecules that have flipped (dashed arrow) into the OM outer leaflet on account of PhoPQ activation, or OM damage inflicted by antimicrobial peptides. The PagP palmitoyltransferase produces lipid A molecules with seven acyl chains with one palmitoyl group, as well as triacylated palmitoyl-PG molecules with a palmitoyl group extending from the sn-3’ head-group position [35]. (B) PhoPQ regulates the chemical structure of S. Typhimurium lipopolysaccharides (LPS). Modifications in red are directly regulated by the PhoPQ system. Those in blue are indirectly regulated by the PhoPQ system through the PmrA response regulator. Dashed lines indicate acyl groups cleaved by PagL and PagP. (C) Chemical structures of specific S. Typhimurium GPL. The PagP enzyme transfers palmitoyl groups from the sn-1 position of GPL donor substrates to the sn-3’ position of PG acceptor substrates by transesterification. The two palmitoyl-PG species depicted are generated by the PagP enzyme within the OM of S. Typhimurium [35]. CL = cardiolipin, or diphosphatidylglycerol, PE = phosphatidylethanolamine, acyl-PG = acylphosphatidyglycerols.
S. Typhimurium regulate the structure of LPS through PhoQ sensing and activation, and these structural alterations contribute to resistance to CAMP. Specific changes in OM lipids regulated by PhoPQ include: reducing average O-antigen chain-length [36,37], acylating, deacylating, and hydroxylating lipid A [28,29], derivitizing lipid A [38] and LPS core phosphates with cationic groups (Fig. 1B) [39], palmitoylating OM PG molecules (Fig. 1C) [35], and increasing cardiolipid content. PhoPQ also activates the synthesis of more than two-dozen unique OM proteins including basic proteins of unknown function, such as PagC, which are basic proteins which are likely to complex with negatively charged lipid molecules as part of the OM barrier [23,40,41]. Therefore, upon PhoPQ activation an extensive alteration of lipopolysaccharides, glycerophospholipids, and proteins elaborates an OM barrier more impermeable to CAMP that promotes survival within acidified host phagosomes.
LPS remodeling increases the OM barrier to CAMP while decreasing innate immune recognition to promote bacterial virulence
Cationic amphipathic antimicrobial peptides have diverse membrane-active structures conserved from bacteria to humans [42,43]. Structural diversity and specific bacterial killing mechanisms of CAMP may have led to mammals evolving multiple sub-types, including amphipathic alpha-helical molecules and beta-sheet structures, the latter whose amphipathic nature is maintained by disulfide bonds. Beta-sheet peptides, such as defensins make up 30% of the dry weight of a neutrophil and are secreted into the intestinal lumen by Paneth cells to protect the intestinal crypts. Defensins are critical innate immune effectors likely targeting Salmonellae during intestinal colonization and inflammation [44,45].
The activity of CAMP requires their attraction to the anionic phosphate groups that flank lipid A and LPS-core sugar molecule on the surface of Gram-negative bacteria (Fig. 1B). Upon surface interaction, amphipathic CAMP hydrophobic faces insert into the lipid bilayer [43]. On penetration, some alpha-helical antimicrobial peptides may form discrete pore-like structures [46], while others can cause acute membrane blistering near the cell poles [47]. Therefore, CAMP may target specific domains within the OM, rather than inserting randomly or uniformly throughout the bilayer. Regardless of their specific mechanism all antimicrobials must penetrate the Gram-negative OM barrier to kill the microbe.
A variety of studies support that resistance to antimicrobial peptides is important for Salmonellae to survive during infection including data demonstrating PhoP-null mutants survive better in macrophages from mice deficient for cathelicidin-related antimicrobial peptide (Cramp), than in macrophages from wild-type mice [48]. Likewise, bacteria with mutations in PhoPQ are attenuated for virulence in a variety of animal and tissue culture models of infection [16] and PhoP-null mutants of Salmonella enterica serovar Typhi are attenuated in human volunteers [49,50]. Therefore, CAMP resistance and OM remodeling is likely critical for both human and animal infections caused by Salmonellae.
Innate immune surveillance pathways detect invading pathogens using pattern recognition receptors that recognize unique microbe associated molecular patterns [51]. Specific PhoPQ-regulated modifications, such as palmitoylation of lipid A, which generates a molecule with seven acyl side chains, one of carbon length sixteen, can reduce LPS activation of the TLR4-containing receptor complex [30]. The human receptor is optimized to recognize lipid A with six fatty acids of carbon length twelve and fourteen corresponding to lauryl and myristoyl groups, respectively [52,53] Lipid A molecules and their regulation by PhoPQ are quite diverse among bacterial species. PhoPQ and the enzymes that modify lipid A are highly conserved and implicated in the pathogenesis of a Legionella pneumophilia, Bordetella spp, Francisella spp., and Yersinia pestis [54]. Specific modifications of lipid A have also been associated with adaptation of Pseudomonas aeruginosa to the airways of individuals with Cystic Fibrosis [55]. Overall understanding of the enzymes that participate in the de-phosphorylation and the acylation state of lipid A has lead to identification of the molecular specificity of the human TLR4 receptor complex and has formed the molecular basis for the use of altered lipid A molecules as important vaccine adjuvants, and therapeutic agents that can activate the receptor without the toxicity of LPS [30, 54].
Outer membrane remodeling mechanisms promoted by the PhoPQ system
Derivitizing LPS phosphates with aminoarabinose and ethanolamine
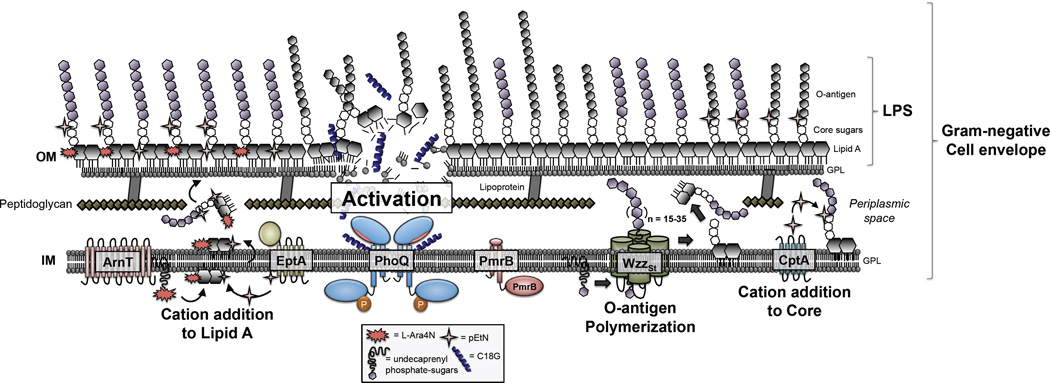
Upon sensing pH and CAMP, PhoPQ increases transcription of pmrD and the pmrCAB operon to activate the PmrA response regulator [24–26]. Activated PmrA induces transcription of genes encoding enzymes that covalently modify lipid A and core sugar phosphates with aminoarabinose (L-Ara4N) and phosphoethanolamine (pEtN) (Fig. 2) [56]. The initial step for L-Ara4N modification is catalyzed by the PhoP and PmrA-regulated UDP-glucose dehydrogenase (PagA, or Ugd) which oxidizes UDP-glucose within the cytosol for amine addition [38]. Remaining components of the L-Ara4N-LPS modification pathway are encoded within an operon, pmrHFIJKLM, which is activated by PhoPQ through PmrA [56]. When L-Ara4N synthesis is mostly complete, the UDP-nucleotide is exchanged for a undecaprenyl-phosphate carrier lipid on the inner leaflet of the IM (Fig. 2) [57]. The carrier and L-Ara4N are then flipped into the outer leaflet of the IM where L-Ara4N is transferred to nascent lipid A phosphates by ArnT, also known as PmrK. Finally, O-antigen is loaded onto the core structure and assembled LPS molecules are transported across the periplasm and inserted into the OM by the Lpt system [32].
Figure 2. Bacterial adaptation to host antimicrobial peptides requires coordinated synthesis of diverse OM structures.
Cationic antimicrobial peptides (CAMP) like C18G, an amphipathic alpha-helical human-derived cathelicidin, damage acute outer membrane (OM) surfaces to expose a thin layer of peptidoglycan, the periplasmic space, and eventually the inner membrane (IM). PhoQ binds CAMP on the outer leaflet of the IM to activate phosphorylation of PhoP, which induces PmrA and its regulon. PmrA regulates: ArnT, an aminoarabinose (L-Ara4N) transferase, EptA, a lipid A phosphoethanolamine (pEtN) transferase, Wzzst, a long-type O-antigen translocase, and CptA, a LPS core pEtN transferase.
Decreased negative charge conferred by cationic L-Ara4N groups on lipid A molecules diminishes CAMP binding sites for drugs like colistins, which are bacterial-derived polymyxins increasingly used as last resort antibiotics against multidrug resistant Gram-negative bacteria, such as P. aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae. Aminoarabinose modification is required for S. Typhimurium to fully colonize the murine small intestine [12,24] and PhoPQ likely senses CAMP within the intestinal lumen to activate the PmrA regulon [12]. Further understanding of the role of L-Ara4N in intestinal colonization and survival may reveal roles for OM remodeling during S. Typhimurium competition with the intestinal microbiota.
Decreasing O-antigen chain length
O-antigens form a discrete heterogeneous layer outside the bacterial cell that is in direct contact with host environments [58]. These immunodominant polysaccharides are under extreme selective pressure and therefore highly variable, likely as a result of selective pressure by bacteriophages. O-antigens are involved in immune evasion [59–61], as well as resistance to killing by host complement [62] and bile acids [15,63,64]. PhoPQ activation reduces the overall number of O-antigen repeats through the action of the PmrA-regulated membrane protein complex, Wzzst. A more uniform and shorter O-antigen, or reduced synthesis of more rare forms of LPS with very long-type O-antigens with greater than one hundred subunits may contribute to O-antigen-related phenotypes [36,37]. Recent structure-function analysis suggests the Wzzst IM protein complex selectively measures shorter long-type O-antigens with between sixteen and thirty-five subunits (Fig. 2) [65]. Mechanistically, it remains unclear how average reductions in O-antigen chain length contribute to the PhoPQ-regulated barrier, but it is plausible that a more uniform shorter O-antigen somehow promotes barrier function while decreasing recognition by the adaptive immune system, analogous to the role of lipid A remodeling in innate immune evasion.
Hydroxylating a lipid A myristoyl group
Initial chemical characterization of PhoPQ-regulated LPS structures measured an abundance of uniquely hydroxylated myristoyl groups [36]. Likewise, lipid A species with 2-hydroxymyristate substitutions are produced by S. Typhimurium during infection of macrophages [66]. Regulated hydroxylation of myristoyl groups is catalyzed by the IM dioxgenase, LpxO [67]. PhoPQ does not regulate transcription of lpxO nor does 2-hydroxymyristate play a defined role in OM barrier function making its physiologic role and mechanism of activation by PhoPQ unknown. The function of LpxO may be linked to VisP. VisP is a periplasmic peptidoglycan binding protein that may physically interact with LpxO [68] and exhibits a genetic relationship with lpxO during infection. Mutations in the S. Typhimurium visP locus result in bacteria that are defective for systemic infection of mice [68]. The visP phenotype is partially synthetic, since systemic defects can be relieved by second site mutations in lpxO. However, since lpxO single mutants show minimal defects in causing systemic disease, it seems likely that the activities of VisP and LpxO are incompatible with growth in the absence of the other protein. It is interesting to speculate that the function of the periplasmic protein VisP is in someway related to specific hydroxylated LPS.
Removing R-3-hydroxy-myristate from lipid A
The outer membrane of Gram-negative bacteria contains few proteins with catalytic activity [69,70]. S. Typhimurium encodes two specialized OM phospholipases whose role in barrier remodeling is fairly well understood. PagL is found in select Gram-negative bacteria including Salmonellae and Pseudomonads [71]. The enzyme hydrolyzes myristate of carbon length fourteen from lipid A to generate pentaacylated structures in S. Typhimurium (Fig. 1B) [28]. Although PagL levels are increased on PhoPQ activation, demyristoylated lipid A is not observed in broth culture [28], or within macrophages [66]. Inhibition of PagL activity by L-Ara4N modified lipid A phosphates may explain why PagL activity is not observed in PhoPQ-activated bacteria. Demyristoylated lipid A structures are produced by S. Typhimurium when genes necessary for L-Ara4N modification are disrupted [72], or when specific residues within the outer leaflet surface loops of PagL are mutated [73,74]. This additional post-translational regulation may suggest that L-Ara4N-modified lipid A molecules bind PagL and render it inactive until membrane damage occurs. Such regulation could be important within host tissues, since demyristoylated lipid A structures have reduced TLR4-activating properties [30].
Transferring palmitoyl groups to lipid A and phosphatidylglycerol within the OM
Most OM phospholipases share an integral membrane beta-barrel structure with an active site exposed to the outer leaflet [73,75,76]. In response to OM damage [77,78], or PhoPQ activation [29,78], the conserved PagP enzyme transfers palmitoyl groups from the sn-1 position of GPL to lipid A using an outer leaflet active site (Fig. 1A) [75,77]. Increased palmitoylation increases the hydrophobicity of the OM and prevents penetration of amphipathic alpha-helical CAMP like C18G and LL-37 [54]. Heptacylated lipid A structures with one palmitoyl group demonstrate reduced activation of TLR4 [52], indicating the effects of lipid A palmitoylation are two-fold. In E. coli and S. Typhimurium, damage to the OM bilayer activates the PagP enzyme [29,77,79]. Penetration of CAMP, depletion of divalent cations, or treatment with detergents can cause LPS molecules to become displaced from the outer leaflet [34,83]. To maintain bilayer integrity, GPL from the inner leaflet flip into the outer leaflet forming GPL microdomains within the OM, which activate PagP palmitoyltransferase activity [77,80–83]. In contrast, when bacteria are not stressed the OM must be predominantly asymmetric [82]. Highly specialized and broadly conserved enzymes like OMPLA specifically degrade GPL that have reached the outer leaflet. Therefore, GPL must aberrantly flip into the outer leaflet at some slow rate during cell division [80,81,83]. Hydrolysis of GPL molecules yields lyso-GPL structures that can act as detergents and disrupt barrier stability. Genetic analysis supports that Gram-negative bacteria have a retrograde transport system that recycles GPL products back to the IM [83]. Recent work demonstrates that PagP has dual substrate specificity within the OM and in addition to lipid A, PagP transfers palmitoyl groups to phosphatidylglycerol on the bacterial surface (Fig. 1A) [35]. Therefore, when the PhoPQ system is activated GPL may be increasingly translocated to the outer leaflet to increase barrier hydrophobicity.
Regulating OM glycerolphospholipids
Recent work from our laboratory indicates that the PhoPQ system regulates the GPL component of the OM. Like for lipid A, the PagP enzyme also catalyzes the transfer of palmitoyl groups to PG within the OM (Fig. 1A) [35]. The synthesis of these unique sn-3’ palmitoylated acylphosphatidylglycerols, referred to as palmitoyl-PG, may contribute to the function of PagP in promoting resistance to antimicrobial peptides. Our group also noted an increase in cardiolipin (CL) content of the OM on activation of PhoPQ (Fig. 1C) [35], though no specific mechanism has been defined to explain this observation. It is interesting to speculate that CL may facilitate specific formation of microdomains within the OM that promote cell division and OM lipid remodeling when PhoPQ is activated [84–86].
Conclusions and future directions
Work over the last 20 years has demonstrated that the OM is not a static organelle but rather a highly regulated and mosaic surface barrier. The regulated lipid and protein components of the OM likely create microdomains for specific barrier functions while allowing multicomponent protein complexes to assemble. In this regard, perhaps the need for a second type III secretion system for S. Typhimurium’s intracellular lifestyle reflects the ability of the remodeled membrane to support the specific assembly and secretion of the phagosome-active, but not the surface-active secretion system. The recent observation that PhoPQ regulate OM GPL suggests that like for lipid A, OM GPL regulation may be important for many Gram-negative pathogens. Future work will define whether mechanisms of OM GPL regulation are conserved in other species and whether specific GPL structures are recognized by innate immune receptors. Lastly, suppression of the host innate immune response is a general principle of Gram-negative bacterial pathogenesis. Recently, it was shown that LPS activates the Nlrc4/Acs inflamasome and Caspase-11 through an unidentified receptor that detects distinct lipid A acylation patterns within the cytoplasm of macrophages [87–91]. Therefore, it is conceivable that regulation of lipid A allows S. Typhimurium to reduce activation of Caspase-11, or alternative cytosolic host-cell death pathways encountered by bacteria during infection of animals and humans.
HIGHLIGHTS.
- -
The Salmonellae PhoPQ virulence system is a major regulator of outer membrane (OM) lipids and proteins. - -
Lipopolysaccharide (LPS) regulation increases bacterial resistance to host killing. - -
PhoPQ regulates modification to LPS phosphates and controls acylation of lipid A. - -
PhoPQ promotes pamitoylation of phosphatidylglycerol (PG) and increases cardiolipin content of the OM. - -
The PhoPQ-regulated OM palmitoyltransferase, PagP, transfers palmitoyl groups to PG within the OM of S. Typhimurium.
Acknowledgments
The authors thank Susana Matamouros, Kevin Hicks, Hemantha and Bridgette Kulasekara, as well as Matthias Christen and Richard Pfuetzner for insightful discussions. Z.D.D was supported by the National Institutes of Health (NIH) Ruth L. Kirschstein National Research Service Award (F32AI096820). S.I.M was supported by the NIH (R01AI030479).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol. 2008;6(1):53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 2.Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, Roth JR, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467(7314):426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiennimitr P, Winter SE, Winter MG, Xavier MN, Tolstikov V, Huseby DL, Sterzenbach T, Tsolis RM, Roth JR, Baumler AJ. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc Natl Acad Sci U S A. 2011;108(42):17480–17485. doi: 10.1073/pnas.1107857108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deriu E, Liu JZ, Pezeshki M, Edwards RA, Ochoa RJ, Contreras H, Libby SJ, Fang FC, Raffatellu M. Probiotic bacteria reduce Salmonella typhimurium intestinal colonization by competing for iron. Cell Host Microbe. 2013;14(1):26–37. doi: 10.1016/j.chom.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steeb B, Claudi B, Burton NA, Tienz P, Schmidt A, Farhan H, Maze A, Bumann D. Parallel exploitation of diverse host nutrients enhances Salmonella virulence. PLoS Pathog. 2013;9(4):e1003301. doi: 10.1371/journal.ppat.1003301. Proteomics, bacterial genetics, competitive infections, and computational analysis obtain a comprehensive overview of Salmonella metabolism and growth in a systemic model of infection. Lipid A and glycerophospholipids are synthesized from sugars, fatty acids, and amino acids. Therefore, studies like these will become increasingly important for under standing outer membrane remodeling mechanisms that occurs within specific host tissues.
- 6.Alpuche Aranda CM, Swanson JA, Loomis WP, Miller SI. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc Natl Acad Sci U S A. 1992;89(21):10079–10083. doi: 10.1073/pnas.89.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin-Orozco N, Touret N, Zaharik ML, Park E, Kopelman R, Miller S, Finlay BB, Gros P, Grinstein S. Visualization of vacuolar acidification-induced transcription of genes of pathogens inside macrophages. Mol Biol Cell. 2006;17(1):498–510. doi: 10.1091/mbc.E04-12-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental Salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J Exp Med. 2000;192(2):227–236. doi: 10.1084/jem.192.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slauch JM. How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol Microbiol. 2011;82(4):952–963. doi: 10.1111/j.1365-2958.2011.07612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberger CM, Gallo RL, Finlay BB. Interplay between antibacterial effectors: A macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc Natl Acad Sci U S A. 2004;101(8):2422–2427. doi: 10.1073/pnas.0304455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valdez Y, Diehl GE, Vallance BA, Grassl GA, Guttman JA, Brown NF, Rosenberger CM, Littman DR, Gros P, Finlay BB. Nramp1 expression by dendritic cells modulates inflammatory responses during Salmonella typhimurium infection. Cell Microbiol. 2008;10(8):1646–1661. doi: 10.1111/j.1462-5822.2008.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards SM, Strandberg KL, Conroy M, Gunn JS. Cationic antimicrobial peptides serve as activation signals for the Salmonella typhimurium phoPQ and pmrAB regulons in vitro and in vivo. Front Cell Infect Microbiol. 2012;2(102) doi: 10.3389/fcimb.2012.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Velkinburgh JC, Gunn JS. PhoP-PhoQ-regulated loci are required for enhanced bile resistance in Salmonella spp. Infect Immun. 1999;67(4):1614–1622. doi: 10.1128/iai.67.4.1614-1622.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crawford RW, Keestra AM, Winter SE, Xavier MN, Tsolis RM, Tolstikov V, Baumler AJ. Very long O-antigen chains enhance fitness during Salmonella-induced colitis by increasing bile resistance. PLoS Pathog. 2012;8(9):e1002918. doi: 10.1371/journal.ppat.1002918. The very long O-antigen chain assembly regulator FepE confers luminal fitness advantage in the mouse colitis model and promotes S. Typhimurium resistance to bile acids. Though the molecular mechanism of this resistance is uncertain, increasing evidence supports that Salmonellae colonize the mammalian gall bladder. Whether specific outer membrane remodeling by PhoPQ is important within this host niche is an interesting question.
- 15.Crawford RW, Rosales-Reyes R, Ramirez-Aguilar Mde L, Chapa-Azuela O, Alpuche-Aranda C, Gunn JS. Gallstones play a significant role in Salmonella spp. Gallbladder colonization and carriage. Proc Natl Acad Sci U S A. 2010;107(9):4353–4358. doi: 10.1073/pnas.1000862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller SI, Kukral AM, Mekalanos JJ. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989;86(13):5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller SI, Mekalanos JJ. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J Bacteriol. 1990;172(5):2485–2490. doi: 10.1128/jb.172.5.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prost LR, Daley ME, Le Sage V, Bader MW, Le Moual H, Klevit RE, Miller SI. Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol Cell. 2007;26(2):165–174. doi: 10.1016/j.molcel.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Bader MW, Sanowar S, Daley ME, Schneider AR, Cho U, Xu W, Klevit RE, Le Moual H, Miller SI. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell. 2005;122(3):461–472. doi: 10.1016/j.cell.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 20.Garcia Vescovi E, Soncini FC, Groisman EA. Mg2+ as an extracellular signal: Environmental regulation of Salmonella virulence. Cell. 1996;84(1):165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 21.Gunn JS, Belden WJ, Miller SI. Identification of phoP-phoQ activated genes within a duplicated region of the Salmonella typhimurium chromosome. Microb Pathog. 1998;25(2):77–90. doi: 10.1006/mpat.1998.0217. [DOI] [PubMed] [Google Scholar]
- 22.Belden WJ, Miller SI. Further characterization of the PhoP regulon: Identification of new PhoP-activated virulence loci. Infect Immun. 1994;62(11):5095–5101. doi: 10.1128/iai.62.11.5095-5101.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guina T, Yi EC, Wang H, Hackett M, Miller SI. A PhoP-regulated outer membrane protease of Salmonella enterica serovar Typhimurium promotes resistance to alpha-helical antimicrobial peptides. J Bacteriol. 2000;182(14):4077–4086. doi: 10.1128/jb.182.14.4077-4086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunn JS, Miller SI. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J Bacteriol. 1996;178(23):6857–6864. doi: 10.1128/jb.178.23.6857-6864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato A, Groisman EA. Connecting two-component regulatory systems by a protein that protects a response regulator from dephosphorylation by its cognate sensor. Genes Dev. 2004;18(18):2302–2313. doi: 10.1101/gad.1230804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fass E, Grosiman EA. Control of Salmonella pathogenecity island-2 gene expression. Current Opin Microbiol. 2009;12(2):199–204. doi: 10.1016/j.mib.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee EJ, Pontes MH, Groisman EA. A bacterial virulence protein promotes pathogenicity by inhibiting the bacterium's own F1Fo ATP synthase. Cell. 2013;154(1):146–156. doi: 10.1016/j.cell.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trent MS, Pabich W, Raetz CR, Miller SI. A PhoP/PhoQ-induced lipase (PagL) that catalyzes 3-O-deacylation of lipid a precursors in membranes of Salmonella typhimurium. J Biol Chem. 2001;276(12):9083–9092. doi: 10.1074/jbc.M010730200. [DOI] [PubMed] [Google Scholar]
- 29.Bishop RE, Gibbons HS, Guina T, Trent MS, Miller SI, Raetz CR. Transfer of palmitate from phospholipids to lipid A in outer membranes of Gram-negative bacteria. EMBO J. 2000;19(19):5071–5080. doi: 10.1093/emboj/cdd507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller SI, Ernst RK, Bader MW. LPS, Tlr4 and infectious disease diversity. Nat Rev Microbiol. 2005;3(1):36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- 31.Parsons JB, Rock CO. Bacterial lipids: Metabolism and membrane homeostasis. Prog Lipid Res. 2013;52(3):249–276. doi: 10.1016/j.plipres.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okuda S, Freinkman E, Kahne D. Cytoplasmic ATP hydrolysis powers transport of lipopolysaccharide across the periplasm in E. coli. Science. 2012;338(6111):1214–1217. doi: 10.1126/science.1228984. This work describes that process by which Gram-negative bacteria transport completed LPS molecules across the periplasm and into the outer membrane by the Lpt system. Multiple rounds of adenosine triphosphate hydrolysis propel a continuous stream of LPS molecules into the outer membrane. Whether analogous systems exist for glycerophospholipid transport to the outer membrane is not understood.
- 33.Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2(5):a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67(4):593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalebroux ZD, Matamouros S, Whittington D, Miller SI. PhoPQ regulates acidic glycerophospholipid content of the Salmonella Typhimurium outer membrane. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1316901111. in press. This recent work is the first to demonstrate specific regulation of outer membrane glycerophospholipids by a bacterial virulence system, and to biochemically show that the PagP enzyme transfers palmitoyl groups to phosphatidylglycerol molecules within the OM of Salmonella. Analogous to lipid A modification, outer membrane glycerophospholipid remodeling may be a broadly conserved mechanism by which bacteria resist innate immunity.
- 36.Guo L, Lim KB, Gunn JS, Bainbridge B, Darveau RP, Hackett M, Miller SI. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science. 1997;276(5310):250–253. doi: 10.1126/science.276.5310.250. [DOI] [PubMed] [Google Scholar]
- 37.Delgado MA, Mouslim C, Groisman EA. The PmrA/PmrB and RcsC/YojN/RcsB systems control expression of the Salmonella O-antigen chain length determinant. Mol Microbiol. 2006;60(1):39–50. doi: 10.1111/j.1365-2958.2006.05069.x. [DOI] [PubMed] [Google Scholar]
- 38.Gunn JS, Lim KB, Krueger J, Kim K, Guo L, Hackett M, Miller SI. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol Microbiol. 1998;27(6):1171–1182. doi: 10.1046/j.1365-2958.1998.00757.x. [DOI] [PubMed] [Google Scholar]
- 39.Tamayo R, Choudhury B, Septer A, Merighi M, Carlson R, Gunn JS. Identification of CptA, a PmrA-regulated locus required for phosphoethanolamine modification of the Salmonella enterica serovar Typhimurium lipopolysaccharide core. J Bacteriol. 2005;187(10):3391–3399. doi: 10.1128/JB.187.10.3391-3399.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller SI, Pulkkinen WS, Selsted ME, Mekalanos JJ. Characterization of defensin resistance phenotypes associated with mutations in the phoP virulence regulon of Salmonella typhimurium. Infect Immun. 1990;58(11):3706–3710. doi: 10.1128/iai.58.11.3706-3710.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pulkkinen WS, Miller SI. A Salmonella typhimurium virulence protein is similar to a Yersinia enterocolitica invasion protein and a bacteriophage lambda outer membrane protein. J Bacteriol. 1991;173(1):86–93. doi: 10.1128/jb.173.1.86-93.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hancock RE, Diamond G. The role of cationic antimicrobial peptides in innate host defences. TrendsMicrobiol. 2000;8(9):402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 43.Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nature Biotechnol. 2006;24(12):1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 44.Gallo RL, Murakami M, Ohtake T, Zaiou M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J Allergy Clin Immunol. 2002;110(6):823–831. doi: 10.1067/mai.2002.129801. [DOI] [PubMed] [Google Scholar]
- 45.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 46.Song C, Weichbrodt C, Salnikov ES, Dynowski M, Forsberg BO, Bechinger B, Steinem C, de Groot BL, Zachariae U, Zeth K. Crystal structure and functional mechanism of a human antimicrobial membrane channel. Proc Natl Acad Sci U S A. 2013;110(12):4586–4591. doi: 10.1073/pnas.1214739110. Numerous antimicrobial peptides have been described, though the structural and mechanistic basis of their action remains speculative. Here, researchers describe the structure of dermicidin in a lipid bilayer and demonstrate the molecular mechanism of action of this peptide as involving formation of high-conductance transmembrane channels that form pores within the inner plasma membrane of bacteria. Future molecular studies of the interactions of antimicrobial peptides with bacterial outer membranes could aid design of peptide antibiotics to treat multidrug resistant Gram-negative pathogens.
- 47.Hartmann M, Berditsch M, Hawecker J, Ardakani MF, Gerthsen D, Ulrich AS. Damage of the bacterial cell envelope by antimicrobial peptides Gramicidin S and Pgla as revealed by transmission and scanning electron microscopy. Antimicrob Agents Chemother. 2010;54(8):3132–3142. doi: 10.1128/AAC.00124-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenberger CM, Gallo RL, Finlay BB. Interplay between antibacterial effectors: A macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc Natl Acad Sci U S A. 2004;101(8):2422–2427. doi: 10.1073/pnas.0304455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hohmann EL, Oletta CA, Killeen KP, Miller SI. phoP/phoQ-deleted Salmonella typhi (ty800) is a safe and immunogenic single-dose typhoid fever vaccine in volunteers. J Infect Dis. 1996;173(6):1408–1414. doi: 10.1093/infdis/173.6.1408. [DOI] [PubMed] [Google Scholar]
- 50.Hohmann EL, Oletta CA, Miller SI. Evaluation of a phoP/phoQ-deleted, aroA-deleted live oral Salmonella typhi vaccine strain in human volunteers. Vaccine. 1996;14(1):19–24. doi: 10.1016/0264-410x(95)00173-x. [DOI] [PubMed] [Google Scholar]
- 51.Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: Discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe. 2009;6(1):10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hajjar AM, Ernst RK, Tsai JH, Wilson CB, Miller SI. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nature Immunol. 2002;3(4):354–359. doi: 10.1038/ni777. [DOI] [PubMed] [Google Scholar]
- 53.Kawasaki K, Ernst RK, Miller SI. 3-O-deacylation of lipid A by PagL, a PhoP/PhoQ-regulated deacylase of Salmonella typhimurium, modulates signaling through Toll-like receptor 4. J Biol Chem. 2004;279(19):20044–20048. doi: 10.1074/jbc.M401275200. [DOI] [PubMed] [Google Scholar]
- 54.Needham BD, Trent MS. Fortifying the barrier: The impact of lipid a remodelling on bacterial pathogenesis. Nat Rev Microbiol. 2013;11(7):467–481. doi: 10.1038/nrmicro3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ernst RK, Yi EC, Guo L, Lim KB, Burns JL, Hackett M, Miller SI. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science. 1999;286(5444):1561–1565. doi: 10.1126/science.286.5444.1561. [DOI] [PubMed] [Google Scholar]
- 56.Chen HD, Groisman EA. The biology of the PmrA/PmrB two-component system: The major regulator of lipopolysaccharide modifications. Annu Rev Microbiol. 2013;67:83–112. doi: 10.1146/annurev-micro-092412-155751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan A, Guan Z, Raetz CR. An undecaprenyl phosphate-aminoarabinose flippase required for polymyxin resistance in Escherichia coli. J Biol Chem. 2007;282(49):36077–36089. doi: 10.1074/jbc.M706172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu B, Knirel YA, Feng L, Perepelov AV, Senchenkova SN, Reeves PR, Wang L. Structural diversity in Salmonella O-antigens and its genetic basis. FEMS microbiology reviews. 2013 doi: 10.1111/1574-6976.12034. [DOI] [PubMed] [Google Scholar]
- 59.Duerr CU, Zenk SF, Chassin C, Pott J, Gutle D, Hensel M, Hornef MW. O-antigen delays lipopolysaccharide recognition and impairs antibacterial host defense in murine intestinal epithelial cells. PLoS Pathog. 2009;5(9):e1000567. doi: 10.1371/journal.ppat.1000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kong Q, Yang J, Liu Q, Alamuri P, Roland KL, Curtiss R., 3rd Effect of deletion of genes involved in lipopolysaccharide core and O-antigen synthesis on virulence and immunogenicity of Salmonella enterica serovar Typhimurium. Infect Immun. 2011;79(10):4227–4239. doi: 10.1128/IAI.05398-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crawford RW, Wangdi T, Spees AM, Xavier MN, Tsolis RM, Baumler AJ. Loss of very-long O-antigen chains optimizes capsule-mediated immune evasion by Salmonella enterica serovar Typhi. mBio. 2013;4(4) doi: 10.1128/mBio.00232-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trebicka E, Jacob S, Pirzai W, Hurley BP, Cherayil BJ. Role of antilipopolysaccharide antibodies in serum bactericidal activity against Salmonella enterica serovar Typhimurium in healthy adults and children in the United States. Clin Vaccine Immunol. 2013;20(10):1491–1498. doi: 10.1128/CVI.00289-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murray GL, Attridge SR, Morona R. Altering the length of the lipopolysaccharide O-antigen has an impact on the interaction of Salmonella enterica serovar Typhimurium with macrophages and complement. J Bacteriol. 2006;188(7):2735–2739. doi: 10.1128/JB.188.7.2735-2739.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crawford RW, Keestra AM, Winter SE, Xavier MN, Tsolis RM, Tolstikov V, Baumler AJ. Very long O-antigen chains enhance fitness during Salmonella-induced colitis by increasing bile resistance. PLoS Pathog. 2012;8(9):e1002918. doi: 10.1371/journal.ppat.1002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Larue K, Kimber MS, Ford R, Whitfield C. Biochemical and structural analysis of bacterial O-antigen chain length regulator proteins reveals a conserved quaternary structure. J Biol Chem. 2009;284(11):7395–7403. doi: 10.1074/jbc.M809068200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gibbons HS, Kalb SR, Cotter RJ, Raetz CR. Role of Mg2+ and pH in the modification of Salmonella lipid A after endocytosis by macrophage tumour cells. Mol Microbiol. 2005;55(2):425–440. doi: 10.1111/j.1365-2958.2004.04409.x. [DOI] [PubMed] [Google Scholar]
- 67.Gibbons HS, Lin S, Cotter RJ, Raetz CR. Oxygen requirement for the biosynthesis of the s-2-hydroxymyristate moiety in Salmonella typhimurium lipid A. Function of LpxO, a new Fe2+/alpha-ketoglutarate-dependent dioxygenase homologue. J Biol Chem. 2000;275(42):32940–32949. doi: 10.1074/jbc.M005779200. [DOI] [PubMed] [Google Scholar]
- 68.Moreira CG, Herrera CM, Needham BD, Parker CT, Libby SJ, Fang FC, Trent MS, Sperandio V. Virulence and stress-related periplasmic protein (VisP) in bacterial/host associations. Proc Natl Acad Sci U S A. 2013;110(4):1470–1475. doi: 10.1073/pnas.1215416110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bishop RE. Structural biology of membrane-intrinsic beta-barrel enzymes: Sentinels of the bacterial outer membrane. Biochim Biophys Acta. 2008;1778(9):1881–1896. doi: 10.1016/j.bbamem.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raetz CR, Reynolds CM, Trent MS, Bishop RE. Lipid A modification systems in Gram-negative bacteria. Annu Rev Biochem. 2007;76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geurtsen J, Steeghs L, Hove JT, van der Ley P, Tommassen J. Dissemination of lipid A deacylases (PagL) among Gram-negative bacteria: Identification of active-site histidine and serine residues. J Biol Chem. 2005;280(9):8248–8259. doi: 10.1074/jbc.M414235200. [DOI] [PubMed] [Google Scholar]
- 72.Kawasaki K, Ernst RK, Miller SI. Inhibition of Salmonella enterica serovar Typhimurium lipopolysaccharide deacylation by aminoarabinose membrane modification. J Bacteriol. 2005;187(7):2448–2457. doi: 10.1128/JB.187.7.2448-2457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rutten L, Geurtsen J, Lambert W, Smolenaers JJ, Bonvin AM, de Haan A, van der Ley P, Egmond MR, Gros P, Tommassen J. Crystal structure and catalytic mechanism of the LPS 3-O-deacylase PagL from Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2006;103(18):7071–7076. doi: 10.1073/pnas.0509392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manabe T, Kawasaki K. Extracellular loops of lipid A 3-O-deacylase PagL are involved in recognition of aminoarabinose-based membrane modifications in Salmonella enterica serovar Typhimurium. J Bacteriol. 2008;190(16):5597–5606. doi: 10.1128/JB.00587-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hwang PM, Choy WY, Lo EI, Chen L, Forman-Kay JD, Raetz CR, Prive GG, Bishop RE, Kay LE. Solution structure and dynamics of the outer membrane enzyme PagP by NMR. Proc Natl Acad Sci U S A. 2002;99(21):13560–13565. doi: 10.1073/pnas.212344499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rutten L, Mannie JP, Stead CM, Raetz CR, Reynolds CM, Bonvin AM, Tommassen JP, Egmond MR, Trent MS, Gros P. Active-site architecture and catalytic mechanism of the lipid a deacylase LpxR of Salmonella typhimurium. Proc Natl Acad Sci U S A. 2009;106(6):1960–1964. doi: 10.1073/pnas.0813064106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jia W, El Zoeiby A, Petruzziello TN, Jayabalasingham B, Seyedirashti S, Bishop RE. Lipid trafficking controls endotoxin acylation in outer membranes of Escherichia coli. J Biol Chem. 2004;279(43):44966–44975. doi: 10.1074/jbc.M404963200. [DOI] [PubMed] [Google Scholar]
- 78.Guo L, Lim KB, Poduje CM, Daniel M, Gunn JS, Hackett M, Miller SI. Lipid a acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell. 1998;95(2):189–198. doi: 10.1016/s0092-8674(00)81750-x. [DOI] [PubMed] [Google Scholar]
- 79.Brozek KA, Bulawa CE, Raetz CR. Biosynthesis of lipid A precursors in Escherichia coli. A membrane-bound enzyme that transfers a palmitoyl residue from a glycerophospholipid to lipid X. J Biol Chem. 1987;262(11):5170–5179. [PubMed] [Google Scholar]
- 80.Boekema EJ, Stuart M, Koning RI, Keegstra W, Brisson A, Verheij HM, Dekker N. A 7.4-a projection structure of outer membrane phospholipase A from Escherichia coli by electron crystallography. J Struct Biol. 1998;123(1):67–71. doi: 10.1006/jsbi.1998.4013. [DOI] [PubMed] [Google Scholar]
- 81.Dekker N. Outer-membrane phospholipase A: Known structure, unknown biological function. Mol Microbiol. 2000;35(4):711–717. doi: 10.1046/j.1365-2958.2000.01775.x. [DOI] [PubMed] [Google Scholar]
- 82.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiological reviews. 1985;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Malinverni JC, Silhavy TJ. An ABC transport system that maintains lipid asymmetry in the Gram-negative outer membrane. Proc Natl Acad Sci U S A. 2009;106(19):8009–8014. doi: 10.1073/pnas.0903229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Renner LD, Weibel DB. Cardiolipin microdomains localize to negatively curved regions of Escherichia coli membranes. Proc Natl Acad Sci U S A. 2011;108(15):6264–6269. doi: 10.1073/pnas.1015757108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Renner LD, Weibel DB. MinD and minE interact with anionic phospholipids and regulate division plane formation in Escherichia coli. J Biol Chem. 2012;287(46):38835–38844. doi: 10.1074/jbc.M112.407817. These two independent studies were the first to biochemically and biophysically demonstrate that cardiolipin localizes to negatively curved regions of the bacterial cell, such as the cell pole, and site membrane fission during cell division. The latter work above demonstrates that the presence of cardiolipin impacts cell division through electrostatic interactions with basic cell division proteins proteins. This work supports the hypothesis that membrane microdomains exist in bacteria and are essential for basic cellular processess.
- 86.Koppelman CM, Den Blaauwen T, Duursma MC, Heeren RM, Nanninga N. Escherichia coli minicell membranes are enriched in cardiolipin. J Bacteriol. 2001;183(20):6144–6147. doi: 10.1128/JB.183.20.6144-6147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, et al. Non-canonical inflammasome activation targets Caspase-11. Nature. 2011;479(7371):117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 88.Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, Monack DM. Caspase-11 increases susceptibility to Salmonella infection in the absence of Caspase-1. Nature. 2012;490(7419):288–291. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, Forsberg LS, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341(6151):1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 90.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates Caspase-11: Implications in TLR4-independent endotoxic shock. Science. 2013;341(6151):1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, Tan MH, Cotter PA, Vance RE, Aderem A, Miao EA. Caspase-11 protects against bacteria that escape the vacuole. Science. 2013;339(6122):975–978. doi: 10.1126/science.1230751. These elegant studies report that cytoplasmic lipopolysaccharides activate macrophage inflamasome components to stimulate cell death pathways in response to invading bacterial pathogens. Although the exact cytoplasmic receptor/complex has not been identified, work from Hagar et al suggests that the undefined receptor distinguishes LPS molecules by detecting their acylation pattern. Therefore, bacteria like Salmonellae may disguise themselves in a manner similar to what has been shown for recognition of LPS by the Toll-like receptor 4 complex. Whether bacterial LPS is transported by the macrophage from the phagosome into the cytoplasm, or whether Salmonellae escape, or secrete LPS into the cytoplasm are important questions for further analysis.