Hereditary spastic paraplegia: clinico-pathologic features and emerging molecular mechanisms (original) (raw)
. Author manuscript; available in PMC: 2014 Jun 4.
Published in final edited form as: Acta Neuropathol. 2013 Jul 30;126(3):307–328. doi: 10.1007/s00401-013-1115-8
Abstract
Hereditary spastic paraplegia (HSP) is a syndrome designation describing inherited disorders in which lower extremity weakness and spasticity are the predominant symptoms. There are more than 50 genetic types of HSP. HSP affects individuals diverse ethnic groups with prevalence estimates ranging from 1.2 to 9.6 per 100,000 [39, 70, 77, 154, 185]. Symptoms may begin at any age. Gait impairment that begins after childhood usually worsens very slowly over many years. Gait impairment that begins in infancy and early childhood may not worsen significantly. Post mortem studies consistently identify degeneration of corticospinal tract axons (maximal in the thoracic spinal cord) and degeneration of fasciculus gracilis fibers (maximal in the cervico-medullary region). HSP syndromes thus appear to involve motor-sensory axon degeneration affecting predominantly (but not exclusively) the distal ends of long central nervous system (CNS) axons. In general, proteins encoded by HSP genes have diverse functions including axon transport (e.g. SPG30/KIF1A, SPG10/KIF5A and possibly SPG4/Spastin); endoplasmic reticulum morphology (e.g. SPG3A/Atlastin, SPG4/Spastin, SPG12/reticulon 2, and SPG31/REEP1, all of which interact); mitochondrial function (e.g. SPG13/chaperonin 60/heat shock protein 60, SPG7/paraplegin; and mitochondrial ATP6; 4) myelin formation (e.g. SPG2/Proteolipid protein and SPG42/Connexin 47); 5) protein folding and ER-stress response (SPG6/NIPA1, SPG8/K1AA0196 (Strumpellin), SGP17/BSCL2 (Seipin) [113-115], “mutilating sensory neuropathy with spastic paraplegia” due to CcT5 mutation and presumably SPG18/ERLIN2); 6) corticospinal tract and other neurodevelopment (e.g. SPG1/L1 cell adhesion molecule and SPG22/thyroid transporter MCT8); 7) fatty acid and phospholipid metabolism (e.g. SPG28/DDHD1, SPG35/FA2H, SPG39/NTE, SPG54/DDHD2, and SPG56/CYP2U1); and 8) endosome membrane trafficking and vesicle formation (e.g. SPG47/AP4B1, SPG48/KIAA0415, SPG50/AP4M1, SPG51/AP4E, SPG52/AP4S1, and VSPG53/VPS37A). The availability of animal models (including bovine, murine, zebrafish, Drosophila, and C. elegans) for many types of HSP permits exploration of disease mechanisms and potential treatments. This review highlights emerging concepts of this large group of clinically similar disorders. For recent review of HSP including historical descriptions, differential diagnosis, and additional references see [78].
Introduction
“Hereditary spastic paraplegia” (HSP) is a clinical diagnostic designation for those neurologic syndromes a) in which bilateral lower extremity weakness and spasticity (each of variable degree) are the predominant (but often not only) manifestations; and b) for which gene mutation is the major causative factor. As with all classifications of clinical syndromes, it may be problematic to decide if a given disorder should be included or excluded in the category of HSP. For example, whereas paraplegin gene mutation, in which spastic paraparesis is often associated with ataxia, is recognized as a form of HSP (SPG7), Friedreich's ataxia and Charlevoix-Saguenay, syndromes in which ataxia is often associated with corticospinal tract deficits are considered forms of spinocerebellar ataxia and not forms of HSP. Although distal axon degeneration of corticospinal tracts is often reported in post mortem examination of HSP (discussed below), classification of a given clinico-genetic syndrome as a form of HSP is based on clinical and genetic features rather than neuropathologic features.
There are more than 50 genetic types of HSP (Table 1). Most types of HSP are designated by their genetic loci (“Spastic parapleGia” [SPG] 1 through 56) that are numbered in order of their discovery. As with other large groups of genetically heterogeneous disorders, variation in severity of the principle syndrome elements (lower extremity spasticity and weakness) together with the variable presence of additional neurologic (and occasionally systemic) abnormalities create wide clinical variation between (and within) genetic types of HSP. Nonetheless, by definition, clinical syndromes of HSP are characterized by lower extremity spasticity and weakness, each of varying degree, variable age-of-symptom onset, and variable degree of progression.
Table 1.
Genetic types of HSP (updated from [79]) (updated from [78])
| Spastic gait (SPG) locus | Protein | Clinical Syndrome | References |
|---|---|---|---|
| Autosomal dominant HSP | |||
| SPG3A (14q11-q21) | Atlastin | Uncomplicated HSP: symptoms usually begin in childhood (and may be non-progressive); symptoms may also begin in adolescence or adulthood and worsen insidiously. Genetic non-penetrance reported. De novo mutation reported presenting as spastic diplegic cerebral palsy. | [99, 184, 259] |
| SPG4 (chr.2p22) | Spastin | Uncomplicated HSP, symptom onset in infancy through senescence, single most common cause of autosomal dominant HSP (~40%); some subjects have late onset cognitive impairment. | [49, 72, 98, 104, 201] |
| SPG6 (15q11.1) | Not imprinted in Prader Willi/Angelman 1 (NIPA1) | Uncomplicated HSP: prototypical late-adolescent, early-adult onset, slowly progressive uncomplicated HSP. Rarely complicated by epilepsy or variable peripheral neuropathy. One subject with uncomplicated HSP later died from amyotrophic lateral sclerosis. | [50, 65, 80, 81, 148, 189, 223] |
| SPG8 (8q23-q24) | KIAA0196 (Strumpellin) | Uncomplicated HSP | [29, 100, 102, 195, 233] |
| SPG9 (10q23.3-q24.2) | Unknown | Complicated: spastic paraplegia associated with cataracts, gastroesophageal reflux, and motor neuronopathy | [155, 214] |
| SPG10 (12q13) | Kinesin heavy chain (KIF5A) | Uncomplicated HSP or complicated by distal muscle atrophy | [76, 196] |
| SPG12 (19q13) | Reticulon 2 (RTN2) | Uncomplicated HSP | [195] [162] |
| SPG13 (2q24-34) | Chaperonin 60 (heat shock protein 60, HSP60) | Uncomplicated HSP: adolescent and adult onset | [82, 95] [41] |
| SPG17 (11q12-q14) | BSCL2/seipin | Complicated: spastic paraplegia associated with amyotrophy of hand muscles (Silver Syndrome) | [19, 183, 249] |
| SPG19 (9q33-q34) | Unknown | Uncomplicated HSP | [234] |
| SPG29 (1p31.1-21.1) | Unknown | Complicated: spastic paraplegia associated with hearing impairment; persistent vomiting due to hiatal hernia inherited | [16] |
| SPG31 (2p12) | Receptor expression enhancing protein 1 (REEP1 | Uncomplicated HSP or occasionally associated with peripheral neuropathy | [24, 263, 264] |
| SPG33 (10q24.2) | ZFYVE27/protrudin} | Uncomplicated HSP | [146] |
| SPG36 (12q23-24) | Unknown | Onset age 14 to 28 years, associated with motor sensory neuropathy | [210] |
| SPG37 (8p21.1-q13.3) | Unknown | Uncomplicated HSP | [91] |
| SPG38 (4p16-p15) | Unknown | One family, 5 affected subjects, onset age 16-21 years. Subjects had atrophy of intrinsic hand muscles (severe in one subject at age 58) | [177] |
| SPG40 (locus unknown) | Unknown | Uncomplicated spastic paraplegia, onset after age 35, known autosomal dominant HSP loci excluded | [221] |
| SPG41 (11p14.1-p11.2 | Unknown | Single Chinese family with adolescent onset, spastic paraplegia associated with mild weakness of intrinsic hand muscles | [257] |
| SPG42 (3q24-q26) | Acetyl CoA transporter (SLC33A1) | Uncomplicated spastic paraplegia reported in single kindred, onset age 4-40 years, possibly one instance of incomplete penetrance. | [136, 137, 209] |
| Autosomal recessive HSP | |||
| SPG5 (8p) | CYP7B1 | Uncomplicated or complicated by axonal neuropathy, distal or generalized muscle atrophy, and white matter abnormalities on MRI | [30, 53, 103, 165, 225, 230, 247] |
| SPG7 (16q) | Paraplegin | Uncomplicated or complicated: variably associated with mitochondrial abnormalities on skeletal muscle biopsy and dysarthria, dysphagia, optic disc pallor, axonal neuropathy, and evidence of “vascular lesions”, cerebellar atrophy, or cerebral atrophy on cranial MRI | [61, 85] |
| SPG11 (15q) | Spatacsin (KIAA1840) | Uncomplicated or complicated: spastic paraplegia variably associated with thin corpus callosum, mental retardation, upper extremity weakness, dysarthria, and nystagmus; may have “Kjellin syndrome”: childhood-onset, progressive spastic paraplegia accompanied by pigmentary retinopathy, mental retardation, dysarthria, dementia, and distal muscle atrophy; juvenile, slowly progressive ALS reported in subjects with SPG11 HSP; 50% of autosomal recessive HSP is considered to be SPG11 | [149, 250] |
| SPG14 (3q27-28) | Unknown | Single consanguineous Italian family, 3 affected subjects, onset age ~30 years; Complicated spastic paraplegia with mental retardation and distal motor neuropathy (sural nerve biopsy was normal) | [236] |
| SPG15 (14q) | Spastizin/ZFYVE26 | Complicated: spastic paraplegia variably associated with associated with pigmented maculopathy, distal amyotrophy, dysarthria, mental retardation, and further intellectual deterioration (Kjellin syndrome). | [92, 111] |
| SPG18 (8p12-p11.21) | Endoplasmic reticulum, lipid raft associated protein 2 (ERLIN2) | Two families described with spastic paraplegia complicated by mental retardation and thin corpus callosum. ERLIN2 mutations also identified in subjects with juvenile primary lateral sclerosis | [7, 8] [5] |
| SPG20 (13q) | Spartin | Complicated: spastic paraplegia associated with distal muscle wasting (Troyer syndrome) | [54, 55, 140, 182, 187] |
| SPG21 (15q21-q22) | Maspardin | Complicated: spastic paraplegia associated with dementia, cerebellar and extrapyramidal signs, thin corpus callosum, and white matter abnormalities (Mast syndrome) | [216] |
| SPG23 (1q24-q32) | Unknown | Complicated: childhood onset HSP associated with skin pigment abnormality (vitiligo), premature graying, characteristic facies; Lison syndrome | [33] |
| SPG24 (13q14) | Unknown | Complicated: childhood onset HSP variably complicated by spastic dysarthira and pseudobulbar signs | [108] |
| SPG25 (6q23-q24.1) | Unknown | Consanguineous Italian family, four subjects with adult (30-46 years) onset back and neck pain related to disk herniation and spastic paraplegia; surgical correction of disk herniation ameliorated pain and reduced spastic paraplegia. Peripheral neuropathy also present. | [262] |
| SPG26 (12p11.1–12q14) | Unknown | Single consanguineous Bedouin family with five affected subjects. Complicated: childhood onset (between 7 and 8 years), progressive spastic paraparesis with dysarthria and distal amyotrophy in both upper and lower limbs, nerve conduction studies were normal; mild intellectual impairment, normal brain MRI | [248] |
| SPG27 (10q22.1-q24.1) | Unknown | Complicated or uncomplicated HSP. Two families described. In one family (7 affected subjects) uncomplicated spastic paraplegia began between ages 25 and 45 years. In the second family (three subjects described) the disorder began in childhood and included spastic paraplegia, ataxia, dysarthria; mental retardation, sensorimotor polyneuropathy, facial dysmorphism and short stature. | [155],[198] |
| SPG28 (14q21.3-q22.3) | DDHD1 | Uncomplicated: pure spastic paraplegia, onset in infancy, childhood, or adolescence, either as an uncomplicated spastic paraplegia syndrome; or variable associated with axonal neuropathy, distal sensory loss, and cerebellar eye movement disturbance | [40] [227] |
| SPG29 (14q) | Unknown | Uncomplicated HSP, childhood onset | |
| SPG30 (2q37.3) | KIF1A | Complicated: spastic paraplegia, distal wasting, saccadic ocular pursuit, peripheral neuropathy, mild cerebellar signs | [127] |
| SPG32 (14q12-q21) | Unknown | Mild mental retardation, brainstem dysraphia, clinically asymptomatic cerebellar atrophy | |
| SPG35 (16q21-q23) | Fatty acid 2-hydroxylase (FA2H) | Childhood onset (6 -11 years), spastic paraplegia with extrapyramidal features, progressive dysarthria, dementia, seizures. Brain white matter abnormalities and brain iron accumulation; an Omani and a Pakistani kindred reported. | [63, 64, 131] |
| SPG39 (19p13) | Neuropathy target esterase (NTE) | Complicated: spastic paraplegia associated with wasting of distal upper and lower extremity muscles | [188] |
| SPG43 (19p13.11-q12 ) | C19orf12 | Two sisters from Mali, symptom onset 7 and 12 years, progressive spastic paraplegia with atrophy of intrinsic hand muscles and dysarthria (one sister) | [156] |
| SPG44 (1q41) | Gap junction protein GJA12/GJC2, also known as connexin47 (Cx47) | Allelic with “Pelizeaus-Merzbacher-like disease” (PMLD, early onset dysmyelinating disorder with nystagmus, psychomotor delay, progressive spasticity, ataxia). GJA/GJC2 mutation I33M causes a milder phenotype: late-onset (first and second decades), cognitive impairment, slowly progressive, spastic paraplegia, dysarthria, and upper extremity involvement. MRI and MR spectroscopy imaging consistent with a hypomyelinating leukoencephalopathy | [178] |
| SPG45 (10q24.3-q25.1) | Unknown | Single consanguineous kindred from Turkey, five subjects described: affected subjects had mental retardation, infantile onset lower extremity spasticity and contractures, one subject with optic atrophy, two subjects with pendular nystagmus; MRI in one subject was normal. | [69] |
| SPG46 (9p21.2-q21.12) | Unknown | Dementia, congenital cataract, ataxia, thin corpus callosum | [38] |
| SPG47 (1p13.2-1p12) | AB4B1 | Two affected siblings from consanguineous Arabic family with early childhood onset slowly progressive spastic paraparesis, mental retardation, and seizures; one subject had ventriculomegaly; the other subject had thin corpus callosum and periventricular white matter abnormalities | [34] |
| SPG48 (7p22.1) | KIAA0415 | Analysis of KIAA0415 gene in 166 unrelated spastic paraplegia subjects (38 recessive, 64 dominant, 64 “apparently sporadic”) and control subjects revealed homozygous mutation in two siblings with late onset (6th decade) uncomplicated spastic paraplegia; and heterozygous mutation in one subject with apparently sporadic spastic paraplegia. | [217] |
| SPG49 (14q32.31) | TECPR2 | 5 subjects from three apparently unrelated families (Jewish Bukharian ancestry) had infantile onset hypotonia, developmental delay with severe cognitive impairment, dysmorphic features (short stature, brady-microcephaly, oral, facial, dental, nuchal abnormalities). Spastic, ataxic, rigid gait developed in childhood; additional features included gastroesophageal reflux, recurrent apneic episodes, mild dysmorphic features. Two subjects had epilepsy and MRI of two subjects showed thin corpus callosum and cerebellar atrophy. | [179] |
| SPG50 (7q22.1) | AP4M1 | Five subjects from one consanguineous Moroccan family exhibited infantile onset, nonprogressive spastic quadriplegic with severe cognitive impairment; variably associated with adducted thumbs. Ventriculomegaly, white matter abnormalities and variable cerebellar atrophy noted on neuroimaging. Neuroaxonal abnormalities, gliosis, and reduced myelin noted on post mortem examination. | [167, 237] |
| SPG51 15q21.2 | AP4E1 | Two siblings from a consanguineous Palestinian Jordanian family and two siblings from a consanguineous Syrian family exhibited microcephaly, hypotonia, psychomotor delay, spastic tetraplegia, marked cognitive impairment with severe language impairment, facial dysmorphic features, abnormal brain MRI showed (including atrophy and diffuse white matter loss). Seizures were variably present. | [2, 163, 167] |
| SPG52 (14q12) | AP4S1 | 5 affected subjects from consanguineous Syrian family exhibited neonatal hypotonia and severe cognitive impairment and progressive, early childhood onset, spastic paraplegia, microcephaly, short stature, facial dysmorphism | [2] |
| SPG52 (14q12) | AP4S1 | Five subjects from a consanguineous Syrian kindred exhibited delayed motor development, and severe cognitive impairment. Neonatal hypotonia was followed by progressive spastic gait with contractures. Dysmorphic features included short stature, microcephaly, and facial abnormalities | [1, 59, 107] |
| SPG53 (8p22) | VSP37A | 9 subjects from two Arab Moslem families exhibited developmental delay, progressive lower extremity spasticity, and subsequently progressive upper extremity involvement; associated with skeletal dysmorphism (kyphosis and pectus carinatum); mild to moderate cognitive impairment; and variable hypertrichosis and impaired vibration sensation. | [261] |
| SPG54 (8p11.23) | DDHD2 | Affected subjects reported from four unrelated families exhibited psychomotor delay, cognitive impairment, progressive spasticity (onset before age 2 years), thin corpus callosum, periventricular white matter abnormalities. Additional clinical features include foot contractures, dysarthria, dysphagia, strabismus, optic hypoplasia | [6, 211] |
| SPG55 | C12ORF65 | 2 Japanese brothers from consanguineous parents exhibited early onset spastic paraplegia variably associated with reduced visual acuity (with central scotoma and optic atrophy), reduced upper extremity strength and dexterity, lower extremity muscle atrophy, and motor sensory neuropathy. | [215] [13] |
| SPG56 4q25 | CYP2U1 | 5 unrelated families were reported with early childhood onset spastic paraplegia, variable upper extremity involvement, upper extremity dystonia, cognitive impairment, thin corpus callosum, brain white matter disturbance, axonal neuropathy, basal ganglia calcifications | [227] |
| 2q31.1 | GAD1 | Four siblings in consanguineous Pakistani family with spastic cerebral palsy, and moderate to severe mental retardation | [161] [153] [141] |
| “SPOAN” syndrome (11q23) | Unknown | Complicated spastic paraplegia associated with optic atrophy, neuropathy (SPOAN) | [142] |
| 5p15.31-14.1 No SPG designation | Epsilon subunit of the cytosolic chaperonin-containing t-complex peptide-1 (Cct5) | Complicated spastic paraplegia associated with mutilating sensory neuropathy. | [36, 37] |
| X-linked HSP | |||
| SPG1 (Xq28) | L1 cell adhesion molecule (L1CAM) | Complicated: associated with mental retardation, and variably, hydrocephalus, aphasia, and adducted thumbs | [122] |
| SPG2 (Xq28) | Proteolipid protein | Complicated: variably associated with MRI evidence of CNS white matter abnormality; may have peripheral neuropathy | [47, 110, 129, 208] |
| SPG16 (Xq11.2-q23) | Unknown | Uncomplicated; or complicated: associated with motor aphasia, reduced vision, nystagmus, mild mental retardation, and dysfunction of the bowel and bladder | [220, 224] |
| SPG22 (Xq21) | Monocarboxylate transport 8 (MCT8) | Complicated (Allan-Herndon-Dudley syndrome): congenital onset, neck muscle hypotonia in infancy, mental retardation, dysarthria, ataxia, spastic paraplegia, abnormal facies | [10, 28, 150] |
| SPG34 | Uncomplicated, onset 12 to 25 years | [143] | |
| Maternal (mitochondrial) inheritance HSP | |||
| No SPG designation | Mitochondrial ATP6 gene | Adult onset, progressive spastic paraplegia, mild to severe symptoms, variably associated with axonal neuropathy, late-onset dementia, and cardiomyopathy. | [238] |
Clinical Classification
HSP syndromes are classified clinically [97] as “uncomplicated” (characterized by lower extremity spasticity and weakness and subtle lower extremity dorsal column impairment) ; and “complicated” (in which spastic paraplegia is associated with additional neurologic or systemic abnormalities including dementia, ataxia, mental retardation, neuropathy, distal wasting, loss of vision, epilepsy, or icthyosis [Sjogren-Larsson syndrome]). While some genetic types of HSP usually (but not invariantly) manifest as “uncomplicated” (e.g.SPG4 HSP due to spastin gene mutation, the single most common form of autosomal dominant HSP), other genetic types usually (but not always) manifest as “complicated HSP syndromes (such as SPG11 HSP, a common autosomal recessive form of HSP frequently associated with mental retardation and thin corpus callosum).
There is imperfect correlation between clinical classification (“uncomplicated” versus “complicated”) and genetic types of HSP. Restated, many genetic types of HSP are associated with both “uncomplicated” and “complicated” HSP syndromes. For example, SPG4 HSP, initially considered to be prototypical of uncomplicated HSP, has been associated with mental retardation and dementia [46, 197, 232, 243], ataxia [169] , thin corpus callosum [174], and muscle wasting [157, 175]. Similarly, SPG3A, which usually manifests as childhood onset, uncomplicated autosomal dominant HSP, has been associated with motor-sensory axonal neuropathy, distal wasting, and thin corpus callosum [56, 90, 116, 176]. Conversely, although autosomal recessive SPG7 (paraplegin mutation) and SPG11 (spatacsin mutation) usually manifest as “complicated HSP syndromes” (with ataxia being frequent in SPG7 HSP and mental retardation being frequent in SPG11 HSP), both of these types of HSP may also manifest as “uncomplicated” spastic paraplegia syndromes.
Some clinical variability within a given genetic type of HSP may be due to the variable consequences of discrete gene mutation (“genotype-phenotype correlation”), about which very little is known in HSP. In some cases however, both “uncomplicated” and “complicated” syndromes occur in the same family in which individuals have the identical gene mutation. These examples highlight the influence of as-yet-unidentified “modifying factors” (including genes and potentially environmental factors) in determining the phenotype.
Clinical presentation
For the vast majority of individuals, HSP presents with gait impairment due to lower extremity weakness and spasticity. Urinary urgency is common in HSP and occasionally may be an early or presenting feature. Symptoms may be first evident at any age, from early childhood through senescence. In general, subjects with “uncomplicated” HSP have normal life expectancy and do not experience loss of dexterity, strength, or coordination in the upper extremities, dysarthria or dysphagia.
Lower extremity spastic weakness (each of variable degree), combined with variable presence of one or more complicating features (discussed above) create numerous clinical HSP syndromes. The following HSP syndromes are commonly recognized (Table 2 [78]): 1) uncomplicated HSP; 2) spastic paraplegia associated with peripheral motor neuropathy and/or distal wasting; 3) spastic paraplegia associated with cognitive impairment; 4) spastic paraplegia associated with ataxia; 5) spastic paraplegia associated with neuroimaging abnormality; 6) spastic paraplegia associated with additional neurologic and systemic abnormalities. Examples of complicated HSP syndromes are summarized in Table 2.
Table 2.
Examples of complicated HSP syndromes*# (from [78])
| Spastic paraplegia and peripheral neuropathy |
|---|
| HSP types SPG2, 3A, 5, 6, 7, 10, 25, 27, 30, 31, 55, 56, SPOAN, Cct5 (epsilon subunit), mutation), mitochondrial ATP6 gene mutation |
| Spastic paraplegia and distal amyotrophy |
| HSP types SPG3A (rare feature of), 4 (rare feature of), 5, 10, 14, 15, 17, 20, 26, 30, 38, 39, 41, 43, 55 |
| Spastic paraplegia and mental retardation |
| HSP types SPG 1, 11, 14, 16,18, 20, 22, 26, 27, 32, 44, 45, 47, 49, 50, 51, 52, 53, 54, 56, GAD1 mutation |
| Spastic paraplegia with dementia |
| SPG4, 15, 21, 35, 46, and Mitochondrial ATP6 gene mutation |
| Spastic paraplegia and vision impairment (including blindness) |
| HSP types SPG15, 16, 45, 46, 54, 55, and SPOAN syndrome |
| Spastic paraplegia and deafness |
| SPG29 |
| Spastic paraplegia with skeletal abnormalities or dysmorphic features |
| HSP types SPG25, 49, 50, 51, 52, 53 |
| Spastic paraplegia and extrapyramidal movement disorder |
| HSP types SPG21, 35, 56 |
| Spastic paraplegia and epilepsy |
| HSP types SPG6, 35, 47, 51 |
| Spastic paraplegia, short stature, seizures, retinal degeneration, ichthyosiform skin changes (Sjogren-Larsson syndrome); autosomal recessive[117], [118] |
| Spastic paraplegia and dysarthria |
| HSP types SPG7, 15, 22, 24, 27, 35, 43, 44, 54 |
| Spastic paraplegia and ataxia |
| HSP types SPG7, 21, 22, 27, 30, 32, 46, 49 |
| Spastic paraplegia and hematologic abnormality |
| Spastic paraplegia and May-Hegglin anomaly: cytoplasmic inclusions in leukocytes, giant platelets, and thrombocytopenia; autosomal dominant[84] |
| Spastic paraparesis and Evan's syndrome (Coombs-positive hemolytic anemia and immune thrombocytopenia without a known underlying etiology); probably autosomal recessive[4] |
| Spastic paraplegia and MRI brain abnormalities |
| HSP types SPG1, 2, 5, 7 (variably abnormal), 11, 15, 18, 21, 32, 35, 44, 46, 47, 49, 50, 54, 56 |
| Spastic paraplegia and skin abnormalities |
| Spastic paraplegia with short stature: seizures, retinal degeneration, ichthyosiform skin changes (Sjogren Larsson syndrome); autosomal recessive[117], [118] |
| SPG23 HSP: Childhood onset spastic paraplegia associated with skin pigment abnormality; autosomal recessive[33] |
| Spastic paraplegia and endocrine disturbance |
| Spastic paraplegia and Kallman syndrome (hypogonadotrophic hypogonadism and anosmia)[231] |
Age of symptom onset may be quite variable even with a given genetic type of HSP. While the average age-of-symptom onset of some genetic types of HSP is much younger (e.g. childhood onset in SPG3A) than in other types (e.g. late-teenage to adult onset in SPG6 HSP), significant overlap between the range of ages at which symptoms first begin limits the ability to predict the genetic type of HSP from age of symptom onset alone.
Progression (rate and degree with which functional disability increases) in HSP is quite variable including relatively static, non-worsening disability, inexorable worsening, and, after a period of insidious decline, reaching an apparent stabilization of disability (apparent “functional plateau”). The fact that many HSP subjects seem to reach a functional plateau beyond which there is very little further disability (beyond that which is attributed to and parallels the approximate rate of normal, age-related decline) may reflect the interplay of multiple factors including a) reduced rate of neurodegeneration; and b) disease-ameliorating processes that promote recovery of damaged axons; and c) functional compensation through neuroplasticity.
When HSP begins in early childhood (first evident as toe-walking by age 2 years, for example), symptoms may be relatively non-progressive even over several decades. This is “relatively non-progressive course” is typical (but not invariant) of SPG3A HSP (the most common cause of early childhood-onset, autosomal dominant HSP) but may also be seen in SPG4 HSP and other types [31, 57]. The syndrome of early childhood onset, relatively non-progressive HSP may be extremely similar to that of spastic diplegic cerebral palsy. Indeed, HSP gene mutations (including de novo mutation for autosomal dominant HSP) have been identified in subjects initially diagnosed as having cerebral palsy [62, 160, 171, 191]. It may be appropriate to consider the recently identified genetic loci for autosomal recessive spastic diplegic cerebral palsy as a form of very early onset HSP [153, 246].
The rate and extent of clinical worsening in HSP is quite variable. For many individuals, gait impairment worsens insidiously over many years. The rate of clinical progression may not be uniform, however. Both more rapid progression during adolescence and slower rate of worsening after 5 to 10 years of symptoms are not uncommon. As noted above, many individuals, after 5 to 10 years of worsening gait impairment, appear to reach a “functional plateau” after which the rate of worsening seems to be reduced.
There are at least four examples in which HSP gene mutations were associated with clinical syndromes other than HSP. First, SPG3A/atlastin mutation (which typically causes early childhood-onset, relatively non-progressive, uncomplicated HSP) was recently identified in a family with autosomal dominant Hereditary Sensory Neuropathy (HSN 1) rather than with the syndrome of HSP [89]. In addition to profound, early sensory loss often associated with painless injuries, impaired healing, and osteomyelitis, HSN1 may be associated with distal motor involvement in advanced cases [89].
Second, BSCL2/Seipin mutations, when heterozygous, cause both autosomal dominant HSP (SPG17), Charcot-Marie-Tooth type 2, and distal hereditary motor neuropathy type V. When homozygous, _BSCL2/_Seipin mutations cause, congenital generalized lipodystrophy type 2 (CGL2), manifesting as severe lipoatrophy, insulin resistance, hypertriglyceridemia, and mental retardation [114, 144].
Third, although SPG11/spatacsin mutations are a frequent cause of autosomal recessive HSP (often, but not invariably associated with thin corpus callosum and mental retardation), they have also presented as levodopa-responsive, juvenile parkinsonism [12, 158].
And finally, although mutation of the SPG20/spartin gene causes autosomal recessive HSP associated with distal muscle wasting (Troyer syndrome), hypermethylation of the SPG20/spartin promoter is an apparent biomarker of colorectal carcinoma [138].
Neurologic examination of subjects with uncomplicated HSP demonstrates variable degrees lower extremity spasticity and weakness. Spasticity is greatest in hamstring, quadriceps, adductor, and gastrocnemius-soleus muscles. Weakness is most prominent in iliopsoas, hamstring, and tibialis anterior muscles. Hypperreflexia, crossed adductor signs, extensor plantar responses are typically present (plantar responses may occasionally be absent). Vibration sensation in the toes is frequently mildly impaired. Mild upper extremity hyperreflexia, without increased muscle tone, weakness, or impaired dexterity is common in subjects with uncomplicated HSP. Although pes cavus is frequent in HSP, it may be absent even in clearly affected subjects.
In addition to variable degrees of lower extremity weakness and spasticity and mild decrease in distal vibration sensation, neurologic examination of subjects with “complicated” HSP syndromes demonstrates additional neurologic or systemic abnormalities such as cognitive impairment, dementia, optic neuropathy, retinitis pigmentosa, extrapyramidal disturbance, ataxia, peripheral neuropathy, distal wasting, or cataracts.
The degree to which muscle weakness and muscle spasticity contribute to functional gait impairment is may be quite variable between individuals [97]. Some individuals have no significant weakness, with gait disturbance resulting primarily from spasticity and slowness of movements. For these individuals, medications that reduce spasticity may be beneficial. For other individuals, muscle weakness is a significant factor in gait disturbance. For individuals with significant weakness, muscle relaxing medications (including Botulinum toxin injections and intrathecal Lioresal pump) offer much less functional improvement.
Treatment
There is no effective treatment to prevent gait impairment in HSP. Spasticity may be reduced with oral or intrathecal Lioresal or oral Dantrolene or Tizanidine and selective injection of botulinum toxin (Botox). Oxybutynin is helpful in reducing urinary urgency. Physical therapy is generally recommended to improve range of motion, maintain and increase lower extremity strength, and increase cardiovascular conditioning. Toe-dragging may be reduced by ankle foot orthotics and by gait-phase dependent, transcutaneous peroneal nerve stimulation.
Genetic classification
There are dominant, recessive, X-linked forms of HSP, each of which is genetically heterogeneous. In addition, mitochondrial ATP6 mutation is an example of an spastic paraplegia syndrome that is transmitted by maternal inheritance [238]. HSP genetic loci are designated SPG (“SPastic parapleGia”) 1 through 56 in order of their discovery. There also are inherited disorders in which lower extremity spastic weakness is the primary clinical symptom that do not have “SPG” designations (e.g., SPOAN syndrome, Epsilon subunit of the cytosolic chaperonin-containing t-complex peptide-1 (Cct5), and mitochondrial ATP6 gene mutation, Table 1).
At least one form of HSP may manifest as both an autosomal recessive and autosomal dominant disorder. SPG7 HSP was originally described as an autosomal recessive disorder due to homozygous or compound heterozygous SPG7/paraplegin gene mutations. Subsequently, there are reports of complicated and uncomplicated HSP syndromes associated with heterozygous SPG7 paraplegin mutation [15, 43, 152]. Some of these individuals have similar histologic features (e.g. ragged red fibers in skeletal muscle biopsy) or biochemical evidence of mitochondrial disturbance similar to that shown in subjects with homozygous (or compound heterozygous SPG7/paraplegin mutations) [43]. It is not known whether autosomal recessive versus autosomal dominant transmission is associated with specific SPG7 mutations. Nor is it known if other types of recessively inherited HSP may also manifest as autosomal dominant disorders. Nonetheless, the fact that SPG7 HSP may manifest as both a dominantly inherited and recessively inherited disorder has significant implications for genetic counseling.
Genetic counseling in HSP must consider the often marked phenotypic variation (including age-of-symptom onset, severity, and the presence of “complicating feature”) between (and often within) different genetic types of HSP, and even between individuals in the same family who share the identical HSP gene mutation. Genetic penetrance in autosomal dominant HSP is age-dependent, may be high (80-90% in SPG4 HSP) or as low as 70% [68]. Apparent genetic anticipation has been reported occasionally in SPG3A and SPG4 HSP [45, 170, 192, 193]. It is interesting that descriptions of apparent genetic mutation for which the causative SPG3A[160] or SPG4 HSP mutations were described did not include trinucleotide repeat (or other tandem nucleotide) expansions that have been associated with other instances of genetic anticipation (such as in Huntington's disease, myotonic dystrophy, and other disorders).
Caution must be exercised in providing genetic counseling and prognosis for many genetic types of HSP because the full phenotypic spectrum and genetic penetrance are often not fully known. This applies particularly to subjects from families for whom the genetic type is not known; and subjects with genetic types of HSP that have been described in only one or several families. With the exception of a minority of HSP types (notably SPG3A, SPG4, SPG7, and SPG11) in which dozens of affected subjects have been reported, clinical descriptions of the majority of genetic types of HSP are limited to one to several families. Moreover, very little is known about mutation-specific genetic penetrance and genotype-phenotype correlation of individual HSP gene mutations. Finally, particular caution should be used when counseling families with SPG7 HSP in light of the observation that some SPG7 may cause autosomal dominant or autosomal recessive HSP.
Neuropathology
Post mortem studies in HSP consistently report axon degeneration involving lateral corticospinal tracts that is most severe at the distal ends (in the thoracic spinal cord) and less severe in the cervical spinal cord [25, 44, 60, 96, 204, 212, 213, 245]. In addition to distal corticospinal tract degeneration, degeneration of axons in fasciculus gracilis fibers is observed consistently and is most prominent in the cervical spinal cord.
Spinal cord axon degeneration may be sufficient to cause mild to marked atrophy of cervical and thoracic segments [67, 100, 101, 130, 218]. In some instances, corticospinal tract degeneration has extended rostrally to include the pons, cerebral peduncles, medulla, and internal capsule. Decreased number of pyramidal (neurons (Betz cells) has been described. Demyelination of degenerating corticospinal tracts and fasciculus gracilis fibers is noted frequently and generally considered to be consistent with the degree of axon degeneration rather than signifying a primary demyelinating process
Caution must be used when generalizing HSP neuropathology because 1) there have been very few autopsies in HSP most of which are from subjects for whom the genetic type of HSP is unknown (e.g. [133]). Further, there may be a bias toward performing and reporting autopsies in unusual or complicated forms of HSP, such as those in which lifespan was reduced (e.g. [148, 172]). For example, the post mortem findings of novel, crystalloid deposits containing oligodendroglial cytoskeletal elements (α- and β-tubulin and TPPP/p25) in a subject with apparently autosomal dominant hereditary spastic paraplegia (genetic type unknown) are of uncertain significance [252].
A number of post mortem studies have been reported for subjects with possible or probable autosomal recessive spastic paraplegia but for whom the specific type of HSP is not known. For example Kuru et al [133] reported a subject with probable autosomal recessive spastic paraplegia associated with thin corpus callosum. Pathologic findings included neurodegeneration involving corticospinal tract, thalamus, cerebral white matter and substantia nigra, anterior horn, and dorsal columns of the spinal cord. There was widespread neuronal lipofuscin and eosinophilic granules; and severe gliosis in the cerebral white matter and substantia nigra. Nomura et al [172] reported a subject with probable autosomal recessive spastic tetraplegia, dysarthria, dementia, leading to death at age 50. Pathologic findings included severe brain atrophy, diffuse spongy changes, neuronal atrophy with lipofuscinosis, degeneration of dorsal spinocerebellar and corticospinal tracts, marked loss of anterior horn cells and nucleus gracilis neurons, and evidence of mitochondrial abnormality in skeletal muscle. Wakabayashi et al [240] reported a subject with probable autosomal recessive spastic paraplegia associated with thin corpus callosum, sensory disturbance, and amyotrophy. Pathologic changes included neuronal loss and gliosis in upper and lower motor neuron pathways, thalamus, lateral geniculate body, dentate nucleus, and posterior column of the spinal cord; many remaining neurons contained ubiquitinated lipofuscin granules; and reduced number of pigmented neurons in the substantia nigra. It is difficult to know the extent to which one or more post mortem observations from individual cases of genetically-undefined spastic paraplegia may be generalized to one or more types of spastic paraplegia of known genetic cause (genetically defined HSP).
Finally, since HSP usually does not shorten lifespan, HSP autopsies may disclose findings related to age or co-occurrence of unrelated, age-dependent disorders (such as Alzheimer's disease and Parkinsons disease). For example, post mortem [245] studies were reported in a subject with SPG4/spastin mutation who, in addition to progressive lower extremity spastic weakness, had dementia, facial bradykinesia and tremor. Corticospinal tract degeneration was maximal in thoracic and lumbar cord and associated with myelin pallor. In addition, neuronal loss was noted in the hippocampus and entorhinal cortex. Frequent neurofibrillary tangles that were immunoreactive for tau were present in remaining neurons. Senile plaques were not observed. Granulovacuolar degeneration and ballooned neurons that immunostained strongly for tau and α–β-crystallin were prominent in limbic areas. The substantia nigra showed moderate loss of pigmented dopaminergic neurons and frequent Lewy bodies and pale bodies. While these latter findings correlated with clinical Parkinsonism, it is not possible to determine whether the entirety of neuropathologic findings is attributable to SPG4/Spastin mutation. Murphy et al described an HSP subject with SPG4/spastin mutation subject who had dementia and for whom post mortem findings included widespread ubiquitin positivity within the neocortex and white matter [166]. These and similar reports illustrate the caution that must be exercised in assessing which post mortem findings in HSP subjects are the consequence of the HSP gene mutation and which findings are attributable to the effects of age and the occurrence of unrelated, age-associated disorders (or theoretically, a combination of these factors).
The predominant distribution of axon degeneration to the distal ends of cortical spinal tracts and fasciculus gracilis fibers has given rise to two widely held concepts: 1) HSP reflects particular vulnerability of the longest, motor and sensory axons in the central nervous system; and 2) that the HSP represents a primary axonopathy. Each of these generalizations is incomplete in the following regards.
First, it is increasingly recognized that neuropathology in many types of HSP extends beyond the corticospinal tracts, dorsal column fibers and even the central nervous system. For example, though formerly considered to represent “uncomplicated” HSP, approximately 17% of subjects with SPG3A HSP have motor-sensory axonal peripheral neuropathy [116]. Peripheral neuropathy has been described as either a rare or common feature in more than a dozen genetic types of HSP (Table 2).
Lower motor neuron involvement, evident as distal muscle wasting is common in a many genetic types of HSP (Tables 1 and 2), notably those due to SPG10/KIF5A, SPG20/Spartin, SPG39/NTE, SPG17/BSCL2 gene mutation. In subjects with SPG39 reflects distal motor neuropathy (not motor sensory neuropathy). Lower motor neuron involvement may also occur in SPG11 HSP (many of whom conform to a clinical syndrome of juvenile ALS [173]); and is a typical feature of SPG17 (Silver syndrome) and SPG20 (Troyer syndrome). Anterior horn cell abnormalities (hyaline inclusions, altered mitochondrial distribution, and altered immunostaining for cytoskeletal proteins [nonphosphorylated neurofilament protein and β-tubulin] were also observed [244] in post mortem studies in SPG4 HSP (the single most common form of dominantly inherited HSP which usually manifests as an uncomplicated spastic paraplegia syndrome.
Second, neurodegeneration in many types of HSP involves neurons that are not among the longest in the CNS (such as the occurrence of dementia in SPG4 HSP, ataxia in SPG7 HSP, and optic neuropathy in SPG7 HSP). For example, post mortem studies in a subjects with SPG4/spastin mutation revealed variable involvement in cerebellum and basal ganglia, Clarke's column, and reduced number of anterior horn cells in some (but not all ) subjects [245].
Third, myelin abnormalities are more common in HSP than previously recognized. At least two types of HSP are due to mutations in genes that are expressed not in neurons but rather in oligodendroglia (SPG2/proteolipid protein, SPG42/Connexin 47 mutation). Some mouse models of SPG2/proteolipid protein mutation HSP have axon degeneration (rather than demyelination), indicating that primary oligodendroglial abnormality may cause axonal degeneration. Post mortem study of a subject with late age-of-symptom onset SPG2 HSP demonstrated moderate cerebral atrophy, widespread pallor of central nervous system myelin preferentially affecting corticospinal tracts, and severe corticospinal tract axon degeneration [222].
In addition to these forms of HSP in which the causative gene mutation is expressed in oligodendroglial, white matter abnormalities have been observed in many forms of complicated HSP (e.g. due SPG5/CYPB7, SPG7/Paraplegin, SPG21/Maspardin, and SPG35/FA2H gene mutations, see Table 1) [61, 64, 131, 216, 242, 258]. It is noteworthy that homozygous mutation of the bovine spastin gene causes congenital bovine dysmyelination [228] rather than primary axonal degeneration.
Fourth, some of the abnormalities in HSP may be developmental rather than degeneration. Vassilopoulos et al [235] showed that the spinal canal diameter was smaller in subjects with HSP. This suggests disturbance in spinal canal development perhaps from altered induction of the developing spinal cord. Thin corpus callosum (associated most often with SPG11 but also may occur in SPG3A [176], SPG4, SPG7, SPG15, SPG21, SPG32, SPG47 , SPG49, SPG54, and SPG56 (see Table 1) is usually considered a developmental abnormality although França et al showed progressive corpus callosum thinning on serial brain magnetic resonance imaging [83].
Analysis of HSP genes and their encoded proteins sheds light on the molecular mechanisms underlying HSP
38 HSP genes have been identified (summarized in Table 3). Identified HSP genes are unique and, with some exceptions, do not conform to members of an extended gene or protein family. Examples of sequence-related proteins include Spastin (SPG4) and Paraplegin (SPG7) which share an AAA domain; and Spartin, whose amino-terminal region is similar to that of Spastin's. Some HSP genes (notably AP4B1 [SPG47], KIAA0415 [SPG48], AP4M1 [SPG50], AP4E1 [SPG51], AP4S1 [SPG52]), VPS37A [SPG53]) while not members of a gene family, encode related elements of a functional protein complex.
Table 3.
HSP proteins (updated from[78])
| HSP gene/protein | Functions | References |
|---|---|---|
| SPG1/L1CAM | Integral membrane glycoprotein, cell adhesion molecule in the immunoglobulin superfamily; mediates cell-to-cell and cell-to-matrix attachment; functions include guidance of neurite outgrowth during development, neuronal cell migration, and neuronal cell survival. Interacts with Bone morphogenic protein (affects L1CAM gene regulation) | [22, 124, 256] |
| SPG2/PLP1 | Proteolipid protein (PLP1) is an integral myelin protein expressed in oligodendroglia and Schwann cells but not in neurons | [219] |
| SPG3A/atlastin | Dynamin family GTPase, interacts with HSP proteins spastin, NIPA1, and REEP1; involved in membrane fusion and severing; contributes to endoplasmic reticulum morphology. Interacts with SPG4/spastin, REEP1, NIPA1, DP1/Yop1p and reticulon families of ER-shaping proteins, HPK/GCK-like kinase (HGK) (a protein kinase in the c-Jun N-terminal kinase signaling pathway; Inhibits BMP signaling in Zebrafish model | [21, 35, 52, 71, 73, 180, 181, 200, 205, 260] |
| SPG4/spastin | Cytosolic (and possibly nuclear) protein with AAA domain; (AAA domain is also present in paraplegin); interacts with microtubules and has microtubule severing properties; interacts with atlastin and REEP1 and contributes to endoplasmic reticulum morphology; mutations affect axonal transport. Interacts with:SPG3A/Atlastin, SPG31/REEP1, SPG33/ZFYVE27, ESCRT-II complex associated CHMP1B, Reticulon 1 (endoplasmic reticulum protein), Reticulon 3, CREL5, COPS5, Tubulin; inhibits bone morphogenic protein signaling | [11, 146, 181, 194, 202, 205, 229] |
| SPG5/CYP7B1 | Cytochrome P450-7B1 (CYP7B1) provides primary metabolic route for cholesterol derivatives dehydroepiandrosterone (DHEA) and related hydroxysteroids via 7α-hydroxylation; and that in the liver; and an alternative route for cholesterol metabolism. | [230] |
| SPG6/NIPA1 | “Not imprinted in Prader Willi/Angelman 1” (NIPA1): 9 alternating hydrophobic-hydrophilic domains predicts integral membrane localization; NIPA1 binds to BMP-II receptor to inhibit BMP signaling; NIPA1 transcription is induced by low extracellular Mg++; NIPA1 expression causes inwardly directly Mg++ conductance. Interacts with Type II bone morphogenic protein (BMP) receptor 2 (BMPRII) to inhibit BMP signaling and SPG3A/atlastin | [229] |
| SPG7/paraplegin | Mitochondrial metalloprotease, involved as protein chaperone in mitochondrial protein quality control. Interacts with AFG3L2 (mutations cause spinocerebellar ataxia type 28) | [145, 203] |
| SPG8/Strumpellin (KIAA0196) | KIAA0196/Strumpellin, mutations may be pathogenic through protein aggregation: Strumpellin binds to valosin-containing protein (VCP; also known as p97, TER ATPase and Cdc48p); VCP-positive inclusions occur in a wide variety of neurodegenerative disorders including Parkinson's disease, Lewy body disease, Huntington's disease, amyotrophic lateral sclerosis and spinocerebellar ataxia type III (Machado-Joseph disease); Strumpellin may also regulate actin dynamics through its interaction with WASH; interacts with Valosin-containing protein (VCP; also known as p97, TER ATPase and Cdc48p); WASH, a Wiskott-Aldrich syndrome protein (WASP) family member, via SWIP (Strumpellin and WASH interacting protein (WASH localizes to endosomal subdomains and regulates endocytic vesicle scission in an Arp2/3-dependent manner). | [119] |
| SPG10/KIF5A | Kinesin heavy chain (KIF5A) a molecular motor subunit that participates in the intracellular movement of organelles and macromolecules. | [253] |
| SPG11/Spatacsin | Unknown function; spatacsin is a component of a protein complex that includes two other HSP proteins, SPG15/ZFYVE26 and SPG48/KIAA0415 [217] suggesting that these forms of HSP may share similar molecular pathogenesis. Interacts with SPG48/KIAA0415, SPG15/ZfyVe26, C200RF29, DKFZp761E198 | [217] |
| SPG12/RTN2 | RTN2 gene encodes reticulon 2, an ER-shaping protein. Reticulon interacts with SPG4/spastin. | [162] |
| SPG13/ HSPD1 | HSPD1 gene encodes Chaperonin 60 (also known as heat shock protein 60, HSP60), the large subunit of the mitochondrial Hsp60/Hsp10 chaperonin complex; functions in mitochondrial protein quality control (binding and sequestration, and refolding of unfolded proteins) | [94] |
| SPG15/Spastizin (ZFYVE26) | FYVE-domain proteins have diverse functions including membrane trafficking, signal transduction, regulating the cytoskeleton, and serving as phosphatidylinositol 3-phosphate (PtdIns3P) phosphatases. Spastizin colocalizes with ER and endosome markers and is a component of a protein complex that includes two other HSP proteins, SPG11/spatacsin and SPG48/KIAA0415. Interacts with SPG11/Spatacsin and SPG48/KIAA0415 | [92, 164, 217] |
| SPG17/BSCL2 | BSCL2/seipin: Endoplasmic reticulum transmembrane protein; mutations appear pathogenic through induction of endoplasmic reticulum stress-mediated apoptosis. | [113-115] |
| SPG18/ERLIN2 | ERLIN2 is a component of lipid rafts that localize to ER and nuclear membranes. ERLIN2 is a mediator of ER stress response to protein misfolding. | [42, 241] |
| SPG20/Spartin | Spartin: N-terminal region similar to spastin; homologous to proteins involved in the morphology and trafficking of endosomes; also localizes to mitochondria; interacts with Eps 15, a protein known to be involved in endocytosis and the control of cell proliferation, suggesting that spartin may be involved in endocytosis, vesicle trafficking, or mitogenic activity; spartin promoter hypermethylation is associated with cytokinesis arrest in colorectal carcinoma. Interacts with GRP78, GRP75, ubiquitin, and the E3 ubiquitin-protein ligases AIP4/Itch and AIP5/WWP1, and; nucleolar protein “nucleolin”; inhibits BMP signaling. | [20, 109, 138, 140, 159, 229] |
| SPG21/Maspardin | Appears to be a cytosolic protein partitioned between the cytosol and vesicles of the endosomal/_trans_-Golgi network; may play a role in endosomal trafficking; may be similar to a noncatalytic α/β hydrolase protein NDRG1, mutations in which cause peripheral neuropathy with early axonal involvement. Interacts with Aldehyde dehyrogenase ALDH16A1 | [93, 216] |
| SPG22/MCT8 | Monocarboxylate transport 8 (MCT8) is a thyroid hormone transporter, results in elevated serum triiodothyronine (T3) levels | [147] |
| SPG28/DDHD1 | DDHD1 is a phosphatidic acid-preferring phospholipase A1 (PA-PIA1). There is evidence that DDHD1 is involved in stimulus-dependent formation of arachidonic acid-containing lysophosphatidyl inositol which plays a role in signal transduction including that of GPR55, a G-protein coupled, putative canabinoid receptor. | [254] |
| SPG30/KIF1A | Microtubule-dependent motor protein (in kinesin-3 family) with apparent role as primary anterograde motor protein for axonal transport of dense-core vesicles | [128, 139] |
| SPG31/REEP1 | Receptor expression enhancing protein 1 (REEP1), structurally related to the DP1/Yop1p family of endoplasmic reticulum -shaping proteins; required for ER network formation in vitro; forms complexes with atlastin and spastin within endoplasmic reticulum; also binds to microtubules and promotes ER alignment along the microtubule cytoskeleton in vitro. Interacts with PG3A/Atlastin and SPG4/Spastin | [181] |
| SPG33/ZFYVE27 | ZFYVE27/protrudin has a role in Rab-11-mediated membrane trafficking and promotes neurite outgrowth. Interacts with SPG4/Spastin and RAB11 | [32, 146] |
| SPG35/FA2H | Fatty acid 2-hydroxylase (FA2H) in oligodendroglia catalyzes the 2-hydroxylation of myelin galactolipids, galactosylceramine, and sulfatide; and therefore, helps determine myelin lipid content. | [86] |
| SPG39/NTE | Neuropathy target esterase (NTE) is a phospholipase B localized to ER membranes; may reduce cytotoxic lysophosphatidylcholine; implicated in toxic organophosphorus compound induced delayed neurodegeneration (OPIDN); may function to regulate cyclic AMP-dependent protein kinase. Interacts with Cyclic AMP-dependent protein kinase (in Drosophila) | [3, 17, 27, 66, 87, 88, 120, 121, 199, 239, 251, 255] |
| SPG42/SLC33A1 | SLC33A1 is an acetyl CoA transporter that moves acetyl-CoA into the Golgi apparatus where it may be transferred to sialyl residues of gangliosides and glycoproteins | [136] |
| SPG43/C19orf12 | Membrane protein of uncertain function | |
| SPG44/GJA12/GJC2 | Gap junction protein (GJA12/GJC2) also known as connexin47 (Cx47); important factor in formation of specialized channels (“gap junctions”) between cells that allow selective movement of ions and metabolites between adjacent intracellular compartments. | [74, 178, 206] |
| SPG47/AP4B1 | Adaptor protein complexes (AP1, AP2, AP3, and AP4) are widely expressed, evolutionarily conserved complexes that participate in vesicle formation and selective inclusion of specific proteins for these vesicles. Homozygous mutations in three AP4 proteins (AP4B1, AP4E1, AP4M1, AP4S1) were identified in subjects with somewhat diverse, autosomal recessive neurologic disorders that share neonatal hypotonia, spastic tetraplegia, marked cognitive impairment associated, and thin corpus callosum. | [1, 23, 59, 106, 107, 237] |
| SPG48/KIAA0415 | KIAA0415 encodes a putative helicase involved in DNA repair; Interacts with SPG11/Spatacsin, SPG15/ZFYVE26, C20ORF29, DKFZp761E198. KIAA0415's function as a subunit for adapter protein 5 (AP-5) suggests a role in endosome membrane dynamics. | [105, 106, 217] |
| SPG49/TECPR2 | Tectonin beta propeller repeat containing protein 2 (TECPR2) appears to participate in autophagy. TECPR2 binds to homologues of autophagy related proteins (Atg8) including MAP1LC3; and upregulates autophagosome accumulation in vitro. | [26, 179] |
| SPG50/ AP4M1 | (see AP4B1) | [1, 23, 59, 107, 237] |
| SPG51/AP4E1 | (see AP4B1) | |
| SPG52/AP4S1 | (see AP4B1) | [59, 107] |
| SPG53/VPS37A | Vacuolar protein sorting 37A encodes a subunit of endosomal sorting complex required for transport (ESCRT-I) complex. ESCRTs regulate trafficking of proteins from endosomes to lysosomes and other vesicles and participate in many other diverse cellular processes including autophagy, membrane scission, and microRNA function. | [135, 261] |
| SPG54/DDHD2 | DDHD2 (DDHD-domain-containing 2) is an intracellular phospholipase A1 (iPLA1). DDHD2 and related proteins (DDHD1 and SEC23IP) also appear to be involved organelle biogenesis and membrane trafficking. | [6, 112, 168, 168, 207, 211, 226] |
| SPG56/CYP2U1 | CYP2U1 can catalyze hydroxylation of arachidonic acid, eicosopentanoic acid, docoshexanoic acid and related long-chain fatty acids that mediate signal transduction pathways. | [227] |
| CcT5 | Epsilon subunit of the cytosolic chaperonin-containing t-complex peptide-1 (Cct5); contributes to the protein folding and assembly of a wide variety of cytosolic proteins including actin and tubulin | [36] |
| Mitochondrial ATP6 gene | Synthesis of ATP (from ADP) in mitochondria is coupled to translocation of protons from the mitochondrial matrix to specific intermembrane sites. Mitochondrial ATP6 forms a channel through which protons flow back from intermembrane sites into the mitochondrial matrix; and is thus an important factor in mitochondrial ATP production. | [151, 238] |
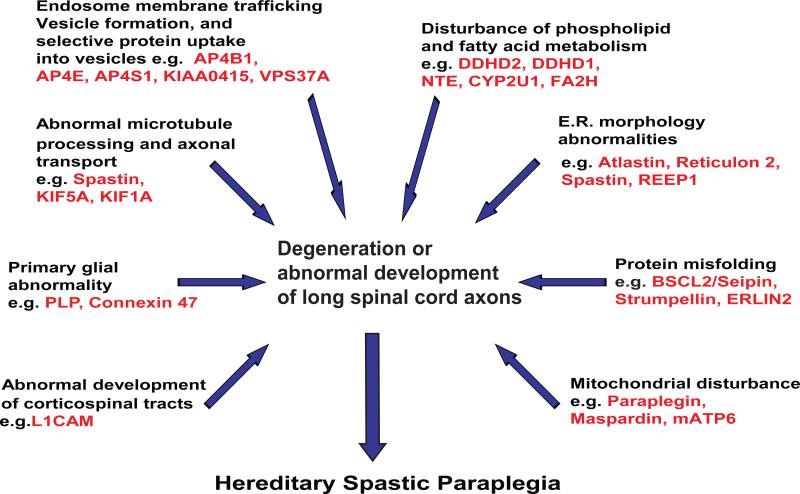
Analysis of the functions of HSP proteins indicates that a variety of primary molecular abnormalities underlie various genetic types of HSP (summarized in Figure). These include 1) axonal transport abnormality e.g. SPG30/KIF1A, SPG10/KIF5A [253] and possibly SPG4/Spastin [123]; 2) disturbance in ER morphology (e.g. SPG12/Reticulon 2, SPG3A/Atlastin, SPG4/Spastin, and SPG31/REEP1); 3) mitochondrial abnormality (e.g. SPG13/chaperonin 60/heat shock protein 60 [95], SPG7/paraplegin; and mitochondrial ATP6 [18, 48, 75, 247]; 4) primary myelin abnormality (e.g. SPG2/Proteolipid protein and SPG42/Connexin 47); 5) abnormal protein conformation leading to ER-stress response (SPG6/NIPA1, SPG8 [51, 258], SGP17/BSCL2, Seipin [113-115], and “mutilating sensory neuropathy with spastic paraplegia” due to CcT5 mutation [36]); 6) disturbance in corticospinal tract and other neurodevelopment (e.g. SPG1/L1 cell adhesion molecule and SPG22/thyroid transporter MCT8); 7) Disturbance in vesicle formation and membrane trafficking including selective uptake of proteins into vesicles (e.g. AP4B1 [SPG47], KIAA0415 [SPG48], AP4M1 [SPG50], AP4E1 [SPG51], AP4S1 [SPG52]), VPS37A [SPG53]); and 8) disturbance of lipid metabolism.
Emerging molecular pathogenesis of the Hereditary Spastic Paraplegias*.
* Some HSP proteins participate in more than one process. In addition, a primary HSP protein abnormality (causative gene mutation) may disturb multiple other cellular and molecular processes.
Among the molecular and cellular categories disturbed in various forms of HSP, it is notable that a subset of HSP types is caused by disturbance in phospholipid, sphingolipid, and fatty acid metabolism (reviewed in Lamari et al [134]). For example DDHD1 (SPG28) and DDHD2 (SPG54) have phospholipase A1 activity and neuropathy target esterase (NTE, SPG39) is a phospholipase B (combined phospholipase A1 and A2 activity). In addition, mutation in a phospholipase A2 gene (PLA2G6) causes autosomal recessive infantile neuroaxonal dystrophy [125] associated with spastic tetraplegia as well as complex neurodegeneration phenotypes associated with brain iron accumulation [132, 134]. CYP2U1 (SPG56) catalyzes hydroxylation of arachidonic acid and similar long-chain fatty acids [227]. Fatty acid amide hydroxylase (FA2H, SPG35) catalyzes 2-hydroxylation of myelin galactolipids, galactosylceramine, and sulfatide. Mutations in fatty acid aldehyde dehydrogenase (FALDH, Sjogren-Larsson syndrome) and fatty acid elongase (ELOVL4) each cause autosomal recessive syndromes in which icthyosis is associated with spastic quadriplegia [9, 58]. The mechanisms (such as the roles of altered lipid-mediated signal transduction or altered membrane rigidity/fluidity) by which these fatty acid and phospholipid disturbances are pathogenic are not clear.
The functional heterogeneity of HSP genes suggests that corticospinal tract axons (and those of dorsal column fibers to a lesser extent) are vulnerable to a number of distinct molecular disturbances. Identification of many HSP proteins, increasing insights into the functions of these proteins, and functional overlap between HSP protein subsets notwithstanding, attempts to integrate functionality of HSP proteins into a single underlying pathogenic mechanism for the myriad forms of HSP is considered both premature and overly simplistic. Though knowledge of HSP is expanding rapidly, there are nearly 20 HSP genes that have been genetically mapped but not yet identified. Moreover, rather than a single, common pathogenic mechanism underlying HSP, it appears likely that disturbance in a number of different molecular processes results in a pattern of relatively selective neurodegeneration involving the corticospinal tracts predominantly. In this regard the HSPs may be similar to the amyotrophic lateral scleroses (plural), a heterogeneous group of motor neuron disorders in which a number of different underlying molecular processes (e.g. [186]) have been implicated (including excitotoxic injury, glial abnormalities, RNA processing disturbance [14], and protein misfolding, for example).
For most HSP gene mutations, the extent to which axon degeneration is “cell autonomous” (due to intrinsic disturbances only in the neurons undergoing degeneration); or “non-cell autonomous” (in which an axon's degeneration depends on its interaction with other cells including glia) is unknown. As noted above, axon degeneration in SPG2 and SPG42 are due to gene mutations that are primarily expressed in oligodendroglia (e.g. proteolipid protein and GJA12/Connexin 47). This indicates that axon degeneration in these types of HSP is “non-cell autonomous and illustrates the importance of oligodendroglia in axon maintenance.
Animal models of HSP (reviewed in [78] and, [190]) include mouse, rat, Drosophila, zebrafish, C. elegans, and cattle. The ability to produce axon degeneration and age-dependent motor impairment in animal models of HSP permits evaluation of genetic mechanisms (such as haploinsufficiency versus “dominant negative” effect) and explore pathogenic mechanisms and potential treatments. Several generalizations may be made. First, vertebrate and invertebrate animal models are useful for demonstrating “proof of concept” that an identified HSP gene mutation is pathogenic. Second, the behavioral phenotype (locomotor impairment) in mouse models of HSP (either spontaneous mutants such as “rumpshaker” model of SPG2 or gene knock-out models), is usually much more mild than humans with the same or equivalent mutations. Preliminary observations in a transgenic rat model of SPG6 mutation suggest that rats may exhibit more severe motor impairment than mice [126]. And finally, the occurrence of congenital dysmyelination in American Brown Swiss cattle that are homozygous for bovine spastin gene mutation [228] suggests that different pathology (demyelination in cattle versus axon degeneration in humans) may be related either to species-specific effects or the nature of the gene mutation (homozygous in cattle, heterozygous in humans).
An important use of HSP animal models will be to determine the extent to which an HSP gene mutation has cell-autonomous pathogenesis. For example, does axon degeneration result when haploinsufficiency is created only in neurons (using Cre-lox cell-type specific gene knock-out, for example)? Studies of mouse models of ALS due to superoxide dismutase 1 (SOD1) gene mutation have highlighted the importance of glia-neuron interaction in determining the onset and rate of progression of neurodegeneration.
Conclusions
Rapid discovery of HSP genes and evaluation of their respective protein functions is reshaping our understanding of the HSPs. Nonetheless, a couple of general concepts remain. First, although lower extremity spastic weakness predominates the clinical syndrome and is the primary factor in functional disability, it is important to note that HSP for the majority of subjects is a motor-sensory disorder. Though usually subtle, dorsal column impairment is common in most types of HSP. And second, axon degeneration particularly involving distal ends of long fibers in the central nervous system is a common pathologic feature.
Beyond these concepts, much of our understanding of HSP is in a state of rapid evolution. For example, though previously considered to be a distal motor-sensory, axonopathy limited to the central nervous system, we now recognize that a majority of genetic forms of HSP involve peripheral neuropathy or other neurologic abnormalities in at least a subset of patients. The concept that HSP exclusively involves length-dependent distal axon degeneration is being modified in light of awareness that neurodegeneration may include shorter axons including those in the cerebellum and those traversing the corpus callosum. And finally, the occurrence of HSP due to mutations in genes that are expressed primarily in oligodendroglia (proteolipid protein and connexin 47) highlights the “non-cell autonomous” pathogenesis of some forms of HSP; and the importance of neuron-glial interaction in axon maintenance and degeneration. The extreme genetic heterogeneity of HSP and the diverse functions of HSP proteins indicate that corticospinal tract axons (and dorsal column axons to a lesser extent) are vulnerable to a variety of primary metabolic impairments. Rather than reflecting disturbance of a single common biochemical pathway, it appears that the common clinical features (i.e. predominated by corticospinal tract degeneration) reflect the limited phenotypic manifestations from relatively uniform degeneration elicited by diverse molecular causes. These advances and the creation of animal and in vitro models permit direct exploration of HSP's pathophysiology and bring us closer to developing treatments for this group of disorders.
Table 5.
Animal models of hereditary spastic paraplegia (see[78] for references)
| HSP Genetic Type | Protein name | Mouse | Drosophila | Zebrafish | C. elegans | Other |
|---|---|---|---|---|---|---|
| SPG1 | L1 cell adhesion molecule (L1CAM) | + | + | |||
| SPG2 | Proteolipid protein | + | ||||
| SPG3A | Atlastin | + | + | + | ||
| SPG4 | Spastin | + | + | + | + | Cattle [228] |
| SPG5 (8p) | CYP7B1 | + | ||||
| SPG6 | “Not imprinted in Prader Willi/Angelman 1” (NIPA1 | + | + | Rat [126] | ||
| SPG7 (16q) | Paraplegin | + | ||||
| SPG8 | KIAA0196/Strumpellin, | + | + | |||
| SPG10 | Kinesin heavy chain (KIF5A | + | ||||
| SPG11 (15q) | Spatacsin (KIAA1840) | + | ||||
| SPG13 | HSPD1 gene encodes Chaperonin 60 (also known as heat shock protein 60, HSP60 | + | ||||
| SPG21 (15q21-q22) | Maspardin | + | ||||
| SPG30 (2q37.3) | KIF1A | + | + | |||
| SPG39 (19p13) | Neuropathy target esterase (NTE) | + | + | |||
| SPG42 (3q24-q26) | Acetyl CoA transporter (SLC33A1) | + | ||||
| SPG53 | Vesicle protein sorting 37A (VPS37A) | + | ||||
| SPG54 | DDHS2 | + |
Acknowledgements
We gratefully acknowledge the support of the National Institutes of Health (R01 NS069700), Department of Veterans Affairs (Merit Review Award), the Spastic Paraplegia Foundation, the Geriatric Research Education and Clinical Center, Ann Arbor Veterans Affairs Medical Center, the generous support from the Katzman Family Fund, and the participation of subjects with hereditary spastic paraplegia and their family members.
References
- 1.Abou Jamra R, Philippe O, Raas-Rothschild A, et al. Adaptor Protein Complex 4 Deficiency Causes Severe Autosomal-Recessive Intellectual Disability, Progressive Spastic Paraplegia, Shy Character, and Short Stature. The American Journal of Human Genetics. 2011;88:788–795. doi: 10.1016/j.ajhg.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abou-áJamra R, Philippe O, Raas-Rothschild A, et al. Adaptor Protein Complex 4 Deficiency Causes Severe Autosomal-Recessive Intellectual Disability, Progressive Spastic Paraplegia, Shy Character, and Short Stature. The American Journal of Human Genetics. 2011;88:788–795. doi: 10.1016/j.ajhg.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abou-Donia MB. Organophosphorus ester-induced delayed neurotoxicity. Ann Rev Pharmacol Toxicol. 1981;21:511–548. doi: 10.1146/annurev.pa.21.040181.002455. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed FE, Qureshi IM, Wooldridge MAW, Pejaver RK. Hereditary spastic paraplegia and Evans's syndrome. Acta Paediat. 1996;85:879–881. doi: 10.1111/j.1651-2227.1996.tb14173.x. [DOI] [PubMed] [Google Scholar]
- 5.Al-Saif A, Bohlega S, Al-Mohanna F. Loss of ERLIN2 function leads to juvenile primary lateral sclerosis. Ann Neurol. 2012;72:510–516. doi: 10.1002/ana.23641. [DOI] [PubMed] [Google Scholar]
- 6.Al-Yahyaee S, Al-Gazali LI, De Jonghe P, et al. A novel locus for hereditary spastic paraplegia with thin corpus callosum and epilepsy. Neurology. 2006;66:1230–1234. doi: 10.1212/01.wnl.0000208501.52849.dd. [DOI] [PubMed] [Google Scholar]
- 7.Al-Yahyaee S, Al-Gazali LI, De JP, et al. A novel locus for hereditary spastic paraplegia with thin corpus callosum and epilepsy. Neurology. 2006;66:1230–1234. doi: 10.1212/01.wnl.0000208501.52849.dd. [DOI] [PubMed] [Google Scholar]
- 8.Alazami AM, Adly N, Al DH, Alkuraya FS. A nullimorphic ERLIN2 mutation defines a complicated Hereditary Spastic Praplegia locus (SPG18). Neurogenetics. 2011;12:333–336. doi: 10.1007/s10048-011-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldahmesh MA, Mohamed J, Alkuraya H, et al. Recessive Mutations in ELOVL4 Cause Ichthyosis, Intellectual Disability, and Spastic Quadriplegia. The American Journal of Human Genetics. 2011;89:745–750. doi: 10.1016/j.ajhg.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allan W, Herndon CN, Dudley FC. Some examples of the inheritance of mental deficiency: apparently sex-linked idiocy and microcephaly. Am J Ment Defic. 1944;48:325–334. [Google Scholar]
- 11.Anderson FH. Nerofibrillary degeneration on Guam. Brain. 1979;102:65–77. doi: 10.1093/brain/102.1.65. [DOI] [PubMed] [Google Scholar]
- 12.Anheim M, Lagier-Tourenne C, Stevanin G, et al. SPG11 spastic paraplegia. A new cause of juvenile parkinsonism. J Neurol. 2009;256:104–108. doi: 10.1007/s00415-009-0083-3. [DOI] [PubMed] [Google Scholar]
- 13.Antonicka H, Oÿstergaard E, Sasarman F, et al. Mutations in C12orf65 in Patients with Encephalomyopathy and a Mitochondrial Translation Defect. The American Journal of Human Genetics. 2010;87:115–122. doi: 10.1016/j.ajhg.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aparicio-Erriu IM, Prehn JH. Molecular mechanisms in Amyotrophic Lateral Sclerosis: The Role of Angiogenin, a Secreted RNase. Front Neurosci. 2012;6:167. doi: 10.3389/fnins.2012.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnoldi A, Tonelli A, Crippa F, et al. A clinical, genetic, and biochemical characterization of SPG7 mutations in a large cohort of patients with hereditary spastic paraplegia. Hum Mutat. 2008;29:522–531. doi: 10.1002/humu.20682. [DOI] [PubMed] [Google Scholar]
- 16.Ashley-Koch A, Kail ME, Nance M, Gaskell P, Svenson I, Marchuck DA, Pericack-Vance MA, Zuchner S. A new locus for autosomal dominant hereditary spastic paraplegia (SPG29) maps to chromosome 2p12. American Journal of Human Genetics . 2005 doi: 10.1007/s10048-006-0029-1. Ref Type: Abstract. [DOI] [PubMed] [Google Scholar]
- 17.Atkins J, Glynn P. Membrane association of and critical residues in the catalytic domain of human neuropathy target esterase. J Biol Chem. 2000;275:24477–24483. doi: 10.1074/jbc.M002921200. [DOI] [PubMed] [Google Scholar]
- 18.Atorino L, Silvestri L, Koppen M, et al. Loss of m-AAA protease in mitochondria causes complex I deficiency and increased sensitivity to oxidative stress in hereditary spastic paraplegia. J Cell Biol. 2003;163:777–787. doi: 10.1083/jcb.200304112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auer-Grumbach M, Schlotter-Weigel B, Lochmuller H, et al. Phenotypes of the N88S Berardinelli-Seip congenital lipodystrophy 2 mutation. Ann Neurol. 2005;57:415–424. doi: 10.1002/ana.20410. [DOI] [PubMed] [Google Scholar]
- 20.Bakowska JC, Jenkins R, Pendleton J, Blackstone C. The Troyer syndrome (SPG20) protein spartin interacts with Eps15. Biochem Biophys Res Comm. 2005;334(4):1042–1048. doi: 10.1016/j.bbrc.2005.06.201. Ref Type: Abstract. [DOI] [PubMed] [Google Scholar]
- 21.Barlowe C. Atlasin GTPases Shape Up ER Networks. Developmental Cell. 2009;17:157–158. doi: 10.1016/j.devcel.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Bateman A, Jouet M, MacFarlane J, Du JS, Kenwrick S, Chothia C. Outline structure of the human L1 cell adhesion molecule and the sites where mutations cause neurological disorders. EMBO J. 1996;15:6050–6059. [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer P, Leshinsky-Silver E, Blumkin L, et al. Mutation in the AP4B1 gene cause hereditary spastic paraplegia type 47 (SPG47). Neurogenetics. 2012;13:73–76. doi: 10.1007/s10048-012-0314-0. [DOI] [PubMed] [Google Scholar]
- 24.Beetz C, Schule R, Deconinck T, et al. REEP1 mutation spectrum and genotype/phenotype correlation in hereditary spastic paraplegia type 31. Brain. 2008;131:1078–1086. doi: 10.1093/brain/awn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behan W, Maia M. Strumpell's familial spastic paraplegia: genetics and neuropathology. J Neurol Neurosurg Psychiatry. 1974;37:8–20. doi: 10.1136/jnnp.37.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature. 2010;466:68–76. doi: 10.1038/nature09204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bettencourt da Cruz A, Wentzell J, Kretzschmar D. Swiss cheese, a protein involved in progressive neurodegeneration, acts as a noncanonical regulatory subunit for PKA-C3. J Neurosci. 2008;28:10885–10892. doi: 10.1523/JNEUROSCI.3015-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bialer MG, Lawrence L, Stevenson RE, et al. Allan-Herndon-Dudley syndrome: clinical and linkage studies on a second family. Am J Med Genet. 1992;43:491–497. doi: 10.1002/ajmg.1320430173. [DOI] [PubMed] [Google Scholar]
- 29.Bian X, Klemm RW, Liu TY, et al. Structures of the atlastin GTPase provide insight into homotypic fusion of endoplasmic reticulum membranes. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1101643108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biancheri R, Ciccolella M, Rossi A, et al. White matter lesions in spastic paraplegia with mutations in SPG5/CYP7B1. Neuromuscul Disord. 2009;19:62–65. doi: 10.1016/j.nmd.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Bien-Willner R, Sambuughin N, Holley H, Bodensteiner J, Sivakumar K. Childhood-onset spastic paraplegia with NIPA1 gene mutation. J Child Neurol. 2006;21:974–977. doi: 10.1177/08830738060210111501. [DOI] [PubMed] [Google Scholar]
- 32.Blair MA, Ma S, Hedera P. Mutation in KIF5A can also cause adult-onset hereditary spastic paraplegia. Neurogenetics. 2006;7:47–50. doi: 10.1007/s10048-005-0027-8. [DOI] [PubMed] [Google Scholar]
- 33.Blumen SC, Bevan S, Abu-Mouch S, et al. A locus for complicated hereditary spastic paraplegia maps to chromosome 1q24-q32. Ann Neurol. 2003;54:796–803. doi: 10.1002/ana.10768. [DOI] [PubMed] [Google Scholar]
- 34.Blumkin L, Lerman-Sagie T, Lev D, Yosovich K, Leshinsky-Silver E. A new locus (SPG47) maps to 1p13.2-1p12 in an Arabic family with complicated autosomal recessive hereditary spastic paraplegia and thin corpus callosum. J Neurol Sci. 2011;305:67–70. doi: 10.1016/j.jns.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Botzolakis EJ, Zhao J, gurba KN, Macdonald RL, Hedera P. The effect of HSP-causing mutations in SPG3A and NIPA1 on the assembly,trafficking, and interaction between atlastin-1 and NIPA1. Mol Cell Neurosci. 2011;46:122–135. doi: 10.1016/j.mcn.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouhouche A, Benomar A, Bouslam N, Chkili T, Yahyaoui M. Mutation in the epsilon subunit of the cytosolic chaperonin- containing t-complex peptide-1 (Cct5) gene causes autosomal recessive mutilating sensory neuropathy with spastic paraplegia. J Med Genet. 2006;43:441–443. doi: 10.1136/jmg.2005.039230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouhouche A, Benomar A, Bouslam N, Ouazzani R, Chkili T, Yahyaoui M. Autosomal recessive mutilating sensory neuropathy with spastic paraplegia maps to chromosome 5p15.31-14.1. Eur J Hum Genet. 2006;14:249–252. doi: 10.1038/sj.ejhg.5201537. [DOI] [PubMed] [Google Scholar]
- 38.Boukhris A, Feki I, Elleuch N, et al. A new locus (SPG46) maps to 9p21.2-q21.12 in a Tunisian family with a complicated autosomal recessive hereditary spastic paraplegia with mental impairment and thin corpus callosum. Neurogenetics. 2010;11:441–448. doi: 10.1007/s10048-010-0249-2. [DOI] [PubMed] [Google Scholar]
- 39.Boukhris A, Stevanin G, Feki I, et al. Tunisian hereditary spastic paraplegias: clinical variability supported by genetic heterogeneity. Clin Genet. 2009;75:527–536. doi: 10.1111/j.1399-0004.2009.01176.x. [DOI] [PubMed] [Google Scholar]
- 40.Bouslam N, Benomar A, Azzedine H, et al. Mapping of a new form of pure autosomal recessive spastic paraplegia (SPG28). Ann Neurol. 2005;57:567–571. doi: 10.1002/ana.20416. [DOI] [PubMed] [Google Scholar]
- 41.Bross P, Naundrup S, Hansen J, et al. The Hsp60-(p.V98I) mutation associated with hereditary spastic paraplegia SPG13 compromises chaperonin function both in vitro and in vivo. J Biol Chem. 2008;283:15694–15700. doi: 10.1074/jbc.M800548200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Browman DT, Resek ME, Zajchowski LD, Robbins SM. Erlin-1 and erlin-2 are novel members of the prohibitin family of proteins that define lipid-raft-like domains of the ER. J Cell Sci. 2006;119:3149–3160. doi: 10.1242/jcs.03060. Ref Type: Journal (Full) [DOI] [PubMed] [Google Scholar]
- 43.Brugman F, Scheffer H, Wokke JHJ, et al. Paraplegin mutations in apparently sporadic adult-onset upper motor neuron syndromes. Neurology. 2008;71:1500–1505. doi: 10.1212/01.wnl.0000319700.11606.21. [DOI] [PubMed] [Google Scholar]
- 44.Buge A, Escourolle R, Rancurel G, Gray F, Pertuiset BF. Strumpell-Lorrains familial spasmodic paraplegia - anatomical and clinical review and report on a new case. Rev Neurol (Paris) 1979;135:329–337. [PubMed] [Google Scholar]
- 45.Burger J, Metzke H, Paternotte C, Schilling F, Hazan J, Reis A. Autosomal dominant spastic paraplegia with anticipation maps to a 4-cM interval on chromosome 2p21-p24 in a large German family. Hum Genet. 1996;98:371–375. doi: 10.1007/s004390050223. [DOI] [PubMed] [Google Scholar]
- 46.Byrne PC, Webb S, McSweeney F, Burke T, Hutchinson M, Parfrey N. Linkage of AD HSP and cognitive impairment to chromosome 2p: haplotype and phenotype analysis indicates variable expression and low or delayed penetrance. Eur J Human Genet. 1998;6:275–282. doi: 10.1038/sj.ejhg.5200185. [DOI] [PubMed] [Google Scholar]
- 47.Cambi F, Tang XM, Cordray P, Fain PR, Keppen LD, Barker DF. Refined genetic mapping and proteolipid protein mutation analysis in X-linked pure hereditary spastic paraplegia. Neurology. 1996;46:1112–1117. doi: 10.1212/wnl.46.4.1112. [DOI] [PubMed] [Google Scholar]
- 48.Casari G, Fusco M, Ciarmatori S, et al. Spastic paraplegia and OXPHOS impairment caused by mutations in paraplegin, a nuclear- encoded mitochondrial metalloprotease. Cell. 1998;93:973–983. doi: 10.1016/s0092-8674(00)81203-9. [DOI] [PubMed] [Google Scholar]
- 49.Charvin d., Fonknechten N, Cifuentes-Diaz C, Joshi V, Hazan J, Melki J, Betuing S. Mutations in SPG4 are responsible for a loss of function of spastin, an abundant neuronal protein localized to the nucleus. American Journal of Human Genetics. 2003;12:71–78. doi: 10.1093/hmg/ddg004. Ref Type: Abstract. [DOI] [PubMed] [Google Scholar]
- 50.Chen S, Song C, Guo H, Xu P, Huang W, et al. Distinct novel mutations affecting the same base in the NIPA1 gene cause autosomal dominant hereditary spastic paraplegia in two Chinese families. Hum Mutat. 2005;25:135–141. doi: 10.1002/humu.20126. [DOI] [PubMed] [Google Scholar]
- 51.Clemen CS, Tangavelou K, Strucksberg KH, et al. Strumpellin is a novel valosin-containing protein binding partner linking hereditary spastic paraplegia to protein aggregation diseases. Brain. 2010;133:2920–2941. doi: 10.1093/brain/awq222. [DOI] [PubMed] [Google Scholar]
- 52.Connell JW, Lindon C, Luzio JP, Reid E. Spastin couples microtubule severing to membrane traffic in completion of cytokinesis and secretion. Traffic. 2009;10:42–56. doi: 10.1111/j.1600-0854.2008.00847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Criscuolo C, Filla A, Coppola G, et al. Two novel CYP7B1 mutations in Italian families with SPG5: a clinical and genetic study. J Neurol. 2009;256:1252–1257. doi: 10.1007/s00415-009-5109-3. [DOI] [PubMed] [Google Scholar]
- 54.Crosby AH, Patel H, Patton MA, Proukakis C, Cross H. Spartin, the Troyer syndrome gene, suggests defective endosomal trafficking underlies some forms of hereditary spastic paraplegia. American Journal of Human Genetics. 2002;71:516. Ref Type: Abstract. [Google Scholar]
- 55.Cross HE, McKusick VA. The Troyer syndrome. A recessive form of spastic paraplegia with distal muscle wasting. Arch Neurol. 1967;16:473–485. doi: 10.1001/archneur.1967.00470230025003. [DOI] [PubMed] [Google Scholar]
- 56.Dalpozzo F, Rossetto MG, Boaretto MS, et al. Infancy onset hereditary spastic paraplegia associated with a novel atlastin mutation. Neurology. 2003;61:580–581. doi: 10.1212/01.wnl.0000078189.73611.df. [DOI] [PubMed] [Google Scholar]
- 57.de Bot ST, van de Warrenburg BP, Kremer HP, Willemsen MA. Child neurology: hereditary spastic paraplegia in children. Neurology. 2010;75:e75–e79. doi: 10.1212/WNL.0b013e3181fc2776. [DOI] [PubMed] [Google Scholar]
- 58.De Laurenzi V, Rogers GR, Hamrock DJ, Marekov LN, Steinert PM, Compton JG, Markova N, Rizzo WB. Sjögren-Larsson syndrome is caused by mutations in the fatty aldehyde dehydrogenase gene. Nat Genet. 1996;12(1):52–57. doi: 10.1038/ng0196-52. Ref Type: Journal (Full) [DOI] [PubMed] [Google Scholar]
- 59.Dell'Angelica EC, Mullins C, Bonifacino JS. AP-4, a Novel Protein Complex Related to Clathrin Adaptors. J Biol Chem. 1999;274:7278–7285. doi: 10.1074/jbc.274.11.7278. [DOI] [PubMed] [Google Scholar]
- 60.Deluca GC, Ebers GC, Esiri MM. The extent of axonal loss in the long tracts in hereditary spastic paraplegia. Neuropathol Appl Neurobiol. 2004;30:576–584. doi: 10.1111/j.1365-2990.2004.00587.x. [DOI] [PubMed] [Google Scholar]
- 61.DeMichele G, DeFusco M, Cavalcanti F, et al. A new locus for autosomal recessive hereditary spastic paraplegia maps to chromosome 16q24.3. Am J Hum Genet. 1998;63:135–139. doi: 10.1086/301930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dennis SC, Green NE. Hereditary spastic paraplegia. J Pediatr Orthop. 1988;8:413–417. doi: 10.1097/01241398-198807000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Dick KJ, Al-Mjeni R, Baskir W, et al. A novel locus for an autosomal recessive hereditary spastic paraplegia (SPG35) maps to 16q21- q23. Neurology. 2008;71:248–252. doi: 10.1212/01.wnl.0000319610.29522.8a. [DOI] [PubMed] [Google Scholar]
- 64.Dick KJ, Eckhardt M, Paisan-Ruiz C, et al. Mutation of FA2H underlies a complicated form of hereditary spastic paraplegia (SPG35). Hum Mutat. 2010;31:E1251–E1260. doi: 10.1002/humu.21205. [DOI] [PubMed] [Google Scholar]
- 65.Du J, Hu YC, Tang BS, et al. Expansion of the phenotypic spectrum of SPG6 caused by mutation in NIPA1. Clin Neurol Neurosurg. 2011;113:480–482. doi: 10.1016/j.clineuro.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 66.Dudek BR, Richardson RJ. Evidence for the existence of neurotoxic esterase in neural and lymphatic tissue of the adult hen. Biochem Pharmacol. 1982;31:1117–1121. doi: 10.1016/0006-2952(82)90351-3. [DOI] [PubMed] [Google Scholar]
- 67.Durr A, Brice A, Serdaru M, et al. The phenotype of “pure” autosomal dominant spastic paraplegia. Neurology. 1994;44:1274–1277. doi: 10.1212/wnl.44.7.1274. [DOI] [PubMed] [Google Scholar]
- 68.Durr A, Davoine C-S, Paternotte C, et al. Phenotype of autosomal dominant spastic paraplegia linked to chromosome 2. Brain. 1996;119:1487–1496. doi: 10.1093/brain/119.5.1487. [DOI] [PubMed] [Google Scholar]
- 69.Dursun U, Koroglu C, Kocasoy OE, Ugur SA, Tolun A. Autosomal recessive spastic paraplegia (SPG45) with mental retardation maps to 10q24.3-q25.1. Neurogenetics. 2009;10:325–331. doi: 10.1007/s10048-009-0191-3. [DOI] [PubMed] [Google Scholar]
- 70.Erichsen AK, Koht J, Stray-Pedersen A, Abdelnoor M, Tallaksen CM. Prevalence of hereditary ataxia and spastic paraplegia in southeast Norway: a population-based study. Brain. 2009;132:1577–1588. doi: 10.1093/brain/awp056. [DOI] [PubMed] [Google Scholar]
- 71.Evans K, Keller C, Gasgow K, Conn B, Lauring B. Interaction of two hereditary spastic paraplegia gene products, spastin and atlastin, suggests a common pathway for axonal maintenance. Proc Natl Acad Sci (USA) 2006;103:10666–10671. doi: 10.1073/pnas.0510863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Evans KJ, Gomes ER, Reisenweber SM, Gundersen GG, Lauring BP. Linking axonal degeneration to microtubule remodeling by Spastin-mediated microtubule severing. J Cell Biol. 2005;168:599–606. doi: 10.1083/jcb.200409058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fassier C, Hutt JA, Scholpp S, et al. Zebrafish atlastin controls motility and spinal motor axon architecture via inhibition of the BMP pathway. Nat Neurosci. 2010;13:1380–1387. doi: 10.1038/nn.2662. [DOI] [PubMed] [Google Scholar]
- 74.Feinstein M, Markus B, Noyman I, et al. Pelizaeus-Merzbacher-like disease caused by AIMP1/p43 homozygous mutation. Am J Hum Genet. 2010;87:820–828. doi: 10.1016/j.ajhg.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferreirinha F, Quattrini A, Pirozzi M, et al. Axonal degeneration in paraplegin-deficient mice is associated with abnormal mitochondria and impairment of axonal transport. J Clin Invest. 2004;113:231–242. doi: 10.1172/JCI20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fichera M, Lo Giudice M, Falco M, et al. Evidence of kinesin heavy chain (KIF5A) involvement in pure hereditary spastic paraplegia. Neurology. 2004;63:1108–1110. doi: 10.1212/01.wnl.0000138731.60693.d2. [DOI] [PubMed] [Google Scholar]
- 77.Filla A, DeMichele G, Marconi R, Busci L, Carillo C, Castellano AE, Iorio L, et al. Prevalence of hereditary ataxias and spastic paraplegias in Molise, a region of Italy. Journal of Neurology. 1992;239:351–353. doi: 10.1007/BF00867594. Ref Type: Abstract. [DOI] [PubMed] [Google Scholar]
- 78.Fink JK. In: Hereditary Spastic Paraplegia. Rimoin D, et al., editors. Churchill Livingstone Elsevier; Philadelphia: 2011. [Google Scholar]
- 79.Fink JK. Hereditary Spastic Paraplegias. In: Schapira AHV, editor. Neurology and Clinical Neurosciences. Mosby Elsevier; Philadelphia: 2007. pp. 899–910. [Google Scholar]
- 80.Fink JK, Sharp G, Lange B, et al. Autosomal dominant hereditary spastic paraparesis, type I: clinical and genetic analysis of a large North American family. Neurology. 1995;45:325–331. doi: 10.1212/wnl.45.2.325. [DOI] [PubMed] [Google Scholar]
- 81.Fink JK, Wu C-TB, Jones SM, et al. Autosomal dominant familial spastic paraplegia: tight linkage to chromosome 15q. Am J Hum Genet. 1995;56:188–192. [PMC free article] [PubMed] [Google Scholar]
- 82.Fontaine B, Davoine C-S, Durr A, et al. A new locus for autosomal dominant pure spastic paraplegia, on chromosome 2q24-q34. Am J Hum Genet. 2000;66:702–707. doi: 10.1086/302776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Franca MC, Jr., D'Abreu A, Maurer-Morelli CV, et al. Prospective neuroimaging study in hereditary spastic paraplegia with thin corpus callosum. Mov Disord. 2007;22:1556–1562. doi: 10.1002/mds.21480. [DOI] [PubMed] [Google Scholar]
- 84.Fujita Y, Fujii T, Nishio A, Tuboi K, Tsuji K, Nakamura M. Familial case of May-Hegglin anomaly associated with familial spastic paraplegia. Am J Hematol. 1990;35:219–221. doi: 10.1002/ajh.2830350317. [DOI] [PubMed] [Google Scholar]
- 85.Garner CC, Garner A, Huber G, Kozak C, Matus A. Molecular cloning of microtubule-associated protein 1 (MAP1A) and microtubule-associated protein 5 (MAP1B): identification of distinct genes and their differential expression in developing brain. J Neurochem. 1990;55:146–154. doi: 10.1111/j.1471-4159.1990.tb08832.x. [DOI] [PubMed] [Google Scholar]
- 86.Gillooly DJ, Simonsen A, Stenmark H. Cellular functions of phosphatidylinositol 3-phosphate and FYVE domain proteins. Biochem J. 2001;355:249–258. doi: 10.1042/0264-6021:3550249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Glynn P. Neuropathy target esterase. Biochem J. 1999;344:625–631. [PMC free article] [PubMed] [Google Scholar]
- 88.Glynn P. Neural development and neurodegeneration: two faces of neuropathy target esterase. Prog Neurobiol. 2000;61:61–74. doi: 10.1016/s0301-0082(99)00043-x. [DOI] [PubMed] [Google Scholar]
- 89.Guelly C, Zhu PP, Leonardis L, et al. Targeted high-throughput sequencing identifies mutations in atlastin-1 as a cause of hereditary sensory neuropathy type I. Am J Hum Genet. 2011;88:99–105. doi: 10.1016/j.ajhg.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haberlova J, Claeys KG, Zamecnik J, De JP, Seeman P. Extending the clinical spectrum of SPG3A mutations to a very severe and very early complicated phenotype. J Neurol. 2008;255:927–928. doi: 10.1007/s00415-008-0598-z. [DOI] [PubMed] [Google Scholar]
- 91.Hanein S, Durr A, Ribai P, et al. A novel locus for autosomal dominant ‘uncomplicated’ hereditary spastic paraplegia maps to chromosome 8p21.1-q13.3. Hum Genet. 2007;122:261–273. doi: 10.1007/s00439-007-0396-1. [DOI] [PubMed] [Google Scholar]
- 92.Hanein S, Martin E, Boukhris A, et al. Identification of the SPG15 gene, encoding spastizin, as a frequent cause of complicated autosomal-recessive spastic paraplegia, including Kjellin syndrome. Am J Hum Genet. 2008;82:992–1002. doi: 10.1016/j.ajhg.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hanna MC, Blackstone C. Interaction of the SPG21 protein ACP33/maspardin with the aldehyde dehydrogenase ALDH16A1. Neurogenetics. 2009;10:217–228. doi: 10.1007/s10048-009-0172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hansen J, Corydon TJ, Palmfeldt J, et al. Decreased expression of the mitochondrial matrix proteases Lon and ClpP in cells from a patient with hereditary spastic paraplegia (SPG13). Neuroscience. 2008;153:474–482. doi: 10.1016/j.neuroscience.2008.01.070. [DOI] [PubMed] [Google Scholar]
- 95.Hansen JJ, Durr A, Cournu-Rebeix I, et al. Hereditary spastic paraplegia SPG13 is associated with a muatation in the gene encoding the mitochondrial chaperonin Hsp60. Am J Hum Genet. 2002;70:1328–1332. doi: 10.1086/339935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harding AE. Hereditary spastic paraplegias. Semin Neurol. 1993;13:333–336. doi: 10.1055/s-2008-1041143. [DOI] [PubMed] [Google Scholar]
- 97.Harding AE. Classification of the Hereditary Ataxias and Paraplegias. Lancet. 1983;1:1151–1155. doi: 10.1016/s0140-6736(83)92879-9. [DOI] [PubMed] [Google Scholar]
- 98.Hazan J, Fontaine B, Bruyn RPM, et al. Linkage of a new locus for autosomal dominant familial spastic paraplegia to chromosome 2p. Hum Mol Genet. 1994;3:1569–1573. doi: 10.1093/hmg/3.9.1569. [DOI] [PubMed] [Google Scholar]
- 99.Hazan J, Lamy C, Melki J, Munnich A, de Recondo J, Weissenbach J. Autosomal dominant familial spastic paraplegia is genetically heterogeneous and one locus maps to chromosome 14q. Nat Genet. 1993;5:163–167. doi: 10.1038/ng1093-163. [DOI] [PubMed] [Google Scholar]
- 100.Hedera P, DiMauro S, Bonilla E, Wald J, Eldevik OP, Fink JK. Phenotypic analysis of autosomal dominant hereditary spastic paraplegia linked to chromosome 8q. Neurology. 1999;53:44–50. doi: 10.1212/wnl.53.1.44. [DOI] [PubMed] [Google Scholar]
- 101.Hedera P, Eldevik OP, Maly P, Rainier S, Fink JK. Spinal cord magnetic resonance imaging in autosomal dominant hereditary spastic paraplegia. Neuroradiology. 2005;47:730–734. doi: 10.1007/s00234-005-1415-3. [DOI] [PubMed] [Google Scholar]
- 102.Hedera P, Rainier S, Alvarado D, et al. Novel locus for autosomal dominant hereditary spastic paraplegia on chromosome 8q. Am J Hum Genet. 1999;64:563–569. doi: 10.1086/302258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hentati A, Pericack-Vance MA, Hung W-Y, Belal S, Laing N, Boustani RM, Hentati F, Hamida MB, Siddique T. Linkage of the “pure” recessive familial spastic paraplegia to chromosome 8 markers and evidence of genetic locus heterogeneity. Human Genetics. 1994;3:1263–1267. doi: 10.1093/hmg/3.8.1263. Ref Type: Abstract. [DOI] [PubMed] [Google Scholar]
- 104.Hentati A, Pericak-Vance MA, Lennon F, et al. Linkage of the late onset autosomal dominant familial spastic paraplegia to chromosome 2p markers. Hum Molec Genet. 1994;3:1867–1871. doi: 10.1093/hmg/3.10.1867. [DOI] [PubMed] [Google Scholar]
- 105.Hirst J, Barlow D, Francisco GC, et al. The Fifth Adaptor Protein Complex. PLoS Biol. 2011;9:e1001170. doi: 10.1371/journal.pbio.1001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hirst J, Irving C, Borner GH. Adaptor protein complexes AP-4 and AP-5: new players in endosomal trafficking and progressive spastic paraplegia. Traffic. 2013;14(2):153–164. doi: 10.1111/tra.12028. Ref Type: Journal (Full) [DOI] [PubMed] [Google Scholar]
- 107.Hirst J, Bright NA, Rous B, Robinson MS. Characterization of a Fourth Adaptor-related Protein Complex. Mol Biol Cell. 1999;10:2787–2802. doi: 10.1091/mbc.10.8.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hodgkinson CA, Bohlega S, Abu-Amero SN, et al. A novel form of autosomal recessive pure hereditary spastic paraplegia maps to chromosome 13q14. Neurology. 2002;59:1905–1909. doi: 10.1212/01.wnl.0000036909.49629.21. [DOI] [PubMed] [Google Scholar]
- 109.Hooper C, Puttamadappa SS, Loring Z, Shekhtman A, Bakowska JC. Spartin activates atrophin-1-interacting protein 4 (AIP4) E3 ubiquitin ligase and promotes ubiquitination of adipophilin on lipid droplets. BMC Biol. 2010;8:72. doi: 10.1186/1741-7007-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hudson LD. Pelizaeus-Merzbacher disease and spastic paraplegia type 2: two faces of myelin loss from mutations in the same gene. J Child Neurol. 2003;18:616–624. doi: 10.1177/08830738030180090801. [DOI] [PubMed] [Google Scholar]
- 111.Hughes CA, Byrne PC, Webb S, et al. SPG15, a new locus for autosomal recessive complicated HSP on chromosome 14q. Neurology. 2001;56:1230–1233. doi: 10.1212/wnl.56.9.1230. [DOI] [PubMed] [Google Scholar]
- 112.Inoue H, Baba T, Sato S, et al. Roles of SAM and DDHD domains in mammalian intracellular phospholipase A1 KIAA0725p. Biochem Biophys Acta. 2012;1823:930–939. doi: 10.1016/j.bbamcr.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 113.Ito D, Fujisawa T, Iida H, Suzuki N. Characterization of seipin/BSCL2, a protein associated with spastic paraplegia 17. Neurobiol Dis. 2008;31:266–277. doi: 10.1016/j.nbd.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 114.Ito D, Suzuki N. [Seipin/BSCL2-related motor neuron disease: Seipinopathy is a novel conformational disease associated with endoplasmic reticulum stress]. Rinsho Shinkeigaku. 2007;47:329–335. [PubMed] [Google Scholar]
- 115.Ito D, Suzuki N. Seipinopathy: a novel endoplasmic reticulum stress-associated disease. Brain. 2009;132:8–15. doi: 10.1093/brain/awn216. [DOI] [PubMed] [Google Scholar]
- 116.Ivanova N, Claeys KG, Deconinck T, et al. Hereditary spastic paraplegia 3A associated with axonal neuropathy. Arch Neurol. 2007;64:706–713. doi: 10.1001/archneur.64.5.706. [DOI] [PubMed] [Google Scholar]
- 117.Jagell S, Gustavson KH, Holmgren G. Sjogren-Larsson syndrome in Sweden: a clinical, genetic and epidemiological study. Clin Genet. 1981;19:233–256. doi: 10.1111/j.1399-0004.1981.tb00704.x. [DOI] [PubMed] [Google Scholar]
- 118.Jagell S, Linden S. Ichtyosis in the Sjogren-Larsson syndrome. Clin Genet. 1982;21:243–252. doi: 10.1111/j.1399-0004.1982.tb00758.x. [DOI] [PubMed] [Google Scholar]
- 119.Jia D, Gomez TS, Metlagel Z, et al. WASH and WAVE actin regulators of the Wiskott-Aldrich syndrome protein (WASP) family are controlled by analogous structurally related complexes. Proc Natl Acad Sci U S A. 2010;107:10442–10447. doi: 10.1073/pnas.0913293107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Johnson MK. The primary biochemical lesion leading to the delayed neurotoxic effects of some organophosphorus esters. Neurochem. 1974;23:785–789. doi: 10.1111/j.1471-4159.1974.tb04404.x. [DOI] [PubMed] [Google Scholar]
- 121.Johnson MK, Glynn P. Neuropathy target esterase. In: Krieger RI, editor. Handbook of Pesticide Toxicology. Academic Press; San Diego: 2001. pp. 953–965. [Google Scholar]
- 122.Jouet M, Rosenthal A, Armstrong G, et al. X-linked spastic paraplegia (SPG1), MASA syndrome and X-linked hydrocephalus result from mutations in the L1 gene. Nat Genet. 1994;7:402–407. doi: 10.1038/ng0794-402. [DOI] [PubMed] [Google Scholar]
- 123.Kasher PR, De Vos KJ, Wharton SB, et al. Direct evidence for axonal transport defects in a novel mouse model of mutant spastin-induced hereditary spastic paraplegia (HSP) and human HSP patients. J Neurochem. 2009;110:34–44. doi: 10.1111/j.1471-4159.2009.06104.x. [DOI] [PubMed] [Google Scholar]
- 124.Kenwrick S, Watkins A, De Angelis E. Neural cell recognition molecule L1: relating biological complexity to human disease mutations. Hum Mol Gen. 2000;9:879–886. doi: 10.1093/hmg/9.6.879. [DOI] [PubMed] [Google Scholar]
- 125.Khateeb S, Flusser H, Ofir R, et al. PLA2G6 Mutation Underlies Infantile Neuroaxonal Dystrophy. The American Journal of Human Genetics. 2006;79:942–948. doi: 10.1086/508572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kisanuki YY, Rainier S, Moore J, Saunders T, Wilkinson JE, Fink JK. Animal model of SPG6 hereditary spastic paraplegia. Am J Hum Genet. 2008:1794/T. [Google Scholar]
- 127.Klebe S, Azzedine H, Durr A, et al. Autosomal recessive spastic paraplegia (SPG30) with mild ataxia and sensory neuropathy maps to chromosome 2q37.3. Brain. 2006;129:1456–1462. doi: 10.1093/brain/awl012. [DOI] [PubMed] [Google Scholar]
- 128.Klebe S, Lossos A, Azzedine H, et al. KIF1A missense mutations in SPG30, an autosomal recessive spastic paraplegia: distinct phenotypes according to the nature of the mutations. Eur J Hum Genet. 2012;20:645–649. doi: 10.1038/ejhg.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kobayashi H, Hoffman EP, Marks HG. The rumpshaker mutation in spastic paraplegia. Nature Genet. 1994;7:351–352. doi: 10.1038/ng0794-351. [DOI] [PubMed] [Google Scholar]
- 130.Krabbe K, Nielsen JE, Fallentin E, Fenger K, Herning M. MRI of autosomal dominant pure spastic paraplegia. Neuroradiology. 1997;39:724–727. doi: 10.1007/s002340050495. [DOI] [PubMed] [Google Scholar]
- 131.Kruer MC, Paisan-Ruiz C, Boddaert N, et al. Defective FA2H leads to a novel form of neurodegeneration with brain iron accumulation (NBIA). Ann Neurol. 2010;68:611–618. doi: 10.1002/ana.22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kurian MA, Morgan NV, MacPherson L, Foster K, Peake D, Gupta R, Philip SG, Hendriksz C, Morton JEV, Kingston HM, Rosser EM, Wassmer E, Gissen P, Maher ER. Phenotypic spectrum of neurodegeneration associated with mutations in the PLA2G6 gene (PLAN). Neurology. 2008;70:1623–1629. doi: 10.1212/01.wnl.0000310986.48286.8e. Ref Type: Journal (Full) [DOI] [PubMed] [Google Scholar]
- 133.Kuru S, Sakai M, Konagaya M, Yoshida M, Hashizume Y. Autopsy case of hereditary spastic paraplegia with thin corpus callosum showing severe gliosis in the cerebral white matter. Neuropathology. 2005;25:346–352. doi: 10.1111/j.1440-1789.2005.00620.x. [DOI] [PubMed] [Google Scholar]
- 134.Lamari F, Mochel F, Sedel F, Saudubray JM. Disorders of phospholipids, sphingolipids and fatty acids biosynthesis: toward a new category of inherited metabolic diseases. J Inherit Metab Dis. 2012:1–15. doi: 10.1007/s10545-012-9509-7. [DOI] [PubMed] [Google Scholar]
- 135.Lee JA, Gao FB. Neuronal Functions of ESCRTs. Exp Neurobiol. 2012;21:9–15. doi: 10.5607/en.2012.21.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lin P, Li J, Liu Q, et al. A missense mutation in SLC33A1, which encodes the acetyl-CoA transporter, causes autosomal-dominant spastic paraplegia (SPG42). Am J Hum Genet. 2008;83:752–759. doi: 10.1016/j.ajhg.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lin P, Mao F, Liu Q, Shao C, Yan C, Gong Y. Prenatal diagnosis of autosomal dominant hereditary spastic paraplegia (SPG42) caused by SLC33A1 mutation in a Chinese kindred. Prenat Diagn. 2010;30:485–486. doi: 10.1002/pd.2485. [DOI] [PubMed] [Google Scholar]
- 138.Lind GE, Raiborg C, Danielsen SA, et al. SPG20, a novel biomarker for early detection of colorectal cancer, encodes a regulator of cytokinesis. Oncogene. 2011;30:3967–3978. doi: 10.1038/onc.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lo KY, Kuzmin A, Unger SM, Petersen JD, Silverman MA. KIF1A is the primary anterograde motor protein required for the axonal transport of dense-core vesicles in cultured hippocampal neurons. Neuro Lett. 2011;491:168–173. doi: 10.1016/j.neulet.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 140.Lu J, Rashid F, Byrne PC. The hereditary spastic paraplegia protein spartin localises to mitochondria. J Neurochem. 2006;98:1908–1919. doi: 10.1111/j.1471-4159.2006.04008.x. [DOI] [PubMed] [Google Scholar]
- 141.Lynex C, Carr I, Leek J, et al. Homozygosity for a missense mutation in the 67 kDa isoform of glutamate decarboxylase in a family with autosomal recessive spastic cerebral palsy: parallels with Stiff-Person Syndrome and other movement disorders. BMC Neurology. 2004;4:20. doi: 10.1186/1471-2377-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Macedo-Souza LI, Kok F, Santos S, et al. Spastic paraplegia, optic atrophy, and neuropathy is linked to chromosome 11q13. Ann Neurol. 2005;57:730–737. doi: 10.1002/ana.20478. [DOI] [PubMed] [Google Scholar]
- 143.Macedo-Souza LI, Kok F, Santos S, et al. Reevaluation of a large family defines a new locus for X-linked recessive pure spastic paraplegia (SPG34) on chromosome Xq25. Neurogenetics. 2008;9:225–226. doi: 10.1007/s10048-008-0130-8. [DOI] [PubMed] [Google Scholar]
- 144.Magre J, Delepine M, Khallouf E, et al. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet. 2001;28:365–370. doi: 10.1038/ng585. [DOI] [PubMed] [Google Scholar]
- 145.Maltecca F, Magnoni R, Cerri F, Cox GA, Quattrini A, Casari G. Haploinsufficiency of AFG3L2, the gene responsible for spinocerebellar ataxia type 28, causes mitochondria-mediated Purkinje cell dark degeneration. J Neurosci. 2009;29:9244–9254. doi: 10.1523/JNEUROSCI.1532-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mannan AU, Krawen P, Sauter SM, et al. ZFYVE27 (SPG33), a novel spastin-binding protein, is mutated in hereditary spastic paraplegia. Am J Hum Genet. 2006;79:351–357. doi: 10.1086/504927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Maranduba CM, Friesema EC, Kok F, et al. Decreased cellular uptake and metabolism in Allan-Herndon-Dudley syndrome (AHDS) due to a novel mutation in the MCT8 thyroid hormone transporter. J Med Genet. 2006;43:457–460. doi: 10.1136/jmg.2005.035840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Martinez-Lage M, Molina-Porcel L, Falcone D, et al. TDP-43 pathology in a case of hereditary spastic paraplegia with a NIPA1/SPG6 mutation. Acta Neuropathol. 2012;124:285–291. doi: 10.1007/s00401-012-0947-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Martinez-Murillo F, Kobayashi H, Pegoraro E, et al. Genetic localization of a new locus for recessive spastic paraplegia to 15q13-15. Neurology. 1999;53:50–56. doi: 10.1212/wnl.53.1.50. [DOI] [PubMed] [Google Scholar]
- 150.Marx J. Alzheimer's research moves to mice. Science. 1991;253:266–267. doi: 10.1126/science.1907022. [DOI] [PubMed] [Google Scholar]
- 151.Mattiazzi M, Vijayvergiya C, Gajewski CD, et al. The mtDNA T8993G (NARP) mutation results in an impairment of oxidative phosphorylation that can be improved by antioxidants. Hum Mol Genet. 2004;13:869–879. doi: 10.1093/hmg/ddh103. [DOI] [PubMed] [Google Scholar]
- 152.McDermott CJ, Dayaratne RK, Tomkins J, et al. Paraplegin gene analysis in hereditary spastic paraparesis (HSP) pedigrees in northeast England. Neurology. 2001;56:476–471. doi: 10.1212/wnl.56.4.467. [DOI] [PubMed] [Google Scholar]
- 153.McHale DP, Mitchell S, Bundey S, et al. A gene for autosomal recessive symmetrical spastic cerebral palsy maps to chromosome 2q24-25. Amer J Hum Genet. 1999;64:526–532. doi: 10.1086/302237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.McMonagle P, Webb S, Hutchinson M. The prevalence of “pure” autosomal dominant hereditary spastic paraparesis in the island of Ireland. J Neurol Neurosurg Psych. 2002;72:43–46. doi: 10.1136/jnnp.72.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Meijer IA, Cossette P, Roussel J, Benard M, Toupin S, Rouleau GA. A novel locus for pure recessive hereditary spastic paraplegia maps to 10q22.1-10q24.1. Ann Neurol. 2004;56:579–582. doi: 10.1002/ana.20239. [DOI] [PubMed] [Google Scholar]
- 156.Meilleur KG, Traore M, Sangare M, et al. Hereditary spastic paraplegia and amyotrophy associated with a novel locus on chromosome 19. Neurogenetics. 2010;11:313–318. doi: 10.1007/s10048-009-0230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Meyer T, Schwan A, Dullinger JS, et al. Early-onset ALS with long-term survival associated with spastin gene mutation. Neurology. 2005;65:141–143. doi: 10.1212/01.wnl.0000167130.31618.0a. [DOI] [PubMed] [Google Scholar]
- 158.Micheli F, Cersosimo MG, Zuniga RC. Hereditary spastic paraplegia associated with dopa-responsive parkinsonism. Mov Disord. 2006;21:716–717. doi: 10.1002/mds.20800. [DOI] [PubMed] [Google Scholar]
- 159.Milewska M, McRedmond J, Byrne PC. Identification of novel spartin-interactors shows spartin is a multifunctional protein. J Neurochem. 2009;111:1022–1030. doi: 10.1111/j.1471-4159.2009.06382.x. [DOI] [PubMed] [Google Scholar]
- 160.Ming L, Rainier S, Mathay J, Fink JK. Hereditary spastic paraplegia with incomplete genetic penetrance and genetic anticipation. 2005 (Manuscript submitted to Neurology, 2005) [Google Scholar]
- 161.Mitchell S, Bundey S. Symmetry of neurological signs in Pakistani patients with probable inherited spastic cerebral palsy. Clin Genet. 1997;51(1):7–14. doi: 10.1111/j.1399-0004.1997.tb02406.x. Ref Type: Journal (Full) [DOI] [PubMed] [Google Scholar]
- 162.Montenegro G, Rebelo AP, Connell J, et al. Mutations in the ER-shaping protein reticulon 2 cause the axon-degenerative disorder hereditary spastic paraplegia type 12. J Clin Invest. 2012;122:538–544. doi: 10.1172/JCI60560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Moreno-De-Luca A, Helmers SL, Mao H, et al. Adaptor protein complex-4 (AP-4) deficiency causes a novel autosomal recessive cerebral palsy syndrome with microcephaly and intellectual disability. J Med Genet. 2011;48:141–144. doi: 10.1136/jmg.2010.082263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mroue RM, El-Sabban ME, Talhouk RS. Connexins and the gap in context. Integr Biol (Camb ) 2011;3:255–266. doi: 10.1039/c0ib00158a. [DOI] [PubMed] [Google Scholar]
- 165.Muglia M, Criscuolo C, Magariello A, et al. Narrowing of the critical region in autosomal recessive spastic paraplegia linked to the SPG5 locus. Neurogenetics. 2004;5:49–54. doi: 10.1007/s10048-003-0167-7. [DOI] [PubMed] [Google Scholar]
- 166.Murphy S, Gorman G, Beetz C, et al. Dementia in SPG4 hereditary spastic paraplegia: Clinical, genetic, and neuropathologic evidence. Neurology. 2009;73:378–384. doi: 10.1212/WNL.0b013e3181b04c6c. [DOI] [PubMed] [Google Scholar]
- 167.Najmabadi H, Hu H, Garshasbi M, et al. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature. 2011;478:57–63. doi: 10.1038/nature10423. [DOI] [PubMed] [Google Scholar]
- 168.Nakajima Ki, Sonoda H, Mizoguchi T, et al. A Novel Phospholipase A1 with Sequence Homology to a Mammalian Sec23p-interacting Protein, p125. J Biol Chem. 2002;277:11329–11335. doi: 10.1074/jbc.M111092200. [DOI] [PubMed] [Google Scholar]
- 169.Nielsen JE, Johnsen B, Koefoed P, et al. Hereditary spastic paraplegia with cerebellar ataxia: a complex phenotype associated with a new SPG4 gene mutation. Eur J Neurol. 2004;11:817–824. doi: 10.1111/j.1468-1331.2004.00888.x. [DOI] [PubMed] [Google Scholar]
- 170.Nielsen JE, Koefoed P, Abell K, et al. CAG repeat expansion in autosomal dominant pure spastic paraplegia linked to chromosome 2p21-p24. Hum Molec Genet. 1997;6:1811–1816. doi: 10.1093/hmg/6.11.1811. [DOI] [PubMed] [Google Scholar]
- 171.Nimityongskul P, Anderson LD, Sri P. Hereditary spastic paraplegia. Orthop Rev. 1992;21:643–646. [PubMed] [Google Scholar]
- 172.Nomura H, Koike F, Tsuruta Y, Iwaki A, Iwaki T. Autopsy case of autosomal recessive hereditary spastic paraplegia with reference to the muscular pathology. Neuropathology. 2001;21:212–217. doi: 10.1046/j.1440-1789.2001.00388.x. [DOI] [PubMed] [Google Scholar]
- 173.Orlacchio A, Babalini C, Borreca A, et al. SPATACSIN mutations cause autosomal recessive juvenile amyotrophic lateral sclerosis. Brain. 2010;133:591–598. doi: 10.1093/brain/awp325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Orlacchio A, Gaudiello F, Totaro A, et al. A new SPG4 mutation in a variant form of spastic paraplegia with congenital arachnoid cysts. Neurology. 2004;62:1875–1878. doi: 10.1212/01.wnl.0000125324.32082.d9. [DOI] [PubMed] [Google Scholar]
- 175.Orlacchio A, Kawarai T, Totaro A, et al. Hereditary spastic paraplegia: clinical genetic study of 15 families. Arch Neurol. 2004;61:849–855. doi: 10.1001/archneur.61.6.849. [DOI] [PubMed] [Google Scholar]
- 176.Orlacchio A, Montieri P, Babalini C, Gaudiello F, Bernardi G, Kawarai T. Late-onset hereditary spastic paraplegia with thin corpus callosum caused by a new SPG3A mutation. J Neurol. 2011;258:1361–1363. doi: 10.1007/s00415-011-5934-z. [DOI] [PubMed] [Google Scholar]
- 177.Orlacchio A, Patrono C, Gaudiello F, et al. Silver syndrome variant of hereditary spastic paraplegia: A locus to 4p and allelism with SPG4. Neurology. 2008;70:1959–1966. doi: 10.1212/01.wnl.0000294330.27058.61. [DOI] [PubMed] [Google Scholar]
- 178.Orthmann-Murphy JL, Salsano E, Abrams CK, et al. Hereditary spastic paraplegia is a novel phenotype for GJA12/GJC2 mutations. Brain. 2009;132:426–438. doi: 10.1093/brain/awn328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Oz-Levi D, Ben-Zeev B, Ruzzo E, et al. Mutation in TECPR2 Reveals a Role for Autophagy in Hereditary Spastic Paraparesis. The American Journal of Human Genetics. 2012;91:1065–1072. doi: 10.1016/j.ajhg.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Park SH, Zhu P-P, Parker RL, Blackstone C. Hereditary spastic paraplegia proteins REEP1, spastin, and atlastin-1 coordinate microtubule interactions with the tubular ER network. J Clin Invest. 2010;120:1097–1110. doi: 10.1172/JCI40979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Park SH, Zhu PP, Parker RL, Blackstone C. Hereditary spastic paraplegia proteins REEP1, spastin, and atlastin-1 coordinate microtubule interactions with the tubular ER network. J Clin Invest. 2010;120:1097–1110. doi: 10.1172/JCI40979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Patel H, Cross H, Proukakis C, et al. SPG20 is mutated in Troyer syndrome, an hereditary spastic paraplegia. Nat Genet. 2002;31:347–348. doi: 10.1038/ng937. [DOI] [PubMed] [Google Scholar]
- 183.Patel H, Hart PE, Warner TT, et al. The silver syndrome variant of hereditary spastic paraplegia maps to chromosome 11q12-q14, with evidence for genetic heterogeneity within this subtype. Am J Hum Genet. 2001;69:209–215. doi: 10.1086/321267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Paternotte C, Rudnicki D, Fizames C, et al. Quality assessment of whole genome mapping data in the refined familial spastic paraplegia interval on chromosome 14q. Genome Research. 1998;8:1216–1227. doi: 10.1101/gr.8.11.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Polo AE, Calleja J, Combarros O, Bericiano J. Hereditary ataxias and paraplegias in Cantabria,Spain: An epidemiological and clinical study. Brain. 1991;114:855–856. doi: 10.1093/brain/114.2.855. [DOI] [PubMed] [Google Scholar]
- 186.Pratt AJ, Getzoff ED, Perry JJP. Amyotrophic lateral sclerosis: update and new developments. Degenr Neurol Neuromuscul Dis. 2012;2:1–14. doi: 10.2147/DNND.S19803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Proukakis C, Cross H, Patel H, Patton MA, Valentine A, Crosby AH. Troyer syndrome revisited. A clinical and radiological study of a complicated hereditary spastic paraplegia. J Neurol. 2004;251:1105–1110. doi: 10.1007/s00415-004-0491-3. [DOI] [PubMed] [Google Scholar]
- 188.Rainier S, Bui M, Mark E, et al. Neuropathy target esterase gene mutations cause motor neuron disease. Am J Hum Genet. 2008;82:780–785. doi: 10.1016/j.ajhg.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rainier S, Chai J-H, Tokarz D, Nicholls RD, Fink JK. NIPA1 gene mutations cause autosomal dominant hereditary spastic paraplegia (SPG6). Am J Hum Genet. 2003;73:967–971. doi: 10.1086/378817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rainier S, Fink JK. Hereditary spastic paraplegia: clinical features and animal models. In: Ledoux M, editor. Animal Models of Movement Disorders. Elseivier Academic Press; New York: 2005. pp. 687–690. [Google Scholar]
- 191.Rainier S, Sher C, Reish O, Thomas D, Fink JK. De novo occurrence of novel SPG3A/atlastin mutation presenting as cerebral palsy. Arch Neurol. 2006;63:445–447. doi: 10.1001/archneur.63.3.445. [DOI] [PubMed] [Google Scholar]
- 192.Raskind WH, Pericak-Vance MA, Lennon F, Wolff J, Lipe HP, Bird TD. Familial spastic paraparesis: evaluation of locus heterogeneity, anticipation and haplotype mapping of the SPG4 locus on the short arm of chromosome 2. Am J Hum Genet. 1997;74:26–36. doi: 10.1002/(sici)1096-8628(19970221)74:1<26::aid-ajmg7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 193.Reddy PL, Seltzer WK, Grewal RP. Possible anticipation in hereditary spastic paraplegia type 4 (SPG4). Can J Neurol Sci. 2007;34:208–210. doi: 10.1017/s0317167100006053. [DOI] [PubMed] [Google Scholar]
- 194.Reid E, Connell J, Edwards S, Duley S, Brown SE, Sanderson CM. The hereditary spastic paraplegia protein spastin interacts with the ESCRT-III complex-associated endosomal protein CHMP1B. Hum Mol Gen. 2005;14:19–38. doi: 10.1093/hmg/ddi003. [DOI] [PubMed] [Google Scholar]
- 195.Reid E, Dearlove AM, Osborn M, Rogers T, Rubinsztein DC. A Locus for Autosomal Dominant “Pure” Hereditary Spastic Paraplegia Maps to Chromosome 19q13. Am J Hum Genet. 2000;66:728–732. doi: 10.1086/302783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Reid E, Dearlove AM, Rhodes M, Rubinsztein DC. A new locus for autosomal dominant ‘pure’ hereditary spastic paraplegia mapping to chromosome 12q13 and evidence for further genetic heterogeneity. Am J Hum Genet. 1999;65:757–763. doi: 10.1086/302555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Reid E, Grayson C, Rubinsztein DC, Rogers MT, Rubinsztein JS. Subclinical cognitive impairment in autosomal dominant ‘pure’ hereditary spastic paraplegia. J Med Genet. 1999;36:797–798. doi: 10.1136/jmg.36.10.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ribai P, Stevanin G, Bouslam N, et al. A new phenotype linked to SPG27 and refinement of the critical region on chromosome. J Neurol. 2006;253:714–719. doi: 10.1007/s00415-006-0094-2. [DOI] [PubMed] [Google Scholar]
- 199.Richardson RJ, Davis CS, Johnson MK. Subcellular distribution of marker enzymes and of neurotoxic esterase in adult hen brain. J Neurochem. 1979;32:607–615. doi: 10.1111/j.1471-4159.1979.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 200.Rismanchi N, Soderblom C, Stadler J, Zhu P-P, Blackstone C. Atlastin GTPases are required for golgi apparatus and ER morphogenesis. Hum Mol Gen. 2008;17:1591–1604. doi: 10.1093/hmg/ddn046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Roll-Mecak A, Vale RD. The drosophila homologue of the hereditary spastic paraplegia protein, spastin, severs and disassembles microtubules. Curr Biol. 2005;15:650–655. doi: 10.1016/j.cub.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 202.Roll-Mecak A, Vale RD. Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin. Nature. 2008;451:363–367. doi: 10.1038/nature06482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ropers F, Derivery E, Hu H, et al. Identification of a novel candidate gene for non-syndromic autosomal recessive intellectual disability: The WASH complex member SWIP. Hum Mol Genet. 2011;20:2585–2590. doi: 10.1093/hmg/ddr158. [DOI] [PubMed] [Google Scholar]
- 204.Sack GH, Huether CA, Garg N. Familial spastic paraplegia: clinical and pathologic studies in a large kindred. Johns Hopkins Med J. 1978;143:117–121. [PubMed] [Google Scholar]
- 205.Sanderson CM, Connell JW, Edwards TL, et al. Spastin and atlastin, two proteins mutated in autosomal dominant hereditary spastic paraplegia, are binding partners. Hum Mol Gen. 2006;15:307–318. doi: 10.1093/hmg/ddi447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sargiannidou I, Markoullis K, Kleopa KA. Molecular mechanisms of gap junction mutations in myelinating cells. Histol Histopathol. 2010;25:1191–1206. doi: 10.14670/HH-25.1191. [DOI] [PubMed] [Google Scholar]
- 207.Sato Si, Inoue H, Kogure T, Tagaya M, Tani K. Golgi-localized KIAA0725p regulates membrane trafficking from the Golgi apparatus to the plasma membrane in mammalian cells. FEBS Lett. 2010;584:4389–4395. doi: 10.1016/j.febslet.2010.09.047. [DOI] [PubMed] [Google Scholar]
- 208.Saugier-Veber P, Munnich A, Bonneau D, et al. X-linked spastic paraplegia and Pelizaeus-Merzbacher disease are allelic disorders at the proteolipid protein locus. Nature Genet. 1994;6:257–262. doi: 10.1038/ng0394-257. [DOI] [PubMed] [Google Scholar]
- 209.Schlipf NA, Beetz C, Schule R, et al. A total of 220 patients with autosomal dominant spastic paraplegia do not display mutations in the SLC33A1 gene (SPG42). Eur J Hum Genet. 2010;18:1065–1067. doi: 10.1038/ejhg.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Schule R, Bonin M, Durr A, et al. Autosomal dominant spastic paraplegia with peripheral neuropathy maps to chr12q23-24. Neurology. 2009;72:1893–1898. doi: 10.1212/WNL.0b013e3181a6086c. [DOI] [PubMed] [Google Scholar]
- 211.Schuurs-Hoeijmakers J, Geraghty M, Kamsteeg EJ, et al. Mutations in DDHD2, Encoding an Intracellular Phospholipase A1, Cause a Recessive Form of Complex Hereditary Spastic Paraplegia. The American Journal of Human Genetics. 2012;91:1073–1081. doi: 10.1016/j.ajhg.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Schwarz GA. Hereditary (familial) spastic paraplegia. AMA Arch Neurol Psychiatry. 1952;68:655–682. doi: 10.1001/archneurpsyc.1952.02320230081010. [DOI] [PubMed] [Google Scholar]
- 213.Schwarz GA, Liu C-N. Hereditary (familial) spastic paraplegia. Further clinical and pathologic observations. AMA Arch Neurol Psychiatry. 1956;75:144–162. doi: 10.1001/archneurpsyc.1956.02330200038005. [DOI] [PubMed] [Google Scholar]
- 214.Seri M, Cusano R, Forabosco P, et al. Genetic mapping to 10q23.3-q24.2, in a large Italian pedigree, of a new syndrome showing bilateral cataracts, gastroesophageal reflux, and spastic paraparesis with amyotrophy. Am J Hum Genet. 1999;64:586–593. doi: 10.1086/302241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Shimazaki H, Takiyama Y, Ishiura H, et al. A homozygous mutation of C12orf65 causes spastic paraplegia with optic atrophy and neuropathy (SPG55). J Med Genet. 2012;49:777–784. doi: 10.1136/jmedgenet-2012-101212. [DOI] [PubMed] [Google Scholar]
- 216.Simpson MA, Cross H, Proukakis C, et al. Maspardin is mutated in Mast Syndrome, a complicated form of hereditary spastic paraplegia associated with dementia. Am J Hum Genet. 2003;73:1147–1156. doi: 10.1086/379522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Slabicki M, Theis M, Krastev DB, et al. A genome-scale DNA repair RNAi screen identifies SPG48 as a novel gene associated with hereditary spastic paraplegia. PLoS Biol. 2010;8:e1000408. doi: 10.1371/journal.pbio.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sperfeld AD, Baumgartner A, Kassubek J. Magnetic resonance investigation of the upper spinal cord in pure and complicated hereditary spastic paraparesis. Eur Neurol. 2005;54:181–185. doi: 10.1159/000090294. [DOI] [PubMed] [Google Scholar]
- 219.Sporkrl O, Uschkureit T, Bussow H, Stoffel W. Oligodendrocytes expressing exclusively the DM20 isoform of the proteolipid protein gene: myelination and development. Glia. 2002;37:19–30. doi: 10.1002/glia.10014. [DOI] [PubMed] [Google Scholar]
- 220.Steinmuller R, Lantingua-Cruz A, Carcia-Garcia R, Kostrzewa M, Steinberger D, Muller U. Evidence of a third locus in X-linked recessive spastic paraplegia [letter]. Hum Genet. 1997;100:287–289. doi: 10.1007/s004390050507. [DOI] [PubMed] [Google Scholar]
- 221.Subramony SH, Nguyen TV, Langford L, Lin X, Parent AD, Zhang J. Identification of a new form of autosomal dominant spastic paraplegia. Clin Genet. 2009;76:113–116. doi: 10.1111/j.1399-0004.2008.01122.x. [DOI] [PubMed] [Google Scholar]
- 222.Suzuki SO, Iwaki T, Arakawa K, Furuya H, Fujii N, Iwaki A. An autopsy case of adult-onset hereditary spastic paraplegia type 2 with a novel mutation in exon 7 of the proteolipid protein 1 gene. Acta Neuropathol. 2011;122:755–781. doi: 10.1007/s00401-011-0916-x. [DOI] [PubMed] [Google Scholar]
- 223.Svenstrup K, Moller RS, Christensen J, Budtz-Jorgensen E, Gilling M, Nielsen JE. NIPA1 mutation in complex hereditary spastic paraplegia with epilepsy. Eur J Neurol. 2013;18:1197–1199. doi: 10.1111/j.1468-1331.2011.03359.x. [DOI] [PubMed] [Google Scholar]
- 224.Tamagaki A, Shima M, Tomita R, et al. Segregation of a pure form of spastic paraplegia and NOR insertion into Xq11.2. Am J Med Genet. 2000;94:5–8. doi: 10.1002/1096-8628(20000904)94:1<5::aid-ajmg2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 225.Tang BS, Chen X, Zhao GH, et al. Clinical features of hereditary spastic paraplegia with thin corpus callosum: report of 5 Chinese cases. Chin Med J (Engl) 2004;117:1002–1005. [PubMed] [Google Scholar]
- 226.Tani K, Mizoguchi T, Iwamatsu A, Hatsuzawa K, Tagaya M. p125 is a novel mammalian Sec23p-interacting protein with structural similarity to phospholipid-modifying proteins. J Bio Chem. 1999;274:20505–20512. doi: 10.1074/jbc.274.29.20505. [DOI] [PubMed] [Google Scholar]
- 227.Tesson C, Nawara M, Salih M, et al. Alteration of Fatty-Acid-Metabolizing Enzymes Affects Mitochondrial Form and Function in Hereditary Spastic Paraplegia. The American Journal of Human Genetics. 2012;91:1051–1064. doi: 10.1016/j.ajhg.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Thomsen B, Nissen PH, Agerholm JS, Bendixen C. Congenital bovine spinal dysmyelination is caused by a missense mutation in the SPAST gene. Neurogenetics. 2010;11:175–183. doi: 10.1007/s10048-009-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tsang HT, Edwards TL, Wang X, et al. The hereditary spastic paraplegia proteins NIPA1, spastin and spartin are inhibitors of mammalian BMP signalling. Hum Mol Genet. 2009;18:3805–3821. doi: 10.1093/hmg/ddp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tsaousidou MK, Ouahchi K, Warner TT, et al. Sequence alterations within CYP7B1 implicate defective cholesterol homeostasis in motor-neuron degeneration. Am J Hum Genet. 2008;82:510–515. doi: 10.1016/j.ajhg.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tuck RR, O'Neill BP, Gharib H, Mulder DW. Familial spastic paraplegia with Kallmann's syndrome. J Neurol, Neurosurg, Psych. 1983;46:671–674. doi: 10.1136/jnnp.46.7.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Uttner I, Baumgartner A, Sperfeld AD, Kassubek J. Cognitive performance in pure and complicated hereditary spastic paraparesis: a neuropsychological and neuroimaging study. Neurosci Lett. 2007;419:158–161. doi: 10.1016/j.neulet.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 233.Valdmanis PN, Meijer IA, Reynolds A, et al. Mutations in the KIAA0196 gene at the SPG8 locus cause hereditary spastic paraplegia. Am J Hum Genet. 2007;80:152–161. doi: 10.1086/510782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Valente EM, Brancati F, Caputo V, Patrono C, Costanti D, Dallapiccola B. Novel locus for autosomal dominant pure heredtiary spastic paraplegia (SPG19) maps to chromosome 9q22-q34. Ann Neurol. 2002;51:681–685. doi: 10.1002/ana.10204. [DOI] [PubMed] [Google Scholar]
- 235.Vassilopoulos D, Spengos M, Zoumbou V, Scarpalezos S. The spinal canal in famlial spastic paraplegia. Eur Neurol. 1981;20:110–114. doi: 10.1159/000115216. [DOI] [PubMed] [Google Scholar]
- 236.Vazza GZM, Boaretto F, Micaglio GF, Sartori V, Mostacciuolo ML. A New Locus for Autosomal Recessive Spastic Paraplegia Associated with Mental Retardation and Distal Motor Neuropathy SPG14, Maps to Chromosome 3q27-q28. Am J Hum Genet. 2000;67:504–509. doi: 10.1086/303017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Verkerk AJ, Schot R, Dumee B, et al. Mutation in the AP4M1 gene provides a model for neuroaxonal injury in cerebral palsy. Am J Hum Genet. 2009;85:40–52. doi: 10.1016/j.ajhg.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Verny C, Guegen N, Desquiret V, et al. Hereditary spastic paraplegia-like disorder due to a mitochondrial ATP6 gene point mutation. Mitochondrion. 2011;11:70–75. doi: 10.1016/j.mito.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 239.Vose SC, Fujioka K, Gulevich AG, Lin AY, Holland NT, Casida JE. Cellular function of neuropathy target esterase in lysophosphatidylcholine action. Toxicol Appl Pharmacol. 2008;232:376–383. doi: 10.1016/j.taap.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 240.Wakabayashi K, Kobayashi H, Kawasaki S, Kondo H, Takahashi H. Autosomal recessive spastic paraplegia with hypoplastic corpus callosum, multisystem degeneration and ubiquitinated eosinophilic granules. Acta Neuropathol. 2001;101:69–73. doi: 10.1007/s004010000255. [DOI] [PubMed] [Google Scholar]
- 241.Wang G, Liu G, et al. ERLIN2 promotes breast cancer cell survival by modulating endoplasmic reticulum stress pathways. BMC Cancer. 2012;12:225. doi: 10.1186/1471-2407-12-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Warnecke T, Duning T, Schirmacher A, et al. A novel splice site mutation in the SPG7 gene causing widespread fiber damage in homozygous and heterozygous subjects. Mov Disord. 2010;25:413–420. doi: 10.1002/mds.22949. [DOI] [PubMed] [Google Scholar]
- 243.Webb S, Coleman D, Byrne P, et al. Autosomal dominant hereditary spastic paraparesis with cognitive loss linked to chromosome 2p. Brain. 1998;121:601–609. doi: 10.1093/brain/121.4.601. [DOI] [PubMed] [Google Scholar]
- 244.Wharton SB, McDermott CJ, Grierson AJ, et al. The cellular and molecular pathology of the motor system in hereditary spastic paraparesis due to mutation of the spastin gene. J Neuropathol Exp Neurol. 2003;62:1166–1177. doi: 10.1093/jnen/62.11.1166. [DOI] [PubMed] [Google Scholar]
- 245.White KD, Ince PG, Lusher M, et al. Clinical and pathologic findings in hereditary spastic paraparesis with spastin mutation. Neurology. 2000;55:89–94. doi: 10.1212/wnl.55.1.89. [DOI] [PubMed] [Google Scholar]
- 246.Wild NJ, Rosenbloom L. Familial cerebral palsy associated with normal intelligence. Postgrad Med J. 1986;62:827–830. doi: 10.1136/pgmj.62.731.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wilkinson PA, Crosby AH, Turner C, et al. A clinical and genetic study of SPG5A linked autosomal recessive hereditary spastic paraplegia. Neurology. 2003;61:235–238. doi: 10.1212/01.wnl.0000069920.42968.8d. [DOI] [PubMed] [Google Scholar]
- 248.Wilkinson PA, Simpson MA, Bastaki L, et al. A new locus for autosomal recessive complicated hereditary spastic paraplegia (SPG26) maps to chromosome 12p11.1-12q14. J Med Genet. 2005;42:80–82. doi: 10.1136/jmg.2004.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Windpassinger C, Auer-Grumbach M, Irobi J, et al. Heterozygous missense mutations in BSCL2 are associated with distal hereditary motor neuropathy and Silver syndrome. Nat Genet. 2004;36:271–276. doi: 10.1038/ng1313. [DOI] [PubMed] [Google Scholar]
- 250.Winner B, Uyanik G, Gross C, et al. Clinical progression and genetic analysis in hereditary spastic paraplegia with thin corpus callosum in spastic gait gene 11 (SPG11). Arch Neurol. 2004;61:117–121. doi: 10.1001/archneur.61.1.117. [DOI] [PubMed] [Google Scholar]
- 251.Winrow CJ, Hemming ML, Allen DM, Quistad GB, Casida JE, Barlow C. Loss of neuropathy target esterase in mice links organophosphate exposure to hyperactivity. Nature Genet. 2003;33:477–485. doi: 10.1038/ng1131. [DOI] [PubMed] [Google Scholar]
- 252.Woehrer A, Laszlo L, Finsterer J, et al. Novel crystalloid oligodendrogliopathy in hereditary spastic paraplegia. Acta Neuropathol. 2012;124:583–591. doi: 10.1007/s00401-012-0965-9. [DOI] [PubMed] [Google Scholar]
- 253.Xia CH, Roberts EA, Her LS, et al. Abnormal neurofilament transport caused by targeted disruption of neuronal kinesin heavy chain K1F5A. J Cell Biol. 2003;161:55–66. doi: 10.1083/jcb.200301026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yamashita A, Kumazawa T, Koga H, Suzuki N, Oka S, Sugiura T. Generation of lysophosphatidylinositol by DDHD domain containing 1 (DDHD1): Possible involvement of phospholipase D/phosphatidic acid in the activation of DDHD1. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2010;1801:711–720. doi: 10.1016/j.bbalip.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 255.Zaccheo O, Dinsdale D, Meacock PA, Glynn P. Neuropathy target esterase and its yeast homologue degrade phosphatidylcholine to glycerophosphocholine in living cells. J Bio Chem. 2004;279:24024–24033. doi: 10.1074/jbc.M400830200. [DOI] [PubMed] [Google Scholar]
- 256.Zhang L. CRASH syndrome: does it teach us about neurotrophic functions of cell adhesion molecules? Neuroscientist. 2010;16:470–474. doi: 10.1177/1073858410365561. [DOI] [PubMed] [Google Scholar]
- 257.Zhao GH, Hu ZM, Shen L, et al. A novel candidate locus on chromosome 11p14.1-p11.2 for autosomal dominant hereditary spastic paraplegia. Chin Med J (Engl) 2008;121:430–434. [PubMed] [Google Scholar]
- 258.Zhao J, Matthies DS, Botzolakis EJ, Macdonald RL, Blakely RD, Hedera P. Hereditary spastic paraplegia-associated mutations in the NIPA1 gene and its Caenorhabditis elegans homolog trigger neural degeneration in vitro and in vivo through a gain-of-function mechanism. J Neurosci. 2008;28:13938–13951. doi: 10.1523/JNEUROSCI.4668-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zhao X, Alvarado D, Rainier S, et al. Mutations in a novel GTPase cause autosomal dominant hereditary spastic paraplegia. Nat Genet. 2001;29:326–331. doi: 10.1038/ng758. [DOI] [PubMed] [Google Scholar]
- 260.Zhu PP, Soderblom C, Tao-Cheng J-H, Stadler J, Blackstone C. SPG3A protein atlastin-1 is enriched in growth cones and promotes axon elongation during neuronal development. Human Mol Gen. 2006;15:1343–1353. doi: 10.1093/hmg/ddl054. [DOI] [PubMed] [Google Scholar]
- 261.Zivony-Elboum Y, Westbroek W, Kfir N, et al. A founder mutation in Vps37A causes autosomal recessive complex hereditary spastic paraparesis. J Med Genet. 2012;49:462–472. doi: 10.1136/jmedgenet-2012-100742. [DOI] [PubMed] [Google Scholar]
- 262.Zortea M, Vettori A, Trevisan CP, et al. Genetic mapping of a susceptibility locus for disc herniation and spastic paraplegia on 6q23.3- q24.1. J Med Genet. 2002;39:387–390. doi: 10.1136/jmg.39.6.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zuchner S, Kail ME, Nance M, et al. A new locus for dominant hereditary spastic paraplegia maps to chromosome 2p12. Neurogenetics. 2006;7:127–129. doi: 10.1007/s10048-006-0029-1. [DOI] [PubMed] [Google Scholar]
- 264.Zuchner S, Wang G, Tran-Viet KN, et al. Mutations in the novel mitochondrial protein REEP1 cause hereditary spastic paraplegia type 31. Am J Hum Genet. 2006;79:365–369. doi: 10.1086/505361. [DOI] [PMC free article] [PubMed] [Google Scholar]