Microbial Translocation and Cardiometabolic Risk Factors in HIV Infection (original) (raw)
Abstract
The widespread access to antiretroviral treatment during the past decades has transformed HIV infection from a lethal disease to a chronic condition, in which the relative burden of non-AIDS-related chronic disorders such as cardiovascular disease, malignancy, renal, liver, and bone disease has increased. The adjusted relative risk for myocardial infarction is reported to be around 2-fold compared to that of the general population, which over time is likely to translate into increased absolute risk in an aging population. Thus, delineating potentially HIV-specific pathogenetic mechanisms is crucial in order to tailor novel strategies for prophylaxis and treatment. This review will focus on advances in the field that possibly link HIV-induced alterations of the gut mucosa and consequent microbial translocation to cardiometabolic risk factors in HIV infection. Recent work suggests that markers of microbial translocation are closely associated with several cardiovascular risk factors such as dyslipidemia, insulin resistance, hypertension, coagulation abnormalities, endothelial dysfunction, and carotid atherosclerosis. Future studies should investigate whether associations between microbial translocation and cardiovascular risk factors will translate into increased risk of acute events, and whether strategies to target gut microbiota and microbial translocation might reduce such a risk.
Introduction
The widespread access to antiretroviral treatment (ART) during the past decades has transformed HIV infection from a lethal disease to a chronic condition. In the same period, the relative burden of non-AIDS-related chronic disorders such as cardiovascular disease (CVD), malignancy, renal, liver, and bone disease has increased.1 Today, non-AIDS-related mortality exceeds AIDS-related mortality in populations with access to ART.2
The extent to which HIV infection contributes to cardiovascular risk in the era of ART is debated. HIV infection has been associated with signs of subclinical atherosclerosis, such as measures of endothelial dysfunction,3–6 increased arterial stiffness,7–10 and increased carotid intima media thickness.11,12 Moreover, compared to the HIV-uninfected population, HIV-infected individuals have increased rates of CVD, including coronary heart disease,13–15 acute myocardial infarction,15–17 cerebrovascular disease,18 and peripheral vascular disease,19 even after adjustment for cardiovascular risk factors. The adjusted relative risk for myocardial infarction is reported to be around 1.5- to 2-fold compared to that of the general population.15,16
Despite an increased relative risk of CVD in the HIV-infected population, the absolute rates of CVD disease in the HIV-infected population are still low, and the incidence of cardiovascular events and mortality has in fact been reported to be stable or declining over the past years.14,20,21 Because 50% of those living with HIV will be older than 50 years by 2015,22 an increased relative risk is likely to translate into increased absolute risk in an aging population.
From a global perspective, HIV-associated cardiomyopathy and pulmonary hypertension are still the leading cardiac manifestations in developing countries with incomplete access to ART.23 However, increased ART coverage in combination with rapid demographic transitions is about to change this picture, as exemplified by Brazil, in which a shift from AIDS to non-AIDS-related mortality is already evident.24
Thus, delineating potentially HIV-specific pathophysiological mechanisms is crucial in order to tailor novel strategies for prophylaxis and treatment in a growing population at risk. This review will focus on recent advances in the field that possibly link HIV-induced alterations of the gut mucosa and consequent microbial translocation to cardiometabolic risk factors in HIV infection.
Traditional and HIV-Specific Risk Factors for CVD
As in the general population, established cardiovascular risk factors such as family history, age, male sex, hypertension, hyperlipidemia, and smoking are leading causes of CVD in the HIV-infected population.15,16,25–27 Certain established risk factors are more prevalent in the HIV-infected population than in the general population, in particular smoking.28–33 Notably, HIV-infected smokers seem to have excess mortality rates compared to HIV-uninfected smokers.34
Furthermore, the use of ART has an unfavorable effect on the lipid profile,16,31,32 at least with earlier regimens, and is associated with increased risk of diabetes mellitus.35–37 Moreover, unhealthy diet, low socioeconomic status, and excessive alcohol intake are likely to be more common in HIV-infected populations,38 as suggested by a Danish study reporting that mothers of HIV-infected patients have an increased risk of myocardial infarction.39
In addition to traditional risk factors, several HIV-specific factors have been proposed to contribute to the increased burden of non-AIDS-related disorders, including consequences of persistent inflammation, immune activation, and immune dysfunction.1 Importantly, incomplete immune recovery in patients on ART is a predictor of both non-AIDS-related morbidity and mortality.40 In addition, advanced immunodeficiency has been associated with an increased burden of non-AIDS morbidities.41
Immune Activation and Microbial Translocation
Importantly, the immune dysfunction of HIV infection is characterized not only by immunodeficiency and loss of CD4+ T cells, but also by chronic immune activation and inflammation.1 Microbial translocation has been proposed as a major driver of the latter.42 During acute HIV infection the gastrointestinal mucosa is depleted of CD4+ T cells, especially Th17 cells. Th17 cells normally promote recruitment of neutrophils, prevent invasion of extracellular microorganisms, and promote epithelial regeneration.43–45 In the course of chronic infection the gastrointestinal mucosa shows evidence of local inflammation, lowered levels of IgA, abnormal enterocyte differentiation and apoptosis, and disruption of tight junctions. These factors enable shifts in the intraluminal microbial composition as well as translocation of microbial products such as lipopolysaccharide (LPS) and flagellin into the systemic circulation.46–50
Translocated microbial products are recognized by Toll-like receptors (TLRs) and other receptors on cells of the innate immune system. To activate TLR4, LPS requires binding by the myeloid differentiation-2 (MD-2) complex, facilitated by CD14-mediated transfer from LPS binding protein.51,52 Recently, it was shown that systemic LPS levels and markers of TLR4 activation (MD-2 and soluble CD14; sCD14) in chronic HIV infection are increased.53 Activated TLRs in turn activate transcription factors that lead to de novo production of proinflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF).42,54 Enhanced microbial translocation has been associated with activated CD4+ and CD8+ T cell phenotypes,42,55,56 and accumulating evidence suggests that microbial translocation is a predictor of disease progression, poor immune restoration, and non-AIDS morbidity in HIV infection.57–59 Notably, chronic immune activation and microbial translocation decrease but persist in the course of effective ART and suppressed viral replication.60–64 This also seems to be true for markers of microbial translocation that decrease upon initiation of ART although they do not reach the level of healthy adults.42,65 Thus, abnormal and in some cases substantially enhanced translocation of microbial products may persist in HIV-infected individuals.66
Microbial Translocation and Cardiovascular Risk in Non-HIV Populations
Several recent studies in HIV-seronegative populations suggest that crosstalk between the gut flora (microbiota) and innate immunity might be involved in the pathogenesis of obesity and cardiovascular risk.67 The gut microbiota contains 10-fold the number of cells and 150 times the number of genes compared to the human body, and is currently being characterized in the Human Microbiome Project and in the MetaHIT program.68 An altered gut microbiota has been linked not only to symptomatic atherosclerosis69 but also insulin resistance, diabetes mellitus,70 and obesity, by increasing the amount of energy harvested from the diet.71 In particular, circulating LPS has been proposed to be an early trigger of inflammation, insulin resistance, and subsequent cardiovascular risk.67 Furthermore, links between inflammation, microbial translocation, and CVD have been reported in patients undergoing peritoneal dialysis and hemodialysis.72,73
An increase in plasma LPS even occurs in HIV-seronegative healthy individuals after a high fat meal,74 probably due to cotransportation over the gut wall together with dietary fat, by incorporation in triglyceride-rich chylomicrons. This “metabolic endotoxemia” has been shown to initiate obesity and insulin resistance.75 Furthermore, low-grade endotoxemia is evident in patients with type 2 diabetes mellitus and the metabolic syndrome75,76 and may predict future atherosclerosis and cardiovascular disease.77 Recently, it was shown that plasma LPS is increased in obese patients and is closely associated with intraabdominal fat volumes and components of the metabolic syndrome.78 Moreover, bacterial DNA in adipose tissue surrounding the gut was found, with concentrations decreasing with increasing distance from the gut, suggesting a “microbial gradient” from mesenteric fat to omental and subcutaneous adipose tissue.78 These findings are well in line with a previous report showing that elevated levels of LPS-binding protein are associated with obesity in otherwise healthy subjects.79
In addition, several mechanistic studies from animal models provide evidence that an altered gut microbiota and translocation of LPS could be early triggers of obesity, insulin resistance, diabetes, and atherosclerosis. First, chronic infusion of endotoxin is sufficient to initiate obesity and insulin resistance in mice, an effect that is almost abolished in CD14 knockout mice.75 Moreover, colonization of germ-free mice with Escherichia coli is sufficient to augment adipose tissue inflammation,80 and traces of gut bacteria are present in mesenteric adipose tissue before the onset of insulin resistance and type 2 diabetes.81 Interestingly, an LPS-producing Enterobacter cloacae isolate from a morbidly obese patient was recently shown to trigger obesity and insulin resistance in germ-free mice.82 Moreover, recurrent exposure to LPS increased mortality and induced cardiac fibrosis in mice.83
Microbial Translocation and Cardiometabolic Risk Factors in HIV Infection
As mentioned above, CVD risk in HIV infection has been linked to failure of CD4+ T cell count reconstitution despite effective ART,84 which, in turn, has been associated with not only a low nadir CD4+ T cell count,85 but also persistent microbial translocation55 and immune activation.86
Data from SIV-infected nonhuman primates suggest that hypercoagulation and CVD in part may be caused by microbial translocation and excessive immune activation,87 supporting translocation as a possible cofactor of the increased cardiovascular risk in HIV infection.66 This is in keeping with recent studies suggesting that microbial translocation is associated with several cardiometabolic risk factors, although direct evidence has been lacking so far.
Hypertension
As in the general population, hypertension is an independent risk factor for myocardial infarction25,29 and cardiovascular events.88 Based on our recent finding of an association between low nadir CD4 count and the development of sustained hypertension,89 we hypothesized that measures of microbial translocation would predict hypertension in ART-naive patients. Indeed, plasma levels of both LPS and sCD14 at the time of nadir were elevated in HIV-infected compared to controls, predicted sustained hypertension, and were intercorrelated in subjects who developed hypertension several years later.90 These data indicate that HIV-related factors including immunodeficiency and microbial translocation could be involved in the development of hypertension.
Dyslipidemia
In the general population, elevated levels of fasting triglycerides and low levels of high-density lipoprotein (HDL) cholesterol are associated with the metabolic syndrome and increased cardiovascular risk. In patients receiving ART, positive associations have recently been reported between plasma LPS and triglycerides, total cholesterol, and LDL cholesterol, respectively.91 These findings are well in line with recent reports of strong correlations between LPS and both elevated fasting triglycerides and low HDL cholesterol,92 as well as low levels of large particle HDL cholesterol.93 Several mechanisms could explain these associations. First, LPS and triglycerides are cotransported in chylomicrons from the gut to circulation94 where LPS is scavenged by HDL cholesterol, which is often low in dyslipidemia.95 Furthermore, LPS down-regulates lipoprotein lipase activity leading to hypertriglyceridemia.96 Finally, plasma metabolomic analyses showed that certain clusters of lipid abnormalities were associated with LPS and LPS-binding protein in patients receiving protease inhibitors.97
Insulin resistance and metabolic syndrome
Interestingly, increased levels of plasma LPS correlate with lower insulin sensitivity in patients on ART.91 Furthermore, in a cohort of ART-naive patients plasma LPS and triglycerides were independently associated and both markers correlated with the development of several factors associated with the metabolic syndrome, including high blood pressure, high body mass index, high levels of glucose and uric acid, as well as low levels of HDL cholesterol.98 However, data on the potential impact of microbial translocation on diabetes mellitus are so far lacking in HIV-infected populations.
Coagulation and platelet dysfunction
In addition to increased cardiovascular risk, HIV-infected patients have an increased risk of venous thrombosis.99 Of note, increased levels of D-dimer, a marker of the coagulation cascade, fibrin formation, and fibrinolysis predict mortality in HIV infection.100 Both LPS and flagellin have been reported to increase the expression of tissue factor, which initiates the coagulation cascade.101 Furthermore, HIV-infected individuals have a higher frequency of activated platelets, which correlated with tissue factor-expressing monocytes and sCD14 levels.102 We have recently found that although HIV-infected individuals had elevated levels of D-dimer, they had paradoxically functional hypocoagulability and platelet dysfunction, where platelet dysfunction was correlated with plasma levels of LPS in untreated patients.103 Hence, we have proposed a model in which chronic endotoxemia and other microbial products over time cause low-grade coagulation and platelet activation, which ultimately leads to platelet exhaustion and dysfunction. The clinical significance of these findings warrants further investigation.
Endothelial dysfunction
Endothelial dysfunction represents early stages of atherosclerosis. The endothelium is normally intact, but might become dysfunctional and leaky by hypertension, hyperglycemia, modified lipids, and free radicals from smoking.104,105 Important in this context is that endothelial cells often express TLR4 as a response to proatherogenic stimuli, and a link between LPS-induced TLR4 activation of endothelial cells and coronary artery disease has been reported.106
Hence, it could be hypothesized that TLR4 stimulation and subsequent endothelial dysfunction might explain the close association between microbial translocation and hypertension previously reported.90 In support of this hypothesis, strong and significant correlations were found between plasma LPS levels and asymmetric dimethylarginine (ADMA), a marker of nitric oxide-dependent endothelial dysfunction and its structural isomer symmetric dimethylarginine (SDMA), a novel biomarker of vascular and renal diseases.107
Endothelial dysfunction can be directly assessed by flow-mediated dilatation (FMD). Interestingly, nadir CD4 count and ADMA were independently associated with impaired FMD in untreated patients.108 In addition, it was recently shown that FMD of the brachial artery was independently associated with plasma LPS levels in patients receiving ART.109 Taken together, these findings suggest that microbial translocation might indeed contribute to endothelial dysfunction in both treated and untreated HIV infection.
Atherosclerosis
Atherosclerotic lesions are asymmetrical focal thickenings of the two innermost layers of the arterial wall, the tunica intima and tunica media, and consist of cells, connective-tissue elements, lipids, and debris.110 The thickness of these two layers of the vessel wall can be measured by ultrasound, and the carotid intima media thickness (CIMT) is an established measurement of subclinical atherosclerosis. In the HIV-infected population, plasma LPS and sCD14 have been related to progression of subclinical atherosclerosis, as measured by yearly change of CIMT.111 Furthermore, HIV-infected individuals receiving ART with pathologically increased CIMT had elevated levels of sCD14 as compared to individuals with normal CIMT.112
Risk of cardiovascular events
Due to the significant associations between LPS levels and different cardiometabolic risk factors in recent studies, we hypothesized that elevated LPS levels would be associated with an increased Framingham risk score, which is a global assessment of the 10-year risk of myocardial infarction and CVD. Indeed, HIV-infected patients within the highest LPS level strata had a significantly higher 10-year predicted risk of myocardial infarction and CVD 91.
However, it remains to be demonstrated in prospective studies whether markers of microbial translocation predict the risk of cardiovascular events, and whether a reduction in microbial translocation would reduce such a risk. In the SMART study, which mostly involved patients on ART, sCD14 but not LPS was predictive of all-cause mortality.57 Importantly, sCD14 is a marker of monocyte activation, which is shed upon several stimuli, including peptidoglycan from Gram-positive bacteria.113 Hence, microbial products beyond LPS might contribute to cardiovascular risk in HIV infection.
Future Perspectives
Gut microbiota-related metabolites
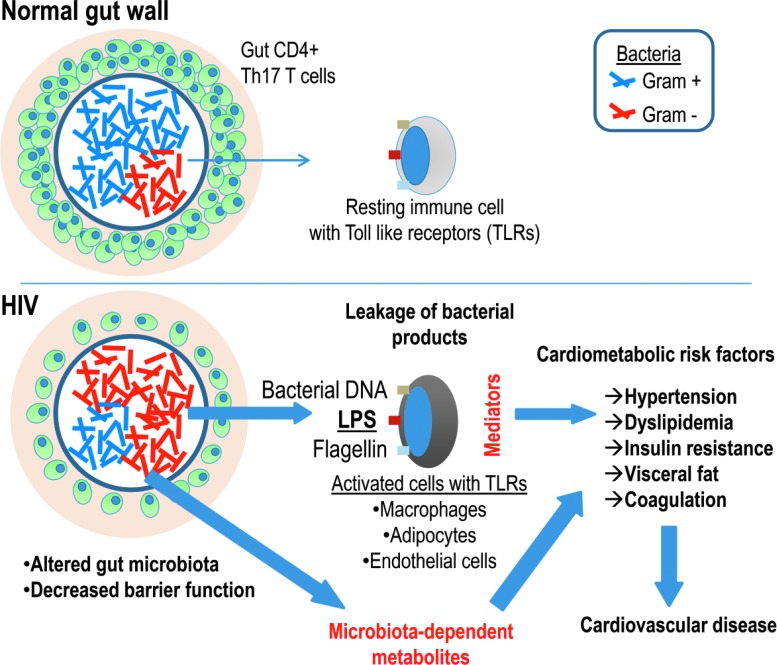
Whereas the gut microbiota has been intensively studied in diseases such as obesity and diabetes,114,115 little work has been done on the potential role of gut microbiota on acute cardiovascular events116 (Fig. 1). Interestingly, recent data show that a gut microbiota-dependent metabolite from dietary carnitine117 and phosphatidylcholine, trimethylamine-_N_-oxide, strongly predicted cardiovascular events, and was markedly reduced after a short course of antibiotics.116
FIG. 1.
Gut microbiota, microbial translocation, and cardiovascular disease. Proposed model, in which an altered gut microbiota is translocated across a damaged gut wall, triggering Toll-like receptors in macrophages, adipocytes, and endothelial cells, with consequent immune activation in relevant end organs. In addition, alterations of gut microbiota, as well as microbiota-dependent metabolites, e.g., resulting from dietary carnitine/phosphatidylcholine116 or increased tryptophan catabolism,119 should be investigated in relation to cardiovascular risk.
Recent data suggest that the gut microbiota often changes during HIV infection,118 and that dysbiotic alterations are associated with disease progression and tryptophan catabolism as measured by the kynurenine-to-tryptophan ratio in plasma.119 In this context it is noteworthy that the kynurenine-to-tryptophan ratio in urine was recently shown to be strongly associated with an adverse prognosis in HIV-seronegative patients with stable coronary artery disease.120 Hence, microbiota-related factors other than direct triggers of inflammation such as LPS should be investigated in relation to cardiovascular risk both in populations with and without HIV.
Is HIV any different?
It remains to be determined whether the potential impact of microbial products on cardiometabolic risk factors is any different in HIV infection compared to other chronic states of inflammation such as diabetes mellitus type 2 and obesity. Whereas the massive Th17 loss in the gut mucosa is probably a relatively HIV-specific event,50 downregulation of tight junction proteins and increased gut permeability also occur in obesity.121 Whatever the cause, this disruption of the gut barrier is likely to contribute to low-grade endotoxemia and possibly cardiometabolic complications in these chronic conditions, yet with substantial individual variation. Thus, comparative studies of microbiota-related factors including microbial translocation and cardiometabolic risk factors in combined conditions such as HIV with and without diabetes or in diabetics with and without HIV could delineate the contribution of HIV per se in these chronic conditions.
Therapeutic options
Given that microbial translocation, and in particular low-grade endotoxemia, could contribute to cardiovascular risk, strategies targeted at reducing microbial translocation or eliminating endotoxins could in theory be prophylactic. These therapeutic options were recently reviewed in detail,66 and we will give a short overview and update.
Sevelamer, which is used to treat hyperphosphatemia in patients with advanced chronic kidney disease, has been found to bind LPS and reduce systemic LPS levels.122 Chloroquine and hydroxychloroquine exert broad inhibition of TLR systems, and have been shown to reduce LPS levels and immune activation in HIV-infected patients on and off ART.123,124 Rifaximin is an orally administered, nonabsorbable antibiotic targeting Gram-negative and Gram-positive bacteria, which has been shown to reduce systemic LPS levels in patients with alcoholic cirrhosis.125 Orally administered bovine immunoglobulin was recently reported to improve intestinal immune reconstitution and absorption in patients with severe HIV enteropathy.126 Prebiotic supplementation with an oligosaccharide mixture has been shown to increase the percentage of bifidobacteria and to decrease sCD14 levels, although LPS levels remained unchanged.127
It should be noted that during CROI 2014, several interventions including Rifaximin, Sevelamer and probiotics failed to show reduction in LPS-levels, although other potentially beneficial effects were reported (poster session # P-E1, posters 337, 339 and 342).
Other ways of targeting the gut microbiota could also be potential therapeutic options in high-risk patients to reduce microbial translocation and thereby cardiovascular risk. It was recently shown that transplantation of healthy gut microbiota from lean donors improved insulin sensitivity in subjects with the metabolic syndrome.128 Given that an altered gut microbiota plays a role in HIV infection, similar strategies could be evaluated to target microbial translocation, cardiovascular risk factors, and immune restoration in HIV-infected individuals.
Finally, the potentially interacting effect of lipids and LPS should be explored in future studies. We recently showed that reduced levels of triglycerides and trunk fat after strength training were associated with a reduction in LPS levels in HIV-infected individuals, most likely mediated through a reduction in triglycerides.129 Notably, 75–97% of circulating endotoxin is bound to lipoproteins, in particular HDL and very-low-density lipoprotein (VLDL).130 Interestingly, LPS promotes VLDL uptake in macrophages to promote foam cell formation in vitro,131 and VLDL-associated LPS activity was recently reported to be associated with systemic inflammation and expression of CD14 on macrophages in patients with periodontitis.132 Hence, delineating to what extent free LPS or lipoprotein-associated LPS has proatherogenic properties will be of interest. In this setting, interventions with lipid-lowering therapies such as statins, fibrates, nicotinic acid, and omega-3 fatty acid supplementation could be applied to investigate the potential effects on total LPS, lipoprotein-bound LPS, and other microbiota-related metabolites. Interestingly, rosuvastatin has been shown to reduce sCD14 levels in HIV-infected individuals on ART independent of its lipid-lowering effect.131
Conclusions
A growing body of evidence suggests that microbiota-related factors including microbial translocation might play a role not only in the pathogenesis of HIV, but possibly even in the development of long-term cardiovascular complications. Prospective studies should investigate whether associations between microbial translocation and cardiometabolic risk factors will translate into an increased risk of cardiovascular events. Finally, clinical trials should be encouraged to test whether strategies that target gut microbiota and microbial translocation might reduce such a risk. Careful selection of high-risk patients into such studies might provide more rapid and definite data.
Acknowledgments
This work was sponsored by Oslo University Hospital and Copenhagen University Rigshospitalet.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Deeks SG: HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011;62:141–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith C, Sabin CA, Lundgren JD, et al. : Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS 2010;24:1537–1548 [DOI] [PubMed] [Google Scholar]
- 3.Wolf K, Tsakiris DA, Weber R, Erb P, and Battegay M: Antiretroviral therapy reduces markers of endothelial and coagulation activation in patients infected with human immunodeficiency virus type 1. J Infect Dis 2002;185:456–462 [DOI] [PubMed] [Google Scholar]
- 4.Solages A, Vita JA, Thornton DJ, et al. : Endothelial function in HIV-infected persons. Clin Infect Dis 2006;42:1325–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torriani FJ, Komarow L, Parker RA, et al. : Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol 2008;52:569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco JJ, Garcia IS, Cerezo JG, et al. : Endothelial function in HIV-infected patients with low or mild cardiovascular risk. J Antimicrob Chemother 2006;58:133–139 [DOI] [PubMed] [Google Scholar]
- 7.Seaberg EC, Benning L, Sharrett AR, et al. : Association between human immunodeficiency virus infection and stiffness of the common carotid artery. Stroke 2010;41:2163–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker JV, Duprez D, Rapkin J, et al. : Untreated HIV infection and large and small artery elasticity. J Acquir Immune Defic Syndr 2009;52:25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schillaci G, De Socio GV, Pucci G, et al. : Aortic stiffness in untreated adult patients with human immunodeficiency virus infection. Hypertension 2008;52:308–313 [DOI] [PubMed] [Google Scholar]
- 10.Schillaci G, De Socio GV, Pirro M, et al. : Impact of treatment with protease inhibitors on aortic stiffness in adult patients with human immunodeficiency virus infection. Arterioscler Thromb Vasc Biol 2005;25:2381–2385 [DOI] [PubMed] [Google Scholar]
- 11.Hulten E, Mitchell J, Scally J, Gibbs B, and Villines TC: HIV positivity, protease inhibitor exposure and subclinical atherosclerosis: A systematic review and meta-analysis of observational studies. Heart 2009;95:1826–1835 [DOI] [PubMed] [Google Scholar]
- 12.Grunfeld C, Delaney JA, Wanke C, et al. : Preclinical atherosclerosis due to HIV infection: Carotid intima-medial thickness measurements from the FRAM study. AIDS 2009;24:1841–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Currier JS, Taylor A, Boyd F, et al. : Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr 2003;33:506–512 [DOI] [PubMed] [Google Scholar]
- 14.Obel N, Thomsen HF, Kronborg G, et al. : Ischemic heart disease in HIV-infected and HIV-uninfected individuals: A population-based cohort study. Clin Infect Dis 2007;44:1625–1631 [DOI] [PubMed] [Google Scholar]
- 15.Klein D, Leyden WA, Xu L X, Chao C, Horberg MA, Towner WJ, et al. : Contribution of immunodeficiency to coronary heart disease: Cohort Study of HIV-infected and HIV-uninfected Kaiser Permanente Members. 18th Conference on Retroviruses and Opportunistic Infections, Boston, MA, 2011. Paper #810 [Google Scholar]
- 16.Triant VA, Lee H, Hadigan C, and Grinspoon SK: Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang S, Mary-Krause M, Cotte L, et al. : Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS 2010;24:1228–1230 [DOI] [PubMed] [Google Scholar]
- 18.Chow FC, Regan S, Feske S, et al. : Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr 2012;60:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Periard D, Cavassini M, Taffe P, et al. : High prevalence of peripheral arterial disease in HIV-infected persons. Clin Infect Dis 2008;46:761–767 [DOI] [PubMed] [Google Scholar]
- 20.Sabin CA, d'Arminio MA, Friis-Moller N, et al. : Changes over time in risk factors for cardiovascular disease and use of lipid-lowering drugs in HIV-infected individuals and impact on myocardial infarction. Clin Infect Dis 2008;46:1101–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bozzette SA, Ake CF, Tam HK, Chang SW, and Louis TA: Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med 2003;348:702–710 [DOI] [PubMed] [Google Scholar]
- 22.High KP, Brennan-Ing M, Clifford DB, et al. : HIV and aging: State of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 2012;60(Suppl 1):S1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thienemann F, Sliwa K, and Rockstroh JK: HIV and the heart: The impact of antiretroviral therapy: A global perspective. Eur Heart J 2013;34:3538–3546 [DOI] [PubMed] [Google Scholar]
- 24.Grinsztejn B, Luz PM, Pacheco AG, et al. : Changing mortality profile among HIV-infected patients in Rio de Janeiro, Brazil: Shifting from AIDS to non-AIDS related conditions in the HAART era. PLoS One 2013;8:e59768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friis-Moller N, Reiss P, Sabin CA, et al. : Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007;356:1723–1735 [DOI] [PubMed] [Google Scholar]
- 26.Iloeje UH, Yuan Y, L'italien G, et al. : Protease inhibitor exposure and increased risk of cardiovascular disease in HIV-infected patients. HIV Med 2005;6:37–44 [DOI] [PubMed] [Google Scholar]
- 27.Ford ES, Greenwald JH, Richterman AG, et al. : Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS 2010;24:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friis-Moller N, Weber R, Reiss P, et al. : Cardiovascular disease risk factors in HIV patients—association with antiretroviral therapy. Results from the DAD study. AIDS 2003;17:1179–1193 [DOI] [PubMed] [Google Scholar]
- 29.Triant VA, Regan S, Lee H, et al. : Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr 2010;55:615–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glass TR, Ungsedhapand C, Wolbers M, et al. : Prevalence of risk factors for cardiovascular disease in HIV-infected patients over time: The Swiss HIV Cohort Study. HIV Med 2006;7:404–410 [DOI] [PubMed] [Google Scholar]
- 31.Saves M, Chene G, Ducimetiere P, et al. : Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis 2003;37:292–298 [DOI] [PubMed] [Google Scholar]
- 32.Kaplan RC, Kingsley LA, Sharrett AR, et al. : Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis 2007;45:1074–1081 [DOI] [PubMed] [Google Scholar]
- 33.Currier JS, Lundgren JD, Carr A, et al. : Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation 2008;118:e29–e35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helleberg M, Afzal S, Kronborg G, et al. : Mortality attributable to smoking among HIV-1-infected individuals: A nationwide, population-based cohort study. Clin Infect Dis 2013;56:727–734 [DOI] [PubMed] [Google Scholar]
- 35.Brown TT, Cole SR, Li X, et al. : Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 2005;165:1179–1184 [DOI] [PubMed] [Google Scholar]
- 36.Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. : HIV infection and the risk of diabetes mellitus. AIDS 2009;23:1227–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De WS, Sabin CA, Weber R, et al. : Incidence and risk factors for new-onset diabetes in HIV-infected patients: The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care 2008;31:1224–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van TH. and Koblin BA: HIV, alcohol, and noninjection drug use. Curr Opin HIV AIDS 2009;4:314–318 [DOI] [PubMed] [Google Scholar]
- 39.Rasmussen LD, Omland LH, Pedersen C, et al. : Risk of myocardial infarction in parents of HIV-infected individuals: A population-based cohort study. BMC Infect Dis 2010;10:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaardbo JC, Hartling HJ, Gerstoft J, and Nielsen SD: Incomplete immune recovery in HIV infection: Mechanisms, relevance for clinical care, and possible solutions. Clin Dev Immunol 2012;2012:670957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guaraldi G, Orlando G, Zona S, et al. : Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011;53:1120–1126 [DOI] [PubMed] [Google Scholar]
- 42.Brenchley JM, Price DA, Schacker TW, et al. : Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006;12:1365–1371 [DOI] [PubMed] [Google Scholar]
- 43.Kao CY, Chen Y, Thai P, et al. : IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol 2004;173:3482–3491 [DOI] [PubMed] [Google Scholar]
- 44.Liang SC, Tan XY, Luxenberg DP, et al. : Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 2006;203:2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salzman NH, Hung K, Haribhai D, et al. : Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol 2010;11:76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Epple HJ, Schneider T, Troeger H, et al. : Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut 2009;58:220–227 [DOI] [PubMed] [Google Scholar]
- 47.Brenchley JM, Paiardini M, Knox KS, et al. : Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 2008;112:2826–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guadalupe M, Reay E, Sankaran S, et al. : Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol 2003;77:11708–11717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehandru S, Poles MA, Tenner-Racz K, et al. : Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med 2004;200:761–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brenchley JM, Schacker TW, Ruff LE, et al. : CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 2004;200:749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.da Silva CJ. and Ulevitch RJ: MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J Biol Chem 2002;277:1845–1854 [DOI] [PubMed] [Google Scholar]
- 52.Latz E, Visintin A, Lien E, et al. : Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem 2002;277:47834–47843 [DOI] [PubMed] [Google Scholar]
- 53.Troseid M, Lind A, Nowak P, et al. : Circulating levels of HMGB1 are correlated strongly with MD2 in HIV-infection: Possible implication for TLR4-signalling and chronic immune activation. Innate Immun 2013;19:290–297 [DOI] [PubMed] [Google Scholar]
- 54.Nowak P, Abdurahman S, Lindkvist A, Troseid M, and Sonnerborg A: Impact of HMGB1/TLR ligand complexes on HIV-1 replication: Possible role for flagellin during HIV-1 infection. Int J Microbiol 2012;2012:263836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang W, Lederman MM, Hunt P, et al. : Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis 2009;199:1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Funderburg N, Luciano AA, Jiang W, et al. : Toll-like receptor ligands induce human T cell activation and death, a model for HIV pathogenesis. PLoS One 2008;3:e1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sandler NG, Wand H, Roque A, et al. : Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011;203:780–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marchetti G, Cozzi-Lepri A, Merlini E, et al. : Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS 2011;25:1385–1394 [DOI] [PubMed] [Google Scholar]
- 59.Dandekar S: Pathogenesis of HIV in the gastrointestinal tract. Curr HIV/AIDS Rep 2007;4:10–15 [DOI] [PubMed] [Google Scholar]
- 60.Deeks SG: Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med 2009;17:118–123 [PubMed] [Google Scholar]
- 61.Autran B, Carcelain G, Li TS, et al. : Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 1997;277:112–116 [DOI] [PubMed] [Google Scholar]
- 62.Douek DC, McFarland RD, Keiser PH, et al. : Changes in thymic function with age and during the treatment of HIV infection. Nature 1998;396:690–695 [DOI] [PubMed] [Google Scholar]
- 63.Hazenberg MD, Stuart JW, Otto SA, et al. : T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: A longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART). Blood 2000;95:249–255 [PubMed] [Google Scholar]
- 64.Pedersen KK, Pedersen M, Gaardbo JC, et al. : Persisting inflammation and chronic immune activation but intact cognitive function in HIV-infected patients after long term treatment with combination antiretroviral therapy. J Acquir Immune Defic Syndr 2013;63:272–279 [DOI] [PubMed] [Google Scholar]
- 65.Troseid M, Nowak P, Nystrom J, et al. : Elevated plasma levels of lipopolysaccharide and high mobility group box-1 protein are associated with high viral load in HIV-1 infection: Reduction by 2-year antiretroviral therapy. AIDS 2010;24:1733–1737 [DOI] [PubMed] [Google Scholar]
- 66.Sandler NG. and Douek DC: Microbial translocation in HIV infection: Causes, consequences and treatment opportunities. Nat Rev Microbiol 2012;10:655–666 [DOI] [PubMed] [Google Scholar]
- 67.Manco M, Putignani L, and Bottazzo GF: Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev 2010;31:817–844 [DOI] [PubMed] [Google Scholar]
- 68.Qin J, Li R, Raes J, et al. : A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010;464:59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karlsson FH, Fak F, Nookaew I, et al. : Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun 2012;3:1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burcelin R, Serino M, Chabo C, Blasco-Baque V, and Amar J: Gut microbiota and diabetes: From pathogenesis to therapeutic perspective. Acta Diabetol 2011;48:257–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Turnbaugh PJ, Ley RE, Mahowald MA, et al. : An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–1031 [DOI] [PubMed] [Google Scholar]
- 72.McIntyre CW, Harrison LE, Eldehni MT, et al. : Circulating endotoxemia: A novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol 2011;6:133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Szeto CC, Kwan BC, Chow KM, et al. : Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol 2008;3:431–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Erridge C, Attina T, Spickett CM, and Webb DJ: A high-fat meal induces low-grade endotoxemia: Evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr 2007;86:1286–1292 [DOI] [PubMed] [Google Scholar]
- 75.Cani PD, Amar J, Iglesias MA, et al. : Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–1772 [DOI] [PubMed] [Google Scholar]
- 76.Pussinen PJ, Havulinna AS, Lehto M, Sundvall J, and Salomaa V: Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care 2011;34:392–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wiedermann CJ, Kiechl S, Dunzendorfer S, et al. : Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: Prospective results from the Bruneck Study. J Am Coll Cardiol 1999;34:1975–1981 [DOI] [PubMed] [Google Scholar]
- 78.Troseid M, Nestvold TK, Rudi K, et al. : Plasma lipopolysaccharide is closely associated with glycemic control and abdominal obesity: Evidence from bariatric surgery. Diabetes Care 2013;36:3627–3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun L, Yu Z, Ye X, et al. : A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care 2010;33:1925–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caesar R, Reigstad CS, Backhed HK, et al. : Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut 2012;61:1701–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amar J, Chabo C, Waget A, et al. : Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. EMBO Mol Med 2011;3:559–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fei N. and Zhao L: An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J 2013;7:880–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lew WY, Bayna E, Molle ED, et al. : Recurrent exposure to subclinical lipopolysaccharide increases mortality and induces cardiac fibrosis in mice. PLoS One 2013;8:e61057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baker JV, Peng G, Rapkin J, et al. : CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS 2008;22:841–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kelley CF, Kitchen CM, Hunt PW, et al. : Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis 2009;48:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hunt PW, Martin JN, Sinclair E, et al. : T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003;187:1534–1543 [DOI] [PubMed] [Google Scholar]
- 87.Pandrea I, Cornell E, Wilson C, et al. : Coagulation biomarkers predict disease progression in SIV-infected nonhuman primates. Blood 2012;120:1357–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lichtenstein KA, Armon C, Buchacz K, et al. : Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis 2010;51:435–447 [DOI] [PubMed] [Google Scholar]
- 89.Manner IW, Troseid M, Oektedalen O, Baekken M, and Os I: Low nadir CD4 cell count predicts sustained hypertension in HIV-infected individuals. J Clin Hypertens (Greenwich) 2013;15:101–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Manner I, Baekken M, Kvale D, et al. : Markers of microbial translocation predict hypertension in HIV-infected individuals. HIV Med 2013;14:354–361 [DOI] [PubMed] [Google Scholar]
- 91.Pedersen KK, Pedersen M, Troseid M, et al. : Microbial translocation in HIV infection is associated with dyslipidemia, insulin resistance, and risk of myocardial infarction. J Acquir Immune Defic Syndr 2013;64:425–433 [DOI] [PubMed] [Google Scholar]
- 92.Byakwaga H, Hsue P, Sambrano E, and Sinclair E: Background subtraction in the limulus subtraction amebocyte assay may underestimate lipopolysaccharide levels in those with the greatest microbial translocation. 20th Conference on Retroviruses and Opportunistic Infections, Atlanta, GA, 2013. Paper #783 [Google Scholar]
- 93.Timmons T: Microbial translocation and metabolic and body composition measures in treated and untreated HIV infection AIDS Res Hum Retroviruses 2014;30:272–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ghoshal S, Witta J, Zhong J, de VW, and Eckhardt E: Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res 2009;50:90–97 [DOI] [PubMed] [Google Scholar]
- 95.Levine DM, Parker TS, Donnelly TM, Walsh A, and Rubin AL: In vivo protection against endotoxin by plasma high density lipoprotein. Proc Natl Acad Sci USA 1993;90:12040–12044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Casanovas A, Carrascal M, Abian J, Lopez-Tejero MD, and Llobera M: Lipoprotein lipase is nitrated in vivo after lipopolysaccharide challenge. Free Radic Biol Med 2009;47:1553–1560 [DOI] [PubMed] [Google Scholar]
- 97.Cassol E, Misra V, Holman A, et al. : Plasma metabolomics identifies lipid abnormalities linked to markers of inflammation, microbial translocation, and hepatic function in HIV patients receiving protease inhibitors. BMC Infect Dis 2013;13:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Manner IW, Baekken M, Oektedalen O, et al. : Plasma LPS and triglycerides are independently associated, and both markers correlate with the development of metabolic syndrome in HIV infection. J Acquir Immune Defic Syndr 2014;65:158–161 [DOI] [PubMed] [Google Scholar]
- 99.Matta F, Yaekoub AY, and Stein PD: Human immunodeficiency virus infection and risk of venous thromboembolism. Am J Med Sci 2008;336:402–406 [DOI] [PubMed] [Google Scholar]
- 100.Kuller LH, Tracy R, Belloso W, et al. : Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008;5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Funderburg NT, Mayne E, Sieg SF, et al. : Increased tissue factor expression on circulating monocytes in chronic HIV infection: Relationship to in vivo coagulation and immune activation. Blood 2010;115:161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mayne E, Funderburg NT, Sieg SF, et al. : Increased platelet and microparticle activation in HIV infection: Upregulation of P-selectin and tissue factor expression. J Acquir Immune Defic Syndr 2012;59:340–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haugaard AK, Lund TT, Birch C, et al. : Discrepant coagulation profile in HIV infection: Elevated D-dimer but impaired platelet aggregation and clot initiation. AIDS 2013;27:2749–2758 [DOI] [PubMed] [Google Scholar]
- 104.Ross R: Atherosclerosis—an inflammatory disease. N Engl J Med 1999;340:115–126 [DOI] [PubMed] [Google Scholar]
- 105.Verma S. and Anderson TJ: Fundamentals of endothelial function for the clinical cardiologist. Circulation 2002;105:546–549 [DOI] [PubMed] [Google Scholar]
- 106.Zeuke S, Ulmer AJ, Kusumoto S, Katus HA, and Heine H: TLR4-mediated inflammatory activation of human coronary artery endothelial cells by LPS. Cardiovasc Res 2002;56:126–134 [DOI] [PubMed] [Google Scholar]
- 107.Manner IW, Baekken M, Os I, Kvale D, Seljeflot I, and Troseid M: Symmetric and asymmetric dimethylarginine are associated with plasma LPS, low nadir CD4 count and future hypertension in HIV-infection. 20th Conference on Retroviruses and Opportunistic Infections, Atlanta, GA, 2013. Abstract #786 [Google Scholar]
- 108.Parikh RV, Scherzer R, Grunfeld C, et al. : Elevated levels of asymmetric dimethylarginine are associated with lower CD4+ count and higher viral load in HIV-infected individuals. Atherosclerosis 2013;229:246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blodget E, Shen C, Aldrovandi G, et al. : Relationship between microbial translocation and endothelial function in HIV infected patients. PLoS One 2012;7:e42624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stary HC, Chandler AB, Dinsmore RE, et al. : A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995;92:1355–1374 [DOI] [PubMed] [Google Scholar]
- 111.Kelesidis T, Kendall MA, Yang OO, Hodis HN, and Currier JS: Biomarkers of microbial translocation and macrophage activation: Association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis 2012;206:1558–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Merlini E, Luzi K, Suardi E, et al. : T-cell phenotypes, apoptosis and inflammation in HIV+ patients on virologically effective cART with early atherosclerosis. PLoS One 2012;7:e46073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weidemann B, Schletter J, Dziarski R, et al. : Specific binding of soluble peptidoglycan and muramyldipeptide to CD14 on human monocytes. Infect Immun 1997;65:858–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Karlsson F, Tremaroli V, Nielsen J, and Backhed F: Assessing the human gut microbiota in metabolic diseases. Diabetes 2013;62:3341–3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Karlsson FH, Tremaroli V, Nookaew I, et al. : Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013;498:99–103 [DOI] [PubMed] [Google Scholar]
- 116.Tang WH, Wang Z, Levison BS, et al. : Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Koeth RA, Wang Z, Levison BS, et al. : Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ellis CL, Ma ZM, Mann SK, et al. : Molecular characterization of stool microbiota in HIV-infected subjects by panbacterial and order-level 16S ribosomal DNA (rDNA) quantification and correlations with immune activation. J Acquir Immune Defic Syndr 2011;57:363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vujkovic-Cvijin I, Dunham RM, Iwai S, et al. : Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med 2013;5:193ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pedersen ER, Svingen GF, Schartum-Hansen H, et al. : Urinary excretion of kynurenine and tryptophan, cardiovascular events, and mortality after elective coronary angiography. Eur Heart J 2013;34:2689–2696 [DOI] [PubMed] [Google Scholar]
- 121.Lam YY, Ha CW, Campbell CR, et al. : Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One 2012;7:e34233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stinghen AE, Goncalves SM, Bucharles S, et al. : Sevelamer decreases systemic inflammation in parallel to a reduction in endotoxemia. Blood Purif 2010;29:352–356 [DOI] [PubMed] [Google Scholar]
- 123.Murray SM, Down CM, Boulware DR, et al. : Reduction of immune activation with chloroquine therapy during chronic HIV infection. J Virol 2010;84:12082–12086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Piconi S, Parisotto S, Rizzardini G, et al. : Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood 2011;118:3263–3272 [DOI] [PubMed] [Google Scholar]
- 125.Kalambokis GN. and Tsianos EV: Rifaximin reduces endotoxemia and improves liver function and disease severity in patients with decompensated cirrhosis. Hepatology 2012;55:655–656 [DOI] [PubMed] [Google Scholar]
- 126.Asmuth DM, Ma ZM, Albanese A, et al. : Oral serum-derived bovine immunoglobulin improves duodenal immune reconstitution and absorption function in patients with HIV enteropathy. AIDS 2013;27:2207–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gori A, Rizzardini G, van't Land B, et al. : Specific prebiotics modulate gut microbiota and immune activation in HAART-naive HIV-infected adults: Results of the “COPA” pilot randomized trial. Mucosal Immunol 2011;4:554–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vrieze A, Van NE, Holleman F, et al. : Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012;143:913–916 [DOI] [PubMed] [Google Scholar]
- 129.Troseid M, Pedersen B, Ditlevsen S, et al. : Decreased trunk fat and triglycerides after strength training are associated with reduced LPS levels in HIV-infected individuals. J Acquir Immune Defic Syndr 2014; in press [DOI] [PubMed] [Google Scholar]
- 130.Kallio KA, Buhlin K, Jauhiainen M, et al. : Lipopolysaccharide associates with pro-atherogenic lipoproteins in periodontitis patients. Innate Immun 2008;14:247–253 [DOI] [PubMed] [Google Scholar]
- 131.Funk JL, Feingold KR, Moser AH, and Grunfeld C: Lipopolysaccharide stimulation of RAW 264.7 macrophages induces lipid accumulation and foam cell formation. Atherosclerosis 1993;98:67–82 [DOI] [PubMed] [Google Scholar]
- 132.Kallio KA, Hyvarinen K, Kovanen PT, Jauhiainen M, and Pussinen PJ: Very low density lipoproteins derived from periodontitis patients facilitate macrophage activation via lipopolysaccharide function. Metabolism 2013;62:661–668 [DOI] [PubMed] [Google Scholar]