Using combination therapy to override stromal-mediated chemoresistance in mutant FLT3-positive AML: Synergism between FLT3 inhibitors, dasatinib/multi-targeted inhibitors, and JAK inhibitors: Novel combination therapy approaches to overriding stromal-mediated chemoresistance (original) (raw)
. Author manuscript; available in PMC: 2014 Jun 12.
Published in final edited form as: Leukemia. 2012 Apr 3;26(10):2233–2244. doi: 10.1038/leu.2012.96
Abstract
Acute myeloid leukemia (AML) progenitors are frequently characterized by activating mutations in the receptor tyrosine kinase FLT3. Protein tyrosine kinases are integral components of signaling cascades that play a role in both FLT3-mediated transformation as well as viability pathways that are advantageous to leukemic cell survival. The bone marrow microenvironment can diminish AML sensitivity to tyrosine kinase inhibitors (TKIs). We hypothesized that inhibition of protein kinases in addition to FLT3 may be effective in overriding drug resistance in AML. We used a cell-based model mimicking stromal protection as part of an unbiased high-throughput chemical screen to identify kinase inhibitors with the potential to override microenvironment-mediated drug resistance in mutant FLT3-positive AML. Several related multi-targeted kinase inhibitors, including dasatinib, with the capability of reversing microenvironment-induced resistance to FLT3 inhibition were identified and validated. We validated synergy in vitro and demonstrated effective combination potential in vivo. In particular Janus kinase (JAK) inhibitors were effective in overriding stromal protection and potentiating FLT3 inhibition in primary AML and cell lines. These results hint at a novel concept of using combination therapy to override drug resistance in mutant FLT3-positive AML in the bone marrow niche and suppress or eradicate residual disease.
Keywords: acute myeloid leukemia, FLT3 inhibitor, multi-targeted kinase inhibitor, mutant FLT3, PKC412, AC220, stromal-mediated chemoresistance, drug resistance, synergy
Introduction
The development of resistance in AML patients to treatment with targeted TKIs is a growing area of concern. A mutated form of the class III receptor tyrosine kinase, FLT3 (_F_ms-_L_ike _T_yrosine kinase-3; STK-1, human _S_tem Cell _T_yrosine _K_inase-1; or FLK-2, _F_etal _L_iver _K_inase-2), is expressed in a subset of AML patients and thus represents an attractive target for small molecule inhibition1. Constitutively activated FLT3 occurs most frequently as internal tandem duplications (ITD) within the juxtamembrane domain, and is observed in approximately 20–25% of AML patients2.
One of the most clinically advanced kinase inhibitors for this population of AML patients is the FLT3 inhibitor, PKC412 (midostaurin)3, which is currently being tested in Phase III clinical trials. In Phase I and II clinical trials, PKC412 was administered in sequential and simultaneous combinations with daunorubicin and cytarabine induction and high-dose cytarabine consolidation and yielded clinical responses with transient and/or reversible side effects4. Of the other FLT3 kinase inhibitors that are in early clinical development, particularly promising is the highly selective and potent FLT3 inhibitor, AC220 (quizartinib)5, which has yielded encouraging results in the form of transient clinical responses as a single agent. The fact, however, that FLT3 inhibitors tested thus far clinically at best induce partial and short-lived responses in patients when used alone suggests a need for development of novel agents that can either be used effectively alone or combined with other agents to suppress disease progression and prolong lifespan.
Clinical trial data with TKIs show that while the peripheral blood of patients responds well, a more modest response is observed in patient bone marrow. It is possible that a small residual pool of leukemic CD34+ cells may persist in the marrow microenvironment of leukemia patients after years of therapy with kinase inhibitors. It is thus likely that the marrow is a sanctuary site for leukemic stem cell, and that the interaction of these quiescent leukemic cells with stroma is sufficient to induce relative drug resistance through activation of alternative viability pathways. While these leukemic cells cannot proliferate in response to oncogene-driven signals, they still survive and form a reservoir of persistent disease.
Bone marrow stromal cells have been implicated in drug resistance, as they are thought to play a role in the long-term survival and growth of normal and leukemia cells and other hematological malignancies, including lymphoma and multiple myeloma6–12. It was recently shown that the activity of first-generation FLT3 inhibitors, such as SU5614 and sorafenib, is diminished against mutant FLT3-expressing cells by contact with stroma13.
We demonstrated stromal protection of leukemic cells from the inhibitory effects of nilotinib, and identified stromal-secreted viability factors, including IL-6 and GM-CSF, as possibly playing a role in stromal-mediated cytoprotection of TKI-treated chronic myeloid leukemia (CML) and AML14–15. In addition, in TKI-treated mice, we identified high tumor burden/residual disease in tissues characterized as significant sources of hematopoiesis-promoting stroma; the pattern of leukemia distribution in mice was consistent with what is observed in imatinib/nilotinib-treated CML patients14.
Protein kinases are intrinsically involved in regulation of signal transduction pathways, and deregulated kinase activity, which affects cell proliferation and apoptosis, often leads to development of cancer. The survival advantage of mutant FLT3-driven leukemia is known to be largely due to mutant FLT3 activation of three major intracellular signaling pathways, PI3K/PTEN/Akt/mTOR, RAS/Raf/MEK/ERK, and Janus kinase/signal transducer and activator of transcription (JAK/STAT)16. Serine/threonine kinases and dual-specificity kinases, such as those comprising the MAP kinase pathway, non-receptor tyrosine kinases (ie Src), receptor tyrosine kinases (ie FLT3), and receptor-associated tyrosine kinases involved in cytokine-mediated signaling (ie JAK, whose effects are mediated by STAT proteins), all represent putative therapeutic targets. As examples, protein kinases, such as SRC, are often activated in malignant cells and contribute to tumor development and are thus viable candidates for targeted drug development. The cross-talk between the three signaling pathways, coupled with the involvement of a variety of signaling mediators in viability pathways conferring a survival advantage to transformed cells in microenvironmental recesses, strongly warrants the use of multi-targeted therapy to treat leukemia and potentially override drug resistance.
We hypothesized that combination therapy geared toward inhibition of diverse protein kinase targets may be effective in potentiating TKI effects on mutant FLT3-positive leukemia cells cultured in a cytoprotective stromal microenvironment. As such, we performed a screen of a kinase inhibitor focused library to identify agents with demonstrated ability to synergize with PKC412 and related inhibitors against stroma-protected, mutant FLT3-expressing cells. Our screen led to the identification of three structurally-related multi-targeted TKIs, including dasatinib, that were able to override the cytoprotective effect of stromal-derived viability factors on FLT3 inhibitor-treated cells. Synergy between these agents was confirmed in vitro, using both stromal-conditioned media (SCM) and adherent stroma, and a positive combination effect was observed in vivo with prolonged survival demonstrated in combination-treated mice. We also independently demonstrated the ability of inhibitors of JAK kinase, a mediator of IL-6 and related growth factor signaling, to similarly potentiate the effects of FLT3 inhibitors, as well as dasatinib and related multi-targeted TKIs, and in effect override stromal-mediated chemoresistance.
Materials and Methods
Kinase Inhibitor Focused Library
We utilized a focused library of kinase inhibitors to screen for inhibitors showing little-to-no appreciable efficacy as single agents, however demonstrating the ability to synergize with PKC412 against human FLT3-ITD-expressing MOLM13 cells cultured in the presence of 50% HS-5 SCM. The library is comprised of approximately 300 publically disclosed kinase inhibitors and approximately 800 novel ATP competitive kinase inhibitors targeting either active or inactive kinase conformations. The chemical screening concentration was 660 nM. Details regarding this are in the supplementary data section.
Cell lines and cell culture
Details are provided as supplementary material17–21.
AML patient cells
Details are provided as supplementary material.
Chemical compounds and biologic reagents
PKC412 and AUZ454 were synthesized by Novartis Pharma AG, Basel, Switzerland, and were dissolved in DMSO to obtain 10 mM stock solutions. Serial dilutions were then made, to obtain final dilutions for cellular assays with a final concentration of DMSO not exceeding 0.1%.
Dasatinib, AZD-1480, AZD-0530, INCB-18,424, CYT387, AC220 and KIN040 were purchased from Haoyuan Chemexpress (Shanghai, China; KIN112, KIN113, were developed in Dr. Gray’s lab (DFCI). Chemical structures are shown in Supplementary Figure 1.
Molecular modeling
Details are provided as supplementary material.
Cell proliferation, cell cycle, and viability analysis
The trypan blue exclusion assay (for proliferation), Annexin-V-Fluos Staining Kit (Boehringer Mannheim, Indianapolis, IN) (for apoptosis), and cell cycle analysis were carried out as previously described3. The Cell Titer Glo assay (Promega, Madison, WI) (for proliferation) was used where indicated, and carried out according to manufacturer instructions.
Antibodies
All antibodies used were purchased from Cell Signaling Technology, Danvers, MA. Phospho-STAT5 Tyr694 (rabbit, #9351S) was used at 1:1000. Total STAT5 (3H7) (rabbit, #9358 mAb) was used at 1:1000. Phospho-AKT (Ser 473) (rabbit, #9271) and total AKT (rabbit, #9272) were used at 1:2500. Anti p-Tyr (clone 4G10, Upstate Biotechnology, NY) was used at 1:1000 for immunoblotting.
Immunoblotting
Protein lysis preparation, immunoprecipitation, and immunoblotting were carried out as previously described3.
Drug combination studies
For drug combination studies, single agents were added simultaneously at fixed ratios to mutant FLT3-expressing cells. Cell viability was determined using the trypan blue exclusion assay, and expressed as the function of growth affected (FA) drug-treated versus control cells; data were analyzed by Calcusyn software (Biosoft, Ferguson, MO and Cambridge, UK), using the Chou-Talalay method22 (Chou and Talalay, 1984). The combination index=[D]1 [Dx]1 + [D]2/[Dx]2, where [D]1 and [D]2 are the concentrations required by each drug in combination to achieve the same effect as concentrations [Dx]1 and [Dx]2 of each drug alone. Calcusyn combination indices can be interpreted as follows: CI <0.1 indicate very strong synergism (a). Values 0.1–0.3 indicate strong synergism (b). Values 0.3–0.7 indicate synergism (c). Values 0.7–0.85 indicate moderate synergism (d). Values 0.85–0.90 indicate slight synergism (e). Values 0.9–1.1 indicate nearly additive effects (f). Values 1.10–1.20 indicate slight antagonism (g). Values 1.20–1.45 indicate moderate antagonism (h). Values 1.45–3.3 indicate antagonism (i). Values 3.3–10 indicate strong antagonism (j). Values >10 indicate very strong antagonism. Note: For some experiments, namely those in which there was no observed single agent activity due to stromal protection, combination indices were not able to be reliably calculated using the Calcusyn software.
Human Stroma Experiments
Details are provided as supplementary material.
Bioluminescent model of progressive FLT3-ITD-driven AML
For administration to 30 female Nu/Nu NCR-nude mice (8 weeks of age; Charles River Laboratories, Wilmington, MA), virus- and _Mycoplasma_-free Ba/F3-FLT3-ITD-luc+ cells were washed in Hank’s Balanced Salt Solution (HBSS; Mediatech, Inc., VA), resuspended in PBS for injection, and administered via intravenous tail vein injection (250 μL, 1.5 x 106 cells). Following administration of Ba/F3-FLT3-ITD-luc+ cells, mice were then imaged 2 days later to determine baseline bioluminescence and quantify tumor-burden as previously described19. Mice were then divided into four treatment groups (n=7 or 8 per group): Vehicle (PKC412 pre-concentrate; 10 μl/g), PKC412 (50 mg/kg), dasatinib (10 mg/kg), and combination. All treatments were dosed PO once daily; mice were not treated on days 8–9 to allow them to gain weight after a significant initial drop (particularly in the dasatinib-treated groups. Groups were imaged every 3–5 days for two weeks, at which time mice started to become moribund. Survival data was collected for all mice. Four whole mice from each group were fixed in 10% neutral-buffered formalin upon sacrifice and sent for histopathological analysis.
Histopathology
Formalin-fixed tissues were dissected and processed routinely for paraffin embedding and sectioning. Micron sections were stained with hematoxylin and eosin.
Drug formulations for in vivo studies
PKC412, synthesized by Novartis Pharma AG, Basel, Switzerland, was supplied as a microemulsion preconcentrate (5% w/v) and diluted with water to achieve the desired final concentrations. Dasatinib was obtained from Haoyuan Chemexpress(Shanghai, China) as a powder. Dasatinib was formulated in 50% propylene glycol, 50% water for in vivo administration.
Results
In vitro chemical screen to identify protein kinase inhibitors able to potentiate the effects of stromal-protected TKIs aimed at AML
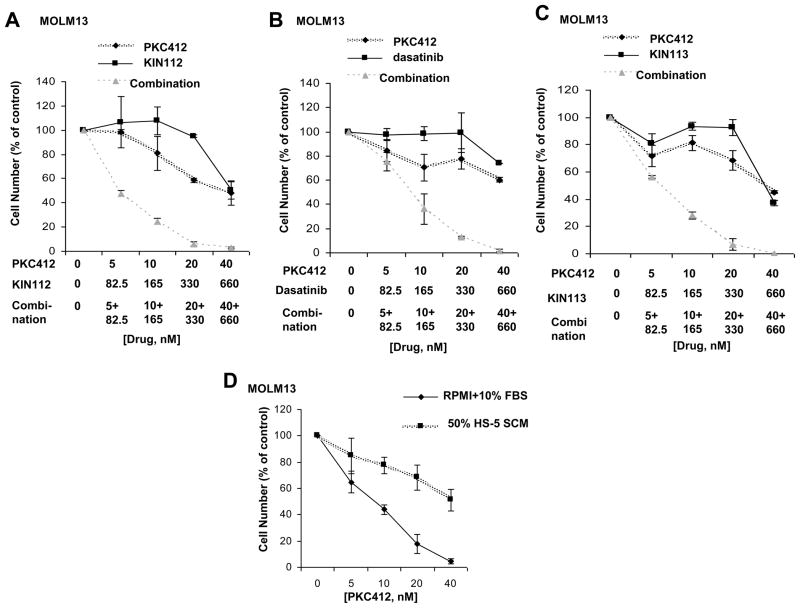
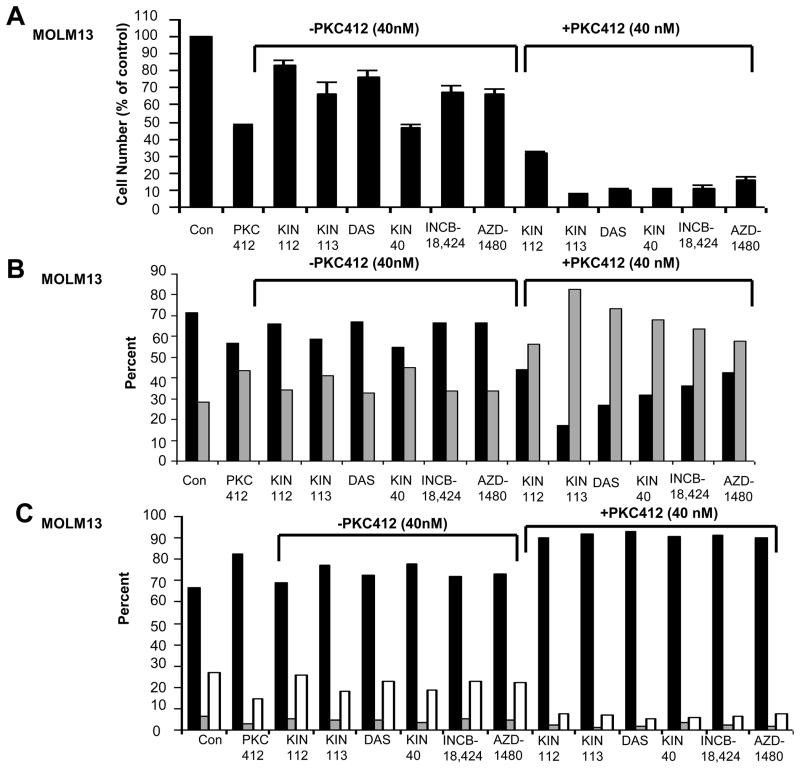
In an attempt to identify protein kinase inhibitors that are able to effectively synergize with standard tyrosine kinase inhibitors, the inhibitory activity of which is diminished in the presence of adherent stroma or stromal-secreted factors, we conducted a combinatorial kinase inhibitor screen in vitro using a chemical library consisting of early-in-development- and FDA-approved kinase inhibitors. As the activity of imatinib and nilotinib against KU812F-luc+ cells has been shown to be diminished in the presence of plated HS-5 stromal cells23 and SCM24, we decided to use this assay system to test the efficacy of PK412, as a single agent, as compared to PKC412 in combination with library compounds. In an unbiased screen of 1100 kinase inhibitors, three library-derived kinase inhibitors were identified, including the BCR/ABL and Src family inhibitor, dasatinib, and the related multi-targeted kinase inhibitors, KIN112 and KIN113. These drugs showed little-to-no single agent activity at the chemical screen concentration of 660 nM, however were able to synergize with PKC412 against MOLM13 cells cultured in the presence of SCM (Figures 1A–C and Supplementary Figure 2). Synergy between PKC412+dasatinib, PKC412+KIN112, and PKC412+KIN113 can be appreciated by the nearly 100% inhibition of cell growth at the highest concentrations of each drug combined, compared to approximately 50% killing of cells by each individual drug at the highest concentrations. The cytoprotective effect of conditioned media derived from two independent human stromal cell lines, HS-5- and HS27a, on PKC412-treated leukemic cells was confirmed in this screening, as was the expected potency of PKC412 in the absence of SCM. Increased inhibition of cell proliferation by drug combination treatments, as compared to single agent treatments, correlated with increased percentages of apoptotic and necrotic cell populations (up to 40% higher in drug combination groups) as well as a higher percentage of cells in the G1 phase of the cell cycle (up to 10–20% higher in drug combination groups) (Figure 2), suggesting that observed synergy is due to both cell killing as well as cell cycle arrest.
Figure 1. Proliferation studies performed with selected chemical library compounds in combination with PKC412 in the presence of HS-5 SCM.
Proliferation studies (trypan blue exclusion assay): Approximately two-day treatment of MOLM13 cells, in the presence of 50% HS-5 SCM, with PKC412, KIN112, or a combination of the two agents (A), PKC412, dasatinib, or a combination of the two agents (B), or PKC412, KIN113, or a combination of the two agents (C). Shown are averages of 2–4 separate calculations of cell viability for each data point. These data are representative of two independent sets of experiments in which similar results were observed (the second data set is shown as supplementary data). (D) Proliferation studies (trypan blue exclusion assay): Approximately two-day treatment of MOLM13 cells, in the absence and presence of 50% HS-5 SCM, with PKC412 (n=3).
Figure 2. Proliferation studies, viability studies, and cell cycle analysis performed with selected chemical library compounds in combination with PKC412 in the presence of HS-5 SCM.
(A) Proliferation studies (Cell Titer Glo assay): Approximately two-day treatment of MOLM13 cells, in the presence of 50% HS-5 SCM. PKC412 was tested at 40 nM. KIN112, KIN113, dasatinib (“DAS”), KIN40, INCB-18,424, and AZD-1480 were tested at 660 nM. Values were calculated as percent of untreated controls and were the average + SEM of duplicate wells per treatment. (B) Viability assays (Annexin-V-Fluos Staining). Black bars represent percent viable. Gray bars represent percent apoptotic/necrotic. (C) Cell cycle analysis. Black bars represent percent G1. Gray bars represent percent G2. White bars represent percent S.
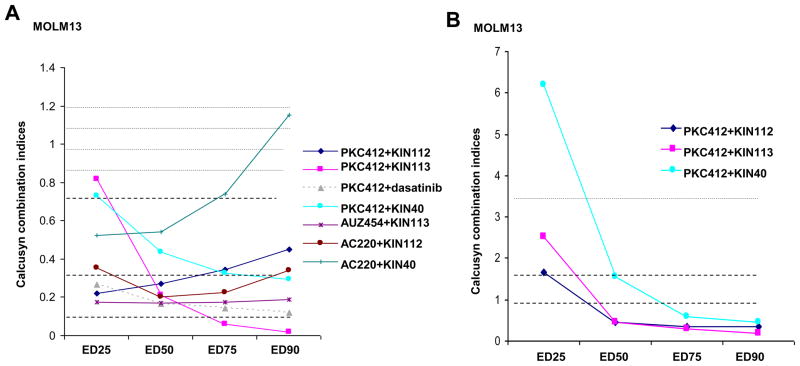
Inhibitors were able to similarly synergize in the presence of SCM with the highly potent and relatively selective FLT3 inhibitors, AUZ45425 and AC220 (Figure 3 and Supplementary Figure 3). Synergy was observed in the presence of SCM derived from HS27a stroma as well as HS-5 stroma (Supplementary Figure 4). Synergy observed between KIN112/KIN113/dasatinib and PKC412 was more pronounced in the presence of SCM than in its absence (Figure 3), suggesting that factors mediating the observed synergy may play a significant role in SCM. Importantly, none of the three identified inhibitors showed appreciable cytotoxicity toward human HS-5 or HS27a bone marrow stromal cells (Supplementary Figure 5).
Figure 3. Calcusyn combination indices generated for combination experiments with FLT3 inhibitors and selected library compounds.
(A) Calcusyn combination indices calculated for proliferation studies performed with FLT3 inhibitors combined with chemical library compounds (KIN112, dasatinib, KIN113) or the pan-Jak inhibitor, KIN40, in the presence of SCM. (B) Calcusyn combination indices calculated for proliferation studies performed with PKC412 combined with chemical library compounds (KIN112, KIN113) or KIN40 in the presence of RPMI+10% FBS.
Synergy between KIN112/KIN113/dasatinib and PKC412 was also observed against MOLM14-luc+ cells co-cultured with adherent HS27a stromal cells (Supplementary Figure 6). Adherent stromal cells conferred a cytoprotective effect on PKC412-treated MOLM14-luc+ cells.
In vivo investigation of the combination of PKC412 and dasatinib against progressive mutant FLT3-positive leukemia
An in vivo study was carried out to investigate both the therapeutic potential as well as toxicity/side effects of the combination of dasatinib and PKC412 in mice harboring mutant FLT3-positive leukemia. This murine model utilized cells expressing FLT3-ITD (which leads to aggressive disease and poor prognosis in AML patients) and was designed to mimic progressive, FLT3-ITD-driven disease.
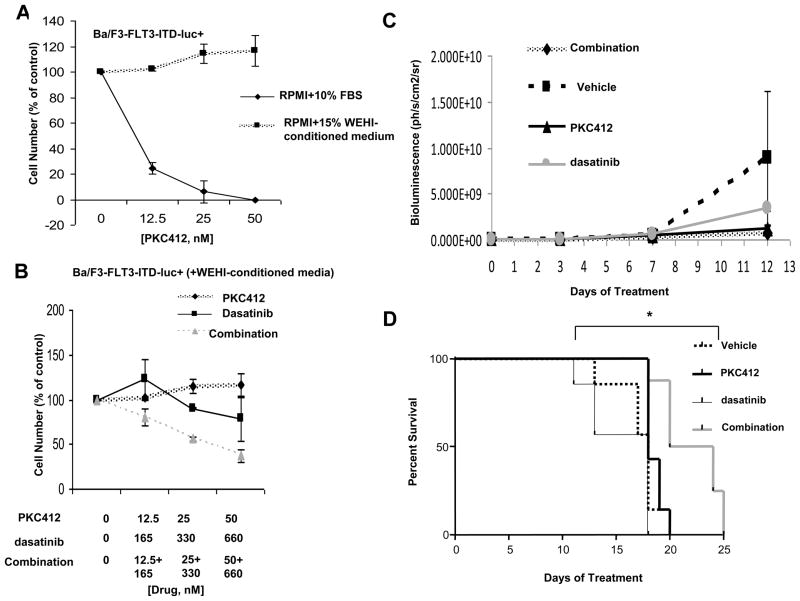
Prior to injection into mice, Ba/F3-FLT3-ITD-luc+ cells were first tested for responsiveness to PKC412, as well as growth factor protection from the cytotoxic effects of PKC412. WEHI-conditioned media (used as a source of IL-3) significantly protected or “rescued” Ba/F3-FLT3-ITD-luc+ cells from the inhibitory effects of PKC412 (Figure 4A and Supplementary Figure 7A). Dasatinib as a single agent does not inhibit growth of this cell line when cultured in the presence of IL-3 (Figure 4B and Supplementary Figure 7B). However, the combination of dasatinib and PKC412 led to substantially more cell killing than either agent alone under the same conditions (Figure 4B and Supplementary Figure 7B).
Figure 4. Investigation of the combination of PKC412 and dasatinib against Ba/F3-FLT3-ITD-luc+ cells in mutant FLT3-harboring mice.
(A) Proliferation study performed in vitro (trypan blue exclusion assay), testing PKC412 against Ba/F3-FLT3-ITD-luc+ cells in the presence and absence of 15% WEHI-conditioned media (used as a source of IL-3). Shown are averages of 2–4 separate calculations of cell viability for each data point. These data are representative of two independent sets of experiments in which similar results were observed (the second data set is shown as supplementary data). (B) Proliferation study performed in vitro (trypan blue exclusion assay), testing the combination of PKC412 and dasatinib against Ba/F3-FLT3-ITD-luc+ cells in the presence of 15% WEHI-conditioned media. Shown are averages of 2–4 separate calculations of cell viability for each data point. These data are representative of two independent sets of experiments in which similar results were observed (the second data set is shown as supplementary data). (C) Total body luminescence: drug treatment effect on tumor burden. 2-way ANOVA analysis revealed no significant difference between groups. (D) Survival curves. *The PKC412+dasatinib combination treatment significantly prolonged survival of mice as compared to the other treatment groups (p-value: 0.0255).
Compared to mice treated for 13 days with vehicle, dasatinib alone, or PKC412 alone, mice treated with a combination of dasatinib and PKC412 trended towards lower tumor burden (bioluminescence) (Figure 4C). As results for the imaging at this point did not reach statistical significance, drug treatments were continued to the time of sacrifice. Whereas monotherapy with PKC412 or dasatinib had no appreciable effects, PKC412+dasatinib combination treatment significantly prolonged survival (Figure 4D) (p-value: 0.0255). These data suggest that the combination of PKC412 and dasatinib may have potential clinical benefit and translate into increased survival.
All mice at the time of sacrifice were determined by histopathology to harbor leukemia. Most of the mice had large numbers of tumor cells in meninges, bone marrow, liver, spleen, and occasionally ovary. As all mice included in this study died from disease and not drug-related toxicities, PKC412 and dasatinib as single agents and in combination were considered to be generally well-tolerated. However, initial weight loss was observed in those treatment groups receiving dasatinib (Supplementary Figure 7C).
Structural information and comparisons between KIN112, dasatinib, and KIN113
The three identified inhibitors, dasatinib, KIN112, and KIN113, from the chemical library screen are all primarily classified as Src-family kinase (SFK) inhibitors, though additional kinase selectivity profiling using KinomeScan against a panel of 442-kinases reveals many additional potential kinase targets (Supplementary Figure 8)26, 27. KIN112 possesses a chemical structure that is predicted to be isosteric with that of dasatinib. KIN112 and KIN113 are very potent SRC inhibitors, with an IC50 for KIN112 against c-SRC of 6 nM, and an IC50 for KIN113 against c-SRC of 102 nM. Molecular modeling studies predict that KIN112 would preferentially bind to SRC in active conformation (DFG-in), analogously to the crystallographic co-structure of dasatinib bound to Src (PDB ID 3G5D), whereas KIN113 is predicted to preferentially bind to the inactive conformation (DFG-out) of Src (Supplementary Figure 9). The highly multi-targeted nature of KIN112, KIN113 and dasatinib make it very difficult to establish whether a particular kinase or a combination of kinases is pharmacologically relevant to the observed synergy with FLT3 inhibition.
Investigation of synergizing potential of JAK inhibitors combined with TKIs against AML
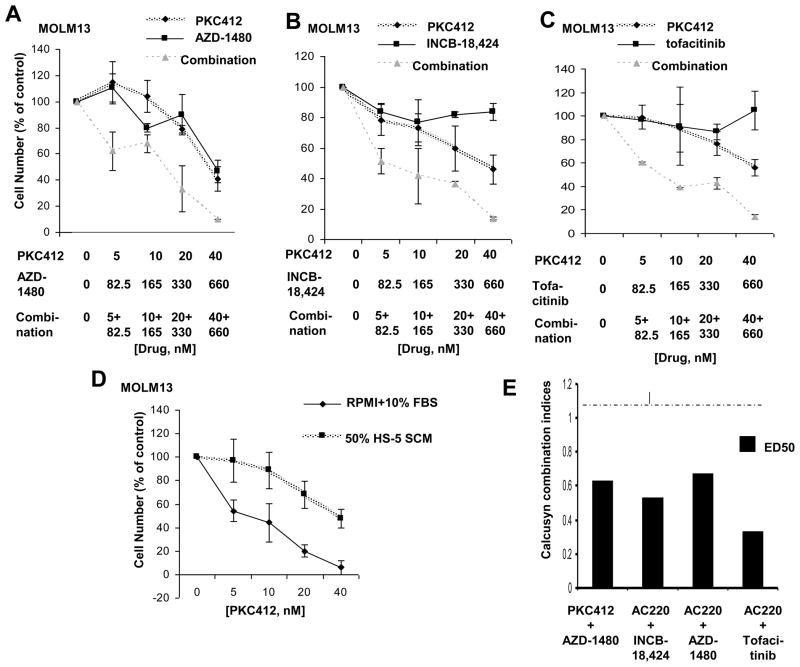
IL-6 and related cytokines exert their effects via by binding to receptors (i.e. glycoprotein 130, OSM receptor, and LIF receptor) that transduce signals to the activation of JAK/STAT and mitogen-activated protein kinase (MAPK) cascades. We hypothesized that inhibition of JAK/STAT signaling may be effective in overriding the viability-inducing effects of stromal-secreted IL-6 and related cytokines on kinase inhibitor-treated leukemic cells. Accordingly, we tested in combination with PKC412 several selective JAK inhibitors, including the JAK1/2 inhibitor, INCB-18,424, selective JAK2 inhibitor, AZD-1480, selective JAK3 inhibitor, Tofacitinib (CP-690550), and the JAK1/2 inhibitor, CYT38728. We also tested in combination with PKC412 the pan-JAK inhibitor, KIN40, and in combination with PKC412 the selective SRC inhibitor, AZD-0530. KinomeScan kinase selectivity profiles for KIN40 and the selective JAK and SRC inhibitors are shown in Supplementary Figure 826,27. The JAK inhibitors significantly enhanced the activity of PKC412 in the presence of SCM and led to more cell killing (Figure 5; Supplementary Figure 10). A more modest positive combination effect was observed between PKC412 and INCB-18,424 with FLT3-ITD-positive MV4,11 cells treated in the presence of SCM (Supplementary Figure 10). Synergy observed between the pan-JAK inhibitor, KIN40, and PKC412 against MOLM13 cells was more pronounced in the presence of SCM than in its absence (Figure 3), suggesting that factors mediating the observed synergy may play a significant role in stromal-mediated chemoresistance.
Figure 5. Proliferation studies performed with FLT3 inhibitors and Jak inhibitors.
Trypan blue exclusion assay: Approximately two-day treatment of MOLM13 cells, in the presence of 50% SCM consisting of 25% HS-5 SCM and 25% HS27a SCM, with PKC412, AZD1480, or a combination of the two agents (A), PKC412, INCB-18, 424, or a combination of the two agents (B), and PKC412, Tofacitinib, or a combination of the two agents (C). Shown are the averages of 2–4 separate calculations of cell viability for each data point. Data are representative of two independent sets of experiments in which similar results were observed (the second data set is shown as supplementary data). (D) Trypan blue exclusion assay: Approximately two-day treatment of MOLM13 cells, in the absence and presence of 50% SCM consisting of 25% HS-5 SCM and 25% HS27a SCM, with PKC412 for each respective study (n=3). (E) Calcusyn combination indices (ED50) calculated for proliferation studies performed with FLT3 inhibitors combined with selective Jak or Src inhibitors. The cut-off for nearly additive effects (C.I.: 1.1) is marked by a dashed line.
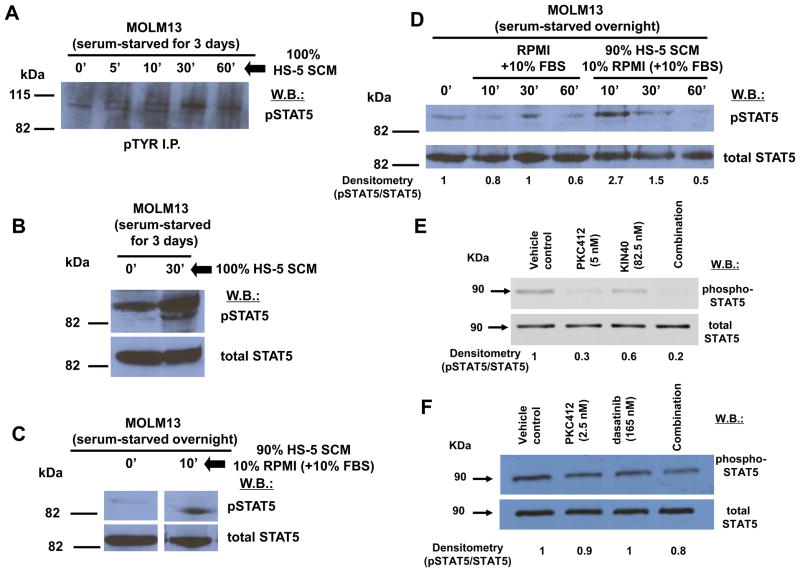
We showed via pTYR immunoprecipitation and whole cell lysate immunoblots that SCM treatment of MOLM13 cells serum-starved for varying lengths of time leads to an increase in tyrosine phosphorylation of STAT5 (Figure 6A–D), suggesting that STAT5 activation may play a role in stromal-mediated chemoresistance. The combination of PKC412 and pan-Jak inhibitor, KIN40, was accompanied by a decrease in levels of phospho-STAT5 that was greater than either drug alone (Figure 6E). This suggests that STAT5, as a key component of both the JAK/STAT signaling pathway and downstream mediator of FLT3 signaling, likely plays a role in synergy between PKC412 and KIN40. Similar results were observed with phospho-STAT5 in MOLM13 cells treated with a combination of dasatinib and PKC412, as compared to either drug alone (Figure 6F). In addition, pAKT levels were inhibited to a greater extent in MOLM cells treated with a combination of dasatinib and PKC412, as compared to either drug alone (Supplementary Figure 11), further suggesting that downstream mediators of FLT3 signaling likely participate in the synergy observed between FLT3 inhibitors combined with dasatinib and related multi-kinase inhibitors.
Figure 6. SCM stimulation of phospho-STAT5 and drug combination effect on phosphorylation of phospho-STAT5.
(A) pTYR immunoprecipitation (I.P.) followed by phospho-STAT5 western blot (w.b.), showing HS-5 SCM stimulation of phospho-STAT5. (B) phospho-STAT5 and total STAT5 whole cell lysate western blots of MOLM13 cells serum-starved for 3 days and treated with 100% HS-5 SCM, showing HS-5 SCM stimulation of phospho-STAT5 activity. (C) phospho-STAT5 and total STAT5 whole cell lysate western blots of MOLM13 cells serum-starved overnight and treated with 90% HS-5 SCM, showing HS-5 SCM stimulation of phospho-STAT5 activity. (D) Comparison of effects of RPMI+10% FBS versus 90% HS-5 SCM on phospho-STAT5 in MOLM13 cells serum-starved overnight. (E) Expression of phospho-STAT5 and total STAT5 in MOLM13 cells treated for 1.5 hr with DMSO vehicle, PKC412 (5 nM), KIN40 (82.5 nM), or a combination of both. Results shown are representative of two independent experiments in which similar results were observed. (E) Expression of phospho-STAT5 and total STAT5 in MOLM13 cells treated for 15 minutes with DMSO vehicle, PKC412 (2.5 nM), dasatinib (165 nM), or a combination of both. Results shown are representative of two independent experiments in which similar results were observed. For all experiments, protein lysates were prepared from MOLM13 cells, and were analyzed via immunoblotting with antibodies to phospho-STAT5 and total STAT5.
Similar to PKC412, AC220 synergized with INCB-18,424, AZD-1480, and Tofacitinib in the presence of SCM (Figure 5). The inhibitory effects of AC220 as a single agent were substantially diminished by the addition of SCM (data not shown).
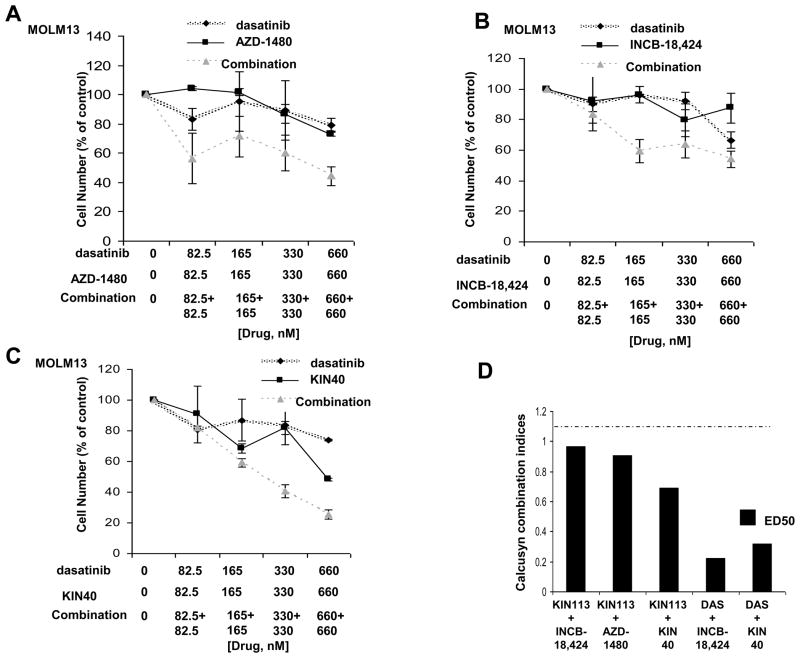
Modest synergy was observed between PKC412 and the selective SRC inhibitor, AZD-0530 (Supplementary Figure 12). In contrast, no apparent positive combination effect was observed between AZD-0530 and the selective JAK inhibitors, AZD-1480 and INCB-18,424 (Supplementary Figure 12). In contrast, the multi-targeted TKIs, dasatinib and KIN113, were observed to synergize with JAK inhibitors (Figure 7 and Supplementary Figure 13). These results suggest that the multiple targets of dasatinib and KIN113 may contribute to the impressive synergy observed with other TKIs in a cytoprotective stromal microenvironment, whereas selective inhibition of SRC may not be sufficient to achieve comparable synergy.
Figure 7. Proliferation studies performed with library-derived multi-targeted inhibitors and Jak inhibitors.
(A–C) Trypan blue exclusion assay: Dasatinib and Jak inhibitor combination studies performed in the presence of 50% HS-5+HS27a SCM. Approximately two-day treatments of MOLM13 cells, in the presence of 50% SCM consisting of 25% HS-5 SCM and 25% HS27a SCM, with dasatinib, AZD-1480, or a combination of the two agents (A), with dasatinib, INCB-18,424, or a combination of the two agents (B), or with dasatinib, KIN40, or a combination of the two agents (C). Shown are averages of 2–4 separate calculations of cell viability for each data point. These data are representative of several independent sets of experiments in which similar results were observed (the second data set is shown as supplementary data). (D) Calcusyn combination indices (ED50) calculated for proliferation studies performed with chemical library compounds combined with selective Jak inhibitors or KIN40. The cut-off for nearly additive effects (C.I.: 1.1) is marked by a dashed line.
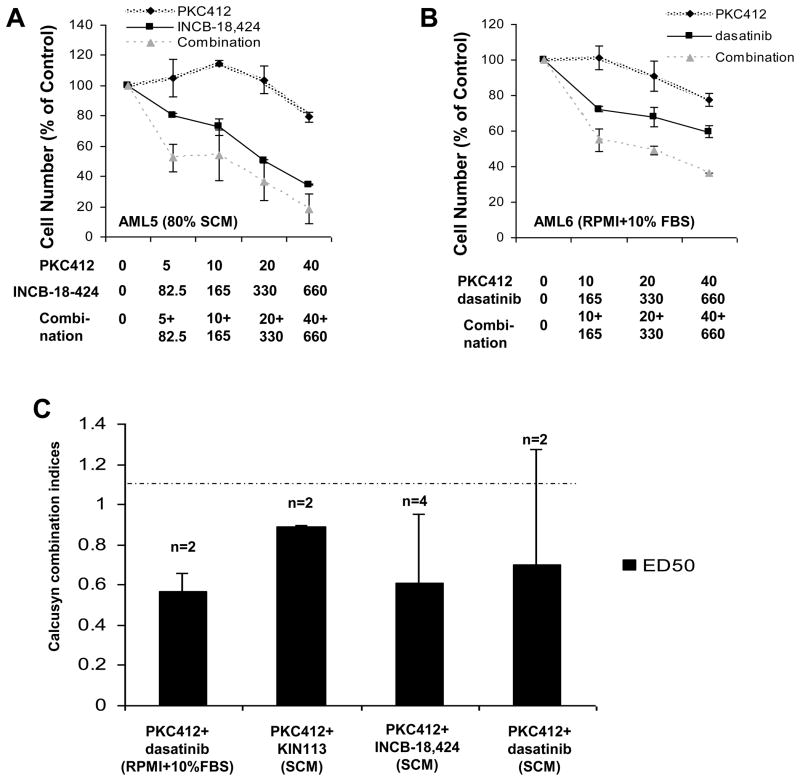
Investigation of drug combination effects against mutant FLT3-positive AML patient cells
PKC412 was tested alone or combined with either multi-targeted TKIs identified in the chemical screen or JAK inhibitors against peripheral blood or bone marrow samples derived from seven AML patients harboring FLT3-ITD. Despite PKC412 responsiveness alone being low in patients that were relapsed or refractory following previous therapies (ie AML 5, AML 6), more AML patient cell killing was still observed when drug combinations, tested in the presence of either RPMI+10% FBS or 50–80% SCM, were used as opposed to single agents (Figure 8). Calcusyn analysis revealed additive to synergistic effects for various combinations, including PKC412+KIN113, PKC412+dasatinib, and PKC412+INCB-18,424 (Figure 8). These results support the positive drug combination effects observed in mutant FLT3-positive cell lines and suggest the possibility of clinical benefit arising from such drug combinations. Patient information is provided in Supplementary Table I.
Figure 8. Combination studies performed with primary mutant FLT3-positive AML patient samples performed in the presence of RPMI+10% FBS or HS-5 SCM.
Cell Titer Glo assay: AML patients were treated in the presence of HS-5 SCM or RPMI+10% FBS. (A–B) Representative dose-response curves. (C) Calcusyn combination indices (ED50) showing effects ranging from nearly additive (1.1) to synergistic (<0.9) are shown for the different combinations. The cut-off for nearly additive effects (C.I.: 1.1) is marked by a dashed line. Patient information is provided as Supplementary Table 1. In addition, AML1 treated in the presence of 50% SCM with PKC412+KIN40 yielded C.I. (ED50) = 0.98283 (suggesting additive effects). AML6 treated in the presence of 80% SCM with PKC412+KIN112 yielded a C.I. (ED50) = 0.76190 (suggesting synergy).
Discussion
The communication between leukemia cells and cells comprising the bone marrow microenvironment influences the growth of transformed cells, as well as their responsiveness to anticancer therapy. There is thus a need for development of novel therapeutic approaches that target not only the leukemic cell, but also the surrounding protective stromal microenvironment.
The association between FLT3-ITD mutations and poor AML patient prognosis29,30 highlights the potential of FLT3-ITD as an important therapeutic target. In addition, FLT3-ITD activation of signal transduction pathways dependent on the interactions of numerous protein kinases underscores the potential therapeutic value of additionally targeting kinase mediators acting downstream of the receptor. Indeed, phosphoproteomic analysis of FLT3 signaling in human leukemia cells performed on primary AML patient bone marrow samples led to identification of over 800 serine/threonine phosphorylation sites and over 200 tyrosine phosphorylation sites31, suggesting the potential importance of a large number of protein kinases as therapeutic targets for overriding cytoprotection conferred by the bone marrow microenvironment of AML patients. In this study, stable isotope labeling by amino acids in cell culture revealed over 400 tyrosine and serine/threonine phosphorylation sites to be responsive to FLT3 inhibition, as well.
Recent studies have implicated kinase activity in stromal-mediated cytoprotection of leukemia cells. For example, in chronic lymphocytic leukemia (CLL), bone marrow stroma, via B-cell receptor activation and induction of chemokines32, protects against purine analog chemotherapy-induced apoptosis33–34. Pharmacologic inhibition of the non-receptor cytoplasmic tyrosine kinase, spleen tyrosine kinase (SYK), which mediates B-cell receptor signaling-induced survival signaling, inhibits the bone marrow survival signals that protect CLL cells35. Another study demonstrated the ability of a selective JAK3 inhibitor to reverse stromal-mediated chemoresistance of CLL cells36.
We have previously shown that inhibitors of PI3K signaling have the ability to effectively combine with PKC412 to effectively kill drug-sensitive and insensitive mutant FLT3-expressing cells37. We have also previously demonstrated the ability of small molecule inhibitors to potentiate the effects of targeted TKIs and in effect override stromal-mediated chemoresistance in leukemia models, such as inhibitor of apoptosis (IAP) inhibitors24,38 and the CXCR4 antagonist, plerixafor39. Thus, TKI-dependent combination therapy appears to represent a potentially effective approach to overriding drug resistance in leukemia patients, due to both factors contributing to aberrant intracellular signaling as well as factors associated with the cytoprotective microenvironment.
Here, in a chemical screen, we identified the dual Src/Abl inhibitor, dasatinib (BMS-354825) and two related multi-targeted TKIs as being able to synergize with FLT3 inhibitors against mutant FLT3-expressing cells cultured in the presence of stroma or SCM. SRC family kinases, which are targets of the three compounds, in addition to displaying increased activity and expression in transformed cells40, have been suggested to play a role in the tumor microenvironment, namely VEGF-induced angiogenesis and vascular permeability41–43.
Dasatinib is also an inhibitor of c-KIT, platelet-derived growth factor receptor (PDGFR) and EphA2 signaling, and was recently FDA-approved for newly diagnosed CML44–46. Dasatinib has been reported to inhibit multiple myeloma-derived angiogenesis via targeting of PDGFRbeta and Src47, and inhibits endothelial and myeloid cell function necessary for sustaining tumor cell growth in vivo48. A recent study showed that the Src family kinase Lyn, in association with CXCR4, is involved in stromal microenvironment resistance of CML to imatinib 49. In this report, imatinib enhancement of CML migration toward stromal cell layers was blocked by inhibition of Lyn or cholesterol depletion, and dasatinib was reported to cause comparatively less CML migration to stroma.
KIN112, another identified multi-targeted TKI possessing a pharmacophore that is isosteric with dasatinib, binds to the active conformation of SRC, whereas the third multi-targeted TKI, KIN113binds to the inactive conformation of SRC. Interestingly, despite the distinct predicted binding modes of KIN112 and KIN113, both compounds exhibited a similar ability to synergize with FLT3 inhibitors in the presence of a cytoprotective stromal microenvironment.
Because the effects of stromal-secreted cytokines, including IL-6, are known to activate JAK/STAT signaling, we investigated the ability of a panel of JAK inhibitors to potentiate the effects of FLT3 inhibition as an attempt to override cytoprotection due to stromal-derived cytokine secretion. We observed that JAK inhibitors synergize with FLT3 inhibitors against mutant FLT3-expressing cells in the presence of stroma or SCM. This appears to involve enhanced inhibition of STAT5 phosphorylation, a finding consistent with the fact that STAT5 has been identified as one of the most important mediators of FLT3-ITD-mediated signaling, and it can also be phosphorylated by JAK50–53. The finding that PKC412 combined with dasatinib similarly causes a more pronounced decrease in STAT5 phosphorylation levels in mutant FLT3-expressing cells is again consistent with the fact that STAT5 is an integral component of mutant FLT3-mediated signaling, as well as the fact that STAT5 can be phosphorylated by SRC kinase52.
In addition, we observed that JAK inhibitors plus dasatinib or KIN113 synergize against mutant FLT3-expressing cells in the presence of a cytoprotective microenvironment. In contrast, a selective SRC inhibitor, AZD0530, was unable to synergize with JAK inhibitors under similar conditions, suggesting that Src inhibition likely needs to be accompanied by inhibition of additional kinase targets for effective reversal of stromal-mediated drug resistance.
Our results are consistent with those of a study that demonstrated increased survival of AML leukemic and progenitor cells in response to the FLT3 inhibitor, AG1296, when cells were cultured under “niche-like” conditions (a microenvironment comprised of serum-free medium supplemented with IL-3, IL-6, SCF, and Ang-1, and including fibronectin)54. In this study, AML cells were more sensitive to inhibitors of signaling pathways, including the JAK/STAT inhibitor, AG490. Our observations also support recent findings showing that JAK2 inhibition may be effective in overriding viability signaling in CML55. Ours, however, is the first to implicate the potential usefulness of JAK1, JAK2, and JAK3 inhibitors- as well as dasatinib and related multikinase inhibitors- as part of combination therapy to override stromal-derived viability signaling in AML.
We thus present for the first time a cell-based chemical screening model designed to mimic bone marrow stroma protection of leukemia. This model was successful in identifying dasatinib and related mutli-targeted inhibitors as potential candidates for clinical testing as part of combination therapy for effectiveness against mutant FLT3-positive leukemia, as well as possible suppression/eradication of minimal residual disease due to stromal protection. In support of this was PKC412+dasatinib-induced prolongation of survival in a mouse model of aggressive, progressive FLT3-ITD-leukemic disease and enhanced killing of stromal-protected primary AML cells by drug combinations versus single agents. We also demonstrated the ability of JAK inhibitors to synergize with FLT3 inhibitors or dasatinib/multi-targeted TKIs against stroma-protected, mutant FLT3-positive AML cells. In conclusion, our data suggest that the drug combinations introduced herein may be beneficial in potentiating the effects of FLT3 inhibitors against mutant FLT3-positive leukemic disease, as well as in potentially overriding drug resistance due to the provision of viability signals from the bone marrow microenvironment.
Supplementary Material
Response Letter
Supplementary Figure Legends
Supplementary Text
Acknowledgments
We wish to thank Feiyang Liu for her technical assistance. We thank DiscoveRx Bioscience for performing KinomeScan™ profiling and the Treespot view image was generated using the web-based TREEspot™ software (DiscoveRx Biosciences). J.D.G is supported by NIH grant CA66996. Q.L. and N.S.G. are supported by NIH LINCS Grant HG006097 and R01 CA130876-02. J.D.G. has a financial interest with Novartis Pharma AG. J.D.G. and A.L.K. have a financial interest with Novartis Pharma AG.
Footnotes
None of the authors included in this manuscript have a financial conflict of interest.
References
- 1.Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3:650–65. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 2.Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–18. [PubMed] [Google Scholar]
- 3.Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitors PKC412. Cancer Cell. 2002;1:433–443. doi: 10.1016/s1535-6108(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 4.Stone RM, DeAngelo DJ, Klimek V, Galinksy I, Estey E, Nimer SD, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 5.Zarrinkar PP, Gunawardane RN, Cramer MD, Gardner MF, Brigham D, Belli B, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML) Blood. 2009;114:2984–2992. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashley DM, Bol SJ, Kannourakis G. Human bone marrow stromal cell contact and soluble factors have different effects on the survival and proliferation of paediatric B-lineage acute lymphoblastic leukaemic blasts. Leuk Res. 1994;18:337–346. doi: 10.1016/0145-2126(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 7.Bradstock K, Bianchi A, Makrynikola V, Filshie R, Gottlieb D. Long-term survival and proliferation of precursor B acute lymphoblastic leukemia cells on human bone marrow stroma. Leukemia. 1996;10:813–820. [PubMed] [Google Scholar]
- 8.Rafii S, Mohle R, Shapiro F, Frey BM, Moore MA. Regulation of hematopoiesis by microvascular endothelium. Leuk Lymphoma. 1997;27:375–386. doi: 10.3109/10428199709058305. [DOI] [PubMed] [Google Scholar]
- 9.Lagneaux L, Delforge A, Bron D, De Bruyn C, Stryckmans P. Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood. 1998;91:2387–2396. [PubMed] [Google Scholar]
- 10.Lagneaux L, Delforge A, De Bruyn C, Bernier M, Bron D. Adhesion to bone marrow stroma inhibits apoptosis of chronic lymphocytic leukemia cells. Leukemia Lymphoma. 1999;35:445–453. doi: 10.1080/10428199909169609. [DOI] [PubMed] [Google Scholar]
- 11.Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713–1724. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 12.Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113:4341–51. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parmar A, Marz S, Rushton S, Holzwarth C, Lind K, Kayser S, et al. Stromal niche cells protect early leukemic FLT3-ITD+ progenitor cells against first-generation FLT3 tyrosine kinase inhibitors. Cancer Res. 2011;71:4696–706. doi: 10.1158/0008-5472.CAN-10-4136. [DOI] [PubMed] [Google Scholar]
- 14.Weisberg E, Wright RD, McMillin DW, Mitsiades C, Ray A, Barrett R, et al. Stromal-mediated protection of tyrosine kinase inhibitor-treated BCR-ABL-expressing leukemia cells. Mol Cancer Ther. 2008a;7:1121–9. doi: 10.1158/1535-7163.MCT-07-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weisberg E, Barrett R, Liu Q, Stone R, Gray N, Griffin JD. FLT3 inhibition and mechanisms of drug resistance in mutant FLT3-positive AML. Drug Resist Updat. 2009;12:81–9. doi: 10.1016/j.drup.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kornblau SM, Womble M, Qiu YH, Jackson CE, Chen W, Konopleva M, et al. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood. 2006;108:2358–65. doi: 10.1182/blood-2006-02-003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuo Y, MacLeod RA, Uphoff CC, Drexler HG, Nishizaki C, Katayama Y, et al. Two acute monocytic leukemia (AML-M5a) cell lines (MOLM13 and MOLM14) with interclonal phenotypic heterogeneity showing MLL-AF9 fusion resulting from an occult chromosome insertion, ins(11;9)(q23;p22p23) Leukemia. 1997;11:1469–77. doi: 10.1038/sj.leu.2400768. [DOI] [PubMed] [Google Scholar]
- 18.Quentmeier H, Reinhardt J, Zaborski M, Drexler HG. FLT3 mutations in acute myeloid leukemia cell lines. Leukemia. 2003;17:120–124. doi: 10.1038/sj.leu.2402740. [DOI] [PubMed] [Google Scholar]
- 19.Kimbrel EA, Davis TN, Bradner JE, Kung AL. In vivo pharmacodynamic imaging of proteosome inhibition. Mol Imaging. 2009;8:140–147. [PubMed] [Google Scholar]
- 20.Armstrong SA, Kung AL, Mabon ME, Silverman LB, Stam RW, Den Boer ML, et al. Validation of a therapeutic target identified by gene expression based classification. Cancer Cell. 2003;3:173–83. doi: 10.1016/s1535-6108(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 21.Kelly LM, Liu Q, Kutok JL, Williams IR, Boulton CL, Gilliland DG. FLT3 internal tandem duplication mutations associated with human acute myeloid leukemias induce myeloproliferative disease in a murine bone marrow transplant model. Blood. 2002;99:310–318. doi: 10.1182/blood.v99.1.310. [DOI] [PubMed] [Google Scholar]
- 22.Chou T-C, Talalay P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv Enz Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 23.McMillin DW, Delmore J, Weisberg E, Negri JM, Geer DC, Klippel S, et al. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat Med. 2010;16:483–9. doi: 10.1038/nm.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisberg E, Ray A, Barrett R, Nelson E, Christie AL, Porter D, et al. Smac mimetics: implications for enhancement of targeted therapies in leukemia. Leukemia. 2010a;25:2100–2109. doi: 10.1038/leu.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weisberg E, Roesel J, Furet P, Bold G, Imbach P, Florsheimer A, et al. Antileukemic effects of novel first- and second-generation FLT3 inhibitors: structure-affinity comparison. Genes Cancer. 2010b;1:1021–32. doi: 10.1177/1947601910396505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fabian MA, Biggs WH, 3rd, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–36. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 27.Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, et al. Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol. 2011;29:1046–51. doi: 10.1038/nbt.1990. [DOI] [PubMed] [Google Scholar]
- 28.Pardanani A, Lasho T, Smith G, Burns CJ, Fantino E, Tefferi A. CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia. 2009;23:1441–1445. doi: 10.1038/leu.2009.50. [DOI] [PubMed] [Google Scholar]
- 29.Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 30.Moreno I, Martin G, Bolufer P, Barragan E, Rueda E, Roman J, et al. Incidence and prognostic value of FLT3 internal tandem duplication and D835 mutations in acute myeloid leukemia. Haematologica. 2003;88:19–24. [PubMed] [Google Scholar]
- 31.Gu TL, Nardone J, Wang Y, Loriaux M, Villen J, Beausoleil S, et al. Survey of activated FLT3 signaling in leukemia. PLoS One. 2011;6:e19169. doi: 10.1371/journal.pone.0019169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burger JA, Quiroga MP, Hartmann E, Burkle A, Wierda WG, Keating MJ, et al. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocyt9ic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood. 2009;113:3050–3058. doi: 10.1182/blood-2008-07-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gobessi S, Laurenti L, Longo PG, Carsetti L, Berno V, Sica S, et al. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia. 2009;23:686–697. doi: 10.1038/leu.2008.346. [DOI] [PubMed] [Google Scholar]
- 34.Kurtova AV, Balakrishnan K, Chen R, Ding W, Schnabl S, Quiroga MP, et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood. 2009;114:4441–4450. doi: 10.1182/blood-2009-07-233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchner M, Fuchs S, Prinz G, Pfeifer D, Bartholome K, Burger M, et al. Spleen Tyrosine Kinase Is Overexpressed and Represents a Potential Therapeutic Target in Chronic Lymphocytic Leukemia. Cancer Res. 2009;69:5424–5432. doi: 10.1158/0008-5472.CAN-08-4252. [DOI] [PubMed] [Google Scholar]
- 36.Steele AJ, Prentice AG, Cwynarski K, Hoffbrand AV, Hart SM, Lowdell MW, et al. The JAK3-selective inhibitor PF-956980 reverses the resistance to cytotoxic agents induced by interleukin-4 treatment of chronic lymphocytic leukemia cells: potential for reversal of cytoprotection by the microenvironment. Blood. 2010;116:4569–77. doi: 10.1182/blood-2009-09-245811. [DOI] [PubMed] [Google Scholar]
- 37.Weisberg E, Banerji L, Wright RD, Barrett R, Ray A, Moreno D, et al. Potentiation of antileukemic therapies by the dual PI3K/PDK-1 inhibitor, BAG956: effects on BCR-ABL- and mutant FLT3-expressing cells. Blood. 2008b;111:3723–34. doi: 10.1182/blood-2007-09-114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weisberg E, Kung AL, Wright RD, Moreno D, Catley L, Ray A, et al. Potentiation of antileukemic therapies by Smac mimetic, LBW242: effects on mutant FLT3-expressing cells. Mol Cancer Ther. 2007;6:1951–61. doi: 10.1158/1535-7163.MCT-06-0810. [DOI] [PubMed] [Google Scholar]
- 39.Weisberg E, Azab AK, Manley PW, Kung AL, Christie AL, Bronson R, et al. Inhibition of CXCR4 in CML cells disrupts their interaction with the bone marrow microenvironment and sensitizes them to nilotinib. Leukemia. doi: 10.1038/leu.2011.360. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–58. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 41.Mukhopadhyay D, Tsiokas L, Zhou XM, Foster D, Brugge JS, Sukhatme VP. Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature. 1995;375:577–81. doi: 10.1038/375577a0. [DOI] [PubMed] [Google Scholar]
- 42.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–24. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 43.Kilarski WW, Jura N, Gerwins P. Inactivation of Src family kinases inhibits angiogenesis in vivo: implications for a mechanism involving organization of the actin cytoskeleton. Exp Cell Res. 2003;291:70–82. doi: 10.1016/s0014-4827(03)00374-4. [DOI] [PubMed] [Google Scholar]
- 44.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-chloro-6-methyl to phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinaes inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–61. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 45.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 46.Schittenhelm MM, Shiraga S, Schroeder A, Corbin A, Griffith D, Lee FY, et al. Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 2006;66:473–81. doi: 10.1158/0008-5472.CAN-05-2050. [DOI] [PubMed] [Google Scholar]
- 47.Coluccia AM, Cirulli T, Neri P, Mangieri D, Colanardi MC, Gnoni A, et al. Validation of PDGFRβ and c-Src tyrosine kinases as tumor/vessel targets in patients with multiple myeloma: preclinical efficacy of the novel, orally available inhibitor dasatinib. Blood. 2008;112:1346–56. doi: 10.1182/blood-2007-10-116590. [DOI] [PubMed] [Google Scholar]
- 48.Liang W, Kujawski M, Wu J, Lu J, Herrmann A, Loera S, et al. Antitumor activity of targeting Src kinases in endothelial and myeloid cell compartments of the tumor microenvironment. Clin Cancer Res. 2010;16:924–35. doi: 10.1158/1078-0432.CCR-09-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tabe Y, Jin L, Iwabuchi K, Wang RY, Ichikawa N, Miida T, et al. Role of stromal microenvironment in nonpharmacological resistance of CML to imatinib through Lyn/CXCR4 interactions in lipid rafts. Leukemia. 2011 doi: 10.1038/leu.2011.291. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 50.Mizuki M, Schwable J, Steur C, Choudhary C, Agrawal S, Sargin B, et al. Suppression of myeloid transcription factors and induction of STAT response genes by AML-specific Flt3 mutations. Blood. 2003;101:3164–3173. doi: 10.1182/blood-2002-06-1677. [DOI] [PubMed] [Google Scholar]
- 51.Spiekermann K, Bagrintseva K, Schwab R, Schmieja K, Hiddemann W. Overexpression and constitutive activation of FLT3 induces STAT5 activation in primary acute myeloid leukemia blast cells. Clin Cancer Res. 2003;9:2140–2150. [PubMed] [Google Scholar]
- 52.Robinson LJ, Xue J, Corey SJ. Src family tyrosine kinases are activated by Flt3 and are involved in the proliferative effects of leukemia-associated Flt3 mutations. Exp Hematol. 2005;33:469–479. doi: 10.1016/j.exphem.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Rocnik JL, Okabe R, Yu JC, Lee BH, Giese N, Schenkein DP, et al. Roles of tyrosine 589 and 591 in STAT5 activation and transformation mediated by FLT3-ITD. Blood. 2006;108:1339–45. doi: 10.1182/blood-2005-11-011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mony U, Jawad M, Seedhouse C, Russell N, Pallis M. Resistance to FLT3 inhibition in an in vitro model of primary AML cells with a stem cell phenotype in a defined microenvironment. Leukemia. 2008;22:1395–401. doi: 10.1038/leu.2008.125. [DOI] [PubMed] [Google Scholar]
- 55.Traer E, MacKenzie R, Snead J, Agarwal A, Eiring AM, O’Hare T, Druker BJ, Deininger MW. Blockade of JAK2-mediated extrinsic survival signals restores sensitivity of CML cells to ABL inhibitors. Leukemia. 2011 doi: 10.1038/leu.2011.325. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Response Letter
Supplementary Figure Legends
Supplementary Text