ERCC1 Isoform Expression and DNA Repair in Non–Small-Cell Lung Cancer (original) (raw)
. Author manuscript; available in PMC: 2014 Jun 12.
Published in final edited form as: N Engl J Med. 2013 Mar 21;368(12):1101–1110. doi: 10.1056/NEJMoa1214271
Abstract
BACKGROUND
The excision repair cross-complementation group 1 (ERCC1) protein is a potential prognostic biomarker of the efficacy of cisplatin-based chemotherapy in non–small-cell lung cancer (NSCLC). Although several ongoing trials are evaluating the level of expression of ERCC1, no consensus has been reached regarding a method for evaluation.
METHODS
We used the 8F1 antibody to measure the level of expression of ERCC1 protein by means of immunohistochemical analysis in a validation set of samples obtained from 494 patients in two independent phase 3 trials (the National Cancer Institute of Canada Clinical Trials Group JBR.10 and the Cancer and Leukemia Group B 9633 trial from the Lung Adjuvant Cisplatin Evaluation Biology project). We compared the results of repeated staining of the entire original set of samples obtained from 589 patients in the International Adjuvant Lung Cancer Trial Biology study, which had led to the initial correlation between the absence of ERCC1 expression and platinum response, with our previous results in the same tumors. We mapped the epitope recognized by 16 commercially available ERCC1 antibodies and investigated the capacity of the different ERCC1 isoforms to repair platinum-induced DNA damage.
RESULTS
We were unable to validate the predictive effect of immunostaining for ERCC1 protein. The discordance in the results of staining for ERCC1 suggested a change in the performance of the 8F1 antibody since 2006. We found that none of the 16 antibodies could distinguish among the four ERCC1 protein isoforms, whereas only one isoform produced a protein that had full capacities for nucleotide excision repair and cisplatin resistance.
CONCLUSIONS
Immunohistochemical analysis with the use of currently available ERCC1 antibodies did not specifically detect the unique functional ERCC1 isoform. As a result, its usefulness in guiding therapeutic decision making is limited. (Funded by Eli Lilly and others.)
In patients with resected stage IB, IIA or IIB, or IIIA non–small-cell lung cancer (NSCLC), platinum-based postoperative chemotherapy is now standard treatment, with an estimated increase in the survival rate of 4 to 5% at 5 years.1,2 The identification of predictive biomarkers of the efficacy of adjuvant chemotherapy could lead to an improved therapeutic index.3 DNA repair capacity is a major determinant of cisplatin resistance; in particular, the excision repair cross-complementation group 1 (ERCC1) protein plays an essential role in nucleotide excision repair. Together with its partner, xeroderma pigmentosum complementation group F (XPF), ERCC1 cleaves DNA structures near the site of the platinum–DNA adduct, thereby allowing elimination of the lesion.4
ERCC1 as a biomarker of patient survival, treatment efficacy, or both has been studied at the genomic level (analysis of single-nucleotide polymorphisms), transcriptional level (reverse-transcriptase–polymerase-chain-reaction [RT-PCR] assay), and protein level (immunohistochemical analysis) in both retrospective and prospective studies. We and others have reported that the level of expression of ERCC1 in NSCLC tumors was prognostic or predictive, or both, of a benefit from cisplatin-based adjuvant chemotherapy (see Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).5–25 The most commonly used technique to evaluate ERCC1 as a biomarker is the assessment of its expression by means of immunohistochemical analysis with the mouse monoclonal antibody 8F1. However, this antibody recognizes a peptide sequence that is still unknown, and the specificity of assessment with 8F1 has been debated.26–29 Recently, data have suggested that it may detect a non-ERCC1 target.30
The ERCC1 gene generates four isoforms (designated 201, 202, 203, and 204) by alternative splicing. Data are lacking on the levels of expression of various ERCC1 transcripts in NSCLC cell lines and tumors, and on their functional capacity in DNA repair. An ERCC1-201–like mutation and the ERCC1-203 isoform have appeared to be nonfunctional in nucleotide excision repair capacity. 31–33 We measured repair of platinum–DNA adducts34 and cisplatin sensitivity in A549-derived cell lines expressing only a single ERCC1 isoform and found that only one of the four ERCC1 isoforms was functional.
In a subsequent study, as part of a Lung Adjuvant Cisplatin Evaluation (LACE) Biology project, we used the 8F1 antibody in a validation series from two independent randomized trials of postoperative adjuvant cisplatin-based chemotherapy in an attempt to confirm that the ERCC1 protein is a predictive marker. The lack of confirmation of previous results prompted us to repeat staining of formerly analyzed samples obtained from the entire International Adjuvant Lung Cancer Trial (IALT) Biology cohort6 and to assess the role of the four protein isoforms and their recognition by currently available commercial antibodies. Here we report the results of this study.
METHODS
PATIENTS AND SAMPLES
Tumor samples from the IALT, the Cancer and Leukemia Group B (CALGB) 9633, and the National Cancer Institute of Canada Clinical Trials Group JBR.10 trials35–37 are included in the LACE Biology biomarker project. These trials compared postoperative platin-based chemotherapy with no chemotherapy.38 The project protocol is available at NEJM.org.
Whole tissue sections were available from all the trials. Samples from the JBR.10 trial (314 patients) and the CALGB 9633 trial (180 patients) were considered to be the validation set. Only samples obtained from 589 patients in the original IALT Biology study could be stained again, since tumor material from 172 patients was no longer available. For analysis of ERCC1 isoform messenger RNA (mRNA), we used frozen samples (obtained from 123 patients) from the Tumour Chemotherapy Resistance (CHEMORES) consortium, as described previously.39
STUDY CONDUCT
No commercial support for this study was sought or provided. The authors designed the study, collected and analyzed the data, wrote the manuscript, and vouch for the integrity of the data and adherence to the study protocol. No one who is not listed as an author contributed to the manuscript.
IMMUNOHISTOCHEMICAL ANALYSIS
Formalin-fixed, paraffin-embedded tissue sections were stained with mouse monoclonal antibody against ERCC1 (Ab-2, clone 8F1, MS-671-P, Neomarkers), as previously described (Fig. S1 in the Supplementary Appendix).6 All 8F1 batches that were used are listed in Table S2 in the Supplementary Appendix. Microscopical evaluation of staining for ERCC1 was performed independently for the validation set and the IALT Biology (second staining) set, and both sets were evaluated by an experienced pathologist in a blinded fashion. To verify the absence of single-reader interpretation biases, a subset of 150 random samples was analyzed again by one of the primary readers from the original study6 and a second pathologist; interobserver concordance with respect to ERCC1 positivity was higher than 95% (kappa = 0.862). Only tumor areas close to internal control cells with valid positive staining for ERCC1 (in the stroma, epithelium, endothelium, or all these areas) were taken into account. We did not select hot spots for assessment, and all representative nuclei were averaged throughout the tumor.
The staining intensity was graded on a scale of 0 to 3 (with a higher number indicating a higher intensity). The percentage of positive tumor nuclei was calculated for each specimen, and a proportion score was assigned (0 for 0%, 0.1 for 1 to 9%, 0.5 for 10 to 49%, and 1.0 for ≥50%). This proportion score was multiplied by the staining intensity of nuclei to obtain a final semiquantitative H score. As previously described,6 tumors with an ERCC1 H score higher than 1 (i.e., tumors with a staining intensity score of 2 and with 50% or more positive nuclei or with a staining intensity score of 3 and 10% or more positive nuclei) were classified as ERCC1-positive.
ANTIBODIES
The antibodies against human ERCC1 that were used for Western blot and epitope mapping analyses are listed in Table S3 in the Supplementary Appendix. An anti–β-actin antibody (A5441, Sigma-Aldrich) was used as the loading control for Western blot analysis. Complete biologic methods are described in the Supplementary Appendix.
STATISTICAL ANALYSIS
Statistical methods for both the clinical and preclinical studies are described in the Supplementary Appendix. The study of the prognostic and predictive value of ERCC1 was based on a multivariable Cox model when stratified according to the trial and adjusted for the state of lung cancer, the type of surgery, the patient’s sex, and histologic findings.
RESULTS
ERCC1 PROTEIN DETECTION WITH 8F1 ANTIBODY
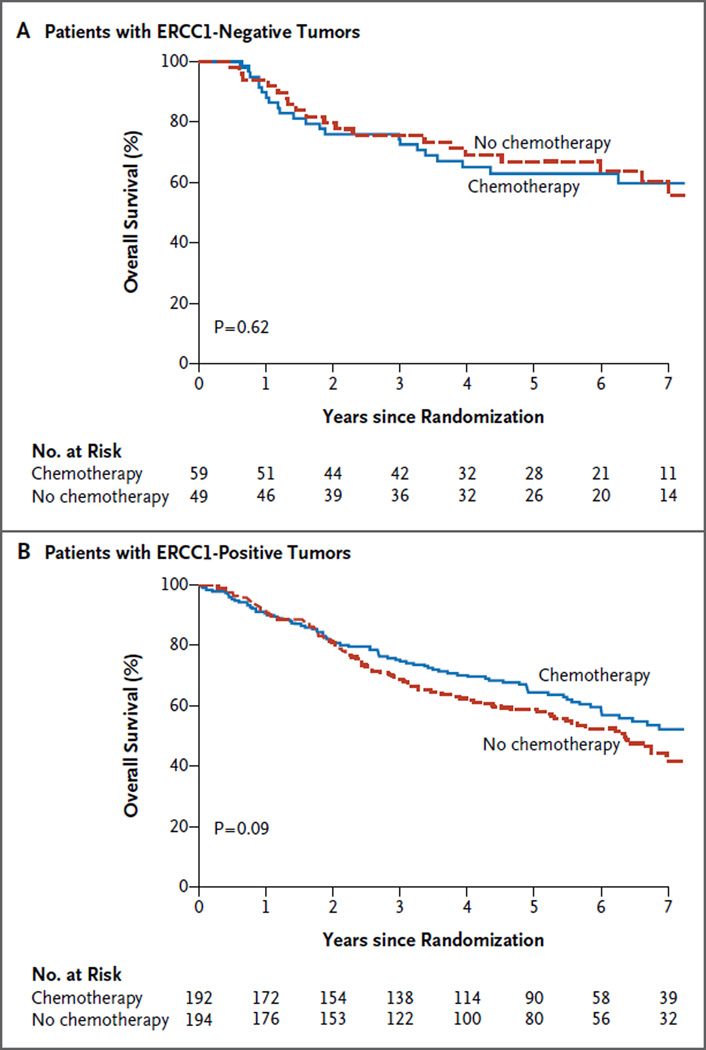
ERCC1 was scored as positive (H score >1) in 78% of samples obtained from the 494 patients in the validation set for whom complete data were available. Among patients with ERCC1-negative tumors, overall survival did not differ significantly between the chemotherapy and control groups (hazard ratio for death, 1.16; 95% confidence interval [CI], 0.64 to 2.10; P = 0.62) (Fig. 1A). Patients with ERCC1-positive tumors appeared to have some benefit from chemotherapy (hazard ratio for death, 0.78; 95% CI, 0.58 to 1.05; P = 0.09) (Fig. 1B), but the predictive effect of ERCC1 was not significant (P = 0.23 for interaction). Analysis according to quartiles did not suggest another relevant cutoff point or the existence of a quantitative effect. Furthermore, the previously reported prognostic effect of ERCC16 was not confirmed (data not shown).
Figure 1. Kaplan–Meier Estimates of Overall Survival in the Validation Cohort.
Overall survival curves are shown according to treatment group for patients with ERCC1-negative tumors (Panel A) and those with ERCC1-positive tumors (Panel B). P values are based on multivariable Cox models. The numbers of events corresponding to the survival curves are shown in the forest plot in Figure S2 in the Supplementary Appendix.
This validation study was performed with a batch of the 8F1 antibody that was different from the batch used in our previous study.6 Although the latter batch was no longer available for repeated staining, in the previous study, ERCC1 was positive in only 44% of the IALT Biology cohort.6 When all the remaining IALT Biology tumor blocks were stained again with the current 8F1 antibody batch, 77% were scored as positive, and ERCC1 was no longer a predictive biomarker of the efficacy of chemotherapy (P = 0.53 for interaction). The hazard ratio for death was 0.81 (95% CI, 0.50 to 1.31; P = 0.39) among patients with ERCC1-negative tumors and 0.96 (95% CI, 0.74 to 1.25; P = 0.78) among those with ERCC1-positive tumors. The hazard ratios for death in the different subgroups and trials (LACE Biology and IALT Biology) are shown in Figure S2A in the Supplementary Appendix, and the hazard ratios for disease recurrence are shown in Figure S2B in the Supplementary Appendix.
DISCREPANCIES BETWEEN 8F1 ANTIBODY BATCHES
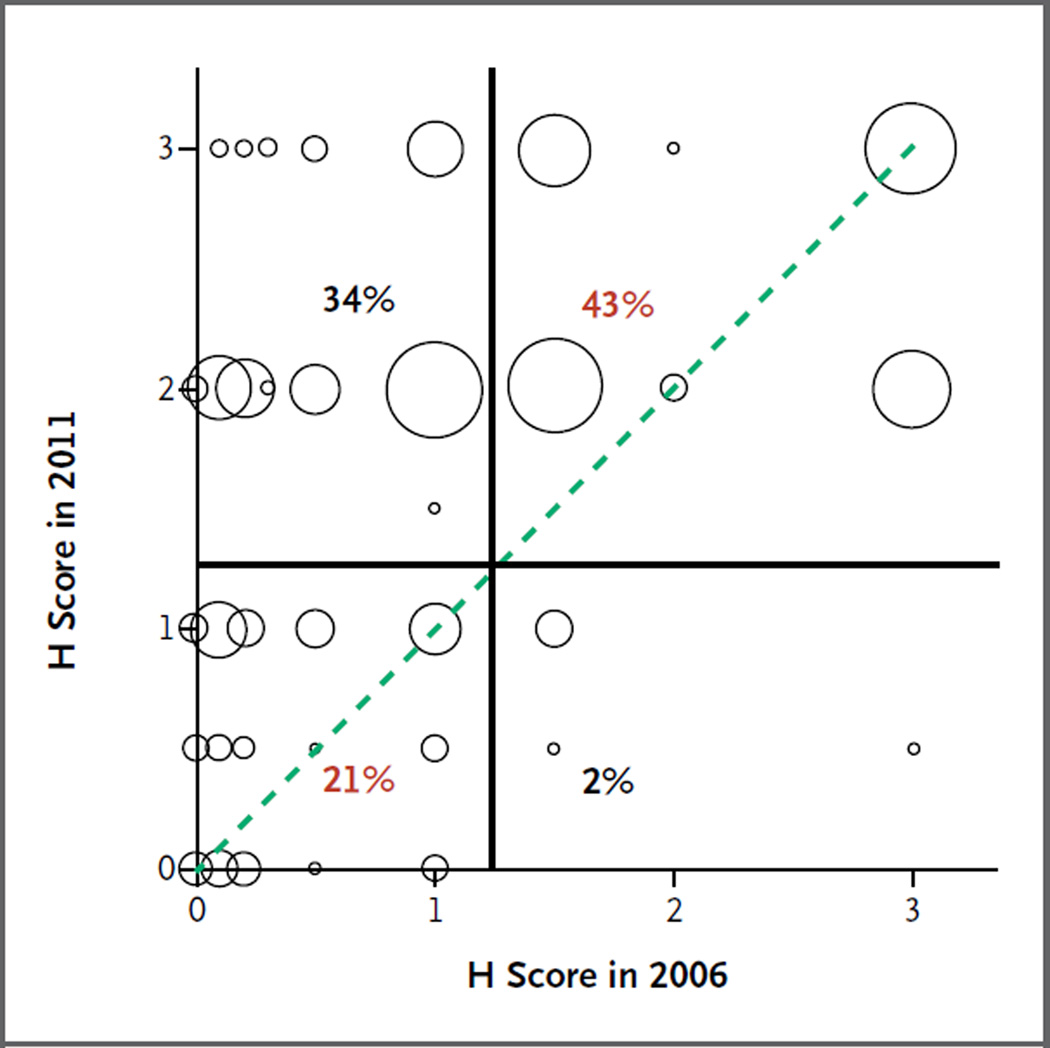
Repeated staining showed important discrepancies between the ERCC1 immunohistochemical results obtained in 2006 and those obtained in 2011 (Fig. S3 in the Supplementary Appendix). Although the overall ERCC1 H scores from the two analyses were concordant (correlation, 0.56), the scores were discordant in samples obtained from 36% of the patients (Fig. 2). This discordance involved mainly tumors classified as negative in the previous batch and as positive in the current batch. Among tumors classified as positive in 2011, the H scores in 2006 covered the whole range of possible values (0 to 3), including tumors classified as having very low levels of ERCC1 expression (Fig. 2).
Figure 2. Discrepancy in Results of Staining of Tumor Samples for ERCC1 between 2006 and 2011.
A correlation plot shows the ERCC1 H scores from our previously reported results in 2006 and from repeated staining with the use of a different 8F1 antibody batch in samples obtained from the same 589 patients in 2011. Each circle is proportional to the number of patients with a particular combination of ERCC1 H scores in the two studies. The circles on the dotted line indicate patients with tumors that had the same H score in the two studies. The H score reflects the staining intensity of nuclei and the percentage of positive nuclei; ERCC1-positive tumors were defined as those with an H score higher than 1. The vertical and horizontal lines represent the cutoff values used to discriminate between patients with tumors that were positive and those with tumors that were negative for ERCC1. Percentages of patients with concordant scores (red) and discordant scores (black) are shown.
Overall, repeated staining of samples from the IALT Biology trial suggested possible changes in the performance of the 8F1 antibody batches (e.g., increased sensitivity) between 2006 and 2011. It was impossible to perform a formal validation of the predictive value of ERCC1 protein reported previously because there were no antibodies left from the previous batch.
PROTEIN ISOFORMS DETECTED BY ERCC1 ANTIBODIES
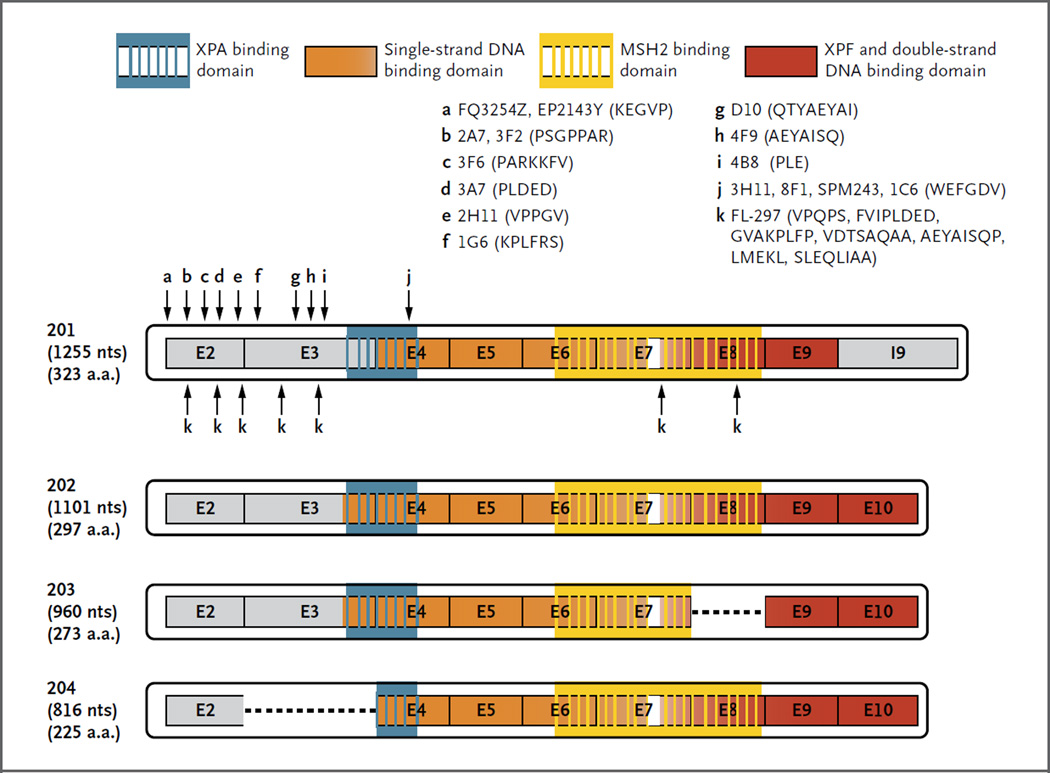
Some investigators have proposed using alternative antibodies to detect ERCC1, such as the rabbit polyclonal FL-297 antibody.26 In addition to 8F1 and FL-297, we investigated 14 other commercially available monoclonal antibodies for the detection of ERCC1. We performed epitope mapping with all 14, and in 4 of them, we tested the ability to discriminate ERCC1 protein isoforms by immunoblotting of isoform-specific protein extracts (Fig. 3, and Fig. S4A in the Supplementary Appendix). Strikingly, the 8F1, 3H11, SPM243, and 1C6 monoclonal antibodies all recognized the same conserved region of the ERCC1 protein (Fig. 3, and Fig. S4B in the Supplementary Appendix). These findings suggest that this region, which is present in all four protein isoforms, is highly immunogenic. The rabbit polyclonal antibody FL-297 bound seven different epitopes of the ERCC1 protein; thus, it also detects all four ERCC1 isoforms. None of the 16 antibodies was specific for only one ERCC1 isoform, and each of them recognized at least three different protein isoforms (Fig. 3).
Figure 3. Mapping of Peptide Sequences Recognized by ERCC1 Antibodies across Different Isoforms.
Recognition sites for 16 commercial antibodies (arrows) and corresponding amino acid sequences of the epitope are shown. XPA denotes xeroderma pigmentosum complementation group A protein, and XPF xeroderma pigmentosum complementation group F protein.
EXPRESSION OF ALL ERCC1 ISOFORMS IN NSCLC CELL LINES AND TUMOR SAMPLES
We analyzed the expression of ERCC1 isoforms using quantitative RT-PCR in various lung-cancer cell lines and in 123 tumor samples from a previous series of patients with resected NSCLC.39 The four isoforms were detected at the mRNA level both in cell lines (Fig. S5A in the Supplementary Appendix) and in the tumor samples (Fig. S5B and Table S4 in the Supplementary Appendix). Because of sequence homologies, it has not been possible to develop ERCC1-202–specific primers; hence, ERCC1-202 and ERCC1-204 isoforms were detected together. The heat map and two-dimensional hierarchical clustering revealed that the relative levels of isoform expression differed among tumors (Fig. S5B in the Supplementary Appendix). An analysis of survival according to the ERCC1-202 and ERCC1-204 mRNA level in the tumors suggested that the mRNA level was not associated with either overall survival (P = 0.76) (Fig. S5C in the Supplementary Appendix) or progression-free survival (P = 0.33) (Fig. S5D in the Supplementary Appendix). No significant interaction was found between ERCC1 isoform mRNA expression and the histologic subtype of NSCLC.
We also explored isoform expression at the protein level with the use of Western blot analysis in cell lines (Fig. S5E in the Supplementary Appendix) and samples obtained from patients with NSCLC (Fig. S5F in the Supplementary Appendix). In these samples, we detected ERCC1-202, ERCC1-203, and ERCC1-204 isoforms, but detection of ERCC1-201 was rare. Our results show that ERCC1 isoform transcripts and proteins are heterogeneously expressed in cell lines and tumor samples. It is therefore crucial to characterize their respective functionality with regard to repair of platinum–DNA adducts in order to avoid misclassification of tumors expressing a high level of nonfunctional ERCC1.
ERCC1-202 AND CELL SENSITIVITY TO CISPLATIN
We first knocked out the ERCC1 gene and obtained seven A549 clones without detectable ERCC1 protein by means of Western blot analysis (an 85 to 95% decrease in ERCC1 expression as compared with wild-type parental A549 cells) (Fig. S6A in the Supplementary Appendix) and with coding mutations in all genomic copies of the ERCC1 gene (Fig. S6B in the Supplementary Appendix). We observed a significant increase (40%) in the accumulation of platinum–DNA adducts in ERCC1-deficient cells as compared with wild-type parental cells, suggesting a reduction in the rate of nucleotide excision repair (Fig. S6C in the Supplementary Appendix). In addition, the 50% inhibitory concentration for cisplatin was reduced by a factor of seven as compared with the value in wild-type cells (Fig. S6D in the Supplementary Appendix). The decreased DNA repair function of ERCC1-deficient clones and subsequent reduction of the 50% inhibitory concentration confirmed the important role of ERCC1 in the repair of platinum–DNA adducts and cisplatin sensitivity.
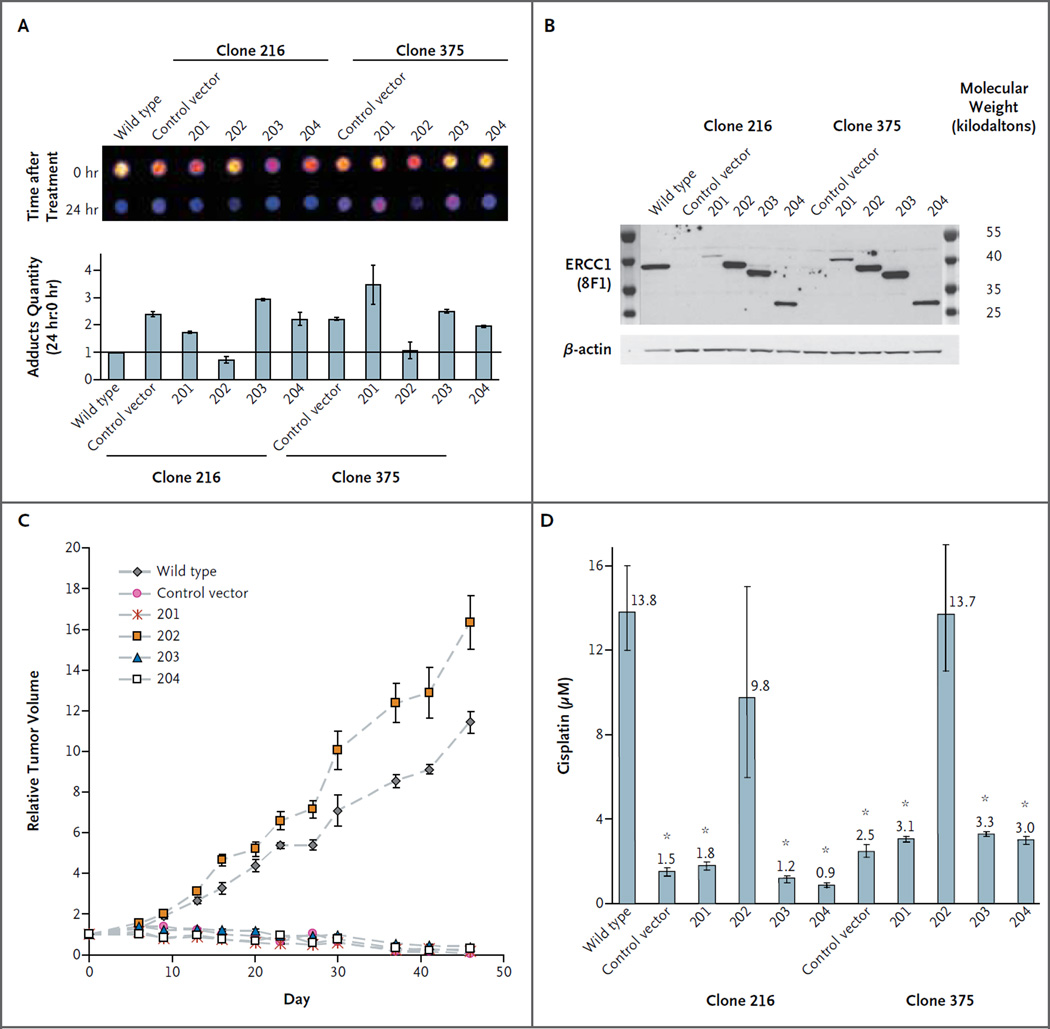
To create cells overexpressing a single isoform, two ERCC1-deficient clones, 216 and 375, were infected with lentiviral constructs coding the different isoforms (201, 202, 203, and 204) or an empty (control) vector. The subsequent evaluation of the efficiency of DNA repair in the isoform-expressing clones showed that the efficiency of repair was maintained only in cells with ectopic reexpression of the ERCC1-202 isoform. The reexpression of other isoforms did not speed up the reduction in the amount of cisplatin–DNA adducts (Fig. 4A).34 Control experiments of single expression of each isoform are shown in Figure 4B. After cisplatin treatment, cell resistance was rescued with ERCC1-202 isoform reexpression in both short-term experiments (48 hours) (Fig. 4C in the Supplementary Appendix) and long-term experiments (15 days) (Fig. S7A in the Supplementary Appendix). These observations were confirmed when we measured cisplatin-induced apoptosis, since only ERCC1-202 isoform reexpression averted activation of caspases 3 and 7 during cisplatin incubation (Fig. S7B in the Supplementary Appendix). The findings were similar with in vivo experiments in which the clones were injected into nude mice. Results in treated mice are shown in Figure 4D, and results in untreated mice are shown in Figure S7C in the Supplementary Appendix. These findings suggest that the ERCC1-202 isoform was the unique functional protein isoform.
Figure 4. Capacity of ERCC1 Protein Isoforms to Allow Nucleotide Excision Repair and Cisplatin Resistance.
Panel A shows the results of dot-blot analysis and quantification of removal of cisplatin–DNA adducts 24 hours after a 2-hour cisplatin treatment at 25 μM in A549 wild-type, ERCC1-deficient clones 216 and 375 (the control vector) and cells expressing single ERCC1 isoforms (201, 202, 203, or 204). The adduct quantity ratio (the ratio of the number of adducts at 24 hours to the number at 0 hours) in A549 wild-type cells was set at 1. Panel B shows control of isoform construct expression in established ERCC1 cell lines with 8F1 and β-actin antibodies. Panel C shows relative tumor volumes over the course of treatment after 106 ERCC1-deficient cells, with single isoform expression, were injected subcutaneously into nude mice. Twice weekly, tumors were measured, and cisplatin was injected intraperitoneally at a dose of 1 mg per kilogram of body weight. The I bars represent 95% confidence intervals. Panel D shows the results of a short-term growth assay for A549 wild-type, ERCC1-deficient, and isoform-expressing cells treated for 48 hours with increasing doses of cisplatin. The numbers above the bars represent the 50% inhibitory concentration for cisplatin. The stars indicate significant differences from wild-type cells (P<0.05). The I bars represent 95% confidence intervals.
DISCUSSION
The prognostic and predictive values of ERCC1 expression have been studied in many solid tumors. A number of clinical studies involving patients with NSCLC suggested that ERCC1 (measured by means of quantitative RT-PCR or immunohistochemical analysis) was a prognostic or a predictive biomarker (Table S1 in the Supplementary Appendix).5–16,18–23,25 In other studies, however, ERCC1 expression was not correlated with overall survival.17,24 In the present study, using immunohistochemical analysis, we were unable to validate ERCC1 protein expression as a predictive marker in a new, independent series of homogeneously treated patients with NSCLC. Three possible explanations for this lack of corroboration were postulated. First, the current tools used to evaluate ERCC1 expression are inadequate. Second, the level of biologic complexity has been underestimated (e.g., the respective roles of the four ERCC1 protein isoforms have not been correctly assessed). Third, the ERCC1 biomarker is not an efficient predictor of the benefit of cisplatin-based chemotherapy in patients.
The first explanation could originate from changes in the staining pattern between the initial and current analyses of samples from the IALT cohort (i.e., the more recent batch [671P907E] of the 8F1 antibody induced higher percentages of stained cells and an increase in the proportion of tumors with high staining intensity). Furthermore, exploratory testing of different cutoff points both in the IALT repeated-staining and validation data sets did not restore ERCC1 prognostic and predictive values. Therefore, ERCC1 validation cannot be obtained by changing the definition of positivity with the use of the ERCC1 H score.
Our own data previously suggested that the 8F1 antibody was specific for ERCC1.27 However, undisclosed characteristics of the antibody might have changed its specificity over the years, and the differences between the two 8F1 batches could be related to distinct antibody titration, affinity, purity, or even epitope recognition. The unavailability of the original batch (671P210) made it impossible to determine the exact nature of changes in antibody performance. Similar discrepancies related to antibody modifications have been described previously for other targets such as human epidermal growth factor receptor type 2, with changes in the concentration of the specific A0485 antibody (Dako) or estrogen receptor with incorrect estrogen-receptor tests in Canada between 1997 and 2005.40 It appears that beyond producer and clone references, batch numbers are also key elements to define an antibody. Clear guidelines that mandate the precise definition of these components are needed for publications on immunohistochemical biomarker studies. Irrespective of production changes in antibodies over the years, recent studies have shown that the current 8F1 antibody detects a non-ERCC1 target.30 Although only nuclear ERCC1 is scored by pathologists, such cross-reactivity could introduce unexpected variability in staining in validation studies.
The present study also showed that most ERCC1 antibodies detected all the identified protein isoforms, and we suspect that the expression of nonfunctional ERCC1 isoforms may lead to potential artifacts, with discrepant results. In the present study, we established ERCC1-deficient cells (in a single cell-line model [A549]) that showed a high sensitivity to cisplatin and a low rate of repair of cisplatin–DNA adducts. Only the reintroduction of the ERCC1-202 isoform rescued nucleotide excision repair activity and the capacity to repair cisplatin-induced DNA damage. These data provide important insights into the relative function of ERCC1 isoforms for removal of cisplatin–DNA adducts and the way in which they might influence patient survival. Since none of the ERCC1 antibodies used by different investigators distinguished functional from nonfunctional isoforms, it might be difficult to validate the correlation between the level of ERCC1 expression and overall survival on the basis of immunohistochemical detection. Abundant expression of one or several nonfunctional isoforms may lead to a false classification of the tumor as ERCC1-positive.
In terms of patient classification and therapeutic applications, our results suggest that evaluation of the expression of the unique functional isoform (ERCC1-202) might constitute a more accurate predictor of cisplatin benefit in patients with NSCLC than any other current approach. However, the development of such a tool that specifically distinguishes and measures the functional ERCC1 protein isoform remains a challenge because of strong homology among the four protein isoforms (Fig. 3), which precludes the generation of an ERCC1-202 isoform–specific antibody and of specific primers and probes for quantitative RT-PCR or in situ hybridization. These results have to be considered carefully in ongoing prospective trials of ERCC1 status in NSCLC.
In conclusion, our study highlights the technical biases that can interfere with the previously reported use of the ERCC1 immunohistochemical signal as a predictive biomarker of adjuvant platinum-based chemotherapy. Currently available antibodies do not have adequate discrimination for therapeutic decision making regarding cisplatin-containing treatment in patients with NSCLC, which requires the specific detection of the unique functional isoform of ERCC1 — ERCC1-202. These results underscore the importance of assessing multiple isoforms and their function in biomarker studies. Functional assays may be required to detect an authentic prognostic influence of DNA repair proteins.3
Supplementary Material
Supplementary Data
Acknowledgments
Supported by an unrestricted grant from Eli Lilly and by grants from Institut National du Cancer Programme National d’Excellence Spécialisé and Programme Hospitalier de Recherche Clinique (to the International Adjuvant Lung Cancer Trial Biology); by La Ligue Nationale Contre le Cancer and an unrestricted grant from Sanofi-Aventis (to the Lung Adjuvant Cisplatin Evaluation Biology project); by a contract (FP7-2007-13) from the European union Seventh Framework Program under a grant agreement (HEALTH-F2-2010-258677-CURELUNG); and by a translational research fellowship from Roche (to Dr. Friboulet).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank Cédric Verjat for assistance with an earlier version of the figures and John Lyons for helpful suggestions.
REFERENCES
- 1.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 2.The NSCLC Meta-analyses Collaborative Group. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet. 2010;375:1267–1277. doi: 10.1016/S0140-6736(10)60059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postel-Vinay S, Vanhecke E, Olaussen KA, Lord CJ, Ashworth A, Soria JC. The potential of exploiting DNA-repair defects for optimizing lung cancer treatment. Nat Rev Clin Oncol. 2012;9:144–155. doi: 10.1038/nrclinonc.2012.3. [DOI] [PubMed] [Google Scholar]
- 4.Bergstralh DT, Sekelsky J. Interstrand crosslink repair: can XPF-ERCC1 be let off the hook? Trends Genet. 2008;24:70–76. doi: 10.1016/j.tig.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Zheng Z, Chen T, Li X, Haura E, Sharma A, Bepler G. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N Engl J Med. 2007;356:800–808. doi: 10.1056/NEJMoa065411. [DOI] [PubMed] [Google Scholar]
- 6.Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 7.Lord RV, Brabender J, Gandara D, et al. Low ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin Cancer Res. 2002;8:2286–2291. [PubMed] [Google Scholar]
- 8.Roth JA, Carlson JJ. Prognostic role of ERCC1 in advanced non-small-cell lung cancer: a systematic review and meta- analysis. Clin Lung Cancer. 2011;12:393–401. doi: 10.1016/j.cllc.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobo M, Isla D, Massuti B, et al. Customizing cisplatin based on quantitative excision repair cross-complementing 1 mRNA expression: a phase III trial in non-small-cell lung cancer. J Clin Oncol. 2007;25:2747–2754. doi: 10.1200/JCO.2006.09.7915. [DOI] [PubMed] [Google Scholar]
- 10.Simon GR, Sharma S, Cantor A, Smith P, Bepler G. ERCC1 expression is a predictor of survival in resected patients with non-small cell lung cancer. Chest. 2005;127:978–983. doi: 10.1378/chest.127.3.978. [DOI] [PubMed] [Google Scholar]
- 11.Choi CM, Yang SC, Jo HJ, et al. Proteins involved in DNA damage response pathways and survival of stage I nonsmall- cell lung cancer patients. Ann Oncol. 2012;23:2088–2093. doi: 10.1093/annonc/mdr606. [DOI] [PubMed] [Google Scholar]
- 12.Seyhan EC, Altin S, Cetinkaya E, et al. Prognostic significance of ERCC1 expression in resected non small cell lung carcinoma. Ann Thorac Cardiovasc Surg. 2011;17:110–117. doi: 10.5761/atcs.oa.09.01526. [DOI] [PubMed] [Google Scholar]
- 13.Okuda K, Sasaki H, Dumontet C, et al. Expression of excision repair cross-complementation group 1 and class III beta-tubulin predict survival after chemotherapy for completely resected non-small cell lung cancer. Lung Cancer. 2008;62:105–112. doi: 10.1016/j.lungcan.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Tseden-Ish M, Choi YD, Cho HJ, et al. Disease-free survival of patients after surgical resection of non-small cell lung carcinoma and correlation with excision repair cross-complementation group 1 expression and genotype. Respirology. 2012;17:127–133. doi: 10.1111/j.1440-1843.2011.02060.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee KH, Min HS, Han SW, et al. ERCC1 expression by immunohistochemistry and EGFR mutations in resected non-small cell lung cancer. Lung Cancer. 2008;60:401–407. doi: 10.1016/j.lungcan.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Vilmar AC, Santoni-Rugiu E, Sørensen JB. ERCC1 and histopathology in advanced NSCLC patients randomized in a large multicenter phase III trial. Ann Oncol. 2010;21:1817–1824. doi: 10.1093/annonc/mdq053. [DOI] [PubMed] [Google Scholar]
- 17.Ota S, Ishii G, Goto K, et al. Immunohistochemical expression of BCRP and ERCC1 in biopsy specimen predicts survival in advanced non-small-cell lung cancer treated with cisplatin-based chemotherapy. Lung Cancer. 2009;64:98–104. doi: 10.1016/j.lungcan.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Zhao J, Yang L, et al. Positive expression of ERCC1 predicts a poorer platinum-based treatment outcome in Chinese patients with advanced non-small-cell lung cancer. Med Oncol. 2010;27:484–490. doi: 10.1007/s12032-009-9239-3. [DOI] [PubMed] [Google Scholar]
- 19.Holm B, Mellemgaard A, Skov T, Skov BG. Different impact of excision repair cross-complementation group 1 on survival in male and female patients with inoperable non-small-cell lung cancer treated with carboplatin and gemcitabine. J Clin Oncol. 2009;27:4254–4259. doi: 10.1200/JCO.2008.18.8631. [DOI] [PubMed] [Google Scholar]
- 20.Hubner RA, Riley RD, Billingham LJ, Popat S. Excision repair cross-complementation group 1 (ERCC1) status and lung cancer outcomes: a meta-analysis of published studies and recommendations. PLoS One. 2011;6(10):e25164. doi: 10.1371/journal.pone.0025164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S, Zhang J, Wang R, Luo X, Chen H. The platinum-based treatments for advanced non-small cell lung cancer, is low/ negative ERCC1 expression better than high/positive ERCC1 expression? A metaanalysis. Lung Cancer. 2010;70:63–70. doi: 10.1016/j.lungcan.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds C, Obasaju C, Schell MJ, et al. Randomized phase III trial of gemcitabine-based chemotherapy with in situ RRM1 and ERCC1 protein levels for response prediction in non-small-cell lung cancer. J Clin Oncol. 2009;27:5808–5815. doi: 10.1200/JCO.2009.21.9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S, Li Q, Zhang Q, et al. Expression of ERCC1 and class III β-tubulin in resected non-small cell lung cancer and its correlation with platinum-based adjuvant chemotherapy. Int J Biol Markers. 2010;25:141–149. doi: 10.1177/172460081002500304. [DOI] [PubMed] [Google Scholar]
- 24.Booton R, Ward T, Ashcroft L, Morris J, Heighway J, Thatcher N. ERCC1 mRNA expression is not associated with response and survival after platinum-based chemotherapy regimens in advanced non-small cell lung cancer. J Thorac Oncol. 2007;2:902–906. doi: 10.1097/JTO.0b013e318155a637. [DOI] [PubMed] [Google Scholar]
- 25.Bepler G, Olaussen KA, Vataire AL, et al. ERCC1 and RRM1 in the International Adjuvant Lung Trial by automated quantitative in situ analysis. Am J Pathol. 2011;178:69–78. doi: 10.1016/j.ajpath.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niedernhofer LJ, Bhagwat N, Wood RD. ERCC1 and non-small-cell lung cancer. N Engl J Med. 2007;356:2538–2541. doi: 10.1056/NEJMc070742. [DOI] [PubMed] [Google Scholar]
- 27.Olaussen KA, Fouret P, Kroemer G. ERCC1-specific immunostaining in non- small-cell lung cancer. N Engl J Med. 2007;357:1559–1561. doi: 10.1056/NEJMc072007. [DOI] [PubMed] [Google Scholar]
- 28.Bhagwat NR, Roginskaya VY, Acquafondata MB, Dhir R, Wood RD, Niedernhofer LJ. Immunodetection of DNA repair endonuclease ERCC1-XPF in human tissue. Cancer Res. 2009;69:6831–6838. doi: 10.1158/0008-5472.CAN-09-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olaussen KA, Soria JC. Validation of ERCC1-XPF immunodetection — letter. Cancer Res. 2010;70:3851–3852. doi: 10.1158/0008-5472.CAN-09-4352. [DOI] [PubMed] [Google Scholar]
- 30.Ma D, Baruch D, Shu Y, et al. Using protein microarray technology to screen anti-ERCC1 monoclonal antibodies for specificity and applications in pathology. BMC Biotechnol. 2012;12:88. doi: 10.1186/1472-6750-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sijbers AM, van der Spek PJ, Odijk H, et al. Mutational analysis of the human nucleotide excision repair gene ERCC1. Nucleic Acids Res. 1996;24:3370–3380. doi: 10.1093/nar/24.17.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dabholkar M, Vionnet J, Parker R, Bostickbruton F, Dobbins A, Reed E. Expression of an alternatively spliced ERCC1 messenger-RNA species, is related to reduced DNA-repair efficiency in human T-lymphocytes. Oncol Rep. 1995;2:209–214. doi: 10.3892/or.2.2.209. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Li T, Ma K, et al. The impacts of ERCC1 gene exon VIII alternative splicing on cisplatin-resistance in ovarian cancer cells. Cancer Invest. 2009;27:891–897. doi: 10.3109/07357900902744536. [DOI] [PubMed] [Google Scholar]
- 34.Liedert B, Pluim D, Schellens J, Thomale J. Adduct-specific monoclonal antibodies for the measurement of cisplatin-induced DNA lesions in individual cell nuclei. Nucleic Acids Res. 2006;34(6):e47. doi: 10.1093/nar/gkl051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non- small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 36.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 37.Strauss GM, Herndon JE, II, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26:5043–5051. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reiman T, Lai R, Veillard AS, et al. Cross-validation study of class III betatubulin as a predictive marker for benefit from adjuvant chemotherapy in resected non-small-cell lung cancer: analysis of four randomized trials. Ann Oncol. 2012;23:86–93. doi: 10.1093/annonc/mdr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friboulet L, Barrios-Gonzales D, Commo F, et al. Molecular characteristics of ERCC1-negative versus ERCC1-positive tumors in resected NSCLC. Clin Cancer Res. 2011;17:5562–5572. doi: 10.1158/1078-0432.CCR-11-0790. [DOI] [PubMed] [Google Scholar]
- 40.Hede K. Breast cancer testing scandal shines spotlight on black box of clinical laboratory testing. J Natl Cancer Inst. 2008;100:836–837. 844. doi: 10.1093/jnci/djn200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data