mTORC1 controls the adaptive transition of quiescent stem cells from G0 to GAlert (original) (raw)
. Author manuscript; available in PMC: 2014 Dec 19.
Published in final edited form as: Nature. 2014 May 25;510(7505):393–396. doi: 10.1038/nature13255
Abstract
A unique property of many adult stem cells is their ability to exist in a non-cycling, quiescent state1. Although quiescence serves an essential role in preserving stem cell function until the stem cell is needed in tissue homeostasis or repair, defects in quiescence can lead to an impairment in tissue function2, the extent to which stem cells can regulate quiescence is unknown. Here, we show that the stem cell quiescent state is composed of two distinct functional phases: G0 and an “alert” phase we term GAlert, and that stem cells actively and reversibly transition between these phases in response to injury-induced, systemic signals. Using genetic models specific to muscle stem cells (or satellite cells (SCs)), we show that mTORC1 activity is necessary and sufficient for the transition of SCs from G0 into GAlert and that signaling through the HGF receptor, cMet is also necessary. We also identify G0-to-GAlert transitions in several populations of quiescent stem cells. Quiescent stem cells that transition into GAlert possess enhanced tissue regenerative function. We propose that the transition of quiescent stem cells into GAlert functions as an ‘alerting’ mechanism, an adaptive response that positions stem cells to respond rapidly under conditions of injury and stress without requiring cell cycle entry or a cell fate commitment.
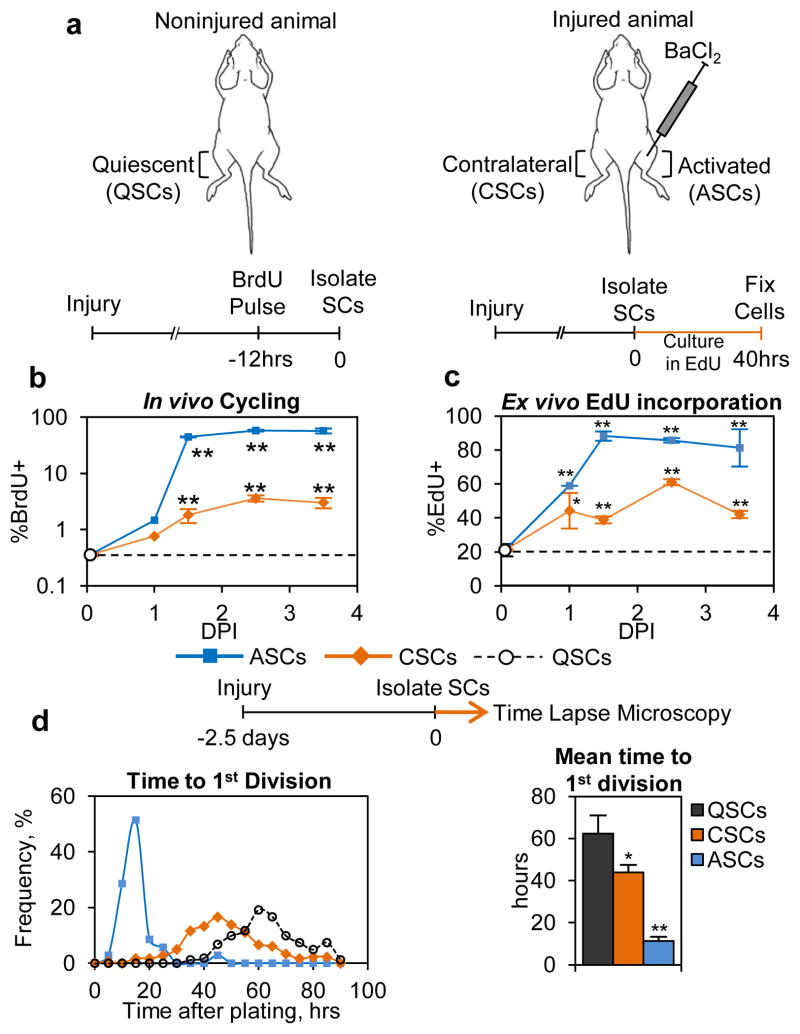
Adult stem cells have historically been presumed to exist in one of two states: 1) the quiescent state in which the cell is not actively cycling; and 2) the activated state where the cell has committed to or is in the cell cycle3–4. In contrast to the cell cycle, which can be sub-divided into distinct phases, quiescence is less well characterized. Emerging data suggest that stem cells can regulate quiescent functional properties 5–6. While studying the regulation of the transition of SCs from the quiescent to the activated state, we made a curious observation: SCs in a muscle contralateral to the muscle in which we induced an injury responded to that distant injury and had cycling properties that were different from those in a noninjured animal (QSCs) and from the injured tissue (ASCs) (Fig. 1a). Using the Pax7CreER driver and Rosa26EYFP lineage tracer to specifically label SCs7–8 (Extended Data Fig. E1a), we found that these contralateral SCs (CSCs) showed markedly increased, but overall still low, propensity to cycle when compared to QSCs, as measured by BrdU incorporation in vivo (Fig. 1b). Upon isolation and culturing ex vivo, CSCs displayed accelerated cell cycle entry as measured by EdU incorporation and time required to complete the first cell division compared to QSCs (Figs. 1c, d). Subsequent cell divisions of progeny of CSCs and QSCs occurred at similar rates to those of ASCs (Fig. E1b). The response of CSCs was not limited to muscle groups directly contralateral to the injury or to the agent of muscle injury (Figs. E1c–e).
Figure 1. Satellite cells distant from the site of injury have different cell cycle kinetics than quiescent and activated satellite cells.
(a) Schematic representation of the location of QSCs, CSCs, and ASCs in relation to muscle injury. (b) CSCs have greater propensity to cycle in vivo than do QSCs (n≥3; significance is versus QSCs). (c) Higher percentages of CSCs incorporate EdU after 40 hrs than QSCs. Data from a representative experiment is presented (n≥2; significance is versus QSCs). (d) CSCs require less time to compete the first division (n=3). Details on data presentation and sample size can be found in the Methods Summary and Supplemental Methods Sections.
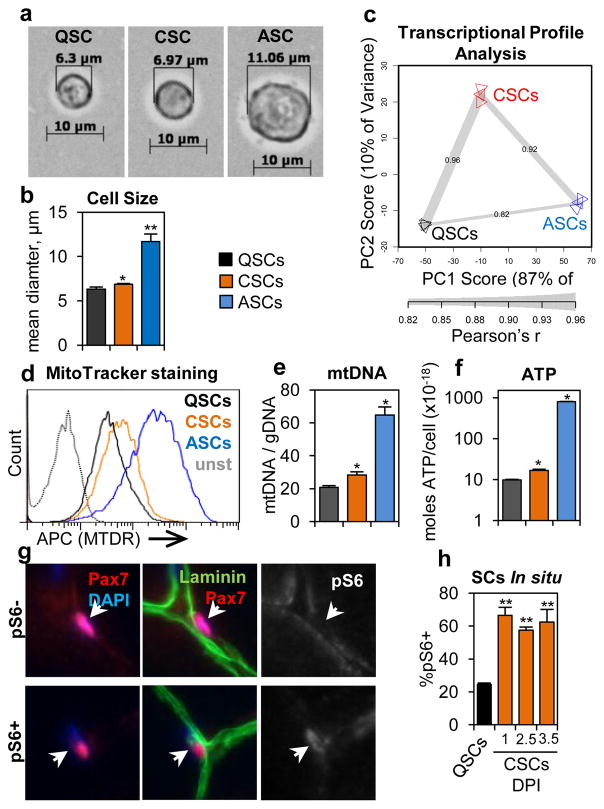
One of the most obvious changes in ASCs is a dramatic increase in cell size relative to QSCs (Fig. 2a). We found that CSCs displayed a very slight, but significant, increase in cell size relative to QSCs (Figs. 2a–b, E2a–b). Similarly, we also observed that CSCs had stronger EYFP intensity from the Rosa26EYFP reporter, elevated levels of Pyronin Y staining, and incorporation of the ribonucleotide EU compared to QSCs (Figs. E2c–e), which suggests increased transcriptional activity. Principle component analysis (PCA) of the transcriptional profiles of QSCs, CSCS, and ASCs showed that CSCs fall between QSCs and ASCs along the first component axis (PC1) (Fig. 2c). Transcriptionally, CSCs were highly correlated with both QSCs and ASCs, more strongly than QSCs and ASCs were correlated (Fig. 2c), which also suggests that CSCs are intermediate between QSCs and ASCs. However, detailed ICC analysis immediately after isolation showed that CSCs are phenotypically more similar to QSCs (Figs. E2f–i). To test if CSCs represent a population of stem cells or a population of committed progenitor cells, we performed transplantation and pulse-chase experiments and found no difference in the engraftment efficiency and capacity for self-renewal between CSCs and QSCs (Fig. E2j, k). Together, these data suggest that CSCs are similar to, but distinct from, QSCs and possess the stem cell characteristics of QSCs.
Figure 2. Satellite cells that are distant from an injury have become ‘alert.’.
(a) Representative images of QSCs, CSCs, ASCs immediately after isolation. (b) CSCs are larger than QSCs (n=3). (c) CSCs have a transcriptional profile that is intermediate between QSCs and ASCs (along PC1) as shown by PCA and Pearson’s r values (n=3). (d) Increased mitochondrial activity in CSCs compared to QSCs. (representative FACS plot, n=4). (e) CSCs have increased mtDNA content relative to QSCs (n≥3). (f) CSCs have more intracellular ATP then QSCs (n=4). (g) IF-IHC staining of TA muscle showing representative pS6− and pS6+ SCs. (h) Quantification of IF-IHC staining for pS6 in SCs (n≥3; significance is versus noninjured).
To gain further insight into what distinguishes CSCs from QSCs, we analyzed the molecular pathways enriched in genes induced in the CSC transcriptome relative to the QSC expression profile. We found that two annotation groups were significantly enriched in genes upregulated in CSCs relative to QSCs: cell cycle and mitochondrial metabolism (Fig. E3a). To further investigate mitochondrial metabolism in CSCs, we performed MitoTracker Deep Red (MTDR) staining and measured mtDNA content and found that, relative to QSCS, CSCs indeed displayed evidence of elevated mitochondrial activity (Figs. 2d, e). Along these lines, and keeping with the increase in cell size, we also found that CSCs have increased levels of cellular ATP (Figs. 2f, E3b–d).
Collectively these data describe a set of properties that distinguishes CSCs from QSCs and ASCs: kinetics of cell cycle entry, propensity to cycle, cell size, transcriptional activity, and mitochondrial metabolism. Importantly, CSCs, like QSCs, are still quiescent in that, as a population, the vast majority of CSCs are not actively cycling. Because the injury-induced phenotype of CSCs is intermediate between QSCs and ASCs, we refer to CSCs as ‘alert’ SCs and the set of properties that distinguishes these cells as the ‘alert’ phenotype. The characteristics of this alert phenotype described above have a common thread in that they have all been previously linked, in other systems, to the mTORC1 signaling pathway (reviewed in9). Indeed, we observed induction of phospho-S6 (pS6), a surrogate of mTORC1 activity, in alert SCs (Figs. 2g–h, E3e–g). Taking this a step further, we found that by sorting SCs for properties of the alert state (Fig. E3h) we enriched for a population of pS6+ SCs that also possessed the other attributes of the alert state (elevated propensity to cycle and reduced time to first division) (Figs. E3i–m). Together these data show that there is a strong correlation between activation of mTORC1 signaling and the alert phenotype in SCs.
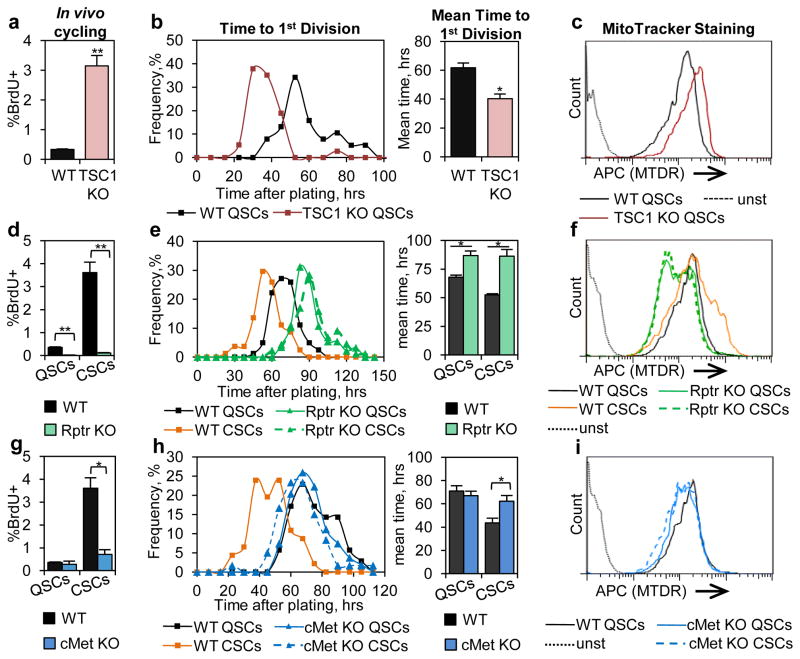
To test if any aspects of the alert response were directly regulated by mTORC1 signaling, we used the Pax7CreER driver to specifically ablate TSC1, an inhibitor of mTORC1 signaling, in SCs. As a genetic model of mTORC1 activation10, TSC1 KO QSCs displayed induction of mTORC1 activity (Figs. E4a–b). Importantly, TSC1 KO QSCs also displayed all aspects of the alert phenotype in an otherwise noninjured context: increased propensity to cycle, accelerated cell cycle entry, increased MTDR staining, and increased cell size (Figs. 3a–c, E4c). To test whether the alert response requires mTORC1, we used a conditional allele of Raptor (Rptr)11, an essential component of the mTORC1 signaling complex, with the Pax7CreER driver to specifically ablate Raptor protein and suppress mTORC1 signaling in SCs (Figs. E4b, E5a–c). Overall, we found that Rptr KO SCs contralateral to a muscle injury were completely unresponsive and did not manifest any characteristics of an alert SC (Figs. 3d–f, E5d–e). These data combined show that mTORC1 signaling in SCs is necessary and sufficient for the alert response.
Figure 3. Activation of mTORC1 is necessary and sufficient for the alert phenotype.
TSC1 KO QSCs display characteristics of alert SCs: (a) increased propensity to cycle in vivo (n≥6); (b) reduced time to first division (n=3); and (c) increased mitochondrial activity (representative FACS plotn=3). Rptr KO suppresses induction of the alert state. Rptr KO CSCs show no differences in: (d) propensity to cycle in vivo (n≥6); (e) time to first division (n=3); and (f) mitochondrial activity (representative FACS plot, n=3). cMet KO CSCs show no injury-induce regulation of: (g) propensity to cycle in vivo (n≥4); (h) time to first division (n≥3); and (i) mitochondrial activity (representative FACS plot, n=3).
Next, we focused on the signals upstream of mTORC1 which initiate the alert response and which are regulated by injury. Latent Hepatocyte Growth Factor (HGF) is found in the extracellular matrix of many tissues, upon injury, it is activated by serum proteases12–13. Active HGF can regulate mTORC1 via PI3K-Akt signaling14. Furthermore HGF is known to influence SC behavior15–16. To test if HGF signaling has a role in the alert response, we used a conditional allele of the HGF receptor, cMet, to suppress HGF signaling in SCs17. Ablation of cMet in SCs completely blocked the activation of mTORC1 signaling, as measured by pS6 staining, in cultured SCs and in vivo in CSCs following injury (Figs. E4b, E5f–g). Consistent with our hypothesis that mTORC1 activation is required for the alert response in SCs, cMet KO CSCs did not exhibit any functional response to injury (Figs. 3g–i, E5h). Collectively, these data suggest that signaling downstream of cMet is critical for the induction of the alert response in SCs.
Following tissue repair after injury, activity of the HGF activation cascade gradually subsides12. We found the frequency of pS6+ CSCs following a distant injury declined to a level similar to that of noninjured animals 28 days after injury (Fig. E6a). Interestingly, we found that, also at 28 DPI, the propensity to cycle and cell cycle entry kinetics of CSCs also returned to those of QSCs (Figs. E6b, c). Furthermore, the transcriptional profile of CSCs 28 DPI had returned to that of QSCs (Fig. E6d). These data suggest that the alert state is reversible and that the functional and transcriptional changes in alert CSCs that occur downstream of mTORC1 revert to the properties of QSCs when mTORC1 activity subsides.
To gain further understanding of the molecular pathways underlying the functional transition into the alert state, we analyzed the transcriptional profiles from the SC-specific genetic models described above. We found that induction of genes involved in mitochondrial metabolism strongly correlated with the ability to transition into the alert state: wild-type CSCs and TSC1 KO QSCs show induction and Rptr KO and cMet KO CSCs do not (Figs. E3a, E7a–e). These data suggest that regulation of mitochondrial metabolism is a crucial aspect of stem cell quiescence.
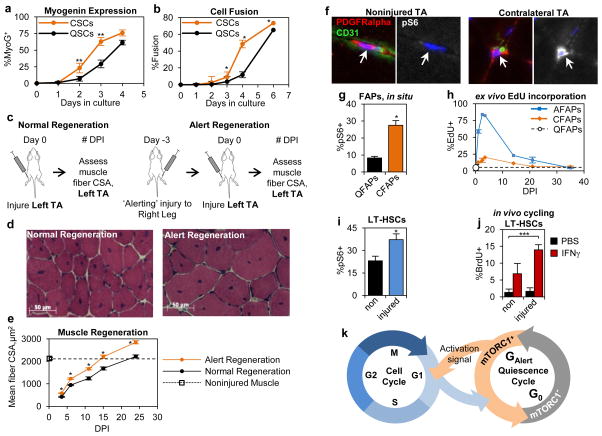
The function of SCs in response to injury is to proliferate, differentiate, and form new muscle tissue18–19. As such, we tested whether the functional changes of CSCs affected their differentiation and muscle regenerative abilities. Following isolation and culturing ex vivo, CSCs displayed enhanced kinetics of differentiation as measured by expression of MyoG and cell fusion (Figs. 4a, b, E8a). To translate these observations in vivo, we assessed the ability of CSCs to participate in muscle regeneration. Three days prior to injury of the left TA muscle, we performed an ‘alerting’ injury to the right limb to transition SCs in the left TA into the alert state (Fig. 4c). We found that animals that received an ‘alerting’ injury displayed dramatically enhanced muscle regeneration at all time points following injury when compared to the normal muscle regenerative process (Figs. 4d, e). These data show that the functional properties of alert SCs translates into enhanced muscle regenerative ability in response to injury.
Figure 4. Stem cells in the alert state have enhanced functional properties.
(a–b) CSCs have enhanced kinetics of myogenic differentiation ex vivo. They rapidly (a) express become MyoG+ and (b) fuse (n=3; significance is versus QSCs at same time point). (c) Schematic depiction of ‘alert’ regeneration experimental design. (d–e) A prior ‘alerting’ injury enhances the progress of muscle regeneration: (d) representative histological section and (e) quantification of nascent, centrally nucleated muscle fiber CSA (n≥3). (f–h) FAPs adopt characteristics of the alert state: (f) higher frequency of pS6+ FAPs in muscles contralateral to injury (representative IF-IHC staining); (g) quantification of pS6 staining (n=4); and (h) accelerated kinetics of cell cycle entry (n≥2). (i–j) LT-HSCs display characteristics of the alert state in response to muscle injury: (i) increased frequency of pS6 staining (n≥4); and (j) enhanced activation response to IFNγ (n≥3; *** p<0.001). (k) Model depicting quiescence cycle of G0 and GAlert phases.
The dramatically enhanced muscle regenerative function of CSCs prompted us to investigate other conditions which may induce the alert state in SCs. We found that SCs adopted functional aspects of the alert response to bone injuries and to minor skin wounds (Figs. E8b, c), injuries for which the role of SCs is not apparent. These data suggest that SCs can adopt the alert state in response to multiple types of injuries and may be a general response of SCs to injury. Therefore, we tested if other populations of quiescent stem cells could similarly adopt properties of the alert state. We found that fibro-adipogenic progenitors (FAPs), a resident mesenchymal stem cell population in skeletal muscle20–21, responded much like SCs. CFAPs (FAPs in muscles of a limb contralateral to the site of muscle injury) displayed an induction of mTORC1 signaling, accelerated cell cycle entry, increased propensity to cycle, and increased cell size when compared to quiescent FAPs from noninjured animals (QFAPs) (Figs. 4f–h, E9a–c). Additionally, we found that long term hematopoietic stem cells (LT-HSCs) displayed activation of mTORC1 signaling in response to muscle injury (Figs. 4i, E9d). To test if mTORC1 activation in LT-HSCs caused increased functional potential, as it does in SCs, we then administered interferon-gamma (IFNγ), to the animals to stimulate LT-HSC activation22. Interestingly and similar to the effect of an ‘alerting’ injury on muscle regeneration, LT-HSCs primed by muscle injury were more sensitive to IFNγ and yielded a more robust response (Fig. 4j). Notably, and similar to what we demonstrated in SCs, the induction of mTORC1 in HSCs increases their mitochondrial activity23–24, which is consistent with a transition into the alert state. Collectively, these data suggest that activation of mTORC1 signaling in quiescent stem cells alters their properties, endowing them with enhanced functional potential, an alerting mechanism, should the stem cell be required in tissue repair.
As it relates to stem cell biology, the data we present here suggest that stem cells undergo dynamic transitions between functional phases in the quiescent state. We propose a model in which GAlert and G0 form a quiescence cycle (Fig. 4k). While it is clear that not all quiescent cells are functionally equivalent25–26, the in vivo relevance and the molecular mechanisms regulating functionally distinct states have not been elucidated. We propose that mTORC1 activity is a distinguishing aspect of at least two distinct phases within quiescence. Here, we demonstrate how these phases of stem cell quiescence in vivo are regulated in the context of physiological conditions by mTORC1 (and, for SCs, by cMet). Most importantly, our data suggest that the ability to transition between G0 and GAlert is critical to the positioning of stem cell populations to be able to respond rapidly in tissue homeostasis and repair while maintaining a pool of deeply quiescent, reserve stem cells. This represents a novel form a cellular memory, an adaptive response akin to that in neuronal or immune cells, in which prior experience influences future responses.
Methods Summary
Unless stated otherwise, in the figure legend, all graphical data are presented as mean ± SEM, except histograms, and significance was calculated using two-tailed unpaired Student _t_-tests: * denoting P < 0.05, ** denoting P < 0.01. Where sample size (n values) are reported as a range, exact sample size values can be found in Supplemental Methods. Time to first division experiments are presented as a representative histogram plotting data from individual cells and, on the right, as a bar graph depicting the quantitative analysis of the mean time to first division in replicate experiments.
Supplementary Material
1
Figure E1. SCs distant from the site of an injury display a functional response to the injury.
(a) Representative FACS plot from isolation of EYFP+ SCs from 10-week-old Pax7CreER/+; Rosa26EYFP/+ mice 3 weeks following TMX. Mononuclear cells from muscle digests are gated in FITC and Pac-Blue (autofluorescence) channels to separate EYFP+ SCs. EYFP+ SCs are typically 2–4% of all events from muscle digestions. (b) Progeny of CSCs and QSCs take comparable times to complete the second cell division. Analysis of the time required to complete the second division (QSCs 10.2±2 hrs; n=148 cells, CSCs 10.9±2 hrs; n=155), following the first cell cycle (Fig. 1d), shows that accelerated cell cycle kinetics of CSCs is limited to the first division. (c) SCs throughout the body increase in propensity to cycle in response to injury. In injured animals, SCs isolated from indicated muscle groups show higher frequency of BrdU incorporation when compared to SCs from the same muscle groups from noninjured mice. (n≥2 animals) (d) Muscle crush injuries increase the in vivo cycling propensity of CSCs. Twelve hours after BrdU pulse labeling, SCs isolated from TA and Gast muscles contralateral to muscle crush injury show elevated BrdU labeling frequency versus SCs from those muscles from noninjured mice. (mean ± SEM; non n=5 animals, muscle crush n=3; ** p<0.01). (e) SCs contralateral to a muscle crush injury have increased cell cycle entry kinetics. 2.5 DPI, SCs contralateral to a muscle crush injury incorporate EdU more rapidly than QSCs when cultured ex vivo for 40 hours (mean ± SEM; n=3 animals; * p<0.05).
Figure E2. CSCs are distinct from QSCs but retain stem cell characteristics.
(a) CSCs are slightly larger than QSCs and much smaller than ASCs. Immediately after isolation, analysis of cell diameters of QSCs, 2.5 DPI CSCs, and 2.5 DPI ASCs, measured by phase contrast microscopy, shows that CSCs have a distribution that is shifted to the right compared to QSCs (histographic representation of data displayed in Figures 2a–b). (b) CSCs are larger than QSCs as measured by the FSC parameter by FACS (representative FACs plot, similar results observed in 4 independent experiments). (c) CSCs have elevated intensity of EYFP reporter. FACS analysis of EYFP intensity in the FITC channel shows that 2.5 DPI CSCs display a slight shift in EYFP distribution relative to QSCs, suggesting increased expression of this reporter from the Rosa26 locus (representative FACS plot, similar results observed in 4 independent experiments). (d) CSCs show elevated levels of pyronin Y staining, suggesting an increased RNA content relative to QSCs, but dramatically less than ASCs (representative FACS plot, similar results observed in 4 independent experiments). (e) CSCs increase global transcriptional activity compared with QSCs. FACS analysis of EU incorporation, following pulse labeling by I.P. injection, shows that 2.5 DPI CSCs have higher levels of EU nucleotide incorporation than QSCs, while ASCs show dramatically elevated incorporation. (f–i) Immunocytochemical (ICC) staining of QSCs, 2.5 DPI CSCs, and 2.5 DPI ASCs immediately after isolation shows that CSCs are highly similar to QSCs in expression of the QSC marker Pax7 (f), as well as markers of SC activation, MyoD (g) and Ki67 (h), and myogenic differentiation, MyoG (i). (mean ± SEM; n=4 animals; * p<0.05, ** p<0.01). (j) CSCs have comparable ability to engraft as QSCs. EYFP+ QSCs and 2.5 DPI CSCs were isolated from donor mice (Pax7CreER/+; Rosa26EYFP/+). 5×104 EYFP+ QSCs were transplanted into the left TAs and 5×104 EYFP+ CSCs were transplanted into the right TAs of host NSG mice. Two weeks after transplantation, EYFP+ SCs were isolated from TA muscles of host mice and SC engraftment efficiency was measured as the number of EYFP+ SCs that were recovered as a percentage of the number of donor SCs that were transplanted (n=4, red line indicates mean). For both donor cell populations, greater than 95% of SCs recovered were found to be Pax7+ as measured by ICC (data not shown). (k) CSCs that incorporate BrdU self-renew. Following injury to one TA muscle, mice were administered BrdU continuously for 4 days followed by 21 days of chase (as diagrammed). IF-IHC analysis of the TA contralateral to the injury revealed BrdU+, Pax7+ cells in the satellite cell position beneath the basal lamina. An example of such a cell is illustrated here (top row of images). On the right is quantification of BrdU+ SCs after 21 days of chase by ICC after FACS isolation, showing that CSCs have self-renewal capacity similar to QSCs (mean ± SEM; n=3 animals; * p<0.05). Below is an example of a BrdU+ myonucleus in the contralateral TA after 21 days of chase, suggesting that CSCs can also fuse with the adjacent fiber following proliferation.
Figure E3. CSCs have elevated mitochondrial and mTORC1 activity.
(e) Induction of genes involved in the cell cycle and mitochondrial metabolism in CSCs. Pathway analysis of genes that were induced in CSCs versus QSCs showed enrichment of genes involved in the cell cycle and mitochondrial metabolism. Redundant KEGG pathways that contain overlapping genes were assembled into annotation groups (details of array and enrichment analysis are found in the methods section). (b) CSCs have slightly increased cell volume compared to QSCs. Cell volume was calculated from cell size measurements (Fig. 2b) (mean ± SEM; n=4 animals; * p<0.05, ** p<0.01 compared to QSCs). (c) CSCs have a slightly greater intracellular ATP concentration than QSCs (mean ± SEM; n=4 animals; * p<0.05 compared to QSCs). (d) Increase in photo emission from CSCs expressing luciferase reporter (LuSEAP). Immediately after isolation and plating, bioluminescence imaging of 1×104 Pax7CreER/+; Rosa26LuSeAP/+ SCs shows that 2.5 DPI CSCs have greater luminescence than QSCs, while ASCs have dramatically elevated luminescence. Activated fibro-adipogenic progenitors (AFAPs) were isolated from the same injured muscle as ASCs and plated as a negative control for LuSEAP expression. Light emission from luciferase is dependent on the amounts of luciferase enzyme, ATP, and luciferin. Increased ATP and increased expression from the Rosa26 locus in CSCs (Figs. 2j and S2c) could both contribute to increased luminescence. Data presented is a representative experiment with similar results observed in two independent experiments. (e) Low magnification image of IF-IHC staining of TA muscle. Boxed areas are of the representative pS6− and pS6+ SCs that are shown in Figure 2g. (f) CSCs have increased levels of pS6 as shown by western blot analysis of whole cells extracts from 1×105 cells of each population harvested immediately after isolation. (g) CSCs show a bimodal distribution of pS6 staining at 1 DPI, with peaks corresponding to the signal in pS6− QSCs and pS6+ ASCs when analyzed by FACS (representative FACS plot, similar results observed in 3 independent experiments). (h) Sorting SCs for properties of the alert state, (i.e. high levels of: MTDR staining and YFP expression) enriches for SCs that display the other properties of alert SCs: elevated mTORC1 activity, reduced time to first division, and increased propensity to cycle. Representative gating of MTDRHi;EYFPHi SCs (Hi) and MTDRLo;EYFPLo SCs (Lo). (i–m) Sorting of Hi SCs reveals a sub-population of QSCs that displays characteristics of the alert state. Hi SC cells have increased mTORC1 activity (i), an increased propensity to cycle in vivo as measured by incorporation of EdU nucleotide 12 hours after pulse labeling (j), and an accelerated time to first division (l). Both Hi and Lo SCs stain positive for the SC marker, Pax7 (l). 12 hours after an in vivo EdU pulse, the majority of SCs that incorporate nucleotide (quantified in panel (j)) stain positive for pS6 (m). Panels (i–m) are displayed as mean ± SEM; n≥3 animals; * p<0.05, ** p<0.01.
Figure E4. TSC1 KO SCs show induction of pS6 and increased cell size
(a) TSC1 KO increases SC pS6 levels. IF-IHC staining shows no pS6 staining of SCs in WT TA muscle and strong staining of SCs in TSC1 KO TA (representative images of low magnification muscle section; numbered boxed regions are shown in high magnification below). (b) Levels of pS6 in SC-specific KO models. TSC1 KO SCs show induction of pS6 when compared to WT QSCs, while Rptr KO QSCs and CSCs show no detectable pS6. cMet KO QSCs show comparable levels of pS6 as WT QSCs. However, unlike WT CSCs, cMet KO CSCs show no induction of pS6. Displayed is western blotting analysis of whole cell extracts from 1×105 cells per each population/genetic model harvested immediately after isolation. The first three lanes (WT: QSCs, CSCS, and ASCs) are the same as Figure E3f and are redisplayed for the purpose of comparison. (c) TSC1 KO SCs are larger than WT SCs (representative FACS plot, similar results observed in 4 independent experiments).
Figure E5. Rptr and cMet KO SCs contralateral to injury display no “alerting” response.
(a) Depletion of Rptr protein in Rptr KO SCs. ICC staining of EYFP+ SCs cultured for 40 hrs after isolation shows that Rptr protein is undetectable in Rptr KO SCs but clearly detectable in WT SCs (b) Absence of pS6 in Rptr KO SCs. ICC staining shows that after 40 hours in culture, EYFP+ WT SCs stain strongly pS6+ whereas EYFP+ Rptr KO SCs do not exhibit any detectable pS6 signal. (c) PCR verification of Rptr exon 6 excision in Rptr KO SCs. Using primers flanking the floxed exon 6 of the Rptr genomic locus, PCR analysis of genomic DNA from SCs isolated from a Rptr conditional KO animal (Rptrflox/flox;Pax7CreER/+;Rosa26EYFP/+) shows efficient recombination of the floxed allele, while analysis of genomic DNA from SCs from a WT animal (Rptr+/+;Pax7CreER/+;Rosa26EYFP/+) and FAPs from a Rptr conditional KO animal shows no recombination. (d) FACS analysis reveals that Rptr KO SCs are slightly smaller and display a slight leftward shift in FSC distribution relative to WT SCs. (e) Rptr KO SCs do not enlarge in response to contralateral injury. 2.5 DPI, Rptr KO CSCs show a nearly identical FSC distribution to that of Rptr KO QSCs and do not increase in size in response to contralateral injury as do WT CSCs (panel (d)). (a–e) Representative data, similar results observed in at least 3 independent experiments. (f) cMet is required for phosphorylation of S6 by HGF. In culture, WT SCs show a robust increase in the frequency of pS6+ SCs in response to a 1 hour stimulation with HGF while cMet KO SCs show no change in pS6 staining frequency (mean ± SEM; n=4; ** p<0.01). (g) cMet KO prevents induction of pS6 in SCs contralateral to injury as measured by IF-IHC (mean ± SEM; n≥3 animals, ≥50 Pax7+ SCs analyzed from each animal; * p<0.05). (h) cMet KO CSCs do not change in size. FACS analysis shows that cMet KO and WT QSCs have similar FSC distributions and that this distribution is not altered in cMet KO SCs contralateral to an injury (a representative FACS plot is shown; similar results were observed in 3 independent experiments).
Figure E6. The functional properties of alert CSCs revert back to the QSC state 28 DPI.
(a) Frequency of pS6+ CSCs returns to noninjured levels 28 DPI. Quantification of the percentage of pS6+ SCs by IF-IHC shows that immediately following injury, the majority of CSCs (orange bars) become pS6+. The frequency of pS6+ CSCs decreases to levels observed in noninjured animals (black bar) by 28 DPI. (mean ± SEM.; n≥3 animals, >50 Pax7+ SCs analyzed from each animal; ** p<0.01 versus noninjured). (b) The propensity of CSCS to cycle returns to the level of QSCs several weeks after injury. At various times after injury, mice were given an I.P. injection of BrdU. SCs were isolated 12 hours later from the injured muscles (ASCs) or from the contralateral muscles (CSCs). The frequency of BrdU incorporation returned to QSC levels (dashed line) by approximately 21 days after injury for both ASCs and CSCs. (mean ± SEM; n≥3 animals). (c) Cell cycle entry kinetics of CSCs returns to the level of QSCs several weeks after injury. At various times after injury, SCs or their progeny were isolated from the injured muscles (ASCs) or from the contralateral muscles (CSCs) and cultured in vitro for 40hrs in the presence of EdU. The frequency of EdU incorporation returned to QSC levels (dashed line) by several weeks after injury for both ASCs and CSCs. (mean ± SEM; n≥2 animals). (d) CSCs isolated 28 DPI have a transcriptional profile very similar to QSCs as shown by PCA and Pearson’s r value. Transcriptome analysis was performed as in Figure 2c, with the addition of data from CSCs 28 DPI.
Figure E7. The ability to adopt the alert state strongly correlates with expression of genes involved in mitochondrial metabolism.
(a) Pathway analysis (as performed in Figure E3a) of the genes induced in TSC1 KO QSCs compared to WT QSCs shows that genes involved in mitochondrial metabolism are significantly enriched. (b) Pathway analyses of the genes induced in Rptr KO CSCs compared to Rptr KO QSCs and (c) cMet KO CSCs compared to cMet KO QSCs show that genes involved in mitochondrial metabolism are not enriched. (d) Expression of genes involved in oxidative phosphorylation (KEGG ID mmu00190) is coupled with the alert state. Heat map of the expression of genes in the oxidative phosphorylation pathway shows that models of the alert state (CSCs and TSC1 KO QSCs) have elevated expression of these genes and that models of non-alert SCs (QSCs, Rptr KO SCs, and cMet KO SCs) have low expression of these genes. Hierarchical clustering (Euclidean distance, complete linkage) shows that models of the alert state (CSCs and TSC1 KO SCs) cluster together and that models of non-alert SCs (QSCs, Rptr KO SCs, and cMet KO SCs) form another cluster. (e) Centroid-based clustering using oxidative phosphorylation genes (KEGG ID mmu00190) shows that grouping SCs into three clusters reveals an “alert” cluster (WT CSCs and TSC1 KO QSCs), a “non-alert” cluster (QSCs, CSCs 28 DPI, Rptr KO QSCs and CSCs, and cMet KO QSCs and CSCs), and an “activated” cluster (ASCs). Ellipses of dispersion show standard deviation (radius) and mean (center) for each cluster using the first two components from PCA. Combined, these data show that induction of genes involved in mitochondrial metabolism strongly and consistently correlates with ability to adopt the alert state: WT CSCs and TSC1 KO QSCs are alert and Rptr KO and cMet KO CSCs are not alert.
Figure E8. SCs enter the alert state in response to multiple types of injuries.
(a) Cultures of CSCs differentiate more quickly than do culture of QSCs (representative ICC staining of MyoG, data quantified in Figures 4a–b). (b) SCs enter the alert state in response to injuries to non-muscle tissue. SCs contralateral to a tibial fracture (Bone Inj) and SCs in an animal that received a skin wound on the abdomen (Skin Inj) increase in propensity to cycle in vivo (mean ± SEM; non n=5 animals, Bone Inj n=2, Skin Inj n=6; ** p<0.01 versus noninjured). (c) SCs increase cycle cell entry kinetics in response to non-muscle injuries. SCs contralateral to a tibial fracture injury and SCs from mice that received a skin injury have increased frequency of EdU incorporation when cultured for 40 hours ex vivo compared to SCs from noninjured animals (mean ± SEM; n=3 animals; * p<0.05 versus noninjured).
Figure E9. FAPs and LT-HSCs adopt an alert state in response to muscle injury.
(a) Increased frequency of pS6+ FAPs contralateral to muscle injury. Representative IF-IHC staining of TA muscle from a noninjured animal (top) or contralateral to injury (bottom) shows that the frequency of pS6+ (PDGFRα+;CD31−) FAPs is increased in contralateral muscle (data are quantified in Figure 4g). Labeled boxes indicate regions for which higher magnification is displayed. (b) CFAPs increase in size. FACS analysis shows that 2.5 DPI CFAPs increase in FSC distribution compared to QFAPs; AFAPs show a greater increase in size (a representative FACs plot is shown; similar results were observed in 3 independent experiments). (c) CFAPs increase in propensity to cycle. Twelve hours following an I.P. injection of BrdU, CFAPs isolated at indicated DPIs have an elevated frequency of BrdU incorporation compared to QFAPs (0 DPI). (d) Muscle injury increases the frequency of phospho-mTOR+ (pmTOR) LT-HSCs. FACS analysis of pmTOR in Lineage−, Sca-1+, cKit+, CD150+ HSCs isolated from bone marrow 1 DPI showed that LT-HSCs induce mTORC1 signaling in response to muscle injury (mean ± SEM; n≥4; * p<0.05).
Acknowledgments
We would like to thank members of the Rando lab for discussions critical to the preparation of this manuscript, especially Tom Cheung, and Stefano Biressi. Lusijah Rott for assistance with FACS. This work was supported by The Glenn Foundation for Medical Research, by a grant from the Department of Veterans Affairs to T.A.R., and grants from the National Institutes of Health to J.T.R. (K99 AG041764), K.Y.K (K08 HL098898), M.A.G. (R01 DK092883) and to T.A.R. (P01 AG036695, R01 AG23806, and R01 AR062185)
Footnotes
Author Contributions
J.T.R. conceived and designed most of the experiments reported. T.A.R. provided guidance throughout. J.T.R., C.B., and N.M. performed experiments and collected data. J.T.R., J.B., and L.L. analyzed the microarray data. J.T.R. and K.K.M. conceived and performed bioluminescence experiments. J.T.R and M.J.C. designed primed regeneration experiments. J.T.R and G.W.C performed and analyzed transplant experiments. K.Y.K., C.R.T, and M.A.G. conceived, performed, and analyzed data from the experiments in HSCs. J.T.R. and T.A.R. analyzed data and wrote the manuscript.
Author Information
Array data is deposited in GEO (Accession GSE 55490 and GSE47177), as previously published27, and as Supplementary Data Set 1. Authors declare no conflicts of interest. Correspondence should be addressed to T.A.R. (rando@stanford.edu).
Reference List
- 1.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossi L, et al. Less is more: unveiling the functional core of hematopoietic stem cells through knockout mice. Cell Stem Cell. 2012;11:302–317. doi: 10.1016/j.stem.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 5.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–360. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung TH, et al. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature. 2012;482:524–528. doi: 10.1038/nature10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishijo K, et al. Biomarker system for studying muscle, stem cells, and cancer in vivo. FASEB J. 2009;23:2681–2690. doi: 10.1096/fj.08-128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seale P, et al. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 9.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwiatkowski DJ, et al. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- 11.Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- 12.Miyazawa K. Hepatocyte growth factor activator (HGFA): a serine protease that links tissue injury to activation of hepatocyte growth factor. FEBS J. 2010;277:2208–2214. doi: 10.1111/j.1742-4658.2010.07637.x. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura T, Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:588–610. doi: 10.2183/pjab.86.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 15.Yamada M, et al. High concentrations of HGF inhibit skeletal muscle satellite cell proliferation in vitro by inducing expression of myostatin: a possible mechanism for reestablishing satellite cell quiescence in vivo. Am J Physiol Cell Physiol. 2010;298:C465–476. doi: 10.1152/ajpcell.00449.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- 17.Huh CG, et al. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci U S A. 2004;101:4477–4482. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brack AS, Rando TA. Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell. 2012;10:504–514. doi: 10.1016/j.stem.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 20.Joe AW, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 22.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C, et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gan B, et al. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc Natl Acad Sci U S A. 2008;105:19384–19389. doi: 10.1073/pnas.0810584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pardee AB. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 26.Soprano KJ. WI-38 cell long-term quiescence model system: a valuable tool to study molecular events that regulate growth. J Cell Biochem. 1994;54:405–414. doi: 10.1002/jcb.240540407. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, et al. Chromatin modifications as determinants of muscle stem cell quiescence and chronological aging. Cell Rep. 2013;4:189–204. doi: 10.1016/j.celrep.2013.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
Figure E1. SCs distant from the site of an injury display a functional response to the injury.
(a) Representative FACS plot from isolation of EYFP+ SCs from 10-week-old Pax7CreER/+; Rosa26EYFP/+ mice 3 weeks following TMX. Mononuclear cells from muscle digests are gated in FITC and Pac-Blue (autofluorescence) channels to separate EYFP+ SCs. EYFP+ SCs are typically 2–4% of all events from muscle digestions. (b) Progeny of CSCs and QSCs take comparable times to complete the second cell division. Analysis of the time required to complete the second division (QSCs 10.2±2 hrs; n=148 cells, CSCs 10.9±2 hrs; n=155), following the first cell cycle (Fig. 1d), shows that accelerated cell cycle kinetics of CSCs is limited to the first division. (c) SCs throughout the body increase in propensity to cycle in response to injury. In injured animals, SCs isolated from indicated muscle groups show higher frequency of BrdU incorporation when compared to SCs from the same muscle groups from noninjured mice. (n≥2 animals) (d) Muscle crush injuries increase the in vivo cycling propensity of CSCs. Twelve hours after BrdU pulse labeling, SCs isolated from TA and Gast muscles contralateral to muscle crush injury show elevated BrdU labeling frequency versus SCs from those muscles from noninjured mice. (mean ± SEM; non n=5 animals, muscle crush n=3; ** p<0.01). (e) SCs contralateral to a muscle crush injury have increased cell cycle entry kinetics. 2.5 DPI, SCs contralateral to a muscle crush injury incorporate EdU more rapidly than QSCs when cultured ex vivo for 40 hours (mean ± SEM; n=3 animals; * p<0.05).
Figure E2. CSCs are distinct from QSCs but retain stem cell characteristics.
(a) CSCs are slightly larger than QSCs and much smaller than ASCs. Immediately after isolation, analysis of cell diameters of QSCs, 2.5 DPI CSCs, and 2.5 DPI ASCs, measured by phase contrast microscopy, shows that CSCs have a distribution that is shifted to the right compared to QSCs (histographic representation of data displayed in Figures 2a–b). (b) CSCs are larger than QSCs as measured by the FSC parameter by FACS (representative FACs plot, similar results observed in 4 independent experiments). (c) CSCs have elevated intensity of EYFP reporter. FACS analysis of EYFP intensity in the FITC channel shows that 2.5 DPI CSCs display a slight shift in EYFP distribution relative to QSCs, suggesting increased expression of this reporter from the Rosa26 locus (representative FACS plot, similar results observed in 4 independent experiments). (d) CSCs show elevated levels of pyronin Y staining, suggesting an increased RNA content relative to QSCs, but dramatically less than ASCs (representative FACS plot, similar results observed in 4 independent experiments). (e) CSCs increase global transcriptional activity compared with QSCs. FACS analysis of EU incorporation, following pulse labeling by I.P. injection, shows that 2.5 DPI CSCs have higher levels of EU nucleotide incorporation than QSCs, while ASCs show dramatically elevated incorporation. (f–i) Immunocytochemical (ICC) staining of QSCs, 2.5 DPI CSCs, and 2.5 DPI ASCs immediately after isolation shows that CSCs are highly similar to QSCs in expression of the QSC marker Pax7 (f), as well as markers of SC activation, MyoD (g) and Ki67 (h), and myogenic differentiation, MyoG (i). (mean ± SEM; n=4 animals; * p<0.05, ** p<0.01). (j) CSCs have comparable ability to engraft as QSCs. EYFP+ QSCs and 2.5 DPI CSCs were isolated from donor mice (Pax7CreER/+; Rosa26EYFP/+). 5×104 EYFP+ QSCs were transplanted into the left TAs and 5×104 EYFP+ CSCs were transplanted into the right TAs of host NSG mice. Two weeks after transplantation, EYFP+ SCs were isolated from TA muscles of host mice and SC engraftment efficiency was measured as the number of EYFP+ SCs that were recovered as a percentage of the number of donor SCs that were transplanted (n=4, red line indicates mean). For both donor cell populations, greater than 95% of SCs recovered were found to be Pax7+ as measured by ICC (data not shown). (k) CSCs that incorporate BrdU self-renew. Following injury to one TA muscle, mice were administered BrdU continuously for 4 days followed by 21 days of chase (as diagrammed). IF-IHC analysis of the TA contralateral to the injury revealed BrdU+, Pax7+ cells in the satellite cell position beneath the basal lamina. An example of such a cell is illustrated here (top row of images). On the right is quantification of BrdU+ SCs after 21 days of chase by ICC after FACS isolation, showing that CSCs have self-renewal capacity similar to QSCs (mean ± SEM; n=3 animals; * p<0.05). Below is an example of a BrdU+ myonucleus in the contralateral TA after 21 days of chase, suggesting that CSCs can also fuse with the adjacent fiber following proliferation.
Figure E3. CSCs have elevated mitochondrial and mTORC1 activity.
(e) Induction of genes involved in the cell cycle and mitochondrial metabolism in CSCs. Pathway analysis of genes that were induced in CSCs versus QSCs showed enrichment of genes involved in the cell cycle and mitochondrial metabolism. Redundant KEGG pathways that contain overlapping genes were assembled into annotation groups (details of array and enrichment analysis are found in the methods section). (b) CSCs have slightly increased cell volume compared to QSCs. Cell volume was calculated from cell size measurements (Fig. 2b) (mean ± SEM; n=4 animals; * p<0.05, ** p<0.01 compared to QSCs). (c) CSCs have a slightly greater intracellular ATP concentration than QSCs (mean ± SEM; n=4 animals; * p<0.05 compared to QSCs). (d) Increase in photo emission from CSCs expressing luciferase reporter (LuSEAP). Immediately after isolation and plating, bioluminescence imaging of 1×104 Pax7CreER/+; Rosa26LuSeAP/+ SCs shows that 2.5 DPI CSCs have greater luminescence than QSCs, while ASCs have dramatically elevated luminescence. Activated fibro-adipogenic progenitors (AFAPs) were isolated from the same injured muscle as ASCs and plated as a negative control for LuSEAP expression. Light emission from luciferase is dependent on the amounts of luciferase enzyme, ATP, and luciferin. Increased ATP and increased expression from the Rosa26 locus in CSCs (Figs. 2j and S2c) could both contribute to increased luminescence. Data presented is a representative experiment with similar results observed in two independent experiments. (e) Low magnification image of IF-IHC staining of TA muscle. Boxed areas are of the representative pS6− and pS6+ SCs that are shown in Figure 2g. (f) CSCs have increased levels of pS6 as shown by western blot analysis of whole cells extracts from 1×105 cells of each population harvested immediately after isolation. (g) CSCs show a bimodal distribution of pS6 staining at 1 DPI, with peaks corresponding to the signal in pS6− QSCs and pS6+ ASCs when analyzed by FACS (representative FACS plot, similar results observed in 3 independent experiments). (h) Sorting SCs for properties of the alert state, (i.e. high levels of: MTDR staining and YFP expression) enriches for SCs that display the other properties of alert SCs: elevated mTORC1 activity, reduced time to first division, and increased propensity to cycle. Representative gating of MTDRHi;EYFPHi SCs (Hi) and MTDRLo;EYFPLo SCs (Lo). (i–m) Sorting of Hi SCs reveals a sub-population of QSCs that displays characteristics of the alert state. Hi SC cells have increased mTORC1 activity (i), an increased propensity to cycle in vivo as measured by incorporation of EdU nucleotide 12 hours after pulse labeling (j), and an accelerated time to first division (l). Both Hi and Lo SCs stain positive for the SC marker, Pax7 (l). 12 hours after an in vivo EdU pulse, the majority of SCs that incorporate nucleotide (quantified in panel (j)) stain positive for pS6 (m). Panels (i–m) are displayed as mean ± SEM; n≥3 animals; * p<0.05, ** p<0.01.
Figure E4. TSC1 KO SCs show induction of pS6 and increased cell size
(a) TSC1 KO increases SC pS6 levels. IF-IHC staining shows no pS6 staining of SCs in WT TA muscle and strong staining of SCs in TSC1 KO TA (representative images of low magnification muscle section; numbered boxed regions are shown in high magnification below). (b) Levels of pS6 in SC-specific KO models. TSC1 KO SCs show induction of pS6 when compared to WT QSCs, while Rptr KO QSCs and CSCs show no detectable pS6. cMet KO QSCs show comparable levels of pS6 as WT QSCs. However, unlike WT CSCs, cMet KO CSCs show no induction of pS6. Displayed is western blotting analysis of whole cell extracts from 1×105 cells per each population/genetic model harvested immediately after isolation. The first three lanes (WT: QSCs, CSCS, and ASCs) are the same as Figure E3f and are redisplayed for the purpose of comparison. (c) TSC1 KO SCs are larger than WT SCs (representative FACS plot, similar results observed in 4 independent experiments).
Figure E5. Rptr and cMet KO SCs contralateral to injury display no “alerting” response.
(a) Depletion of Rptr protein in Rptr KO SCs. ICC staining of EYFP+ SCs cultured for 40 hrs after isolation shows that Rptr protein is undetectable in Rptr KO SCs but clearly detectable in WT SCs (b) Absence of pS6 in Rptr KO SCs. ICC staining shows that after 40 hours in culture, EYFP+ WT SCs stain strongly pS6+ whereas EYFP+ Rptr KO SCs do not exhibit any detectable pS6 signal. (c) PCR verification of Rptr exon 6 excision in Rptr KO SCs. Using primers flanking the floxed exon 6 of the Rptr genomic locus, PCR analysis of genomic DNA from SCs isolated from a Rptr conditional KO animal (Rptrflox/flox;Pax7CreER/+;Rosa26EYFP/+) shows efficient recombination of the floxed allele, while analysis of genomic DNA from SCs from a WT animal (Rptr+/+;Pax7CreER/+;Rosa26EYFP/+) and FAPs from a Rptr conditional KO animal shows no recombination. (d) FACS analysis reveals that Rptr KO SCs are slightly smaller and display a slight leftward shift in FSC distribution relative to WT SCs. (e) Rptr KO SCs do not enlarge in response to contralateral injury. 2.5 DPI, Rptr KO CSCs show a nearly identical FSC distribution to that of Rptr KO QSCs and do not increase in size in response to contralateral injury as do WT CSCs (panel (d)). (a–e) Representative data, similar results observed in at least 3 independent experiments. (f) cMet is required for phosphorylation of S6 by HGF. In culture, WT SCs show a robust increase in the frequency of pS6+ SCs in response to a 1 hour stimulation with HGF while cMet KO SCs show no change in pS6 staining frequency (mean ± SEM; n=4; ** p<0.01). (g) cMet KO prevents induction of pS6 in SCs contralateral to injury as measured by IF-IHC (mean ± SEM; n≥3 animals, ≥50 Pax7+ SCs analyzed from each animal; * p<0.05). (h) cMet KO CSCs do not change in size. FACS analysis shows that cMet KO and WT QSCs have similar FSC distributions and that this distribution is not altered in cMet KO SCs contralateral to an injury (a representative FACS plot is shown; similar results were observed in 3 independent experiments).
Figure E6. The functional properties of alert CSCs revert back to the QSC state 28 DPI.
(a) Frequency of pS6+ CSCs returns to noninjured levels 28 DPI. Quantification of the percentage of pS6+ SCs by IF-IHC shows that immediately following injury, the majority of CSCs (orange bars) become pS6+. The frequency of pS6+ CSCs decreases to levels observed in noninjured animals (black bar) by 28 DPI. (mean ± SEM.; n≥3 animals, >50 Pax7+ SCs analyzed from each animal; ** p<0.01 versus noninjured). (b) The propensity of CSCS to cycle returns to the level of QSCs several weeks after injury. At various times after injury, mice were given an I.P. injection of BrdU. SCs were isolated 12 hours later from the injured muscles (ASCs) or from the contralateral muscles (CSCs). The frequency of BrdU incorporation returned to QSC levels (dashed line) by approximately 21 days after injury for both ASCs and CSCs. (mean ± SEM; n≥3 animals). (c) Cell cycle entry kinetics of CSCs returns to the level of QSCs several weeks after injury. At various times after injury, SCs or their progeny were isolated from the injured muscles (ASCs) or from the contralateral muscles (CSCs) and cultured in vitro for 40hrs in the presence of EdU. The frequency of EdU incorporation returned to QSC levels (dashed line) by several weeks after injury for both ASCs and CSCs. (mean ± SEM; n≥2 animals). (d) CSCs isolated 28 DPI have a transcriptional profile very similar to QSCs as shown by PCA and Pearson’s r value. Transcriptome analysis was performed as in Figure 2c, with the addition of data from CSCs 28 DPI.
Figure E7. The ability to adopt the alert state strongly correlates with expression of genes involved in mitochondrial metabolism.
(a) Pathway analysis (as performed in Figure E3a) of the genes induced in TSC1 KO QSCs compared to WT QSCs shows that genes involved in mitochondrial metabolism are significantly enriched. (b) Pathway analyses of the genes induced in Rptr KO CSCs compared to Rptr KO QSCs and (c) cMet KO CSCs compared to cMet KO QSCs show that genes involved in mitochondrial metabolism are not enriched. (d) Expression of genes involved in oxidative phosphorylation (KEGG ID mmu00190) is coupled with the alert state. Heat map of the expression of genes in the oxidative phosphorylation pathway shows that models of the alert state (CSCs and TSC1 KO QSCs) have elevated expression of these genes and that models of non-alert SCs (QSCs, Rptr KO SCs, and cMet KO SCs) have low expression of these genes. Hierarchical clustering (Euclidean distance, complete linkage) shows that models of the alert state (CSCs and TSC1 KO SCs) cluster together and that models of non-alert SCs (QSCs, Rptr KO SCs, and cMet KO SCs) form another cluster. (e) Centroid-based clustering using oxidative phosphorylation genes (KEGG ID mmu00190) shows that grouping SCs into three clusters reveals an “alert” cluster (WT CSCs and TSC1 KO QSCs), a “non-alert” cluster (QSCs, CSCs 28 DPI, Rptr KO QSCs and CSCs, and cMet KO QSCs and CSCs), and an “activated” cluster (ASCs). Ellipses of dispersion show standard deviation (radius) and mean (center) for each cluster using the first two components from PCA. Combined, these data show that induction of genes involved in mitochondrial metabolism strongly and consistently correlates with ability to adopt the alert state: WT CSCs and TSC1 KO QSCs are alert and Rptr KO and cMet KO CSCs are not alert.
Figure E8. SCs enter the alert state in response to multiple types of injuries.
(a) Cultures of CSCs differentiate more quickly than do culture of QSCs (representative ICC staining of MyoG, data quantified in Figures 4a–b). (b) SCs enter the alert state in response to injuries to non-muscle tissue. SCs contralateral to a tibial fracture (Bone Inj) and SCs in an animal that received a skin wound on the abdomen (Skin Inj) increase in propensity to cycle in vivo (mean ± SEM; non n=5 animals, Bone Inj n=2, Skin Inj n=6; ** p<0.01 versus noninjured). (c) SCs increase cycle cell entry kinetics in response to non-muscle injuries. SCs contralateral to a tibial fracture injury and SCs from mice that received a skin injury have increased frequency of EdU incorporation when cultured for 40 hours ex vivo compared to SCs from noninjured animals (mean ± SEM; n=3 animals; * p<0.05 versus noninjured).
Figure E9. FAPs and LT-HSCs adopt an alert state in response to muscle injury.
(a) Increased frequency of pS6+ FAPs contralateral to muscle injury. Representative IF-IHC staining of TA muscle from a noninjured animal (top) or contralateral to injury (bottom) shows that the frequency of pS6+ (PDGFRα+;CD31−) FAPs is increased in contralateral muscle (data are quantified in Figure 4g). Labeled boxes indicate regions for which higher magnification is displayed. (b) CFAPs increase in size. FACS analysis shows that 2.5 DPI CFAPs increase in FSC distribution compared to QFAPs; AFAPs show a greater increase in size (a representative FACs plot is shown; similar results were observed in 3 independent experiments). (c) CFAPs increase in propensity to cycle. Twelve hours following an I.P. injection of BrdU, CFAPs isolated at indicated DPIs have an elevated frequency of BrdU incorporation compared to QFAPs (0 DPI). (d) Muscle injury increases the frequency of phospho-mTOR+ (pmTOR) LT-HSCs. FACS analysis of pmTOR in Lineage−, Sca-1+, cKit+, CD150+ HSCs isolated from bone marrow 1 DPI showed that LT-HSCs induce mTORC1 signaling in response to muscle injury (mean ± SEM; n≥4; * p<0.05).