BONE MARROW STROMA SECRETED CYTOKINES PROTECT JAK2V617F-MUTATED CELLS FROM THE EFFECTS OF A JAK2 INHIBITOR (original) (raw)
. Author manuscript; available in PMC: 2014 Jun 23.
Abstract
Signals emanating from the bone marrow microenvironment, including stromal cells, are thought to support the survival and proliferation of the malignant cells in patients with myeloproliferative neoplasms (MPN). To examine this hypothesis we established a co-culture platform (cells co-cultured directly [cell-on-cell] or indirectly [separated by micropore membrane]) designed to interrogate the interplay between JAK2V617F–positive cells and the stromal cells. Treatment with atiprimod, a potent JAK2 inhibitor, caused marked growth inhibition and apoptosis of human (SET2) and mouse (FDCP-EpoR) JAK2V617F–positive cells, and of primary blood or bone marrow mononuclear cells from patients with polycythemia vera, but these effects were attenuated when any of these cell types were co-cultured (cell-on-cell) with human marrow stromal cell lines (HS5, NK.tert, or TM-R1). Co-culture with stromal cells hampered the ability of atiprimod to inhibit the phosphorylation of JAK2 and the downstream signal transducer and activators of transcription (STAT) 3, and STAT5. This protective effect was maintained in non-contact co-culture assays (JAK2V617F–positive cells separated by 0.4 μm micropore membranes from stromal cells), suggesting a paracrine effect. Cytokine profiling of supernatants from non-contact co-culture assays detected distinctly high levels of IL-6, FGF, and CXCL10/IP10. Anti-IL-6, -FGF, or -CXCL10/IP10 neutralizing antibodies ablated the protective effect of stromal cells and restored atiprimod-induced apoptosis of JAK2V617F–positive cells. Thus, our results suggest that humoral factors secreted by stromal cells protect MPN clones from JAK2 inhibitor therapy, underscoring the importance of targeting the marrow niche in MPN for therapeutic purposes.
Keywords: myeloproliferative neoplasms, myelofibrosis, polycythemia vera, stroma, cytokines, JAK2
INTRODUCTION
The myeloproliferative neoplasms (MPNs) polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), arise from the clonal transformation of hematopoietic stem cells (HSCs)/progenitors (HPs), which gives rise to abnormal proliferation of one or several hematopoietic lineages driven by hypersensitivity to regulatory growth factors.(1) Deregulation of kinase activity has emerged as a major mechanism by which cancer cells evade normal physiological constraints on growth and survival. In MPNs, the gain-of-function JAK2V617F mutation is present in almost all patients with PV and in approximately 50% of patients with ET or PMF.(1-5) JAK2V617F activates several signaling pathways crucial for cellular survival and proliferation. The putative role of JAK2V617F in the pathogenesis of MPNs provided the rationale for the development of JAK2 inhibitors for the treatment of patients with MPNs. Clinical trials testing the activity of several JAK2 inhibitors are underway, particularly in MF.(6, 7) Even though preliminary results show significant clinical benefit of therapy, these agents have shown no activity in correcting the fibrosis, osteosclerosis, and neoangiogenesis that characterizes the bone marrow of patients with MF, and no elimination of malignant clone as judged by the continuous presence of JAK2V617F– positive cells in patients on therapy.
Several lines of evidence suggest that in MF stromal cells are primed by the malignant hematopoietic clone which, in turn, conditions the stroma to create a “favorable” pathologic microenvironment that nurtures and protects the malignant cells. In MF both cellular and extracellular levels of various fibrogenic and angiogenic cytokines are increased, thus supporting the notion that the bone marrow histologic changes that characterized MF are reactive and mediated by cytokines such as transforming growth factor beta (TGF-ß), platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), and vascular endothelial growth factor (VEGF), among others.(8) The net result is a tumor niche that provides environmental cues which contribute to the proliferation, maintenance, and (potentially) resistance to therapy of the malignant clone. Indeed, marrow stromal cells have been shown to protect chronic lymphocytic leukemia (CLL) cells from spontaneous or drug-induced apoptosis in vitro and to confer resistance to therapy in CLL and other B-cell malignancies, like acute lymphoblastic leukemia (ALL).(9-11) Understanding the information exchange between the malignant clone and the bone marrow milieu may shed light on how to eliminate malignant MPN cells that reside in protective stromal niche within the marrow. We herein present evidence supporting a protective effect of the stromal bone marrow niche against JAK2 inhibitor therapy via stroma cell-secreted humoral factors. The manipulation of these contextual cues potentially might be exploited therapeutically for the eradication of JAK2V617F– positive clones.
MATERIALS AND METHODS
Cells, monoclonal antibodies, and chemicals
Murine FDCP (factor dependent cell Patersen) cells transfected with the erythropoietin receptor harboring the human JAK2V617F mutant allele (henceforth referred to as FDCP-EpoRV617F cells), a kind gift from Dr. Joseph Prchal (University of Utah, Salt Lake City, UT), were cultured at 37°C in a humidified 5% CO2 atmosphere using RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 5% WEHI conditioned media. Human SET2 leukemia cell line with JAK2V617F mutation was purchased from DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen Braunschweig, Germany), and maintained in RPMI1640 medium supplemented with 20% FCS. Human stromal NK.tert cell line (derived from human bone marrow cells immortalized with hTERT) containing exogene MFG-tsT-IRES-neo was obtained from the RIKEN Cell Bank (Sapporo Medical University, Japan)(12) and cultured in alpha-Minimum Essential Medium Eagle with Earl salts and L-glutamine (α-MEM; Invitrogen) supplemented with 12.5% FCS (HyClone), 12.5% human serum (Cellgro), 1 μm hydrocortisone (Sigma-Aldrich), and 100 μm 2-mercaptoethanol (Sigma-Aldrich). Human stromal cells HS5 (CRL-11882, ATCC, Manassas, VA) were maintained in alpha-MEM medium containing 10% FCS. The primary stromal cell line TM-R1 (Taghi Manshouri-Rob1) was established in our laboratory by culturing bone marrow mononuclear cells from a patient with PMF in α-MEM medium containing 20% FCS. Bone marrow aspirate samples and peripheral blood samples from patients with PV (none receiving PV-directed therapy) were derived according to an IRB approved laboratory protocol, from leftover material obtained from specimens used for clinical purposes: mononuclear cells were isolated as previously published and used in experiments without further isolation of specific cell types.(13, 14)
The monoclonal antibodies used were: mouse anti-phosopho-STAT3 (05-485) and -STAT5 (06-553); mouse anti-phosphotyrosine clone-4G10 (05321); rabbit anti-JAK2 (06-255); rabbit anti-total-STAT3 (06-596) and -STAT5 (05-533); all from Upstate Biotechnology (Lake Placid, NY). Goat anti-human-interleukin-6 (IL6, AF-206-NA), -chemokine C-X-C-motif ligand 10 (CXCL10/IP10, AF-266-NA), and –fibroblast growth factor basic/2 (FGF2, AF233-NA) were obtained from R&D Systems (Minneapolis, MN). Mouse anti-β-actin (A5441) was purchased from Sigma-Aldrich (St. Louis, MO). Conjugated horse radish peroxidase sheep anti-mouse (NA931V) from GE-Healthcare, and horse radish peroxidase donkey anti-rabbit (711-035-15) from Jakson ImmunoReseach (West Grove, PA). Cytokines used were: recombinant human anti-IL8 (208-IL010), -fibroblast growth factor (hFGF, 233-FA-025), and –vascular endothelial growth factor (hVEGF, 4644-VS/CF) from R&D Systems. The JAK2 inhibitor atiprimod (Callisto Pharmaceutical, New York, NY), was dissolved in phosphate-buffered solution (PBS, Gibco BRL, Grand Island, NY) to a final concentration of 8mM. The stock solution was kept at 4°C and further diluted in tissue cultured medium just before use.
Growth inhibition MTT assay
MTT assays were performed as previously described.(13, 14) Results shown represent the average standard deviation of three independent experiments, each done in sextuplets.
Co-culture assays
Stromal cell suspensions were prepared at 4×10_5_ cells/ml, and then seeded onto 6-well culture plates (3046, Falcon) at 2ml/well, or onto 96-well assay black plates (3603, Corning INC, Corning, NY) at 50μl/well. After overnight incubation, the non-adherent cell fraction was removed and adherent stromal confluent monolayers were washed three times with PBS. FDCP-EpoRV617F or SET2 cells were then added to prepared adherent stroma (8×105 cells/4ml/well into 6-well plates; 1×104 cells/50μl/well into 96-well plates) either directly (cell-on-cell) or indirectly (separated by 0.4μm micropore membranes, Falcon 35-3493). Atiprimod was added at different concentrations as indicated. FDCPEpoRV617F or SET2 were then assayed for cell proliferation in 96-well plates after 72hrs of incubation, as described above (MTT assay). Alternatively, cell aliquots from 6-well plates were collected after 4, 24, and 48hrs of incubation to examine apoptosis or were washed three times with cold PBS and frozen at −80°C for western blot analysis. Same type of experiments was done using primary bone marrow mononuclear cells from PV patients instead of FDCP-EpoRV617F or SET2 cells.
In additional experiments, supernatants were collected from co-culture assays described above, and plated in new plates, and fresh FDCP-EpoRV617F cells added with 1μm atiprimod for 48 hours; induction of apoptosis was then assessed by flow cytometry.
Apoptosis assays
Apoptosis of FDCP-EpoRV617F and SET2 cells, obtained from co-culture assays, was detected by flow cytometer using recombinant human Annexin-V conjugated with Allophycocyamin (APC, CALTAG, CA; used for FDCP cells) or with FITC (51-6710AK, BD Pharmingen, San Diego, CA; used for SET2 cells). Analysis was done on a FACSort flow cytometer (Becton Dickinson Systems, San Jose, CA), and results analyzed using FlowJo Software version 7.2.5 software (Ashland, OR).
JAK2 immunoprecipitation and western blotting
JAK2 immunoprecipitation and western blotting were performed as previously described.(15, 16) Quantitative densitometry has been used to determine fold change in protein content in western blots.
Cytokine multiplexed Bio-Plex assay
FDCP-EpoRV617F and SET-2 cells were washed three times with PBS at room temperature and resuspended in serum-free medium (StemCell Technology) and 2×105 cells/ml were dispensed onto 6-well plates coated or uncoated with stroma (separated by micropore membranes), with or without 1μM atiprimod and co-cultured for 4, 24, and 48hrs. Supernatants were collected for cytokine measurements. Bio-Plex human cytokine 27-plex panel assay (171-A11127, Bio-Rad) was used for simultaneous quantitation of 27 cytokines, according to the manufacturer's instructions. After washing, beads were resuspended in 125μl of Bio-Plex assay buffer and analyzed on the Bio-Plex system using Bio-Plex Manager software with 5PL curve fitting. The reader was set to read a minimum of 100 beads; results were expressed as median fluorescent intensity (MFI). After centering each treated sample on the corresponding control sample from the same time point, the treated and control groups were compared, using two-sided paired t-test.
Cytokine neutralization
Stromal cell monolayers were prepared in 6-well plates as described above. After overnight incubation at 37°C, they were washed with PBS three times at room temperature and either normal goat serum (1μg/ml as a control), goat anti-human-IL6 (0.3μg), -CXCL10/IP10 (0.3μg), or -FGF4 (0.3μg) antibodies (individually or in combination) were added to culture together with culture medium at 37°C for 4hrs. FDCP-EpoR JAK2V617F cells (2×105/ml) were then dispensed onto all wells (separated by micropore membranes from stroma), and cultured in the presence of 1μm atiprimod. After 48hrs of incubation at 37°C, FDCP-EpoRV617F cells were collected and apoptosis was quantitated by flow cytometry.
RESULTS
Stroma attenuated the anti-proliferative effect of JAK2 inhibitor therapy on JAK2V617F-positive cells
We have recently reported atiprimod to be a potent inhibitor of JAK2 tyrosine kinase.(13) It inhibited the growth and caused apoptosis of JAK2V617F–positive cells, both of cell lines and primary MPN patient blood or bone marrow mononuclear cells, in short-term assays. It also suppressed (but not completely abrogated) the growth of BFU-E colonies from primary PV patient blood or bone marrow mononuclear cells in 14-day clonogenic assays.(13) Indeed, treatment with atiprimod induced only a moderate reduction in the JAK2V617F allele burden of the proliferating BFU-E colonies. Because colonies were grown in the presence of growth factors and accessory bone marrow cells were present in all cultures, we hypothesized that these factors, either alone or in combination, prevented complete growth inhibition of primary JAK2V617F colony-forming cells. To explore this possibility we designed subsequent experiments.
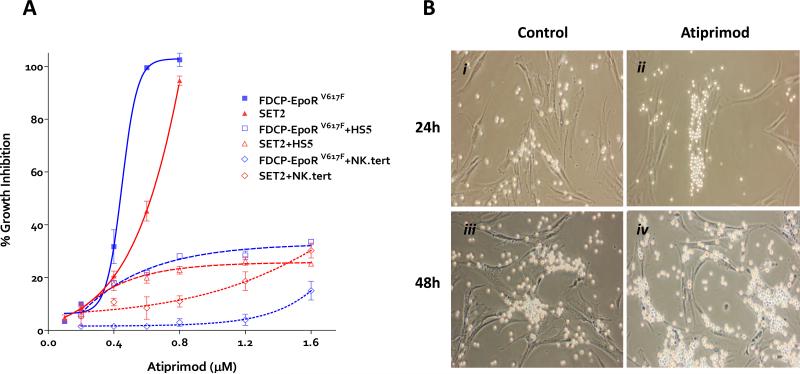
The effect of atiprimod on the viability of FDCP-EpoR cells transduced with either mutant (JAK2V617F) or wild-type (JAK2WT) human JAK2 and on the JAK2V617F–positive SET2 cell line was investigated using the MTT assay. Exposure to increasing concentrations of atiprimod for 72hrs resulted in a dose-dependent inhibition of proliferation (IC50 of 397nM for FDCP-EpoRV617F, 544nM for SET2, and 810nM for FDCP-EpoRWT cells (Figure 1A). To test the effect of stroma presence in the culture on the activity of JAK2 inhibitor, we co-cultured FDCP-EpoRV617F and SET2 cells with the human stromal cell lines HS5 or NK.tert, with or without atiprimod. Atiprimod had no effect on the proliferation of either stromal cell line (data not shown). The activity of atiprimod on the proliferation of FDCP-EpoRV617F or SET2 cells was significantly reduced in the presence of either HS5 or NK.tert stromal cells (Figure 1A). The same protective effect was seen when primary JAK2V617F–positive mononuclear blood cells from PV patients were exposed to atiprimod in a co-culture with stromal cells: they continued proliferating over time even in the presence of 1μm atiprimod (Figure 1B).
Figure 1. The effect of atiprimod and stromal cells on the proliferation of FDCP-EpoR JAK2V617F and SET-2 cells and primary polycythemia vera cell colonies.
(A) Both JAK2V617F-transduced FDCP-EpoR cells and human SET-2 cells were treated with increasing concentrations of atiprimod for 72 hours. The MTT assay was used to assess cell proliferation. Data points represent the mean ±SD from three independent measurements. A non-linear regression model was applied to the MTT results to estimate the IC50 values. (B) Monolayers of adherent primary TM-R1 stromal cells were grown and washed with PBS to remove the non-adherent cell fraction. Then, bone marrow mononuclear cells (4×10_5_ per 2 ml) from a JAK2V617F–positive PV patient were seeded on top and co-cultured at 37°C in the absence (i and iii) or in the presence (ii and iv) of 1μm atiprimod. Co-cultures were imaged at 24 hours (i and ii) and at 48 hours (iii and iv). Shortly after seeding, JAK2V617F–positive cells cluster over and proliferate in intimate contact with TM-R1 cells. Primary JAK2V617F–positive cells continue proliferating even in the presence of 1μm atiprimod when co-cultured with TM-R1 stromal cells. Images were captured using a Nikon ELWD 0.3 phase-contrast microscope (10/0.25 objective lens) and a Nikon digital camera (D40, Nikon Corp, Japan) with camera control pro software.
Stroma protects JAK2V617F-positive cells from atiprimod-induced apoptosis
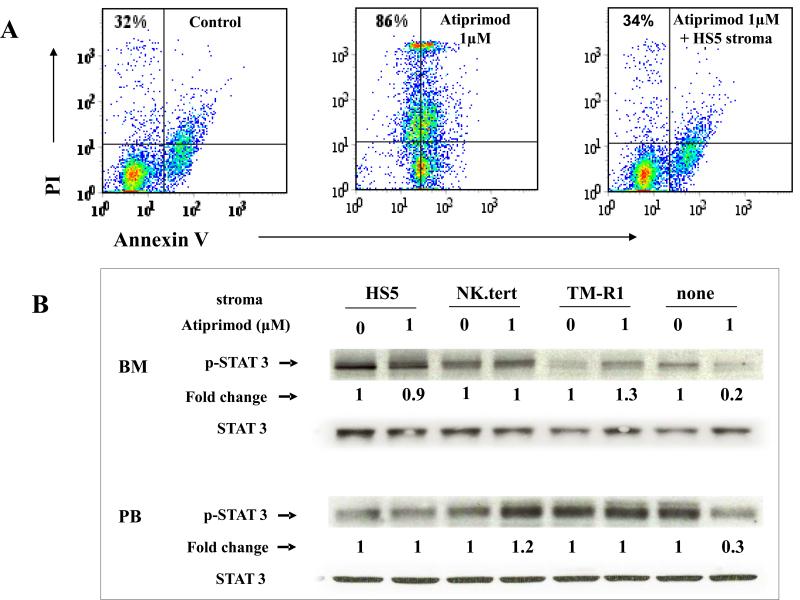
To understand the “protective effect” of the stroma on the _JAK2V617F_positive cells, we first determined whether atiprimod-mediated growth inhibition of JAK2V617F– positive cells was due to drug-induced apoptosis and whether stroma can prevent it. Treatment of FDCP-EpoRV617F cells with 1μM atiprimod resulted in significant apoptosis, but almost no atiprimod-induced apoptosis of FDCP-EpoRV617F cells was observed in the presence of HS5 stromal cells (Figure 2A). The experiment was then expanded to include both FDCP-EpoRV617F and SET2 cells treated with atiprimod in co-cultures with 3 different stromal cell lines, HS5, NK.tert or TM-R1 (Figure 2B). Co-culture of either JAK2V617F–positive cell line on any monolayer of different stromal cells almost completely abrogated the pro-apoptotic activity of atiprimod on JAK2V617F–positive cells.
Figure 2. The effect of stromal cells on the atiprimod-induced apoptosis of cells expressing mutant JAK2V617F.
(A) FDCP-EpoRV617F cells were cultured alone (control), or with atiprimod, with or without HS5 stromal cells. Following 48 hour culture cells were harvested, stained with annexing V and percentage of apoptotic cells determined by flow cytometry. Data from representative experiment are shown. Atiprimod-induced apoptosis was dramatically impaired when JAK2V617F-positive cells were co-cultured with stromal cells (B) FDCP-EpoRV617F and SET-2 cells were cultured without (control) or with three different stromal cell monolayers (HS5, NK.tert, TM-R1) in the absence or presence of atiprimod for 48 hours. The induction of apoptosis was then assessed by flow cytometry. Results represent the mean ± SD from 3 independent experiments.
Stroma impedes atiprimod-induced inhibition of JAK2, STAT3 and STAT5 phosphorylation in JAK2V617F-–positive cells
FDCP-EpoRV617F cells were exposed to atiprimod in the presence or absence of stroma and after 4, 24, and 48hrs of culture, cell aliquots were collected and subjected to immunoprecipitation and western blot analysis. While untreated FDCP-EpoRV617F cells consistently at every time point showed phosphorylation of JAK2, atiprimod exposure resulted in significant reduction of JAK2 phosphorylation without altering total JAK2 levels: JAK2 remained dephosphorylated even 48hrs post atiprimod treatment (Figure 3A). However, if FDCP-EpoRV617F were grown with HS5 or NK.tert stromal cells while treated with atiprimod, this pattern was significantly altered. In the presence of stroma in culture, after 24hrs phosphorylation of JAK2 was partially restored and after 48hrs the phosphorylation of JAK2 returned to a level similar to that of untreated cells (Figure 3).
Figure 3. Stromal cells prevent phosphorylation of the JAK-STAT axis by atiprimod.
FDCP-EpoRV617F cells were exposed to 1 μm atiprimod alone (control) or in the presence of HS5 stromal cells (upper panel) or NK.tert stromal cells (lower panel) for 4, 24, and 48 hours. Cells were then lysed and whole cell lysates were immunoprecipitated with a rabbit anti-JAK2 antibody for detection of p-JAK2. Western blot analysis using anti-phosphotyrosine antibody was performed. Then, membranes were stripped and reprobed with anti-JAK2.
Since co-culturing with stroma prevented the long-lasting inhibitory effect of atiprimod on JAK2 phosphorylation and because in vitro expression of JAK2V617F results in constitutive activation of the downstream signaling effectors STAT3 and STAT5, we next examined the status of the latter under the same culture conditions. FDCPEpoRV617F and SET2 cells were exposed to 1μm atiprimod in the presence or absence of HS5 (Supplemental Figure 1A) or NK.tert (Supplemental Figure 1B) stromal cells for 4, 24, and 48hrs. Atiprimod caused complete, long-lasting inhibition of STAT3 and STAT5 phosphorylation in both FDCP-EpoRV617F and SET2 cells. However, if FDCP-EpoRV617F and SET2 cells were treated with atiprimod in the presence of stroma, the atiprimod-induced dephosphorylation of STAT3 and STAT5 was transient, with phosphorylation fully restored by 48hrs (Supplemental Figures 1A-B). Therefore, atiprimod inhibits signaling through the JAK-STAT axis in JAK2V617F–positive cells (causes dephosphorylation of JAK2, STAT3 and STAT5) but this effect is over time, by 48hrs, abrogated if JAK2V617F–positive cells are co-cultured with stroma.
Next, we repeated above experiments using peripheral blood and bone marrow mononuclear cells from PV patients instead of JAK2V617F–positive cell lines. Treatment of PV cells with 1μm atiprimod for 48hrs resulted in their apoptosis (Figure 4A). However, their treatment in the presence of HS5 stroma completely abrogated the pro-apoptotic activity of atiprimod (Figure 4A). When primary PV cells were treated with 1μm atiprimod in the presence of either HS5, NK.tert, or TM-R1 stroma, STAT3 phosphorylation was preserved (Figure 4B), while it was significantly reduced if stromal cells were not present.
Figure 4. The effect of stromal cells on primary bone marrow and peripheral blood cells treated with atiprimod.
(A) Peripheral blood mononuclear cells from PV patients were cultured alone (control), or with atiprimod, with or without HS5 stromal cells. Following 48 hour culture cells were harvested, stained with annexing V FITC and percentage of apoptotic cells determined by flow cytometry. Data from representative experiment is shown. (B) Ex vivo bone marrow and peripheral blood mononuclear cells from patients with PV were co-cultured with or without HS5, NK.tert, or TM-R1 stromal cells, with or without of 1 μm atiprimod, for 48 hours. Total protein was extracted and subjected to 4-12% NuPAGE® gel and immunoblotting membranes were stained with phospho-STAT3. Membranes were then stripped and reprobed with rabbit anti-total STAT3.
Stroma abrogates atiprimod-induced apoptosis of JAK2V617F-cells in a cell contact-independent manner
Given the protective effect of stroma on atiprimod-induced apoptosis and JAK2-STAT pathway dephosphorylation, we next explored the nature of the molecular crosstalk between JAK2V617F–positive cells and stromal cells. In order to determine whether the protective effect of stroma on JAK2V617F-carrying cells in co-culture assays was dependent on cell-to-cell interactions, we carried out co-cultures in which JAK2V617F– positive cells (FDCP-EpoRV617F and SET2) were cultured either in direct contact with stromal cell monolayers (HS5, NK.tert, or TM-R1) or were separated from stroma by a 0.4μm micropore membrane that interrupted cell-to-cell contact but allowed them to be bathed by the same culture medium. Atiprimod 1μm induced apoptosis in approximately 90% of JAK2V617F–positive cells but caused minimal to no apoptosis of any of the stromal cell lines (data not shown). We exposed to atiprimod for 48hrs FDCP-EpoRV617F and SET2 cells co-cultured either directly onto HS5, NK.tert, or TM-R1 stromal cell monolayers or separated from them by 0.4μm micropore membranes, to prevent direct cell-to-cell contact. Atiprimod failed to induce significant apoptosis regardless of whether JAK2V617F–positive cells were co-cultured in direct contact with stroma or separated from stromal cells by a membrane (Figure 5 and Supplemental Figure 2).
Figure 5. Evaluation of atiprimod-induced apoptosis in JAK2 mutant cells co-cultured with stromal cells separated by micropore membranes.
Human SET2 cells were co-cultured directly (cell on cell) or indirectly (separated by 0.4 μm micropore membranes) with HS5, NK.tert, or TM-R1 stromal cells with or without 1μm atiprimod for 48 hours. Induction of apoptosis was assessed by flow cytometry to compare the direct or indirect effect of stromal monolayers.
Quantitation of secreted cytokines in co-culture systems
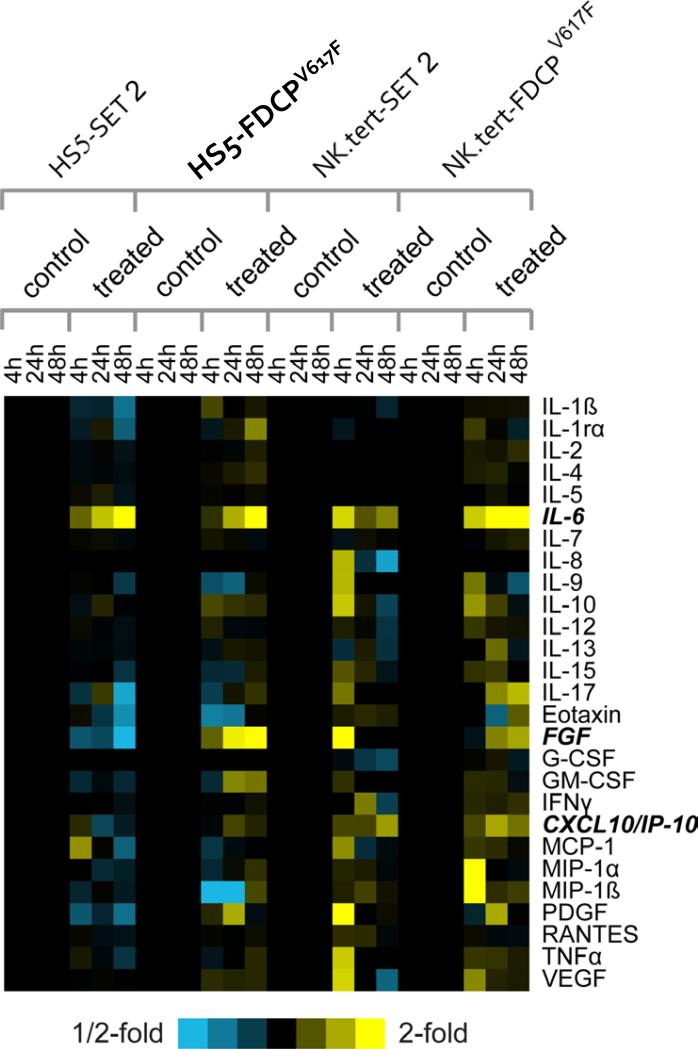
The fact that stroma protects JAK2V617F–positive cells from apoptosis without the requirement for direct cell-to-cell contact led us to hypothesize that humoral factors secreted by stromal cells may underpin such phenomenon. To investigate this hypothesis, the levels of 27 cytokines were measured in serum-free supernatants from co-culture assays involving FDCP-EpoRV617F or SET2 cells and HS5 or NK.tert stromal cells, separated by a membrane, in the absence or presence of 1μm atiprimod (Figure 6). IL-6 levels changed significantly over time in the co-culture system (P<0.00002) in the presence of atiprimod; CXCL10/IP10 levels also changed (P<0.05), though the fold increase observed was more modest. In addition, we noticed that FGF showed a marked upward trend in some of the cultures, but varied depending on the cell types involved (Figure 6). We therefore, examined the role of these 3 cytokines in subsequent experiments, as representative of the changes in cytokine levels observed in our experiments.
Figure 6. Quantitative measurement of soluble cytokines in serum free media from co-cultures.
Heat map depicting the changes in levels of 27 secreted cytokines after 4, 24, and 48 hours of co-culture (stromal cells and JAK2-mutation positive cells separated by membrane) with or without atiprimod. Rows, cytokines; columns, profiled samples. Yellow, high expression; blue, low expression; black, no change. Each treated sample is centered on the corresponding control sample from the same time point. Cytokines chosen for further functional studies are highlighted (bold italics).
Neutralization of selected cytokines abrogates the protective effect of stroma on JAK2V617F-positive cells
To determine whether elevated cytokines detected in supernatants from co-culture assays were stroma-derived, and to confirm the potential paracrine protective effect of stroma on JAK2V617F–positive cells, we carried out several experiments.
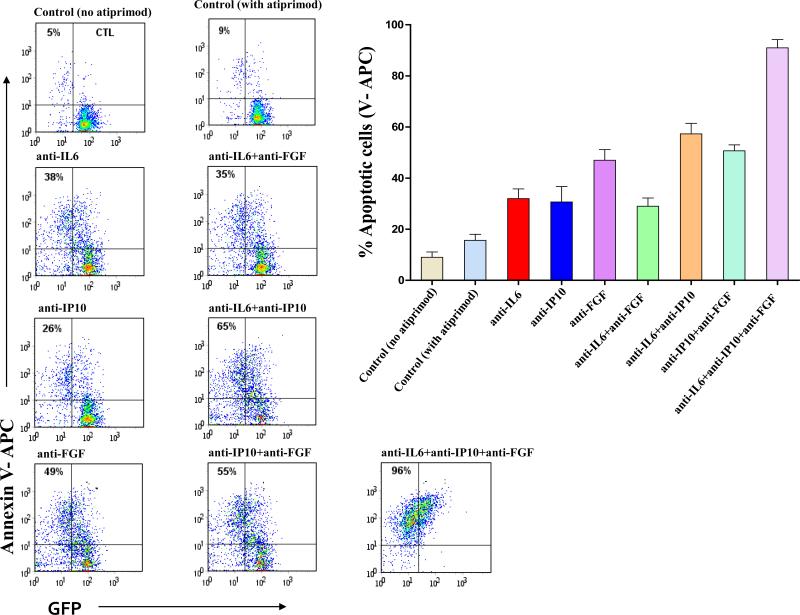
First, given that IL-6, CXCL10/IP10, and FGF appeared elevated in supernatants from co-culture assays, stromal monolayers (HS5, NK.tert, and TM-R1) were treated for 4hrs with monoclonal antibodies against IL-6, CXCL10/IP10 and/or FGF. Then, FDCPEpoRV617F cells were exposed to 1μm atiprimod for 48hrs and cultured with each one of the three pretreated stromal monolayers separated by 0.4μm micropore membranes. Pretreatment of stroma with IL6, CXCL10/IP10, or FGF antibodies diminished the protective effect of stroma on FDCP-EpoRV617F cells against atiprimod-induced apoptosis (Figure 7 and Supplemental Figures 3A-B). Pretreatment of stroma with a combination of two or three monoclonal antibodies resulted in an additive effect, with the highest neutralizing effect observed when all three antibodies were used simultaneously. We replicated these results using IL-6, CXCL10/IP10, and/or FGF antibodies in co-cultures of human SET2 and TM-R1 cell lines (data not shown).
Figure 7. Antibody-mediated neutralization of IL6, CXCL10/IP10, and FGF restores atiprimod-induced apoptosis of FDCP-EpoR JAK2V617F cells in co-cultures.
HS5 stromal cells were plated and cultured for 24 hours. Then, stromal cell monolayers were pre-treated with normal goat serum as a control (1 μg), or with goat anti-hIL6, anti-hCXCL10/IP10, and/or anti-hFGF antibodies (0.3 μg), for 4 hours at 37°C. Then, 2×10_5_/mL mouse FDCP-EpoRV617F cells were seeded in the presence of 1μM atiprimod. Control culture without atiprimod has also been done. After 48hrs, JAK2V617F-expressing cells were collected and apoptotic cells were detected by flow cytometry using recombinant human Annexin-V conjugated-APC. Data from representative experiment out of 3 independent experiments is presented; summary results of all 3 experiments are shown too.
Second, we addressed a question whether supernatants from co-cultures of stromal and JAK2V617F cells would still be protective for JAK2V617F cells in the absence of stromal cells. FDCP-EpoRV617F cells were co-cultured indirectly (separated by 0.4 μm micropore membranes) with HS5, NK.tert, or TM-R1 stromal cells with or without 1μm atiprimod for 48 hours. Supernatants were then collected from different co-cultures and plated in new plates, and fresh FDCP-EpoRV617F cells added with 1μm atiprimod for 48 hours; induction of apoptosis was then assessed by flow cytometry (Supplemental Figure 4). Supernatants from co-cultures containing stromal cells, FDCP-EpoRV617F cells, and atiprimod (all together) protected FDCP-EpoRV617F cells the most from atiprimod-induced apoptosis.
DISCUSSION
Given that forced expression of JAK2V617F in cell lines confers constitutive STAT5 activation, cytokine-independent cell proliferation and enhanced cytokine sensitivity, targeted pharmacological inhibition of JAK2V617F kinase is an attractive therapeutic strategy for patients with MPNs.(2) However, experimental evidence suggests that MPNs do not evolve simply from HSC intrinsic defects. Rather, they are heavily influenced by genetic and epigenetic events affecting the bone marrow niche.(17) Alteration of the extrinsic regulation of HSC function have been shown to be able to override intrinsic cues in vivo and give rise to MPNs as shown in mice deficient for retinoic acid receptor gamma and in those null for the cell cycle regulator retinoblastoma protein.(18) Therefore, in addition to genetic events in the malignant clone, non-genetically determined alterations of the bone marrow niche in patients with MPNs may be responsible for response and resistance to treatment.(17) Clonal MPN cells engage in a constant crosstalk with the surrounding marrow niche directly via adhesion molecules (e.g. N-cadherin, CD44) or indirectly via humoral factors.(17, 19) Among the latter, several circulating cytokines and chemokines involved in HSC/HP proliferation and mobilization, as well as promotion of fibrosis and angiogenesis (e.g. VEGF, TGF-β, IL-6), have been shown to be elevated in patients with PMF.(8, 17) While disrupting the crosstalk between the malignant clone and its milieu is an attractive therapeutic strategy in MPNs, little is known about the role of the above-mentioned humoral factors in the pathogenesis of MPNs or in the response to MPN-directed therapy. In order to gain further insights into the biology of the MPN-stroma crosstalk, we explored the interaction between the bone marrow stromal cells and JAK2V617F-positive cells in the context of JAK2 inhibitor therapy. First, we established culture conditions to model the effect of the marrow microenvironment on therapy response in MPNs by using co-culture assays that employed different marrow stromal cells involving both murine cell lines as well as human primary cells from patients with MPNs. Potent JAK2 kinase inhibition with atiprimod suppressed very effectively the growth and induced apoptosis of murine FDCP-EpoRV617F as well as of JAK2V617F-carrying human SET2 cells while exerting negligible inhibition on stromal cells. However, when JAK2V617F-positive cells were cultured on monolayers of stroma the atiprimod-induced antiproliferative and proapoptotic effects were dramatically impaired, suggesting a protective effect of the stroma on JAK2V617F-positive cells. It is important to emphasize that our co-culture assays obviated the potential confounding bias of using cells of murine origin as well as that of using stromal cell lines, in light of the fact that we observed similar results when primary MPN cells and stromal cells derived from patients with MPNs were employed in the assays. In addition, similar results were observed when co-cultures of malignant and stromal cells were conducted in a species-mismatched manner.
We noted that the protective effect of stroma on the malignant clone during JAK2 inhibitor therapy relates to the fact that the former prevent the sustained inhibition of phosphorylation of JAK2, STAT3, and STAT5 observed when MPN cells are cultured in the absence of stroma. The full extent of the stromal-mediated abrogation of JAK2 inhibitor-induced apoptosis of MPN cells was observed when JAK2V617F-positive cells were separated from stroma by micropore filters that prevented cell-cell contact while allowing both cell types to be exposed to the same medium in co-culture assays. Thus, cell-cell contact does not appear to be required for marrow stroma to extend its protective effect on the malignant clone. These results led us to hypothesize that the protection against therapy-induced apoptosis exerted by bone marrow stroma could be paracrine in nature. To test this hypothesis, we profiled a large panel of soluble factors in serum-free supernatants generated in co-cultures of JAK2V617F-positive cells on a variety of stromal cells, separated by membranes, both prior and at different time points during atiprimod treatment. While the levels of some cytokines varied depending on the cell types involved in the coculture, three cytokines, IL-6, CXCL10/IP10, and FGF, exhibited increasing levels over time in the presence of atiprimod. IL-6 is a pleiotropic cytokine that, upon binding to its receptor, causes activation of the JAK2/STAT3 and ERK-1/2 pathways.(20) Serum levels of IL-6 were increased during progression of patients with ET and PV to MF.(21) FGF, a potent pro-angiogenic factor, has been implicated in the pathogenesis of MPNs.(8, 22) While the role of the chemokine CXCL10/IP10 is MPNs is yet to be defined, it has been shown to be constitutively secreted by acute myeloid leukemia blasts.(23) To support a putative role of these soluble factors in treatment resistance in our experiments, we utilized neutralizing monoclonal antibodies in co-culture assays. Notably, pretreatment of stromal monolayers with neutralizing antibodies against IL-6, FGF, or CXCL10/IP10 diminished markedly protective stromal effect and restored atiprimod-induced apoptosis of JAK2V617F-positive cells. Our experiments demonstrated a role for stroma-secreted cytokines (of which IL-6, FGF, and CXCL10/IP10 are representative from our experiments) in response to JAK2 inhibitor therapy. It is hypothesized that the inhibition of JAK2 in our co-culture systems altered its intracellular pathway activation and that alternative pathways might have been engaged and, as a result, cellular interaction and cytokine production modified.
In summary, we have standardized an in vitro culture system that allows the interrogation of the crosstalk between the malignant MPN clones and the stroma, as well as the influence of the latter on the former during therapy. This system has allowed us to identify a role for stroma-derived cytokines in protecting the malignant clones against JAK2-directed therapy. The therapeutic implications of these findings are of importance and may apply to other myeloid hematologic malignancies, as has already been shown for lymphoid malignancies such as CLL and ALL.(9-11) Further work is warranted to dissect the interactions between the non-hematopoietic marrow niche and the malignant hematopoietic clones in MPD. The development of suitable in vivo model would be of immense importance due to limitations of in vitro/ex vivo laboratory evaluations.
Supplementary Material
1
2
ACKNOWLEDGEMENTS
This work was supported in part by the Chambers Medical Foundation, the Joe W. and Dorothy Dorsett Brown Foundation, and the Marshall Heritage Foundation (all to SV).
Footnotes
Conflict of interest: none
REFERENCES
- 1.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 2.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–8. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 3.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 4.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–97. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Zhao R, Xing S, Li Z, Fu X, Li Q, Krantz SB, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280:22788–92. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–27. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos FP, Kantarjian HM, Jain N, Manshouri T, Thomas DA, Garcia-Manero G, et al. Phase 2 study of CEP-701, an orally available JAK2 inhibitor, in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. Blood. 2010;115:1131–6. doi: 10.1182/blood-2009-10-246363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tefferi A. Pathogenesis of myelofibrosis with myeloid metaplasia. J Clin Oncol. 2005;23:8520–30. doi: 10.1200/JCO.2004.00.9316. [DOI] [PubMed] [Google Scholar]
- 9.Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23:43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- 10.Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114:3367–75. doi: 10.1182/blood-2009-06-225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayala F, Dewar R, Kieran M, Kalluri R. Contribution of bone microenvironment to leukemogenesis and leukemia progression. Leukemia. 2009;23:2233–41. doi: 10.1038/leu.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawano Y, Kobune M, Yamaguchi M, Nakamura K, Ito Y, Sasaki K, Takahashi S, et al. Ex vivo expansion of human umbilical cord hematopoietic progenitor cells using a coculture system with human telomerase catalytic subunit (hTERT)-transfected human stromal cells. Blood. 2003;101:532–40. doi: 10.1182/blood-2002-04-1268. [DOI] [PubMed] [Google Scholar]
- 13.Quintas-Cardama A, Manshouri T, Estrov Z, Harris D, Zhang Y, Gaikwad A, et al. Preclinical characterization of atiprimod, a novel JAK2 AND JAK3 inhibitor. Invest New Drugs. 2010 Apr 7; doi: 10.1007/s10637-010-9429-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verstovsek S, Manshouri T, Quintas-Cardama A, Harris D, Cortes J, Giles FJ, et al. WP1066, a novel JAK2 inhibitor, suppresses proliferation and induces apoptosis in erythroid human cells carrying the JAK2 V617F mutation. Clin Cancer Res. 2008;14:788–96. doi: 10.1158/1078-0432.CCR-07-0524. [DOI] [PubMed] [Google Scholar]
- 15.Nussenzveig RH, Swierczek SI, Jelinek J, Gaikwad A, Liu E, Verstovsek S, et al. Polycythemia vera is not initiated by JAK2V617F mutation. Exp Hematol. 2007;35:32–8. doi: 10.1016/j.exphem.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Manshouri T, Quintas-Cardama A, Nussenzveig RH, Gaikwad A, Estrov Z, Prchal J, et al. The JAK kinase inhibitor CP-690,550 suppresses the growth of human polycythemia vera cells carrying the JAK2V617F mutation. Cancer Sci. 2008;99:1265–73. doi: 10.1111/j.1349-7006.2008.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lataillade JJ, Pierre-Louis O, Hasselbalch HC, Uzan G, Jasmin C, Martyré MC, et al. Does primary myelofibrosis involve a defective stem cell niche? From concept to evidence. Blood. 2008;112:3026–35. doi: 10.1182/blood-2008-06-158386. [DOI] [PubMed] [Google Scholar]
- 18.Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007;129:1081–95. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rameshwar P, Chang VT, Gascon P. Implication of CD44 in adhesion-mediated overproduction of TGF-beta and IL-1 in monocytes from patients with bone marrow fibrosis. Br J Haematol. 1996;93:22–9. doi: 10.1046/j.1365-2141.1996.4631004.x. [DOI] [PubMed] [Google Scholar]
- 20.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–56. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 21.Panteli KE, Hatzimichael EC, Bouranta PK, Katsaraki A, Seferiadis K, Stebbing J, et al. Serum interleukin (IL)-1, IL-2, sIL-2Ra, IL-6 and thrombopoietin levels in patients with chronic myeloproliferative diseases. Br J Haematol. 2005;130:709–15. doi: 10.1111/j.1365-2141.2005.05674.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang JC, Chang TH, Goldberg A, Novetsky AD, Lichter S, Lipton J. Quantitative analysis of growth factor production in the mechanism of fibrosis in agnogenic myeloid metaplasia. Exp Hematol. 2006;34:1617–23. doi: 10.1016/j.exphem.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Olsnes AM, Motorin D, Ryningen A, Zaritskey AY, Bruserud O. T lymphocyte chemotactic chemokines in acute myelogenous leukemia (AML): local release by native human AML blasts and systemic levels of CXCL10 (IP-10), CCL5 (RANTES) and CCL17 (TARC). Cancer Immunol Immunother. 2006;55:830–40. doi: 10.1007/s00262-005-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2