Variation in the intensity of hematuria evaluation: a target for primary care quality improvement (original) (raw)
. Author manuscript; available in PMC: 2015 Jul 1.
Abstract
Background
Hematuria is a common clinical finding and represents the most frequent presenting sign of bladder cancer. The American Urological Association recommends cystoscopy and abdomino-pelvic imaging for patients over 35 years. Nonetheless, fewer than half of patients presenting with hematuria undergo proper evaluation. We sought to identify clinical and non-clinical factors associated with evaluation of persons with newly diagnosed hematuria.
Methods
Retrospective cohort study, using claims data and laboratory values. The primary exposure was practice site, as a surrogate for non-clinical, potentially modifiable sources of variation. Primary outcomes were cystoscopy and/or abdomino-pelvic imaging within 180 days following hematuria diagnosis. We modeled the association between clinical and non-clinical factors and appropriate hematuria evaluation.
Results
We identified 2,455 primary care patients 40 years of age or older diagnosed with hematuria between 2004 and 2012 in the absence of other explanatory diagnosis. 13.7% of patients underwent cystoscopy within 180 days. Multivariate logistic regression revealed significant variation between those who did and did not undergo evaluation in age, gender and anti-coagulant use (p<0.001, p=0.036, p=0.028). Addition of practice site improved the predictive discrimination of each model (p<0.001). Evaluation was associated with higher rates of genitourinary neoplasia diagnosis.
Conclusions
Patients with hematuria rarely underwent complete evaluation. While established risk factors for malignancy were associated with increasing use of diagnostic testing, factors unassociated with risk, such as practice site, also accounted for significant variation. Inconsistency across practice sites is undesirable and may be amenable to quality improvement interventions.
Keywords: hematuria, diagnostic test, routine, guideline adherence, quality of health care, urinary bladder neoplasms
Introduction
Hematuriais a common clinical finding and represents the most frequent presenting symptom/sign of urothelial carcinoma of the bladder, particularly among persons 40 years and older.1 Proper evaluation of hematuria is necessary to identify the one person in ten who may have a life-threatening malignancy or other treatable condition.2 The American Urological Association (AUA) Best Practice Guidelines recommend that all patients 35 years and older, presenting with asymptomatic hematuria (>3 red blood cells per high-power field), for which benign causes have been ruled out, undergo cystoscopy. Additionally, these guidelines recommend evaluation of the renal parenchyma and urothelium, with CT urography (CTU) being the preferred imaging modality.3 Current literature suggests that fewer than half of patients diagnosed with hematuriaare subsequently referred to a urologist for evaluation.4–6 While urinary tract infection, benign prostatic hyperplasia, andurolithiasis represent common nonmalignant etiologies of hematuria7 failure to adequately evaluate patients with hematuria risks delaying the diagnosis of potentially lethal malignancies such as bladder cancer, and is therefore a significant quality of care concern.
Few studies have investigated sources of variation in the evaluation of patients presenting with asymptomatic hematuria.6, 8 The prevalence of microscopic hematuria in the adult population ranges from 2.4% to 31.1%, and up to 3.3% of these individuals will have an underlying urothelial malignancy.9 Conversely, between 9% and 18% of patients with hematuria will have no underlying pathology, drawing into question the utility of ubiquitous hematuria evaluations.10 The potential impact of addressing variation at this proximal point in the care pathway could be substantial both in terms of quality of care and optimizing healthcare value.11–15
We sought to identify both relevant clinical factors (e.g., age and smoking status) and non-clinical factors (e.g., practice setting and payer) associated with timely cystoscopic and/or radiologic evaluation of persons with newly diagnosed hematuria in a major academic health system. We hypothesized that both clinically relevant and nonclinical factors would influence the likelihood and intensity of hematuria evaluation.
Methods
After receiving approval from the Vanderbilt University Medical Center (VUMC) Institutional Review Board, we performed a retrospective cohort study to identify variation in patterns and intensity of hematuria evaluation among patients age 40 and over with a first diagnosis of hematuria in a primary care setting. As a surrogate for non-clinical, potentially modifiable sources of variability, the exposure of interest was practice site. The primary outcome of interest was receipt of cystoscopy and/or imaging evaluation, performed within 180 days of hematuria diagnosis.
Data Source and Management
We accessed VUMC’s Research Derivative (RD), an enterprise-wide data repository that contains administrative and clinical information including a complete record of visits and admissions, laboratory data, and diagnosis and procedure codes, on every patient treated in the Vanderbilt health system. We obtained additional data points, such as smoking history, marital status, and insurance coverage, through manual chart review of the electronic medical record. Study data were stored and managed using the secure REDCap electronic data capture platform hosted at VUMC.16
Cohort Definition
We identified 6,585 patients in the RD who were 40 years of age or older and were diagnosed with a first episode of hematuria between 2004 and 2012 either by urinalysis (>3 RBCs per high power field) or International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes for hematuria (599.7, 599.70, 599.71 or 599.72), at one of Vanderbilt’s 19 primary care clinics. To be included in the study, patients must have had records for one year prior to the date of hematuria diagnosis.
Patients were excluded if they had a urinary tract infection (defined as a urinalysis positive for both leukocyte esterase and urine nitrites, or a positive urine culture) within four weeks prior to or one week following the index hematuria episode (n=590, 9.0%). We convened a panel of content experts to develop a set of explanatory diagnoses and procedures that would preclude the need for a hematuria evaluation (for a complete list, see Supplemental Table 1). We then used Physicians Current Procedural Terminology Coding System, 4th edition (CPT-4) and ICD-9 codes to exclude patients with an explanatory diagnosis or procedure within 180 days preceding their hematuria diagnosis (n=3,540, 53.8%). This yielded a final cohort of 2,455 patients.
Independent Variables
The 19 primary care clinics were condensed into 8 clusters based on geographic proximity, in order to facilitate statistical analysis. Specifically, all primary care clinics at the main campus were clustered together, and those in outlying sites were grouped together if they were within the same zip code or same town. Additional covariates included patient age, gender, race (categorized as white, black or all other), comorbidity (modified Charlson comorbidity index),17,18 distance from the provider (expressed in miles based on the difference between ZIP code centroids of patient residence and practice site),19 long-term anticoagulant use (determined by ICD-9 codes), smoking history, marital status and insurance coverage. Patients were classified as never smokers, former smokers (had quit using tobacco products for at least 30 days prior to diagnosis of microhematuria), or current smokers.
Outcome Definition
Primary outcomes were receipt of cystoscopy and/or abdomino-pelvic imaging within 180 days of hematuria diagnosis, as determined by CPT codes. Secondary outcomes included diagnoses of genitourinary neoplasms, infection, urolithiasis, prostatic enlargement, trauma, medical renal disease, hematologic/thrombotic diseases, and anatomic abnormalities within 180 days after hematuria diagnosis, as determined by ICD-9 codes.
Statistical Analysis
Descriptive statistics were calculated for patient demographics and each outcome variable, and relevant parametric or nonparametric tests were performed to identify differences in patient characteristics across practice sites and across outcome categories. Missing data were considered missing at random. Multivariable logistic regression models were used to evaluate the relationship between practice site and cystoscopy and/or imaging within 180 days of hematuria diagnosis, adjusting for patient age, gender, race, Charlson comorbidity index, insurance type, marital status, smoking history, anticoagulant use, and distance from the provider. Models with and without indicator variables for practice site were compared using area under the receiver operator characteristic curve (AUC) and likelihood ratio tests. All analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC) and R version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria), with additional functions (called “rms” and “Hmisc”) added. All p-values are two-sided, and a p value of < 0.05 was considered statistically significant.
Results
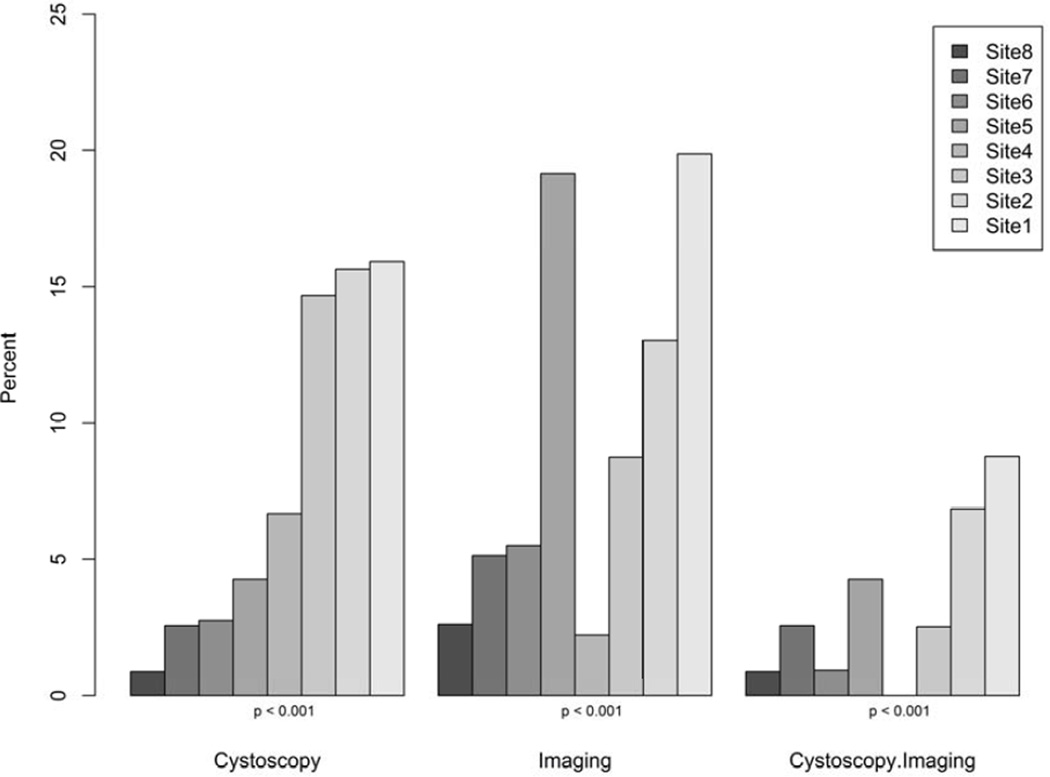
The cohort consisted of 2,455 patients (Table 1) who were 40 years of age or older at the time of initial hematuria diagnosis in a primary care clinic and did not have a pre-existing cause of hematuria, recent urologic procedure, or recent infection. Females outnumbered males 2:1. Overall, Whites outnumbered African-Americans 6:1, with considerable between-site variation. Private insurance was most common, comprising between 50% and 75% of the patient sample depending on site. Nonsmokers comprised approximately two thirds of the cohort, with minimal variation between practice sites. Practice sites 1-8 accounted for 1,118, 307, 675, 45, 47, 109, 39, and 115 patients, respectively.
Table 1.
Characteristics of all patients, and those who underwent follow up diagnostic testing after initial diagnosis of hematuria
| Proportion Receiving | |||||||
|---|---|---|---|---|---|---|---|
| Cystoscopy | Imaging Studies | Cystoscopy + Imaging | |||||
| All(n=2455) | 13.7%(n = 335) | P value | 13.9%(n=342) | P value | 5.7%(n=141) | P value | |
| Age, median (IQR)* | 58 (49, 68) | 62(53, 71) | <0.001 | 59 (50, 68) | 0.333 | 63 (54, 72) | <0.001 |
| Gender, n(%) | <0.001 | <0.001 | <0.001 | ||||
| Male | 724 (29.5) | 19.1% (138) | 21.0% (152) | 10.4% (75) | |||
| Female | 1731 (70.5) | 11.4% (197) | 11.0% (190) | 3.8% (66) | |||
| Race, n(%) | 0.026 | 0.416 | 0.475 | ||||
| White | 1949(84.3) | 14.6% (285) | 13.9% (270) | 5.9% (115) | |||
| Black | 312(13.5) | 11.2% (35) | 16.7% (52) | 6.7% (21) | |||
| Other | 51 (2.2) | 3.9% (2) | 13.7% (7) | 1.9% (1) | |||
| CCI**, median (IQR) | 0(0,1) | 0(0, 1) | 0.711 | 0 (0, 2) | <0.001 | 0(0,1) | 0.098 |
| '0 | 1501 (61.1) | 13.7% (205) | 0.084 | 11.7% (176) | <0.001 | 5.0% (75) | 0.048 |
| '1-2 | 622 (25.3) | 15.4% (96) | 16.9% (105) | 7.7% (48) | |||
| '3+ | 332(13.5) | 10.2% (34) | 18.4% (61) | 5.4% (18) | |||
| Anticoagulant use, n(%) | 113(4.6) | 19.5% (22) | 0.069 | 23.9% (27) | 0.003 | 9.7% (11) | 0.062 |
| Smoking history, n(%) | 0.403 | 0.01 | 0.522 | ||||
| Current smoker | 406(17.0) | 14.5% (59) | 16.0% (65) | 6.4% (26) | |||
| Former smoker | 473(19.8) | 15.6% (74) | 17.8% (84) | 6.8% (32) | |||
| Non-smoker | 1514(63.3) | 13.3% (201) | 12.6% (191) | 5.5% (83) | |||
| Total Pack-Years, n(%) | 0.070 | 0.647 | 0.389 | ||||
| > 20 pks/yr | 336 (55.5) | 18.4% (62) | 18.2% (61) | 7.7% (26) | |||
| <= 20 pks/yr | 269 (44.5) | 13.0% (35) | 16.7% (45) | 5.9% (16) | |||
| Married, n (%) | 0.806 | 0.085 | 0.270 | ||||
| No | 792 (35.7) | 14.4% (114) | 15.9% (126) | 6.7% (53) | |||
| Yes | 1427(64.3) | 14.0% (200) | 13.2% (189) | 5.5% (79) | |||
| Primary insurance, n (%) | 0.016 | 0.24 | 0.108 | ||||
| Private | 1319(53.8) | 11.8% (155) | 12.7% (168) | 5.1% (67) | |||
| Medicare | 842 (34.3) | 15.8% (133) | 15.2% (128) | 6.3% (53) | |||
| MCare HMO | 203 (8.3) | 17.7% (36) | 16.7% (34) | 8.9% (18) | |||
| Other | 88 (3.6) | 12.5% (11) | 11 (3.2) | 3(2.1) | |||
| Distance (miles), median (IQR) | 11.4 (4.8, 26.6) | 11.9 (5.4,36.1) | 0.023 | 10.5 (4.8,26.1) | 0.522 | 10.2 (3.9, 27.4) | 0.485 |
Overall, 13.7% of patients underwent cystoscopy within 180 days, and there were significant differences between those who did and did not undergo cystoscopy with respect to age, gender, race, payer and distance from clinic (Table 1). 13.9% of patients underwent abdomino-pelvic imaging, with 9.3% of patients undergoing CT, 4.4% ultrasound, 1.5% intravenous pyeolography, 3.0% X-ray and 0.4% MRI. Use of imaging varied significantly by gender, Charlson Comorbidity Index, anticoagulant use, and smoking history. Only 5.7% of patients underwent both imaging and cystoscopy, with variability by age and gender on univariate analysis. We observed statistically significant variation across practice sites for receipt of cystoscopy, imaging, and both cystoscopy and imaging (p<0.001 for each) (Figure 1).
Figure 1.
Use of cystoscopy and/or Imaging Study for each practice site
We fit individual multivariate logistic regression models for each outcome category: cystoscopy, imaging, and complete evaluation (Supplemental Table 2). Females were significantly less likely to undergo cystoscopy (OR 0.48, 95% CI [0.37,0.62], p<0.001), imaging (OR 0.47, 95% CI [0.36, 0.61], p<0.001) or both (OR 0.31, 95% [0.21, 0.45], p<0.001). Older age was a significant predictor of cystoscopy (OR 1.01 [1.00, 1.03], p=0.036) and of a complete workup (OR 1.03 [1.01, 1.05], p=0.014), but not of imaging. Smoking was not associated with follow-up diagnostics and there was an association between anticoagulant use and imaging (OR 1.72, [1.06, 2.78]), but not other outcomes.
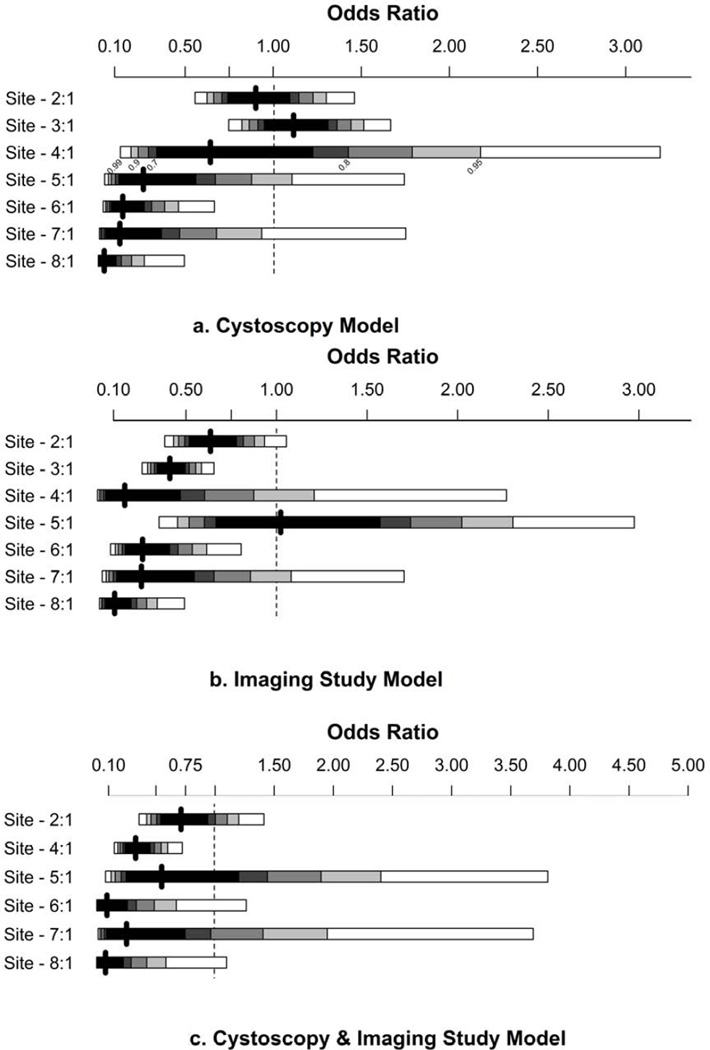
The addition of practice site improved predictive discrimination of each model. With the addition of practice site, the area under the curve for the cystoscopy model improved from 0.622 to 0.681, improved from 0.640 vs. 0.696 for the imaging model, and from 0.685 vs. 0.741 for the complete evaluation model (p<0.001 for each comparison by likelihood ratio test). The odds ratios for practice site, with the largest site as the referent, are presented in Figure 2. The effect size and significance of the clinically relevant factors (e.g., age and gender) diminished with the addition of practice site (Supplemental Table 2).
Figure 2.
Forrest Plots of odds ratios for practice site in the cystoscopy, imaging study, and cystoscopy + imaging study models
The distribution of diagnoses following hematuria evaluation is displayed in Table 2. Sixty-six patients (2.7%)were diagnosed with a genitourinary neoplasm within 180 days of their hematuria diagnosis, with bladder cancer being the most common. Infection was identified in 2.4%, stone disease in 4.6%, and 2.5% of patients were found to have BPH. On unadjusted analysis, receipt of cystoscopy or imaging was associated with higher rates of diagnosis. Ultrasound had lower diagnostic yield of neoplasms compared to CT (n=10 [9.3%] vs. n=28 [14.6%], p<0.001). Similarly, patients who underwent cystoscopy + ultrasound had lower diagnostic yield of neoplasms compared to cystoscopy + CT (n=6 [16.2%] vs. n=18 [19.3%], p<0.001).Complete data regarding distribution of diagnosis stratified by intensity of hematuria evaluation is presented in Supplemental Table 3.
Table 2.
Explanatory diagnoses identified within 180 days of initial hematuria diagnosis
| Total, n(%) | Cystoscopy, n (%) | Imaging studies, n(%) | Cystoscopy + Imaging, n(%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No (n=2120) | Yes (n=335) | Pvalue | No (n=2113) | Yes (n=342) | Pvalue | No (n=2314) | Yes (n=141) | Pvalue | ||
| Neoplasm | 66 (2.7) | 23 (1.1) | 43 (12.8) | <0.001 | 25 (1.2) | 41 (12.0) | <0.001 | 40 (1.7) | 26 (18.4) | <0.001 |
| Prostate cancer | 15 (0.6) | 9 (0.4) | 6 (1.8) | 0.010 | 7 (0.3) | 8 (2.3) | <0.001 | 11 (0.5) | 4 (2.8) | 0.009 |
| Renal cell carcinoma | 16 (0.6) | 6 (0.3) | 10 (3.0) | <0.001 | 2 (0.1) | 14 (4.1) | <0.001 | 7 (0.3) | 9 (6.4) | <0.001 |
| Bladder cancer | 33 (1.3) | 1 (0.05) | 32 (9.6) | <0.001 | 16 (0.8) | 17 (5.0) | <0.001 | 16 (0.7) | 17 (12.1) | <0.001 |
| Other | 9 (0.4) | 8 (0.4) | 1 (0.3) | 1.000 | 1 (0.05) | 8 (2.3) | <0.001 | 8 (0.4) | 1 (0.7) | 0.413 |
| Infection (UTI)* | 59 (2.4) | 29 (1.4) | 30 (9.0) | <0.001 | 34 (1.6) | 25 (7.3) | <0.001 | 44 (1.9) | 15 (10.6) | <0.001 |
| Urolithiasis | 113 (4.6) | 55 (2.6) | 58 (17.3) | <0.001 | 36 (1.7) | 77 (22.5) | <0.001 | 67 (2.9) | 46 (32.6) | <0.001 |
| Other | 133 (5.4) | 73 (3.4) | 60 (17.9) | <0.001 | 56 (2.6) | 77 (22.5) | <0.001 | 91 (3.9) | 42 (29.8) | <0.001 |
| Any explantory diagnosis | 292 (11.9) | 158 (7.4) | 134 (40.0) | <0.001 | 134 (6.3) | 158 (46.2) | <0.001 | 208 (9.0) | 84 (59.6) | <0.001 |
Discussion
Our study has several notable findings. First, only 13.7% of patients diagnosed with hematuria underwent cystoscopy, and a similar proportion (13.9%) underwent radiographic evaluation. Furthermore, only 5.7% of the overall cohort underwent both cystoscopic and radiologic evaluation, what the AUA considers standard practice in patients over 35 years of age. We identified several clinically relevant factors to be associated with follow-up evaluation. Increasing age and male gender, known risk factors for urothelial carcinoma, predicted a greater likelihood of undergoing cystoscopy. While the overall rates of follow-up testing in this study were low, we identified higher rates of evaluation in patients with these known risk factors for malignancy indicating that providers are, to an extent, using clinical information to risk stratify patients.8
However, practice site, which presumably bears little influence on the risk of malignancy, was also found to be a significant predictor of receipt of diagnostic studies. Patients who underwent follow-up testing were far more likely to be diagnosed with malignancy (12.8% vs. 1.1% for cystoscopy; 12.0% vs. 1.2% for imaging; 18.4% vs. 1.7% for complete workup; p<0.001 for each) and other urologic/nephrologic conditions within 6 months than those who did not. While this finding is expected given that patients with known malignancy risk factors were more likely to undergo further evaluation, it is an important observation because a measurable proportion of patients presenting with hematuria will have a genitourinary malignancy (2.7% in this series), and delays in diagnosis could lead to later stage at diagnosis, higher treatment burden, and less favorable cancer control outcomes.20 Furthermore, variation based on practice site may be amenable to quality improvement interventions, such as comparative performance feedback or education, or process improvement interventions to overcome logistical barriers.21
Hematuria is associated with underlying malignancy in 2–4% of adults,9 depending on risk factors, and the often short therapeutic window for patients with urothelial carcinoma necessitates early diagnosis and intervention.22 Unfortunately, most patients presenting with hematuria do not receive appropriate evaluation, regardless of the presence of risk factors for malignancy. A recent retrospective study of patients with hematuria at two treatment sites found that only 12.8% of high-risk patients underwent cystoscopy.23 We found clinically appropriate variation in use of follow up testing based on age and gender. While age- and gender-based variation is consistent with prior findings24 and offers some reassurance that providers are incorporating clinical risk factors into clinical decision-making, these differences may partially explain why women with newly diagnosed urothelial tumors are diagnosed at higher stage compared to their male counterparts.25 In our study, prior smoking history did not increase the likelihood of cystoscopic evaluation, as one might have expected, but was associated with higher use of radiologic evaluation following hematuria diagnosis. Since urothelial carcinoma is the second leading cause of tobacco-related cancer mortality,26–27 this inconsistency in diagnostic intensity among tobacco users represents an opportunity for quality improvement.
Asymptomatic hematuria effects up to one-fifth of the adult population, and is the most common presenting symptom of urothelial malignancy.3 As such, professional organizations such as the AUA and American Academy of Family Practitioners have issued practice guidelines on the appropriate evaluation of microscopic hematuria.9,28 Yet there is legitimate debate about the widespread application of diagnostic testing for hematuria, since the yield of such testing is low in some settings.6,8,29–30 Perhaps this uncertainty regarding the appropriate candidates for and intensity of evaluation for patients with hematuria underlies the low rates of testing seen in the literature,4,6 and may explain the variability across practice sites observed in this study. Yet one of the six pillars for improving the delivery of healthcare in the US, according to the Institute of Medicine, is Equitable Care, defined as “consistent care regardless of patient characteristics and demographics.”31 Consistency is a major component of quality of care, and as such, the identification of variation attributed to practice setting raises serious questions about the quality of care being delivered.
There are many potential explanations for differences in utilization of follow up testing across practice sites. It is possible that there are structural differences between practice sites that either facilitate or impede the receipt of appropriate evaluation. Such structural factors may include availability of on-site urology or imaging services. In fact, we found that the site with the highest proportion of patients undergoing complete evaluation was the main hospital campus at which both urology and imaging services are available. Alternatively, our results could reflect differences in provider knowledge and behavior, factors that could be amenable to educational and feedback interventions. Finally, some of the variability observed may be due to unmeasured between-site differences in patient-level factors. Such factors may include financial barriers including co-pays for additional testing or cost of travel to distant sites with urology and/or radiology services. Improving guideline-adherence due to structural differences and limitations in patient self-efficacy could entail process improvements (e.g., free shuttles between sites), addressing logistic barriers to specialist care (e.g., providing contact information for the specialty clinic or having office staff schedule the Urology visit) or reducing patient-level barriers (e.g., identifying low-cost specialty providers). Regardless of the method(s) pursued, improving adherence to published hematuria guidelines addresses numerous quality dimensions including care coordination, clinical care, and patient experience and engagement.32–33 Quality improvement efforts in the evaluation of hematuriamay not only serve to improve care at the patient level, but may also serve as a model for improving interaction between primary care physicians and specialists.
Our findings must be interpreted in the context of the current study design. A significant challenge of the current study was refining the cohort to exclude those patients with pre-existing diagnoses or recent procedures that could explain their hematuria. We sought to exhaustively exclude patients with pre-existing diagnoses. The rate of subsequent diagnoses of malignancy, stone, etc. are consistent with prior studies, suggesting that we achieved that goal. Nonetheless, the strict inclusion criteria we applied may reduce the external validity of our study. Additionally, we did not have complete data on risk factors for malignancy (e.g., gross vs. microscopic hematuria, urinary symptoms, chemical and occupational exposures), which could have influenced use of diagnostic testing. Finally, it is possible that some patients may have gone outside our health system for diagnostic care resulting in underestimation of cystoscopy and/or imaging rates. We believe that the magnitude of attrition from our system is small based both upon internal data as well as known referral patterns in middle Tennessee.
Conclusions
Across our large academic health system, only a small proportion of patients presenting with new-onset hematuria underwent complete evaluation. While we observed expected variation in use of diagnostic testing by factors associated with risk of malignancy, we also found clinically and statistically significant variation by factors not classically associated with risk, such as practice site. Such inconsistency across practice sites is undesirable and may be amenable to quality improvement interventions. Improvements in our understanding of the barriers to adherence to evidence-based guidelines for the evaluation of hematuria could improve care of diseases such as urothelial carcinoma. More generally, understanding the sources of non-clinical variability in the application of evidence-based guidelines is a first step toward improving guideline implementation.
Supplementary Material
01
Clinical Significance.
- A minority of patients with hematuria underwent appropriate evaluation consisting of cystoscopy and abdomino-pelvic imaging.
- While established risk factors for malignancy were associated with increasing use of diagnostic testing, factors unassociated with risk, such as practice site, also accounted for significant variation.
- Such inconsistency across practice sites is undesirable and may be amenable to quality improvement interventions, thereby improving care of hematuria-associated diseases such as urothelial carcinoma.
Acknowledgment
Funding Support: This study was funded by a grant from the National Cancer Institute to study variation in evaluation for hematuria (1R03CA173807-01; Barocas PI), and a Vanderbilt Institute for Clinical and Translational Research grant (VR4961). The data was requested from the Research Derivative and data management was facilitated by the use of Vanderbilt University’s Research Electronic Data Capture (REDCap) system, which are both supported by the Vanderbilt Institute for Clinical and Translational Research grant (UL1TR000445 from NCATS/NIH). Dr. Resnick was supported by the Veterans Affairs National Quality Scholars Program with use of facilities at Veterans Health Administration Tennessee Valley Healthcare System and in part by a grant from the Urology Care Foundation Research Scholars Program and the AUA Southeastern Section Research Scholar Fund
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All authors have made a substantial contribution to this study, have access to the data, have read and approved the final manuscript, and have no direct or indirect commercial, financial incentive associated with publishing the article. Our manuscript is original and has not been published by any other journal.
Contributors:
Tom Elasy, MD MPH
Prior presentations:
American College of Surgeons Annual Meeting; Washngton, DC; October 8, 2013
References
- 1.Konety BR, Joyce GF, Wise M. Chapter 7: Bladder and Upper Tract Urothelial Cancer. In: Litwin MS, Saigal CS, editors. Urologic Diseases in America. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Printing Office; 2007. pp. 223–279. NIH Publication No. 07-5512. [Google Scholar]
- 2.Mariani AJ, Mariani MC, Macchioni C, Stams UK, Hariharan A, Moriera A. The significance of adult hematuria: 1,000 hematuria evaluations including a risk-benefit and cost-effectiveness analysis. J Urol. 1989;141:350–355. doi: 10.1016/s0022-5347(17)40763-4. [DOI] [PubMed] [Google Scholar]
- 3.Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy–part I: definition, detection, prevalence, and etiology. Urology. 2001;57:599–603. doi: 10.1016/s0090-4295(01)00919-0. [DOI] [PubMed] [Google Scholar]
- 4.Nieder AM, Lotan Y, Nuss GR, et al. Are patients with hematuria appropriately referred to urology? A multi-institutional questionnaire based survey. Urol Oncol. 2010;28:500–503. doi: 10.1016/j.urolonc.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Yafi FA, Aprikian AG, Tanguay S, et al. Patients with microscopic and gross hematuria: Practice and referral patterns among primary care physicians in a universal health care system. Can Urol Assoc J. 2011;5:97–101. doi: 10.5489/cuaj.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buteau A, Seideman CA, Svatek RS, et al. What is evaluation of hematuria by primary care physicians: Use of electronic medical records to assess practice patterns with intermediate follow-up. Urologic Oncology: Seminars and Original Investigations. 2012 doi: 10.1016/j.urolonc.2012.07.001. In Press. [DOI] [PubMed] [Google Scholar]
- 7.Margulis V, Sagalowsky AI. Assesment of Hematuria. Med Clin N Am. 2011;95:153–159. doi: 10.1016/j.mcna.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Loo RK, Lieberman SF, Slezak JM, et al. Stratifying Risk of Urinary Tract Malignant Tumors in Patients With Asymptomatic Microscopic Hematuria. Mayo Clin Proc. 2013;88:129–138. doi: 10.1016/j.mayocp.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Davis R, Jones JS, Barocas DA, et al. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol. 2012;188:2473. doi: 10.1016/j.juro.2012.09.078. [DOI] [PubMed] [Google Scholar]
- 10.Tomson C, Porter T. Asymptomatic microscopic or dipstick haematuria in adults: Which investigations for which patients? A review of the evidence. BJU Int. 2002;90:185–198. doi: 10.1046/j.1464-410x.2002.02841.x. [DOI] [PubMed] [Google Scholar]
- 11.Ritchie CD, Bevan EA, Collier SJ. Importance of occult haematuria found at screening. Br Med J. 1986;292:681–683. doi: 10.1136/bmj.292.6521.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topham PS, Jethwa A, Watkins M, Rees Y, Feehally J. The value of urine screening in a young adult population. Fam Pract. 2004;21:18–21. doi: 10.1093/fampra/cmh105. [DOI] [PubMed] [Google Scholar]
- 13.Shimakuro T, Naito K. Evaluation of hematuria and proteinuria positivity in relation to ageing in 6,651 apparently healthy men and women. Hinyokika Kiyo. 2007;53:783–788. [PubMed] [Google Scholar]
- 14.Carel RS, Silverberg DS, Kaminsky R, Aviram A. Routine urinalysis (dipstick) findings in mass screening of healthy adults. Clin Chem. 1987;33:2106–2108. [PubMed] [Google Scholar]
- 15.Steiner H, Bergmeister M, Verdorfer I, et al. Early results of bladder-cancer screening in a high-risk population of heavy smokers. BJU Int. 2008;102:291–296. doi: 10.1111/j.1464-410X.2008.07596.x. [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J ClinEpidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 18.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 19.Guagliardo MF. Spatial accessibility of primary care: concepts, methods and challenges. Int J Health Geogr. 2004;26:3. doi: 10.1186/1476-072X-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollenbeck BK, Dunn RL, Ye Z, Hollingsworth JM, Lee CT, Birkmeyer JD. Racial differences in treatment and outcomes among patients with early stage bladder cancer. Cancer. 2010;116:50–56. doi: 10.1002/cncr.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller DC, Murtagh DS, Suh RS, et al. Regional collaboration to improve radiographic staging practices among men with early stage prostate cancer. J Urol. 2011 Sep;186:844–849. doi: 10.1016/j.juro.2011.04.078. [DOI] [PubMed] [Google Scholar]
- 22.Sánchez-Ortiz RF, Huang WC, Mick R, Van Arsdalen KN, Wein AJ, Malkowicz SB. An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. J Urol. 2003;169:110. doi: 10.1016/S0022-5347(05)64047-5. [DOI] [PubMed] [Google Scholar]
- 23.Elias K, Svatek RS, Gupta S, Ho R, Lotan Y. High-risk patients with hematuria are not evaluated according to guideline recommendations. Cancer. 2010;116:2954–2959. doi: 10.1002/cncr.25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson EK, Daignault S, Zhang Y, Lee CT. Patterns of Hematuria Referral to Urologists: Does a Gender Disparity Exist? Urology. 2008;72:498–503. doi: 10.1016/j.urology.2008.01.086. [DOI] [PubMed] [Google Scholar]
- 25.Mungan NA, Aben KK, Schoenberg MP, et al. Gender differences in stage-adjusted bladder cancer survival. Urology. 2000;55:876–880. doi: 10.1016/s0090-4295(00)00523-9. [DOI] [PubMed] [Google Scholar]
- 26.Gandini S, Botteri E, Iodice S, et al. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122:155–164. doi: 10.1002/ijc.23033. [DOI] [PubMed] [Google Scholar]
- 27.Vineis P, Alavanja M, Buffler P, et al. Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst; 2004;96:99–106. doi: 10.1093/jnci/djh014. [DOI] [PubMed] [Google Scholar]
- 28.McDonald MM, Swagerty D, Wetzel L. Assessment of microscopic hematuria in adults. Am Fam Physician. 2006;73:1748–1754. [PubMed] [Google Scholar]
- 29.Malmstrom PU. Time to abandon testing for microscopic haematuria in adults? BMJ. 2003;326:813–815. doi: 10.1136/bmj.326.7393.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou R, Dana T. Screening adults for bladder cancer: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:461–468. doi: 10.7326/0003-4819-153-7-201010050-00009. [DOI] [PubMed] [Google Scholar]
- 31.Committee on Quality of Health Care in America of the Institute of Medicine. Washington, DC: National Academy Press; 2001. Crossing the Quality Chasm: A New Health System for the 21st Century; p. 337. [PubMed] [Google Scholar]
- 32.US Dept of Health and Human Services. [accessed June 12 2013];Annual Progress Report to Congress: National Strategy for Quality Improvement in Health Care. 2012 http://www.ahrq.gov/workingforquality/nqs/nqs2012annlrpt.pdf.
- 33.Conway PH, Mostashari F, Clancy C. The Future of Quality Measurement for Improvement and Accountability. JAMA. 2013;309:2215–2216. doi: 10.1001/jama.2013.4929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01