HIV-1–specific immune responses in subjects who temporarily contain virus replication after discontinuation of highly active antiretroviral therapy (original) (raw)
Abstract
Therapeutic intervention with highly active antiretroviral therapy (HAART) can lead to suppression of HIV-1 plasma viremia to undetectable levels for 3 or more years. However, adherence to complex drug regimens can prove problematic, and subjects may temporarily discontinue HAART for variable periods. We studied 6 HIV-1–infected individuals who stopped therapy. Off HAART, levels of viremia were suppressed to fewer than 500 copies/mL in 2 subjects for more than 12 and more than 24 months, respectively, and in 1 subject for 4 months on 1 occasion. Three subjects failed to contain plasma viremia. Broad and strong HIV-1–specific immune responses were detected in subjects with prolonged suppression of viral replication. This longitudinal study suggests that containment of HIV-1 replication to low or undetectable levels after discontinuation of HAART is associated with strong virus-specific immune responses. Boosting of HIV-1–specific immune responses should be considered as an adjunctive treatment strategy for HIV-1–infected individuals on HAART.
Introduction
With the discovery of the relationship between a high level of plasma HIV-1 RNA and an increased rate of disease progression (1, 2), therapeutic emphasis in HIV-1 infection has been to reduce plasma viremia to the lowest level possible. Treatment with highly active antiretroviral therapy (HAART) results in a rapid reduction of plasma viremia in most subjects, and suppression of plasma viremia can be maintained for more than 3 years with adherence to the prescribed antiretroviral drug regimen. However, the discovery of a long-lived reservoir of infectious HIV-1 and evidence for ongoing, low-level viral replication in subjects on HAART suggest that subjects would have to take suppressive therapy for many years to eradicate HIV-1 (3–7). HAART is not without potential pitfalls. Adherence to the complex drug regimens can be difficult, with adverse events causing serious problems (8, 9). In addition, HAART is unaffordable for most individuals in developing countries (10).
We report here on 6 subjects who had variable adherence to their prescribed drug regimens. After cessation of drug therapy, 3 of the 6 subjects temporarily contained plasma viremia for 4 to more than 24 months. We have quantified HIV-1–specific immune responses in these individuals and show that suppression of viral replication in the absence of HAART is associated with strong virus-specific immune responses.
Methods
Subjects.
Four of the 6 subjects were enrolled in trial “313,” which provided therapy within 120 days of infection with the combination of ritonavir, zidovudine, and lamivudine (3TC). The clinical characteristics of this cohort have been described elsewhere (11). Subject no. 12 formally withdrew from the trial after 15 months. Further follow-up was limited to visits in December 1997 and September 1998. One subject (no. 3005) was enrolled in trial MMA-197, which provided therapy within 90 days of HIV-1 infection with d4T, ritonavir, saquinavir, and 3TC. The remaining subject, no. 31, was chronically infected with HIV-1 (for approximately 27 months) when enrolled into trial 509. Subject no. 31 was treated with d4T, ritonavir, saquinavir, and ddI. All studies had ethical approval from the Rockefeller University Institutional Review Board.
Adherence to HAART.
Estimates of adherence are based on the percentage of total recommended drug doses taken by the subject during each interval between visits and calculated by the clinical staff. These estimates were corroborated with direct subject interviews.
Quantifying plasma HIV-1 RNA.
Levels of plasma HIV-1 RNA were quantified with an RT-PCR assay (Roche Diagnostics Inc., Branchberg, New Jersey, USA) with a lower limit of detection of 50 copies/mL (with dilution), or with a branched DNA assay (Chiron Corp., Emeryville, California, USA) with a lower limit of 500 copies/mL (for subject no. 31).
Immunological measurements.
The limiting dilution assay, used for quantifying the HIV-1–specific cytotoxic T lymphocyte precursor (CTLp) frequency, has been described previously (12). Results are expressed as CTLp/106 PBMCs.
The enzyme-linked immunoassay (ELISPOT), used for quantifying the release of cytokines from antigen-specific CD8+ T cells, has also been described previously (13). Results are expressed as spot-forming cells (SFCs)/106 PBMCs.
From our previous studies of HIV-1–infected untreated subjects, we have established a descriptive range of strength of CTL responses: low (CTLp, 11–50/106 PBMCs; SFC, 10–200/106 PBMCs), moderate (CTLp, 51–100/106 PBMCs; SFC, 201–500/106 PBMCs), and high (CTLp > 101/106 PBMCs; SFC >501/106 PBMCs) (11–14).
Isolation and characterization of virus isolates.
Viruses were isolated from plasma by coculture with activated donor PBMCs (15). Virus stocks were propagated on stimulated PBMCs. The 50% tissue culture infectious doses (TCID50) were determined as described previously (16).
Binding antibody titers.
The midpoint titers of binding antibody to recombinant, monomeric gp120 from the HIV-1 JR-FL strain were determined by ELISA, as described previously (17). The JR-FL gp120 protein was a gift from P. Maddon (Progenics Inc., Tarrytown, New York, USA).
Neutralization assay.
Neutralization assays were performed as described previously (18), with some modifications (A. Trkola et al., manuscript in preparation). The calculated inhibitory doses (serum neutralization titers) are reported as titers and refer to the reciprocal of the dilution of sera in this final mixture. The titers causing 50% and 90% reduction in p24 production were determined by linear regression analysis. If the appropriate degree of inhibition was not achieved at the lowest serum dilution (1:50), a value of less than 1:50 was recorded.
Results
Plasma viremia and adherence to HAART therapy.
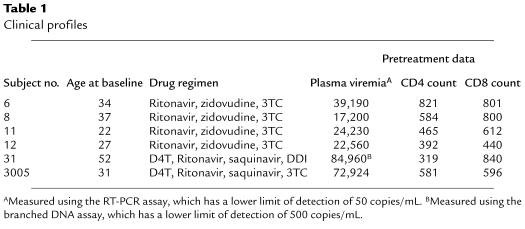
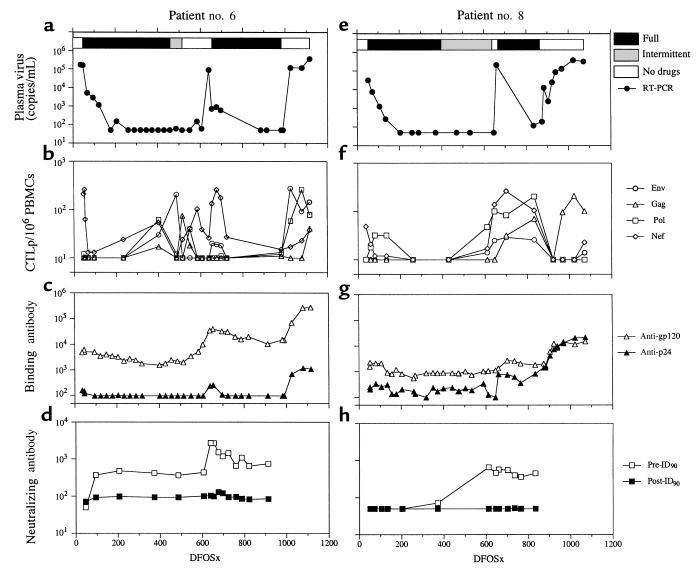
Age at study entry, drug therapy, and pretreatment virological and immunologic data are shown in Table 1. Subjects nos. 11 and 12 had a rapid reduction in plasma viremia, whereas the rate was slower in nos. 6 and 8 (Figure 1, a–e; and Figure 2, a–e). Subjects nos. 6 and 8 were fully adherent to therapy for 13 and 12 months, respectively, whereas nos. 11 and 12 had begun intermittent dosing by 1 month after starting HAART (Figure 1; and Figure 2, a and e). At 692DFOSx, subject no. 11 discontinued HAART completely. Strikingly, plasma viremia remained low for more than 12 months (Figure 2a). Subject no. 12 stopped HAART at 421DFOSx, after intermittent adherence and a 1-month temporary discontinuation (297–325DFOSx) with plasma virus rebound (Figure 2e). After final discontinuation, he had a transient rebound in plasma viremia, which was reduced to fewer than 300 copies/mL without antiretroviral drug therapy. Plasma viremia remained at low or undetectable levels for longer than 24 months (Figure 2e), although relatively few sampling points were available for analysis.
Table 1.
Clinical profiles
Figure 1.
Longitudinal study of virological and immunological parameters in subjects nos. 6 and 8. Plasma viremia is shown in a and e. The horizontal bars at the top of a and e represent the estimation of adherence to HAART. Filled bars reflect full adherence; gray bars intermittent adherence; and open bars no drug therapy. The frequency of HIV-1–specific CTLp/106 PBMCs is shown in b and f. Binding antibody titers to gp120 and p24 are shown in c and g. Neutralizing antibody titers to previral isolates (pre-ID90) and postviral isolates (post-ID90) are shown in d and h.
Figure 2.
Longitudinal study of virological and immunological parameters in subjects nos. 11 and 12. Plasma viremia, measured by RT-PCR, is shown in a and e. The horizontal bars at the top of a and e represent the estimation of adherence to HAART. Filled bars reflect full adherence; gray bars intermittent adherence; and open bars no drug therapy. The frequency of HIV-1–specific CTLp/106 PBMCs is shown in b and f. Binding antibody titers to gp120 and p24 are shown in c and g. Neutralizing antibody titers to previral isolates (pre-ID90) and postviral isolates (post-ID90) are shown in d and h.
Subjects nos. 6 and 8 were fully adherent to HAART for 1 year or more before becoming intermittent with dosings. Subject no. 6 was intermittently adherent to HAART for 2 months and then discontinued therapy at 514DFOSx. His viremia remained at fewer than 150 copies/mL for 4 months (514–639DFOSx) before rebounding to 9 × 104 copies/mL. He resumed HAART at 676DFOSx and remained fully adherent to the drug regimen for the ensuing 10 months, before final discontinuation at 982DFOSx. His plasma viremia rebounded to around 105 copies/mL by 1026DFOSx (Figure 1a). Subject no. 8 discontinued HAART for 1 month at 638DFOSx. Viral replication to a level of 2 × 105 copies/mL was detected at 659DFOSx (Figure 1e) but dropped to low levels with resumption of therapy. At 865DFOSx, he stopped taking all drugs owing to acute hepatitis A infection, and plasma viremia increased to around 3 × 105 copies/mL (Figure 1e).
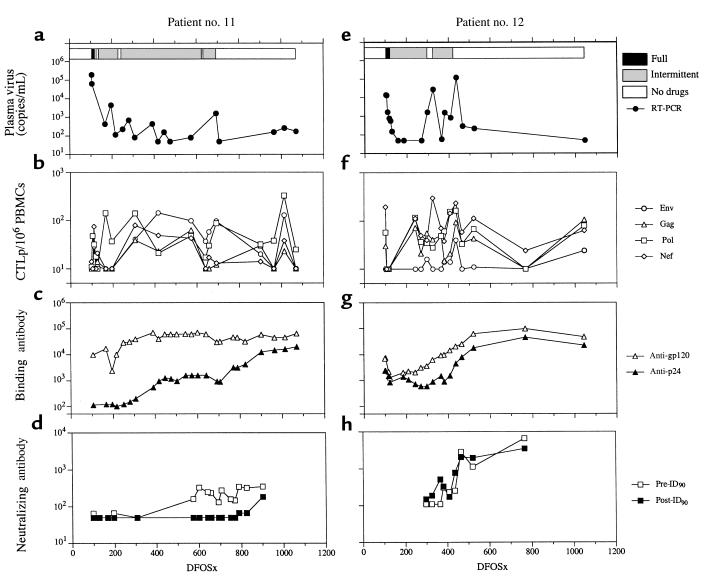
Subject no. 3005 was fully adherent to HAART from 90 to 130DFOSx and was then intermittent for 1 month. He discontinued HAART at 158DFOSx and had a rebound to 72,133 copies/mL. He resumed therapy, and viremia was reduced to 16,193 copies/mL at 228DFOSx (Figure 3c). Subject no. 31 started on HAART therapy in February 1996. He was fully adherent to HAART for 340 days after initiation of treatment (DFIRx), but he temporarily discontinued for 3 days at 340DFIRx. He resumed drugs at full dosing for 275 days and then discontinued all drugs at 618DFIRx. He resumed HAART at 701DFIRx (Figure 3e).
Figure 3.
Longitudinal study of virological and immunological parameters in subjects nos. 11, 3005, and 31. Plasma viremia was measured by RT-PCR (a and c) or bDNA (e). The horizontal bars at the top of a, c, and e represent the estimation of adherence to HAART. Filled bars reflect full adherence; gray bars intermittent adherence; and open bars no drug therapy. The frequency of HIV-1–specific SFC/106 PBMCs is shown in b, d, and f.
HIV-1–specific cellular immune responses.
Before treatment, subjects nos. 11 and 12 had broad HIV-1–specific CTL responses directed to several viral antigens (Figure 2, b and f). In contrast, nos. 6 and 8 had a monospecific-Nef CTL response (Figure 1, b and f). After starting HAART, decay half-lives (_t_1/2) for CTLp ranged from 3 to 8 days, except in subject no. 8, in whom the _t_1/2 was considerably slower (Figure 1f).
When subject no. 11 discontinued HAART at 692DFOSx, he had moderate to strong Env- and Pol-specific CTLp responses, which were maintained for 12 months (Figure 2b). Quantification of antigen-reactive CD8+ T-cell responses using ELISPOT also showed high levels before and after drug discontinuation (Figure 3b). In subject no. 12, plasma viremia rebounded by 436DFOSx, and all HIV-1–specific CTL responses increased (Figure 2f). Plasma viremia was contained to fewer than 300 copies/mL without HAART (465DFOSx), and HIV-1–specific CTLp was maintained at moderate frequency for longer than 24 months (Figure 2, e and f).
HIV-1–specific CTLp responses were at a low level in subject no. 6 but increased when he became intermittent with HAART therapy. At the time of the first HAART cessation (514DFOSx), he had broad and strong CTLp responses. These declined, and only a low Nef-specific CTLp response was present when viral rebound occurred at 639DFOSx (Figure 1b). CTL responses increased with this rebound in viremia (654–696DFOSx). When he stopped therapy for the second time (982DFOSx), his CTLp responses had declined. Viral rebound occurred, and all CTLp responses increased in temporal association. Similarly, HIV-1–specific CTLp responses were at low or undetectable levels in subject no. 8 when he stopped HAART for the first time (638DFOSx). Viremia was not contained, and he resumed therapy. He then stopped all drugs because of acute hepatitis A (865DFOSx), after which all CTLp responses transiently fell below detection (923DFOSx). When viral replication increased, his Gag-specific CTLp response also rose (Figure 1f).
Two other subjects (nos. 31 and 3005) failed to contain viral replication after HAART discontinuation. Quantification of the HIV-1–specific CD8+ T-cell response in subject no. 3005 showed a moderate level of HIV-1 specific SFC at time of intermittent therapy 130–144DFOSx. After discontinuation of therapy, plasma viremia rose to 72,133 copies/mL (200DFOSx); no HIV-1–specific CD8+ T cells were detected (Figure 3, c and d). Subject no. 31 was fully adherent to drug therapy between 343 and 618DFIRx, and HIV-1–specific CD8+ T-cell responses were low or absent. As in subject no. 3005, viremia was not contained and antigen-reactive cells were not detected (Figure 3, e and f).
HIV-1–specific humoral immune responses.
Before therapy, subjects nos. 6, 8, and 12 had moderate anti-gp120 binding antibody titers (midpoint titers of 103–104), whereas subject no. 11 had a higher titer (>104) (Figure 1, c and g; and Figure 2, c and g). Subject no. 11 had gradual increases in both gp120 and p24 binding antibody titers during the first 1–2 years. Anti-p24 titers continued increasing after HAART discontinuation (Figure 2c). The pattern of anti-gp120 and gp-p24 responses observed in subject no. 12 was broadly similar to that seen in no. 11. Subject no. 6 had an increase in anti-gp120 titer to 1:40,000 and a smaller, transient increase in anti-p24 titer at the time of peak plasma virus rebound (639DFSOx). Upon recommencing HAART, anti-gp120 titers slowly declined to 1:10,000. At the second discontinuation, anti-gp120 and anti-p24 binding antibody titers again increased significantly to 1:275,000 and 1:1,100, respectively. A broadly similar pattern of binding antibody responses was observed in no. 8 (Figure 1, c and g).
Before therapy, neutralizing antibody titers were undetectable in subjects nos. 6, 8, and 11. After starting HAART, 90% inhibitory doses of neutralizing antibody to pretreatment viral isolates (pre-ID90) rose in subject no. 6 (from <1:50 to 1:371). The pre-ID90 spiked to 1:2,726 at 639DFOSx when plasma virus peaked, but afterward, it returned to moderate levels (1:745) (Figure 1d). Neutralization titers in subject no. 8 also increased to moderate levels (from 1:72 to 1:652) near the time of detectable plasma virus despite low anti-gp120 titers (never >1:5,000) (Figure 1, g and h). Neutralization titers in subject no. 12 increased to an unusually high level of 1:2,868 after the peak in plasma viremia at 465DFOSx (Figure 2h), whereas neutralization titers remained moderate (<1:350) in subject no. 11 (Figure 2d). Of note, the neutralization antibody titers were lower against post-treatment viral isolates in all 4 subjects (Figure 1; and Figure 2, d and h), suggesting that some degree of viral escape from HIV-1–specific humoral responses may have taken place.
Discussion
Three of 6 HIV-1–infected study subjects suppressed plasma viremia for 4 to more than 24 months after HAART discontinuation. This is surprising, as a majority of subjects who cease HAART experience rapid viral rebound to pretherapy levels (19, 20) with rare exceptions (21, 22). In this study, we assessed virological and immunological factors to determine correlates of viral suppression. The most striking association with suppression of plasma viremia after HAART cessation was the presence of broad and strong HIV-1–specific immune responses.
Previous evidence that HIV-1–specific immune responses contribute to the containment of HIV-1 replication come from both in vitro and in vivo studies. The decline in primary viremia is temporally associated with the HIV-1–specific CTL response (23, 24), and, in some individuals who develop CTL escape mutants in primary infection, viremia rapidly increases (25). However, the maintenance of the HIV-1–specific immune response appears to depend on antigenic stimulation, and these responses decline during suppressive HAART (17, 26–28). In the subjects studied here, intermittent adherence to the drug regimen appeared to stimulate HIV-1–specific immune responses, even at low levels of plasma viremia. Their intermittent adherence seems to have boosted their immune responses by providing a low but sufficient level of antigen, thereby increasing the “immunological set point” — the level of HIV-1–specific immune response in equilibrium with viral replication after seroconversion. This autoimmunization would be a powerful stimulus to boost CTL and antibody responses. The threshold level of viral replication required to boost immune responses seems greater than the low levels of ongoing replication seen in patients with plasma viremia levels below detection (6, 29).
Two patterns of suppression of viral replication upon HAART discontinuation were noted. In the first, exemplified by subjects nos. 11 and 6, discontinuation was not followed by immediate viral rebound. Viral suppression lasted for more than 12 months in subject no. 11 and for 4 months in subject no. 6. At the time of drug discontinuation, broad and strong HIV-1–specific CTL responses were present, along with moderate neutralizing antibody titers. In these cases, we hypothesize that HIV-1–specific CTL responses were of sufficient breadth and strength to maintain viral replication at a low level. In the second pattern, as seen in subject no. 12, viral rebound occurred but was contained to a low level without restarting drug therapy. Neutralizing antibody titers were boosted to high levels, as were HIV-1–specific CTL responses. We postulate that in this case, HIV-1–specific CTL and neutralizing antibodies controlled viral replication, and viremia was reduced to below detection. The autologous neutralization titer seen in subject no. 12 was exceptionally high. We hypothesize that the strength and breadth of HIV-1–specific immune responses in subject no. 8 were not sufficient to contain viral replication.
Why did suppression of viral replication fail in subjects nos. 6, 31, and 3005? In subject no. 6, viremia was suppressed for 4 months and his CTL responses waned, leaving a low monospecific-Nef response. Putative mechanisms for the reduction in frequency or effectiveness of CTL include virus escape from CTL recognition (25, 30), clonal exhaustion, lack of CD4+ help (31), and anergic antigen-reactive T cells (32). When subject no. 6 resumed HAART after breakthrough viremia, his HIV-1–specific immune responses again declined, resulting in low CTLp levels when he stopped again. This may explain why he failed to control viremia upon second discontinuation. Subjects nos. 31 and 3005 had low or absent HIV-1–specific CD8+ T-cell responses before HAART discontinuation, failed to contain viremia, and, interestingly, had no increase in frequency of HIV-1–specific SFC with viral rebound.
The majority of patients who are poorly adherent to their drug regimens and discontinue drugs fail to experience prolonged suppression of plasma viremia as observed in the 3 patients reported here. Our results suggest that the patients who contained viral replication had higher levels of immune responses than those who did not. However, it is uncertain how this may be achieved, and uncontrolled discontinuation of HAART may have serious consequences, including the development of drug resistance. Structured therapeutic intervention studies designed to test the hypothesis that the immunological set point can be increased and plasma viremia reduced for prolonged periods should therefore proceed with caution. Our data also support the exploration of an alternative approach: boosting HIV-1–specific immune responses in HAART-treated individuals by postinfection therapeutic immunization.
Acknowledgments
We gratefully acknowledge the assistance of the nursing staff at the Rockefeller University General Clinical Research Center Hospital; J. Jin and J. Song for processing PBMC samples; J. Hu, M. Larsson, and N. Bhardwaj for advice on ELISPOT assays; and M. Small and W. Chen for expert administrative help. This work was supported by grants from the National Institutes of Health (NIH; U01AI41534, MO1-RR00102, R01 AI44595, and R37 AI36082). Gabriel Ortiz is a student in the Tri-Institutional M.D.-Ph.D. Program (Rockefeller University, Cornell University Medical College at New York Hospital, and Memorial Sloan Kettering Cancer Center; New York, New York, USA) and is supported by NIH Medical Scientists Training Program (grant GM 0773) and Minority Pre-doctoral Fellowship (F31 GM20068-01).
Footnotes
Gabriel M. Ortiz and Douglas F. Nixon contributed equally to this work.
References
- 1.Mellors J, et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien T, et al. Serum HIV-1 RNA levels and time to development of AIDS in the multicenter hemophilia cohort study. JAMA. 1996;276:105–110. [PubMed] [Google Scholar]
- 3.Chun T, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong J, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 5.Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, et al. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 7.Finzi D, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 8.Stephenson J. AIDS researchers target poor adherence. JAMA. 1999;281:1069. doi: 10.1001/jama.281.12.1069. [DOI] [PubMed] [Google Scholar]
- 9.Stephenson J. Studies reveal early impact of HIV infection, effects of treatment. JAMA. 1998;279:641–642. [PubMed] [Google Scholar]
- 10.Arya S. Antiretroviral therapy in countries with low health expenditure. Lancet. 1998;351:1433–1434. doi: 10.1016/S0140-6736(05)79479-4. [DOI] [PubMed] [Google Scholar]
- 11.Markowitz M, et al. The effect of commencing antiretroviral therapy soon after HIV-1 infection on viral replication and antiviral immune responses. J Infect Dis. 1999;179:525–537. doi: 10.1086/314628. [DOI] [PubMed] [Google Scholar]
- 12.Jin X, et al. Longitudinal and cross-sectional analysis of CTL responses and their relationship to vertical HIV transmission. J Infect Dis. 1998;178:1317–1326. doi: 10.1086/314455. [DOI] [PubMed] [Google Scholar]
- 13.Larsson M, et al. A recombinant vaccinia virus based ELISPOT assay detects high frequencies of Pol-specific CD8 T cells in HIV-1-positive individuals. AIDS. 1999;13:767–777. doi: 10.1097/00002030-199905070-00005. [DOI] [PubMed] [Google Scholar]
- 14.Nixon DF, et al. Molecular tracking of an HIV-1 nef specific CTL clone shows persistence of clone-specific T-cell receptor DNA but not mRNA following early combination antiretroviral therapy. Immunol Lett. 1999;66:219–228. doi: 10.1016/s0165-2478(98)00162-x. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y, Qin L, Zhang L, Safrit J, Ho D. Characterization of long-term survivors of HIV-1 infection. Immunol Lett. 1996;51:7–13. doi: 10.1016/0165-2478(96)02548-5. [DOI] [PubMed] [Google Scholar]
- 16.Purtscher M, et al. Restricted antigenic variability of the epitope recognized by the neutralizing gp41 antibody 2F5. AIDS. 1996;10:587–593. doi: 10.1097/00002030-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Morris L, et al. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J Exp Med. 1998;188:233–245. doi: 10.1084/jem.188.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connor R, et al. Immunological and virological analyses of persons infected by HIV-1 while participating in trials of recombinant gp120 subunit vaccines. J Virol. 1998;72:1552–1576. doi: 10.1128/jvi.72.2.1552-1576.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jubault V, et al. High rebound of plasma and cellular HIV load after discontinuation of triple combination therapy. AIDS. 1998;12:2358–2359. [PubMed] [Google Scholar]
- 20.Staszewski S, et al. Rebound of HIV-1 viral load after suppression to very low levels. AIDS. 1998;12:2360. [PubMed] [Google Scholar]
- 21.Vila J, et al. Absence of viral rebound after treatment of HIV-infected patients with didanosine and hydroxycarbamide. Lancet. 1997;350:635–636. doi: 10.1016/S0140-6736(97)24035-3. [DOI] [PubMed] [Google Scholar]
- 22.Lisziewicz J, Jessen H, Finzi D, Siliciano R, Lori F. HIV-1 suppression by early treatment with hydroxyurea, didanosine, and a protease inhibitor. Lancet. 1998;352:199–200. doi: 10.1016/S0140-6736(98)24029-3. [DOI] [PubMed] [Google Scholar]
- 23.Koup R, et al. Temporal association of cellular immune responses with the initial control of viremia in primary HIV-1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borrow P, Lewicki H, Hahn B, Shaw G, Oldstone M. Virus-specific CD8+ CTL activity associated with control of viremia in primary HIV-1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borrow P, et al. Antiviral pressure exerted by HIV-1–specific CTL during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 26.Ogg G, et al. Quantitation of HIV-1-specific CTL and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 27.Ogg G, et al. Decay kinetics of HIV-specific effector CTL after combination antiretroviral therapy. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray C, et al. Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy. J Immunol. 1999;162:1780–1788. [PubMed] [Google Scholar]
- 29.Furtado M, et al. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N Engl J Med. 1999;340:1614–1622. doi: 10.1056/NEJM199905273402102. [DOI] [PubMed] [Google Scholar]
- 30.Price D, et al. Positive selection of HIV-1 CTL escape variants during primary infection. Proc Natl Acad Sci USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg E, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 32.Zajac A, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]