A proinflammatory role for IL-18 in rheumatoid arthritis (original) (raw)
Abstract
IL-18 is a novel cytokine with pleiotropic activities critical to the development of T-helper 1 (Th1) responses. We detected IL-18 mRNA and protein within rheumatoid arthritis (RA) synovial tissues in significantly higher levels than in osteoarthritis controls. Similarly, IL-18 receptor expression was detected on synovial lymphocytes and macrophages. Together with IL-12 or IL-15, IL-18 induced significant IFN-γ production by synovial tissues in vitro. IL-18 independently promoted GM-CSF and nitric oxide production, and it induced significant TNF-α synthesis by CD14+ macrophages in synovial cultures; the latter effect was potentiated by IL-12 or IL-15. TNF-α and IFN-γ synthesis was suppressed by IL-10 and TGF-β. IL-18 production in primary synovial cultures and purified synovial fibroblasts was, in turn, upregulated by TNF-α and IL-1β, suggesting that monokine expression can feed back to promote Th1 cell development in synovial membrane. Finally, IL-18 administration to collagen/incomplete Freund’s adjuvant–immunized DBA/1 mice facilitated the development of an erosive, inflammatory arthritis, suggesting that IL-18 can be proinflammatory in vivo. Together, these data indicate that synergistic combinations of IL-18, IL-12, and IL-15 may be of importance in sustaining both Th1 responses and monokine production in RA.
J. Clin. Invest. 104:1393–1401 (1999).
Introduction
A critical recent advance in cellular immunology has been the discovery of functionally distinct T-cell subsets, Th1 and Th2, separated on the basis of their cytokine expression. That such functional dichotomy has direct relevance to autoimmune disease has been recognized with the demonstration of distinct roles for Th1 and Th2 responses in several autoimmune models, including collagen-induced arthritis (CIA) and nonobese diabetic (NOD) mice (1–3). Reversal of such T-cell polarization likely confers therapeutic advantage, as immunopathology can be ameliorated or abrogated by administration of cytokines of reciprocal activity (2, 4).
Several data suggest that rheumatoid arthritis (RA) is a “Th1-associated” disease (5–8). However, the factors that initiate and sustain Th1 responses in RA synovium are unclear. Animal and human in vitro studies have demonstrated a critical role for macrophage-derived IL-12 in Th1 cell development (9). Although IL-12 may be detected in RA synovium, the absolute levels present remain unclear (9–11). Recently, a novel cytokine, IL-18, has been cloned that exhibits powerful Th1-promoting activities in synergy with IL-12 (12). Pro–IL-18 is cleaved by IL-1β–converting enzyme (ICE, caspase 1) to yield an active 18-kDa glycoprotein with significant structural similarity to IL-1 (13). IL-18 induces proliferation, upregulates IL-2Rα expression, and promotes IFN-γ, TNF-α, and GM-CSF production by Th1 clones (14, 15). IL-18 enhances T-cell and natural killer–cell (NK-cell) cytotoxicity and directly induces IFN-γ production by NK cells (16). Synthesis of IL-18 has been described in macrophages, Kupffer cells, keratinocytes, articular chondrocytes, and osteoblasts (15, 17, 18). IL-18 mRNA is upregulated in NOD mice and the murine IL-18 gene maps to the Idd2 susceptibility locus, suggesting a potential role in predisposition to autoimmunity (19). Moreover protective Th1 responses during murine Cryptococcus neoformans or yersinia infections may be abrogated or enhanced by manipulation of IL-18 expression (20, 21).
We therefore sought to identify IL-18 expression in tissues from patients with RA, to establish its potential functional effects within synovial membrane, and to determine those factors that regulate its expression therein. We report here that IL-18 is present in significantly elevated levels in the synovium of patients with RA but not in that of those with osteoarthritis (OA). Together with IL-12 and IL-15, IL-18 induced and sustained articular Th1 cell responses, manifest as IFN-γ production. Furthermore, IL-18 independently promoted monokine production, through a direct effect on synovial macrophages.
Methods
Reagents.
DMEM, RPMI 1640, glutamine, penicillin/streptomycin, FCS, LPS, and trypsin/EDTA were obtained from Sigma (Poole, United Kingdom), and collagenase was obtained from Worthington Scientific (Lakewood, New Jersey, USA). Recombinant human TNF-α and IFN-γ were gifts from G.R Adolf (Bender Wien, Vienna, Austria). Recombinant human IL-1β, IL-10, IL-12, TGF-β, and murine IL-18 were from R&D Systems (Abingdon, United Kingdom).
Cloning, expression, purification, and biologic activity of human IL-18.
Total RNA was extracted from THP-1 cells (stimulated for 18 hours with human IFN-γ [100 U/mL] and LPS [1 μg/mL]) using the TRIzol Reagent (Life Technologies, Paisley, United Kingdom). RNA was transcribed into cDNA using SuperScript II RT (Life Technologies) according to a standard protocol. Primer set pairs designed from human IL-18 sequence data were used to clone hIL-18 from the cDNA: sense 5′ATCAGGATCCTTTGGCAAGCTTGAATCTAAATTATC3′ antisense 5′ATAGGTCGACTTCGTTTTGAACAGTGAACATTATAG3′ (product 487 bp). The PCR product was confirmed by sequencing, cloned into the pQE-30 expression vector (QIAGEN, Dorking, United Kingdom), and expressed in Escherichia coli M15 (QIAGEN). After induction with isopropyl-D-thiogalactoside (BioLine, London, United Kingdom), IL-18 was extracted under native conditions and purified as a 6× histidine-tagged fusion protein using a nickel agarose purification system (QIAGEN) according to the manufacturer’s recommendations. Purity was analyzed by SDS-PAGE and Coomassie blue staining, which showed a single-protein band at approximately 23,000. Biologic activity was determined by IFN-γ induction from PBMC cultures (12). Cytokines used for in vitro assays were free of LPS as assessed by the limulus amoebocyte assay (Sigma).
Generation of anti–IL-18 antibodies.
Monoclonal anti-human IL-18 antibodies were generated as described previously (22). Briefly, after immunization and boosting of BALB/c mice with recombinant IL-18, spleen cells were removed for fusion with NS0 cells. Positive clones were selected and checked for specificity by ELISA. Clone D3B6 (IgG1) was chosen for routine use. A polyclonal sheep anti-human IL-18 was raised by standard methods (SAPU, Carluke, United Kingdom). Confirmatory monoclonal anti-human IL-18 mAb’s (clones 1.51E3E1, 3.51D3D4, and 6.42D4D1) were a gift from A. Jackson (Imperial Cancer Research Fund, Leeds, United Kingdom). Antibody specificity was confirmed by Western blotting. Affinity-purified material electrophoresed in 15% SDS-PAGE was transferred onto nitrocellulose (Sigma). After blocking overnight, the membrane was incubated with D3B6, followed by sheep anti-mouse IgG-HRP (Amersham Life Sciences, Little Chalfont, United Kingdom), and developed by enhanced chemiluminescence (Amersham International, United Kingdom). D3B6 bound IL-18 at molecular weight 23,000, but not IL-1β (data not shown).
Clinical materials and cell preparation.
Synovial membranes (arthroplasty specimens), fluids, and blood samples were obtained from patients with RA and patients with OA satisfying American College of Rheumatology criteria (23). Single-cell suspensions were prepared from membranes as described previously (24). Briefly, tissue fragments were digested with collagenase (2.5 mg/mL) at 37°C for 2 hours, filtered through gauze, and washed. If required, synovial fibroblasts (SFBs) were grown out of primary cell suspensions in 25-cm2 flasks and used from third to fifth passages (>99% AS02+ by flow cytometry; Binding Site, Birmingham, United Kingdom). Blood and synovial fluid (SF) mononuclear cell fractions were obtained by density gradient centrifugation through Lymphoprep (Nycomed, Oslo, Norway). Culture was performed at 37°C/5%CO2 using Nunclon tissue culture plastics (Life Technologies) in RPMI-1640 supplemented with 10% FCS, 2 mM glutamine, 100 IU/mL penicillin, 100 mg/mL streptomycin, and 1.25 μg/mL amphotericin B. Cells at 1 × 106/mL were cultured for 48 hours unless otherwise stated. Supernatants were stored at –70°C. SF mononuclear cells for intracellular TNF-α expression were cultured for 4 hours with 10 μg/mL Brefeldin A (Sigma).
Cytokine and cytokine receptor estimation.
IL-18 was measured by ELISA, using either antibodies from R&D Systems or using the mAb D3B6 in conjunction with sheep anti-human IL-18 raised in our laboratories. Briefly, Immulon4 microtiter plates (Dynex, Billingshurst, United Kingdom) were coated with murine monoclonal anti-human IL-18 antibody (R&D Systems or D3B6). Recombinant IL-18 or samples were incubated and detected with either biotin-conjugated goat anti-human IL-18 (R&D Systems) or biotin-conjugated sheep anti-human IL-18 and developed with Streptavidin-HRP (SAPU) and TMB substrate (Dynex). Rheumatoid factor (RF) was removed from SF by prior incubation with RapiTex beads (Behring Diagnostics, Marburg, Germany). As an additional specificity control and to minimize RF contamination, monoclonal murine anti–IL-1β (R&D Systems) was used as an irrelevant capture antibody, followed by sample, secondary antibodies, and detection system as before. IFN-γ was measured by ELISA as already described here, using paired anti-human IFN-γ antibodies (gift of T. Meager, National Institute for Biological Standards and Control, Potters Bar, United Kingdom), detection with horseradish peroxidase–conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA), and TMB substrate. GM-CSF was measured by ELISA (Genzyme, Cambridge, Massachusetts, USA). Intracellular TNF-α expression was identified in permeabilized synovial mononuclear populations by FACS analysis (Becton Dickinson, Cowley, United Kingdom) using PE-conjugated monoclonal anti–TNF-α antibody (R&D Systems), together with FITC anti-CD3 or FITC anti-CD14 antibodies (Becton Dickinson). IL-18 receptor expression was determined by FACS analysis using monoclonal anti-human IL-18 receptor antibody (R&D Systems).
Expression of IL-18 and IL-18R mRNA in synovial tissue.
cDNA was prepared from synovial tissue and fibroblasts using TRIzol extraction (Life Technologies) and RT. IL-18 mRNA was detected using primers described previously for cloning IL-18. Primers for IL-18R were: sense 5′CCCAACGATAAAGAAGAACGC C3′ antisense 5′TGTCTGTGCCTCCCGTGCTGGC3′ (product 419 bp), and for β-actin: sense 5′GTGGGGCGCCCCAGGCACCA3′ antisense 5′CTCCTTAATGTCAC GCACG ATTTC3′ (product 548 bp).
Nitric oxide estimation.
Nitric oxide (NO) was measured as its oxidized product, nitrite, using the Griess reaction (24).
Immunohistochemistry.
Formaldehyde-fixed paraffin sections (3 μm) were stained by a standard Streptavidin-HRP protocol. Product was viewed with 3,3′-dia-minobenzidine tetrachloride (DAB; Sigma) and counterstained with hematoxylin (Sigma). IL-18 expression was quantified by 2 independent observers by light microscopy. Staining was quantified from 0 to 4 as follows. Lining layer (LL)/interstitial (IS) areas: 0, no staining; 1, <5% positive cells/nuclei per high power field (HPF; ×400); 2, 5–30%; 3, 30–90%; 4, >90%. Lymphocytic aggregate (LA) area: 0, no staining; 1, <5%; 2, 5–20%; 3, 20–40%; 4, >40%. Positive vessels were expressed as percent of total vessels per HPF. For double staining, sections were first stained with anti–IL-18 and developed with SG peroxidase substrate (blue; Vector Laboratories Ltd., Peterborough, United Kingdom). After hydrogen peroxide (Sigma) treatment to quench residual HRP activity, sections were further stained with anti-CD68 or anti-CD3 antibody (DAKO, Ely, United Kingdom) and developed with DAB (brown), without nuclear counterstain. Negative controls, including the omission of 1 or both primary antibodies or the addition of isotype-matched control antibodies of irrelevant specificity, confirmed the absence of cross-reactivity between anti–IL-18, anti-CD3, or anti-CD68 antibodies. SFBs were formaldehyde fixed in situ in Lab-Tek chamber slides (Nunclon) and stained as already described here.
Induction and assessment of CIA.
CIA was induced as described previously (25). Briefly, male DBA/1 mice (Harlan Olac, Bicester, United Kingdom) were immunized (intradermally) with 200 μg of type II bovine collagen (CII; Sigma) in CFA or incomplete adjuvant (IFA; Difco Laboratories, Detroit, Michigan, USA). After intraperitoneal CII (200 μg in PBS) challenge on day 21, arthritis development was scored by erythema, swelling, or loss function per paw (scale 0–3; maximum, 12 per mouse) by a treatment-blind observer. Mice were sacrificed, hind limbs were fixed in 10% neutral-buffered formalin, and 5-μm sections were stained with hematoxylin and eosin (Sigma). Mice immunized with CII/IFA also received intraperitoneal injections of either murine IL-18 (100 ng) in PBS/0.1% BSA or PBS/0.1% BSA from day –1 to day 4 and from day 20 to day 24.
Statistical analysis.
This was performed on Minitab software, (State College, Pennsylvania, USA) using paired t test and Mann-Whitney analyses as appropriate.
Results
IL-18 and IL-18 receptor expression in RA synovial membrane.
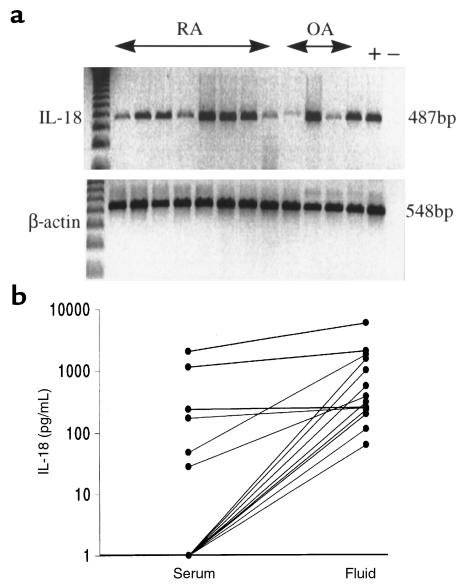
We sought to identify IL-18 mRNA and protein expression within RA synovial tissues. Using RT-PCR, we demonstrated constitutive IL-18 mRNA expression in 20 of 20 RA synovial samples (Figure 1a) and in 8 of 10 OA samples examined. IL-18 was detected by ELISA in 12 of 18 SFs obtained from patients with RA (Figure 1b), whereas matched sera typically contained low levels of IL-18 only (SF vs. serum: P < 0.005). Prior removal of rheumatoid factor did not alter the levels of IL-18 detected. Moreover, no significant signal was obtained when 8 SFs were assayed using anti–IL-1β antibody as a capture antibody in an otherwise identical ELISA protocol. Thus, neither cross-reactivity with IL-1 nor RF contamination is likely to explain our observations. The median concentration detected in positive samples was 342 pg/mL (intraquartile range, 210–1,606), comparable to TNF-α concentrations normally detected in SF (11). IL-12 (p40) concentrations in the SF (median, 82 pg/mL; IQ range, 0–313) did not correlate with those of IL-18. These data clearly indicate that IL-18 is expressed in RA synovial membrane, and, because secretion of IL-18 requires precursor cleavage (13), they strongly suggest that mature IL-18 is present in the synovial compartment.
Figure 1.
(a) IL-18 mRNA was detected in RA and OA synovial membranes by RT-PCR (representative of 20 RA and 10 OA tissues). (b) Significantly higher levels of IL-18 were detected in RA SF than in sera (18 matched samples assayed after removal of rheumatoid factor).
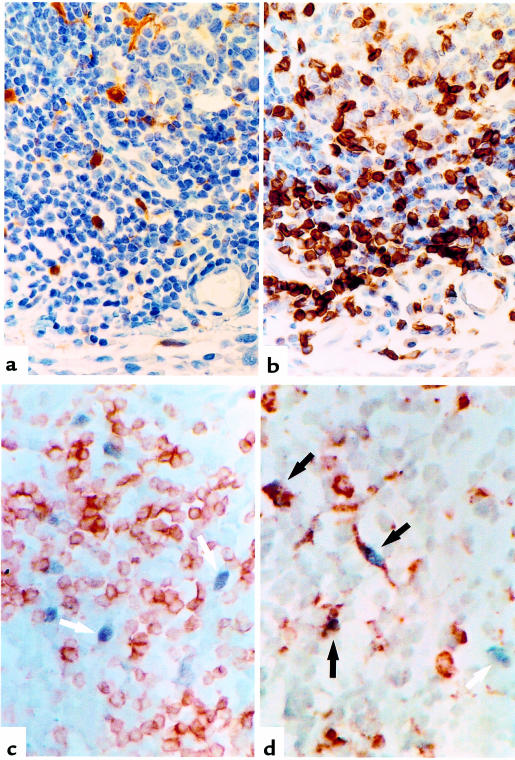
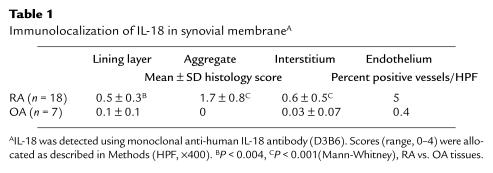
To determine the distribution of IL-18 expression, we generated an mAb to human IL-18 (clone D3B6) that exhibited a cytoplasmic staining pattern in RA synovial tissues (Figure 2a). Staining was neutralized by prior incubation with recombinant IL-18, but not with the structurally related cytokine IL-1β (data not shown). IL-18 staining was detected in 16 of 18 RA synovia examined. IL-18 expression was most prominent within and adjacent to lymphocytic aggregates (Table 1) and was demonstrated using double staining to reside primarily in CD68+ macrophages. A small population of CD68–, IL-18+ cells were also detected, likely representing fibroblasts (Figure 2d). These appearances were similar to those in germinal centers in inflamed human tonsil (data not shown). IL-18 staining was also detected within and adjacent to the lining layer, albeit at lower levels (Table 1; Figure 2a). Double staining indicated that this IL-18 expression was predominantly, but not exclusively, found within CD68+ macrophages. CD3+ lymphocytes were consistently negative (Figure 2, c and d). This distribution is in contrast to that of other monokines such as IL-1β, TNF-α, or IL-12, which are usually prominent in lining and sublining layer macrophages (10, 11). Expression of IL-18 in endothelial cells was present in 9 of 18 tissues examined at low levels only (5 ± 7% vessels per HPF). Little IL-18 protein was detected in OA synovial membranes (Table 1). Confirmatory immunohistology was performed using a further series of 3 anti-human IL-18 mABs, raised from discrete clones, which revealed an identical, neutralizable staining pattern in parallel RA synovial tissues (data not shown).
Figure 2.
(a) IL-18 was detected immunochemically (brown) in synovial membrane using anti–IL-18 antibody (D3B6). (b) CD3 localization (brown) in parallel sections indicated IL-18 expression in T cell–rich aggregates. Double staining with anti–IL-18 (blue/black) and (c) anti-CD3 (brown) or (d) anti-CD68 (brown) antibodies, without nuclear counterstain, localized IL-18 mainly to CD68+ macrophages. (Black arrow, double positive; white arrow, IL-18 single positive).
Table 1.
Immunolocalization of IL-18 in synovial membraneA
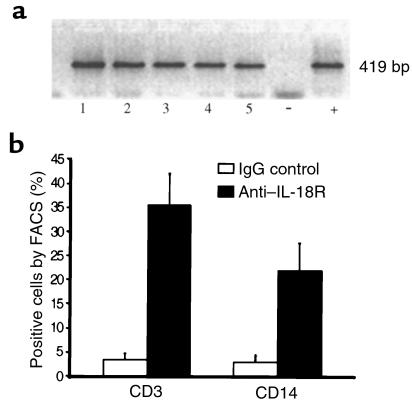
We next sought evidence for IL-18R expression using reagents specific for its unique α chain. IL-18Rα mRNA was detected by RT-PCR in 10 of 10 RA synovial membranes examined (Figure 3), with product specificity confirmed by sequencing. Cellular expression of IL-18Rα protein was characterized by FACS analysis of nonpermeabilized SF mononuclear cells. Significant subpopulations of CD3+ SF lymphocytes and CD14+ macrophages expressed IL-18R (Figure 3).
Figure 3.
(a) IL-18 receptor mRNA expression was detected by RT-PCR in 10 of 10 RA synovial membranes (5 representative data are shown in lanes 1–5). LPS-stimulated THP-1 cells served as positive control. (b) Pooled data (mean ± SEM) to characterize IL-18R+ cells in RA synovial fluid mononuclear cells (n = 8 patients).
IL-18 induced IFN-γ production in RA synovial membrane.
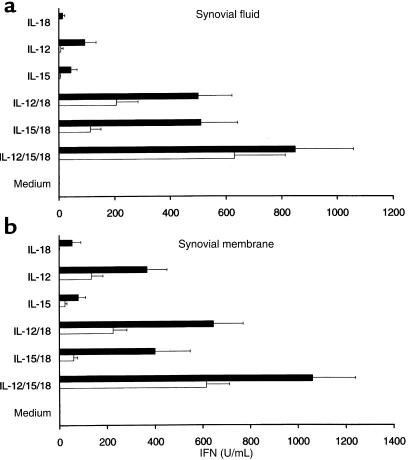
The described biologic effects of IL-18 (26, 27) suggested that it might promote synovial inflammation. We therefore investigated the effects of IL-18 on cytokine synthesis by RA synovial tissues in vitro. IL-18 and IL-12 alone induced low levels of IFN-γ synthesis by cultures of either SF mononuclear cells or synovial membrane–derived single-cell suspensions (Figure 4). Such synthesis was consistently augmented by addition of IL-18 in combination with IL-12 (Figure 4; P = 0.005 for SF mononuclear cells; P = 0.0002 for synovial membrane–derived single-cell suspensions). Other proinflammatory cytokines may independently interact with IL-18. We therefore tested the ability of IL-15, a T-cell activatory cytokine that we previously detected in synovial membrane (28, 29), to synergize with IL-18. IL-15 appeared as potent as IL-12 in combining with IL-18 to promote IFN-γ production by synovial tissues in vitro (Figure 4). Moreover, in all synovial tissues tested (n = 10), the combination of IL-12, IL-15, and IL-18 proved optimal in inducing IFN-γ production. The absolute levels of IFN-γ detected ex vivo in synovial tissues, however, are usually low (30), although intracellular FACS and ELISPOT analyses indicate that a majority of synovial T cells exhibit the potential for IFN-γ expression (7, 8). Thus, we investigated the effect of coincident IL-10 and TGF-β addition on IFN-γ production in vitro. IFN-γ levels induced by IL-18 in combination with IL-12 or IL-15 were consistently suppressed in the presence of IL-10 and TGF-β (Figure 4; P = 0.002 for SF mononuclear cells; P = 0.038 for synovial membrane–derived single-cell suspensions). These data indicate that combinations of T-cell activatory cytokines, which may be expressed individually at low concentrations, can sustain synovial Th1 responses. Moreover, their functional effects are likely modified by the presence of anti-inflammatory cytokine activities.
Figure 4.
Recombinant IL-18 (100 ng/mL), IL-12 (5 ng/mL), and/or IL-15 (100 ng/mL) were added to SF (a) and synovial membrane (b) cultures for 48 hours in the presence (empty boxes) or absence of IL-10 (20 ng/mL)/TGF-β (5 ng/mL) (filled boxes), and IFN-γ production was determined by ELISA. Data (mean ± SEM) are pooled from 10 individual RA patient samples.
Enhanced synovial monokine and NO expression by IL-18.
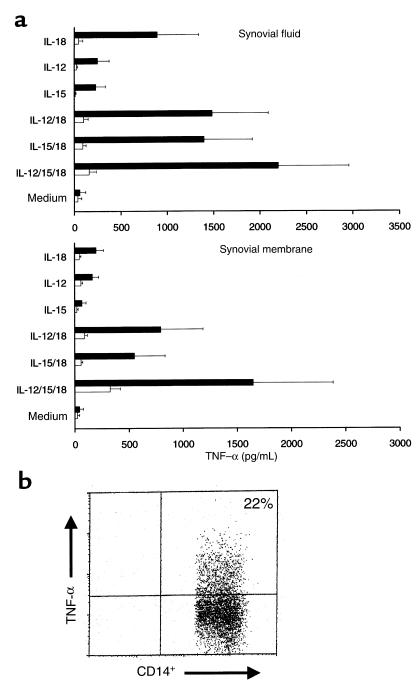
Clinical intervention studies have clearly established the importance of TNF-α in RA pathogenesis (31). We therefore determined the effect of IL-18 on TNF-α production. IL-18 and IL-12 individually induced TNF-α production by both SF mononuclear cells and synovial membrane cultures (Figure 5). Combination of IL-12 with IL-18 induced synergistic enhancement of TNF-α production in both culture systems (P = 0.002 for both versus medium cultures). Addition of IL-15 alone induced modest levels of TNF-α production, which were significantly enhanced by combination with IL-18 (P = 0.03 for SF mononuclear cells; P = 0.013 for synovial membrane cultures) or IL-12/IL-18 (P = 0.01 and P = 0.002, respectively) (Figure 5). The presence of IL-10 and TGF-β in combination effectively abrogated the synthesis of TNF-α in these cultures. To determine the primary cellular source of TNF-α production, we performed intracellular FACS analysis in a subset of SF cultures, which consistently revealed TNF-α expression in CD14+ macrophages, but not in CD3+ T lymphocytes, within 4 hours of addition of IL-18 and Brefeldin A (Figure 5c). Similar data were obtained when IL-12, IL-15, and IL-18 were added in combination (data not shown). To determine potential effects on other monokines, we investigated the ability of IL-18 to upregulate GM-CSF synthesis in synovial cultures, as GM-CSF can be independently induced by IL-18 in PBMCs (27). Significant upregulation of GM-CSF production was observed in 9 of 9 synovial membrane cultures after addition of 100 ng/mL IL-18 (medium 270 ± 58 pg/mL vs. IL-18 1,889 ± 242 pg/mL; P < 0.004). No further enhancement was induced by coincident addition of either IL-12 or IL-15. These data clearly indicate that the functional potential of IL-18 in synovial membrane extends beyond Th1 regulation to include direct effects on macrophages to promote cytokine expression.
Figure 5.
(a) TNF-α production in cultures, identical to those described in Figure 4, was determined by ELISA. Data (mean ± SEM) are pooled from 10 individual RA patients, each cultured in triplicate. (b) Enhanced intracellular TNF-α expression in CD14+ monocytes within synovial fluid mononuclear cells measured by FACS after addition of 100 ng/mL IL-18. Data are representative of 10 similar experiments. Isotype-matched control antibody staining was less than 1%.
NO production is a feature of inflamed synovial membrane, where it is produced by SFBs and by macrophages (24). Because NO may regulate Th1 responses (32), we tested the ability of IL-18 to promote NO production in synovial membrane cultures. Significant induction of nitrite production was observed in 9 of 9 synovial membranes examined (medium 1 μM vs. IL-18 14 ± 5.6 μM; P < 0.008). Maximal nitrite production observed with the cytokine cocktail, IL-18/IL-12/IL-15 (29 ± 7.4 μM), was significantly higher than that obtained with IL-18 alone (P = 0.03).
Proinflammatory effects of IL-18 in vivo.
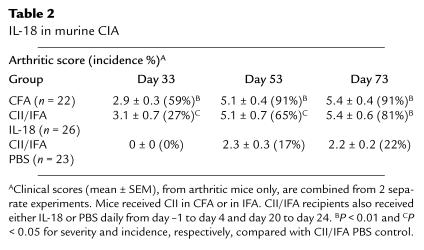
Murine CIA is a useful model in which the critical role of cytokines, such as TNF-α and IL-1, have been characterized in vivo (11, 31). We used CIA to investigate whether IL-18 could promote articular inflammation. DBA/1 mice immunized with CII in IFA developed low incidence of mild arthritis. In contrast, mice in which recombinant murine IL-18 was coadministered during CII/IFA priming (day –1 to day 4) and challenge (day 20 to day 24) developed high incidence and severity of inflammatory arthritis (P < 0.01; Table 2), which was comparable in severity by day 33, and in incidence by day 73, to that achieved in positive control animals that received CII in CFA. Histological examination confirmed the presence of synovial hyperplasia and inflammation and cartilage destruction (data not shown). These data clearly demonstrate that IL-18 is capable of promoting destructive inflammatory arthritis.
Table 2.
IL-18 in murine CIA
Regulation of IL-18 production in synovial tissues.
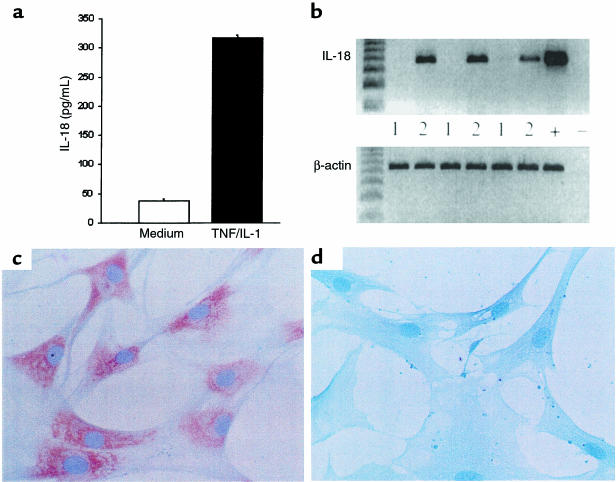
IL-18 synthesis has been described mainly from monocytic cells responding to bacterial products and proinflammatory cytokines. Although IL-18 mRNA was easily detected by RT-PCR, unstimulated primary synovial membrane cultures did not consistently produce IL-18 protein at levels detectable by ELISA (sensitivity, 35 pg/mL) (Figure 6a), although IL-6 was always present (data not shown). Addition of IL-1β and TNF-α induced significant IL-18 production in 4 of 6 synovial membrane cultures (Figure 6a). Because IL-18 mRNA was constitutively expressed in macrophages and in primary synovial cultures, we next sought IL-18 mRNA-negative cells of synovial origin. Histological examination had suggested that a small population of SFBs might express IL-18 in situ. RA SFB cultures were therefore established that did not express IL-18 mRNA, enabling us to examine directly those factors required to upregulate novel IL-18 gene expression. Addition of TNF-α and IL-1β induced IL-18 mRNA expression in 10 of 10 such SFB lines tested (Figure 6b). Induction of IL-18 protein expression was identified immunohistochemically using D3B6 (Figure 6, c and d), the specificity of which was confirmed in this culture system by effective prior neutralization with recombinant IL-18. Parallel experiments demonstrated upregulation of IL-15, but not IL-12, and mRNA and protein expression by TNF-α and IL-1β (data not shown). Together, these data suggest that monokines, themselves upregulated by IL-18, can in turn promote IL-18 gene expression and protein secretion. Moreover, under the influence of TNF-α and IL-1β, SFBs may contribute to this process, although their quantitative effect may be minor.
Figure 6.
(a) IL-18 from synovial membrane cultures representative of 4 positive cultures: 2 were negative. (b) Upregulation of IL-18 mRNA in SFB cell lines (3rd passage, n = 10 RA patients) by TNF-α and IL-1β in vitro. Immunohistological localization in SFBs using D3B6 mAb showed that IL-18 was present after (c), but not before (d), stimulation with TNF-α and IL-1β.
Discussion
The present study set out to examine the role of IL-18 in regulating Th1 responses in RA synovial membrane. Our data suggest that IL-18 can indeed promote articular Th1 responses but may also act directly on macrophages to induce proinflammatory cytokine production. Moreover, IL-18 promoted CIA in vivo, suggesting that these in vitro observations are of pathophysiological importance.
RA is most likely a Th1-associated disease. Those T cell–derived cytokines that can be detected in synovial membrane, albeit at low levels, are of Th1 type, with relative absence of IL-4 or IL-5 (5). T-cell clones grown out of synovial tissues are similarly usually of Th1 phenotype (6). Up to 80% of synovial T cells are CCR5+ (33), and when stimulated and analyzed by immunofluorescence or ELISPOT (7, 8) are found to express predominantly IFN-γ, but not IL-4. Moreover, recent preliminary data suggest that initial peripheral blood Th1/Th2 ratios determined by intracellular FACS may predict responsiveness to disease-modifying antirheumatic drug therapy 9 months later (34). Those factors that regulate such Th1 responses, still poorly understood in RA, are therefore of considerable interest. The present report demonstrates that IL-18 can sustain Th1 cell responses in synovial membrane, through synergy with other T-cell activatory cytokines. Because variable levels of IL-12 have been detected in the RA synovium, a crucial role for IL-18 in synovitis may be to promote IL-12–dependent Th1 responses in the context of relatively low levels of IL-12 expression. Our data further suggest that IL-15 can contribute to this process and may replace the requirement for IL-12, should the latter be limiting.
The paradoxical failure to detect high levels of IFN-γ in situ in synovial membrane in the presence of IL-18 may reflect several factors. Anti-inflammatory cytokines, such as IL-10 and TGF-β, are widely expressed in synovial membrane and clearly suppressed IFN-γ synthesis in vitro. The duration of sustained IFN-γ expression within a single Th1 cell after stimulation in the presence of such factors is unclear. Synovial lymphocytes, however, clearly retain their functional maturation status as exemplified by their subsequent IFN-γ expression ex vivo. The inflammatory potential of IL-18 may therefore reflect a balance of pro- versus anti-inflammatory cytokines in the local milieu, in which an equilibrium can sustain Th1 phenotype without inducing persistently high levels of IFN-γ production. An IL-18–binding protein (IL-18BP) has recently been described that is constitutively expressed in lymphoid tissues and that can regulate the contribution of IL-18 to Th1 responses in vivo (35). IL-18BP expression, if confirmed in synovial membrane, could further limit IFN-γ upregulation by locally produced IL-18. Nevertheless, low numbers of IFN-γ–expressing cells are consistently detected in RA synovium (5, 7). Our data indicate that IL-18, together with IL-12 and IL-15, can perpetuate such Th1 responses. Furthermore, IL-18R was detected on CD3+ cells, likely representing a mature Th1 subset (36), and on macrophages, indicating that endogenous IL-18 expression can be biologically active. In a murine model of inflammatory arthritis, IL-18 was capable of promoting a severe, erosive inflammatory polyarthropathy. Moreover, as IL-18, but not IL-12, activates the IFN-γ promoter directly through an AP-1 site, independent of T-cell receptor signaling (37), it is possible that IL-18, like IL-15, IL-1β, and IL-6, can contribute to antigen-independent T-cell activation in chronic inflammatory responses (29, 38).
Monocyte-derived cytokines occupy a central position in the pathogenesis of RA (11). Therapeutic blockade of TNF-α and IL-1β, using soluble receptors or mAb, suppresses murine CIA and RA itself (39). Clinical effects are transient, however, suggesting that critical pathways that maintain synovitis persist. Factors that upregulate TNF-α production include positive feedback from monokines, immune complexes, and cell contact with “cytokine-activated” T cells (38, 40). IL-18 induced high levels of TNF-α and GM-CSF production by synovial tissues, in synergy with IL-12 and IL-15. Single-cell staining revealed TNF-α protein in CD14+ macrophages after IL-18 addition to Brefeldin A–treated synovial cells. Because no IFN-γ secretion occurred in these cultures, a direct effect of IL-18 on TNF-α production by synovial macrophages is probable. IL-18 induces IL-1β mRNA expression directly in purified macrophages, whereas TNF-α production by PBMCs has been attributed to CD3/CD4+ cells (27, 41). Whether this minor discrepancy is a function of the activation/maturation status of synovial versus blood macrophages requires clarification. Together these data clearly indicate that the proinflammatory activities of IL-18 in synovial membrane extend beyond Th1 cells to include macrophages.
IL-18 expression in synovial tissues in vitro was enhanced by TNF-α and IL-1β, raising the possibility of a positive feedback loop that could lead to reciprocal amplification of Th1 responses and monocyte production. Moreover, IL-18 inhibited TGF-β–induced proliferation, induced matrix metalloproteinase and inducible NO synthase (NOS) gene expression, and enhanced glycosaminoglycan release in chondrocytes in vitro (18). IL-18 could therefore contribute to cartilage degradation, although whether synovium-derived IL-18 can contribute locally to this process remains to be determined. We have now also shown that IL-18 induced nitrite synthesis in synovial membrane cultures. Given that NO reduces IL-18 cleavage through inhibition of ICE (42), upregulation of NO release by IL-18, IL-12, or IL-15 in synovial cultures may represent an endogenous regulatory pathway for IL-18 (and IL-1β) production. Compatible with this, we previously demonstrated dysregulated Th1 responses in iNOS-deficient mice (32).
IL-18 is a member of the IL-1 cytokine family that is widely expressed in RA synovium. IL-1β and IL-18 gene expression is differentially regulated (26). The pattern of distribution of IL-18 in synovial membrane appears distinct from that of IL-1β. Whereas IL-1β is highly expressed throughout the lining layer, interstitium, and vasculature, IL-18 is localized primarily to lymphocytic aggregates. Because ICE regulates the processing and secretion from intracellular precursors of both IL-1β and IL-18 (26), it seems likely that other distinct mechanisms will contribute to differential cytokine expression in discrete parts of the synovium. ICE inhibition remains an enticing therapeutic target. ICE inhibitors effectively suppress murine CIA (43). Whether such effects are associated with suppression of IL-18–dependent effects remains unclear.
A further implication of this study is that detection of a cytokine and determination of its absolute concentration in SF is unlikely to correlate directly to its relative functional importance, but should rather reflect its potential for synergy. We have identified an important role for IL-18, together with IL-15 and IL-12, in such a network within the synovial membrane. Although the mechanism of such synergy is yet to be defined, the present study provides rationale for identifying functional cytokine “cassettes” that together may be amenable to inhibition.
Acknowledgments
This work received financial support from the Arthritis Research Campaign, United Kingdom; The Medical Research Council; The Wellcome Trust; The Nuffield Foundation; and the MacFeat Bequest, University of Glasgow. Clinical samples were kindly provided by J. Hunter, A. Kinninmonth, I. Stother, R.D. Sturrock, H.A. Capell, and R. Madhok.
References
- 1.Mauri C, Williams RO, Walmsley M, Feldmann M. Relationship between Th1/Th2 cytokine patterns and the arthritogenic response in collagen induced arthritis. Eur J Immunol. 1996;26:1511–1518. doi: 10.1002/eji.1830260716. [DOI] [PubMed] [Google Scholar]
- 2.Racke MK, et al. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med. 1994;180:1961–1966. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin dependent diabetes. Science. 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 4.Mosmann TR, Sad S. The expanding universe of T cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:13–17. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 5.Ulfgren A-K, Lindblad S, Klareskog L, Andersson J, Andersson U. Detection of cytokine producing cells in the synovial membrane from patients with rheumatoid arthritis. Ann Rheum Dis. 1995;54:654–661. doi: 10.1136/ard.54.8.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miltenburg AMM, van Laar JM, De Kuiper R, Daha MR, Breedveld FC. T cells cloned from human rheumatoid synovial membrane functionally represent the Th1 subset. Scand J Immunol. 1992;35:603–610. doi: 10.1111/j.1365-3083.1992.tb03260.x. [DOI] [PubMed] [Google Scholar]
- 7.Dolhain RJEM, Heiden AN, Haar NT, Breedveld FC, Miltenburg AMM. Shift towards T lymphocytes with a T helper 1 cytokine secretion profile in the joints of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1961–1969. doi: 10.1002/art.1780391204. [DOI] [PubMed] [Google Scholar]
- 8.Ronnelid J, et al. Production of T cell cytokines at the single cell level in patients with inflammatory arthritides: enhanced activity in synovial fluid compared to blood. Br J Rheum. 1998;37:7–14. doi: 10.1093/rheumatology/37.1.7. [DOI] [PubMed] [Google Scholar]
- 9.Gately MK, et al. The interleukin-12/interleukin-12 receptor system. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 10.Morita Y, et al. Expression of interleukin-12 in synovial tissue from patients with rheumatoid arthritis. Arthritis Rheum. 1998;41:306–314. doi: 10.1002/1529-0131(199802)41:2<306::AID-ART15>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Feldmann F, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 12.Ushio S, et al. Cloning of the cDNA for human IFNγ-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J Immunol. 1996;156:4274–4279. [PubMed] [Google Scholar]
- 13.Gu Y, et al. Activation of interferon-gamma inducing factor mediated by interleukin-1β converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 14.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-γ production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259–264. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 15.Kohno K, Kurimoto M. Interleukin 18, a cytokine which resembles IL-1 structurally and IL-12 functionally but exerts its effect independently of both. Clin Immunol Immunopathol. 1998;86:11–15. doi: 10.1006/clin.1997.4475. [DOI] [PubMed] [Google Scholar]
- 16.Takeda K, et al. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 17.Udagawa N, et al. Interleukin-18 (interferon-gamma–inducing factor) is produced by osteoblasts and acts via granulocyte/macrophage colony-stimulating factor and not via interferon-gamma to inhibit osteoclast formation. J Exp Med. 1997;185:1005–1012. doi: 10.1084/jem.185.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olee T, Hashimoto S, Quach J, Lotz M. IL-18 is produced by articular chondrocytes and induces proinflammatory and catabolic responses. J Immunol. 1999;162:1096–1100. [PubMed] [Google Scholar]
- 19.Rothe H, Jenkins N, Copeland N, Kolb H. Active stage of autoimmune diabetes is associated with the expression of a novel cytokine, IGIF, which is located near Idd2. J Clin Invest. 1997;99:469–474. doi: 10.1172/JCI119181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawakami K, et al. IL-18 protects mice against pulmonary and disseminated infection with Cryptococcus neoformans by inducing IFN-γ production. J Immunol. 1997;159:5528–5534. [PubMed] [Google Scholar]
- 21.Bohn E, et al. IL-18 (IFN-gamma–inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J Immunol. 1998;160:299–307. [PubMed] [Google Scholar]
- 22.Chan WL, Mitchison NA. Use of somatic cell hybrids for production of monospecific viral antibodies. Lab Res Methods Biol Med. 1982;15:125–141. [PubMed] [Google Scholar]
- 23.Arnett FC, Edworthy SM, Block DA. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 24.McInnes IB, et al. Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J Exp Med. 1997;184:1519–1524. doi: 10.1084/jem.184.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruchatz H, Leung BP, Wei X-Q, McInnes IB, Liew FY. Soluble IL-15 receptor alpha-chain administration prevents murine collagen-induced arthritis: a role for IL-15 in development of antigen-induced immunopathology. J Immunol. 1998;160:5654–5660. [PubMed] [Google Scholar]
- 26.Puren AJ, Fantuzzi G, Dinarello CA. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1β are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc Natl Acad Sci USA. 1999;96:2256–2261. doi: 10.1073/pnas.96.5.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinarello CA, et al. Overview of interleukin-18: more than an interferon-gamma inducing factor. J Leukoc Biol. 1998;63:658–664. [PubMed] [Google Scholar]
- 28.McInnes IB, et al. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996;2:175–182. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- 29.McInnes IB, Liew FY. Interleukin 15: a proinflammatory role in rheumatoid arthritis synovitis. Immunol Today. 1998;19:75–79. doi: 10.1016/s0167-5699(97)01205-x. [DOI] [PubMed] [Google Scholar]
- 30.Firestein GS, Zvaifler NJ. Peripheral blood and synovial fluid monocyte activation in inflammatory arthritis. II. Low levels of synovial fluid and synovial tissue interferon suggest that gamma-interferon is not the primary macrophage activating factor. Arthritis Rheum. 1987;30:864–871. doi: 10.1002/art.1780300804. [DOI] [PubMed] [Google Scholar]
- 31.Feldmann M, Charles P, Taylor P, Maini RN. Biological insights from clinical trials with anti-TNF therapy. Springer Semin Immunopathol. 1998;20:21–28. doi: 10.1007/BF00832008. [DOI] [PubMed] [Google Scholar]
- 32.McInnes, I.B., and Liew, F.Y. 1998. Nitric oxide and immune response. In Nitric oxide and bone disease. M. Hukkanen, J.M. Polak, and S.P.F. Hughes, editors. Cambridge University Press. Cambridge, United Kingdom. 8–20.
- 33.Loetscher P, et al. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 34.van der Graaff WL, Prins AP, Dijkmans BA, van Lier RA. Prognostic value of Th1/Th2 ratio in rheumatoid arthritis. Lancet. 1998;351:1931. doi: 10.1016/s0140-6736(05)78615-3. [DOI] [PubMed] [Google Scholar]
- 35.Novick D, et al. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity. 1999;10:127–136. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- 36.Xu D, et al. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not Th2 cells. J Exp Med. 1998;188:1485–1492. doi: 10.1084/jem.188.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbulescu K, et al. IL-12 and IL-18 differentially regulate the transcriptional activity of the human IFN-gamma promoter in primary CD4+ T lymphocytes. J Immunol. 1998;160:3642–3647. [PubMed] [Google Scholar]
- 38.McInnes IB, Leung BP, Sturrock RD, Field M, Liew FY. Interleukin-15 mediates T cell–dependent regulation of tumour necrosis factor-alpha production in rheumatoid arthritis. Nat Med. 1997;3:189–195. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- 39.Williams RO, Feldmann M, Maini RN. Anti-tumour necrosis factor ameliorates joint disease in murine collagen induced arthritis. Proc Natl Acad Sci USA. 1994;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vey E, Zhang J, Dayer J-M. IFNγ and 1,25(OH) D3 induce on THP-1 cells distinct patterns of cell surface antigen expression, cytokine production and responsiveness to contact with activated T cells. J Immunol. 1992;149:2040–2046. [PubMed] [Google Scholar]
- 41.Puren AJ, Fantuzzi G, Gu Y, Su MS, Dinarello CA. Interleukin-18 (IFNγ-inducing factor) induces IL-8 and IL-1β via TNF-α production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998;101:711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim YM, Talanian RV, Li J, Billiar TR. Nitric oxide prevents IL-1β and IFN-—inducing factor (IL-18) release from macrophages by inhibiting caspase-1 (IL-1β-converting enzyme) J Immunol. 1998;161:122–128. [PubMed] [Google Scholar]
- 43.Ku G, Faust T, Lauffer LL, Livingston DJ, Harding MW. Interleukin-1β converting enzyme inhibition blocks progression of type II collagen–induced arthritis in mice. Cytokine. 1996;8:377–386. doi: 10.1006/cyto.1996.0052. [DOI] [PubMed] [Google Scholar]