Acute Alcohol Intake Induces SOCS1 and SOCS3 and Inhibits Cytokine-Induced STAT1 and STAT3 Signaling in Human Monocytes (original) (raw)
. Author manuscript; available in PMC: 2014 Jul 31.
Abstract
Background
Acute alcohol consumption is associated with induction of immuno-inhibitory cytokines and down-regulation of pro-inflammatory responses to various pathogens. We previously reported that alcohol activates janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling leading to IL-10 induction. The JAK-STAT pathway also activates its own negative regulators, suppressors of cytokine signaling (SOCS) 1 and SOCS3. SOCS proteins are inducible inhibitors that negatively regulate STAT3/STAT1 signaling pathways induced by cytokines, IL-6 or IFNs. Here we aimed to explore the effect of acute alcohol on induction of SOCS1/SOCS3 and regulation of STAT3/STAT1 pathways induced by IL-6 or IFNs in human monocytes.
Methods
Blood samples from normal volunteers were collected before and 24 hours after consumption of 2 ml vodka/kg body weight. For in vitro experiments human monocytes were pretreated with ethanol (EtOH) followed by stimulation with cytokines; proteins were analyzed by Western blot, nuclear protein binding to DNA by EMSA, and RNA by real time PCR.
Results: Acute in vivo or in vitro alcohol treatment increased both SOCS1 and SOCS3 RNA expression in monocytes. Alcohol treatment resulted in increased STAT3 and STAT1 DNA binding capacity. Activation of both STAT1 and STAT3 has been shown to induce SOCS1/3. We hypothesized that induction of SOCS proteins by alcohol in turn may lead to modulation of cytokine signaling through STAT1 and STAT3. Indeed, we observed significant down-regulation of IL-6-, IFNα- and IFNγ-induced STAT1 DNA binding as well as inhibition of IL-6- and IFNγ-induced STAT3 when alcohol was added to monocytes 3 hours prior to the cytokine stimulation. Consistent with inhibition of IL-6-induced STAT3 DNA binding in alcohol-pretreated cells, the levels of IL-6-dependent genes, MCP-1 and ICAM-1, was reduced after IL-6 stimulation. Similar to EtOH alone, combined EtOH+IL-6 simulation resulted in increased expression of both SOCS3 and SOCS1 genes.
Conclusion
While acute alcohol treatment alone activates STAT1/3 signaling pathways and induces SOCS3 and SOCS1 levels in monocytes, alcohol also leads to down-regulation of IL-6-, IFNα-, and IFNγ-induced signaling via STAT1/STAT3 pathways, likely through excessive SOCS activation.
Keywords: Toll-Like Receptor 4, Lipopolysacharide (LPS), In Vivo, In Vitro, Ethanol
Alcohol affects multiple functions of the immune system; however the most detrimental effects are seen in innate immunity related to antimicrobial defense and inflammation. Associations between alcohol consumption and increased risk of infections due to immunosuppression were found in numerous studies (Boe et al., 2003; Happel and Nelson, 2005; Mandrekar et al., 2006; Szabo et al., 1999). We previously reported that moderate acute alcohol consumption induces IL-10, a potent anti-inflammatory cytokine, via activation of the Src-STAT3 pathway (Norkina et al., 2007). Signal transducers and activators of transcription (STAT) family proteins play an essential role in signal transduction of cytokines (Takeda and Akira, 2000). STATs are present in the cytoplasm in a latent form and become activated through phosphorylation of tyrosine residues via cytokine receptor associated tyrosine kinases and nonreceptor tyrosine kinases, JAKs and Src kinases, respectively (Murray, 2007; Takeda and Akira, 2000). We had recently reported that STAT proteins play a critical role in alcohol-initiated production of IL-10 (Norkina et al., 2007). Further, we identified implications of Src kinases in alcohol-induced STAT3 activation in human monocytes (Norkina et al., 2007), however the mechanisms of STAT regulation during acute alcohol treatment are currently unknown.
Cytokine-initiated signaling in immune cells must be under stringent control to ensure an appropriate cellular response. Suppressors of cytokine signaling (SOCS) are one of 3 major families of regulatory proteins aimed at attenuation of signal transduction induced by cytokines (Alexander and Hilton, 2004; Rakesh and Agrawal, 2005). SOCS proteins provide a negative feedback loop aimed to attenuate signal transduction through the JAK, TYK/STAT pathway and the mechanisms of SOCS proteins activity have been widely investigated in recent years. SOCS1 interacts with tyrosine in the activation loop of JAKs through its SH2 domain (Murray, 2007; Takeda and Akira, 2000). Phospho-tyrosine residues in the cytoplasmic domains of cytokine receptors serve as a site for interaction with SOCS3 (Alexander and Hilton, 2004; Murray, 2007; Takeda and Akira, 2000). Both SOCS1 and SOCS3 carry an N-terminal domain, known as the kinase inhibitory region (KIR), that acts as a pseudo-substrate for JAKs (Rakesh and Agrawal, 2005). SOCS3 is a negative regulator of signaling induced by cytokines such as Interferon (IFN) γ and Interleukin (IL)-6, while SOCS1 attenuates signaling induced by IFNγ, IL-2, IL-6, IL-7, IL-12, and IL-15 (Murray, 2007). Both SOCS1 and SOCS3 inhibit IFN-α-induced expression of antiviral proteins (Vlotides et al., 2004). Besides the limiting effects on STAT-dependent signaling, SOCS proteins themselves are regulated through activation of STAT-dependent elements (Gatto et al., 2004; He et al., 2003; Ramana et al., 2005; Venkataraman et al., 1999). More recently, it has been reported that SOCS gene expression can be induced by chemokines, but also by some Toll-like receptor (TLR) agonists, such as LPS (via TLR4) and CpG-DNA (via TLR9) (Hu et al., 2007;Mellor et al., 2005). Previous reports from us, and others, demonstrated that alcohol alters TLR4- and TLR9-induced signaling (Goral and Kovacs, 2005; Goral et al., 2004; Mandrekar et al., 2006; Pruett et al., 2004). Further, in vivo alcohol consumption leads to STAT3 activation (Norkina et al., 2007).
In this study, we investigated the effect of alcohol on STAT protein activation in order to evaluate the hypothesis that moderate acute alcohol intake may result not only in activation of STATs, but also in STAT-dependent induction of suppressors of cytokine signaling (SOCS). Finally, we addressed the question, whether moderate acute alcohol exposure could influence subsequent cytokine signaling induced by inflammatory cytokines IL-6, IFNα, and IFNγ, which are targets of SOCS proteins in humanmonocytes.
MATERIALS AND METHODS
Reagents
Lipopolysaccharide (from E. coli O111:B4) was from Sigma- Aldrich. Inc (St. Louis, MO), fetal calf serum (FCS) from HyClone (Logan, UT), culture media RPMI1640 from Gibco (Grand Island, NY), human recombinant IFNα-A/D was from Sigma-Aldrich, Inc. (Saint Luis, MO), IFNγ and IL-6 were from PeproTech Inc. (Rocky Hill, NJ).
Blood Donors, Cell Separation and Cell Stimulation
Healthy individuals with no history of alcohol abuse or other co-morbidities were enrolled in the study after informed consent was obtained. The study was approved by the Committee for the Protection of Human Subjects in Research at the University of Massachusetts Medical School. Alcohol use habits of blood donors were determined by a questionnaire that incorporated the Alcohol Use Disorders Identification Test and CAGE tests. To qualify for the study, males had alcohol use of fewer than 12 drinks/wk, females fewer than 9 drinks/wk, and all abstained from alcohol for at least 48 hours before participation. Blood was collected from arm vein and preserved from coagulation using heparine sulfate (10 U/ml). To study the in vivo effect of alcohol on monocyte function, the donors consumed alcohol (2 ml vodka/kg body weight in a total volume of 300 ml orange juice) while in the Clinical Research Center (CRC) at the UMass Medical Center. Blood samples were obtained before and 24 hours after the alcohol consumption. Control blood samples were collected from age- and gender-matched alcohol-abstinent individuals.
Monocytes were separated from peripheral blood mononuclear cells (PBMCs) by centrifugation in Ficoll gradient and adherence to plastic, and cultured in RPMI1640 with 10%FBS and MEM supplements, as previously described (Mandrekar et al., 2006; Norkina et al., 2007). For the in vitro experiments ethanol (EtOH) was used at 50 mM concentration, IL-6 at 5 ng/ml, IFNγ at 0.1 ng/ml, IFNα at 10 U/ml.
Preparation of Nuclear and Cytoplasmic Extracts
For collection of subcellular fractions, the monocytes were washed in ice-cold PBS and scraped in cold buffer A (10 mMHepes, pH 7.9, 10 mM KCl, 0.1 mM EGTA, 1 mM DTT, 1 mM PMSF, 0.1 mM EDTA, and 0.1 µg/ml protease inhibitors (aprotinin, antipain, and leupeptin, all from SIGMA, St Louis, MO). Cells were then lysed with 0.5% NP-40 (SIGMA). The lysate was centrifuged at 12,000 × g for 30 seconds to pellet the nuclei and the supernatant was stored at −80°C as the cytoplasmic extract. The nuclear pellet was washed twice with ice-cold buffer A and later re-suspended in ice-cold buffer B (20 mM Hepes, pH 7.9, 400 mM KCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, 20% glycerol, and 10 µg/ml protease inhibitors). All tubes were kept on a shaker at 4°C for 30 minutes. The lysate was then centrifuged at 12,000 × g for 15 minutes and the supernatant was stored at −80°C as the nuclear extract. Protein content was determined in all extracts using the Protein Dye Reagent Assay from BioRad (Hercules, CA).
Detection of DNA-Binding Capacity of Transcriptional Factors
The STAT3 DNA binding capacity was measured in conventional electromobility shift assays (EMSA), as we previously described (Mandrekar et al., 2006; Norkina et al., 2007). Briefly, 5 mg of nuclear protein from each sample were incubated with consensus32P-labeled double-stranded oligonucleotide (STAT3: 5′-GAT CCT TCT GGG AAT TCC TAG ATC-3′; mutant STAT3: 5′-GAT CCT TCT GGG AAT TCC TAG ATC-3′; STAT1: 5′-CAT GTT ATG CAT ATT CCT GTA AGT-3′; mutant STAT1 5′-CAT GTT ATG CAT ATT CCT GTA AGT G-3′; all from Santa Cruz Biotechnology Inc, Santa Cruz, CA) in a binding reaction mixture containing 20 mM Hepes, pH 7.9, 10 mM KCl, 2.5 mM EDTA, 1 mM DTT, 5% glycerol, 200 µg/ml BSA, and 2 µg/ml poly(dI–dC). Cold competition was achieved by addition of a 20-fold excess of specific unlabeled double-stranded probe to the reaction mixture 20 minutes prior to addition of radioactive-labeled oligonucleotide; mutant oligonucleotides were employed as controls in similar conditions. Samples were incubated at room temperature for 30 minutes. All protein/oligonucleotide complexes were separated on a 5% polyacrylamide gel. The gels were dried and exposed to autoradiography. The densitometric analysis of specific bands was performed using GDS-800 system and Labwork 4.0 program (UVP BioImaging Systems).
RNA Isolation and Real-Time PCR
Total RNA was isolated from monocytes using the RNeasy Kit from Qiagen (Valencia, CA), according to manufacturers instructions. Reverse transcriptions were performed using the First Strand cDNA Synthesis Kit (Promega, Madison, WI) according to manufacturers instructions. One µg of total RNA was transcribed to cDNA in a 20 µl reaction volume. For transcript quantification purposes by real-time PCR, the SYBR Green Mix containing Thermo-Start DNA Polymerase was used according to manufacture instructions (Eurogentech, San Diego, CA). All primers were from IDT (Coralville, IN); sequences and PCR conditions are shown in Table 1. The PCR reaction using 1 µl of cDNA was carried out in iCycler Thermal Cycler (Bio-Rad, Hercules, CA). A hot-start phase was applied at 95°C for 10 minutes for all primers. At each cycle, accumulation of PCR products was detected by monitoring the increase in fluorescence by dsDNA-binding SYBR Green. A dissociation/melting curve of the PCR product was constructed in the range of 55°C to 95°C. Data were analyzed using the Biorad ICycler software and comparative Ct method with the following formula: ΔCt= Ct (target gene) - Ct (normalizer, 18S). Fold increase in the expression of gene of interest in experimental groups compared to media control was calculated as 2−(Δ ΔCt).
Table 1.
Real Time PCR Primers
| Gene | Direction | Primer sequence | Amplification parameters |
|---|---|---|---|
| SOCS1 | Forward | GAA CTG CTT TTT CGC CCT TA | 45 cycles at 95°C for 60 seconds, 56°C for 60 seconds, 72°C for 60 seconds |
| Reverse | CTC GAA GAG GCA GTC GAA G | ||
| SOCS3 | Forward | GCA CAA GCA CAA GAA GCC AAC C | 45 cycles at 95°C for 60 seconds, 56°C for 60 seconds, 72°C for 60 seconds |
| Reverse | TCC CTC CAA CAC ATT CCA GGT C | ||
| MCP-1 | Forward | TGG CTG TGT TTG CTT CTG TC | 35 cycles at 95°C for 15 seconds, 60°C for 15 seconds, 72°C for 15 seconds |
| Reverse | TCT CAC TGC CCT ATG CCT CT | ||
| ICAM-1 | Forward | CAG GGA ATA TGC CCA AGC TA | 35 cycles at 95°C for 15 seconds, 60°C for 15 seconds, 72°C for 15 seconds |
| Reverse | TGA ACC ATG ATT GCA CCA CT | ||
| 18S | Forward | GTA ACC CGT TGA ACC CCA TT | 25 cycles at 95°C for 15 seconds, 60°C for 15 seconds, 72°C for 15 seconds |
| Reverse | CCA TCC ATT CGG TAG TAG CG |
Statistical Analysis
The statistical significance of the data was calculated using Student’s t-test and nonparametric Wilcoxon tests. A value of p < 0.05 was considered significant.
RESULTS
Acute Alcohol Induces SOCS1 and SOCS3 Expression in Vivo and in Vitro
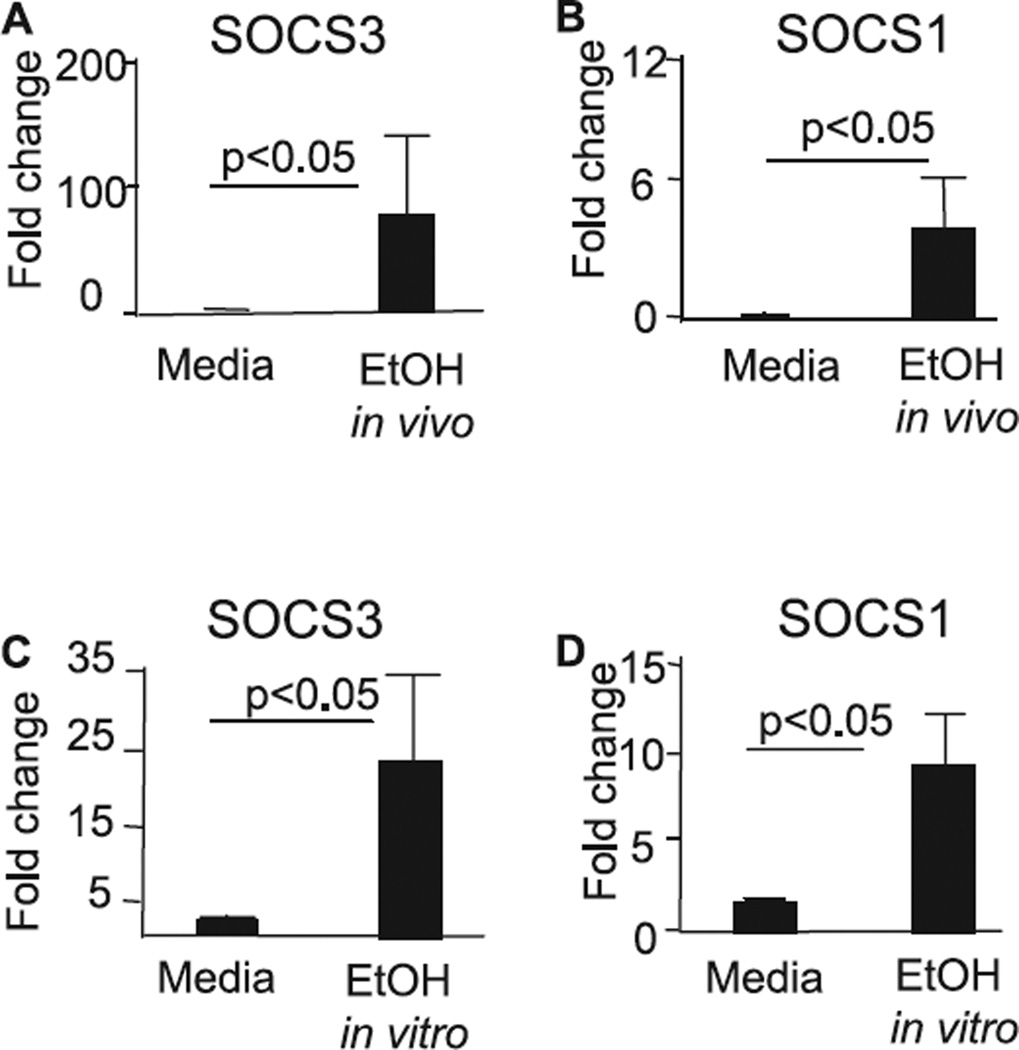
Alcohol consumption in humans or mice or ethanol administration to cells in vitro is associated with suppression of innate immunity (Boe et al., 2003; Goral and Kovacs, 2005; Goral et al., 2004; Happel and Nelson, 2005; Mandrekar et al., 2006; Norkina et al., 2007; Pruett et al., 2004; Szabo et al., 1999). Suppressive effects of ethanol on autocrine amplification loop of type 1 IFNs (Vlotides et al., 2004), TLR signaling (Goral and Kovacs, 2005; Goral et al., 2004; Mandrekar et al., 2006; Pruett et al., 2004), NFκB activation (Norkina et al., 2007; Vlotides et al., 2004), and cellular response to IFNγ were reported (Happel et al., 2007), however the mechanisms of the inhibitory action of ethanol on cell signaling are not fully understood. Recently, we showed that acute ethanol treatment induces expression of IL-10 via activation of Src kinases and involves STAT3, AP-1, and Sp-1 transcription factors in human monocytes (Norkina et al., 2007). Suppressors of the cytokine signaling (SOCS) control cytokine-induced STAT activation, thus preventing excessive stimulation of the immune cell (Alexander and Hilton, 2004; Murray, 2007; Rakesh and Agrawal, 2005; Takeda and Akira, 2000). To address the question whether moderate alcohol intake can elicit expression of SOCS1 and SOCS3, we measured SOCS3 (Fig. 1_A_) and SOCS1 (Fig. 1_B_) RNA expression in monocytes after in vivo alcohol intake. We found significantly higher levels of both SOCS3 (Fig. 1_A_) and SOCS1 (Fig. 1_B_) in monocytes after alcohol consumption compared to levels of monocytes obtained before alcohol consumption in the same individuals. Similar to the in vivo findings, we found up-regulation of expression of SOCS3 (Fig. 1_C_) and SOCS1 (Fig. 1_D_) RNA levels in human monocytes after in vitro treatment with EtOH. These data suggested that the mechanisms of the inhibitory activity of acute alcohol involves induction of suppressors of the cytokine signaling, SOCS3 and SOCS1, in monocytes.
Fig. 1.
Acute alcohol exposure induces SOCS1 and SOCS3 expression in human monocytes in vivo and in vitro. (A, B) Human monocytes were collected before and 24 hours after in vivo alcohol exposure. The mRNA expression of SOCS3 (A) and SOCS1 (B) was quantified using specific primers in real-time PCR and normalized for housekeeping gene 18S. The results are shown as mean ± SE from n = 4. (C, D) Monocytes from normal alcohol-naïve individuals were stimulated in vitro with EtOH (25 mM) for 5 hours. The mRNA of SOCS3 (C) and SOCS1 (D) was quantified as above and is shown as fold change compared to control cells from n = 4.
Acute Moderate Alcohol Intake Activates STAT3 DNA Binding
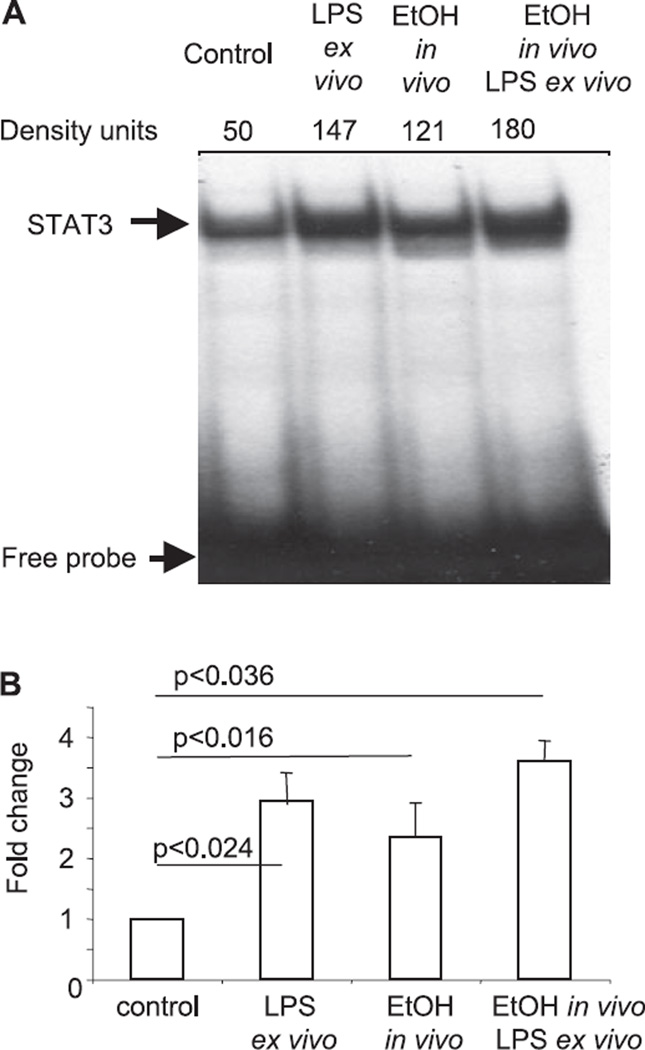
Our previous data indicated that in vitro ethanol treatment of monocytes leads to production of IL-10 in a STAT3-dependent manner (Norkina et al., 2007). Here we analyzed whether alcohol intake in vivo will elicit activation of STAT3 DNA binding, similar to that seen in the in vitro system. As shown in Fig. 2, in vivo alcohol intake induced STAT3 DNA binding. Further, the effect of in vivo alcohol intake was maintained ex vivo, as indicated by increased STAT3 DNA binding ex vivo in LPS-stimulated cells of in vivo alcohol-exposed individuals. Ex vivo LPS stimulation alone also induced STAT3 activation. Collectively, these data provided evidence that ethanol intake in vivo activates STAT3, which is an important upstream activator of SOCS3 and SOCS1.
Fig. 2.
Acute alcohol induces STAT3 DNA binding in vivo. Monocytes were separated from donors before and after in vivo alcohol consumption. Some cells were stimulated ex vivo, as indicated, with LPS (1 µg/ml) for 45 minutes. Five µg of the nuclear extract was subjected to the conventional EMSA with radioactive-labeled consensus STAT3 oligonucleotide. The protein-STAT3 oligonucleotide complexes were separated on polyacrylamide gel, dried and exposed to autoradiography. One representative gel (A) and densitometric analysis from n = 3 is shown.
Ethanol Attenuates Cytokine-Induced STAT Protein Activation in Monocytes
Multiple activators, including IL-6 and IFNs, induce STAT1 and STAT3 activation upon binding to their respective cytokine receptor complexes (Murray, 2007; Takeda and Akira, 2000). Type 1 interferon (IFNα) and type 2 IFN (IFNγ), modulate immune functions and induce antiviral responses by promoting or suppressing inflammation (Stetson and Medzhitov, 2006). The pleiotropic action of IFNs can be partially attributed to diverse effects of STAT1 and STAT3 activation in the immune cell. Similar to IFNγ, IFNα induces STAT1 (Grace and Cutler, 2004; Ramana et al., 2005; Venkataraman et al., 1999), thus eliciting expression of inflammatory genes and enhancing antiviral responses. The role of IFNα-induced STAT3 is poorly studied, but it was suggested that IFNα-induced STAT3 restrains inflammation and attenuates STAT1 function (Ho and Ivashkiv, 2006). Here we investigated the effect of ethanol on IL-6 and IFNs-induced activation of STAT proteins in human monocytes.
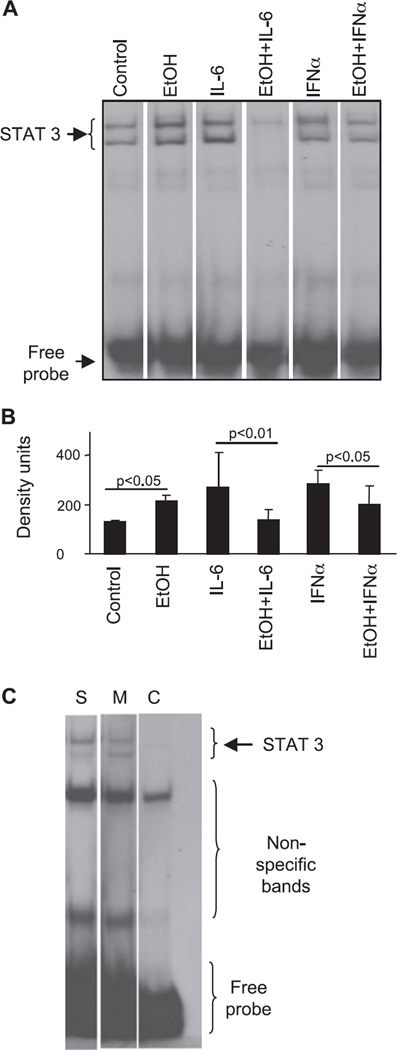
STAT3 acts as a master switch in the establishment of pro-and anti-inflammatory balance in macrophages (Murray, 2007). Similar to our previous reports (Norkina et al., 2007), here we found that ethanol alone induced STAT3 activation, as indicated by increased DNA binding to consensus STAT-specific oligonucleotide (Fig. 3_A,B_). In contrast, exposure to alcohol led to significantly attenuated cytokine-induced STAT3 DNA binding in response to either IL-6 or IFNα (Fig. 3_A,B_). STAT3 binding was competed off with unlabeled consensus STAT3-specific oligonucleotide while STAT3 mutant oligonucleotide did not affect the binding (Fig. 3_C_), suggesting STAT3 reagent specificity.
Fig. 3.
Ethanol attenuates cytokine-induced STAT3 DNA binding in human monocytes in vitro. Human monocytes were stimulated with IL-6 (5 ng/ml) or IFNα (10 U/ml) for 45 minutes without or with ethanol (50 mM) pretreatment for 3 hours prior to the cytokine stimulation, as indicated. Five µg of nuclear protein were analyzed for STAT3 DNA binding capacity using a consensus specific radioactive labeled oligonucleotide. One representative gel (A) and the densitometric analysis (B) from n = 5 are shown. To confirm the reagent specificity (C), nuclear extracts were incubated with a consensus specific radioactive labeled STAT3 oligonucleotide (line S-sample) in the presence of mutant STAT3 (line M-mutant) or cold consensus STAT3 (line C-cold competition) oligonucleotide.
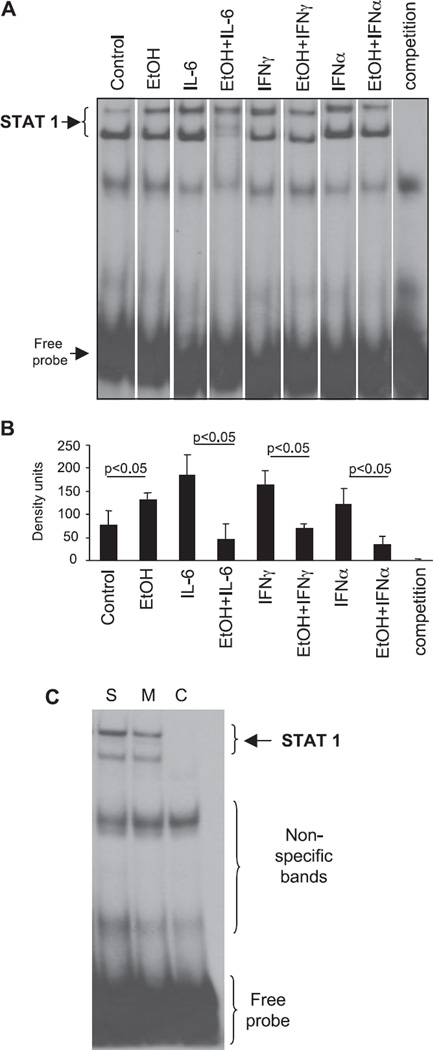
Most of the antiviral and antitumor effects of IFNs are attributed to activation of STAT1 (Grace and Cutler, 2004; Ho and Ivashkiv, 2006; Stetson and Medzhitov, 2006). Here, we show the novel finding that similar to its effect on STAT3, acute ethanol treatment alone induces STAT1 DNA binding in human monocytes (Fig. 4). More importantly, we report that alcohol pretreatment modulates cell signaling by significantly inhibiting IL-6 and IFNα-induced STAT1 activation in monocytes (Fig. 4_A,B_). IFNγ, a classical activator of STAT1, was used as a control for STAT1 DNA binding. Similar to IL-6- and IFNα-induced STAT1 activation, IFNγ-induced STAT1 DNA binding was significantly attenuated by acute alcohol pretreatment (Fig. 4). To confirm STAT1 reagent specificity, we show that STAT1 binding was competed off with unlabeled consensus STAT1-specific oligonucleotide while STAT1 mutant oligonucleotide did not affect the binding (Fig. 4_C_). Collectively, these findings indicated that moderate doses of ethanol can induce or inhibit STAT1 activation depending on the activation status of monocytes.
Fig. 4.
Ethanol induces STAT1 DNA binding and attenuates cytokine-elicited STAT1 DNA binding in human monocytes. Human monocytes were stimulated with IL-6 (5 ng/ml), IFNγ (0.1 ng/ml) or IFNα (10 U/ml) for 45 minutes without or with ethanol (50 mM) pretreatment for 3 hours prior to cytokine stimulation, as indicated. Five µg of nuclear protein were analyzed for STAT1 DNA binding capacity using a specific radioactive labeled oligonucleotide. As a specificity control, IL-6-stimulated sample was preincubated with 100x cold STAT1 oligonucloitide (competition) prior to radioactive STAT1 oligonucleotide. One representative gel (A) and the densitometric analysis (B) from n = 5 are shown. To confirm the reagent specificity (C), nuclear extracts were incubated with a consensus specific radioactive labeled STAT1 oligonucleotide (line S-sample) in the presence of mutant STAT1 (line M-mutant) or cold consensus STAT1 (line C-cold competition) oligonucleotide.
Ethanol Treatment Inhibits IL-6-Induced RNA Expression of ICAM-1 and MCP-Genes in Human Monocytes
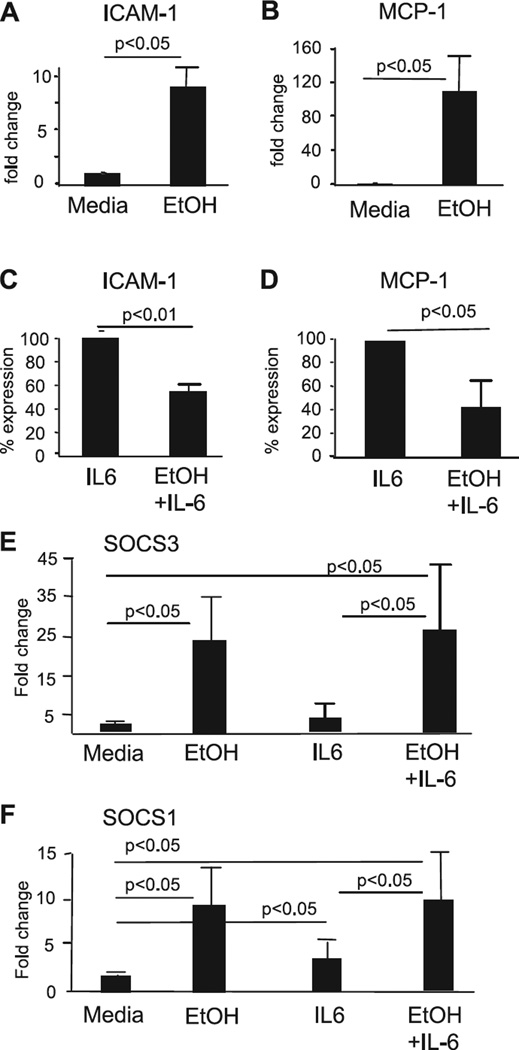
To investigate the downstream effects of inhibition of STAT signaling, we measured RNA expression of chemoattractant peptide-1 (MCP-1) and intracellular adhesion molecule- 1 (ICAM-1) in human monocytes. Both MCP-1 and ICAM-1 are induced by IL-6 and regulated in a STAT/SOCS-dependent manner (Burysek et al., 2002; Coccia et al., 1999; Federici et al., 2002). In agreement with our findings of STAT activation by ethanol treatment alone (Figs 2 and 3), we found that expression of both ICAM-1 (Fig. 5_A_) and MCP-1 (Fig. 5_B_) genes was up-regulated in monocytes upon exposure to ethanol. Further, expression of ICAM-1 (Fig. 5_C_) and MCP-1 (Fig. 5_D_) in response to IL-6 stimulation was attenuated in monocytes after treatment with ethanol. Similar to EtOH alone (Fig. 1), combined EtOH+IL-6 stimulation resulted in increased expression of both SOCS3 (Fig. 5_E_) and SOCS1 (Fig. 5_F_) genes compared to the corresponding control groups. These data suggested that the effect of alcohol on induction of cytokine-dependent genes, such as MCP-1 and ICAM-1, followed the patterns of activation of STAT proteins (Figs 3 and 4) as well as of SOCS3 and SOCS1 genes (Fig. 1).
Fig. 5.
Ethanol induces mRNA expression of ICAM-1 and MCP-1, but inhibits IL-6-induced mRNA expression of these genes. Monocytes were analyzed untreated (control) or after pretreatment with ethanol (50 mM) for 5 hours, for ICAM-1 (A) or MCP-1 (B) mRNA expression using real time PCR. Gene expression was normalized to 18S housekeeping control and the data presented as fold change compared to control (n = 4). (C, D) Monocytes were treated with IL-6 (5 ng/nm) for 5 hours without or with ethanol (50 mM) pretreatment prior to stimulation (for 3 hours) with IL-6 (as indicated). ICAM-1 (C) or MCP-1 (D) gene expression was quantified using real time PCR, as above. The data are presented as percent of IL-6-induced level of ICAM-1 or MCP-1 expression shown as 100% (n = 4). (E, F) The samples from panels A–D were analyzed for SOCS3 (E) and SOCS1 (F) expression, as above. Gene expression was normalized to 18S housekeeping control and the data presented as fold change compared to control (n = 4).
DISCUSSION
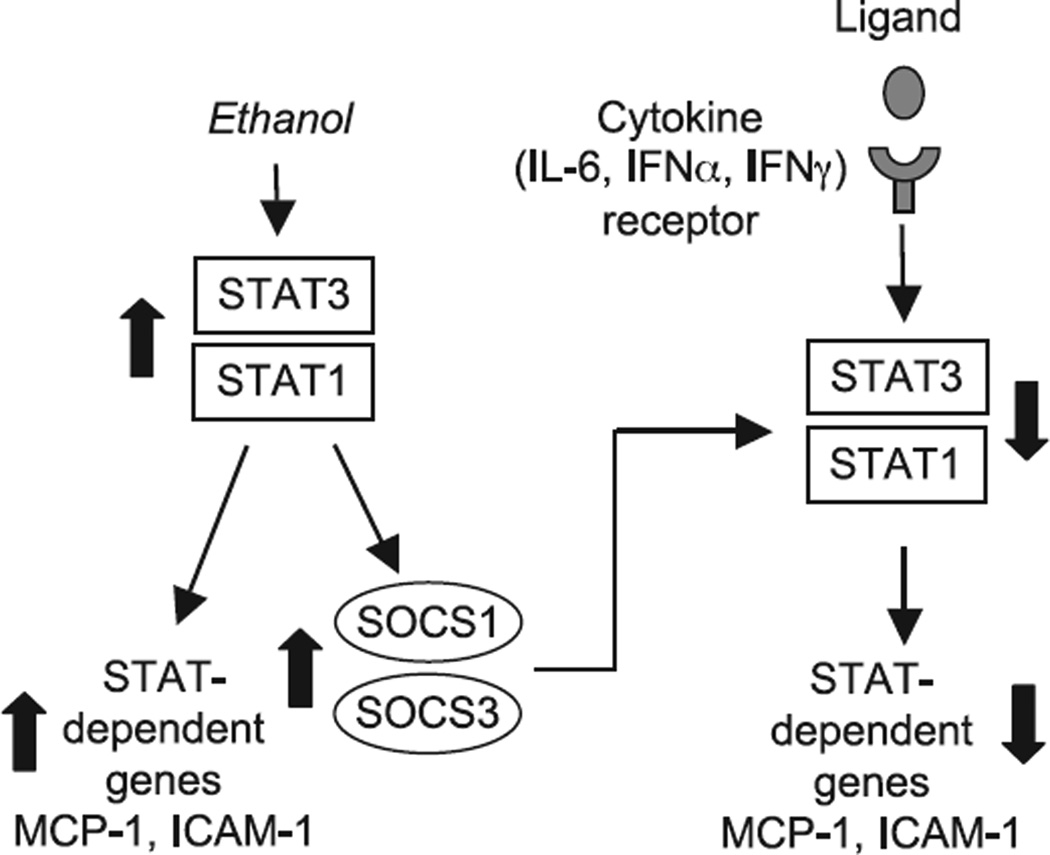
Acute alcohol consumption is associated with both beneficial and detrimental effects on human health. While an anti-inflammatory component is the major contributor to the beneficial effect of acute alcohol consumption on the cardiovascular system in humans, the same anti-inflammatory effects are disadvantageous for the immune responses to various pathogens or injury (Boe et al., 2003; Happel and Nelson, 2005; Szabo et al., 1999). The mechanisms of such versatile effects of acute alcohol treatment are not fully understood. Here we report novel observations that alcohol modulates the STAT/SOCS system to regulate innate immune responses. Further, we show that the effects of acute alcohol treatment are differential depending on the activation state of the cells. In quiescent cells acute alcohol treatment activates STAT proteins and triggers expression of STAT-dependent proteins, including the negative regulators of the STAT pathway, the SOCS proteins. In contrast, acute alcohol pretreatment impairs cytokine-induced activation of STATs (Fig. 6). Taking into account the broad utilization of the STAT proteins in signaling from distinct cytokine receptors, our observation of alcohol-induced inhibition of all major STAT activators, including IL-6 and both type I and type II interferons, may account for the deleterious effects of acute alcohol on cytokine responses.
Fig. 6.
The model of ethanol-induces modulation of STAT/SOCS signaling pathways. Ethanol alone induces activation of STAT3/STAT1, which triggers transcription of STAT-dependent genes, including SOCS1 and SOCS3. Pretreatment with ethanol prior to stimulation with cytokines leads to down-regulation of IL-6-, IFNα-, and IFNγ-induced signaling via STAT1/-STAT3 pathways, likely through excessive SOCS activation. The arrows indicate the effect of ethanol.
Our findings on the inhibitory effects of acute alcoholinduced modulation of STAT/SOCS pathway in monocytes are consistent with the previously reported role of ethanol in STAT1 activation and attenuation of IFNα-induced STAT1 activation in the Huh human hepatoma cell line (Szabo et al., 2006). Close monitoring of the changes in gene expression induced by JAK-STAT signaling have revealed that both cell-type specific transcription and core, or stereotypic, RNA profiles are induced by activated cytokine receptors in different cell types (Murray, 2007). For example, IFNγ, via STAT1, induces the expression of a similar cohort of genes regardless of the cell type tested (van Boxel-Dezaire et al., 2006). These genes are often termed the “IFN signature” and overlap with the gene expression pattern induced by IFNα/β signaling that also involves STAT1, in cooperation with STAT2 and IRF9 (van Boxel-Dezaire et al., 2006).
STAT3 signaling illustrates a more complex system and includes common and distinct features between IL-6, IL-10 and IFNs-induced signaling (Murray, 2007). We chose to employ IL-6 as a prototype system in order to analyze the effects of acute alcohol treatment on gene expression downstream of cytokine-induced STAT activation due to the fact that first, IL-6 employs both STAT1 and STAT3 proteins in its signaling pathway, and second, IL-6 signaling functions under the tight control of SOCS proteins (Heinrich et al., 1998; Kimura et al., 2005; Murray, 2007; Zhang et al., 2003). We identified that similar to regulation of STAT proteins, alcohol alone activated IL-6 dependent genes but inhibited them when it was combined with IL-6. Such activity pattern was consistent with the induction of SOCS1 and SOCS3 in the presence of alcohol both in vitro and ex vivo. Our findings are relevant in vivo where acute alcohol consumption significantly abolishes plasma levels of MCP-1 during inflammation (Pedersen et al., 2004; Szabo et al., 1999). Given the employment of common JAK/STAT signaling pathway by multiple cytokines, including type I and type II interferons, it is conceivable that their down-stream genes may be regulated in a similar matter to IL-6-dependent genes MCP-1 and ICAM-1. Indeed, decreased efficacy of IFNα therapy in alcohol consuming patients with chronic hepatitis C infection (HCV) has been reported (Okazaki et al., 1994). Further, in vivo acute ethanol administration prior to challenge with LPS significantly abrogated production of IFNγ-dependent chemokines MIG, IP-10, and I-TAC (Happel et al., 2007). While the in vivo dissection of the primary cytokine induction and their self-amplification loops is difficult, finding of impaired in vivo LPS-mediated IFNγ induction in the presence of alcohol suggests an additional loop of regulation. Further, SOCS1 selectively inhibits LPS-induced IL-6 production by regulating JAK-STAT pathway even in the absence of ethanol (Kimura et al., 2005). In support of data from Happel et al. (Happel et al., 2007), our in vitro and ex vivo experiments indicate to opposite effect of ethanol on LPS- and cytokine (IL-6, IFN)-induced, STAT/SOCS-mediated activation of monocytes/macrophages.
Multiple differences exist in LPS- compared to cytokine (IL-6 and IFN)-induced STAT/SOCS signaling cascade that could account for the maintenance of LPS stimulated STAT proteins despite the increase in SOCS expression. LPS-induced signaling via TLR4 results in a powerful downstream activation of 3 major families of proteins important in activating inflammatory gene expression: NFκB/Rel proteins, IFN regulatory factors (IRFs), and MAPKs, but also activates tyrosine kinase cascades and calcium signaling (Pålsson-McDermott and O’Neill, 2004). Cytokines, including IFNs and IL-6, are also potent endogenous macrophage-activating factors. However, TLR and cytokine-induced macrophage activation differ in their kinetic, the signaling pathways employed and in the outcome of activation. In contrast to TLRs, the cytokines are relatively weak activators of NFκB and MAPKs (Hu et al., 2007). The binding of cytokines to their specific receptors leads to activation of receptor-associated Jak protein tyrosine kinases, followed by tyrosine phosphorylation and activation of latent cytoplasmic STAT proteins, which in turn dimerize and translocate to the nucleus where they bind to promoter sequences and activate transcription (Hu et al., 2007; Murray, 2007). STAT transcriptional activity is potentiated by serine phosphorylation of transcription activation domains, which can be mediated by multiple kinases, including MAPKs, PKC, and calcium-triggered calmodulin-dependent protein kinase II. Alcohol is a known modulator of both early and late steps of the TLR4-mediated macrophage activation, and the crosstalk between the TLR4- and IFNγ-triggered pathways is well documented (Schroder et al., 2006), thus suggesting the possibility of direct and/or indirect effects of alcohol on cytokine-induced Mf activation. The SOCS family brings an additional layer of complexity to the negative regulation of macrophages. SOCS1 binds to the p65 subunit of NF-κB and facilitates ubiquitylation and degradation of p65 (Ryo et al., 2003). SOCS1 also binds to tyrosine phosphorylated myeloid differentiation primary-response gene 88 (MyD88)-adaptor-like protein (MAL) through its interaction with BTK (Bruton’s tyrosine kinase) (Mansell et al., 2006), and induces ubiquitylation and degradation of MAL, thereby leading to the suppression of MAL-dependent p65 phosphorylation and transactivation of NF-κB (Mansell et al., 2006). In addition to the NF-κB pathway, SOCS1 might regulate the stress-activated mitogen-activated protein kinases (MAPKs) JUN N-terminal kinase (JNK) and p38 by binding to apoptosis signal-regulating kinase 1 (ASK1), which is an upstream activator of both the JNK and p38 MAPK cascades (He et al., 2006). The effect of SOCS3 on TLR signaling is thought to occur indirectly through STAT1 activation (Ogata et al., 2006; Prele et al., 2006; Wang et al., 2004). In contrast, SOCS3 is a key regulator for the divergent activity of cytokine-induced macrophage activation, and IL-6 in particular. More recently Murray proposed that the generic effect of STAT3 on IL-6-triggered pathway is anti-inflammatory, but the IL-6R in the presence of SOCS3 generates a signal that suppresses the expression of anti-inflammatory genes (Murray, 2007). However, it has not been clarified what kind of signal is generated (or suppressed) upon IL-6R/SOCS3 association. The complexity of the signaling, the crosstalk between different upstream activators of Jak/STAT/SOCS cascade, and the functional redundancy within the SOCS family members are several levels where the differences between TLR4 and cytokine-mediated macrophage activation could be identified. Our manuscript points to the particularities of the differential regulation of the STAT/SOCS signaling cascade in the presence of alcohol, that also depends on the upstream triggers of this pathway.
Although the suppressor of cytokine signaling (SOCS) proteins are the best understood negative regulators of the JAKSTAT pathway, the biochemical mechanism of SOCS-mediated inhibition is currently unexplained. SOCS proteins regulate both the quality and the quantity of STAT signals from cytokine receptors thus it is conceivable that they would play a major role in the ethanol-induced regulation of STAT (Murray, 2007). However, many other possibilities may also contribute to ethanol inhibition of cytokine activation of STAT signaling in monocytes. It was reported that acute ethanol inhibits IL-6- and IFN-activation of STATs in hepatocytes, and ethanol modulation of the activity of PKC and MAPK kinases was observed in hepatocytes and macrophages (Chen et al., 2001; D’Souza et al., 1992; Kato et al., 2005; Nguyen et al., 2000; Oak et al., 2006). Ethanol treatment may also increase the stability of SOCS1 and SOCS3 protein via inhibition of proteasome activity (Osna et al., 2005). It is certain that the effect of alcohol is multifactorial and the exact levels of interplay of all these pathways are yet to be fully understood.
To date modulation of STAT/SOCS signaling pathway is reality in biomedical research. Recently Deng et al. (2007) reported that resveratrol, a major component of natural alcoholic beverages, exhibits anti-inflammatory effects and inhibit STAT3-mediated cell activation. Our findings indicate differential effect of acute alcohol treatment on the STAT/SOCS system. More importantly, our findings suggest that modulation of the STAT/SOCS signaling pathway could constitute a potential therapeutic target for acute alcohol-induced immunodepression during infections and trauma.
ACKNOWLEDGMENTS
We thank CFAR facility at University of Massachusetts Medical School for excellent support. This research was supported by NIH grants AA 008577 and AA011576.
REFERENCES
- Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004;22:503–529. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- Boe DM, Nelson S, Zhang P, Quinton L, Bagby GJ. Alcohol-induced suppression of lung chemokine production and the host defense response to Streptococcus pneumoniae. Alcohol Clin Exp Res. 2003;27:1838–1845. doi: 10.1097/01.ALC.0000095634.82310.53. [DOI] [PubMed] [Google Scholar]
- van Boxel-Dezaire AH, Rani MR, Stark GR. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity. 2006;25:361–372. doi: 10.1016/j.immuni.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Burysek L, Syrovets T, Simmet T. The serine protease plasmin triggers expression of MCP-1 and CD40 in human primary monocytes via activation of p38 MAPK and janus kinase (JAK)/STAT signaling pathways. J Biol Chem. 2002;277:33509–33517. doi: 10.1074/jbc.M201941200. [DOI] [PubMed] [Google Scholar]
- Chen J, Clemens DL, Cederbaum AI, Gao B. Ethanol inhibits the JAK-STAT signaling pathway in freshly isolated rat hepatocytes but not in cultured hepatocytes or HepG2 cells: evidence for a lack of involvement of ethanol metabolism. Clin Biochem. 2001;34:203–209. doi: 10.1016/s0009-9120(01)00216-8. [DOI] [PubMed] [Google Scholar]
- Coccia EM, Del Russo N, Stellacci E, Testa U, Marziali G, Battistini A. STAT1 activation during monocyte to macrophage maturation: role of adhesion molecules. Int Immunol. 1999;11:1075–1083. doi: 10.1093/intimm/11.7.1075. [DOI] [PubMed] [Google Scholar]
- D’Souza NB, Bautista AP, Lang CH, Spitzer JJ. Acute ethanol intoxication prevents lipopolysaccharide-induced down regulation of protein kinase C in rat Kupffer cells. Alcohol Clin Exp Res. 1992;16:64–67. doi: 10.1111/j.1530-0277.1992.tb00637.x. [DOI] [PubMed] [Google Scholar]
- Deng J, Grande F, Neamati N. Small molecule inhibitors of Stat3 signaling pathway. Curr Cancer Drug Targets. 2007;7:91–107. doi: 10.2174/156800907780006922. [DOI] [PubMed] [Google Scholar]
- Federici M, Giustizieri ML, Scarponi C, Girolomoni G, Albanesi C. Impaired IFN-gamma-dependent inflammatory responses in human keratinocytes overexpressing the suppressor of cytokine signaling 1. J Immunol. 2002;169:434–442. doi: 10.4049/jimmunol.169.1.434. [DOI] [PubMed] [Google Scholar]
- Gatto L, Berlato C, Poli V, Tininini S, Kinjyo I, Yoshimura A, Cassatella MA, Bazzoni F. Analysis of SOCS-3 promoter responses to interferon gamma. J Biol Chem. 2004;279:13746–13754. doi: 10.1074/jbc.M308999200. [DOI] [PubMed] [Google Scholar]
- Goral J, Choudhry MA, Kovacs EJ. Acute ethanol exposure inhibits macrophage IL-6 production: role of p38 and ERK1/2 MAPK. J Leukoc Biol. 2004;75:553–559. doi: 10.1189/jlb.0703350. [DOI] [PubMed] [Google Scholar]
- Goral J, Kovacs EJ. In vivo ethanol exposure down-regulates TLR2-, TLR4-, and TLR9-mediated macrophage inflammatory response by limiting p38 and ERK1/2 activation. J Immunol. 2005;174:456–463. doi: 10.4049/jimmunol.174.1.456. [DOI] [PubMed] [Google Scholar]
- Grace MJ, Cutler D. Pegylating IFNs at his-34 improves the in vitro antiviral activity through the JAK?STAT pathway. Antivir Chem Chemother. 2004;15:287–297. doi: 10.1177/095632020401500601. [DOI] [PubMed] [Google Scholar]
- Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2:428–432. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- Happel KI, Rudner X, Quinton LJ, Movassaghi JL, Clark C, Odden AR, Zhang P, Bagby GJ, Nelson S, Shellito JE. Acute alcohol intoxication suppresses the pulmonary ELR-negative CXC chemokine response to lipopolysaccharide. Alcohol. 2007;41:325–333. doi: 10.1016/j.alcohol.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, You L, Uematsu K, Matsangou M, Xu Z, He M, McCormick F, Jablons DM. Cloning and characterization of a functional promoter of the human SOCS-3 gene. Biochem Biophys Res Commun. 2003;301:386–391. doi: 10.1016/s0006-291x(02)03071-1. [DOI] [PubMed] [Google Scholar]
- He Y, Zhang W, Zhang R, Zhang H, Min W. SOCS1 inhibits tumor necrosis factor-induced activation of ASK1–JNK inflammatory signaling by mediating ASK1 degradation. J Biol Chem. 2006;281:5559–5566. doi: 10.1074/jbc.M512338200. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130?Jak?STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HH, Ivashkiv LB. Role of STAT3 in type I interferon responses: negative regulation of STAT1-dependent inflammatory gene activation. J Biol Chem. 2006;281:14111–14118. doi: 10.1074/jbc.M511797200. [DOI] [PubMed] [Google Scholar]
- Hu X, Chen J, Wang L, Ivashkiv LB. Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. J Leukoc Biol. 2007;82:237–243. doi: 10.1189/jlb.1206763. [DOI] [PubMed] [Google Scholar]
- Kato H, Negoro M, Wakabayashi I. Effects of acute ethanol administration on LPS-induced expression of cyclooxygenase-2 and inducible nitric oxide synthase in rat alveolar macrophages. Alcohol Clin Exp Res. 2005;29(12 Suppl):285S–293S. doi: 10.1097/01.alc.0000191809.29775.41. [DOI] [PubMed] [Google Scholar]
- Kimura A, Naka T, Muta T, Takeuchi O, Akira S, Kawase I, Kishimoto T. Suppressor of cytokine signaling-1 selectively inhibits LPS-induced IL-6 production by regulating JAK-STAT. Proc Natl Acad Sci U S A. 2005;102:17089–17094. doi: 10.1073/pnas.0508517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, White B, Szabo G. Moderate alcohol intake in humans attenuates monocyte inflammatory responses: inhibition of nuclear regulatory factor kappa B and induction of interleukin 10. Alcohol Clin Exp Res. 2006;30:135–139. doi: 10.1111/j.1530-0277.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Mansell A, Smith R, Doyle SL, Gray P, Fenner JE, Crack PJ, Nicholson SE, Hilton DJ, O’Neill LA, Hertzog PJ. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nature Immunol. 2006;7:148–155. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. Cutting edge: CpG oligonucleotides induce splenic CD19 + dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol. 2005;175:5601–5605. doi: 10.4049/jimmunol.175.9.5601. [DOI] [PubMed] [Google Scholar]
- Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2639. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- Nguyen VA, Chen J, Hong F, Ishac EJ, Gao B. Interferons activate the p42?44 mitogen-activated protein kinase and JAK-STAT (Janus kinase-signal transducer and activator transcription factor) signalling pathways in hepatocytes: differential regulation by acute ethanol via a protein kinase C-dependent mechanism. Biochem J. 2000;349:427–434. doi: 10.1042/0264-6021:3490427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkina O, Dolganiuc A, Shapiro T, Kodys K, Mandrekar P, Szabo G. Acute alcohol activates STAT3, AP-1, and Sp-1 transcription factors via the family of Src kinases to promote IL-10 production in human monocytes. J Leukoc Biol. 2007;82:752–762. doi: 10.1189/jlb.0207099. [DOI] [PubMed] [Google Scholar]
- Oak S, Mandrekar P, Catalano D, Kodys K, Szabo G. TLR2- and TLR4-mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J Immunol. 2006;176:7628–7635. doi: 10.4049/jimmunol.176.12.7628. [DOI] [PubMed] [Google Scholar]
- Ogata H, Chinen T, Yoshida T, Kinjyo I, Takaesu G, Shiraishi H, Iida M, Kobayashi T, Yoshimura A. Loss of SOCS3 in the liver promotes fibrosis by enhancing STAT3-mediated TGF-beta1 production. Oncogene. 2006;25:2520–2530. doi: 10.1038/sj.onc.1209281. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Yoshihara H, Suzuki K, Yamada Y, Tsujimura T, Kawano K, Yamada Y, Abe H. Efficacy of interferon therapy in patients with chronic hepatitis C. Comparison between non-drinkers and drinkers. Scand J Gastroenterol. 1994;29:1039–1043. doi: 10.3109/00365529409094883. [DOI] [PubMed] [Google Scholar]
- Osna NA, Clemens DL, Donohue TM., Jr Ethanol metabolism alters interferon gamma signaling in recombinant HepG2 cells. Hepatology. 2005;42:1109–1117. doi: 10.1002/hep.20909. [DOI] [PubMed] [Google Scholar]
- Pålsson-McDermott EM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N, Larsen S, Seidelin JB, Nielsen OH. Alcohol modulates circulating levels of interleukin-6 and monocyte chemoattractant protein-1 in chronic pancreatitis. Scand J Gastroenterol. 2004;39:277–282. doi: 10.1080/00365520310008296. [DOI] [PubMed] [Google Scholar]
- Prele CM, Keith-Magee AL, Yerkovich ST, Murcha M, Hart PH. Suppressor of cytokine signalling-3 at pathological levels does not regulate lipopolysaccharide or interleukin-10 control of tumour necrosis factor-alpha production by human monocytes. Immunology. 2006;119:8–17. doi: 10.1111/j.1365-2567.2006.02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett SB, Zheng Q, Fan R, Matthews K, Schwab C. Acute exposure to ethanol affects Toll-like receptor signaling and subsequent responses: an overview of recent studies. Alcohol. 2004;33:235–239. doi: 10.1016/j.alcohol.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Rakesh K, Agrawal DK. Controlling cytokine signaling by constitutive inhibitors. Biochem Pharmacol. 2005;70:649–657. doi: 10.1016/j.bcp.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Ramana CV, Kumar A, Enelow R. Stat1-independent induction of SOCS-3 by interferon-gamma is mediated by sustained activation of Stat3 in mouse embryonic fibroblasts. Biochem Biophys Res Commun. 2005;327:727–733. doi: 10.1016/j.bbrc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- Ryo A, Suizu F, Yoshida Y, Perrem K, Liou YC, Wulf G, Rottapel R, Yamaoka S, Lu KP. Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65?RelA. Mol Cell. 2003;12:1413–1422. doi: 10.1016/s1097-2765(03)00490-8. [DOI] [PubMed] [Google Scholar]
- Schroder K, Sweet MJ, Hume DA. Signal integration between IFNgamma and TLR signalling pathways in macrophages. Immunobiology. 2006;211:511–524. doi: 10.1016/j.imbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Szabo G, Aloman C, Polyak SJ, Weinman SA, Wands J, Zakhari S. Hepatitis C infection and alcohol use: a dangerous mix for the liver and antiviral immunity. Alcohol Clin Exp Res. 2006;30:709–719. doi: 10.1111/j.1530-0277.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- Szabo G, Chavan S, Mandrekar P, Catalano D. Acute alcohol consumption attenuates interleukin-8 (IL-8) and monocyte chemoattractant peptide-1 (MCP-1) induction in response to ex vivo stimulation. J Clin Immunol. 1999;19:67–76. doi: 10.1023/a:1020518703050. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. STAT family of transcription factors in cytokinemediated biological responses. Cytokine Growth Factor Rev. 2000;11:199–207. doi: 10.1016/s1359-6101(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Venkataraman C, Leung S, Salvekar A, Mano H, Schindler U. Repression of IL-4-induced gene expression by IFN-gamma requires Stat1 activation. J Immunol. 1999;162:4053–4061. [PubMed] [Google Scholar]
- Vlotides G, Sorensen AS, Kopp F, Zitzmann K, Cengic N, Brand S, Zachoval R, Auernhammer CJ. SOCS-1 and SOCS-3 inhibit IFN-alpha-induced expression of the antiviral proteins 2,5-OAS and MxA. Biochem Biophys Res Commun. 2004;320:1007–1014. doi: 10.1016/j.bbrc.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, Dalton W, Jove R, Pardoll D, Yu H. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nature Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Li Y, Ren YF, Shen BF. Comparison of IL-6-induced STAT1 activation in different responsive cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2003;19:106–108. [PubMed] [Google Scholar]