Transport along the dendritic endoplasmic reticulum mediates the trafficking of GABAB receptors (original) (raw)
ABSTRACT
In neurons, secretory organelles within the cell body are complemented by the dendritic endoplasmic reticulum (ER) and Golgi outposts (GOPs), whose role in neurotransmitter receptor trafficking is poorly understood. γ-aminobutyric acid (GABA) type B metabotropic receptors (GABABRs) regulate the efficacy of synaptic transmission throughout the brain. Their plasma membrane availability is controlled by mechanisms involving an ER retention motif and assembly-dependent ER export. Thus, they constitute an ideal molecular model to study ER trafficking, but the extent to which the dendritic ER participates in GABABR biosynthesis has not been thoroughly explored. Here, we show that GABAB1 localizes preferentially to the ER in dendrites and moves long distances within this compartment. Not only diffusion but also microtubule and dynein-dependent mechanisms control dendritic ER transport. GABABRs insert throughout the somatodendritic plasma membrane but dendritic post-ER carriers containing GABABRs do not fuse selectively with GOPs. This study furthers our understanding of the spatial selectivity of neurotransmitter receptors for dendritic organelles.
KEY WORDS: GABA, Dendrite, Endoplasmic reticulum, Golgi outposts, Neuron, Trafficking
INTRODUCTION
The control of the availability of neurotransmitter receptors at appropriate subdomains of the plasma membrane is crucial for neuronal function and synaptic plasticity. Availability is governed by mechanisms of intracellular trafficking that deliver newly synthesized receptors to the plasma membrane and remove them for storage, recycling or degradation. These mechanisms determine the activity-dependent strengthening or weakening of synapses (Ehlers, 2000; Clem and Barth, 2006).
Two major secretory pathways operate in dendrites (Horton and Ehlers, 2003). In a canonical pathway, proteins are synthesized and post-translationally modified in the somatic ER, transported to a perinuclear Golgi for additional modifications and targeted distally in long-haul post-Golgi vesicles. The distribution of the continuous ER throughout dendrites (Broadwell and Cataldo, 1983; Spacek and Harris, 1997), and the localization of functional Golgi outposts (GOPs), which are defined as discrete Golgi structures discontinuous with the somatic organelle (Horton and Ehlers, 2003; Horton and Ehlers, 2004; Ye et al., 2007), suggest that local secretory pathways also exist in neurons. Thus, proteins can be synthesized and transported by diffusion along the somatodendritic ER and exported through specialized ER exit sites (ERES) in dendrites for local Golgi trafficking and delivery to the plasma membrane (Aridor et al., 2004; Cui-Wang et al., 2012; Chen et al., 2013). Importantly, glutamate receptors (AMPARs and NMDARs) are differentially sorted to the perinuclear Golgi or GOPs (Jeyifous et al., 2009). However, the role of dendritic organelles in the trafficking of neurotransmitter receptors is not fully understood.
γ-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the central nervous system, and GABA type B metabotropic receptors (GABABRs) mediate the slow and prolonged phase of inhibitory postsynaptic potentials (Bettler et al., 2004). The receptors are found in both somatodendritic and axonal compartments, and are heteromers composed of two subunits, GABAB1 and GABAB2, encoded by the Gabbr1 and Gabbr2 genes, respectively. GABAB1 contains an arginine-X-arginine (RXR)-type ER retention motif in the carboxyl-terminus that is inactivated by GABAB2, resulting in the assembly of a heterodimer and delivery to the plasma membrane (Margeta-Mitrovic et al., 2000). In spite of their key function in regulating synaptic transmission, the mechanisms that control their availability remain for the most part unexplored, especially the mechanisms that regulate their exocytic route in dendrites.
Conventional vesicular mechanisms regulate multiple stages of GABABR trafficking (Pooler et al., 2009; Vargas et al., 2008; Grampp et al., 2007; Biermann et al., 2010). However, segregated GABAB1 and GABAB2 subunits are detected by light and electron microscopy in hippocampal and visual cortex neurons (Gonchar et al., 2001; Ramírez et al., 2009; Biermann et al., 2010). Because GABAB1 is exported from the ER only upon binding to GABAB2 this evidence suggests that they utilize an alternative ER transport pathway into dendrites during the early stages of their biosynthetic itinerary. However, the roles of dendritic organelles in the trafficking of GABABRs are not well delineated. Here, we combine fixed and live-cell confocal and super-resolution imaging of intracellular GABAB subunits with the use of organelle reporters and trafficking blockers to explore the contribution of the dendritic ER and GOPs to the exocytic pathway of GABABRs in cultured hippocampal neurons.
RESULTS
GABAB1 colocalizes with the ER in dendrites
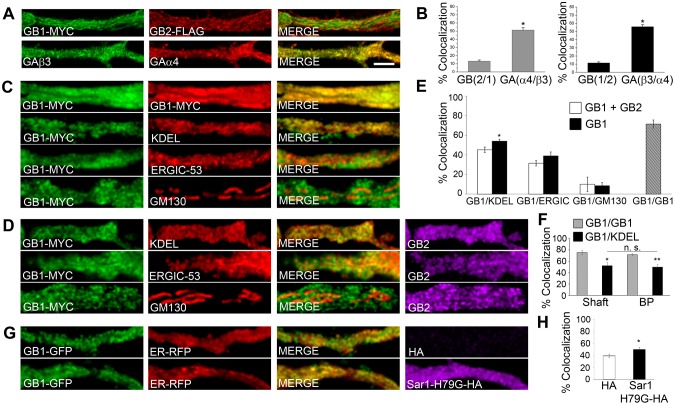
Local dendritic trafficking requires newly synthesized membrane proteins to dwell in, assemble in and exit from the somatodendritic ER. Because GABABR assembly is required for the export of the receptor from the ER, the existence of segregated subunits in dendrites is a good reporter of residence in the ER (Ramírez et al., 2009). To investigate this, we compared the colocalization of transfected GABAB1 and GABAB2, the two subunits of the GABABR, to that of recombinant GABAAα4 and GABAAβ3 (encoded by Gabra4 and Gabrb3, respectively), two subunits of the non-related ionotropic GABAA receptor (GABAAR). Colocalization was significantly lower for GABAB than for GABAA subunits (Fig. 1A,B). These observations are consistent with previous reports (Gonchar et al., 2001; Ramírez et al., 2009) and indicate that segregated GABAB subunits are abundant in dendrites, possibly revealing dendritic ER transport and assembly.
Fig. 1.
GABAB1 is enriched in and traffics through the ER in dendrites. (A) Rat hippocampal neurons were co-transfected with GABAB1–Myc (GB1–MYC, green) and GABAB2–FLAG (GB2–FLAG, red) or with the GABAA subunit fusion proteins pH/BBSβ3 (GAβ3, green) and RFP-BBSα4 (GAα4, red). Proximal dendrites are shown. Scale bar: 10 µm. (B) Left plot, percentage colocalization of GABAB2 with GABAB1 and of RFP-BBSα4 with pH/BBSβ3. Right plot, percentage colocalization of GABAB1 with GABAB2 or of pH/BBSβ3 with RFP-BBSα4. n = 4–8 neurons. (C) Neurons were transfected with GABAB1–Myc (green) and labeled with antibodies against Myc and KDEL (ER marker), ERGIC-53 (ERGIC marker) or GM130 (cis-Golgi marker) (red). The top panels are positive controls with GABAB1–Myc and two different secondary antibodies. (D) Same as in C for neurons co-transfected with GABAB1–Myc (red) and GABAB2 (purple). (E) Percentage of colocalization of GABAB1–Myc with KDEL, ERGIC-53 and GM130 (black bars) or of GABAB1–Myc with the same organelle markers in the presence of GABAB2 (white bars). GABAB1–Myc was used as positive control (hatched bar). n = 5–7 neurons. (F) Percentage colocalization of GABAB1–Myc with KDEL in dendritic shafts and branching points (BP). GABAB1–Myc was used as positive control (gray bar). n = 4–6 neurons. (G) Neurons from transgenic mice expressing GABAB1–GFP (green) were transfected with ER–RFP (red) in the presence or absence of Sar1-H79G–HA (purple). (H) Percentage of colocalization of GABAB1–GFP with ER–RFP. n = 6–7 neurons. All quantitative data show the mean±s.e.m.; *P<0.05; **P<0.01; n.s., non-significant; Student's _t_-test.
To determine whether segregated GABAB1 subunits are enriched in the dendritic ER, we carried out quantitative colocalization with markers of secretory organelles. GABAB1, overexpressed either alone or in combination with GABAB2, colocalized preferentially with the ER and, to a lesser degree, with the ER-to-Golgi intermediate compartment (ERGIC) and GOPs (Fig. 1C–E). In agreement with the role of the GABAB2 subunit in controlling receptor export from the ER, the colocalization of GABAB1 with the ER decreased significantly in the presence of GABAB2 (GABAB1, 54±2%; GABAB1+GABAB2, 45±3%; P = 0.025, Student's _t_-test; ±s.e.m.) (Fig. 1D,E). The colocalization of GABAB1 with the ER was comparable in regions of low and high network complexity, such as dendritic shafts and branching points, respectively (Cui-Wang et al., 2012) (shaft, 52±6%; branching points, 50±4%; P = 0.758, Student's _t_-test) (Fig. 1F). To rule out the possibility of artificial colocalization due to overexpression, we analyzed neurons derived from GABAB1–EGFP transgenic mice, which express GABAB1–EGFP at endogenous levels (Casanova et al., 2009). Colocalization between GABAB1 and the ER in this model was comparable to that observed with overexpressed subunits (Fig. 1G,H). More importantly, blocking ER export in transgenic neurons with Sar1-H79G–HA, a dominant negative form of Sar1 that is locked in a GTP-bound state and blocks export from the ER (Ward et al., 2001), increased the colocalization of GABAB1 with the ER (control, 39±3%; Sar1-H79G–HA, 50±4%; P = 0.04, Student's _t_-test) (Fig. 1G,H), indicating that GABAB1 accumulates in the dendritic ER when export is prevented. These results are consistent with GABAB1 ER retention and export (Margeta-Mitrovic et al., 2000).
GABAB1 transports within the ER
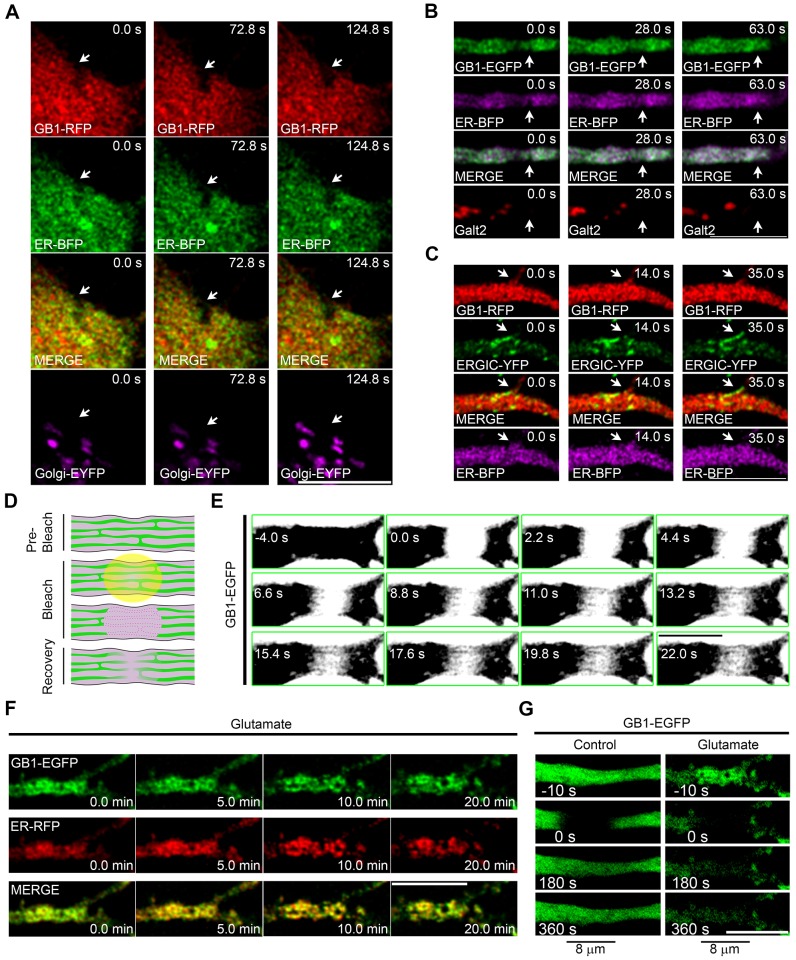
We then explored whether the ER functions as a transport organelle for GABAB1. We first carried out live-cell imaging to characterize the dynamic relationship between intracellular GABAB1 and the ER in the soma and dendrites of neurons co-transfected with GABAB1–mRFP or GABAB1–EGFP and ER–BFP, a vector that encodes a blue fluorescent protein fused to the human ER resident protein Sec61β – the encoded fusion protein strongly labels the ER (Zurek et al., 2011). Although the spatial resolution was not sufficient to visualize the tubular network because of its densely packed nature (Spacek and Harris, 1997), punctate and irregular structures containing fluorescent GABAB1 were observed in the somatic and dendritic compartments, and their distribution patterns changed over time (Fig. 2A,B, top panels, arrows). Not every puncta of GABAB1 colocalized with the ER, in agreement with the results presented in Fig. 1, but the overall structure often rearranged in synchrony. By contrast, the dynamics of the Golgi, labeled with either Golgi–EYFP (EYFP fused to the Golgi-targeted membrane-anchoring signal peptide from human β-1,4-galactosyltransferase; labels Golgi in the soma) or Galt2–mCherry (Galt2 is officially known as galactosyltransferase 2; labels Golgi in the dendrites) and the ERGIC, labeled with ERGIC-53–YFP (Ward et al., 2001), were noticeably different from the dynamics of GABAB1 in both compartments (Fig. 2A–C).
Fig. 2.
GABAB1 is mobile within the neuronal ER. (A) Neurons were co-transfected with GABAB1–mRFP (GB1–RFP, red), ER–BFP (green) and Golgi–EYFP (purple) and were imaged live. A somatic region is shown. Arrows show synchronous rearrangement of GABAB1–mRFP and ER–BFP signals. (B) Neurons were co-transfected with GABAB1–EGFP (green), ER–BFP (purple) and Galt2–mCherry (red) and were imaged live. A dendrite is shown. Arrows show synchronous rearrangement of GABAB1–EGFP and ER–BFP signals. (C) Neurons were co-transfected with GABAB1–mRFP (red), ERGIC–YFP (green) and ER–BFP (purple), and imaged live. A dendrite is shown. Arrows show the asynchronous rearrangement of GABAB1–mRFP and ERGIC–YFP signals. (D) Diagram illustrating the FRAP experiments carried out for ER proteins in dendrites. (E) The ER mobility of GABAB1–EGFP was evaluated by FRAP in a thick dendrite. The image was saturated to allow visualization of the tubular ER structure. (F) Dendrites of neurons transfected with GABAB1–EGFP (green) and ER–RFP (red) and imaged live in the absence or presence of 100 µM glutamate (20 min). (G) The mobility of GABAB1–EGFP was evaluated by FRAP (bleached region 8 µm) before (left) or after (right) the glutamate treatment. Scale bars: 10 µm.
Next, we explored the diffusion of GABAB1 within the dendritic ER using fluorescence recovery after photobleaching (FRAP, Fig. 2D) on an 8-µm dendritic segment of neurons transfected with GABAB1–EGFP. ER-retained GABAB1–EGFP was mobile in dendrites, and lateral mobility in ER tubules was observed by high-rate imaging in thick dendrites (Fig. 2E). Prolonged NMDA receptor activation with glutamate produces reversible ER fission and disrupts the transport of luminal ER proteins (Kucharz et al., 2009; Ramírez et al., 2011). Thus, we used this treatment in neurons that were co-transfected with GABAB1–EGFP and ER–RFP to confirm the ER mobility of GABAB1–EGFP. As expected, glutamate receptor activation significantly altered the structure of the ER and resulted in the co-rearrangement of ER–RFP and GABAB1–EGFP (Fig. 2F). More importantly, the mobility of GABAB1–EGFP was severely impaired (Fig. 2G).
These results demonstrate that GABAB1 localizes to the ER, which allows the transport of unassembled GABAB1 in dendrites. Because the subunit is not an ER-resident protein but a trafficking cargo we will refer to it as ‘ER-associated GABAB1’ throughout the rest of the text.
Characterization of GABAB1 transport within the dendritic ER
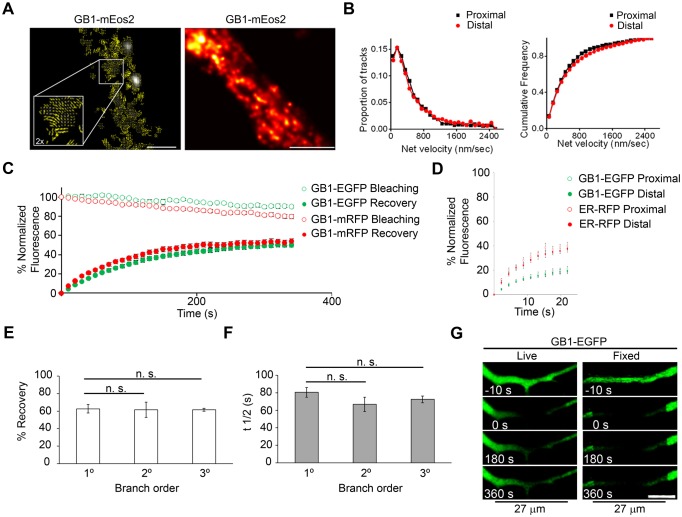
To gain a better understanding of ER-associated GABAB1 subunit transport we first used single-particle tracking photoactivated localization microscopy (sptPALM) (Manley et al., 2008; Frost et al., 2010). This technique is well suited to examine the dynamic properties of single intracellular molecules within small regions, and allows a spatial resolution of ∼10–50 nm (Sengupta et al., 2012). To track receptor subunits, we generated mEos2-tagged GABAB1 (GABAB1–mEos2). mEos2 is a bright and photostable photoconvertible fluorescent protein that is switched from green to red fluorescence by UV irradiation (McKinney et al., 2009). This construct was retained intracellularly and maintained the characteristic trafficking properties of GABAB1 (data not shown). Individual molecular locations within transfected neurons were recorded in a 20,000-frame series, and the summation of these positions revealed that GABAB1–mEos2 was distributed throughout a complex heterogeneous structure (Fig. 3A, right panel), similar to that reported for the dendritic ER using structured illumination or electron microscopy (Cui-Wang et al., 2012; Broadwell and Cataldo, 1983; Spacek and Harris, 1997). Single photoswitched GABAB1–mEos2 molecules were tracked over multiple image frames and were found to be highly mobile, and their motility was not oriented in relation to the axis of the dendritic shaft (Fig. 3A, left panel; Fig. 3B), consistent with the expected organization of the ER. The molecular velocity spanned a range from nearly immobile to 1 µm/s, with a fraction of the population moving at rates >2 µm/s. Notably, the distribution of velocities was not different in proximal and distal segments of a dendrite (Fig. 3B, right panel). Because most molecules were tracked for only brief periods over which the influence of directed transport or confinement is minimized (<4 frames or 200 ms), the molecular velocity can be used to estimate an effective diffusion coefficient D, given that the mean squared molecular displacement increases as 4_D_Δ_t_, where Δ_t_ is the observation interval. Thus, the modal velocity of 200 nm/s observed here indicates that a substantial proportion of GABAB1 moves within the dendritic ER with a relatively slow effective D, ∼0.01 µm2/s, whereas the subpopulation of molecules undergoing rapid motion does so with D surpassing 1 µm2/s. These values are consistent with the general expectation for motion of transmembrane proteins in the lipid bilayer (Saxton and Jacobson, 1997) and are comparable to those of ER-resident proteins (Bannai et al., 2004). It should be noted that because each molecule is tracked only briefly, it is not possible to determine with this approach how frequently molecules transition from one rate of mobility to another, and thus for how long they remain immobile. These observations suggest that two or more subpopulations of different mobility contribute to the ER transport of GABAB1 in dendrites.
Fig. 3.
Characterization of the ER transport of GABAB1. (A) The mobility of GABAB1–mEos2 was evaluated by using sptPALM in dendrites. Left panel, quiver plots show GABAB1–mEos2 (GB1−mEos2) molecules mobilizing in multiple directions (inset is 2× magnification). Scale bar: 1 µm. Right panel, GABAB1–mEos2 was imaged in a live neuron for 10 seconds. Scale bar: 2 µm. (B) Net velocity of GABAB1–mEos2 single molecules at initial and distal segments of the dendrite (black and red curves, respectively). (C) FRAP of GABAB1–EGFP (green symbols) and GABAB1–mRFP (red symbols) in dendrites. The upper curves (empty symbols) correspond to the experimental bleaching without FRAP. n = 14–28 neurons. (D) FRAP of GABAB1–EGFP (green symbols) and ER–RFP (red symbols) in dendrites. n = 8 neurons. (E) Percentage recovery of GABAB1–EGFP in primary (1°), secondary (2°) and tertiary (3°) dendritic branches. n = 3–5 neurons. (F) t1/2 of recovery of GABAB1–EGFP in primary, secondary and tertiary dendritic branches. n = 3–5 neurons. All quantitative data show the mean±s.e.m.; n.s., non-significant. (G) The long-range mobility of GABAB1–EGFP was evaluated by bleaching a 27-µm dendritic region in live (left) or fixed (right) cells. Scale bar: 10 µm.
We used FRAP to further examine the transport of ER-associated GABAB1 fused to EGFP or mRFP. We used the percentage recovery as an indication of the mobile fraction, and recovery rate (_t_1/2) was used as an indication of transport velocity within the ER. The mobile fraction of GABAB1–EGFP was 50±2% (±s.e.m.) and _t_1/2 was 75±2 s, with minimal interference from acquisition bleaching (Fig. 3C). The percentage recovery and _t_1/2 were not affected by the fluorescent tag because the same results were obtained with GABAB1–mRFP (Fig. 3C). The transport of GABAB1 was bidirectional and significantly slower than that of ER–RFP (Fig. 3D). The percentage recovery and _t_1/2 were identical in primary, secondary and tertiary branches (percentage recovery of primary branches versus secondary, P = 0.919; versus tertiary, P = 0.833; _t_1/2 of primary branches versus secondary, P = 0.242; versus tertiary, P = 0.275, Student's _t_-test) (Fig. 3E,F). Significant recovery was observed after bleaching a large 27-µm stretch of dendritic volume, indicating that the ER allows long-distance protein transport (Fig. 3G).
Dendritic transport of ER-associated GABAB1 is controlled by multiple components
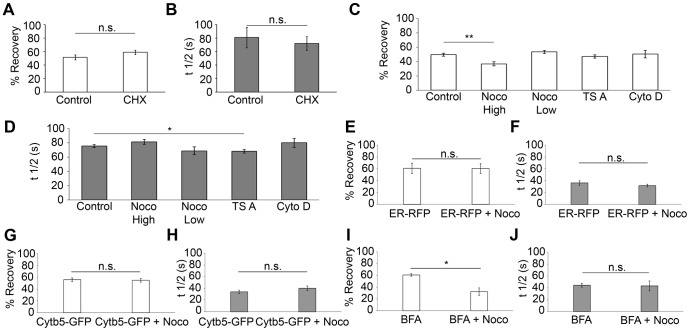
Previous studies have suggested that the transport of neurotransmitter receptors within the dendritic ER is controlled by lateral diffusion (Cui-Wang et al., 2012). Long-distance transport of ER-associated GABAB1 raises the possibility that simple diffusion is not sufficient to explain its mobility. First, to confirm that our FRAP-based transport assay was not confounded by the appearance of newly synthesized GABAB1, we blocked protein synthesis by treating neurons with cycloheximide (CHX, 0.1 µg/µl, 90 min). Importantly, the percentage recovery and _t_1/2 were unaffected by cycloheximide (Fig. 4A,B, GABAB1–EGFP±CHX percentage recovery, P = 0.166; _t_1/2, P = 0.642, Student's _t_-test). Next, taking into account the fact that multiple cytoskeletal mechanisms drive protein transport in neurons, we examined their contribution to the ER-mediated transport of GABAB1 in proximal dendrites. The mobile fraction of GABAB1 decreased significantly after high doses of nocodazole (Noco High, 100 µM, 4 h), but was unaffected by low doses of nocodazole (Noco Low, 100 nM, 5–70 min), trichostatin A (TSA, 125 nM, 30 min) or cytochalasin D (cytoD, 5 µM, 2 h) (Noco High, 37±3%, P = 0.002; Noco Low, 53±2%, P = 0.214; TSA, 47±2%, P = 0.397; cytoD, 50±5%, P = 0.901, Student's _t_-test; ±s.e.m.) (Fig. 4C). These results indicate that, in addition to diffusion, a proportion of subunit transport depends on polymerized microtubules (which are affected by high doses of nocodazole), but not on dynamic microtubules (which are affected by low doses of nocodazole), acetylated microtubules (the levels of which are increased by TSA) or the actin cytoskeleton (which is disrupted by cytochalasin D). Interestingly, luminal ER–RFP and the ER membrane protein cytochrome-b5–EGFP (Cytb5–GFP) were unaffected by high doses of nocodazole (percentage recovery of ER–RFP±Noco High, P = 0.966; percentage recovery of Cytb5–GFP±Noco High, P = 0.827. _t_1/2 for ER–RFP±Noco High, P = 0.361; for Cytb5–GFP±Noco High, P = 0.239, Student's _t_-test) (Fig. 4E–H) arguing against a global effect of nocodazole in the transport of proteins through the ER. To confirm that nocodazole affected ER-retained GABAB1, we preincubated neurons with brefeldin A (BFA), a treatment that results in the dispersal of the Golgi and accumulation of cargo in the ER. Under these conditions, high doses of nocodazole still decreased the transport of GABAB1 (control BFA, 61±2%; BFA+Noco High, 32±6%, P = 0.016, Student's _t_-test) (Fig. 4I,J). Although nocodazole might be affecting a minor BFA-independent component of post-ER COPII-coated carriers, the robust effect of BFA confirms that microtubule dependence occurs mostly on ER-associated GABAB1. Control experiments demonstrated the effectiveness of all pharmacological treatments (data not shown).
Fig. 4.
The ER transport of dendritic GABAB1 is controlled by multiple components. (A) Percentage recovery of GABAB1–EGFP for control samples or after treatment with 0.1 µg/µl cycloheximide (CHX) for 90 min. n = 4 and 5 neurons, respectively. (B) Same as above for the t1/2. (C,D) Same as in A,B for neurons under control conditions or treated with 100 µM nocodazole for 4 h (Noco High), 100 nM nocodazole for 5–70 min (Noco Low), 125 nM TSA for 30 min or 5 µM cytochalasin D for 2 h (Cyto D). n = 28, 9, 8, 20 and 4 neurons, respectively. At least three independent experiments were performed per condition. (E,F) Same as in A,B for neurons transfected with ER–RFP under control conditions or following treatment with 100 µM nocodazole for 4 h (ER–RFP+Noco). n = 4 and 7 neurons, respectively. (G,H) Same as in A,B for neurons transfected with Cytb5–GFP under control conditions or following treatment with 100 µM nocodazole for 4 h (Cytb5–GFP+Noco). n = 5 and 6 neurons, respectively. (I,J) Same as in A,B for neurons treated with 0.5 µg/ml BFA for 20 h or 0.5 µg/ml BFA for 20 h+100 µM nocodazole for 4 h (BFA+Noco). n = 3 and 4 neurons, respectively. All data show the mean±s.e.m.; *P<0.05; **P<0.01; n.s., non-significant; Student's _t_-test.
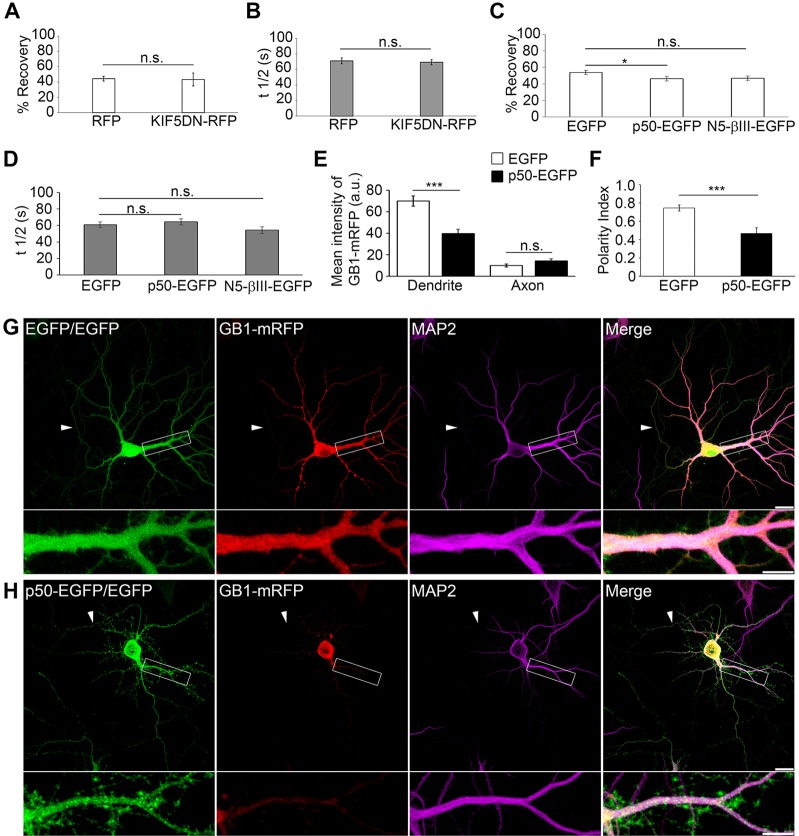
Interestingly, only TSA decreased _t_1/2 significantly _(t_1/2, 68±2 s; P = 0.026, Student's _t_-test) (Fig. 4D). Although this experiment does not directly identify a specific transport mechanism, tubulin acetylation could decrease GABAB1 confinement by increasing the recruitment of the molecular motors to microtubules (Reed et al., 2006; Dompierre et al., 2007; Cai et al., 2009) or by decreasing ER complexity (Friedman et al., 2010; Cui-Wang et al., 2012). Because the former interpretation involves molecular motors and we have demonstrated that a component of transport is microtubule dependent, we evaluated the role of kinesin-1 and dynein in the transport of dendritic GABAB1. Kinesin-1 co-immunoprecipitates with GABAB1 in brain extracts (Vidal et al., 2007), participates in the axonal localization of GABAB1 (Valdés et al., 2012) and has been implicated in ER dynamics (Woźniak et al., 2009). However, the expression of a dominant-negative version of kinesin-1 comprising the cargo-binding domain of Kif5C that interferes with endogenous kinesin–cargo interactions (KIF5CDN–RFP, data not shown) (Falley et al., 2009) had no effect on the GABAB1 mobile fraction (control RFP, 44±3%; KIF5CDN–RFP, 43±8%; P = 0.872, Student's _t_-test) or recovery kinetics in dendrites (control RFP, 71±4 s; KIF5CDN–RFP, 69±3 s; P = 0.290, Student's _t_-test) (Fig. 5A,B).
Fig. 5.
Dendrite-specific ER transport and targeting of GABAB1 depends on dynein. (A) Percentage recovery of GABAB1–EGFP fluorescence for neurons transfected with RFP or KIF5DN–RFP. n = 17 and 8 neurons, respectively. Three independent experiments were performed per condition. (B) Same as in A for the t1/2. (C,D) Same as in A,B for neurons co-transfected with GABAB1–mRFP and EGFP, dynamitin (p50–EGFP) or N5-βIII-spectrin (N5-βIII-EGFP). n = 13–26 neurons. Four independent experiments were performed per condition. (E) Quantification of GABAB1–mRFP fluorescence intensities in axons and dendrites of neurons transfected with control EGFP or p50–EGFP. n = 16 neurons per condition. (F) Polarity index of GABAB1–mRFP for neurons transfected with EGFP or p50–EGFP. n = 16 neurons per condition. All quantitative data show the mean±s.e.m.; *P<0.05; ***P<0.001; n.s., non-significant; Student's _t_-test. (G,H) Representative images of neurons co-transfected with GABAB1–mRFP (GB1–mRFP, red) and a double dose of control EGFP plasmid (EGFP/EGFP, green) (G) or p50–EGFP plus EGFP (p50–EGFP/EGFP, green) (H) and labeled with the dendritic marker MAP2 (purple). Dendritic segments outlined in white are enlarged below the main image. Arrowheads mark the axon. Scale bars: 10 µm.
Because the mixed microtubule orientation in proximal dendrites allows minus-end-directed dynein to drive transport bidirectionally (Kapitein et al., 2010), we used overexpression of GFP-tagged p50 (also known as dynamitin or DCTN2) (p50–EGFP) to inhibit dynein function (Echeverri et al., 1996; Burkhardt et al., 1997; Presley et al., 1997). Importantly, p50–EGFP overexpression significantly decreased the percentage recovery of GABAB1 (control EGFP, 57±4%; p50–EGFP, 46±3%; P = 0.049, Student's _t_-test), whereas it had no effect on _t_1/2 (Fig. 5C,D). GFP-tagged N5-βIII-spectrin (N5-βIII–EGFP), an established blocker of the βIII-spectrin–dynein complex (Devarajan et al., 1997), produced a mild but non-significant decrease in the percentage recovery (47±3%; P = 0.077, Student's _t_-test) and did not alter _t_1/2 (Fig. 5C,D).
A prediction of our FRAP experiments is that dynein inhibition could limit the ER distribution of GABAB1 in dendrites. Thus, we tested the intracellular distribution of transfected GABAB1–mRFP in the presence of p50–EGFP. As previously reported, dendritic morphology was altered by p50–EGFP (Kapitein et al., 2010) (Fig. 5H). More importantly, the distribution of GABAB1 in p50–EGFP-expressing neurons was dramatically different from that of control neurons. Specifically, the distribution of ER-associated GABAB1 was restricted to the proximal regions in dendrites, whereas it was unaltered in axons [GABAB1–mRFP mean intensity (arbitrary units) in dendrites for control EGFP, 69.9±4.8; for p50–EGFP, 39.8±3.8, P = 0.00003. In axons for control EGFP, 10.1±1.3; for p50–EGFP, 14.3±2.0, P = 0.095, Student's _t_-test] (Fig. 5E,G,H). By contrast, dynein inhibition did not affect EGFP or MAP2 distribution in dendrites or axons (MAP2 mean intensity of control EGFP versus p50–EGFP for dendrites, P = 0.309; for axons, P = 0.144. EGFP mean intensity of control EGFP versus p50–EGFP for dendrites, P = 0.334; for axons, P = 0.101, Student's _t_-test). To further analyze the effect of p50–EGFP on the intracellular distribution of GABAB1–mRFP, we calculated a polarity index, where an index of >0 or <0 indicates polarization towards dendrites or axons, respectively (Kapitein et al., 2010). The polarity index of ER-associated GABAB1 in control neurons was 0.75±0.03 (Fig. 5F), consistent with the preferential somatodendritic distribution of GABAB1 subunits (Biermann et al., 2010; Valdés et al., 2012). By contrast, upon expression of p50−EGFP, the polarity index of GABAB1 decreased significantly (0.47±0.06; P = 0.0005, Student's _t_-test) (Fig. 5F). As expected, the polarity index of MAP2 and EGFP were unaffected by dynein inhibition (MAP2, P = 0.111; EGFP, P = 0.386, Student's _t_-test). Taken together, these data demonstrate that dynein is essential for one component of dendritic ER transport and for the targeting of GABAB1 subunits.
GABABRs traffic through canonical and local secretory routes
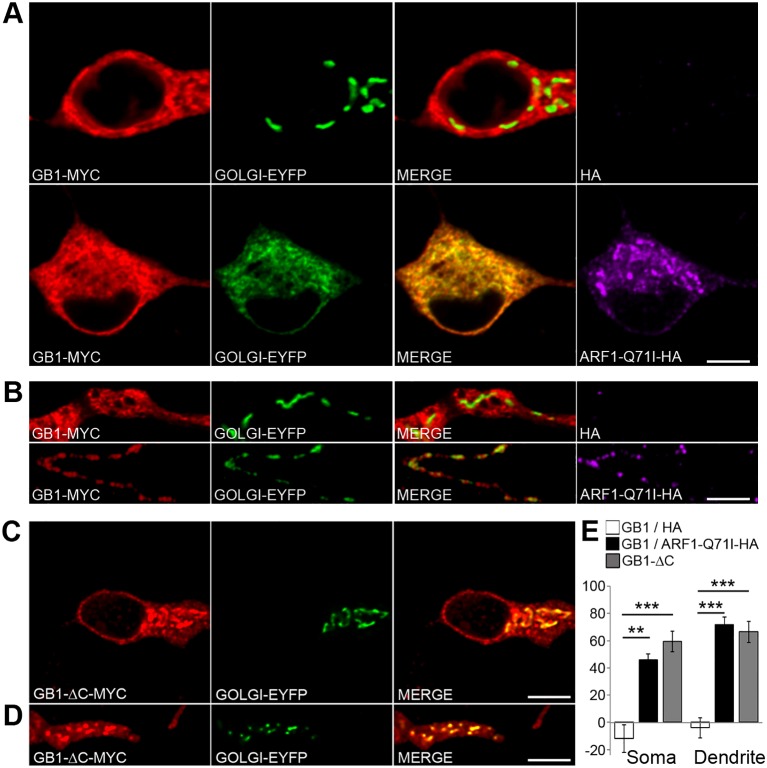
Transport through a subcompartment of the dendritic ER determines the specific sorting of NMDARs to GOPs (Jeyifous et al., 2009). Likewise, the localization and transport of nascent GABAB1 subunits within the dendritic ER might result in local trafficking through GOPs. To examine whether GABABRs traffic through GOPs, we co-transfected GABAB1–Myc and GABAB2–FLAG, which assemble and traffic to the plasma membrane, together with ARF1-Q71I–HA, a constitutively active mutant of the small GTPase ARF1 – involved in COPI recruitment – that prevents export from the Golgi and produces the accumulation of cargo in the somatic Golgi and GOPs in neurons (Zhang et al., 1994; Jeyifous et al., 2009). Under steady-state conditions, GABAB1 was mostly excluded from the Golgi in the perinuclear region and in dendrites (Fig. 6A,B). Upon expression of ARF1-Q71I–HA, GABAB1 accumulated significantly in both somatic and dendritic Golgi structures (P = 0.0061 and P = 0.0001, respectively, Student's _t_-test) (Fig. 6A,B,E). These results indicate that newly synthesized receptors traffic through the perinuclear Golgi and GOPs.
Fig. 6.
GABABRs traffic through satellite GOPs. (A) Neurons were co-transfected with GABAB1-Myc (GB1–MYC, red) and Golgi–EYFP (green), or with GABAB1–Myc (red), Golgi–EYFP (green) and ARF1-Q71I–HA (purple) to block exit from the Golgi. GABAB2–FLAG is not shown. (B) Same as in A for proximal dendrites and GOPs. (C) Somas of neurons co-transfected with GABAB1-ΔC–Myc (GB1-ΔC–MYC, red) and Golgi–EYFP (green). (D) Same as in C for proximal dendrites and GOPs. (E) Percentage of colocalization of GABAB1–Myc with Golgi–EYFP in the absence (white bars) or presence of ARF1-Q71I–HA (black bars) or GABAB1-ΔC–Myc (gray bars) in the soma (left) and dendrites (right). Data show the mean±s.e.m.; n = 4–8 neurons; **P<0.01; ***P<0.001; Student's _t_-test. Scale bars: 10 µm.
It has been suggested that dwell times and diffusion within the ER define the exploration volumes of nascent proteins and, therefore, spatially determine ER export domains (Herpers and Rabouille, 2004; Cui-Wang et al., 2012). Thus, we reasoned that GABAB1 subunits with long ER residency would preferably feed GOPs, whereas rapidly exported subunits might preferentially use the perinuclear Golgi. To test this hypothesis, we used GABAB1-ΔC–Myc, a mutant subunit that contains a deletion of 104 amino acids in its carboxyl terminus. This mutant protein lacks the ER retention domain, does not need GABAB2 for ER export and is efficiently transported to the plasma membrane (Restituito et al., 2005). Unexpectedly, even under basal conditions, the colocalization of this mutant subunit with GOPs was 70% higher than that of full-length GABAB1 (percentage colocalization with Golgi–EYFP for GABAB1–Myc+GABAB2, −4±8%; for GABAB1-ΔC–Myc, 66±8%; P = 0.0002, Student's _t_-test) (Fig. 6C–E). These results indicate that dendritic ER transport does not restrict the sorting of GABABRs to GOPs.
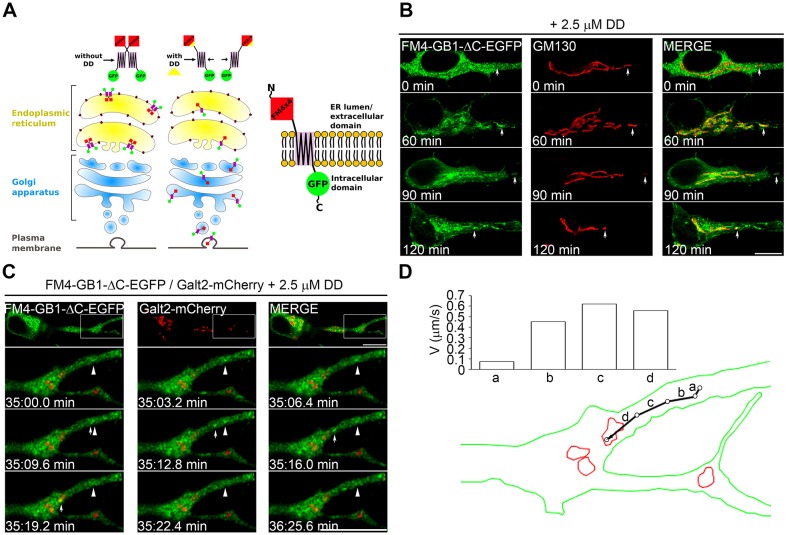
To further explore the ER-to-Golgi trafficking of GABABRs, we used a recently described pulse-chase system based on the fusion of four repeats of mutant FKBP12 (each repeat is referred to as an FM domain) to a transmembrane protein of interest. This fusion results in ER retention due to aggregation of the FM domains (Al-Bassam et al., 2012). Addition of the rapamycin analog D/D solubilizer allows disaggregation of the FM domains, release from the ER and visualization of a synchronous pool of trafficking cargo. We engineered the FM domains at the N-terminus of GABAB1-ΔC (FM–GABAB1-ΔC–EGFP), which is exposed to the ER lumen during trafficking (Fig. 7A). After addition of D/D solubilizer, FM–GABAB1-ΔC–EGFP was efficiently released from the ER and reached the plasma membrane (data not shown). Importantly, in neurons, FM–GABAB1-ΔC–EGFP was released from the ER after the addition of D/D solubilizer and subsequently condensed in the somatic Golgi and in GOPs (Fig. 7B). Individual carriers of ER-released GABAB1-ΔC–EGFP traveled bidirectionally in dendrites with a mild retrograde bias and merged with stationary GOPs that were at variable distances from their site of appearance (the percentage of carriers that merged with GOPs that traveled <2.5 µm or >2.5 µm was 60% and 40%, respectively. Retrograde transport, 70%; anterograde transport, 30%; six neurons, three independent experiments) (Fig. 7C). Instantaneous velocities of post-ER carriers were in the range of those reported for carriers of the ERGIC (Appenzeller-Herzog and Hauri, 2006) (Fig. 7D). Taken together, these results indicate that GABAB1 traffics through both the perinuclear Golgi and GOPs, and that their use is not determined by ER residency times.
Fig. 7.
GABAB1 carriers merge with GOPs after ER export. (A) Diagram of the FM-based pulse-chase system implemented for a GABAB1 construct with EGFP fused to the C-terminus and four FM domains fused to the N-terminus (FM4x4). (B) Neurons were transfected with FM4-GB1-ΔC–EGFP (green) and fixed before or after a 60–120-min treatment with D/D solubilizer (DD). The Golgi was stained with GM130 (red). Arrows point to Golgi structures that show little colocalization before the addition of D/D solubilizer (0 min), but precise colocalization after incubation with D/D solubilizer (60–120 min). Scale bar: 10 µm. (C) Neurons were co-transfected with FM4-GB1-ΔC–EGFP (green) and Galt2–mCherry (red) to visualize GOPs in dendrites (upper panels). After 12 h of expression, protein synthesis was inhibited with cycloheximide and neurons were incubated with D/D solubilizer for 30 min at 37°C and imaged live. Lower panels show a time series of the boxed dendritic segment. Arrows indicate moving carriers that merge with GOPs. Arrowheads show a static GABAB1 structure. Scale bar: 5 µm. (D) Diagram of the trajectory of the moving carrier visualized in C. The graph shows velocities in each interval.
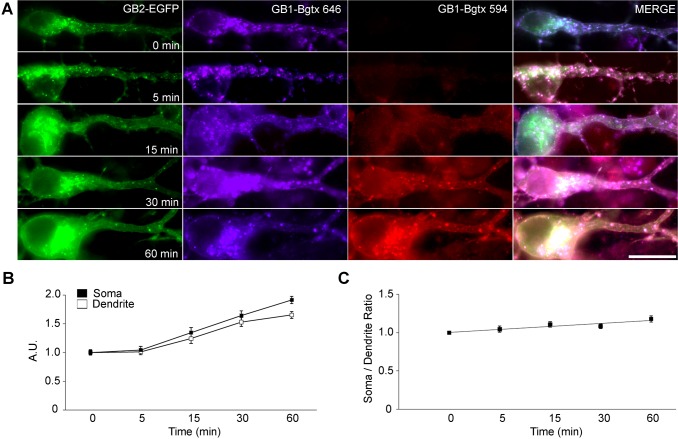
Finally, we speculated that the use of dendritic organelles might influence the site of delivery at the neuronal plasma membrane. To spatially characterize the insertion of newly synthesized GABABRs, the kinetics of de novo insertion were evaluated by quantifying the appearance of plasma membrane bgtx-Myc–GABAB1a, a modified version of the GABAB1a subunit (one of the two alternative isoforms of GABAB1) containing an α-bungarotoxin-binding site engineered at the extracellular N-terminus. De novo exocytosed bgtx-Myc–GABAB1a subunits were specifically and irreversibly labeled with fluorescent α-bungarotoxin (Bgtx-594) after saturating the recycling pool (Bgtx-646) as reported previously (Wilkins et al., 2008; Terunuma et al., 2010). We measured the mean intensity of Bgtx-594 at the outline of the soma or dendrite to estimate the number of newly inserted receptors. In neurons transfected with bgtx-Myc–GABAB1a and GABAB2–EGFP, de novo insertion was evident after 15 min of Bgtx-594 labeling, and gradually increased over 60 min (Fig. 8A). The timecourses of de novo insertion of bgtx-Myc–GABAB1a in the soma and in dendrites were remarkably similar (Fig. 8B). Indeed, the ratio of somatic∶dendritic insertion revealed only a mild positive slope (Fig. 8C). Overall these data do not support an early exocytosis event in the cell body followed by subsequent diffusion and redistribution to dendrites (Rosenberg et al., 2001), but rather the simultaneous delivery of newly synthesized receptors to the entire somatodendritic compartment. These data are in agreement with the use of the entire ensemble of exocytic organelles.
Fig. 8.
GABAB1 insertion is somatodendritic. (A) Neurons were co-transfected with bgtx-Myc–GABAB1a (GB1-Bgtx) and GABAB2–EGFP (GB2–EGFP, green). The population of recycling receptors was saturated with Bgtx-646 (blue). _De_-_novo_-inserted receptors were visualized by binding to Bgtx-594 (red) between 0 and 60 min. Scale bar: 20 µm. (B) The intensity of red fluorescence (de novo insertion) was measured at the somas (black) and proximal dendrites (white) and plotted against time. A.U., arbitrary units. n = 3 independent experiments; data show the mean±s.e.m.; 17–26 neurons; *P<0.05; ***P<0.001; Student's _t_-test. (C) The ratio of somatic versus dendritic intensity was calculated from the data in B.
DISCUSSION
The large size and morphological complexity of neurons introduce significant challenges to the delivery of membrane cargoes, which might be difficult to convey exclusively through long-haul post-Golgi vesicles. Thus, local trafficking involving the dendritic ER and GOPs provides an alternative means to regulate the supply of membrane components, but its role in the delivery of neuronal proteins is still poorly understood (Horton and Ehlers, 2003). Here, we demonstrate that GABAB1 transport in dendrites is independent of COPI vesicle formation and takes place within the dendritic ER. The transport of ER-associated GABAB1 is not controlled by diffusion alone, but also by an active mechanism involving microtubules and dynein. GABABRs traffic through both the perinuclear Golgi and GOPs, and insert at the plasma membrane throughout the somatodendritic compartment. We conclude that GABABRs utilize a long-distance ER transport mechanism and both perinuclear and local secretory routes prior to reaching the plasma membrane. Our observations argue against the view that dendritic ER transport restricts the trafficking of neurotransmitter receptors to GOPs, and they are consistent with the hypothesis that ER assembly of GABABRs is the rate-limiting step for receptor trafficking.
ER localization of GABAB1 in dendrites
We demonstrate that the ER retention of GABAB1 produces a broad intracellular ER-enriched distribution in dendrites, with a small proportion localizing at the ERGIC and Golgi, possibly revealing an ER–ERGIC–Golgi retrieval mechanism (Ramírez et al., 2009; Biermann et al., 2010; Brock et al., 2005). Other neurotransmitter receptors and ion channels are also enriched in the ER and display a variety of ER retention signals (Ramírez and Couve, 2011) but it is still unclear to what extent ER retention is related to local dendritic checkpoints and how these regulate later trafficking stages.
ER cargoes in neurons might be confined by regions of high network complexity, especially those at dendritic branching points (Cui-Wang et al., 2012). However, the continuity of the ER allows proteins to disperse throughout the organelle. In our experiments, the time of expression was sufficient to distribute GABAB1 through regions of low and high complexity and to localize the subunit intracellularly within the entire somatodendritic compartment. This phenomenon was not an artifact of overexpression, because the colocalization with the ER in dendrites was similar in transgenic neurons that expressed GABAB1–EGFP at endogenous levels and increased after blocking ER export. By contrast, NMDARs accumulate exclusively in the soma after blockade of ER export, providing evidence that multiple ER subcompartments control the trafficking of different neurotransmitter receptors (Jeyifous et al., 2009).
ER transport in dendrites
The intracellular velocities of GABAB1 are comparable to those reported for ER resident proteins (GFP–KDEL, RFP–KDEL, GFP–SERCA2a and GFP–IP3R1) (Bannai et al., 2004). Although diffusion has been elegantly characterized for other neurotransmitter receptors – namely, ER-retained AMPA subunits (Cui-Wang et al., 2012) – lateral diffusion alone is not sufficient to explain the ER transport of GABAB1 in neurons. Three pieces of evidence strongly support this conclusion: the microtubule-dependent transport of GABAB1, the microtubule dependence even after BFA-induced ER retention, and the dynein-dependent transport and localization of ER-associated GABAB1.
Regarding the underlying transport mechanism, our results show that a component of ER-associated GABAB1 transport is impaired after depolymerization of microtubules. However, our data do not support the idea that microtubule-dependent ER remodeling controls the mobility of GABAB1, because the transport of ER–RFP and Cytb5–GFP were unchanged by nocodazole. Others have proposed that vesicular-like carriers mediate a motor-assisted component of ER transport in dendrites, potentially identifying an active mechanism (Bannai et al., 2004; Jeyifous et al., 2009). With our available data we cannot conclude whether the mobility of GABAB1 is mediated by a vesicular-like component or by transport along the continuous ER network. Although it is unusual to envisage a motor dragging cargo within the plane of the bilayer, we favor this view, and we speculate that the diffusion of neurotransmitter receptors within the plane of the ER membrane provides a basal dynamics that might be suitable for modification by molecular motors. Supporting this notion, microtubule and motor-driven motility of ERES occurs independently of the movement of ER membranes (Gupta et al., 2008).
We also speculate that the active component of ER-retained GABAB1 introduces an additional mechanism for the confinement of ER cargoes that might counteract the dispersing tendency of diffusion. As a matter of fact, our data suggest that microtubule acetylation increases the recruitment of a higher proportion of faster molecular motors that decrease the confinement of GABABRs.
Are canonical and local trafficking in dendrites mutually exclusive?
Our data show that GABAB1 localizes to the dendritic ER even after co-transfection with GABAB2, that GABABRs accumulate in GOPs and that they are delivered to the plasma membrane throughout the somatodendritic compartment. Thus, the evidence suggests that a local secretory route operates for GABABRs. Other receptors also employ local routes, but differences in how these routes are utilized are already apparent. For instance, endogenous and overexpressed NMDARs accumulate exclusively on GOPs upon expression of ARF1-Q71I–HA (Jeyifous et al., 2009), whereas GABABRs accumulate in the somatic Golgi and in GOPs. Although the intrinsic limitations of overexpression should be considered, the differences between GABABRs and NMDARs suggest that distinct neurotransmitter receptors display different degrees of spatial selectivity for organelles.
ER export kinetics and lateral diffusion determine the spatial range over which export occurs (Herpers and Rabouille, 2004; Cui-Wang et al., 2012). Although most of our findings are consistent with this proposal, our results with GABAB1-ΔC indicate to the contrary that an improved ability to exit the ER increases trafficking through GOPs. One interpretation of these observations is that post-ER carriers fuse indiscriminately with the perinuclear Golgi and GOPs, which act in concert to increase the trafficking capacity of the neuron. Interestingly, upon ER release, the temperature-sensitive VSVG mutant or BDNF use both the perinuclear Golgi and GOPs (Horton and Ehlers, 2003). Our experiments using GABAB1 are consistent with this view of non-selective processing and suggest that, for a subset of neuronal cargoes, ER transport does not restrict sorting to GOPs and that selected proteins use the complete ensemble of Golgi structures. In this context, the absence of dendritic Golgi in ∼30% of neurons (Horton and Ehlers, 2003; Horton et al., 2005) and the presence of GOPs in only ∼18% of mature dendrites (Horton et al., 2005), in a scenario where all dendrites contain neurotransmitter receptors, suggest that local trafficking cannot be exclusive. However, metabotropic glutamate receptor (mGluR) activity confines ER transport (Cui-Wang et al., 2012) and enhances the formation of ERES containing NMDARs (Aridor et al., 2004). Therefore, it is still unknown how, or if, the balance of canonical and local trafficking is modified by activity, and whether preferential use of local routes influences the local availability of receptors at synapses in response to activity.
Local supply of GOPs
The dendritic distribution of the ER and GOPs suggests that intermediate transport carriers might operate locally. Indeed, VSVG carriers that merge with GOPs have been reported (Horton and Ehlers, 2003). Additionally, packets containing VSVG become concentrated at branching points, presumably GOPs, after local ER export (Chen et al., 2013). However, a relevant issue that remains to be addressed is to what extent the local ER contributes to supply nearby GOPs. We have observed post-ER carriers merging with GOPs after both long and short trajectories, suggesting that dendritic ER export is not necessarily coupled to local trafficking. Therefore, although our results indicate that the dendritic ER is a crucial component for the transport of GABAB subunits, they do not support the notion that GOPs feed exclusively on the local ER, presenting a more complex scenario of local trafficking in dendrites.
MATERIALS AND METHODS
Animals
Adult pregnant female Sprague-Dawley rats were purchased from the Central Animal Facility at Universidad Católica de Chile and were euthanized by asphyxia in a CO2 chamber according to the Guide for Care and Use of Laboratory Animals of The National Research Council (1996). GABAB1–EGFP mice were kindly provided by Bernhard Bettler (University of Basel, Switzerland). They carry a bacterial artificial chromosome containing GABAB1-EGFP in a homozygous knockout background for GABAB1. The construct is expressed under control of GABAB1 promoter elements (Schuler et al., 2001; Casanova et al., 2009).
Cells
COS-7 cells were transfected by electroporation (Couve et al., 1998). Primary hippocampal neurons were cultured from embryonic day (E)18 rats or E18 GABAB1–EGFP transgenic mice (Goslin and Banker, 1991) and transfected by using Ca2+ phosphate at 7–15 days in vitro (Valdés et al., 2012; Jiang and Chen, 2006). Endogenous subunits do not assemble with recombinant subunits until 4–5 days post-transfection (Valdés et al., 2012); therefore, all experiments were analyzed between 12 and 48 h post-transfection.
Reagents and DNA plasmids
BFA, cycloheximide, cytochalasin D, TSA and nocodazole were purchased from Sigma (St Louis, MO). D/D Solubilizer was purchased from Clontech (Mountain View, CA). Myc–GABAB1, FLAG–GABAB2, HA–GABAB2, Myc–GABAB1-ΔC, GABAB1a–EGFP, GABAB2–EGFP, GABAB1a–mRFP and Bgtx-Myc–GABAB1 were as described previously (Couve et al., 1998; Calver et al., 2001; Couve et al., 2002; Restituito et al., 2005; Ramírez et al., 2009; Terunuma et al., 2010). To generate GABAB1a–mEos2, rat GABAB1a was subcloned into pmEos2-N1 (between the _Spe_I and _BamH_I sites). mEos2, kindly provided by Sean McKinney (Howard Hughes Medical Institute, Janelia Farm Research Campus, MD), is fused to the intracellular carboxyl terminus of GABAB1a. To generate FM–GABAB1-ΔC–EGFP, four FM domains were fused to the N-terminus of GABAB1-ΔC by amplifying GABAB1-ΔC using the primers 5′-ATCTCGAGGAGCAGAAGCTGATCTCAG-3′ and 3′-CTTTTGTCCTAGTAGGTGGTCTTAAGCG-5′, and subcloning the resulting amplicon into the Type I FM4-EGFP vector (Thuenauer et al., 2004) between the _Xho_I and _EcoR_I sites.
The GABAA subunits RFP-BBSα4 and pH/BBSβ3 have been described previously (Abramian et al., 2010; Bogdanov et al., 2006). pDsRed-C1 (RFP), pEYFP-Golgi, pEYFP-ER and pDsRed2-ER (ER-RFP) were obtained from Clontech (Mountain View, CA). Galt2–mCherry was kindly provided by Alfredo Cáceres (Instituto de Investigación Médica Mercedes y Martín Ferreyra, Argentina). Cytb5–EGFP was kindly provided by Claudio Hetz (Universidad de Chile, Chile). BFP–Sec61β (ER–BFP), ARF1-Q71I–HA and Sar1-H79G–HA have been described previously (Ward et al., 2001; Jeyifous et al., 2009; Zurek et al., 2011). Kif5CDN–RFP corresponds to amino acids 678–955 of KIF5C (accession number NM_001107730). This construct is also referred to as DN2 (Falley et al., 2009). Dynamitin subunit p50–EGFP and N5-βIII-spectrin were kindly provided by Anna Akhmanova (Erasmus Medical Center, The Netherlands) and Michael Stankewich (Yale University, New Haven, CT), respectively (Devarajan et al., 1997; Splinter et al., 2012). All manipulations and the fidelity of DNA constructs were verified by sequencing.
Antibodies
Antibodies against Myc and ERGIC-53 were purchased from Sigma. Antibodies against KDEL and GM130 were purchased from StressGen (Ann Arbor, MI). Antibodies against HA were purchased from Roche (Indianapolis, IN). Anti-GFP antibodies (ab6556) were purchased from Abcam (Cambridge, UK). Fluorescently labeled secondary antibodies were purchased from Jackson Immuno Research Laboratories (West Grove, PA).
Immunofluorescence, time-lapse microscopy and FRAP
Immunofluorescence was performed in permeabilized neurons (Ramírez et al., 2009). Fixed and live-cell imaging was performed on a spectral confocal microscope Olympus FluoView FV1000 (Center Valley, PA) with a UPLSAPO 60×/1.35 objective and 3× digital zoom, in Tyrode medium (124 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 30 mM d-glucose and 25 mM HEPES pH 7.4) in an equilibrated microscopy suite at 23°C. For FRAP experiments, dendritic regions of interest (ROIs) of 60×60 pixels (small ROI) or 200×200 pixels (large ROI), ∼15 µm away from the soma, were bleached for 2 s using the tornado mode with the argon 473-nm laser at 100% power for EGFP-tagged proteins or with the argon 405-nm and 559-nm laser at 100% power for mRFP-tagged proteins. Fluorescence recovery was measured every 10 s at 20% laser power using KALMAN2 by imaging the entire field. Boxes of 8 µm2 were selected within the photo-bleached ROI to quantify recovery using ImageJ. Individual FRAPs were fitted to a first-order exponential decay curve using established criteria, except for those for Cytb5–EGFP, which were best fitted to an asymptotic type I curve (Snapp et al., 2003), and selected by fitting (R2≧0.95).
The average intensity of the fluorescent signals in dendrites and axons was determined using ImageJ (method modified from Kapitein et al., 2010). Briefly, confocal images were acquired with a UPLSAPO 60×/1.35 objective using sequential acquisition settings and KALMAN2 at 2048×2048 pixel resolution. Each image was a _z_-series of 5–10 images of which the maximum projection was used. Lines of width 1 pixel were traced along axons and the major dendrite. Dendrites and axons were identified based on their morphology and by immunostaining for MAP2. Average pixel intensities were determined along the traced lines and the background was subtracted. The average dendrite (Id) and axonal (Ia) intensities were used to calculate the polarity index (PI) using PI = (Id−Ia)/(Id+Ia).
Colocalization
Colocalization analysis was performed as described previously (Ramírez et al., 2010). All parameters were kept constant for a particular type of experiment. Two-channel fluorescent image stacks [intensity I(x,y,z), ∈ (0,255), voxel size Δx/Δy/Δz = 137/137/250 nm] were recorded in the multi-track mode and KALMAN4. Four optical slices per neuron were selected and deconvolved by Huygens Scripting software (Scientific Volume Imaging, Hilversum, The Netherlands) using an algorithm based on the Classic Maximum Likelihood Estimator. Image processing routines for segmentation, visualization, calculation of colocalization coefficients and statistical validation were based on Interactive Data Language (ITT, Boulder, CO). Percentage colocalization for Manders coefficients (M1 and M2) was calculated using the CDA (confined displacement algorithm) plugin for ImageJ, followed by interpolation of the Manders coefficients using the formula: Percentage colocalization = [M(0)−M(random)/M(100)−M(random)]×100, where M(0) is Manders at the original position, M(100) is the theoretical maximum (1), and M(random) is the corresponding value for the random. Statistical validation was performed using CDA.
sptPALM
sptPALM was carried out by modifying a previously reported approach (Frost et al., 2010). Briefly, imaging was conducted on an Olympus IX81 inverted microscope with a 100×/1.45 total internal reflection fluorescence (TIRF) oil-immersion objective. Output from a set of diode lasers (Coherent, Santa Clara, CA) was directed to the rear of the microscope using a custom optical path. Molecules were simultaneously photoconverted and excited using 405-nm (<100 µW) and 561-nm (20 mW) light through oblique illumination (near total internal reflection) to reduce background fluorescence. A total of 10,000–20,000 frames were collected at 5 Hz with 10-ms laser pulses to minimize receptor motion during the frame (Frost et al., 2012). Fluorescence was detected by an iXon+ 897 EM-CCD camera (Andor Technology, South Windsor, CT) placed after a 1.6× magnifying optic, resulting in a pixel size of 100 nm. Following acquisition, molecules were localized by fitting a two-dimensional elliptical Gaussian function to a 9×9 pixel array surrounding the peak, and locations were assembled into tracks using available algorithms (Manley et al., 2008). Molecules were segregated into those that were tracked over multiple frames and those that appeared in a single frame. The distribution of tracked molecules was calculated based on the density of molecules within a search radius of 500 nm. Local net velocities were calculated using the first and last points of tracks originating within this radius. Velocities and vectors were mapped only in pixels with at least three tracks originating within the search radius.
α-bungarotoxin labeling
Neurons were co-transfected with bgtx-Myc–GABAB1a and GABAB2–EGFP. At 48 h post-transfection, live neurons were incubated for 30 min with 13 µM (+)-tubocurarine chloride hydrate (Sigma). They were then incubated with α-bungarotoxin (Invitrogen, Eugene, OR) conjugated to Alexa Fluor® 646 for 120 min at 37°C, washed with PBS and incubated with α-bungarotoxin conjugated to Alexa Fluor® 594 for different periods of time at 37°C. Neurons were fixed and imaged on an Olympus BX61WI upright microscope, an Olympus disk-scanning unit and an UPlanFLN 60×/1.25. Mean intensity was quantified using ImageJ in a 2×1-µm ROI at the outline of the somatic or dendritic (20 µm from the soma) regions to estimate the fluorescence associated with the plasma membrane.
Acknowledgments
We thank Richard Saliba (Tufts University, Boston, USA), Viviana Valdés (Universidad Mayor, Santiago, Chile), José Cánovas and María A. Juricic (both Universidad de Chile, Santiago, Chile) for technical assistance and for critical reading of the manuscript.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
A.C, J.I.V. and T.A.B. conceived and designed the experiments. J.I.V. and M.J-B performed the experiments and analyzed the data. J.I.V., A.C. and T.A.B. interpreted the data. H.L. and O.R. contributed image analysis routines. V.H.C. and D.S. generated reagents. J.I.V. and A.C. wrote the manuscript.
Funding
This work was supported by Iniciativa Científica Milenio [grant number P09-015-F to A.C. and J.I.V.]; National Institutes of Health [grant number MH80046 to H.L. and T.A.B.]; Fondo Nacional de Desarrollo Científico y Tecnológico [grant number 3110157 to O.R., 1100137 and 1140617 to A.C. and M.J.-B.]. D.S., J.I.V. and V.H.C. were supported by Comisión Nacional de Investigación Científica y Tecnológica. Deposited in PMC for release after 12 months.
References
- Abramian A. M., Comenencia-Ortiz E., Vithlani M., Tretter E. V., Sieghart W., Davies P. A., Moss S. J. (2010). Protein kinase C phosphorylation regulates membrane insertion of GABAA receptor subtypes that mediate tonic inhibition. J. Biol. Chem. 285, 41795–41805 10.1074/jbc.M110.149229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Bassam S., Xu M., Wandless T. J., Arnold D. B. (2012). Differential trafficking of transport vesicles contributes to the localization of dendritic proteins. Cell Rep. 2, 89–100 10.1016/j.celrep.2012.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenzeller-Herzog C., Hauri H. P. (2006). The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J. Cell Sci. 119, 2173–2183 10.1242/jcs.03019 [DOI] [PubMed] [Google Scholar]
- Aridor M., Guzik A. K., Bielli A., Fish K. N. (2004). Endoplasmic reticulum export site formation and function in dendrites. J. Neurosci. 24, 3770–3776 10.1523/JNEUROSCI.4775-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai H., Inoue T., Nakayama T., Hattori M., Mikoshiba K. (2004). Kinesin dependent, rapid, bi-directional transport of ER sub-compartment in dendrites of hippocampal neurons. J. Cell Sci. 117, 163–175 10.1242/jcs.00854 [DOI] [PubMed] [Google Scholar]
- Bettler B., Kaupmann K., Mosbacher J., Gassmann M. (2004). Molecular structure and physiological functions of GABA(B) receptors. Physiol. Rev. 84, 835–867 10.1152/physrev.00036.2003 [DOI] [PubMed] [Google Scholar]
- Biermann B., Ivankova-Susankova K., Bradaia A., Abdel Aziz S., Besseyrias V., Kapfhammer J. P., Missler M., Gassmann M., Bettler B. (2010). The Sushi domains of GABAB receptors function as axonal targeting signals. J. Neurosci. 30, 1385–1394 10.1523/JNEUROSCI.3172-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov Y., Michels G., Armstrong-Gold C., Haydon P. G., Lindstrom J., Pangalos M., Moss S. J. (2006). Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 25, 4381–4389 10.1038/sj.emboj.7601309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwell R. D., Cataldo A. M. (1983). The neuronal endoplasmic reticulum: its cytochemistry and contribution to the endomembrane system. I. Cell bodies and dendrites. J. Histochem. Cytochem. 31, 1077–1088 10.1177/31.9.6309951 [DOI] [PubMed] [Google Scholar]
- Brock C., Boudier L., Maurel D., Blahos J., Pin J. P. (2005). Assembly-dependent surface targeting of the heterodimeric GABAB Receptor is controlled by COPI but not 14-3-3. Mol. Biol. Cell 16, 5572–5578 10.1091/mbc.E05-05-0400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt J. K., Echeverri C. J., Nilsson T., Vallee R. B. (1997). Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 139, 469–484 10.1083/jcb.139.2.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D., McEwen D. P., Martens J. R., Meyhofer E., Verhey K. J. (2009). Single molecule imaging reveals differences in microtubule track selection between Kinesin motors. PLoS Biol. 7, e1000216 10.1371/journal.pbio.1000216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calver A. R., Robbins M. J., Cosio C., Rice S. Q., Babbs A. J., Hirst W. D., Boyfield I., Wood M. D., Russell R. B., Price G. W. et al. (2001). The C-terminal domains of the GABA(b) receptor subunits mediate intracellular trafficking but are not required for receptor signaling. J. Neurosci. 21, 1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova E., Guetg N., Vigot R., Seddik R., Julio-Pieper M., Hyland N. P., Cryan J. F., Gassmann M., Bettler B. (2009). A mouse model for visualization of GABA(B) receptors. Genesis 47, 595–602 10.1002/dvg.20535 [DOI] [PubMed] [Google Scholar]
- Chen D., Gibson E. S., Kennedy M. J. (2013). A light-triggered protein secretion system. J. Cell Biol. 201, 631–640 10.1083/jcb.201210119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem R. L., Barth A. (2006). Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron 49, 663–670 10.1016/j.neuron.2006.01.019 [DOI] [PubMed] [Google Scholar]
- Couve A., Filippov A. K., Connolly C. N., Bettler B., Brown D. A., Moss S. J. (1998). Intracellular retention of recombinant GABAB receptors. J. Biol. Chem. 273, 26361–26367 10.1074/jbc.273.41.26361 [DOI] [PubMed] [Google Scholar]
- Couve A., Thomas P., Calver A. R., Hirst W. D., Pangalos M. N., Walsh F. S., Smart T. G., Moss S. J. (2002). Cyclic AMP-dependent protein kinase phosphorylation facilitates GABA(B) receptor-effector coupling. Nat. Neurosci. 5, 415–424 [DOI] [PubMed] [Google Scholar]
- Cui-Wang T., Hanus C., Cui T., Helton T., Bourne J., Watson D., Harris K. M., Ehlers M. D. (2012). Local zones of endoplasmic reticulum complexity confine cargo in neuronal dendrites. Cell 148, 309–321 10.1016/j.cell.2011.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarajan P., Stabach P. R., De Matteis M. A., Morrow J. S. (1997). Na,K-ATPase transport from endoplasmic reticulum to Golgi requires the Golgi spectrin-ankyrin G119 skeleton in Madin Darby canine kidney cells. Proc. Natl. Acad. Sci. USA 94, 10711–10716 10.1073/pnas.94.20.10711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dompierre J. P., Godin J. D., Charrin B. C., Cordelières F. P., King S. J., Humbert S., Saudou F. (2007). Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington's disease by increasing tubulin acetylation. J. Neurosci. 27, 3571–3583 10.1523/JNEUROSCI.0037-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverri C. J., Paschal B. M., Vaughan K. T., Vallee R. B. (1996). Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 132, 617–633 10.1083/jcb.132.4.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers M. D. (2000). Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron 28, 511–525 10.1016/S0896-6273(00)00129-X [DOI] [PubMed] [Google Scholar]
- Falley K., Schütt J., Iglauer P., Menke K., Maas C., Kneussel M., Kindler S., Wouters F. S., Richter D., Kreienkamp H. J. (2009). Shank1 mRNA: dendritic transport by kinesin and translational control by the 5′untranslated region. Traffic 10, 844–857 10.1111/j.1600-0854.2009.00912.x [DOI] [PubMed] [Google Scholar]
- Friedman J. R., Webster B. M., Mastronarde D. N., Verhey K. J., Voeltz G. K. (2010). ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J. Cell Biol. 190, 363–375 10.1083/jcb.200911024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost N. A., Shroff H., Kong H., Betzig E., Blanpied T. A. (2010). Single-molecule discrimination of discrete perisynaptic and distributed sites of actin filament assembly within dendritic spines. Neuron 67, 86–99 10.1016/j.neuron.2010.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost N. A., Lu H. E., Blanpied T. A. (2012). Optimization of cell morphology measurement via single-molecule tracking PALM. PLoS ONE 7, e36751 10.1371/journal.pone.0036751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonchar Y., Pang L., Malitschek B., Bettler B., Burkhalter A. (2001). Subcellular localization of GABA(B) receptor subunits in rat visual cortex. J. Comp. Neurol. 431, 182–197 [DOI] [PubMed] [Google Scholar]
- Goslin K., Banker G. (1991). Rat hippocampal neurons in low-density cultures. Culturing Nerve Cells Banker G, Goslin K, ed251–281Cambridge: MA, MIT [Google Scholar]
- Grampp T., Sauter K., Markovic B., Benke D. (2007). Gamma-aminobutyric acid type B receptors are constitutively internalized via the clathrin-dependent pathway and targeted to lysosomes for degradation. J. Biol. Chem. 282, 24157–24165 10.1074/jbc.M702626200 [DOI] [PubMed] [Google Scholar]
- Gupta V., Palmer K. J., Spence P., Hudson A., Stephens D. J. (2008). Kinesin-1 (uKHC/KIF5B) is required for bidirectional motility of ER exit sites and efficient ER-to-Golgi transport. Traffic 9, 1850–1866 10.1111/j.1600-0854.2008.00811.x [DOI] [PubMed] [Google Scholar]
- Herpers B., Rabouille C. (2004). mRNA localization and ER-based protein sorting mechanisms dictate the use of transitional endoplasmic reticulum-golgi units involved in gurken transport in Drosophila oocytes. Mol. Biol. Cell 15, 5306–5317 10.1091/mbc.E04-05-0398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton A. C., Ehlers M. D. (2003). Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J. Neurosci. 23, 6188–6199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton A. C., Ehlers M. D. (2004). Secretory trafficking in neuronal dendrites. Nat. Cell. Biol. 6, 585–591 [DOI] [PubMed] [Google Scholar]
- Horton A. C., Rácz B., Monson E. E., Lin A. L., Weinberg R. J., Ehlers M. D. (2005). Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron 48, 757–771 10.1016/j.neuron.2005.11.005 [DOI] [PubMed] [Google Scholar]
- Jeyifous O., Waites C. L., Specht C. G., Fujisawa S., Schubert M., Lin E. I., Marshall J., Aoki C., de Silva T., Montgomery J. M. et al. (2009). SAP97 and CASK mediate sorting of NMDA receptors through a previously unknown secretory pathway. Nat. Neurosci. 12, 1011–1019 10.1038/nn.2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., Chen G. (2006). High Ca2+-phosphate transfection efficiency in low-density neuronal cultures. Nat. Protoc. 1, 695–700 10.1038/nprot.2006.86 [DOI] [PubMed] [Google Scholar]
- Kapitein L. C., Schlager M. A., Kuijpers M., Wulf P. S., van Spronsen M., MacKintosh F. C., Hoogenraad C. C. (2010). Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr. Biol. 20, 290–299 10.1016/j.cub.2009.12.052 [DOI] [PubMed] [Google Scholar]
- Kucharz K., Krogh M., Ng A. N., Toresson H. (2009). NMDA receptor stimulation induces reversible fission of the neuronal endoplasmic reticulum. PLoS ONE 4, e5250 10.1371/journal.pone.0005250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley S., Gillette J. M., Patterson G. H., Shroff H., Hess H. F., Betzig E., Lippincott-Schwartz J. (2008). High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat. Methods 5, 155–157 10.1038/nmeth.1176 [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M., Jan Y. N., Jan L. Y. (2000). A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron 27, 97–106 10.1016/S0896-6273(00)00012-X [DOI] [PubMed] [Google Scholar]
- McKinney S. A., Murphy C. S., Hazelwood K. L., Davidson M. W., Looger L. L. (2009). A bright and photostable photoconvertible fluorescent protein. Nat. Methods 6, 131–133 10.1038/nmeth.1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooler A. M., Gray A. G., McIlhinney R. A. (2009). Identification of a novel region of the GABA(B2) C-terminus that regulates surface expression and neuronal targeting of the GABA(B) receptor. Eur. J. Neurosci. 29, 869–878 10.1111/j.1460-9568.2009.06636.x [DOI] [PubMed] [Google Scholar]
- Presley J. F., Cole N. B., Schroer T. A., Hirschberg K., Zaal K. J., Lippincott-Schwartz J. (1997). ER-to-Golgi transport visualized in living cells. Nature 389, 81–85 10.1038/38891 [DOI] [PubMed] [Google Scholar]
- Ramírez O. A., Couve A. (2011). The endoplasmic reticulum and protein trafficking in dendrites and axons. Trends Cell Biol. 21, 219–227 10.1016/j.tcb.2010.12.003 [DOI] [PubMed] [Google Scholar]
- Ramírez O. A., Vidal R. L., Tello J. A., Vargas K. J., Kindler S., Härtel S., Couve A. (2009). Dendritic assembly of heteromeric gamma-aminobutyric acid type B receptor subunits in hippocampal neurons. J. Biol. Chem. 284, 13077–13085 10.1074/jbc.M900575200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez O., García A., Rojas R., Couve A., Härtel S. (2010). Confined displacement algorithm determines true and random colocalization in fluorescence microscopy. J. Microsc. 239, 173–183 10.1111/j.1365-2818.2010.03369.x [DOI] [PubMed] [Google Scholar]
- Ramírez O. A., Härtel S., Couve A. (2011). Location matters: the endoplasmic reticulum and protein trafficking in dendrites. Biol. Res. 44, 17–23 10.4067/S0716-97602011000100003 [DOI] [PubMed] [Google Scholar]
- Reed N. A., Cai D., Blasius T. L., Jih G. T., Meyhofer E., Gaertig J., Verhey K. J. (2006). Microtubule acetylation promotes kinesin-1 binding and transport. Curr. Biol. 16, 2166–2172 10.1016/j.cub.2006.09.014 [DOI] [PubMed] [Google Scholar]
- Restituito S., Couve A., Bawagan H., Jourdain S., Pangalos M. N., Calver A. R., Freeman K. B., Moss S. J. (2005). Multiple motifs regulate the trafficking of GABA(B) receptors at distinct checkpoints within the secretory pathway. Mol. Cell. Neurosci. 28, 747–756 10.1016/j.mcn.2004.12.006 [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Meier J., Triller A., Vannier C. (2001). Dynamics of glycine receptor insertion in the neuronal plasma membrane. J. Neurosci. 21, 5036–5044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J., Jacobson K. (1997). Single-particle tracking: applications to membrane dynamics. Annu. Rev. Biophys. Biomol. Struct. 26, 373–399 10.1146/annurev.biophys.26.1.373 [DOI] [PubMed] [Google Scholar]
- Schuler V., Lüscher C., Blanchet C., Klix N., Sansig G., Klebs K., Schmutz M., Heid J., Gentry C., Urban L. et al. (2001). Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)). Neuron 31, 47–58 10.1016/S0896-6273(01)00345-2 [DOI] [PubMed] [Google Scholar]
- Sengupta P., Van Engelenburg S., Lippincott-Schwartz J. (2012). Visualizing cell structure and function with point-localization superresolution imaging. Dev. Cell 23, 1092–1102 10.1016/j.devcel.2012.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapp E. L., Altan N., Lippincott-Schwartz J. (2003). Measuring protein mobility by photobleaching GFP chimeras in living cells. Curr. Protoc. Cell Biol. 19, 21.1.1–21.1.24 [DOI] [PubMed] [Google Scholar]
- Spacek J., Harris K. M. (1997). Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J. Neurosci. 17, 190–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splinter D., Razafsky D. S., Schlager M. A., Serra-Marques A., Grigoriev I., Demmers J., Keijzer N., Jiang K., Poser I., Hyman A. A. et al. (2012). BICD2, dynactin, and LIS1 cooperate in regulating dynein recruitment to cellular structures. Mol. Biol. Cell 23, 4226–4241 10.1091/mbc.E12-03-0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma M., Vargas K. J., Wilkins M. E., Ramírez O. A., Jaureguiberry-Bravo M., Pangalos M. N., Smart T. G., Moss S. J., Couve A. (2010). Prolonged activation of NMDA receptors promotes dephosphorylation and alters postendocytic sorting of GABAB receptors. Proc. Natl. Acad. Sci. USA 107, 13918–13923 10.1073/pnas.1000853107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuenauer R., Hsu Y. C., Carvajal-Gonzalez J. M., Deborde S., Chuang J. Z., Römer W., Sonnleitner A., Rodriguez-Boulan E., Sung C. H. (2014). Four-dimensional live imaging of apical biosynthetic trafficking reveals a post-Golgi sorting role of apical endosomal intermediates. Proc. Natl. Acad. Sci. USA 111, 4127–4132 10.1073/pnas.1304168111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés V., Valenzuela J. I., Salas D. A., Jaureguiberry-Bravo M., Otero C., Thiede C., Schmidt C. F., Couve A. (2012). Endoplasmic reticulum sorting and kinesin-1 command the targeting of axonal GABAB receptors. PLoS ONE 7, e44168 10.1371/journal.pone.0044168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas K. J., Terunuma M., Tello J. A., Pangalos M. N., Moss S. J., Couve A. (2008). The availability of surface GABA B receptors is independent of gamma-aminobutyric acid but controlled by glutamate in central neurons. J. Biol. Chem. 283, 24641–24648 10.1074/jbc.M802419200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal R. L., Ramírez O. A., Sandoval L., Koenig-Robert R., Härtel S., Couve A. (2007). Marlin-1 and conventional kinesin link GABAB receptors to the cytoskeleton and regulate receptor transport. Mol. Cell. Neurosci. 35, 501–512 10.1016/j.mcn.2007.04.008 [DOI] [PubMed] [Google Scholar]
- Ward T. H., Polishchuk R. S., Caplan S., Hirschberg K., Lippincott-Schwartz J. (2001). Maintenance of Golgi structure and function depends on the integrity of ER export. J. Cell Biol. 155, 557–570 10.1083/jcb.200107045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins M. E., Li X., Smart T. G. (2008). Tracking cell surface GABAB receptors using an alpha-bungarotoxin tag. J. Biol. Chem. 283, 34745–34752 10.1074/jbc.M803197200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woźniak M. J., Bola B., Brownhill K., Yang Y. C., Levakova V., Allan V. J. (2009). Role of kinesin-1 and cytoplasmic dynein in endoplasmic reticulum movement in VERO cells. J. Cell Sci. 122, 1979–1989 10.1242/jcs.041962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B., Zhang Y., Song W., Younger S. H., Jan L. Y., Jan Y. N. (2007). Growing dendrites and axons differ in their reliance on the secretory pathway. Cell 130, 717–729 10.1016/j.cell.2007.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. J., Rosenwald A. G., Willingham M. C., Skuntz S., Clark J., Kahn R. A. (1994). Expression of a dominant allele of human ARF1 inhibits membrane traffic in vivo. J. Cell Biol. 124, 289–300 10.1083/jcb.124.3.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurek N., Sparks L., Voeltz G. (2011). Reticulon short hairpin transmembrane domains are used to shape ER tubules. Traffic 12, 28–41 10.1111/j.1600-0854.2010.01134.x [DOI] [PMC free article] [PubMed] [Google Scholar]