Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations (original) (raw)
. Author manuscript; available in PMC: 2015 Feb 1.
Abstract
Pancreatic ductal adenocarcinoma is refractory to available therapies. We have previously shown that these tumors have elevated autophagy and inhibition of autophagy leads to decreased tumor growth. Using an autochthonous model of pancreatic cancer driven by oncogenic Kras and the stochastic LOH of p53, we demonstrate that while genetic ablation of autophagy in the pancreas leads to increased tumor initiation, these premalignant lesions are impaired in their ability to progress to invasive cancer, leading to prolonged survival. Additionally, mouse pancreatic cancer cell lines with differing p53 status are all sensitive to pharmacologic and genetic inhibition of autophagy. Lastly, a mouse pre-clinical trial using cohorts of genetically characterized patient derived xenografts treated with hydroxychloroquine showed responses across the collection of tumors. Together our data support the critical role of autophagy in pancreatic cancer and that inhibition of autophagy may have clinical utility in the treatment of these cancers, independent of p53 status.
Keywords: autophagy, pancreatic cancer, p53, chloroquine, metabolism, Atg5
Introduction
Pancreatic ductal adenocarcinoma (PDAC) accounts for 95% of pancreatic cancer, and is one of the most lethal cancers with a five year survival rate of ~6% (1). More than 80% of patients are diagnosed at an advanced stage and treatment is very limited. Previously we identified that human PDAC have elevated basal autophagy and either genetic or pharmacological inhibition of autophagy (latter with chloroquine (CQ) treatment) showed robust responses in human and mouse PDAC cell lines and tumor models (2). Because of its extensive use in human patients for other indications, multiple clinical trials have opened using the CQ derivative, hydroxychloroquine (HCQ) to inhibit autophagy in pancreatic cancer patients (www.clinicaltrials.gov).
Autophagy is a catabolic mechanism that recycles cellular components and the level is dynamically controlled to maintain cell function. When cells are under stress such as starvation, autophagy is elevated to redistribute energy to sustain cell survival; however it could also lead to cell death when the attempts to sustain viability have failed (3). The dual role of autophagy also applies to tumorigenesis as it can both serve as a barrier to cancer initiation, as well as promote cancer growth by providing energy for advanced malignancies (4). This dynamic role of autophagy in cancer has been supported using various mouse models. Mosaic deletion of Atg5 predisposes mice to benign liver adenomas which do not progress to malignant tumors (5). Previous data have also shown that autophagy may play a particularly important role in cancers driven by oncogenic Kras (2, 6, 7). Indeed, mice engineered to express oncogenic Kras in the lung with concurrent Atg5 or Atg7 loss develop increased benign lesions (adenomas) but fail to progress to malignancy (8, 9).
Sequence analysis of PDAC has shown that over 90% of tumors possess KRAS mutations (10) and mouse models have validated the critical role of this oncogene in driving PDAC formation (11, 12) and in tumor maintenance (13). In addition, PDAC demonstrate a mixture of tumor suppressor gene mutations at varying frequencies which have been shown to constrain tumor progression, including p53, CDKN2A, and SMAD4 (14). p53 is one of the most commonly altered genes in all of human cancer and it is found mutated or lost in the majority (~75%) of PDAC (15). In most cases, these genetic mutations, including the tumor suppressor gene p53, are acquired via loss of heterozygosity (LOH) during tumor development (15). As a result, a conditional p53 deletion allele or a p53 mutant allele is often crossed to an activated Kras allele to accelerate tumorigenesis in PDAC genetically engineered mouse models (GEMM). This is typically done as a single copy, as mice with homozygous p53 deletion have rapidly progressive disease that is non-metastatic (16) and for these reasons most treatment studies using PDAC GEMMs have focused on heterozygous p53 models (17). The heterozygous p53 mice develop tumors dependent on the stochastic LOH at the p53 locus, analogous to human PDAC (18).
Recently, the role of autophagy in PDAC progression was explored using a GEMM (19). Although loss of Atg5 or Atg7 in the pancreas with an activating Kras mutation completely inhibited progression of pre-malignant lesions to invasive cancer, in the context of an embryonic homozygous p53 deletion in the pancreas, autophagy loss appeared to accelerate the progression to invasive cancer (19). However, due to the nature of the p53 homozygous model used, the conclusion generated from this model might not be fully representative of human tumors where p53 alterations occur by LOH. In this study, we utilized a p53 heterozygous PDAC GEMM to study how loss of autophagy impacts tumor development in a situation where p53 is present before tumor initiation and lost in fully developed tumors, mimicking human PDAC development. We also explored how tumor cell lines with p53 deletions or mutations responded to autophagy inhibition. Finally we determined how human patient-derived xenografts responded to autophagy inhibition using HCQ treatment to assess the clinical significance of p53 genotypes on human tumor response.
Results
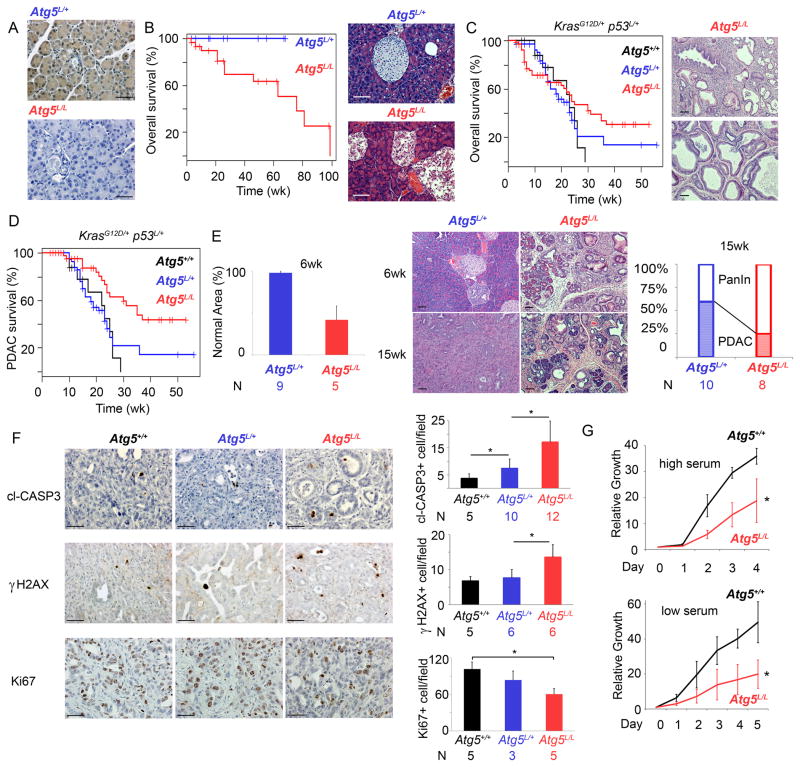
To study the function of autophagy in pancreatic cancer development, we generated pancreatic conditional Atg5 knockout mice using a Pdx1 promoter-driven Cre recombinase (20) (Fig. 1A). We first assessed the impact of homozygous deletion of Atg5 (Atg5L/L) in the pancreas. Pancreatic deletion of Atg5 was not embryonic lethal and did not cause signs of malignant transformation, however it caused a cellular disruption in endocrine tissues starting at 12 weeks leading to a reduction in insulin producing β-cells as had been shown previously with Atg7 deletion in that compartment (21) (Supplementary Fig. S1A and S1B). Thus, Atg5L/L mice had a reduced long-term survival that was consistent with previous reports (Fig. 1B) (19). Given the relatively restricted phenotype of the Atg5L/L mice and the long survival of this cohort, we crossed Atg5L/L mice into the PDAC GEMM (Pdx1Cre+ ; lslKrasG12D/+ ; p53L/+) to assess the role of autophagy in PDAC development. To this end we generated three cohorts with differing Atg5 allelic status: Atg5+/+, Atg5L/+ and Atg5L/L. This GEMM recapitulates the human condition beginning from pre-malignant PanIN to invasive and malignant PDAC (16, 18). We first compared the overall survival between the groups and found that the Atg5L/L cohort had the longest median survival (Fig. 1C). Indeed, there were significantly more long-term survivors (>30 weeks of age) in this group compared to either the Atg5L/+ or Atg5+/+ cohorts (p<0.0005). Interestingly, we observed ~20% of Atg5L/L mice died prior to 6 weeks of age, which was not seen in the Atg5+/+ cohort (0%) and infrequently (4%) in the Atg5L/+ group. Analysis of this small population of Atg5L/L mice revealed that they did not die of PDAC but due to acinar destruction as shown by massive acinar to ductal metaplasia leading to the disruption of most of the exocrine pancreas and left on average less than 10% normal pancreas (Supplementary Fig. S2A and S2B). The majority of the Atg5L/L cohort survived past this early insult and when death due to PDAC was assessed, the Atg5L/L mice had a significantly prolonged survival compared to the Atg5+/+ and Atg5L/+ cohorts (p=0.0032) (Fig. 1D). To investigate the mechanism behind the prolonged survival in the Atg5L/L mice, we performed a detailed histological assessment of pancreata at an early time point (6 weeks) and a later time point (15 weeks) to determine how Atg5 loss impacts both tumor initiation, as well as progression. At 6 weeks, there was a marked difference in formation of non-invasive precursor lesions, pancreatic intraepithelial neoplasia or PanIN between the cohorts, with the Atg5L/L group having on average more than 50 PanIN per mouse vs. the Atg5L/+ which showed only 2–5 PanINs per mouse (Fig. 1E). The difference in PanIN formation was further quantified by comparing the fraction of normal pancreas remaining to that containing PanIN and surrounding inflammation (22). The Atg5L/L cohort showed 41.7% normal pancreatic parenchyma vs. 98.6% in the Atg5L/+ group (p=0.0015) (Fig. 1E). At 15 weeks the normal pancreatic parenchyma in the Atg5L/L group further decreased to 31.7%, but interestingly only 25% of the mice (2 out of 8) mice had invasive PDAC while 60% of Atg5L/+ mice (6 out of 10) developed PDAC (Fig. 1E). Together, these data indicate that although autophagy deficiency increased PanIN development (tumor initiation), it inhibits PanIN progression to PDAC. IHC for Atg5 and LC3 confirmed that all tumors developed in Atg5L/L mice lacked Atg5 expression and had absent autophagy (Supplementary Fig. S3A and S3B). Interestingly, cell lines derived from two of the deleted tumors re-established Atg5 expression after a few passages, indicating that rare subclones escaped Pdx1-cre mediated excision and can grow out in a small fraction of the tumors (Supplementary Fig. S4A). As expected, tumors from all three genotypes showed LOH of the remaining p53 allele and did not express p53 protein (Supplementary Fig S4B). We also assessed tumors from the three groups for expression of cleaved caspase-3, γH2AX, and Ki-67 (Fig. 1F) to measure apoptosis, DNA damage, and proliferation respectively and found that there was elevation of both apoptosis and DNA damage as well as a decrease in proliferation in the Atg5L/L group compared to the Atg5+/+ or Atg5L/+ cohorts (Fig. 1F). Therefore in the tumors that were able to form in the setting of autophagy loss, there was increased DNA damage and cell death. This is consistent with the fact that cell lines derived from these autophagy incompetent tumors proliferate at a significantly slower rate than Atg5+/+ tumors under both basal and low serum conditions (Fig. 1G). Together our data suggest that autophagy is required for proper progression of premalignant lesions to invasive PDAC and those tumors that do progress are less robust.
Figure 1.
Loss of autophagy extends PDAC survival. (A) Pancreatic sections stained by IHC for Atg5 expression, showing Pdx1 driven Cre mediated deletion of Atg5. Scale bar: 50μm. (B) Kaplan-Meier analysis comparing overall survival of Pdx1Cre+ Atg5L/+ (blue) and Atg5L/L (red) mice. Representative sections stained with H&E illustrating the β-islet disruption in the Atg5L/L mice. Atg5L/+ mice are shown as controls. Scale bar: 100μm. (C) Kaplan-Meier analysis comparing overall survival of p53L/+ lslKrasG12D/+ Pdx1Cre+ mice with Atg5+/+ (black), Atg5L/+ (blue) and Atg5L/L (red). There are significantly more Atg5L/L mice with long-term survival (>30wks) than in the Atg5+/+ or Atg5L/+ cohorts (p<0.0005 Fisher’s Exact Test). H&E staining of representative p53L/+ lslKrasG12D/+ Pdx1Cre+ Atg5L/L mice that died prior to 6 weeks of each due to pancreatic disruption. (D) Kaplan- Meier analysis comparing PDAC-specific survival of Atg5+/+ (black), Atg5L/+ (blue) and Atg5L/L (red) mice with p53L/+ lslKrasG12D/+ Pdx1Cre+. The survival of the Atg5L/L cohort was significantly longer than the Atg5+/+ cohort (*p=0.003, log-rank test). (E) Quantification of normal pancreas area of Atg5L/+ and Atg5L/L mice at 6 weeks. H&E staining of representative Atg5L/+ and Atg5L/L pancreata at 6 and 15 weeks. Proportion of mice that developed PDAC (versus only PanIN) at 15 wks in Atg5L/+ (n=10) and Atg5L/L (n=8) mice. Scale bar: 100μM. (F) Pancreatic tumors from indicated genotypes stained with cl-CASPASE3, γH2AX and Ki67 antibody. Number of cl-CASPASE3 or γH2AX positive cells per field was counted from five random fields per mouse. Scale bar: 50μm. *p<0.05 by T-test. (G) Growth curve comparing proliferation of Atg5+/+ (black, n=4) and Atg5L/L (red, n=4) tumor cell lines at both high and low serum condition. Error bars represent standard deviations of the results from 4 cell lines of each genotype. *p<0.05 by T-test.
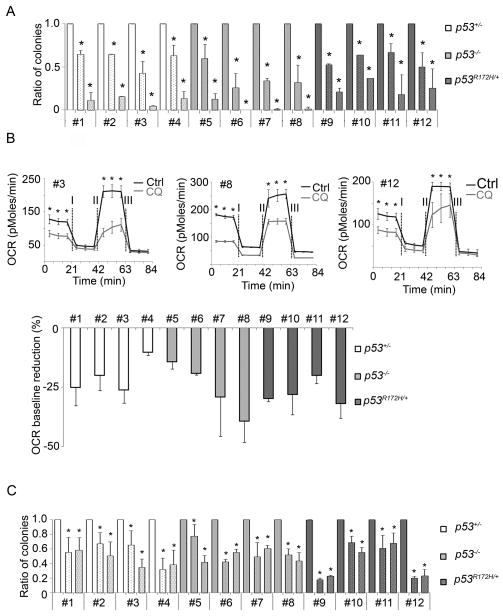
Previously we reported that human PDAC cell lines and tumors have elevated basal autophagy and CQ treatment, as well as suppression of autophagy using RNAi against critical autophagy genes, could inhibit cell growth in vitro as well as in xenografts. Additionally, CQ-treatment significantly prolonged survival in the lsl_KrasG12D_; p53L/+ PDAC GEMM (2). A recent study using a _Kras_-driven PDAC GEMM with an embryonic p53 homozygous deletion showed that either Atg5 or Atg7 deletion promoted tumor progression, thus reducing mouse survival. In their model, CQ treatment appeared to have a modest reduction in lifespan as well. Given the differences between our prior and current data using the p53L/+ PDAC GEMM, and that reported by Rosenfeldt et al using the p53L/L GEMM (19), we examined the impact of autophagy inhibition in a panel of murine PDAC cell lines with varying p53 genotypes (p53L/+, p53L/L and p53R172H/+). As the majority of the human clinical trials are utilizing CQ or its derivative, hydroxychloroquine (HCQ), we focused on response to CQ given its clinical relevance. As has been reported in the past (18), the WT allele of p53 was lost in p53L/+, p53L/L and the p53R172H/+ lines as confirmed by PCR (Supplementary Fig. S5A). Western blot showed loss of p53 expression in p53L/+ and p53L/L lines, while p53 mutations were detected in the p53R172H/+ lines (DNA point mutation confirmed by sequencing) (Supplementary Fig. S5B and C). All PDAC lines, independent of p53 genotype showed a significant, dose-dependent reduction in clonogenic growth when treated with CQ (Fig. 2A). We had previously shown that one of the consequences of autophagy inhibition in human PDAC cells was a decrease in oxidative phosphorylation (measured by oxygen consumption) (2). Consistent with these findings, all cell lines independent of p53 status had a significantly reduced baseline oxygen consumption rate (OCR) (Fig. 2B). To validate the CQ data, we repeated the clonogenic assays using shRNAs to either Atg5 or Atg7. Suppression of expression of both ATG genes as well as autophagy inhibition was confirmed by western blotting (Supplementary Fig. S6). Similar to the CQ data, suppression of autophagy via RNAi significantly attenuated clonogenic growth independent of p53 genotype (Fig. 2C).
Figure 2.
Autophagy inhibition reduces colony formation and reduces base-line oxygen consumption rate independent of p53 status. (A) Chloroquine (CQ) reduces clonogenic growth (0 μM CQ -blank bar, 7.5 μM CQ -10% dotted bar and 15 μM CQ -25% dotted bar). Error bars represent the standard deviation of a representative experiment performed in triplicate. *p<0.005 by T-test. (B) CQ reduces baseline OCR (oxygen consumption rate) in tumor cell lines. A representative OCR plot is shown for one cell line of each genotype. Error bars represent standard deviations of triplicate wells. *p<0.05 by T-test. Below is quantification of the reduction in baseline OCRs from cell lines of each indicated p53 genotype. Error bars represent the standard deviation from three experiments. (C) Clonogenic assay shows that knocking down autophagy reduces colony formation independent of p53 genotype. shGFP (blank bar), shAtg5 (10% dotted bar) and shAtg7 (25% dotted bar). Error bars represent the standard deviation of a representative experiment performed in triplicate. *p<0.05 by T-test.
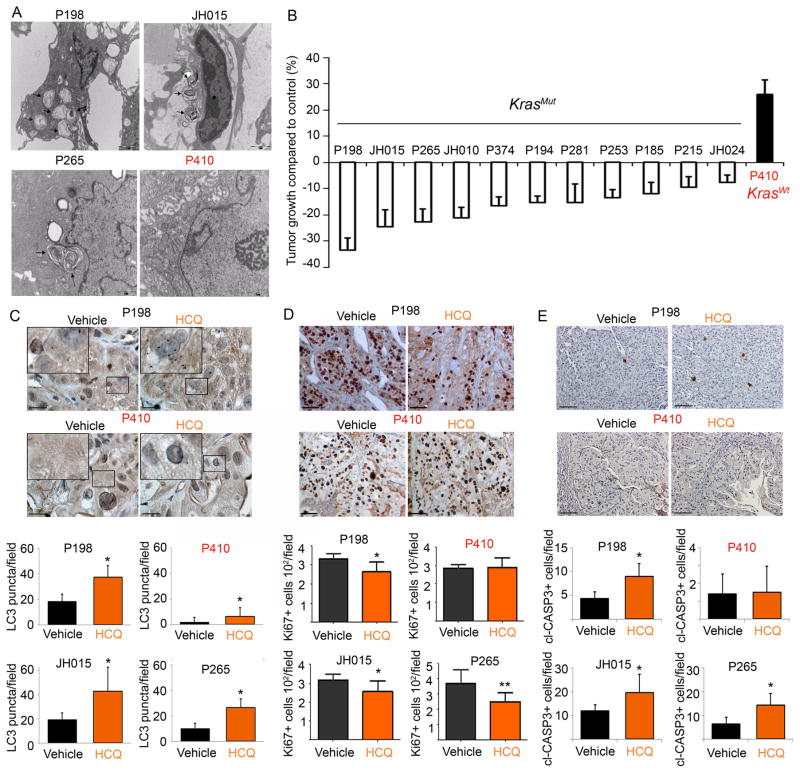
Lastly, to model the therapeutic situation that is occurring in ongoing human clinical trials, we performed efficacy trials using 12 individual human patient-derived pancreatic cancer xenografts (PDX) treated with HCQ or saline control. Mice with established pancreatic tumors were treated with HCQ, and tumor growth was compared to the vehicle treated mice. The overwhelming majority of the PDX lines showed a reduction in tumor volume compared to controls (p<0.05), with a third of the PDX lines showing over 20% inhibition of tumor growth compared to the tumors in the vehicle treated mice (Fig. 3B). All tumors had KRAS mutations (except P410) and p53 mutations (except JH024) (Supplementary Table S1). Interestingly, consistent with previous findings of the role of autophagy in KRAS mutant cancers (2, 8) the KRAS WT tumor did not appear to have elevated autophagy by transmission electron microscopy (TEM) and did not respond to HCQ treatment (Fig. 3A and B). IHC for LC3 in the treated tumors showed that HCQ increased the LC3 punctate staining in the HCQ treated samples, consistent with effective autophagy inhibition. In line with the TEM data, the KRAS WT tumor had the lowest amount of basal puncta which did show an increase upon HCQ treatment. (Fig. 3C). Additionally, the fact that all the p53 mutant tumors showed varying degrees of response further supports the fact that disruption of the p53 axis does not impact response to anti-autophagy therapies. Ki-67 and cleaved caspase-3 staining of the three best responders versus the KRAS WT non-responder was consistent with its effect on tumor volume: HCQ treatment significantly inhibited tumor cell proliferation and increased apoptosis in the responders while had minimal impact on the non-responder (Fig. 3D and E).
Figure 3.
HCQ treatment selectively suppressed tumor growth and proliferation in pancreatic cancer xenografts with active baseline autophagy.
(A) Representative TEM photomicrographs of autophagy in pancreatic cancer xenografts. Arrowheads point towards autophagosomes. (B) Efficacy of HCQ in a panel of 12 individual patient-derived pancreatic cancer xenografts (PDX) (n=8–10 tumors per PDX). (C) LC3 staining of PDX tumors treated with vehicle or HCQ. Small black squared area was enlarged in the left corner. Scale bar: 20 μm. Numbers of LC3 puncta per field were quantified (* p<0.05, T-test). (D) Representative photomicrographs of Ki-67 stained tumor sections in pancreatic cancer xenografts sensitive or resistant to HCQ treatment. Number of Ki67+ cells was quantified for each line. Five fields were counted from two tumors for each PDX (* p<0.05, **p<0.01, T-test). Scale bar: 50μm. (E) cl-CASP3 staining of PDX tumors and positive cells per field were quantified (* p<0.05). Scale bar: 50μm.
Discussion
In this study, we have utilized multiple orthogonal approaches (autochthonous models, cell lines and human tumor xenografts) to demonstrate that disruption of the p53 axis (a finding observed in 75% of PDAC) has no impact on the efficacy of autophagy inhibition. We utilized a PDAC GEMM (Pdx1Cre+; lslKrasG12D/+; p53L/+) where p53 is lost by stochastic LOH as seen in human tumors and determined the role of autophagy in PDAC progression using a conditional Atg5 allele. We found that deletion of Atg5 predisposed mice to pre-malignant pancreatic lesions as evidenced by the increased occurrence of PanINs. On the other hand, mice with Atg5 deletion were significantly less likely to develop PDAC and therefore had improved survival.
The role of autophagy in tumorigenesis is controversial because there are studies supporting it both being a suppressor and a promoter. Evidence to support autophagy as a tumor suppressor comes from studies where autophagy genes were deleted in mice. With the exception of Beclin1, where the heterozygote was used and autophagy was only partially attenuated, these studies have shown that loss of autophagy predisposes mice to benign tumors (4, 23, 24). In contrast, evidence to support autophagy as a tumor promoter comes from studies of advanced tumors, where blocking autophagy inhibits tumor growth and can synergize with chemotherapy in mouse models (2, 25). Our data suggest a dual role of autophagy in PDAC development, whereby autophagy loss increases the initiation of tumors, but abrogates the efficient progression to invasive cancer.
Several prior studies have shown that autophagy deletion attenuates malignant tumor formation in lslKrasG12D/+ tumor models (8, 9). However, the role of p53 in this process is complex, with studies reporting results that differ in terms of how tumorigenesis is impacted upon autophagy loss with concurrent p53 deletion (9, 19). A recent study using a PDAC GEMM showed that the impact of autophagy inhibition differed depending on whether p53 was concurrently deleted or not (19). Some of these differences could be due to the models used or alternatively that particular ATG genes may have non-overlapping functions that are independent of autophagy. Additionally, there are likely differences on whether p53 is deleted homozygously in the germline, or if one copy is lost by somatic LOH (mimicking the cognate human phenomenon). Our data are consistent with prior reports showing that loss of autophagy can promote the initiation of tumorigenesis in the pancreas, and prevent the progression to cancer in the setting of oncogenic Kras mutations (19). However, unlike the data from Rosenfeldt and colleagues, our work shows that Atg5 deletion impairs the progression of pre-malignant PanIN to invasive PDAC in the setting of p53 loss. We believe this difference stems from the fact that in their study, p53 was homozygously deleted during embryogenesis. Therefore, in the physiological setting of p53 loss during tumor progression, autophagy appears to be required for optimal PDAC development. The intricate and complex relationship of autophagy and p53 is of great importance and awaits further study (26).
Perhaps most relevant to cancer treatment, we showed that acute inhibition of autophagy by CQ-treatment or RNAi inhibited growth of murine PDAC cell lines with various p53 alterations and is consistent with our prior data using human PDAC cell lines, which almost all harbor p53 mutations (2). Lastly, we utilized a large panel of patient derived PDAC xenografts and performed treatment studies using HCQ. HCQ-treatment attenuated the growth of the majority of primary patient derived PDAC xenografts that harbor p53 mutations.
Together, our data continue to support the integration of anti-autophagy therapies into the treatment of PDAC. Ongoing human clinical trials in PDAC will determine whether this approach is feasible and effective in patients.
Methods
Genetically Engineered Mice
Atg5L/L mice were kindly provided by Dr. Noboru Mizushima (27). p53L/L mice were obtained from Anton Berns (28). _Pdx1_-Cre was obtained from Doug Melton (20). All animal experiments were approved by the Institutional Animal Care and Use Committee under protocol 10–055 in Dana Farber Cancer Institute. Mice were maintained on a mixed background. Survival was determined by humane endpoints as specified by protocol including showing signs of being moribund, significant weight loss, skin ulceration or in rare cases found dead. All mice with PDAC-specific death were histologically confirmed.
Histology
All tissues were fixed in 10% formalin overnight and embedded in paraffin. For IHC, tumors were deparaffinized, rehydrated. After antigen retrieval in citrate buffer (pH6), tumors were labeled with primary antibody overnight and then detected using vectastain Elite ABC kit (pk-6100, Vector labs) and DAB (sk-4100, Vector labs). Antibodies used for immunohistology are ATG5 (1:200, NB110-53818, Novus Biological), cleaved-Caspase3 (1:200, D175, Cell signaling), γH2AX (1:100, clone JBW301, Millipore), LC3 (1:100, NBP1-19167, Novus Biologicals). Ki-67 staining was performed using an anti-MIB-1 (Ki-67) antibody (Ventana Medical Systems; clone K2, 1:100 dilutions) as previously described (29). Sections from two tumors per treatment group were examined microscopically and more than five representative fields from each slide were photographed under 200× magnifications (except LC3 which taken at 1000× magnification). (30).
Cell culture
All cell lines were derived from mouse primary tumors and grown in DMEM (11965, invitrogen) with 10% FBS and 1% Penstrip. p53L/L and p53R172H lines were obtained from Dr H. Ying (MD Anderson) and Dr. D. Tuveson (Cold Spring Harbor Laboratory). Cells were re-genotyped to verify p53 status and western blots used to confirm p53 protein expression. p53 mutant cell lines were sequenced to verify the presence of the mutation. Atg5L/L lines were harvested from ~5mm2 chunk of tumor, minced and digested in 4% collagenase/dispase for 1hr. Cell lines were routinely tested and all negative for mycoplasma infection.
Clonogenic assay
Cells were plated in 6-cm dishes at 500 cells per dish in growth medium with 10% FBS and treated with CQ the day after seeding. After 7 d, cells were fixed in 80% methanol and stained with 0.2% crystal violet and colonies were counted. The surviving fraction was calculated using the plating efficiency.
Growth Curves
Cells were plated in 24-well plates at 3,000 cells per well in 1 ml of media. Media was not changed throughout the course of the experiment. At the indicated time points, cells were fixed in 10% formalin and stained with 0.1% crystal violet. Dye was extracted with 10% acetic acid and the relative proliferation was determined by attenuance (D) at 595 nm.
OCR
Oxygen consumption measurements: 1.5×104 cells were seeded in a 96-well Seahorse plate, and oxygen consumption rates (OCRs) were measured using the Seahorse XF96 instrument (Seahorse Biosciences). Basal mitochondrial respiration (3 mM glucose) and ATP production (2 mM oligomycin) were measured. Maximal respiration was obtained by FCCP (0.5 mM) and non-mitochondrial OCRs were obtained by adding 2 mM antimycin A. Values were normalized by protein concentration to account for cell number.
Western Blot
Proteins were extracted by RIPA buffer and separated on 4–12% stacking SDS–PAGE gel. Proteins were then transferred to PVDF membrane (Biorad). Membranes were blocked with 5% non-fat dry and then incubation with the primary antibody overnight at 4°C. Following TBST washing, membranes were incubated with peroxidase-conjugated secondary antibody for 1_h and exposed on film using the enhanced chemiluminescence (ECL) detection system (Thermo Scientific). Antibodies used were: ATG5 (1:500, NB110-53818, Novus Biological), ATG7 (1:300, A2856, Sigma), LC3B (1:500, NB600-1384, Novus Biological), p53 (1:1000, FL-393, Santa Cruz Biotechnology) and β-Actin (1:3000, A2066, Sigma).
Lentivirus-mediated shRNAs
All shRNA vectors were obtained from the RNA Interference Screening Facility of Dana Farber Cancer Institute. Atg5 (TRCN0000375819) and Atg7 (TRCN0000092163). shGFP:
- forward5′ CCGGCGCAAGCTGACCCTGAGTTCATTCAAGAGATGAACTTCAGGGTCAGCTTGCTTTTT
- reverse5′ AATTAAAAAGCAAGCTGACCCTGAAGTTCATCTCTTGAATGAACTCAGGGTCAGCTTGCG (31). Lentivirus was produced using 293T cells as previously described (2).
Electron microscopy
Freshly harvested tumors (subcutaneous) from mice were fixed immediately with 3% paraformaldehyde, 1.5% glutaraldehyde, 2.5% sucrose in 0.1 M Sodium Cacodylate and cut into 1 mm3 squares. After post-fixation in 1% osmium tetroxide for 1 h on ice and dehydration, the samples were embedded in a mixture of Epon–araldite. Thin sections from four blocks were collected on uncoated grids, stained with uranil and lead citrate. Samples were sectioned and examined using a FEI Tecnai 12 transmission electron microscope equipped with 16 bit 2K x2K FEI Eagle bottom mount camera and SIS Megaview III wide-angle camera. Images were captured in 12,000x magnifications (32).
In vivo efficacy of HCQ in human pancreatic cancer xenografts
Animal experiments were conducted following approval and in accordance with the Institutional Animal Care and Use Committee guidelines of the Johns Hopkins University under protocol MO06M385. Total of twelve human pancreatic cancer xenografts established from the primary tumors, resected from pancreatic cancer patients in Johns Hopkins Hospital was used for the study (33). The mutational status of these tumors were previously reported (14) and shown as supplementary Table 1. Fresh tumors resected from mice were cut into cubes of 2 mm3, and were subcutaneously implanted on both flanks of 6-week-old female nu/nu athymic mice (Harlan). When cohorts of tumors reached ~150 mm3, animals (5 mice/group each group with 8–10 tumors) were randomly assigned to receive vehicle or HCQ (60 mg/kg, i.p., once daily for 4 weeks) treatments (2). Tumors were measured twice per week and tumor volumes were calculated using the formula: V = a × b2/2, a being the largest dimension and b the smallest. Tumor growth in HCQ treated animals were compared to that vehicle treated mice.
Statistics
Overall survival events included death as defined by protocol with censoring for alive at last follow-up. Events for PDAC-specific survival included deaths attributable to PDAC with uninformative censoring for deaths related to other causes or at last follow-up. Survival plots were generated using the Kaplan-Meier method. The log-rank test was used to compare survival distributions between groups. The proportion of mice alive at 30 weeks or longer in the Atg5L/L group was compared to the other two groups using a Fisher’s exact test. Statistical analyses were performed using R version 3.0.2 (34).
Supplementary Material
1
2
3
4
5
6
7
Statement of significance.
Recently, a mouse model with embryonic homozygous p53 deletion showed paradoxical effects of autophagy inhibition. We used a mouse model with p53 LOH (similar to human tumors), tumor cell lines, and patient derived xenografts to show that p53 status does not impact response to autophagy inhibition. These findings have important implications on ongoing clinical trials.
Acknowledgments
Support by National Cancer Institute Grant R01CA157490, ACS Research Scholar Grant RSG-13-298-01-TBG, and the Lustgarten Foundation, to A.C.K. AM, RNV and DVH supported by a Stand Up to Cancer Dream Team Translational Research Grant, a Program of the Entertainment Industry Foundation (SU2C-AACR-DT0509).
We thank David Tuveson and Haoqiang Ying for providing mouse PDAC lines; Noboru Mizushima for the conditional Atg5 null mice. A.C.K is a consultant for Forma Therapeutics.
Footnotes
Conflict of interest: There are no conflicts of interest to declare.
References
- 1.Society AC. Cancer Facts & Figures 2013. 2013 [Google Scholar]
- 2.Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, et al. Pancreatic cancers require autophagy for tumor growth. Genes & development. 2011;25:717–29. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimmelman AC. The dynamic nature of autophagy in cancer. Genes & development. 2011;25:1999–2010. doi: 10.1101/gad.17558811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, et al. Autophagy-deficient mice develop multiple liver tumors. Genes & development. 2011;25:795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes & development. 2011;25:460–70. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lock R, Roy S, Kenific CM, Su JS, Salas E, Ronen SM, et al. Autophagy facilitates glycolysis during Ras-mediated oncogenic transformation. Molecular biology of the cell. 2011;22:165–78. doi: 10.1091/mbc.E10-06-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo JY, Karsli-Uzunbas G, Mathew R, Aisner SC, Kamphorst JJ, Strohecker AM, et al. Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes & development. 2013;27:1447–61. doi: 10.1101/gad.219642.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao S, Tortola L, Perlot T, Wirnsberger G, Novatchkova M, Nitsch R, et al. A dual role for autophagy in a murine model of lung cancer. Nat Commun. 2014;5:3056. doi: 10.1038/ncomms4056. [DOI] [PubMed] [Google Scholar]
- 10.Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–54. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 11.Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes & development. 2003;17:3112–26. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer cell. 2003;4:437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 13.Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–70. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K, et al. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989;342:705–8. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- 16.Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF, et al. Both p16(Ink4a) and the p19(Arf)- p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci U S A. 2006;103:5947–52. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westphalen CB, Olive KP. Genetically engineered mouse models of pancreatic cancer. Cancer journal. 2012;18:502–10. doi: 10.1097/PPO.0b013e31827ab4c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Rosenfeldt MT, O’Prey J, Morton JP, Nixon C, MacKay G, Mrowinska A, et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504:296–300. doi: 10.1038/nature12865. [DOI] [PubMed] [Google Scholar]
- 20.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–57. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 21.Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell metabolism. 2008;8:325–32. doi: 10.1016/j.cmet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 22.von Figura G, Wagner M, Nalapareddy K, Hartmann D, Kleger A, Guachalla LM, et al. Regeneration of the exocrine pancreas is delayed in telomere-dysfunctional mice. PloS one. 2011;6:e17122. doi: 10.1371/journal.pone.0017122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–20. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077–82. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117:326–36. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maiuri MC, Galluzzi L, Morselli E, Kepp O, Malik SA, Kroemer G. Autophagy regulation by p53. Curr Opin Cell Biol. 2010;22:181–5. doi: 10.1016/j.ceb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–9. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 28.Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes & development. 2000;14:994–1004. [PMC free article] [PubMed] [Google Scholar]
- 29.Yabuuchi S, Pai SG, Campbell NR, de Wilde RF, De Oliveira E, Korangath P, et al. Notch signaling pathway targeted therapy suppresses tumor progression and metastatic spread in pancreatic cancer. Cancer Lett. 2013;335:41–51. doi: 10.1016/j.canlet.2013.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizuma M, Rasheed ZA, Yabuuchi S, Omura N, Campbell NR, de Wilde RF, et al. The gamma secretase inhibitor MRK-003 attenuates pancreatic cancer growth in preclinical models. Mol Cancer Ther. 2012;11:1999–2009. doi: 10.1158/1535-7163.MCT-12-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart SA, Dykxhoorn DM, Palliser D, Mizuno H, Yu EY, An DS, et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. Rna. 2003;9:493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCaffery JM, Farquhar MG. Localization of GTPases by indirect immunofluorescence and immunoelectron microscopy. Methods Enzymol. 1995;257:259–79. doi: 10.1016/s0076-6879(95)57031-4. [DOI] [PubMed] [Google Scholar]
- 33.Hidalgo M, Bruckheimer E, Rajeshkumar NV, Garrido-Laguna I, De Oliveira E, Rubio-Viqueira B, et al. A pilot clinical study of treatment guided by personalized tumorgrafts in patients with advanced cancer. Mol Cancer Ther. 2011;10:1311–6. doi: 10.1158/1535-7163.MCT-11-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Team RDC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2
3
4
5
6
7