Cardiovascular Phenotype in Patients with Heart Failure and Preserved Ejection Fraction with or without Diabetes: A RELAX Trial Ancillary Study (original) (raw)
. Author manuscript; available in PMC: 2015 Aug 12.
Published in final edited form as: J Am Coll Cardiol. 2014 Aug 12;64(6):541–549. doi: 10.1016/j.jacc.2014.05.030
Abstract
Background
RELAX was a multicenter randomized trial of sildenafil versus placebo in heart failure and preserved ejection fraction (HFpEF) with rigorous entry criteria and extensive phenotypic characterization of participants.
Objectives
To characterize clinical features, exercise capacity, and outcomes in patients with HFpEF with or without diabetes and gain insight into contributing pathophysiologic mechanisms.
Methods
RELAX enrolled 216 stable outpatients with heart failure, EF ≥50%, elevated natriuretic peptide or intracardiac pressures, and reduced exercise capacity. Prospectively collected data included echocardiography, cardiac magnetic resonance imaging, a comprehensive biomarker panel, exercise testing, and clinical events over 6 months.
Results
Compared with non-diabetics (n=123), diabetic (n=93) HFpEF patients were younger, more obese, more often male, and had a higher prevalence of hypertension, renal dysfunction, pulmonary disease, and vascular disease (p<0.05 for all). Uric acid, C-reactive protein, galectin-3, carboxy-terminal telopeptide of collagen type I, and endothelin-1 levels were higher in diabetics (p<0.05 for all). Diabetic patients had more ventricular hypertrophy but systolic and diastolic ventricular function parameters were similar in diabetics and non-diabetics except for a trend toward higher filling pressures (E/e′) in diabetics. Diabetics had worse maximal (peak oxygen uptake) and submaximal (6-minute walk distance) exercise capacity (p<0.01 for both). Diabetic patients were more likely to have been hospitalized for HF in the year prior to study entry (47% vs 28%, p=0.004) and had a higher incidence of cardiac or renal hospitalization at 6 months after enrollment (23.7% vs 4.9%, p<0.001).
Conclusions
HFpEF patients with diabetes are at increased risk of hospitalization and have reduced exercise capacity. Multi-morbidity, impaired chronotropic reserve, left ventricular hypertrophy and activation of inflammatory, pro-oxidative, vasoconstrictor, and pro-fibrotic pathways may contribute to adverse outcomes in HFpEF patients with diabetes. (NCT00763867)
Keywords: heart failure with preserved ejection fraction, diabetes mellitus, biomarkers, exercise capacity, left ventricular structure
Introduction
Diabetes adversely affects outcomes for all types of cardiovascular diseases (1). In particular, diabetes is associated with a 70–80% increase in mortality and hospitalizations in patients with heart failure and preserved ejection fraction (HFpEF) (2–4), but the underlying mechanisms for this relationship are unclear. Few studies provide detailed phenotypic comparison of diabetic and non-diabetic patients with HFpEF. Notably, while improving exercise capacity is an important treatment goal and common endpoint in clinical trials in HFpEF, the impact of diabetes on exercise capacity and the pathophysiologic mechanisms driving such differences have not been evaluated in patients with HFpEF (5,6). Because 30–40% of patients with HFpEF have diabetes (2,5,7), understanding whether diabetic HFpEF patients have distinctive characteristics and outcomes may have important implications for clinical management and identification of effective medical therapies for this large patient subgroup.
The Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure with Preserved Ejection Fraction (RELAX) trial enrolled both diabetic and non-diabetic patients with HFpEF (5). This rigorously characterized HFpEF cohort, including detailed measurements of exercise capacity, provides the opportunity to evaluate the diabetic HFpEF phenotype. We hypothesized that HFpEF patients with diabetes represent a more severe subgroup of the disease with more severe reduction in exercise capacity, in association with evidence of distinctive pathophysiologic mechanisms.
Methods
Patient population
The rationale, design, inclusion and exclusion criteria, and primary results of the RELAX trial have been reported (5,8). RELAX was a multicenter, randomized 24-week trial of sildenafil versus placebo in 216 stable outpatients with heart failure. Patients were eligible for enrollment if they had an EF ≥50%, NYHA class II-IV symptoms, stable medical therapy, and objective evidence of heart failure. Patients also had to meet 2 screening criteria: peak VO2 ≤60% of age and sex-adjusted normal value (with a respiratory exchange ratio ≥1.0) (9); and either elevated natriuretic peptide (NT-proBNP ≥400 pg/mL or BNP ≥200 pg/mL) or elevated pulmonary capillary wedge pressure (rest >20 mmHg or exertional >25 mmHg). The enrolling sites determined diagnosis of diabetes and other clinical characteristics. The institutional review board at each enrolling site approved the study protocol, and all patients provided written informed consent.
Study procedures
Baseline testing included a history and physical examination, phlebotomy for biomarkers, Minnesota Living with Heart Failure Questionnaire, measurement of 6-minute walk distance (6MWD), echocardiogram, cardiac magnetic resonance imaging (CMR) (for those in sinus rhythm), and cardiopulmonary exercise testing (CPET).
Biomarker assessment
Assays were performed at the Heart Failure Network (HFN) biomarker core laboratory (University of Vermont, Burlington, VT) and included measures of renal function (creatinine and cystatin-C), markers of neurohumoral activation (N-terminal pro-B-type natriuretic peptide [NT-proBNP], endothelin-1, and aldosterone), fibrosis related markers (amino-terminal propeptide of procollagen type III [PIIINP], galectin-3, and carboxy-terminal telopeptide of collagen type I [CITP]), and markers of myocardial necrosis (high sensitivity cardiac troponin I [hs-cTnI]), oxidative stress (uric acid), and inflammation (C-reactive protein [CRP]).
Doppler echocardiography
Brachial blood pressure (BP) and heart rate (HR) were measured while the echocardiogram was being recorded. Left ventricular (LV) cavity dimension and wall thicknesses were measured from 2D images. LV mass was calculated using the formula recommended by the American Society of Echocardiography (ASE) and indexed to height 1.7 (10,11). Reported EF preferentially used biplane Simpson’s method, modified Quinones formula, single plane volumetric or visual estimate (10). Midwall fractional shortening (mFS) and end systolic wall stress (cESS) were measured as previously described (12). Contractility (sc-mFS) was assessed by indexing mFS to (log transformed) cESS. Stroke volume was calculated from the time velocity integral of the pulsed wave Doppler signal of LV outflow tract (LVOT) flow and LVOT area. Pulmonary artery systolic pressure was calculated from the peak tricuspid regurgitant velocity and the estimated right atrial pressure using the simplified Bernoulli equation. Early diastolic medial and lateral mitral annular tissue velocity (e′), early mitral inflow deceleration time, and the ratio of the early transmitral flow velocity (E) to e′ (E/e′) were used to estimate LV relaxation, LV stiffness, and LV filling pressure, respectively. Pulse pressure (PP) and mean arterial pressure (MAP) were calculated using standard formulae. End-systolic pressure (ESP) was estimated as 0.9*systolic BP (SBP) (13). Effective arterial elastance (Ea; ESP/SV), systemic arterial compliance (SAC; SV/PP) and systemic vascular resistance (SVR; (MAP/[cardiac output])*80) were derived as previously described (13). The HFN core echocardiography laboratory (Mayo Clinic, Rochester, MN) completed all measurements according to the American Society of Echocardiography recommendations (10,14).
Cardiac magnetic resonance (CMR)
Brachial BP and HR were measured during the CMR. Aortic distensibility was measured using aortic maximal (CSAmax) and minimal cross sectional area (CSAmin) as (aortic CSAmax − aortic CSAmin)/(aortic CSAmin x (PP)) (15). Calculation of volumes and mass were performed according to Simpson’s rule on traced endocardial and epicardial short axis LV images by the HFN core CMR laboratory (Duke University, Durham, NC).
Cardiopulmonary exercise testing
A detailed description of the RELAX CPET protocol, methodologies and calculations has been published (5,8). All measurements were performed by the HFN core CPET laboratory (Harvard University, Cambridge, MA).
Statistical analysis
Data are reported as median (25th, 75th percentile) or frequency (%) as appropriate. Between group comparisons used Wilcoxon rank and chi-square tests. Linear regression models were used to evaluate the relationship between diabetes and peak VO2 or 6 minute walk distance, adjusting for factors known to influence exercise capacity in other studies and in RELAX (exercise modality, age, sex, body size, chronotropic response, and hemoglobin) (16). Chronotropic incompetence was determined as described previously and chronotropic index = [peak HR − rest HR]/[(220-age) − rest HR] (17). The association between diabetes and hospitalization (one or more times) for cardiovascular or renal causes during the 6-month study period was assessed by chi-square and a multivariable Cox proportional hazards model which adjusted for known predictors (age, NYHA class, and GFR) of hospitalization in patients with heart failure. We assessed for a potential interaction between diabetes status and treatment group (sildenafil vs placebo) with respect to change (from baseline to 24 weeks) in peak VO2 and 6-minute walk distance, and the number of patients with one or more hospitalizations during the study period using linear or logistic regression as appropriate. A p-value ≤0.05 was considered significant for all analyses. All statistical analyses were performed with SAS software, version 9.2 (SAS, Cary, NC).
Results
Clinical characteristics
Of the 216 patients with HFpEF enrolled in RELAX, there were 93 (43%) with diabetes. Compared to non-diabetic patients, diabetic patients were younger, more obese, more often male, and had a higher prevalence of co-morbidities, including hypertension, ischemic heart disease, peripheral vascular disease, and obstructive lung disease (Table 1). Anemia tended to be more common in diabetic patients and renal function was more impaired in diabetic patients with higher creatinine and cystatin-C levels and lower estimated glomerular filtration rate. At study entry, heart failure signs and symptoms and heart failure related quality of life were similar between the groups. Diabetic patients were more often taking calcium channel blockers, statins, and diuretics, but there was no difference in the use of β-blockers, ACE inhibitors, or angiotensin receptor blockers. Most patients with diabetes were taking insulin or oral agents for glycemic control.
Table 1.
Baseline characteristics of HFpEF patients with and without diabetes
| Non-DM (n=123) | DM (n=93) | p-value | |
|---|---|---|---|
| Demographic/Clinical | |||
| Age | 71 (63,79) | 66 (62,73) | 0.003 |
| Female | 57.7% | 35.5% | 0.001 |
| Self-reported white race | 93.5% | 88.2% | 0.17 |
| BSA | 2.02 (1.86,2.21) | 2.23 (2.06,2.47) | <0.001 |
| BMI | 30.7 (27.6,34.2) | 37.1 (32.3, 42.0) | <0.001 |
| Heart rate | 68 (61,78) | 70 (62,78) | 0.74 |
| Systolic blood pressure | 126 (113, 138) | 128 (114,137) | 0.97 |
| Comorbidities | |||
| Ischemic heart disease | 33% | 47% | 0.03 |
| Hypertension | 77% | 95% | <0.001 |
| Peripheral vascular disease | 7% | 25% | <0.001 |
| Obstructive lung disease | 12% | 29% | 0.002 |
| Hyperlipidemia | 70% | 80% | 0.11 |
| Atrial fibrillation or flutter | 55% | 46% | 0.19 |
| Anemia* | 30% | 42% | 0.06 |
| Hemoglobin (g/dL) | 13.0 (12.1, 14.0) | 12.8 (11.7, 13.7) | 0.10 |
| BUN (mg/dL) | 22 (16, 25) | 28 (20, 39) | 0.001 |
| Creatinine | 1.05 (0.83,1.29) | 1.21 (0.89,1.70) | <0.001 |
| Glomerular filtration rate (ml/min/1.73m2) | 67.5 (51.1,83.6) | 57.1 (39.2,78.9) | 0.02 |
| Cystatin C | 1.19 (1.03,1.55) | 1.59 (1.14,1.97) | <0.001 |
| Heart failure | |||
| Jugular venous pressure ≥8 cm | 47% | 44% | 0.68 |
| Edema | 16% | 26% | 0.09 |
| NYHA functional class | 0.34 | ||
| II | 50% | 43% | |
| III | 50% | 57% | |
| MLHFQ total score | 42 (30,58) | 47 (26,68) | 0.20 |
| Medications at enrollment | |||
| βblocker | 76% | 76% | 0.90 |
| ACE-I or ARB | 67% | 75% | 0.17 |
| Aldosterone antagonist | 11% | 11% | 0.97 |
| Calcium channel blocker | 24% | 40% | 0.01 |
| Statin | 54% | 76% | <0.001 |
| Loop diuretic | 72% | 83% | 0.07 |
| Any diuretic | 82% | 91% | 0.046 |
| Diabetes therapy | |||
| Insulin treated | --- | 42% | --- |
| Oral medications alone | --- | 47% | --- |
| Diet alone | --- | 11% | --- |
Biomarker profile
HFpEF patients with or without diabetes had similar elevation of NT-proBNP but diabetic patients had higher levels of endothelin-1, a potent endogenous vasoconstrictor (Table 2). With respect to pro-fibrotic markers, diabetic patients had higher galectin-3 and CITP levels, but increases in PIIINP did not achieve statistical significance. Diabetic patients had higher levels of uric acid and CRP, suggesting greater oxidative stress and inflammation, as well as a trend towards higher levels of hs-cTnI, suggesting more ongoing myocardial necrosis.
Table 2.
Biomarker profile of HFpEF patients with and without diabetes
| Non-DM (n=123) | DM (n=93) | p-value | |
|---|---|---|---|
| Biomarkers (core lab) | |||
| NT-proBNP | 713 (303,1593) | 648 (280,1553) | 0.73 |
| cGMP | 77 (58, 102) | 79 (56, 101) | 0.99 |
| Endothelin-1 | 2.3 (1.8,3.0) | 2.5 (2.0,3.5) | 0.05 |
| Aldosterone | 182 (122,286) | 202 (117,274) | 0.97 |
| PIIINP | 7.5 (6.1,8.9) | 8.2 (6.0,10.9) | 0.11 |
| Galectin-3 | 13.1 (10.6,16.0) | 15.5 (12.2,21.4) | <0.001 |
| CITP | 5.7 (4.5,7.5) | 7.8 (5.5,12.4) | <0.001 |
| Uric acid | 6.8 (5.5,8.5) | 7.8 (6.3,9.4) | 0.005 |
| C-reactive protein | 3.3 (1.6,7.3) | 4.5 (2.1,10.0) | 0.015 |
| hs-cTnI | 8.3 (4.7,16.5) | 10.6 (6.5,20.9) | 0.10 |
Ventricular and vascular remodeling and function
By echocardiography, LV mass index tended to be higher in diabetic patients, but relative wall thickness was similar in diabetic and non-diabetic patients (Table 3). Unadjusted LV cavity dimensions were similar, but LV end-diastolic dimension was smaller in diabetic patients when indexed for body surface area (BSA). Systolic performance was similar in diabetic and non-diabetic patients. Most diastolic function parameters were similar in diabetic and non-diabetic patients although diabetic patients tended to have higher E/e′ suggesting higher LV filling pressures. While unadjusted left atrial volumes were similar, left atrial volume indexed to BSA was smaller in diabetic patients.
Table 3.
Ventricular and vascular remodeling and function in HFpEF patients with and without diabetes.
| Non-Diabetic (n=123) | Diabetic (n=93) | p-value | |||
|---|---|---|---|---|---|
| n | Median (25th, 75th) | n | Median (25th, 75th) | ||
| Echocardiographic Data | |||||
| LV structure | |||||
| LV mass (g/ht1.7) | 92 | 63 (50, 81) | 66 | 69 (57, 88) | 0.12 |
| LV end-diastolic dimension (cm) | 98 | 4.6 (4.2,5.1) | 66 | 4.6 (4.3,5.2) | 0.46 |
| LV end-diastolic dimension/BSA (cm/m2) | 98 | 2.3 (2.1,2.5) | 66 | 2.1 (1.9,2.3) | 0.001 |
| Relative wall thickness | 92 | 0.40 (0.35,0.45) | 66 | 0.41 (0.38,0.50) | 0.11 |
| LV systolic function | |||||
| EF (%) | 123 | 60 (56,65) | 90 | 60 (55,65) | 0.47 |
| Stress corrected mFS | 78 | 14.2 (12.5,16.3) | 58 | 14.7 (12.7,16.5) | 0.50 |
| Cardiac index (L/min/m2) | 102 | 2.5 (2.1,2.8) | 73 | 2.4 (2.0,2.9) | 0.78 |
| LV diastolic function | |||||
| Medial e′ | 113 | 0.06 (0.04,0.08) | 84 | 0.06 (0.05,0.08) | 0.64 |
| Lateral e′ | 113 | 0.08 (0.06,0.10) | 79 | 0.09 (0.06,0.11) | 0.91 |
| E/e′ (medial) | 108 | 14.6 (11,22) | 80 | 18.0 (13,25) | 0.054 |
| E/A ratio | 78 | 1.5 (1.0, 2.3) | 64 | 1.4 (1.0, 2.0) | 0.70 |
| Deceleration time (ms) | 110 | 185 (152,216) | 83 | 187 (157,233) | 0.32 |
| LA volume (ml) | 90 | 94 (75,121) | 59 | 89 (70,112) | 0.34 |
| LA volume index (ml/m2) | 90 | 47 (39,60) | 59 | 41 (32,55) | 0.02 |
| Systemic and pulmonary artery function | |||||
| Pulse pressure (mmHg) | 118 | 55 (47,68) | 89 | 58 (48,69) | 0.32 |
| Arterial elastance (mmHg/ml) | 103 | 1.6 (1.3,1.9) | 75 | 1.4 (1.2,1.9) | 0.10 |
| SVR (dyne/sec/cm−5) | 102 | 1453 (1150,1699) | 73 | 1322 (1085,1638) | 0.16 |
| SAC (ml/mmHg) | 103 | 1.3 (1.1,1.7) | 75 | 1.5 (1.1,1.9) | 0.17 |
| PA systolic pressure (mmHg) | 87 | 41 (34,49) | 51 | 43 (32, 54) | 0.87 |
| Cardiac Magnetic Resonance (CMR) Data | |||||
| EF (%) | 69 | 66 (60,72) | 48 | 65 (54,69) | 0.10 |
| Cardiac index (L/min/m2) | 69 | 2.3 (1.9,2.7) | 46 | 2.4 (2.0,2.8) | 0.87 |
| LV mass (g/ht1.7) | 69 | 50 (43, 58) | 48 | 65 (53, 76) | <0.001 |
| LV end-diastolic volume (ml) | 69 | 110 (91,133) | 48 | 128 (104,156) | 0.002 |
| LV end-diastolic volume/BSA (ml/m2) | 69 | 55 (48,66) | 48 | 58 (46,69) | 0.44 |
| Aortic elastance (mmHg/ml) | 69 | 1.6 (1.4,1.9) | 44 | 1.5 (1.1,1.8) | 0.17 |
| Aortic distensibility (10−3 mmHg) | 54 | 1.1 (0.6,1.5) | 32 | 1.5 (0.9,2.3) | 0.051 |
| SVR (dyne/sec/cm−5) | 69 | 1488 (1274, 1880) | 44 | 1388 (1132, 1698) | 0.10 |
Echocardiographic measurements of pulmonary and systemic vascular function were similar between diabetic and non-diabetic patients.
In the subgroup of patients who underwent CMR (n=117), indexed LV mass was higher in diabetic than non-diabetic patients while LV volumes (indexed to BSA) were similar. Aortic stiffness and systemic vascular resistance were similar between diabetic and non-diabetic patients.
Exercise capacity
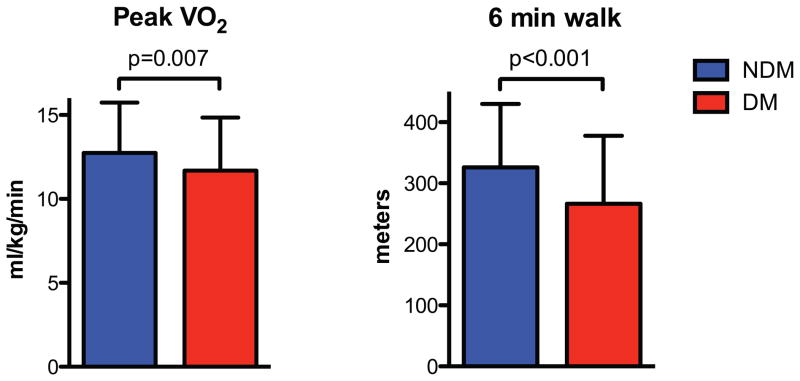
Compared to non-diabetic patients, diabetic patients had a lower peak VO2 (absolute and percent predicted) (Figure 1; Online Supplement), despite similar effort (similar respiratory exchange ratio). Submaximal exercise performance also was impaired in diabetic patients as evidenced by lower VO2 at the ventilatory anaerobic threshold and absolute and percent predicted 6-minute walk distance (p<0.05 for all) (Online Supplement, Table 1). Among diabetic patients, there was a trend toward a lower peak VO2 in those treated with versus without insulin (10.9 [9.4, 12.9] vs 11.6 [10.1, 13.8] ml/kg/min, p=0.07). The chronotropic index was lower and the prevalence of chronotropic incompetence was higher in diabetic than non-diabetic patients while peak systolic blood pressure was similar between groups. After adjusting for age, sex, and exercise modality, diabetes was associated with a 2.09 ml/kg/min lower peak VO2 (p<0.001) (Online Supplement, Table 2). After additional, sequential adjustment for factors (BMI, hemoglobin, and chronotropic index) that are known to influence peak exercise performance and potential mechanisms for the adverse effect of diabetes on exercise performance, the relationship between diabetes and a lower peak VO2 was attenuated but remained significant (Online Supplement, Table 2). Similar findings were observed in multivariable analyses evaluating the relationship between diabetes and 6-minute walk distance (Online Supplement, Table 2).
Clinical outcomes
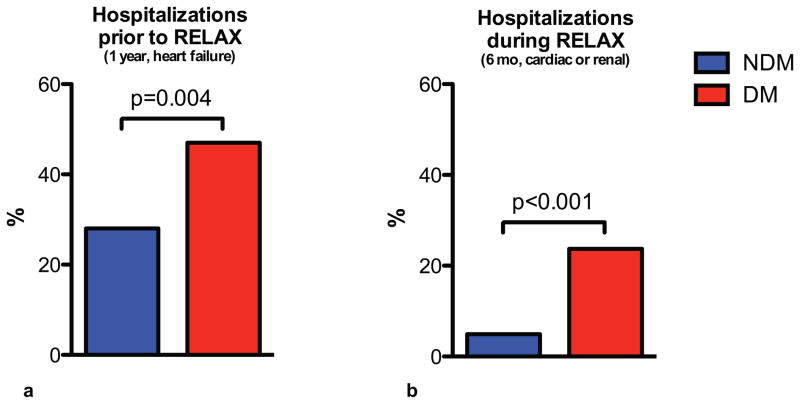
Compared to non-diabetic patients, at study enrollment, diabetic patients had more frequently been hospitalized at least once for heart failure over the preceding 12 months (47% vs 28%, p=0.004) (Figure 2). During the 6-month study period, diabetic patients were more likely to be hospitalized (one or more times) for cardiac or renal causes than non-diabetic patients (23.7% vs 4.9%, p<0.001) (Figure 1). After adjustment for age, NYHA class, and glomerular filtration rate, diabetes remained a significant predictor of hospitalization for cardiac or renal causes during the 6-month study period (HR 4.08, 95% CI 1.60–10.36, p=0.003).
Figure 2. Hospitalizations in HFpEF patients with and without diabetes.
Data are shown for HFpEF patients with and without diabetes for the prevalence of at least 1 heart failure hospitalization during the 12-month period prior to enrollment in RELAX (a) and for the percent of patients with one or more hospitalizations for cardiac or renal causes during the 6-month trial period (b).
Figure 1. Exercise capacity in HFpEF patients with and without diabetes.
Data shown as mean and SD.
Response to sildenafil
There was no interaction between diabetes status and treatment group (sildenafil vs placebo) with respect to the RELAX primary endpoint of change in peak VO2 (interaction p=0.49) from baseline to 24 weeks or change in 6-minute walk distance (interaction p=0.30). There was also no interaction between diabetes status and treatment group with respect to hospitalizations for cardiac or renal causes during the study period (interaction p=0.80).
Discussion
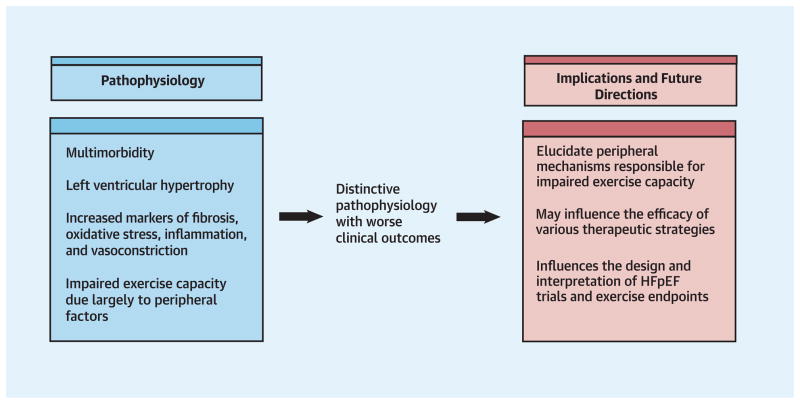
We found that in a cohort of patients with objective evidence of HFpEF and reduced exercise capacity, diabetic patients had a more severe disease phenotype characterized by more numerous co-morbidities, increased left ventricular hypertrophy, and increased circulating markers of vasoconstriction, oxidative stress, inflammation, and fibrosis. Adjusting for known determinants of peak exercise capacity, diabetic patients had a significantly lower peak VO2 and 6-minute walk distance. Patients with diabetes had more hospitalizations both before and after study entry. These findings are consistent with a recent novel HFpEF paradigm described by Paulus and Tschöpe and underscore that the diabetic HFpEF patient is at particularly high risk given their comorbidity burden and somewhat distinctive pathophysiology (18). These data are relevant to the design and interpretation of clinical trials enrolling HFpEF patients and support the need for therapeutic strategies targeting the pathophysiology of diabetic HFpEF patients. Analyses from several other studies have compared diabetic to non-diabetic patients with HFpEF and report worse outcomes in diabetic patients, including increased mortality and hospitalizations (3,4,13). Among patients with preserved EF (>40%) in CHARM, diabetes was associated with a 2-fold increase in cardiovascular death or hospitalization for heart failure after multivariable adjustment and an 80% increase in the hazard of all-cause mortality (3). In a secondary analysis of the DIG study, diabetic patients with EF >45% had an adjusted hazard ratio of 1.68 for heart failure death or hospitalization (4). We confirm the adverse prognostic impact of diabetes in HFpEF and extend these previous studies in several ways. The RELAX trial differed from other comparable studies in that it was restricted to patients with ejection fraction >50% and had rigorous entry criteria to select patients with documented cardiac limitation to exercise and elevated filling pressures by biomarker, invasive, or echocardiographic criteria (7,8). The RELAX trial also included formal, detailed assessment of exercise capacity, distinguishing it from these prior studies. Finally, diabetes was also more common in the RELAX cohort (43%) than other comparable studies (17–32%), a fact that may reflect the entry criteria for RELAX which resulted in a cohort with more advanced HFpEF (5–7,19).
Biomarker profile in HFpEF patients with diabetes
Prior studies comparing diabetic and non-diabetic patients with HFpEF have not included a detailed biomarker profile. Utilizing a panel of biomarkers that evaluated multiple biological pathways, we found that diabetic HFpEF patients have increased mediators of vasoconstriction (endothelin-1) and fibrosis (CITP, galectin-3), increased oxidative stress (uric acid), and inflammation (CRP), and a suggestion of greater ongoing myocardial necrosis (hs-cTnI). Diabetes is characterized by a complex milieu of hyperinsulinemia, insulin resistance, hyperglycemia, and increased circulating and intramyocardial nonesterified fatty acids (20); these biomarker data provide insight into the ways in which diabetes may exacerbate and intensify the pathophysiology of HFpEF, which adversely impacts clinical outcomes. Despite a modestly higher LV filling pressure in diabetic patients as evidenced by a higher E/e′, there was no difference in NT-proBNP levels, which may have been due to a greater prevalence of obesity in the diabetic patients.
Ventricular and vascular structure and function in diabetic patients with HFpEF
Of the prior studies that have compared diabetic and non-diabetic patients with HFpEF (3,4,13), only one has provided a detailed comparison of ventricular and vascular structure and function (13). Consistent with Mohammed et al., we observed that systolic function is similar between diabetic and non-diabetic patients; indices of LV relaxation and stiffness were also similar, whereas LV filling pressures (E/e′) tended to be higher in diabetic patients. We also observed increased LV hypertrophy in diabetic patients. Surprisingly, left atrial volume and LV end-diastolic dimension indexed to BSA were smaller in diabetic patients, which may reflect “over correction” by indexing to BSA in obese patients. Resting measures of vascular function, including pulsatile and resistive load, were similar between diabetic and non-diabetic patients.
Exercise capacity in diabetic patients with HFpEF
Diabetic patients had worse maximal and submaximal exercise performance, which had not been previously evaluated in patients with HFpEF. Although we cannot determine the causative mechanisms for the worse exercise performance exhibited by diabetic patients due the correlative study design employed, our data do provide important insights to be further examined in future studies. We observed significant differences in exercise capacity despite a lack of difference in resting systolic or diastolic cardiac function (except for a trend for E/e′) or systemic or peripheral vascular pressures or function. In contrast, despite a similar resting heart rate and similar prevalence of β-blocker usage, there was a marked difference in the chronotropic index between diabetic and non-diabetic patients. Recent studies have demonstrated the important contribution of peripheral, non-cardiac, factors to exercise performance (21,22). An increase in heart rate is a critical part of increasing cardiac output and has been shown to be an important contributor to exercise capacity (21–23). Cardiovascular autonomic neuropathy is a known complication of diabetes, which includes diminished exercise capacity due to impaired parasympathetic and sympathetic responses that would normally increase cardiac output and blood flow to exercising muscles (24). The withdrawal and reactivation of vagal tone is thought to be an important underlying mechanism for heart rate changes during exercise (25). HFpEF patients have a slower heart rate rise, lower peak heart rate, and impaired recovery (22). An attenuated heart rate response to exercise is associated with increased mortality as is slower heart recovery after exercise, which was more common in diabetic patients (25,26).
Beyond differences in heart rate, there may be differences in peripheral oxygen utilization that may explain, in part, the reduced exercise capacity in diabetic compared to non-diabetic patients with HFpEF. A significant contributor to reduced peak VO2 in patients with HFpEF is reduced arterial-venous oxygen content difference, which is due to reduced O2 delivery or reduced O2 extraction in exercising muscles (21,27). Decreased O2 delivery may occur in diabetic patients with HFpEF due to a greater prevalence of anemia (a trend was seen in our data) or less vasodilator reserve due to autonomic dysfunction, an increased prevalence of peripheral vascular disease, or impaired endothelial function from oxidative stress, inflammation, and vasoconstriction (consistent with the biomarker elevations we observed in diabetic patients with HFpEF). Abnormalities in oxygen delivery and extraction have been observed in diabetic patients (28,29).
Additionally, older and emerging studies demonstrate that differences in skeletal muscle function, composition, and strength explain important differences in exercise capacity in heart failure patients with reduced and preserved EF (30–32). Although not examined in this study, diabetes has been shown to affect skeletal muscle composition and function likely due to multiple factors including inflammation, obesity, insulin resistance, fatty acid oxidation, oxidative stress, and impaired mitochondrial function (33–35). Obesity also impairs exercise capacity and often coexists with diabetes. While there is likely some overlap in the mechanisms by which diabetes and obesity adversely affect exercise capacity, we found that diabetes was still associated with reduced exercise performance even after controlling for a large difference in BMI. Other studies have demonstrated that diabetes, particularly the degree of insulin resistance, has a larger adverse effect on exercise capacity than obesity (35,36).
Collectively, these data suggest that the reduced exercise capacity of diabetic patients with HFpEF is largely due to peripheral factors, including impaired chronotropic reserve, reduced peripheral oxygen utilization, and altered skeletal muscle function. Further studies are needed to carefully examine these mechanisms, as well as to evaluate the contractility and vasodilator reserve of diabetic patients with HFpEF. Given the more marked impairment in exercise capacity among diabetic patients with HFpEF, it seems even more important to encourage exercise training in these individuals, which has been shown to improve exercise capacity and quality of life in patients with HFpEF largely through its effect on peripheral mechanisms (37,38).
Limitations
Our study has several limitations to consider when interpreting the results. The enrolling sites determined the diagnosis of diabetes, and it was not verified by other mechanisms. We do not have access to data on the severity or duration of diabetes, microvascular complications, or glucose control. While the detailed phenotyping of patients was a strength of the study, the relatively small number of patients included may prevent us from detecting significant, smaller magnitude, differences between the diabetic and non-diabetic patients. Further, the small number of patients and clinical events limit the power of our interaction analyses and our ability to adjust for confounders in the Cox model for hospitalization. Finally, we do not have detailed hemodynamic and echocardiographic data at peak exercise that would provide insight into the effect of diabetes on cardiac and vascular reserve function.
Conclusions
In a carefully phenotyped population of patients with HFpEF, those with diabetes were at increased risk of hospitalization. Multi-morbidity, impaired chronotropic reserve, LV remodeling and activation of inflammatory, pro-oxidative, vasoconstrictor, and pro-fibrotic pathways may contribute to adverse outcomes in HFpEF patients with diabetes. The mechanisms for the impaired exercise performance in diabetic patients are multifactorial, but appear to be largely due to peripheral factors. Our findings support the need for therapeutic strategies targeting the distinctive pathophysiology of diabetes in HFpEF (Central Illustration) and have implications for the design of clinical trials evaluating the HFpEF population.
Supplementary Material
Perspectives.
Competency in Medical Knowledge 1
Among patients with heart failure who have preserved left ventricular ejection fraction, those with diabetes have a more severe disease phenotype, more extensive comorbidities, greater left ventricular hypertrophy, and higher circulating markers of vasoconstriction, oxidative stress, inflammation, and fibrosis.
Competency in Medical Knowledge 2
More severely impaired exercise tolerance in diabetic patients with heart failure and preserved left ventricular ejection fraction is due mainly to extracardiac factors.
Translational Outlook 1
Future studies should be directed toward understanding the distinctive pathophysiology and extracardiac mechanisms that contribute to reduced exercise capacity in diabetic patients who have heart failure with preserved left ventricular ejection fraction and addressing these in the design of clinical trials.
Acknowledgments
Funding: This study was supported by the National Institutes of Health grants U10HL084904 (data coordinating center), U10HL084907 (MMR), and U10HL110309 (DLM, VDR, LdlF, SMJ, JV). BRL was supported by K23 HL116660 and Washington University Institute of Clinical and Translational Sciences grants UL1 TR000448 and KL2 TR000450 from the National Center for Advancing Translational Sciences of the National Institutes of Health. VDR was supported in part by the Barnes-Jewish Hospital Foundation.
Abbreviations
CMR
cardiac magnetic resonance imaging
CPET
cardiopulmonary exercise testing
DM
diabetes mellitus
EF
ejection fraction
HFpEF
heart failure with preserved ejection fraction
LV
left ventricular
NDM
non-diabetic
NT-proBNP
N-terminal pro B-type natriuretic peptide
NYHA
New York Heart Association
VO2
oxygen uptake
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–8. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald MR, Petrie MC, Varyani F, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377–85. doi: 10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]
- 4.Aguilar D, Deswal A, Ramasubbu K, et al. Comparison of patients with heart failure and preserved left ventricular ejection fraction among those with versus without diabetes mellitus. Am J Cardiol. 2010;105:373–7. doi: 10.1016/j.amjcard.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–77. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edelmann F, Wachter R, Schmidt AG, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309:781–91. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 7.Campbell RT, Jhund PS, Castagno D, et al. What have we learned about patients with heart failure and preserved ejection fraction from DIG-PEF, CHARM-preserved, and I-PRESERVE? J Am Coll Cardiol. 2012;60:2349–56. doi: 10.1016/j.jacc.2012.04.064. [DOI] [PubMed] [Google Scholar]
- 8.Redfield MM, Borlaug BA, Lewis GD, et al. PhosphdiesteRasE-5 Inhibition to Improve CLinical Status and EXercise Capacity in Diastolic Heart Failure (RELAX) trial: rationale and design. Circ Heart Fail. 2012;5:653–9. doi: 10.1161/CIRCHEARTFAILURE.112.969071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher GF, Balady G, Froelicher VF, et al. Exercise standards. A statement for healthcare professionals from the American Heart Association. Writing Group. Circulation. 1995;91:580–615. doi: 10.1161/01.cir.91.2.580. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Chirinos JA, Segers P, De Buyzere ML, et al. Left ventricular mass: allometric scaling, normative values, effect of obesity, and prognostic performance. Hypertension. 2010;56:91–8. doi: 10.1161/HYPERTENSIONAHA.110.150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borlaug BA, Lam CS, Roger VL, et al. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–8. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammed SF, Borlaug BA, Roger VL, et al. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: a community-based study. Circ Heart Fail. 2012;5:710–9. doi: 10.1161/CIRCHEARTFAILURE.112.968594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Hundley WG, Kitzman DW, Morgan TM, et al. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. doi: 10.1016/s0735-1097(01)01447-4. [DOI] [PubMed] [Google Scholar]
- 16.Mohammed SF, Borlaug BA, McNulty S, et al. Resting Ventricular-Vascular Function and Exercise Capacity in Heart Failure with Preserved EF (HFpEF): A RELAX Trial Ancillary Study. Circ Heart Fail. 2014 doi: 10.1161/CIRCHEARTFAILURE.114.001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (metoprolol or atenolol) Am J Cardiol. 2005;96:1328–33. doi: 10.1016/j.amjcard.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 18.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 19.Shah AM, Shah SJ, Anand IS, et al. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2014;7:104–15. doi: 10.1161/CIRCHEARTFAILURE.113.000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res. 2006;98:596–605. doi: 10.1161/01.RES.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- 21.Haykowsky MJ, Brubaker PH, John JM, et al. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–74. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borlaug BA, Melenovsky V, Russell SD, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–47. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 23.Borlaug BA, Olson TP, Lam CS, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–54. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115:387–97. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 25.Cole CR, Blackstone EH, Pashkow FJ, et al. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–7. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 26.Lauer MS, Okin PM, Larson MG, et al. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation. 1996;93:1520–6. doi: 10.1161/01.cir.93.8.1520. [DOI] [PubMed] [Google Scholar]
- 27.Esposito F, Mathieu-Costello O, Shabetai R, et al. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol. 2010;55:1945–54. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu YW, Hsu CL, Wang SS, et al. Impaired exercise capacity in diabetic patients after coronary bypass surgery: effects of diastolic and endothelial function. Cardiology. 2008;110:191–8. doi: 10.1159/000111929. [DOI] [PubMed] [Google Scholar]
- 29.Bauer TA, Reusch JE, Levi M, et al. Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care. 2007;30:2880–5. doi: 10.2337/dc07-0843. [DOI] [PubMed] [Google Scholar]
- 30.Harrington D, Anker SD, Chua TP, et al. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997;30:1758–64. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- 31.Haykowsky MJ, Kouba EJ, Brubaker PH, et al. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–6. doi: 10.1016/j.amjcard.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitzman DW, Nicklas B, Kraus WE, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–H1370. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park SW, Goodpaster BH, Strotmeyer ES, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care. 2007;30:1507–12. doi: 10.2337/dc06-2537. [DOI] [PubMed] [Google Scholar]
- 34.Piepoli MF, Coats AJ. The ‘skeletal muscle hypothesis in heart failure’ revised. Eur Heart J. 2013;34:486–8. doi: 10.1093/eurheartj/ehs463. [DOI] [PubMed] [Google Scholar]
- 35.Regensteiner JG, Bauer TA, Reusch JE, et al. Abnormal oxygen uptake kinetic responses in women with type II diabetes mellitus. J Appl Physiol. 1998;85:310–7. doi: 10.1152/jappl.1998.85.1.310. [DOI] [PubMed] [Google Scholar]
- 36.Seibaek M, Vestergaard H, Burchardt H, et al. Insulin resistance and maximal oxygen uptake. Clin Cardiol. 2003;26:515–20. doi: 10.1002/clc.4960261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haykowsky MJ, Brubaker PH, Stewart KP, et al. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–8. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitzman DW, Brubaker PH, Morgan TM, et al. Exercise training in older patients with heart failure and preserved ejection fraction: a rlandomized, controlled, single-blind trial. Circ Heart Fail. 2010;3:659–67. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.