Phase IIB Trial of Oral Midostaurin (PKC412), the FMS-Like Tyrosine Kinase 3 Receptor (FLT3) and Multi-Targeted Kinase Inhibitor, in Patients With Acute Myeloid Leukemia and High-Risk Myelodysplastic Syndrome With Either Wild-Type or Mutated FLT3 (original) (raw)
Abstract
Purpose
Mutations leading to constitutive activation of the FMS-like tyrosine kinase 3 receptor (FLT3) occur in blasts of 30% of patients with acute myeloid leukemia (AML). Midostaurin (PKC412; N-benzoylstaurosporin) is a multitargeted tyrosine kinase inhibitor, with demonstrated activity in patients with AML/myelodysplastic syndrome (MDS) with FLT3 mutations.
Patients and Methods
Ninety-five patients with AML or MDS with either wild-type (n = 60) or mutated (n = 35) FLT3 were randomly assigned to receive oral midostaurin at 50 or 100 mg twice daily. The drug was discontinued in the absence of response at 2 months, disease progression, or unacceptable toxicity. Response was defined as complete response, partial response (PR), hematologic improvement, or reduction in peripheral blood or bone marrow blasts by ≥ 50% (BR).
Results
The rate of BR for the population in whom efficacy could be assessed (n = 92) was 71% in patients with FLT3-mutant and 42% in patients with FLT3 wild-type. One PR occurred in a patient with FLT3-mutant receiving the 100-mg dose regimen. Both doses were well-tolerated; there were no differences in toxicity or response rate according to the dose of midostaurin.
Conclusion
These results suggest that midostaurin has hematologic activity in both patients with FLT3-mutant and wild-type. The degree of clinical activity observed supports additional studies that combine midostaurin and other agents such as chemotherapy especially in FLT3-mutant AML.
INTRODUCTION
Only 70% of younger adults and 40% to 50% of older adults with acute myeloid leukemia (AML) achieve complete remission using standard therapy.1,2 Standard therapy is nonspecific and of limited efficacy with a cure rate no higher than 40% in younger adults and lower than 10% in older adults.2 Therapeutic need and an enhanced understanding of AML biology have prompted the clinical development of molecularly targeted therapies including inhibitors of the FMS-like tyrosine kinase 3 receptor (FLT3).
FLT3 plays a critical role during hematopoeisis by regulating proliferation, differentiation, and apoptosis of cell progenitors. Approximately 30% of patients with AML harbor a mutation in FLT3, which results in constitutive activation. These mutations include internal tandem duplications (ITD), in which three to 60 amino acids are repeated in the juxtamembrane domain or in the tyrosine kinase domain 1 (occurring in approximately 25% of patients), and point mutations in the activating loop region (occurring in approximately 5% of patients).3–6 These mutations are capable of mediating growth factor independence in factor-dependent leukemia cell lines, and the FLT3-ITD produces a fatal myeloproliferative syndrome in murine models.7–9
Small molecule inhibitors of FLT3 are capable of specifically killing factor-independent transformed cell lines7 as well as increasing the survival of FLT3-mutant myeloproliferative syndrome mice.8 There are several small molecule inhibitors of FLT3 in development which demonstrate various degrees of FLT3 inhibition.10 However, potency alone may not be the key to antileukemic effect. Inhibition of other enzymes/pathways, a need for sufficient exposure to the leukemic stem cell and disease state (newly diagnosed or relapsed) may be equally important factors.10
A phase I study employing midostaurin in patients with solid tumors demonstrated a tolerable dose which led to enzyme inhibition in vivo.11 Subsequent studies determined that midostaurin inhibited activated FLT3 in the nanomolar range.7 A phase II trial wherein patients with FLT3-mutant were treated with midostaurin at a dose of 75-mg orally three times daily demonstrated a ≥ 50% reduction from baseline in peripheral blood and/or bone marrow blast count in 14 (70%) of 20 patients.12 Seven patients achieved a greater than 2-log reduction in blast count for more than 4 weeks; one patient achieved a near complete remission (bone marrow cellularity was 10%). Overall, midostaurin was generally well-tolerated.
Ancillary studies documented inhibition of autophosphorylation of FLT3; however, the primary antileukemia mechanism of action of midostaurin, which also inhibits other tyrosine kinases, such as c-kit and platelet-derived growth factor receptor,13 remains uncertain. As FLT3 is overexpressed in many patients with AML, it is possible that inhibition of FLT3 could have therapeutic benefit in patients with either mutant or wild-type FLT3. In an effort to further understand the efficacy of this agent in a wide variety of patients with AML or myelodysplastic syndrome (MDS), we conducted a phase IIB, randomized trial at two dose levels (50- or 100-mg orally twice daily) in patients with both mutant and wild-type FLT3. Pharmacokinetic studies showed that the more convenient twice daily dosing schedule would achieve sufficient plasma concentrations to inhibit the target.14
PATIENTS AND METHODS
Objectives
The primary objective of the study was to determine the safety, tolerability, and efficacy of two doses of midostaurin in patients with AML and high-risk MDS with either wild-type or mutated FLT3. Secondary objectives included an evaluation of the pharmacokinetics of midostaurin and its metabolites in peripheral blood.
Trial Design
This trial (NCT00045942) available at www.clinicaltrials.gov was an open-label, randomized, phase II study of midostaurin monotherapy in patients with AML/high-risk MDS with either wild-type or mutated FLT3. Patients were randomly assigned to either 50- or 100-mg orally twice daily. Patients continued therapy until the occurrence of disease progression or unacceptable toxicity. All authors had access to the primary clinical trial data.
Study Population
Patients with AML were required to have relapsed/refractory AML or be ineligible to receive standard chemotherapy. Patients with MDS with subtypes refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, or chronic myelomonocytic leukemia were eligible. Patients were required to have their FLT3 mutation status determined before enrollment and not have been previously treated with a FLT3 inhibitor. Further, patients were required to be ≥ 18 years of age, have a WHO performance state of 0 to 2 and life expectancy of at least 3 months, be recovered from prior cytotoxic chemotherapy (treatment with hydroxyurea was allowed up to 7 days before day 1 of study drug administration), have a serum creatinine of ≤ 1.5 × upper limit of normal (ULN), AST/ALT ≤ 3 × ULN, and total bilirubin ≤ 2.0 × ULN, and provide written informed consent. In addition, patients must not have had a prior allogeneic, syngeneic, or autologous bone marrow transplant within 2 months of enrolling in the study; female patients or adults of child-bearing age not employing an effective method of birth control were not eligible. Grounds for ineligibility also included severe and/or uncontrolled medical or psychiatric conditions, impairment of GI function or GI disease that could significantly alter the absorption of midostaurin, more than two prior regimens for the current relapse or primary refractory disease, an active uncontrolled infection, or any pulmonary infiltrate on baseline chest x-ray known to be new in the previous 4 weeks. The study protocol was approved by institutional review boards and conducted in accordance with good clinical practice and the guiding principles of the Declaration of Helsinki. Written informed consent was obtained from all subjects at prescreening.
Treatment Plan
Midostaurin was administered orally in 25-mg capsules. Patients with wild-type FLT3 were randomly assigned to receive midostaurin by continuous oral twice daily doses of 50 or 100 mg. Patients were enrolled in cohorts of 10 to a maximum of 30 patients. Patients with mutant FLT3 were randomly assigned to receive midostaurin by continuous oral twice daily doses of 50 or 100 mg. Patients were enrolled in cohorts of 10 followed by two cohorts of five to a maximum of 20 patients. The smaller size of the cohort of patients with mutant blasts was planned due to the longer expected timeframe required to accrue patients. The decision to continue or terminate accrual was made after evaluating each cohort.15,16 Midostaurin was continued until disease progression or unacceptable toxicity and was withheld in the event of a grade 3 or 4 nonhematologic toxicity considered to be study drug-related. If this toxicity occurred at 100-mg twice daily, dose reduction to 50-mg twice daily was allowed once toxicity resolved to grade 1 within 14 days. Patients who experienced grade 3 or 4 nonhematologic toxicities at the lower dose which did not resolve within 14 days were taken off the study. There were no dose modifications for hematologic toxicity. No other medicine for AML (except for hydroxyurea as noted above) or MDS was allowed during the study (4 weeks before the first dose of study drug to the last day of study medication). Prophylaxis was allowed for prevention of nausea and vomiting. Any drug known to inhibit or induce CYP3A4 could not be used due to possible pharmacologic interaction with midostaurin.
Pharmacokinetic data were obtained during 4 days of the first 5 weeks of therapy. Disease assessment by means of bone marrow examination was carried out monthly. Clinical response included complete remission (CR), partial remission (PR), hematologic improvement (HI), and blast response (BR). Definitions of CR and PR were per the International Working Group guidelines for AML.17 Definitions of HI were taken from the guidelines for response in MDS.18 BR was defined as a reduction of 50% or greater in the percentage of bone marrow blasts or the absolute number of peripheral blood blasts, although these two types of BR are reported separately.
Pharmacokinetics
Blood samples were drawn on day 1 (before the first dose and 4 hours after the first dose), day 2 (24 hours after the first dose), and before dosing on days 3 and 8 of cycle 1 and on day 1 of each subsequent cycle. The concentrations of midostaurin and metabolites CGP52421 (e1 and e2 epimers of the monohydroxy metabolite) and CGP62221 (desmethyl metabolite) in plasma samples were determined using the liquid chromatography tandem mass spectrometry method (limits of quantification = 9 nmol/L) at Novartis societe anonyme, Rueil-Malmaison, France.
RESULTS
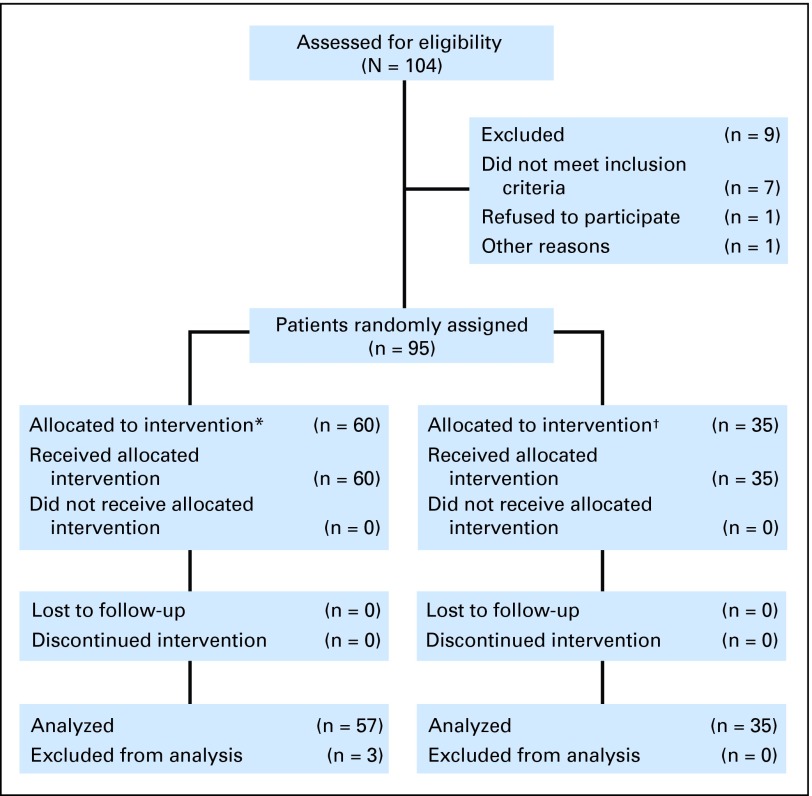
Of the 95 enrolled patients, 85 (89%) had AML, 49 (52%) were male, 84 (88%) were white, and 61 (64%) were at least 65 years old (Table 1). The blasts of 35 patients (37%) harbored FLT3 mutations, and of these, ITDs were detected in 26 (74%). The myeloid malignancies in three (9%) of the FLT3 mutated and 23 (38%) of the patients with wild-type FLT3 were previously untreated (Table 1). All 95 enrolled patients received at least one dose of study drug and are included in the analysis of toxicity. Three patients with FLT3 wild-type treated at 100 mg were not included in the efficacy population; one had a major protocol violation and the other two failed to complete 8 days of cycle 1 due to adverse events. Therefore, 92 patients were analyzed for efficacy (35 FLT3-mutant, 57 wild-type; Tables 2, 3 and Figs 1 and 2).
Table 1.
Patient Characteristics
| Characteristic | FLT3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutant | Wild-Type | |||||||||||
| 50 mg Twice Daily | 100 mg Twice Daily | Total | 50 mg Twice Daily | 100 mg Twice Daily | Total | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| No. of patients | 18 | 17 | 35 | 31 | 29 | 60 | ||||||
| Male sex | 13 | 72 | 8 | 47 | 21 | 60 | 15 | 48 | 13 | 45 | 28 | 47 |
| White race/ethnicity | 16 | 89 | 15 | 88 | 31 | 89 | 28 | 90 | 25 | 86 | 53 | 88 |
| Age ≥ 65 years | 7 | 39 | 9 | 53 | 16 | 46 | 24 | 77 | 21 | 72 | 45 | 75 |
| Performance status of 0 or 1 | 15 | 83 | 11 | 65 | 26 | 74 | 27 | 87 | 23 | 79 | 50 | 83 |
| MDS | 3 | 17 | 0 | 3 | 9 | 3 | 10 | 4 | 14 | 7 | 12 | |
| Previously treated* | 18 | 100 | 14 | 82 | 32 | 91 | 18 | 58 | 19 | 66 | 37 | 62 |
| WBC > 50, ×109/L | 4 | 22 | 4 | 24 | 8 | 23 | 0 | 2 | 7 | 2 | 3 | |
| WBC median, ×109/L | 15.4 | 25.1 | 18.10 | 4.2 | 3.2 | 3.9 | ||||||
| Minimum-maximum | 1.1-85.5 | 0.7-97.1 | 0.7-97.1 | 0.9-49.7 | 0.9-69.8 | 0.9-69.8 | ||||||
| Abnormal cytogenetics | 10 | 56 | 8 | 47 | 18 | 51 | 19 | 61 | 16 | 55 | 35 | 58 |
| FLT3 ITD | 12 | 67 | 14 | 82 | 26 | 74 | NA | NA | NA | |||
| FLT3 D835Y | 6 | 33 | 3 | 18 | 9 | 26 | NA | NA | NA |
Table 2.
Clinical Response
| Response* | FLT3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutant | Wild-Type | |||||||||||
| 50 mg Twice Daily | 100 mg Twice Daily | Total | 50 mg Twice Daily | 100 mg Twice Daily | Total | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| No. | 18 | 17 | 35 | 30 | 27 | 57 | ||||||
| PR | 0 | 1 | 6 | 1 | 3 | 0 | 0 | 0 | ||||
| Hematologic improvement | 9 | 50 | 7 | 41 | 16 | 46 | 13 | 43 | 7 | 26 | 20 | 35 |
| Blast response | ||||||||||||
| Total | 12 | 67 | 13 | 76 | 25 | 71 | 15 | 50 | 9 | 33 | 24 | 42 |
| PB and bone marrow | 5 | 42 | 1 | 8 | 6 | 24 | 4 | 27 | 2 | 22 | 6 | 25 |
| Bone marrow only | 0 | 4† | 24 | 4 | 11 | 2 | 13 | 1 | 11 | 3 | 13 | |
| PB only | 7 | 58 | 8 | 62 | 15 | 60 | 9 | 60 | 6 | 67 | 15 | 63 |
| 2-log PB blast reduction | 8 | 44 | 9 | 53 | 17 | 49 | 11 | 37 | 8 | 30 | 19 | 33 |
| Overall response | ||||||||||||
| (CR, PR, HI, BR) | 12 | 67 | 13 | 76 | 25 | 71 | 20 | 67 | 12 | 44 | 32 | 56 |
Table 3.
Blast Response Rates
| Baseline Characteristic: Blast Response | FLT3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutant | Wild-Type | |||||||||||
| 50 mg Twice Daily | 100 mg Twice Daily | Total | 50 mg Twice Daily | 100 mg Twice Daily | Total | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| No. | 18 | 17 | 35 | 30 | 27 | 57 | ||||||
| Previously treated* | 12 of 18 | 67 | 10 of 14 | 71 | 22 of 32 | 69 | 8 of 17 | 47 | 5 of 18 | 28 | 13 of 35 | 37 |
| Previously untreated | 0 | 3 of 3 | 100 | 3 of 3 | 100 | 7 of 13 | 54 | 4 of 9 | 44 | 11 of 22 | 50 | |
| FLT3 ITD | 8 of 12 | 67 | 11 of 14 | 79 | 19 of 26 | 73 | NA | NA | NA | |||
| FLT3 D835Y | 4 of 6 | 67 | 2 of 3 | 67 | 6 of 9 | 67 | NA | NA | NA | |||
| AML | 11 of 15 | 73 | 13 of 17 | 76 | 24 of 32 | 75 | 13 of 27 | 48 | 9 of 24 | 38 | 22 of 51 | 43 |
| MDS | 1 of 3 | 33 | 0 | 1 of 3 | 33 | 2 of 3 | 67 | 0 of 3 | 0 | 2 of 6 | 33 |
Fig 1.
CONSORT diagram.
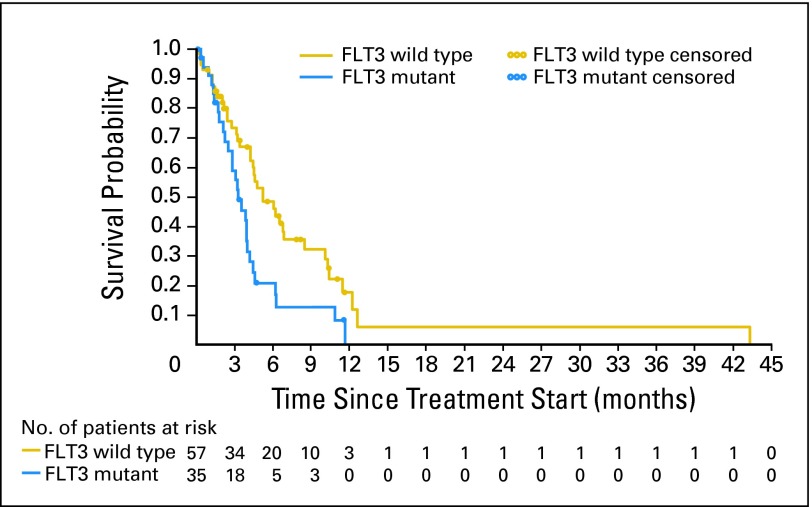
Fig 2.
Kaplan-Meier plot of overall survival according to FMS-like tyrosine kinase 3 receptor (FLT3) mutation status. All reported deaths that occurred in the efficacy population after first dose of study drug are included. Censoring (26 patients) took place within a period of 9 months from study completion with a mean post-treatment follow-up of 41 days (standard deviation, 75).
Both patients with FLT3-mutant and wild-type responded to midostaurin (Table 2). Only one patient (1%) experienced a PR, as previously defined.18 HI was achieved in 16 (46%) of 35 patients with FLT3-mutant and 20 (35%) of 57 patients with FLT3 wild-type. Blast reductions were frequently observed, occurring in 71% of patients with FLT3-mutant and 42% of patients with FLT3 wild-type (Table 2). Peripheral blood blast reductions of 2-logs or greater were seen in 49% of patients with FLT3-mutant and 33% of patients with FLT3 wild-type (Table 2). Within the FLT3-mutant group, 22 of 32 (69%) relapsed or refractory (ie, previously treated) patients displayed a BR, compared to three (100%) of three who were previously untreated (Table 3). Within the FLT3 wild-type group, 13 (37%) of 35 relapsed or refractory patients displayed a BR, compared with 11 (50%) of 22 who were previously untreated. There were no clear differences in the blast responses according to type of FLT3 mutation or dose of study drug (Table 3).
Although patients' blasts typically decreased within 1 week of starting treatment, onset of blast response (reaching 50% reduction below baseline) occurred at a median of 29 days for both FLT3-mutant (96% occurred on cycle 2 day 1 or before) and wild-type (83% occurred on cycle 2 day 1 or before) groups. Kaplan-Meier estimates for median time to treatment failure, defined as disease progression or study discontinuation for reasons potentially related to treatment failure (ie, death or adverse event) were 50 days (95% CI, 36 to 65) in the FLT3-mutant group, and 56 days (95% CI, 38 to 68) in the FLT3 wild-type group. Median time to treatment failure for the blast responders in the FLT3-mutant group was 60 days (95% CI, 44 to 85) compared to 83 days (95% CI, 57 to 115) in the wild-type group.
Based on post-treatment follow-up data, the Kaplan-Meier estimate for median survival for the entire group was 130 days (95% CI, 105 to 159), which is a reflection of the poor prognosis of the patients with AML and MDS treated in this study. Figure 2 depicts the overall survival by FLT3 status and includes all reported deaths that occurred in the efficacy population after first dose of study drug. Twenty-eight deaths (80%) occurred in the mutant population with a median survival of 100 days (95% CI, 76 to 121) and 38 deaths (67%) occurred in the wild-type population with median survival of 159 days (95% CI, 130 to 209). Twenty-six patients were censored (censoring occurred at the date the patient was last known to be alive) within a period of 9 months from study completion with mean post treatment follow-up of 41 (standard deviation, 75) days.
Midostaurin was generally well-tolerated at both dose levels (Table 4). It is difficult to attribute adverse events (AEs) and particularly hematologic AEs, to drug or disease due to the poor baseline condition of this patient population. Mild to moderate nausea and vomiting was common; however, there was no apparent association between AEs and dose or the patient's mutational status. Within the overall population, 28 deaths (18 in the 50-mg twice daily dose group; 10 in the 100-mg twice daily group) occurred within 28 days after last dose of study treatment. The most common causes of death were disease progression (n = 17), sepsis (n = 3), and one report each of renal insufficiency, cardiac failure, splenic infarct, general health deterioration, intracranial bleed, liver failure, multisystem organ failure, and unknown cause.
Table 4.
Adverse Events
| Event* | 50 mg Twice Daily (n = 49) | 100 mg Twice Daily (n = 46) | Total (N = 95) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1-2 | Grade 3-4 | Grade 1-2 | Grade 3-4 | Grade 1-2 | Grade 3-4 | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Nausea | 29 | 59 | 1 | 2 | 28 | 61 | 0 | 57 | 60 | 1 | 1 | |
| Vomiting | 24 | 49 | 0 | 22 | 48 | 1 | 2 | 46 | 48 | 1 | 1 | |
| Diarrhea | 13 | 27 | 2 | 4 | 23 | 50 | 3 | 7 | 36 | 38 | 5 | 5 |
| Fatigue | 19 | 39 | 1 | 2 | 14 | 30 | 1 | 2 | 33 | 35 | 2 | 2 |
| Pyrexia | 13 | 27 | 2 | 4 | 12 | 26 | 5 | 11 | 25 | 26 | 7 | 7 |
| Dyspnea | 11 | 22 | 3 | 6 | 9 | 20 | 4 | 9 | 20 | 21 | 7 | 7 |
| Hypokalemia | 13 | 27 | 2 | 4 | 7 | 15 | 2 | 4 | 20 | 21 | 4 | 4 |
| Febrile neutropenia | 3 | 6 | 9 | 18 | 2 | 4 | 9 | 20 | 5 | 5 | 18 | 19 |
| Pneumonia | 8 | 16 | 6 | 12 | 5 | 11 | 3 | 6 | 13 | 14 | 9 | 10 |
| Peripheral edema | 10 | 20 | 0 | 9 | 20 | 2 | 4 | 19 | 20 | 2 | 2 | |
| Asthenia | 8 | 16 | 2 | 4 | 8 | 17 | 0 | 16 | 17 | 2 | 2 | |
| Constipation | 4 | 8 | 0 | 12 | 26 | 2 | 4 | 16 | 17 | 2 | 2 | |
| Cough | 10 | 20 | 1 | 2 | 6 | 13 | 1 | 2 | 16 | 17 | 2 | 2 |
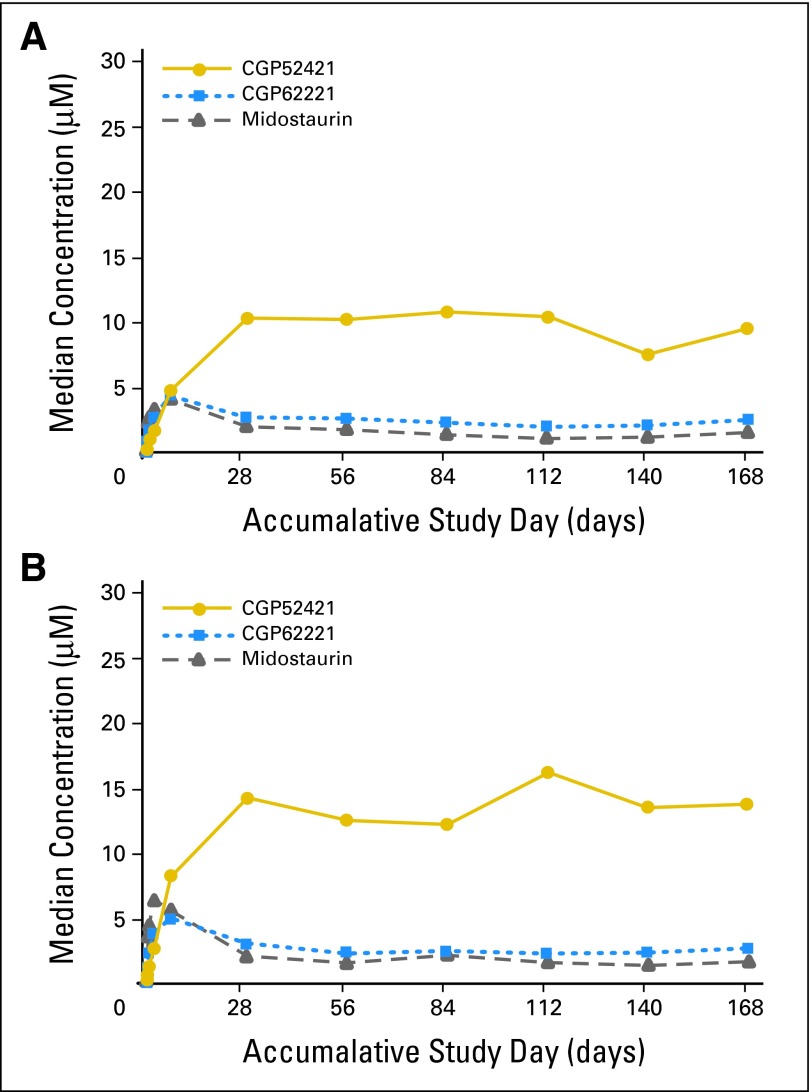
Once in the liver, midostaurin is metabolized by CYP3A4 into two major metabolites, CGP62221 (via O-demethylation) and CGP52421 (via 7-hydroxylation). Blood samples for pharmacokinetic analysis were collected from 45 (50 mg) and 42 (100 mg) patients. After multiple oral doses of 50- or 100-mg twice daily, midostaurin and CGP62221 concentrations accumulated significantly in the first 3 to 5 days, and then declined by approximately 40% to 80% before reaching a new steady-state between 2 and 3 weeks post dose (Fig 3). CGP52421 concentrations accumulated continuously through day 28 and remained stable thereafter, achieving concentrations roughly 7 times higher than that of either the parent drug or CGP62221 (Fig 3). The average trough concentrations observed at the 50- and 100-mg doses were as follows (± standard deviation): midostaurin (1.25 μmol/L ± 0.35, 1.48 μmol/L ± 0.34), CGP62221 (2.08 μmol/L ± 0.29, 2.27 μmol/L ± 0.30), and CGP52421 (9.61 μmol/L ± 1.16, 13.6 μmol/L ± 1.42). Midostaurin trough concentrations were less than dose proportional between the 50- and 100-mg twice daily doses.14 However, the steady-state levels of midostaurin and the metabolites reached at 2 to 3 weeks were above the 50% inhibitory concentration for FLT3 inhibition obtained in cell-based assays.13 Although CGP52421 is believed to induce metabolism of midostaurin and CGP62221,19 this is not considered to interfere with activity of midostaurin. The time-dependent kinetics of midostaurin may have resulted from an induction of CYP3A4 by midostaurin and/or its metabolites.19 However, a protein binding displacement mechanism cannot be excluded. The blast response correlated both with midostaurin plasma levels alone and summed together with its two major metabolites, corrected for their in vitro potency differences. Midostaurin plasma concentration declined over time, which could contribute to disease relapse during drug exposure.20 The pharmacokinetic data will be reported separately in more detail.
Fig 3.
Plasma concentrations of midostaurin and metabolites. Median trough plasma concentration-time profiles of midostaurin and its metabolites, CGP62221 and CGP52421, after oral dose of (A) 50 mg (n = 45) and (B) 100 mg (n = 42) midostaurin twice a day in patients with wild-type or mutated FMS-like tyrosine kinase 3 receptor acute myeloid leukemia.
DISCUSSION
This study corroborates and expands the results of a 20-patient proof-of-concept trial of midostaurin treatment in patients with FLT3-mutant AML.12 An activating mutation of FLT3 appears to contribute to leukemogenesis in these patients, and also confers a negative prognostic impact. It is therefore significant that the majority of patients with FLT3-mutant treated with midostaurin responded with reductions in blast counts. Biologic responses also occurred in patients with no documented mutation of FLT3, but with a lower frequency compared with the FLT3-mutant population. The responses seen were not sustained or profound enough to reach a CR status or be associated with major clinical benefit in the wild-type–FLT3 patient groups. Nonetheless, this study provides important lessons that may be useful in the development of FLT3 inhibitors as therapeutic agents in myeloid leukemias. First, the drug is relatively well-tolerated at these two doses. There was no apparent difference in toxicity between patient groups receiving either 50- or 100-mg orally twice daily. As midostaurin can inhibit multiple enzyme pathways presumptively involved in the control of cell proliferation, including c-kit, platelet-derived growth factor receptor, and protein kinase C,13 there was a concern that toxicity might be more widespread. In particular, interstitial lung disease has occurred with the epidermal growth factor kinase inhibitors used in lung cancer,18 and unexplained severe pulmonary events occurred in the proof-of-concept trial.12 It was thus reassuring that severe pulmonary toxicity was rare in this 95-patient trial.
Several hypotheses may explain why midostaurin's effect is mainly limited to BR in patients with FLT3-mutant and wild-type with AML. First, there may be alternative pathways that promote survival even when FLT3 signaling is inhibited. Second, locally elaborated survival signals within the stem-cell niche may operate to protect leukemia clones.21 Finally, sufficient free drug may not be available due to a high level of protein binding. Nevertheless, the consistently high BR in patients with FLT3-mutant AML observed in the proof-of-concept12 and this study indicate midostaurin may have important biologic activity in FLT3-mutant AML. As suggested by the higher response rate in patients who were previously untreated in this trial, midostaurin might be particularly effective in the newly diagnosed patient population. This finding is not surprising in light of in vitro studies which suggest that agents with broader activity, such asmidostaurin, may have greater clinical utility in newly diagnosed patients whose blasts tend to be less addicted to FLT3-mediated signaling than those in relapsed patients.10
Data in support of the importance of FLT3 inhibition in mediating a response to midostaurin, at least in patients with mutant FLT3 has been previously reported. First, the ability of plasma obtained from patients on FLT3 inhibitor therapy to inhibit FLT3 autophosphorylation (plasma inhibitory activity) in vitro correlates with decline in blasts based on data derived in part from the proof-of concept12 part of this trial.22 An in vivo pharmacodynamic assessment of FLT3 inhibition in blasts obtained serially from patients on this trial was only technically possible in such a small subset of patients that conclusions were not possible, though in the proof-of concept trial, downregulation of FLT3 autophosphorylation was observed in some patients whose blasts declined.12 A successful pharmacodynamic study and/or a thorough analysis of the correlation between response and plasma inhibitory activity in both FLT3-mutant and FLT3 wild-type AML (adjusted for allelic burden which could also play a role in response) would have been ideal in this trial. Secondly, a responding patient who subsequently lost his response developed a new FLT3 mutation incapable of binding midostaurin.23 Although we did not perform expression profiling in this study, AML in certain patients with FLT3–wild-type may depend on FLT3-mediated signaling as shown by the presence of an FLT3 activation signature similar to what is seen in most, but not all, patients with a FLT3 ITD.24 Preclinical studies documenting a synergistic antileukemia effect of chemotherapy with FLT3 inhibitors suggests utility in combining these treatments in clinical studies.25 Final results from a study26 examining the treatment of patients with AML using standard chemotherapy in combination with midostaurin will soon be available.
Acknowledgment
We thank Susanna Hamilton and Sigitas Verselis of the Dana-Farber Cancer Institute for determining the FLT3 status in patient samples. Medical writing assistance was provided by Michelle Boehm of Articulate Science, LLC.
Footnotes
See accompaning article on page 4333
Supported by Novartis Pharmaceuticals.
Presented in part at the 45th Annual Meeting of the American Society of Hematology, December 6-9, 2003, San Diego, CA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00045942.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: D. Gary Gilliland, Merck (C); Catherine Dutreix, Novartis (C); Jodi Virkus, Novartis (C) Consultant or Advisory Role: Richard M. Stone, Novartis (C), Genzyme (C), Celgene (C); Daniel J. DeAngelo, Novartis (C); Gary J. Schiller, Vion (C), Millenium (C), Genzyme (C), Novartis (C); Francis J. Giles, Novartis (C) Stock Ownership: Alice Huntsman-Labed, Novartis; Jodi Virkus, Novartis Honoraria: Thomas Fischer, Novartis; Daniel J. DeAngelo, Novartis; Gerhard Ehninger, Novartis; Francis J. Giles, Novartis Research Funding: Richard M. Stone, Novartis; Gary J. Schiller, Novartis, Celgene, Millenium; Virginia M. Klimek, Novartis; Francis J. Giles, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Richard M. Stone, Daniel J. DeAngelo, Gerhard Ehninger, Francis J. Giles
Provision of study materials or patients: Thomas Fischer, Richard M. Stone, Daniel J. DeAngelo, Ilene Galinsky, Elihu Estey, Gerhard Ehninger, Eric J. Feldman, Gary J. Schiller, Virginia M. Klimek, Stephen D. Nimer, Francis J. Giles
Collection and assembly of data: Thomas Fischer, Richard M. Stone, Ilene Galinsky, Edward Fox, Virginia M. Klimek, D. Gary Gilliland
Data analysis and interpretation: Thomas Fischer, Richard M. Stone, Carlo Lanza, Edward Fox, Gerhard Ehninger, Stephen D. Nimer, D. Gary Gilliland, Catherine Dutreix, Alice Huntsman-Labed, Francis J. Giles
Manuscript writing: Thomas Fischer, Richard M. Stone, Daniel J. DeAngelo, Carlo Lanza, Alice Huntsman-Labed, Jodi Virkus, Francis J. Giles
Final approval of manuscript: Thomas Fischer, Richard M. Stone, Daniel J. DeAngelo, Ilene Galinsky, Elihu Estey, Carlo Lanza, Edward Fox, Gerhard Ehninger, Eric J. Feldman, Gary J. Schiller, Virginia M. Klimek, Stephen D. Nimer, D. Gary Gilliland, Catherine Dutreix, Alice Huntsman-Labed, Jodi Virkus, Francis J. Giles
REFERENCES
- 1.Burnett AK, Goldstone AH, Stevens RM, et al. Randomised comparison of addition of autologous bone-marrow transplantation to intensive chemotherapy for acute myeloid leukaemia in first remission: Results of MRC AML 10 trial: UK Medical Research Council Adult and Children's Leukaemia Working Parties. Lancet. 1998;351:700–708. doi: 10.1016/s0140-6736(97)09214-3. [DOI] [PubMed] [Google Scholar]
- 2.Stone RM. The difficult problem of acute myeloid leukemia in the older adult. CA Cancer J Clin. 2002;52:363–371. doi: 10.3322/canjclin.52.6.363. [DOI] [PubMed] [Google Scholar]
- 3.Breitenbuecher F, Schnittger S, Grundler R, et al. Identification of a novel type of ITD mutations located in nonjuxtamembrane domains of the FLT3 tyrosine kinase receptor. Blood. 2009;113:4074–4077. doi: 10.1182/blood-2007-11-125476. [DOI] [PubMed] [Google Scholar]
- 4.Kayser S, Schlenk RF, Londono MC, et al. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood. 2009;114:2386–2392. doi: 10.1182/blood-2009-03-209999. [DOI] [PubMed] [Google Scholar]
- 5.Gilliland DG, Griffin JD. Role of FLT3 in leukemia. Curr Opin Hematol. 2002;9:274–281. doi: 10.1097/00062752-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Levis M, Small D. Novel FLT3 tyrosine kinase inhibitors. Expert Opin Investig Drugs. 2003;12:1951–1962. doi: 10.1517/13543784.12.12.1951. [DOI] [PubMed] [Google Scholar]
- 7.Weisberg E, Boulton C, Kelly LM, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1:433–443. doi: 10.1016/s1535-6108(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 8.Kelly LM, Yu JC, Boulton CL, et al. CT53518, a novel selective FLT3 antagonist for the treatment of acute myelogenous leukemia (AML) Cancer Cell. 2002;1:421–432. doi: 10.1016/s1535-6108(02)00070-3. [DOI] [PubMed] [Google Scholar]
- 9.Kelly LM, Liu Q, Kutok JL, et al. FLT3 internal tandem duplication mutations associated with human acute myeloid leukemias induce myeloproliferative disease in a murine bone marrow transplant model. Blood. 2002;99:310–318. doi: 10.1182/blood.v99.1.310. [DOI] [PubMed] [Google Scholar]
- 10.Pratz KW, Sato T, Murphy KM, et al. FLT3 mutant allelic burden and clinical status are predictive of response to FLT3 inhibitors in AML. Blood. 2009;115:1425–1432. doi: 10.1182/blood-2009-09-242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Propper DJ, McDonald AC, Man A, et al. Phase I and pharmacokinetic study of PKC412, an inhibitor of protein kinase C. J Clin Oncol. 2001;19:1485–1492. doi: 10.1200/JCO.2001.19.5.1485. [DOI] [PubMed] [Google Scholar]
- 12.Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 13.Manley PW, Boulton C, Caravatti G, et al. Preclinical profile of PKC412 (midostaurin) as an FLT3 inhibitor for the therapy of AML. Presented at the American Association for Cancer Research Annual Meeting; July 11-14, 2003; Washington, DC. poster 1004. [Google Scholar]
- 14.Yin O, Wang Y, Lanza C, et al. Pharmacokinetics (PK) and pharmacodynamics (PD) of midostaurin (PKC412) in patients with acute myeloid leukemia (AML) J Clin Oncol. 2008;26:388s. abstr 7064. [Google Scholar]
- 15.Estey EH, Thall PF. New designs for phase 2 clinical trials. Blood. 2003;102:442–448. doi: 10.1182/blood-2002-09-2937. [DOI] [PubMed] [Google Scholar]
- 16.Thall PF, Estey EH. A bayesian strategy for screening cancer treatments prior to phase II clinical evaluation. Stat Med. 1993;12:1197–1211. doi: 10.1002/sim.4780121303. [DOI] [PubMed] [Google Scholar]
- 17.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Bennett JM, Kantarjian H, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96:3671–3674. [PubMed] [Google Scholar]
- 19.Yin OQ, Wang Y, Schran H. A mechanism-based population pharmacokinetic model for characterizing time-dependent pharmacokinetics of midostaurin and its metabolites in human subjects. Clin Pharmacokinet. 2008;47:807–816. doi: 10.2165/0003088-200847120-00005. [DOI] [PubMed] [Google Scholar]
- 20.Dutreix C, Huntsman Labed A, Roesel J, et al. Midostaurin: Review of pharmacokinetics (PK) and PK/pharmacodynamic (PD) relationship in AML/MDS patients. J Clin Oncol. 2009;27 abstr e14540. [Google Scholar]
- 21.Andreeff M, McQueen T, Williams C, et al. Clonal abnormalities in MSC derived from AML bone marrows. Blood. 2008:112. abstr 2428. [Google Scholar]
- 22.Levis M, Brown P, Smith BD, et al. Plasma inhibitory activity (PIA): A pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood. 2006;108:3477–3483. doi: 10.1182/blood-2006-04-015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heidel F, Solem FK, Breitenbuecher F, et al. Clinical resistance to the kinase inhibitor PKC412 in acute myeloid leukemia by mutation of asn-676 in the FLT3 tyrosine kinase domain. Blood. 2006;107:293–300. doi: 10.1182/blood-2005-06-2469. [DOI] [PubMed] [Google Scholar]
- 24.Bullinger L, Dohner K, Kranz R, et al. An FLT3 gene-expression signature predicts clinical outcome in normal karyotype AML. Blood. 2008;111:4490–4495. doi: 10.1182/blood-2007-09-115055. [DOI] [PubMed] [Google Scholar]
- 25.Levis M, Pham R, Smith BD, et al. In vitro studies of a FLT3 inhibitor combined with chemotherapy: Sequence of administration is important to achieve synergistic cytotoxic effects. Blood. 2004;104:1145–1150. doi: 10.1182/blood-2004-01-0388. [DOI] [PubMed] [Google Scholar]
- 26.Stone RM, Fischer T, Paquette R, et al. A phase 1b study of midostaurin (PKC412) in combination with daunorubicin and cytarabine induction and high-dose cytarabine consolidation in patients under age 61 with newly diagnosed de novo acute myeloid leukemia: Overall survival of patients whose blasts have FLT3 mutations is similar to those with wild-type FLT3. Blood. 2009:114. abstr 634. [Google Scholar]