Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells (original) (raw)
Significance
Recent clinical trials have shown highly promising responses in a subset of patients treated with immune checkpoint inhibitory anti–programmed cell death-1, anti–programmed cell death ligand-1, and anti–cytotoxic T-lymphocyte-associated antigen-4 antibodies, but the majority of patients in these trials remained unresponsive. Our results show that elevated myeloid-derived suppressor cells(MDSCs) are responsible for the resistance and that elimination of MDSCs can lead to cures of experimental, metastatic tumors.
Keywords: 5-azacytidine, entinostat, methyltransferase, HDAC, exome
Abstract
Impressive responses have been observed in patients treated with checkpoint inhibitory anti–programmed cell death-1 (PD-1) or anti–cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) antibodies. However, immunotherapy against poorly immunogenic cancers remains a challenge. Here we report that treatment with both anti–PD-1 and anti–CTLA-4 antibodies was unable to eradicate large, modestly immunogenic CT26 tumors or metastatic 4T1 tumors. Cotreatment with epigenetic-modulating drugs and checkpoint inhibitors markedly improved treatment outcomes, curing more than 80% of the tumor-bearing mice. Functional studies revealed that the primary targets of the epigenetic modulators were myeloid-derived suppressor cells (MDSCs). A PI3K inhibitor that reduced circulating MDSCs also eradicated 4T1 tumors in 80% of the mice when combined with immune checkpoint inhibitors. Thus, cancers resistant to immune checkpoint blockade can be cured by eliminating MDSCs.
The mammalian immune system is delicately regulated, allowing it to mount an effective attack against foreign invaders such as bacteria and viruses with minimal bystander casualties. This requires functionally redundant regulatory mechanisms to ensure safety (1–3). Cancers appear able to hijack these mechanisms to avoid immune destruction. Several of the regulatory mechanisms exploited by cancer have been identified. These include regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages and neutrophils, immune checkpoint pathways, and immunosuppressive cytokines (4–8). Most recently, the checkpoints guarded by the programmed cell death-1 (PD-1) and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) receptors have been under intense investigation because of the availability of antibodies that can inhibit their function. Recent clinical trials with anti–CTLA-4, anti–PD-1, and anti–PD-L1 monoclonal antibodies showed remarkable therapeutic responses (9–12), underscoring the idea that disruption of immune checkpoints can be therapeutically useful. However, the objective responses were observed in a minority of the treated patients and tumor types, and the reasons why certain tumors respond and others do not are mysterious. CT26 and 4T1 are among the most popular syngeneic tumor models used for assessing novel therapeutic approaches. CT26 was derived from an undifferentiated colorectal carcinoma induced in a BALB/c mouse by repeated intrarectal instillations of _N_-nitroso-_N_-methylurethan and shown to be modestly immunogenic (13, 14), whereas 4T1 originated from a spontaneous mammary tumor in a BALB/c mouse (15). 4T1 is poorly immunogenic and highly metastatic, characteristics shared with advanced human cancers (16). Despite the extensive use of these tumor cell lines in cancer research, little genetic characterization is available for either of them.
In the current study, we evaluated both models with respect to their responses to the immune checkpoint inhibitors alone and combined with other agents. We also determined the sequences of their coding genes and mutant peptides that bind to the class I major histocompatibility complex (MHC-I). We found that the more immunogenic CT26 cells had considerably more mutations than 4T1 cells, and that the majority of the tumors derived from CT26 cells could be cured by anti–PD-1 and anti–CTLA-4 antibodies. In contrast, 4T1-derived tumors could not generally be cured by these antibodies. Mechanistic studies led to the conclusion that MDSCs were interfering with our therapeutic attempts; reduction of these MDSCs allowed frequent cures of 4T1 tumors by PD-1/CTLA-4 inhibition, even when they were advanced and metastatic.
Results
Genetic Analysis.
We first sequenced the exomes (24,306 genes) of both CT26 and 4T1 cells. Eight and 3.5 Gb of generated sequence were mapped to the genome for CT26 and 4T1, respectively; 83.5% (CT26) and 72.3% (4T1) of bases in the targeted regions were covered by at least 10 unique reads in tumor DNA. Sequencing of the exomes revealed 683 and 47 somatic mutations in CT26 and 4T1, respectively (Dataset S1).
It has been shown that ∼10% of the mutant amino acids created by somatic mutations in human colorectal and breast cancers give rise to epitopes that are predicted to be recognized by the patient’s MHC-I alleles (17). To determine whether this was true for the murine colorectal (CT26) and breast (4T1) tumors, we mapped the somatically mutated epitopes to BALB/c MHC-I using established algorithms. Because such predictions are meaningful only if the mutant genes are expressed, we determined the transcriptomes of the two cell lines using RNA sequencing. Three hundred and fourteen of the 683 mutations detected in CT26 occurred in expressed genes, with 28 mutated epitopes predicted to bind with at least moderate affinity to H2-(d) MHC-I alleles found in BALB/c mice (Table S1 and Dataset S1). The 4T1 cells harbored 27 mutations in expressed genes, with only one predicted to bind to H2-(d) MHC-I alleles. These data are consistent with the suggestion that CT26 is more immunogenic than 4T1 because the former has more mutant epitopes. It is also consistent with the observation that human tumors associated with environmental mutagens (such as UV light and cigarette smoke) have more mutations than other tumors (18).
Effects of Immune Checkpoint Blockade.
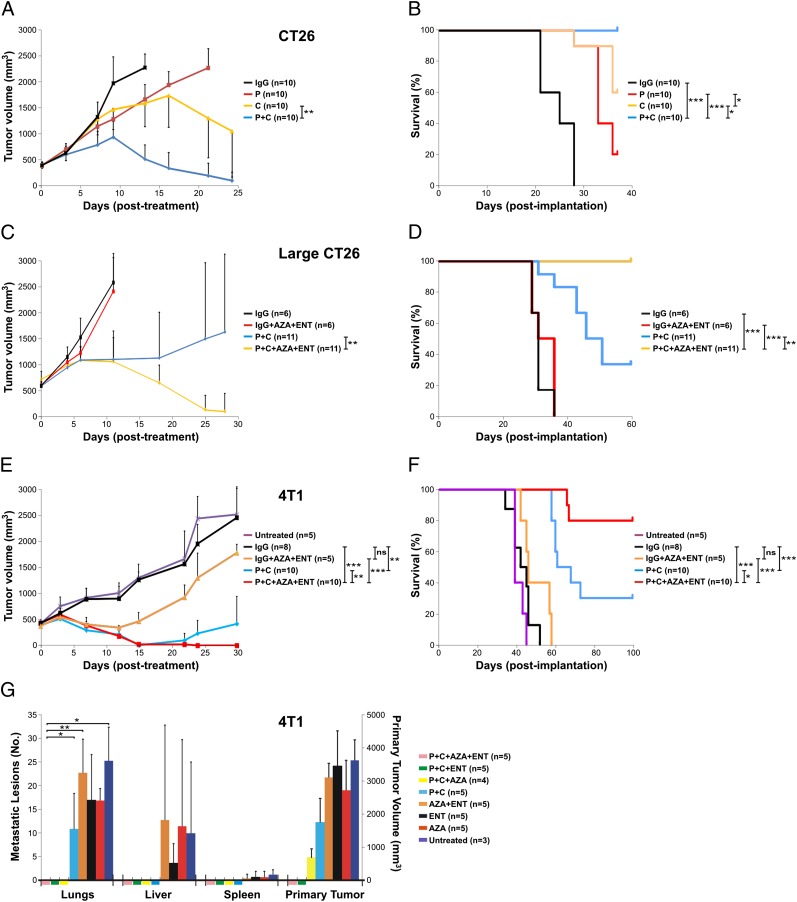
We then tested the effects of immune checkpoint-blocking antibodies on the tumors derived from these cells in mice. BALB/c mice bearing s.c. CT26 tumors of moderate sizes (∼400 mm3) were used for the initial experiments. Although repeated treatment with anti–CTLA-4 or anti–PD-1 antibodies as single agents retarded tumor growth, tumor eradication was not observed (Fig. 1 A and B). Combination therapy with both antibodies resulted in eradication of tumors in the vast majority of the mice. Conversely, tumors larger than 600 mm3 (large CT26) did not respond very well to the combined anti–PD-1/anti–CTLA-4 treatment (Fig. 1_C_), with only 4 out of 11 animals showing long-term survival (Fig. 1_D_).
Fig. 1.
Therapeutic response of tumor-bearing mice. BALB/c mice bearing different tumors were treated with various therapeutic modalities as indicated. C, anti–CTLA-4 antibody; IgG, IgG control; P, anti–PD-1 antibody. Tumor volumes (A, C, and E) and animal survival (B, D, and F) were recorded. (A and B) BALB/c mice with CT26 tumors of moderate sizes. (C and D) BALB/c mice with large CT26 tumors. (E and F) BALB/c mice with metastatic 4T1 tumors. (G) 4T1 tumor-bearing mice were treated as indicated and euthanized 6 wk after tumor implantation. The primary tumor from each mouse was measured and metastatic lesions in different organs were counted. Means and SDs are shown. The number of animals used in each experimental arm and P values are also indicated. *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant.
Next, BALB/c mice with well-established 4T1 tumors (∼400 mm3) were evaluated; these tumors spontaneously metastasize to the lungs and other organs. The 4T1 tumor model is highly recalcitrant to most therapeutic agents, including immunotherapy (16). The animals generally succumb to metastatic disease, even when the primary tumor is surgically removed (19). Only a small fraction of the primary tumors showed durable response to the antibody treatment: 3 out of 10 animals showed complete regression of their primary tumors when treated with both anti–PD-1 and anti–CTLA-4 antibodies, and these were the only long-term survivors (Fig. 1 E and F).
Epigenetic Modulation.
We hypothesized that the tumors in the animals that had not been cured might have down-regulated the expression of MHC-I–related genes through epigenetic silencing in tumor cells. Indeed, this hypothesis forms the basis for therapies involving epigenetic modulation (20), using inhibitors of either DNA methyltransferase or histone deacetylase (HDAC). To evaluate this possibility, we treated animals bearing large CT26 tumors (>600 mm3) as described above with anti–PD-1/anti–CTLA-4 antibodies as well as 5-azacytidine (AZA; a DNA methyltransferase inhibitor) and entinostat (ENT; a class I HDAC inhibitor). The tumors responded to this regimen remarkably well, with eradication of primary tumors in 10 out of 11 mice and 100% survival 60 d after tumor implantation (Fig. 1_D_). Similarly, in response to the anti–PD-1/anti–CTLA-4 plus AZA/ENT treatment, mice with 4T1 tumors (∼400 mm3) showed complete regression of all primary tumors 3 wk after treatment initiation and 80% survival 100 d after tumor implantation (Fig. 1 E and F). Temporary self-limiting toxicity, as indicated by body weight changes, was observed when ENT was used (Fig. S1). However, the addition of anti–PD-1/anti–CTLA-4 antibodies did not add to the toxicity.
In parallel experiments, we treated 4T1 tumor-bearing mice as described above but killed them 6 wk after tumor implantation. We then examined their primary tumors as well as lungs and other organs for metastasis. The primary tumors were eradicated in all five mice treated with anti–PD-1/anti–CTLA-4 antibodies plus AZA/ENT, and none of them showed any metastases (Fig. 1_G_ and Table S2). In contrast, all five mice with anti–PD-1/anti–CTLA-4 treatment alone still had large primary tumors and an average of 11 lung metastases. We also treated the tumor-bearing mice with anti–PD-1/anti–CTLA-4 antibodies plus either ENT or AZA. No primary tumors or metastases were found in any of the mice treated with anti–PD-1/anti–CTLA-4 antibodies plus ENT, suggesting that when combined with PD-1/CTLA-4 double blockade, class I HDAC inhibitors alone (without DNA methylation inhibitors) were sufficient to eradicate both primary tumors and metastasis (Fig. 1_G_ and Table S2). In the mice treated with anti–PD-1/anti–CTLA-4 antibodies plus AZA, the primary tumors were not eradicated, although no metastases were observed. Without PD-1/CTLA-4 inhibition, ENT and AZA, alone or in combination, were unable to eradicate either primary tumor or metastasis (Fig. 1_G_ and Table S2). When PD-1/CTLA-4 inhibition was not applied, metastatic lesions were observed in multiple organs in addition to those in the lungs.
Mechanistic Studies.
As noted above, we expected that the epigenetic modulators were increasing the expression of MHC-I–related genes, thereby making the cancer cells more susceptible to killing by T cells. To test this expectation, we analyzed the expression of genes involved in MHC-I presentation by RT-PCR in CT26 and 4T1 cells treated with AZA, ENT, or the combination of the two. Expression of the MHC-I, β-2 microglobulin (B2M), and transporter associated with antigen processing 1 (TAP1) genes was detected in both tumor cell lines in the absence of treatment. However, exposure to epigenetic modulators did not significantly increase the expression (Fig. S2).
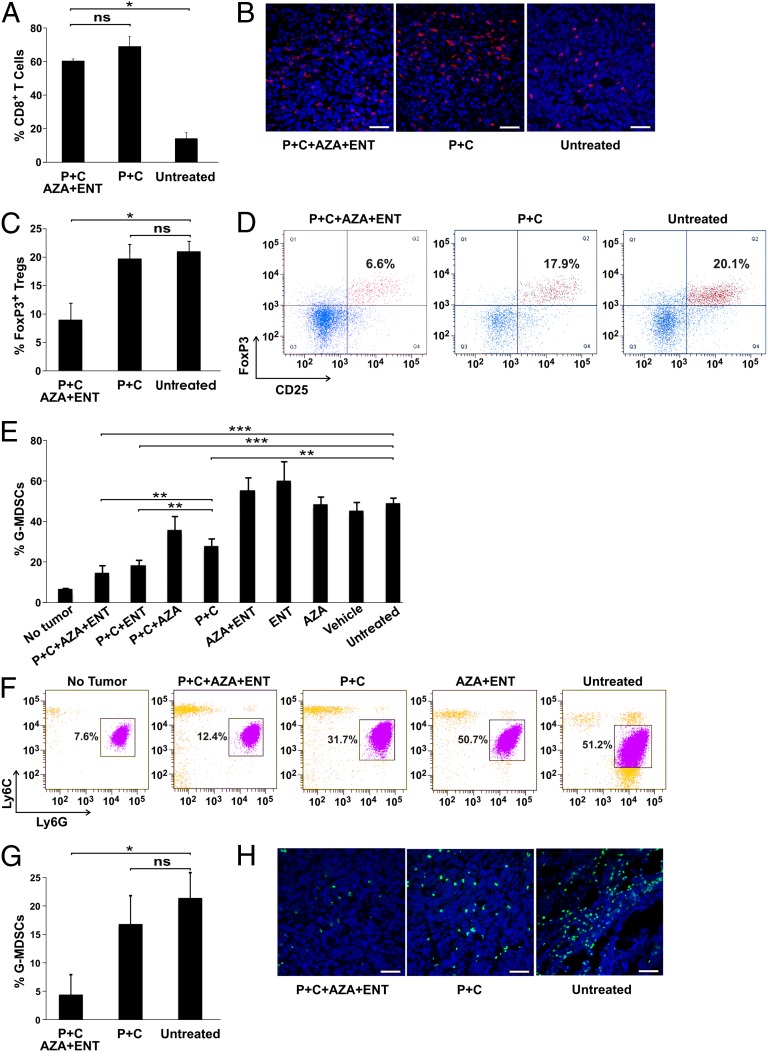
We then determined whether the epigenetic modulators affected T-cell accumulation within the tumors. As assessed by flow cytometry and immunohistofluorescence, tumor-infiltrating CD8+ T cells increased by approximately fourfold after PD-1/CTLA-4 inhibition (Fig. 2 A and B). The addition of AZA and ENT did not increase tumor-infiltrating CD8+ T cells further. However, inclusion of AZA and ENT in the treatment regimen resulted in a significant decrease in tumor-infiltrating FoxP3+ Tregs compared with either untreated tumors or tumors treated with anti–PD-1/anti–CTLA-4 antibodies (Fig. 2 C and D).
Fig. 2.
Response of immune cells following immune checkpoint blockade and epigenetic modulation. BALB/c mice bearing metastatic 4T1 tumors were treated with the indicated therapeutic modalities, followed by FACS and immunohistofluorescence analyses to assess tumor-infiltrating and circulating immune cells. Means and SDs are shown, with P values indicated. (A) FACS result for tumor-infiltrating CD8+ T cells. (B) Representative immunohistofluorescence staining of tumor-infiltrating CD8+ T cells. (Scale bars, 50 μm.) (C) FACS result for tumor-infiltrating CD4+CD25+FoxP3+ Tregs. (D) Representative FACS data showing percentages of FoxP3 and CD25 double positive cells in CD45+CD3+CD4+ gated tumor-infiltrating cells. (E) FACS result for circulating G-MDSCs. (F) Representative FACS data showing percentages of Ly6G+Ly6Clo cells (G-MDSCs) in CD45+CD11b+F4/80−MHC-II− gated circulating cells. (G) FACS result for tumor-infiltrating G-MDSCs. (H) Representative immunohistofluorescence staining of tumor-infiltrating Ly6G+ cells. (Scale bars, 50 μm.) *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant.
We next analyzed MDSCs by flow cytometry, because these myeloid-derived immature cells are often elevated in tumor-bearing hosts and have potent immunosuppressive activities (21, 22). We found that 4T1 tumor-bearing mice had a five- to sevenfold increase in circulating granulocytic MDSCs (G-MDSCs; defined as CD11b+Ly6G+Ly6CloMHC-II−) compared with non–tumor-bearing animals (Fig. 2_E_ and Fig. S3 A and B). Large numbers of G-MDSCs were also observed in the spleen and tumor (Fig. S3_B_). Addition of ENT or AZA/ENT to PD-1/CTLA-4 inhibition resulted in a striking reduction in the number of circulating G-MDSCs, bringing them down to a level similar to that observed in non–tumor-bearing mice (Fig. 2 E and F). Interestingly, the epigenetic modulators alone or AZA plus anti–PD-1/anti–CTLA-4 antibodies failed to abate the G-MDSCs. The epigenetic modulators substantially reduced the number of tumor-infiltrating G-MDSCs as well when combined with immune checkpoint blockade (Fig. 2 G and H).
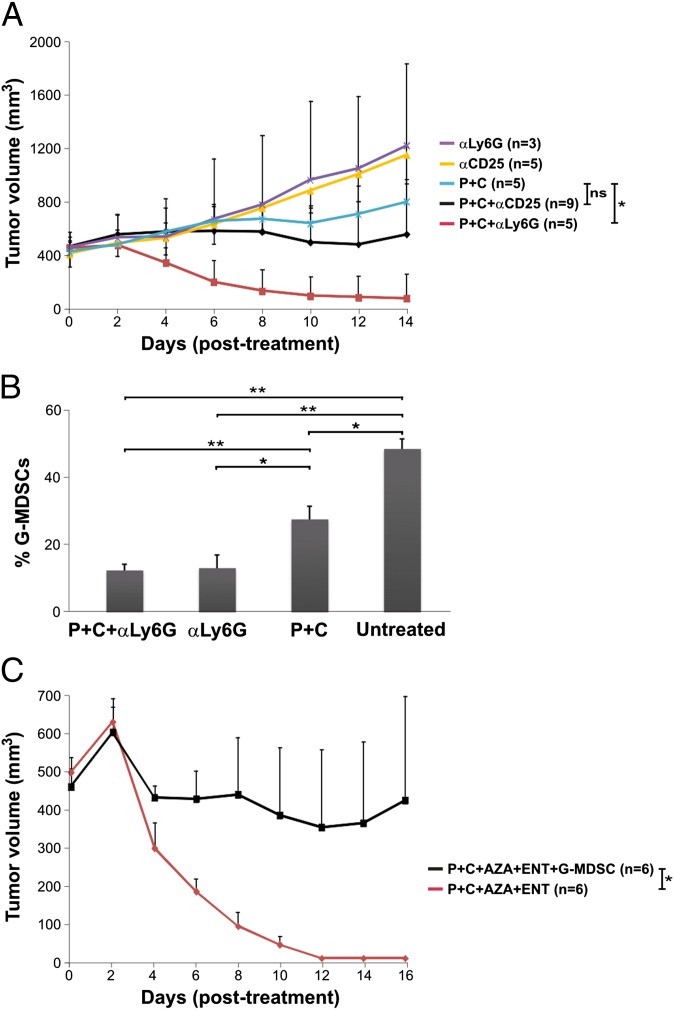
These data are consistent with the hypothesis that immune checkpoint blockade leads to expansion of cytotoxic effector T cells (Teffs), but Teffs are not fully functional unless immune suppressor cells are reduced by treatment with epigenetic modulators. To further test this hypothesis, we used neutralizing antibodies against CD25 or Ly6G to deplete Tregs or G-MDSCs, respectively, in mice bearing 4T1 tumors (23–25). We found that anti-Ly6G, when used in combination with anti–PD-1/anti–CTLA-4 antibodies, was as effective as the epigenetic modulators (Fig. 3_A_). Flow cytometry showed a substantial reduction in G-MDSC levels after anti-Ly6G treatment (Fig. 3_B_). In contrast, anti-CD25 treatment only showed marginal improvement in efficacy when combined with immune checkpoint blockade (Fig. 3_A_). However, it should be noted that the anti-CD25 treatment may also affect activated Teffs, which can transiently express CD25. As expected, without immune checkpoint blockade, both anti-CD25 and anti-Ly6G were ineffective (Fig. 3_A_).
Fig. 3.
Myeloid-derived Ly6G+ cells are responsible for resistance to immune checkpoint blockade. (A) BALB/c mice bearing 4T1 tumors were treated with various antibodies or antibody combinations as indicated and tumor volumes were recorded over time. αCD25, anti-CD25 antibody; αLy6G, anti-Ly6G antibody. (B) FACS result for circulating G-MDSCs after treatment with different antibodies or antibody combinations. (C) 4T1 tumor-bearing mice were treated with anti–PD-1/anti–CTLA-4 antibodies plus epigenetic modulators with or without adoptive transfer of MDSCs isolated by affinity purification from the 4T1 tumor-bearing animals. Tumor volumes were recorded following the treatments. Means and SDs are shown, with P values indicated. *P < 0.05, **P < 0.01; ns, not significant.
To directly evaluate the ability of the tumor-induced G-MDSCs to interfere with the effects of immune checkpoint blockade, we isolated them from 4T1 tumor-bearing mice by affinity purification. We then injected the purified G-MDSCs into 4T1 tumor-bearing mice treated with anti–PD-1/anti–CTLA-4 antibodies plus AZA/ENT. The adoptive transfer of G-MDSCs significantly attenuated the response to the combination therapy (Fig. 3_C_). Based on the above results, we concluded that the effects of epigenetic modulation were more likely the result of depletion of G-MDSCs than of direct depletion of Tregs.
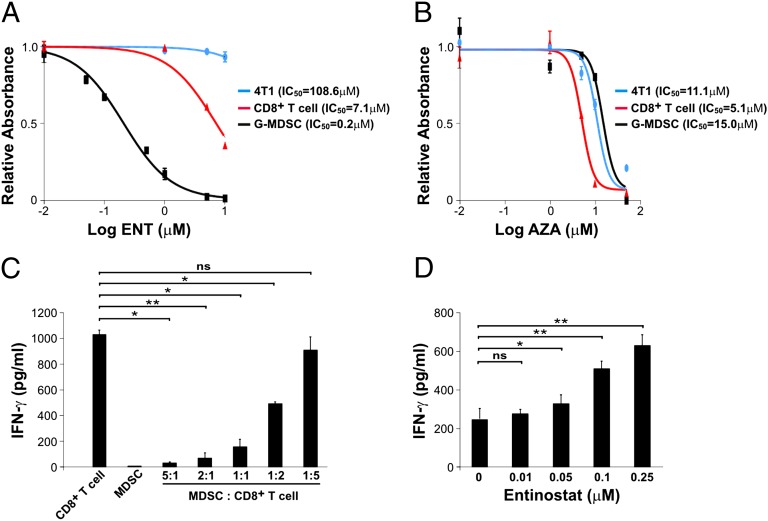
To investigate whether epigenetic modulation directly affected G-MDSCs, we purified these cells from 4T1 tumor-bearing mice as described above and treated them with ENT or AZA in vitro. G-MDSCs showed markedly reduced viability after ENT treatment in a dose-dependent fashion (Fig. 4_A_). Conversely, AZA had no effect at comparable concentrations (Fig. 4_B_). We also treated 4T1 tumor cells with the same concentrations of ENT or AZA and found them unresponsive (Fig. 4 A and B). Importantly, ENT had only modest effects on CD8+ T cells (Fig. 4_A_), creating a large therapeutic window in which G-MDSCs can be depleted while sparing CD8+ T cells. Finally, we cocultured CD8+ T cells with G-MDSCs and analyzed the concentration of IFN-γ in culture medium by ELISA following T-cell activation with CD3 and CD28 antibodies. G-MDSCs inhibited IFN-γ secretion (Fig. 4_C_), whereas inclusion of ENT in the culture medium reverted the inhibition in a dose-dependent manner (Fig. 4_D_). These data support the notion that G-MDSCs directly inhibit the function of CD8+ T cells and that ENT alleviates the inhibition by directly suppressing G-MDSCs.
Fig. 4.
Direct effects of epigenetic modulators on cultured cells. (A and B) 4T1 cells, purified CD8+ T cells, or G-MDSCs were treated with different concentrations of ENT (A) or AZA (B). Cell viability was assessed using a metabolism-based colorimetric assay. (C) Conditioned media from cocultures of G-MDSCs and CD8+ T cells at different ratios were analyzed for IFN-γ concentration. (D) Conditioned media from cocultures at a G-MDSC-to-CD8+ T-cell ratio of 1:1 were collected after treatment with ENT at increasing doses for 24 h and analyzed for IFN-γ concentration. Means and SDs of data from at least triplicate wells are shown, with P values indicated. *P < 0.05, **P < 0.01; ns, not significant.
To further confirm this conclusion, as well as to provide additional therapeutic approaches to achieve the same goal, we searched for other therapeutic agents that might suppress G-MDSC function. Phosphatidylinositide 3-kinases (PI3Ks) are known to play important roles in hematopoietic cell biology and can activate Gr1+/CD11b+ myeloid cells (26). We had previously developed a diverse array of PI3K inhibitors and chose to test one (J32) with high cellular potency (27–29). J32 proved to be cytotoxic to G-MDSCs at nanomolar concentrations (IC50 of 14.3 nM) and much less toxic to CD8+ T cells (IC50 of 94.6 nM) (Fig. S4_A_). Treatment of 4T1 tumor-bearing mice with a relatively low dose of J32 (22 mg/kg) in combination with anti–PD-1/anti–CTLA-4 antibodies resulted in a marked reduction in circulating G-MDSCs (Fig. S4_B_) and eradication of 4T1 tumors in 80% of the animals (Fig. S4_C_). Alone, J32 had no appreciable effect on 4T1 tumor growth.
Discussion
Metastatic cancers that are minimally immunogenic are very difficult to eradicate, in either mice or humans. In this study, we show that immune checkpoint blockade with anti–PD-1 plus anti–CTLA-4 antibodies has impressive therapeutic effects against both tumor types used in this study but cannot cure either large, modestly immunogenic CT26 tumors or poorly immunogenic metastatic 4T1 tumors of moderate sizes. The use of both antibodies together was inspired by clinical results showing that anti–PD-1 plus anti–CTLA-4 exerted greater effects in melanoma patients than anti–CTLA-4 alone (12). Similarly, we found that the dual-antibody treatment was superior to treatment with anti–CTLA-4 or anti–PD-1 alone in mice (Fig. 1 A and B).
DNA methyltransferase and HDAC inhibitors have been in clinical development as single agents for a number of years (30–32). Two such agents, 5-azacytidine (Vidaza) and 5-aza-2′-deoxyazacytidine (decitabine), have been approved for the therapy of neoplastic diseases (myelodysplasia, a precursor of leukemia, and chronic myelomonocytic leukemia) (33). The presumptive mechanism of action of these drugs is the activation of tumor suppressor genes or immunity-associated genes silenced in tumor cells (34, 35). However, our studies indicate an additional mechanism: In addition to acting on tumor cells, these agents also act on host cells in the immune system such as MDSCs. When used in conjunction with immune checkpoint blockade, the latter mechanism seems very important, based on the following lines of evidence: The epigenetic modulators kill MDSCs at much lower concentrations than required for killing tumor cells in vitro; the epigenetic modulators have only a marginal effect at best on tumor cells in vivo at the doses used in this study; reduction of MDSCs using antibodies directed against them has similar antitumor effects to those observed with the epigenetic modulators; in adoptive transfer experiments, MDSCs purified from nontreated tumor-bearing mice can abolish the therapeutic effects of epigenetic modulation; and inhibition of MDSCs with an agent (a PIK3 inhibitor) of a completely different class has similar effects to those of epigenetic modulators.
A recent clinical study demonstrated that epigenetic modulation exerted major therapeutic effects on a small fraction of patients with non–small-cell lung cancer (NSCLC) (36). Other studies have suggested that 5-azacytidine up-regulates genes and pathways related to both innate and adaptive immunity and genes related to immune evasion in NSCLC lines (35). These important studies as well as recent clinical trials with immune checkpoint blockade have led to the initiation of a clinical trial combining anti–PD-1 antibody, 5-azacytidine, and entinostat in NSCLC patients (http://clinicaltrials.gov/ct2/show/NCT01928576?term=entinostat+pd-1&rank=1). It will be interesting to determine the importance of both changes in gene expression in the tumor cells and changes in the number and function of MDSCs in this trial.
Our observations raise a number of questions. For example, what are the mechanisms underlying the selective suppression of MDSCs by epigenetic and PI3K inhibitors? Would other approaches (e.g., myelosuppressive agents) targeting immune suppressor cells synergize with immune checkpoint blockade for complete eradication of solid tumors and their metastases? Would priming with epigenetic inhibitors before immune checkpoint blockade work as well as concomitant administration of the two, as done in the current study? Experiments addressing these questions may lead to the development of more effective therapies harnessing the power of immunity.
Materials and Methods
Animal study was approved and overseen by The Johns Hopkins University Institutional Animal Care and Use Committee. Standard protocols with modifications were followed in preparation of genomic and cDNA libraries, exome capture, sequence analysis, immunological assays, and animal experiments performed in this study. Detailed methods and associated references are available in SI Materials and Methods.
Supplementary Material
Supporting Information
Acknowledgments
The authors thank Surojit Sur for thoughtful discussions, Lillian Dasko-Vincent and Evangeline Watson for expert technical assistance with cell imaging and animal experiments, and Mark Kelman for logistic support. This project was supported by The Virginia and D. K. Ludwig Fund for Cancer Research, a research fund from BioMed Valley Discoveries, and Grants CA062924 and CA043460 from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
References
- 1.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pentcheva-Hoang T, Corse E, Allison JP. Negative regulators of T-cell activation: Potential targets for therapeutic intervention in cancer, autoimmune disease, and persistent infections. Immunol Rev. 2009;229(1):67–87. doi: 10.1111/j.1600-065X.2009.00763.x. [DOI] [PubMed] [Google Scholar]
- 3.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagaraj S, Youn JI, Gabrilovich DI. Reciprocal relationship between myeloid-derived suppressor cells and T cells. J Immunol. 2013;191(1):17–23. doi: 10.4049/jimmunol.1300654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13(10):739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lippitz BE. Cytokine patterns in patients with cancer: A systematic review. Lancet Oncol. 2013;14(6):e218–e228. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 8.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 9.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolchok JD, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corbett TH, Griswold DP, Jr, Roberts BJ, Peckham JC, Schabel FM., Jr Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res. 1975;35(9):2434–2439. [PubMed] [Google Scholar]
- 14.Belnap LP, Cleveland PH, Colmerauer ME, Barone RM, Pilch YH. Immunogenicity of chemically induced murine colon cancers. Cancer Res. 1979;39(4):1174–1179. [PubMed] [Google Scholar]
- 15.Dexter DL, et al. Heterogeneity of tumor cells from a single mouse mammary tumor. Cancer Res. 1978;38(10):3174–3181. [PubMed] [Google Scholar]
- 16.Pulaski BA, Ostrand-Rosenberg S. Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res. 1998;58(7):1486–1493. [PubMed] [Google Scholar]
- 17.Segal NH, et al. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68(3):889–892. doi: 10.1158/0008-5472.CAN-07-3095. [DOI] [PubMed] [Google Scholar]
- 18.Vogelstein B, et al. Cancer genome landscapes. Science. 2013;339(6127):1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rashid OM, et al. Resection of the primary tumor improves survival in metastatic breast cancer by reducing overall tumor burden. Surgery. 2013;153(6):771–778. doi: 10.1016/j.surg.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lampen MH, van Hall T. Strategies to counteract MHC-I defects in tumors. Curr Opin Immunol. 2011;23(2):293–298. doi: 10.1016/j.coi.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: Linking inflammation and cancer. J Immunol. 2009;182(8):4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couper KN, et al. Anti-CD25 antibody-mediated depletion of effector T cell populations enhances susceptibility of mice to acute but not chronic Toxoplasma gondii infection. J Immunol. 2009;182(7):3985–3994. doi: 10.4049/jimmunol.0803053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Setiady YY, Coccia JA, Park PU. In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. Eur J Immunol. 2010;40(3):780–786. doi: 10.1002/eji.200939613. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava MK, et al. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS ONE. 2012;7(7):e40677. doi: 10.1371/journal.pone.0040677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmid MC, et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3Kγ, a single convergent point promoting tumor inflammation and progression. Cancer Cell. 2011;19(6):715–727. doi: 10.1016/j.ccr.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt-Kittler O, et al. PI3Kalpha inhibitors that inhibit metastasis. Oncotarget. 2010;1(5):339–348. doi: 10.18632/oncotarget.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandelker D, et al. A frequent kinase domain mutation that changes the interaction between PI3Kalpha and the membrane. Proc Natl Acad Sci USA. 2009;106(40):16996–17001. doi: 10.1073/pnas.0908444106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Z, et al. Definition of the binding mode of a new class of phosphoinositide 3-kinase α-selective inhibitors using in vitro mutagenesis of non-conserved amino acids and kinetic analysis. Biochem J. 2012;444(3):529–535. doi: 10.1042/BJ20120499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: Overview and perspectives. Mol Cancer Res. 2007;5(10):981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- 31.Khan O, La Thangue NB. HDAC inhibitors in cancer biology: Emerging mechanisms and clinical applications. Immunol Cell Biol. 2012;90(1):85–94. doi: 10.1038/icb.2011.100. [DOI] [PubMed] [Google Scholar]
- 32.Lyko F, Brown R. DNA methyltransferase inhibitors and the development of epigenetic cancer therapies. J Natl Cancer Inst. 2005;97(20):1498–1506. doi: 10.1093/jnci/dji311. [DOI] [PubMed] [Google Scholar]
- 33.Griffiths EA, Gore SD. DNA methyltransferase and histone deacetylase inhibitors in the treatment of myelodysplastic syndromes. Semin Hematol. 2008;45(1):23–30. doi: 10.1053/j.seminhematol.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baylin SB, Jones PA. A decade of exploring the cancer epigenome—Biological and translational implications. Nat Rev Cancer. 2011;11(10):726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wrangle J, et al. Alterations of immune response of non-small cell lung cancer with azacytidine. Oncotarget. 2013;4(11):2067–2079. doi: 10.18632/oncotarget.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juergens RA, et al. Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer. Cancer Discov. 2011;1(7):598–607. doi: 10.1158/2159-8290.CD-11-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information