Morpholino antisense oligonucleotides targeting intronic repressor Element1 improve phenotype in SMA mouse models (original) (raw)
Abstract
Spinal muscular atrophy (SMA) is a neurodegenerative disease caused by the loss of Survival Motor Neuron-1 (SMN1). In all SMA patients, a nearly identical copy gene called SMN2 is present, which produces low levels of functional protein owing to an alternative splicing event. To prevent exon-skipping, we have targeted an intronic repressor, Element1 (E1), located upstream of SMN2 exon 7 using Morpholino-based antisense oligonucleotides (E1MO-ASOs). A single intracerebroventricular injection in the relatively severe mouse model of SMA (SMNΔ7 mouse model) elicited a robust induction of SMN protein, and mean life span was extended from an average survival of 13 to 54 days following a single dose, consistent with large weight gains and a correction of the neuronal pathology. Additionally, E1MO-ASO treatment in an intermediate SMA mouse (SMNRT mouse model) significantly extended life span by ∼700% and weight gain was comparable with the unaffected animals. While a number of experimental therapeutics have targeted the ISS-N1 element of SMN2 pre-mRNA, the development of E1 ASOs provides a new molecular target for SMA therapeutics that dramatically extends survival in two important pre-clinical models of disease.
INTRODUCTION
Spinal muscular atrophies (SMAs) are collectively the second most common autosomal recessive neurodegenerative group of disorders with an incidence of 1 in 6000 (1) and a carrier frequency of ∼1 in 35 (2). The diseases are caused by the loss of α-motor neurons resulting in subsequent atrophy of voluntary muscle groups leading to paralysis and eventually to premature infantile death. Genetically, the types of SMA result from a homozygous loss or mutation in the telomeric copy of the Survival Motor Neuron-1 (SMN1) gene. All SMA patients rely on the nearly identical copy gene, SMN2, which produces low levels of functional SMN protein. SMN is ubiquitously expressed and is a critical factor in a variety of RNA pathways. The best characterized SMN activity is in the assembly and maturation of the spliceosomal UsnRNPs (3,4). Even though the SMN2 gene is 99% identical in nucleotide sequence and is completely identical in amino acid sequence, ∼90% of SMN2-derived transcripts are alternatively spliced and encode a truncated protein lacking the final coding exon (exon 7). This aberrant splicing event is the result of a silent, non-polymorphic C-to-T nucleotide transition 6 nucleotides within exon 7 (5,6). SMN2, however, is an excellent target for therapeutic intervention. Previously, _cis_-acting negative regulatory regions that surround SMN2 exon 7 have been identified and described (5,7–14). In particular, ISS-N1 has been a hotspot for experimental therapeutics, especially antisense oligonucleotides (ASOs). ASO molecules of various lengths and backbone chemistries have been used to inhibit the repressor activity of ISS-N1, leading to an increase in SMN protein and significant extensions of survival in SMA animal models. Presently, the 2′-MOE chemistry used by ISIS Pharmaceuticals in the development of their ASO, SMN-Rx, is in Phase II clinical trials (15,16). Similar Morpholino-based ASOs have shown excellent pre-clinical promise in severe SMA mice and are under further development.
Currently, no effective treatment exists for SMA, and the complexity and expansive clinical spectrum strongly argue that a single compound will be insufficient to address the needs of all SMA patients. Going forward, it is essential to broaden the range of tractable genetic targets for SMA. To this end, we have focused upon a genetic region upstream of SMN2 exon 7 called Element1 (E1). Using a mini-gene system, E1 was shown to function as a potent repressor of SMN2 exon 7 splicing (14,17) and was bound by splicing repressor proteins (17). Native RNA antisense molecules expressed from a viral backbone modulated SMN2 exon 7 splicing and elevated SMN protein levels, although the inherent instability of the native RNA molecules likely accounted for the poor impact upon disease severity and poor extensions in survival (17).
In this report, Morpholino-based ASOs targeting the E1 region have been developed and examined in two important animal models of disease: the ‘gold standard’ SMNΔ7 mouse, which is a very severe model living only ∼14 days and a recently developed model called SMNRT, in which animals live ∼35 days and represent a less severe population. Using a relatively low dose of the Element1 ASO (E1MO-ASO), the SMA phenotype at the molecular, cellular and organismal levels were largely rescued, including a 300–700% extension in survival for the two mouse models. From a pre-clinical perspective, there is excellent target engagement (SMN2 splicing), molecular efficacy (SMN protein production) and robust phenotypic rescue in two complementary models of disease. Collectively, this work identifies a lead ASO candidate that targets a distinct region of the SMN2 pre-mRNA.
RESULTS
Element1 as an ASO target
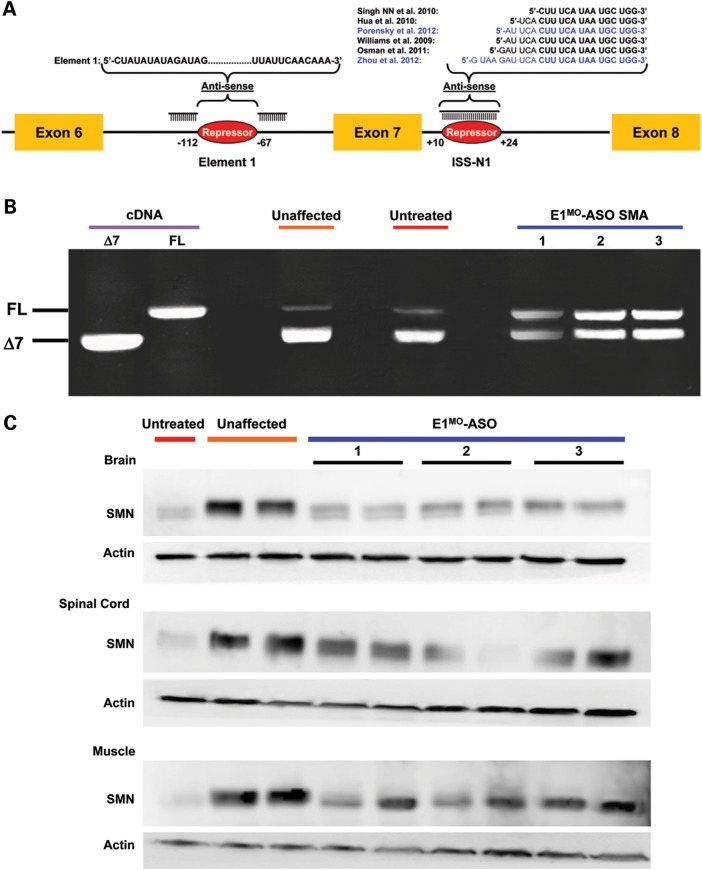
Previously, we have developed antisense-based oligonucleotides termed bifunctional RNAs, which targeted the E1 intronic repressor sequence (17). Using 2′-O-methyl chemistry, bifunctional RNA activity in vivo was statistically significant, but only marginally increased survival and a change in SMN2 splicing was not detected. Recently, Morpholino-based antisense oligonucleotides targeting the ISS-N1 splicing repressor in intron 7 have been shown to be effective at modulating SMN2 splicing in vivo (18,19). To expand the repertoire of potential targets for SMA therapeutics, Morpholino-modified ASOs (E1MO-ASOs) were developed that target the E1 repressor, a region distinct from the ISS-N1 repressor that has been the focus of the overwhelming majority of ASO strategies (Fig. 1A). Following a single injection into the central nervous system via intracerebroventricular (ICV) delivery of the E1MO-ASO, pre-mRNA exon-skipping from the SMN2 transgene was significantly reduced in total RNA isolated from brain tissue, resulting in a nearly 3- to 4-fold increase in full-length SMN transcript in each of three animals examined (Fig. 1B and Supplementary Material, Fig. S1). The mice used in this experiment were phenotypically wild type (unaffected) but carried the human genomic SMN2 gene. To determine whether SMN levels increased similarly, a single ICV injection was delivered to SMNΔ7 (SMNΔ7+/+;SMN2+/+;_Smn_−/−) pups on P2 and protein extracts were collected from brain, spinal cord and skeletal muscle (Musculus gastrocnemius) on P7. The SMNΔ7 model, which has an average life span of 12–14 days, has been extensively characterized and utilized for a number of translational studies (20–22). In each treated animal examined, SMN protein levels were elevated several fold compared with untreated SMNΔ7 animals, although wild-type tissue still contained slightly higher levels of SMN (Fig. 1C and Supplementary Material, Fig. S2). Spinal cord and muscle displayed the greatest percentage increase (Supplementary Material, Fig. S2). Interestingly, the ASO was able to enter peripheral tissue following an ICV injection, potentially due in part to a disease-compromised vascular system or blood–brain barrier.
Figure 1.
Targeting the intronic repressor Element1 with Morpholino modified ASOs. (A) Schematic representation of specific Morpholino-modified ASO targeting the intronic repressor Element1 (E1MO-ASO). The specific design of the E1MO-ASO is illustrated with the antisense domains consisting of two non-sequential target antisense sequences targeting the intronic Element1 repressor. In addition, several previously published ASO sequences with different modified chemistries and targeting the intronic silencer ISS-N1 are also shown. (B) Increase in full-length SMN transcript after E1MO-ASO treatment. RT–PCR image showing full-length SMN in three individual animals. Specific plasmids, pCIExSkip and pCIFL, were used for cDNA controls. (C) Injection of Morpholino-based ASO targeting the Element1 repressor increases total SMN protein in SMNΔ7 mouse model. Single ICV injection of E1MO-ASO increases SMN protein levels. Western blots (n = 5) for each treatment group were performed on brain, spinal cord and muscle tissues at P7.
Phenotypic correction in severe SMA mice
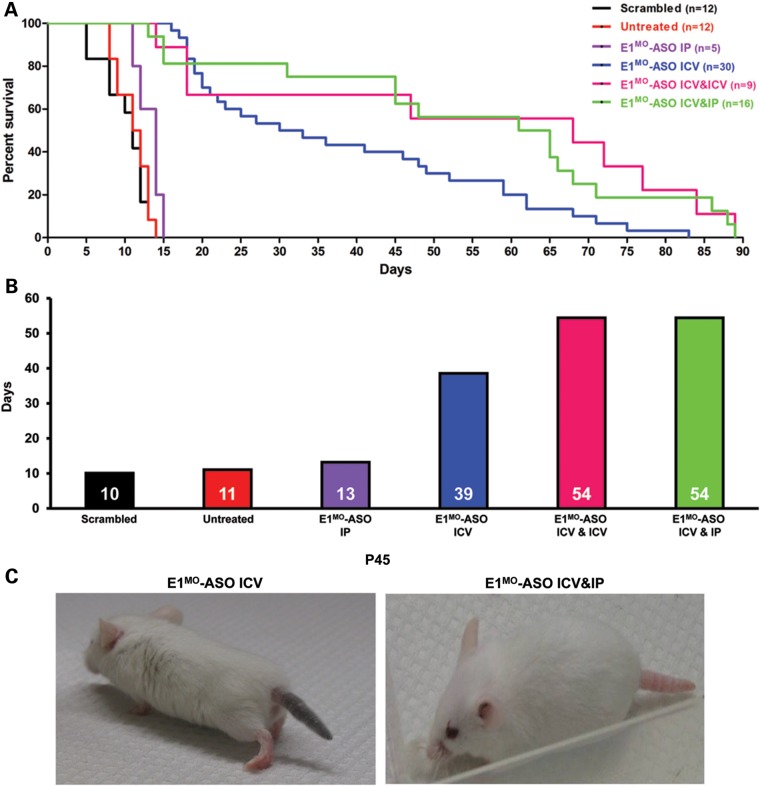
To determine whether delivery of the E1MO-ASO to SMA mice on P2 improved the phenotype, survival, weight gain, righting reflexes and strength measurements were collected. A relatively low dose of ASO (2 µl of 2 mm) was selected, comparable with the ‘low’ dose from a previously published report examining ISS-N1 ASOs (18). Untreated SMA mice lived <2 weeks, similar to a cohort treated with a control ASO consisting of the scrambled sequence. Similarly, delivery of the E1MO-ASO via a single intraperitoneal (IP) injection failed to extend survival beyond 1–2 days. However, a single ICV injection of E1MO-ASO led to nearly a 400% improvement in life span, with more than one-third of the treated animals living beyond 50 days. When we looked at the life span of the negative control group animals, we observed no difference between our untreated and the scrambled Morpholino-injected animals. SMA animal models exhibit extensive peripheral defects, particularly in severe models. To determine whether E1MO-ASO treatment would rescue peripheral defects, a combinatorial treatment of ICV and IP injections was performed: two injections of the 2 mm ASO were delivered via ICV and an IP injection. In a separate cohort, two ICV injections were administered separated by 12 h. The double-dosing resulted in a similar extension in survival, out to an average of 54 days, with more than one-quarter of the animals living beyond 70 days (Fig. 2A and B). While the treated mice were highly ambulatory, distal necrosis, particularly of the tail, was observed in nearly all of the longer lived animals. In the ICV-treated cohort, necrosis initiated at approximately P40–45, whereas ICV/IP treatment delayed necrosis onset to approximately P60 (Fig. 2C and Supplementary Material, Fig. S3). Collectively, these results demonstrate that the E1MO-ASO can significantly extend survival in a severe mouse model of SMA.
Figure 2.
Phenotypic improvements in the severe SMNΔ7 mouse models of SMA after E1MO-ASO treatment. (A) Severe SMNΔ7 SMA mice showed significant improvement in survival and longevity after injections with E1MO-ASO oligos. Kaplan–Meier survival curves were constructed from the various treatment groups and the routes of delivery as indicated. Log-rank (Mantel–Cox) statistics were applied for comparisons between groups where P < 0.0001 for all treatment groups compared with untreated animals, with the exception of E1MO-ASO IP-injected animals when compared with the untreated controls (P = 0.0526). (B) The survival curves depict a significant increase in life expectancy for E1MO-ASO ICV-, ICV&ICV- and ICV&IP-injected animals with increases in median survival to 39, 54 and 54 days, respectively. (C) Some tail necrosis was displayed by the ICV-injected SMA animals around Days 40–45.
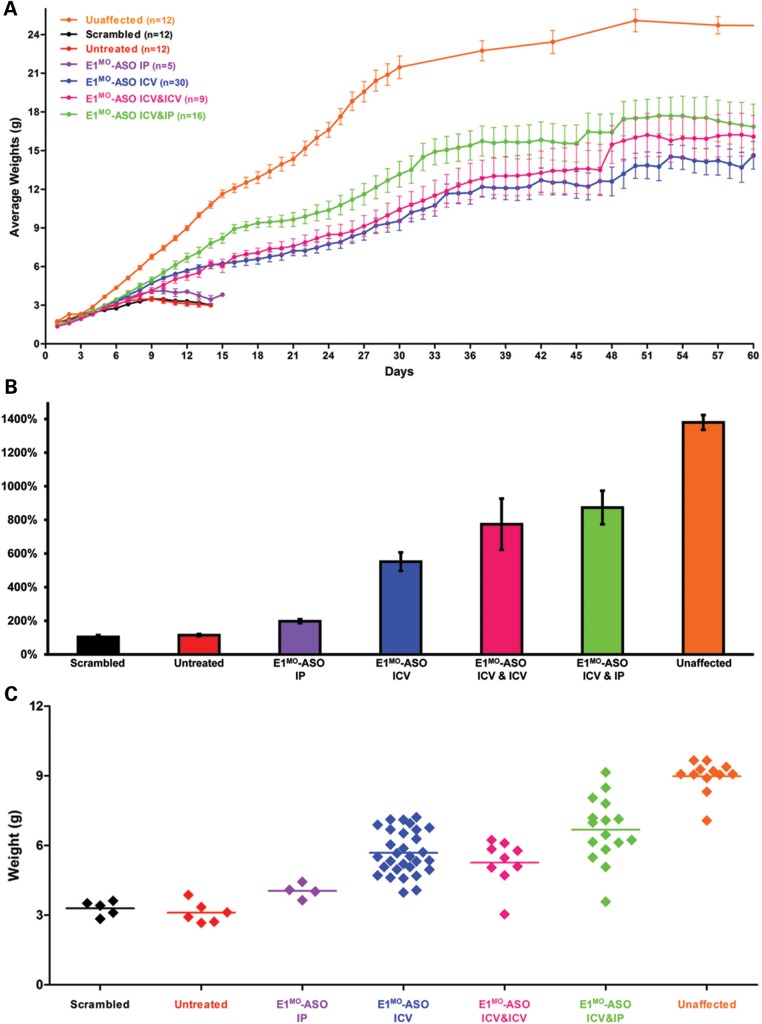
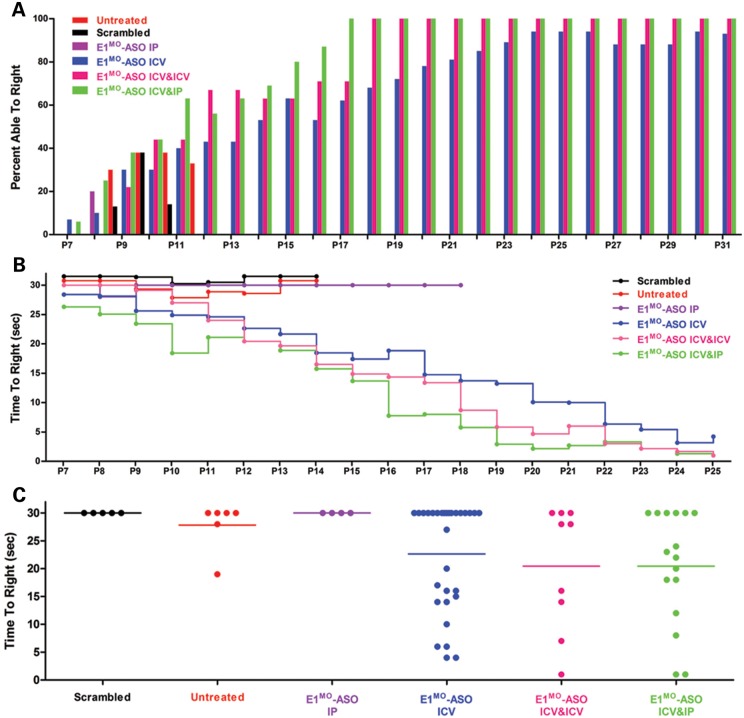
E1MO-ASO treatment resulted in significant weight gain compared with either untreated, scramble or IP-only ASO-treated cohorts. The ICV/IP-treated animals gained the most weight compared with the ICV or ICV/ICV groups, resulting in animals that achieved 15–18 g. This was in stark contrast to the untreated, scramble or IP-only ASO-treated cohorts that failed to thrive and were unable to achieve 5 g (Fig. 3A–C). An additional measure of phenotypic correction used in the SMA field is the timed-righting response. Animals are placed on their backs, and the time it takes to stand on four legs is recorded, as well as failed attempts. In the ICV, ICV/ICV and ICV/IP cohorts, SMNΔ7-treated animals improved significantly based upon the percentage of animals that could successfully turn over as well as the speed at which the animals successfully righted themselves (Fig. 4A–C).
Figure 3.
E1MO-ASO treatment results in a significant weight gain**.** (A) Starting at P7, E1MO-ASO-injected animals were heavier than untreated, scrambled and IP only-injected SMA controls. Total body weight was measured daily for all animal groups post-injection. (B) The percent weight gained from birth to peak was also compared between groups treated. For statistical significance between each treatment group, see Supplementary Material, Figure S4. (C) Individual weights on P12 for all controls and animals treated with E1MO-ASO.
Figure 4.
Assessment of overall fitness, ambulatory abilities and muscle strength of treated SMNΔ7 animals. (A) Bar graph showing the percent of all tested animal groups able to right themselves from a prone position. By P14, >50% of all ICV-, ICV&ICV- and ICV&IP-injected mice were able to right themselves. (B) Graph representing raw data of the average time to right from P7 to P25. E1MO-ASO ICV-, E1MO-ASO ICV&ICV- and E1MO-ASO ICV&IP-injected animals were able to right themselves within 20 s after 2 weeks. (C) Scatter plot of TTR performance of mice injected with E1MO-ASO. To highlight the performance of individual mice, TTR values are shown for P12. (D) Grip strength measurements in grams. Treated animals were compared with their unaffected littermates. Measurements were taken from P25 through P77. (E) Rotarod performance test. The test was used to measure riding time parameter (in seconds) of the E1MO-ASO-treated animals and compared with the times of their age-matched unaffected controls. Measurements were taken from P25 through P77.
Typically, SMA animals do not live long enough to perform gross motor function tests such as rotarod and grip strength; however, E1MO-ASO-treated mice lived long enough and were healthy enough to perform these assays. Following a 1-week period to acclimate to the equipment, grip strength and rotarod performance was collected for 16 consecutive trials. Grip strength analysis revealed that the SMA E1MO-ASO-treated animals performed consistently, albeit with less force, compared with wild-type animals (Fig. 4D). Rotarod performance also demonstrated that the ASO-rescued animals were not fully corrected compared with wild-type animals especially at later time points. While the treated animals were never fully corrected compared with wild-type animals, it is important to stress that their untreated (or scramble-treated cohorts) were dead weeks prior to these studies. This increasing discrepancy could in part be due to the development of tail necrosis as flexibility and/or loss of the tail would impact balance and rotarod performance (Fig. 4E).
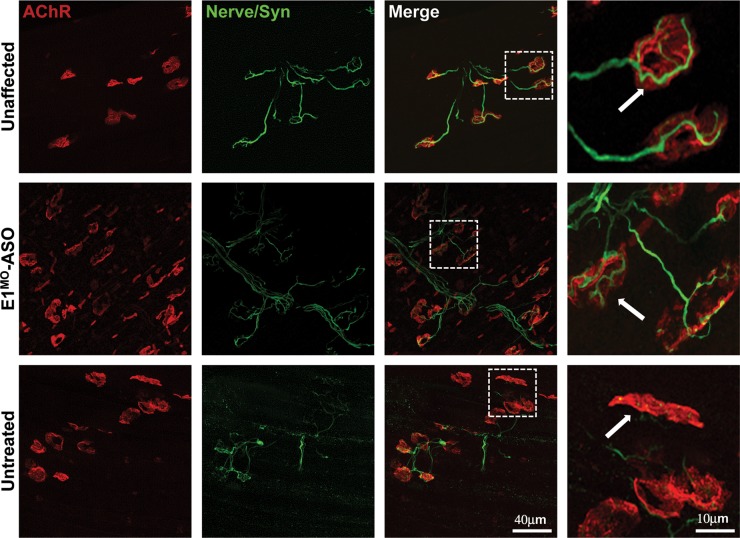
An important hallmark of the SMA phenotype that directly relates to disease pathogenesis is the integrity of the neuromuscular junctions (NMJs). As expected, NMJs from untreated SMA mice appear immature, poorly developed, and there was little overlap between the pre- and post-synaptic endplates. In contrast, the wild-type and E1MO-ASO-treated tissues exhibit well-developed NMJs with a high degree of connectivity between the axons and the post-synaptic endplate (Fig. 5). These results are consistent with the significant correction of the SMA phenotype at the organismal level and provide evidence that a molecular correction of SMN2 splicing using an E1MO-ASO can profoundly reverse the severe SMA phenotype observed in SMNΔ7 mice.
Figure 5.
Improvement in NMJs pathology. The longissimus capitis (LC) muscles from ICV-injected and control animals at P12 were immunostained for nerve terminals with anti-neurofilament/anti-synaptophysin (Nerve/Syn) (in green) and motor endplates with α-bungarotoxin (in red). While the untreated SMNΔ7 mice displayed typical severe denervation, E1MO-ASO treatment substantially restored NMJ's pretzel-like structures. Selected high-magnification images of NMJs in LC muscles of control and SMNΔ7 mice are shown (last column). Arrows indicate occupied endplates in both treated and unaffected animals contrasting the empty endplate seen in untreated controls.
Phenotypic correction in intermediate SMA mice
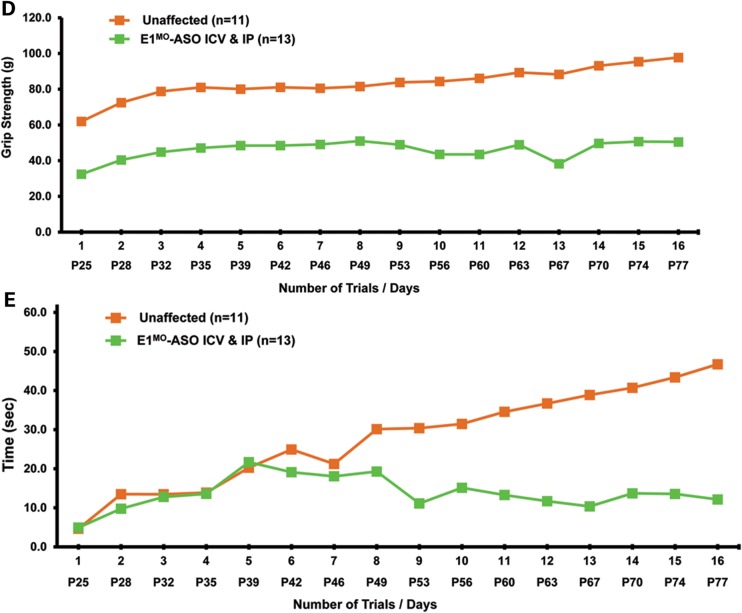
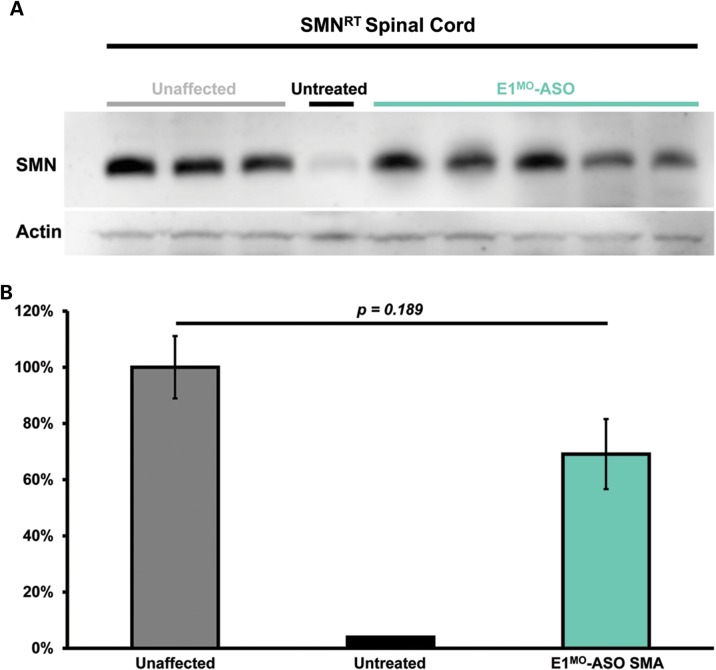
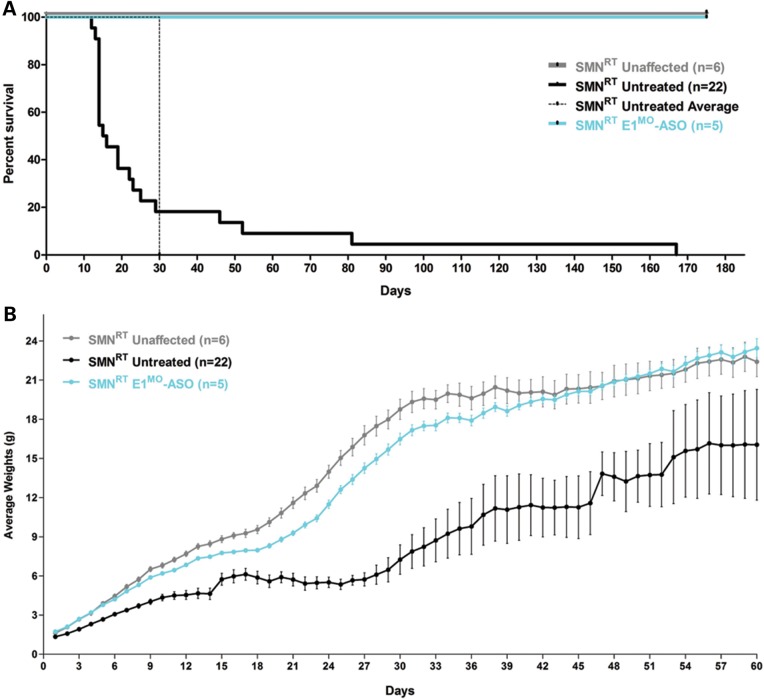
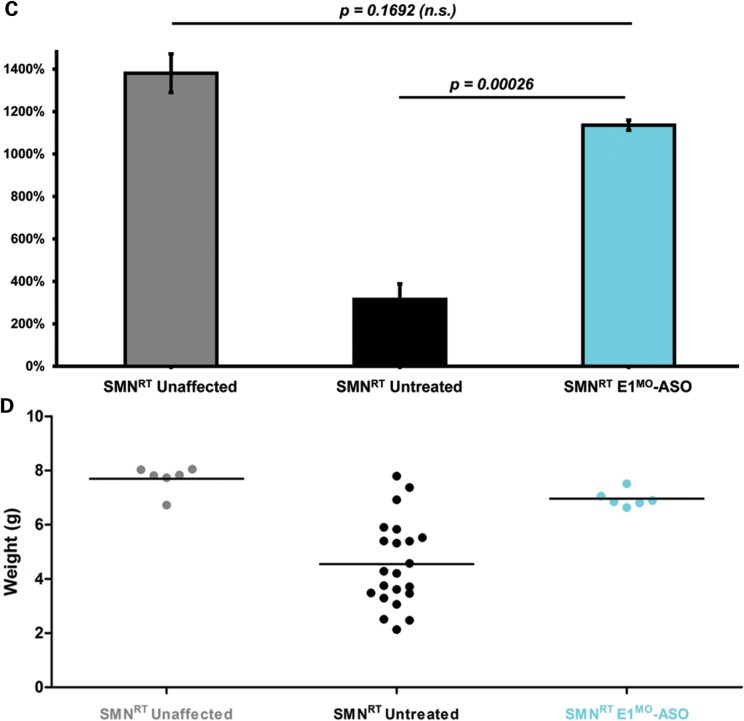
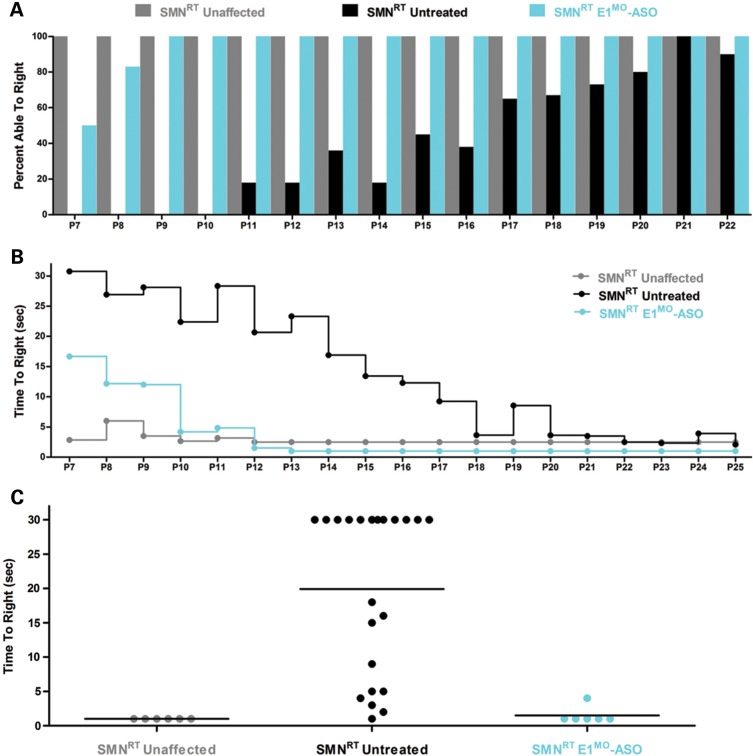
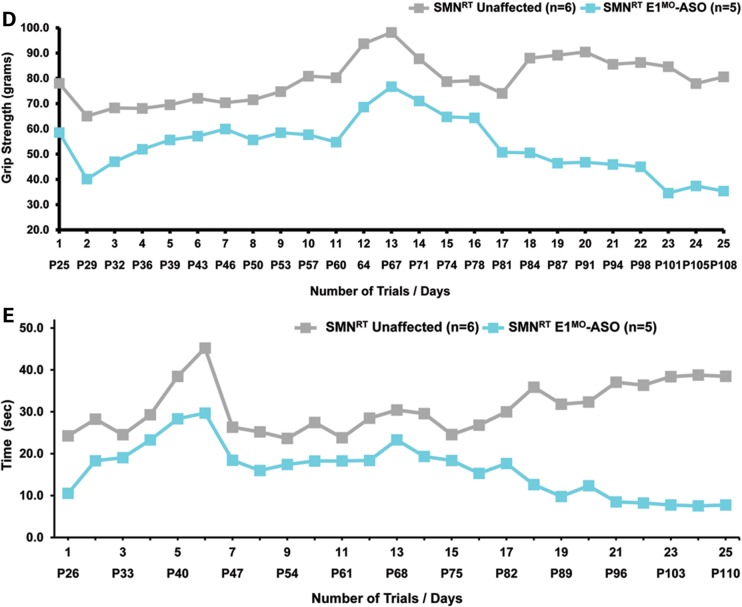
Testing therapeutics in more than one model of disease validates the molecular engagement of a specific target, demonstrates applicability to a broader range of the patient population and sheds light upon the biology of the disease. To address these important parameters, a newly developed intermediate model of disease was examined. This model, SMNRT, expresses low levels of SMN, lives ∼32 days and exhibits an intermediate phenotype in most cellular and organismal parameters of disease (23). Following a single ICV injection of the E1MO-ASO (2 mm), SMN protein was increased significantly, ∼8- to 10-fold above untreated levels in spinal cord extracts (Fig. 6A and B). To verify whether ICV delivery of E1MO-ASO also extended survival of the milder form SMA animals (SMNRT mouse model), lifespan for the treatment groups were analyzed by Kaplan–Meier survival curve and compared with the lifespan of the animals from the control groups. ICV injections of the E1MO-ASO significantly increased the average lifespan of the SMNRT mice, compared with their aged-matched untreated control animals (Fig. 7A). In fact, all E1MO-ASO-treated animals were still alive at P175, at which point the experiment was stopped and animals were euthanized. Remarkably, the treated SMNRT mice were phenotypically indistinguishable from their unaffected age-matched littermates within the first 2 months of their life span (Supplementary Material, Video). Consistent with an early and robust increase of SMN, treated SMNRT mice gained weight to near wild-type levels during the first 4–5 weeks, and treated animals weighed as much as wild-type animals beyond ∼40 days (Fig. 7B–D). At nearly all time points, SMNRT-treated mice were able to right themselves more rapidly than untreated mice and were as efficient as the wild-type animals at time points beyond P10 (Fig. 8A–C). Differences in grip strength and rotarod performance were detected over the trial period, and as the animals aged, a greater disparity was observed between SMNRT-treated mice and unaffected animals. Similar to the SMNΔ7 experiments, the initiation of tail necrosis later in life (at approximately P70–80 for the SMNRT mice) may have negatively impacted their ability to perform in these assays (Fig. 8D and E).
Figure 6.
SMN protein induction in the milder mouse model of SMA (SMNRT). (A) Western blot (n = 3) showing increased SMN in spinal cord tissue of five (5) ICV-injected animals with E1MO-ASO. (B) Western blot quantification. Bar graph showing no significant difference in protein induction between unaffected and treated milder type SMART mice.
Figure 7.
SMNRT mice injected with E1MO-ASO showed significant improvement in survival and weight gain. (A) The treated SMNRT animals (labeled ‘SMNRT E1MO-ASO’) and the unaffected littermates (labeled ‘SMNRT Unaffected’) were substantially more vigorous and lived >180 days compared with the untreated mice (labeled ‘SMART Untreated’). The Kaplan–Meier survival curve depicts an identical in life expectancy for unaffected and treated SMNRT mice. Animals were culled after 180 days. (B) Significant increase in the average weight of SMNRT animal model after treatment with E1MO-ASO. (C) Comparison of the percent weight gained from birth to peak between animal groups. For statistical significance between each treatment group, Student's _t_-test was used and _P_-values are shown for E1MO-ASO SMNRT animals. (D) Individual weights on P12. Weights of treated mice are comparable with the weights of unaffected age-matched littermates.
Figure 8.
TTR, muscle strength, balance and motor-planning measurements show significant improvement in E1MO-ASO-treated SMNRT mice. (A) Percent of the tested animals able to right themselves compared with untreated mice where the TTR reflex is delayed. By P9, all treated and unaffected animals were able to right themselves. (B) Average time to right from P7 to P25 for all experimental groups. SMNRT mice injected with E1MO-ASO were able to right themselves within 10 s after 10 days. (C) Scatter plot of TTR performance of SMNRT mice injected with E1MO-ASO. Time-to-right performance of individual mice at age P12 shows that treated animals can successfully turn themselves within 5 s. (D) Grip strength measurements in grams. Treated animals were compared with their unaffected littermates. Measurements were taken from P25 through P108. (E) Rotarod performance test. The test was used to measure riding time parameter (in seconds) of the E1MO-ASO-treated animals and compared with the times of their age-matched unaffected controls. Measurements were taken from P26 through P110.
DISCUSSION
The presence of SMN2 has provided researchers with an array of therapeutic opportunities, including increasing promoter activity, stabilizing SMN2 RNA and/or protein and modulating SMN2 exon 7 splicing (22,24,25). ASOs have become important tools to modulate splicing in SMA with the overall goal of moving these compounds from pre-clinical models to the clinic. To date, the ASOs have exhibited a number of important properties that are common to successful candidate compounds, including compound/ASO stability, target specificity and a well-defined molecular pathway (SMN2 exon 7 splicing). To this end, in this report, we have developed a Morpholino-based ASO that targets the E1 regulatory region, which is upstream of SMN2 exon 7 and is distinct from the target of other ASO strategies, ISS-N1. While we have previously examined the utility of the E1 region as a potential therapeutic target using bi-functional RNAs, these RNAs were not chemically modified and therefore were not intrinsically stable, consistent with an initial burst in SMN protein levels that quickly waned and failed to elicit a robust extension in survival (17). Using the nuclease-resistant Morpholino backbone, the E1MO-ASO was not only able to modulate SMN2 exon 7 splicing and increase SMN in disease-relevant tissues such as the spinal cord, but E1MO-ASO was also able to largely correct the SMA pathology in two important models of disease: the severe SMNΔ7 and the ‘intermediate’ SMNRT mouse models.
The SMNΔ7 model is the gold standard in the SMA field; however, the inclusion of the SMNRT model was important as it likely represents a different SMA population and it also serves to validate the target engagement of the E1MO-ASO. While all of the E1MO-ASO SMNRT mice were alive at P175, it is noteworthy that all of the animals presented internal organ abnormalities, in particular swollen and enlarged bladders (Supplementary Material, Fig. S5). As there was only a single injection at birth of the ASO, the organ damage is likely not a consequence of the treatment because wild-type mice treated with the E1MO-ASO were completely normal, but rather the ASO activity was reduced over time owing to turnover of the cerebral spinal fluid, stability of the compound and normal tissue growth. SMNΔ7 mice treated with E1MO-ASO did also exhibit organ pathology; however, this was less frequent and was restricted to the longer lived animals (∼60+ days).
The dose that was initially administered was a 2 mm (35.15 µg ASO) ICV injection. However, a doubling of this dose via two ICV injections or an ICV+IP dosing further enhanced survival. Interestingly, the ICV+IP dosing was the most efficacious presumably because the IP administration allowed for a greater distribution to peripheral tissues. Similar results have been observed using ISS-N1 inhibiting ASOs (18,19) when delivered to the periphery via ICV injections and using scAAV9-SMN when delivered to the periphery via intramuscular injections (26). However, it is also important to stress that the SMA mouse models do not necessarily reflect the frequency of peripheral complications in most SMA patients. Currently, all of the SMA models exhibit profound peripheral organ defects, whereas peripheral organ damage in SMA patients is largely restricted to very severe SMA cases (27).
The overwhelming majority of ASO strategies have focused upon the intronic repressor downstream of SMN2 exon 7, ISS-N1. The exceptions include antisense molecules targeting the intron 7/exon 8 junction (28–30) and our previous report developing bifunctional RNAs that target E1 (17). Currently, the ASO developed by Isis Pharmaceuticals and colleagues has entered Phase II/III clinical trials. Additional Morpholino-based ASOs targeting the same genetic region have shown considerable promise in the SMNΔ7 model. Morpholino-based ASOs that induce exon-skipping in other disease contexts have already entered clinical trials, such as for Duchene Muscular Dystrophy (31), demonstrating the practicality of the Morpholino backbone and the relatively low toxicity associated with systemic administration of Morpholino-based ASOs. The current work targeting E1 is by no means designed to diminish these efforts that focus upon ISS-N1; rather, for such a complex and devastating disease like SMA, it is imperative to have as many well-validated pre-clinical successes as possible considering at this juncture there are no proven or approved SMA treatments. It will be particularly interesting going forward to determine whether combinations of ASOs elicit great responses, perhaps even lowering the doses for total ASO per individual. Alternatively, combining ASOs with small molecules may prove to be a potent combination. For example, ASOs have an exceptionally well-defined mode-of-action: SMN2 exon 7 splicing. By combining this activity with a small molecule that increases promoter activity or enhances SMN2 pre-mRNA stability, a synergistic activity could be observed. While it is clear that additional work needs to be done, ongoing efforts to successfully translate the exceptionally detailed and mechanistic understanding of SMN2 pre-mRNA splicing has reached the point at which bona fide candidate compounds are entering clinic and providing hope for SMA patients.
MATERIALS AND METHODS
Animal procedures and ASO delivery
All animals were housed and treated in accordance with Animal Care and Use Committee guidelines of the University of Missouri, Columbia, MO, USA. The colony was maintained as heterozygote breeding pairs under specific pathogen-free conditions. SMNΔ7 (SMNΔ7+/+;SMN2+/+;_Smn_−/−) and SMNRT (_SMN_RT+;SMN2+/+;_Smn_−/−) mice were genotyped on the day of birth (P1) using standard PCR protocol (JAX® Mice Resources) on tail tissue material. The following primer sets were used: for the mouse Smn gene, mSmn-WT forward (5′ tctgtgttcgtgcgtggtgacttt 3′) and mSmn-WT reverse (5′ cccaccacctaagaaagcctcaat 3′) and for the Smn knockout SMN1-KO forward (5′ ccaacttaatcgccttgcagcaca 3′) and SMN1-KO reverse (5′ aagcgagtggcaacatggaaatcg 3′). ICV injections were performed on P2, as previously described (32–35). Briefly, mice were immobilized via cryoanesthesia and injected using microliter-calibrated sterilized glass micropipettes. The injection site was ∼0.25 mm lateral to the sagittal suture and 0.50–0.75 mm rostral to the neonatal coronary suture. The needles were inserted perpendicular to the skull surface using a fiber-optic light (Boyce Scientific, Inc.) to aid in illuminating pertinent anatomical structures. Needles were removed after 10 s of discontinuation of plunger movement to prevent backflow. Treated animals were placed in a warmed container for recovery (5–10 min) until movement was restored. Single injections of 2 µl of the Morpholino-modified E1MO-ASOs were delivered via ICV injections as described earlier for all mice. Time-to-right (TTR) reflex tests were conducted as previously described (36). Each pup was placed onto its back, and the time it takes to right itself on the ground was recorded. The test was terminated at 30 s, and if an animal had not turned by this time, it was recorded as ‘Failure’. Righting reflex measurements were done daily starting at P7 because unaffected animals start to turn over at this time. For grip strength assessment, a grasping response test was utilized. Each pup's front paws were placed on a wire mesh (1-cm2 grids) and gently dragged horizontally along the mesh (BioSeb Model BP32025, Vitrolles, FR, EU & Pinellas Park, FL, USA). Any resistance felt was scored as a positive response. The strength of the animal holding onto the mesh before release was recorded in grams. Grip strength was measured every 3–4 days starting on P25. Motor activity and coordination were measured by utilizing rotarod treadmill for mice (IITC Rotarod Series 8, IITC Life Science, Inc., CA, USA). The animals were placed on textured drums to avoid slipping. When the tested animal dropped onto the individual sensing platform below, the test results were recorded in seconds. Measurements were performed every 3–4 days starting on P25.
Element1 antisense oligonucleotides (E1MO-ASO)
The following oligos were modified at every base with Morpholino chemistry groups (GeneTools L.L.C., Philomath, OR 97370, USA): E1MO-ASO (26-mer) 5′ CUA UAU AUA GAU AGU UAU UCA ACA AA 3′, and negative scrambled control provided and tested by GeneTools L.L.C. (25-mer), 5′ CCU CUU ACC UCA GUU ACA AUU UAU A 3′.
Immunohistochemistry of neuromuscular junctions
Immunochemistry and NMJ analysis were performed following a modified protocol described in detail previously (23,37). Three animals from each treatment and control groups at age P12 were anesthetized by anesthetic inhalant Isoflurane® USP, VetOne™ (1-chloro-2, 2,2-trifluoroethyl difluoromethyl ether; 50 mg/kg) followed by transcardiac perfusion with Phosphate-Buffered Saline solution (Dulbecco's, Gibco®, LifeTechnologies™, Carlsbad, CA, USA) and fixed with 4% paraformaldehyde (Sigma–Aldrich, St. Louis, MO, USA). Whole-mount preparations were done by dissecting and examining the longissimus capitis muscle. Tissues were stained using specific antibodies including anti-neurofilament (1:2000; Chemicon®, EMD Millipore, Billerica, MA, USA) and anti-synaptophysin (1:200, LifeTechnologies™). Acetylcholine receptors (AChRs) were labeled with Alexa Fluor 594-conjugated α-bungarotoxin (LifeTechnologies™). Muscle preparations were viewed using a laser scanning confocal microscope (40× objective; 0.8NA; Zeiss LSM Model 510 META, Carl Zeiss, Jena, Germany, EU). From the confocal microscopy, Z-series stack images of immunostained whole-mount muscles were obtained at sequential focal planes 1 μm apart and merged using microscope-integrated software and despeckled by ImageJ software, Fiji (38).
RT–PCR assays
Total RNA was isolated from brain tissues harvested on P7 and homogenized using TRIzol reagent (LifeTechnologies™). Two micrograms of total RNA was used to generate first-strand cDNA by using 100 ng of random primers, 2 µl dNTP (10 mm) Mix, 4 µl of 5× first-strand buffer, 1.0 µl DTT (0.1 m) and 1 µl SuperScript™ III reverse transcriptase (200U/µl) (LifeTechnologies™) at 50°C/50 min followed by reaction inactivation at 70°C/15 min. Cycling conditions were an initial denaturation step (94°C/3 min), 30 cycles (94°C/30 s, 60°C/0.5 min, 72°C/1 min) and a final extension step (72°C/10 min). Reaction products were resolved by electrophoresis through a 2.0% agarose gel (GeneMate, BioExpress, Kaysville, UT, USA) and visualized by ethidium bromide staining on FOTODYNE™ Imaging Systems (Hartland, WI, USA). For cDNA controls, specific plasmids pCIExSkip and pCIFL were used (5).
Western blots
For the SMNΔ7 mouse Western blots, indicated tissues were collected at selected time points (P7) and immediately frozen in liquid nitrogen. Tissue samples were placed at −80°C until ready for analysis. Roughly 100 mg of tissue was homogenized in JLB buffer [50 mm Tris–HCl, pH 7.5, 150 mm NaCl, 20 mm NaH2(PO4), 25 mm NaF, 2 mm EDTA, 10% glycerol, 1% Triton X-100 and protease inhibitors (Roche, Indianapolis, IN, USA)]. Equal amounts of protein were separated on 12% SDS–PAGE gels. SMN immunoblots were performed using a mouse SMN-specific monoclonal antibody (BD Biosciences, San Jose, CA, USA) diluted 1:300 in TBST [Tris-buffered Saline Tween20 (10 mm Tris–HCl, pH7.5, 150 mm NaCl, 0.2% Tween20)] in 1.5% dry milk. Then, blots were visualized by chemiluminescence on a FujiFilm imager LAS-3000 (FujiFilmUSA, Hanover Park, IL, USA) and the corresponding software. To verify equal loading, the Western blots were then stripped using H2O2 for 30 min at room temperature and re-probed with anti-β-actin rabbit and anti-rabbit HRP secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). Western blots were performed in quadruplicate or more and representative blots are shown. The signal intensity was measured for each band on an immunoblot, normalized to the loading control, and the fold increase was determined in relation to the appropriate control. Data were quantified utilizing FUJI FILM MultiGauge v. 2.0.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG online.
FUNDING
This work was supported by a grant from NIH/NINDS R54NS41584 (C.L.L.), a NIH Training Grant (M.R.M.) T32 GM008396 and a fellowship from the Howard Hughes Medical Institute to the University of Missouri SciXchange program (A.M.L.).
Supplementary Material
Supplementary Data
ACKNOWLEDGEMENTS
The authors thank John Marston (Department of Veterinary Pathobiology, University of Missouri, Columbia, MO, USA) for expert technical assistance in animal husbandry.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Crawford T.O., Pardo C.A. The neurobiology of childhood spinal muscular atrophy. Neurobiol. Dis. 1996;3:97–110. doi: 10.1006/nbdi.1996.0010. [DOI] [PubMed] [Google Scholar]
- 2.Feldkotter M., Schwarzer V., Wirth R., Wienker T.F., Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am. J. Hum. Genet. 2002;70:358–368. doi: 10.1086/338627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meister G., Eggert C., Fischer U. SMN-mediated assembly of RNPs: a complex story. Trends Cell Biol. 2002;12:472–478. doi: 10.1016/s0962-8924(02)02371-1. [DOI] [PubMed] [Google Scholar]
- 4.Pellizzoni L., Baccon J., Rappsilber J., Mann M., Dreyfuss G. Purification of native survival of motor neurons complexes and identification of Gemin6 as a novel component. J. Biol. Chem. 2002;277:7540–7545. doi: 10.1074/jbc.M110141200. [DOI] [PubMed] [Google Scholar]
- 5.Lorson C.L., Hahnen E., Androphy E.J., Wirth B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. USA. 1999;96:6307–6311. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monani U.R., Lorson C.L., Parsons D.W., Prior T.W., Androphy E.J., Burghes A.H., McPherson J.D. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 1999;8:1177–1183. doi: 10.1093/hmg/8.7.1177. [DOI] [PubMed] [Google Scholar]
- 7.Miyaso H., Okumura M., Kondo S., Higashide S., Miyajima H., Imaizumi K. An intronic splicing enhancer element in survival motor neuron (SMN) pre-mRNA. J. Biol. Chem. 2003;278:15825–15831. doi: 10.1074/jbc.M209271200. [DOI] [PubMed] [Google Scholar]
- 8.Chen H.H., Chang J.G., Lu R.M., Peng T.Y., Tarn W.Y. The RNA binding protein hnRNP Q modulates the utilization of exon 7 in the survival motor neuron 2 (SMN2) gene. Mol. Cell. Biol. 2008;28:6929–6938. doi: 10.1128/MCB.01332-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh N.K., Singh N.N., Androphy E.J., Singh R.N. Splicing of a critical exon of human survival motor neuron is regulated by a unique silencer element located in the last intron. Mol. Cell. Biol. 2006;26:1333–1346. doi: 10.1128/MCB.26.4.1333-1346.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kashima T., Manley J.L. A negative element in SMN2 exon 7 inhibits splicing in spinal muscular atrophy. Nat. Genet. 2003;34:460–463. doi: 10.1038/ng1207. [DOI] [PubMed] [Google Scholar]
- 11.Young P.J., DiDonato C.J., Hu D., Kothary R., Androphy E.J., Lorson C.L. SRp30c-dependent stimulation of survival motor neuron (SMN) exon 7 inclusion is facilitated by a direct interaction with hTra2 beta 1. Hum. Mol. Genet. 2002;11:577–587. doi: 10.1093/hmg/11.5.577. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann Y., Wirth B. hnRNP-G promotes exon 7 inclusion of survival motor neuron (SMN) via direct interaction with Htra2-beta1. Hum. Mol. Genet. 2002;11:2037–2049. doi: 10.1093/hmg/11.17.2037. [DOI] [PubMed] [Google Scholar]
- 13.Lorson C.L., Androphy E.J. An exonic enhancer is required for inclusion of an essential exon in the SMA-determining gene SMN. Hum. Mol. Genet. 2000;9:259–265. doi: 10.1093/hmg/9.2.259. [DOI] [PubMed] [Google Scholar]
- 14.Miyajima H., Miyaso H., Okumura M., Kurisu J., Imaizumi K. Identification of a cis-acting element for the regulation of SMN exon 7 splicing. J. Biol. Chem. 2002;277:23271–23277. doi: 10.1074/jbc.M200851200. [DOI] [PubMed] [Google Scholar]
- 15.Hua Y., Sahashi K., Rigo F., Hung G., Horev G., Bennett C.F., Krainer A.R. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature. 2011;478:123–126. doi: 10.1038/nature10485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rigo F., Hua Y., Krainer A.R., Bennett C.F. Antisense-based therapy for the treatment of spinal muscular atrophy. J. Cell Biol. 2012;199:21–25. doi: 10.1083/jcb.201207087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baughan T.D., Dickson A., Osman E.Y., Lorson C.L. Delivery of bifunctional RNAs that target an intronic repressor and increase SMN levels in an animal model of spinal muscular atrophy. Hum. Mol. Genet. 2009;18:1600–1611. doi: 10.1093/hmg/ddp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porensky P.N., Mitrpant C., McGovern V.L., Bevan A.K., Foust K.D., Kaspar B.K., Wilton S.D., Burghes A.H. A single administration of morpholino antisense oligomer rescues spinal muscular atrophy in mouse. Hum. Mol. Genet. 2012;21:1625–1638. doi: 10.1093/hmg/ddr600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou H., Janghra N., Mitrpant C., Dickinson R.L., Anthony K., Price L., Eperon I.C., Wilton S.D., Morgan J., Muntoni F. A novel morpholino oligomer targeting ISS-N1 improves rescue of severe spinal muscular atrophy transgenic mice. Hum. Gene. Ther. 2013;24:331–342. doi: 10.1089/hum.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le T.T., Pham L.T., Butchbach M.E., Zhang H.L., Monani U.R., Coovert D.D., Gavrilina T.O., Xing L., Bassell G.J., Burghes A.H. SMNDelta7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum. Mol. Gen. 2005;14:845–857. doi: 10.1093/hmg/ddi078. [DOI] [PubMed] [Google Scholar]
- 21.Osborne M., Lutz C. Reference Guide to Mouse Models of Spinal Muscular Atophy. The Jackson Laboratory Manual. 2013;17 [Epub ahead of print] [Google Scholar]
- 22.Lorson M.A., Lorson C.L. SMN-inducing compounds for the treatment of spinal muscular atrophy. Future Med. Chem. 2012;4:2067–2084. doi: 10.4155/fmc.12.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cobb M.S., Rose F.F., Rindt H., Glascock J.J., Shababi M., Miller M.R., Osman E.Y., Yen P.F., Garcia M.L., Martin B.R., et al. Development and characterization of an SMN2-based intermediate mouse model of Spinal Muscular Atrophy. Hum. Mol. Genet. 2013;22:1843–1855. doi: 10.1093/hmg/ddt037. [DOI] [PubMed] [Google Scholar]
- 24.Arnold W.D., Burghes A.H. Spinal muscular atrophy: development and implementation of potential treatments. Ann. Neurol. 2013;74:348–362. doi: 10.1002/ana.23995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porensky P.N., Burghes A.H. Antisense oligonucleotides for the treatment of spinal muscular atrophy. Hum. Gene. Ther. 2013;24:489–498. doi: 10.1089/hum.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benkhelifa-Ziyyat S., Besse A., Roda M., Duque S., Astord S., Carcenac R., Marais T., Barkats M. Intramuscular scAAV9-SMN injection mediates widespread gene delivery to the spinal cord and decreases disease severity in SMA mice. Mol. Ther. 2013;21:282–290. doi: 10.1038/mt.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shababi M., Lorson C.L., Rudnik-Schoneborn S.S. Spinal muscular atrophy: a motor neuron disorder or a multi-organ disease? J. Anat. 2014;224:15–28. doi: 10.1111/joa.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim S.R., Hertel K.J. Modulation of survival motor neuron pre-mRNA splicing by inhibition of alternative 3′ splice site pairing. J. Biol. Chem. 2001;276:45476–45483. doi: 10.1074/jbc.M107632200. [DOI] [PubMed] [Google Scholar]
- 29.Madocsai C., Lim S.R., Geib T., Lam B.J., Hertel K.J. Correction of SMN2 Pre-mRNA splicing by antisense U7 small nuclear RNAs. Mol. Ther. 2005;12:1013–1022. doi: 10.1016/j.ymthe.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 30.Dickson A., Osman E., Lorson C.L. A negatively acting bifunctional RNA increases survival motor neuron both in vitro and in vivo. Hum. Gene. Ther. 2008;19:1307–1315. doi: 10.1089/hum.2008.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cirak S., Arechavala-Gomeza V., Guglieri M., Feng L., Torelli S., Anthony K., Abbs S., Garralda M.E., Bourke J., Wells D.J., et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 2011;378:595–605. doi: 10.1016/S0140-6736(11)60756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glascock J.J., Osman E.Y., Coady T.H., Rose F.F., Shababi M., Lorson C.L. Delivery of therapeutic agents through intracerebroventricular (ICV) and intravenous (IV) injection in mice. J. Vis. Exp. 2011;3 doi: 10.3791/2968. pii: 2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osman E.Y., Yen P.F., Lorson C.L. Bifunctional RNAs targeting the intronic splicing silencer N1 increase SMN levels and reduce disease severity in an animal model of spinal muscular atrophy. Mol. Ther. 2012;20:119–126. doi: 10.1038/mt.2011.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Passini M.A., Bu J., Richards A.M., Kinnecom C., Sardi S.P., Stanek L.M., Hua Y., Rigo F., Matson J., Hung G., et al. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci. Transl. Med. 2011;3:72ra18. doi: 10.1126/scitranslmed.3001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coady T.H., Baughan T.D., Shababi M., Passini M.A., Lorson C.L. Development of a single vector system that enhances trans-splicing of SMN2 transcripts. PLoS ONE. 2008;3:e3468. doi: 10.1371/journal.pone.0003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butchbach M.E., Edwards J.D., Burghes A.H. Abnormal motor phenotype in the SMNDelta7 mouse model of spinal muscular atrophy. Neurobiol. Dis. 2007;27:207–219. doi: 10.1016/j.nbd.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ling K.K., Gibbs R.M., Feng Z., Ko C.P. Severe neuromuscular denervation of clinically relevant muscles in a mouse model of spinal muscular atrophy. Hum. Mol. Genet. 2012;21:185–195. doi: 10.1093/hmg/ddr453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data