Fragile hearts: New insights into translational control in cardiac muscle (original) (raw)
. Author manuscript; available in PMC: 2014 Nov 1.
Published in final edited form as: Trends Cardiovasc Med. 2013 Apr 10;23(8):275–281. doi: 10.1016/j.tcm.2013.03.003
Abstract
Current investigations focused on RNA-binding proteins in striated muscle, which provide a scenario whereby muscle function and development are governed by the interplay of post-transcriptional RNA regulation, including transcript localization, splicing, stability, and translational control. New data have recently emerged, linking the RNA-binding protein FXR1 to the translation of key cytoskeletal components such as talin and desmoplakin in heart muscle. These findings, together with a plethora of recent reports implicating RNA-binding proteins and their RNA targets in both basic aspects of muscle development and differentiation as well as heart disease and muscular dystrophies, point to a critical role of RNA-based regulatory mechanisms in muscle biology. Here we focus on FXR1, the striated muscle-specific member of the Fragile X family of RNA-binding proteins and discuss its newly reported cytoskeletal targets as well as potential implications for heart disease.
Controlling gene expression via RNA regulation
The processes of RNA splicing, export from the nucleus, transport, and translation are collectively referred to as RNA regulatory mechanisms and have been increasingly recognized as key steps in controlling gene expression during normal development and in disease states. Of these, localized translation is a gene expression mechanism initially described in developing oocytes, where axis polarity is controlled through protein synthesis of localized mRNAs (Wilhelm and Smibert, 2005). In neurons, local protein synthesis has been demonstrated to be a key mechanism for synaptic plasticity: mRNAs whose cognate proteins are required for the remodeling of synapses are localized at subsynaptic sites and translated locally, in response to synaptic input (Martin and Zukin, 2006). Similar mechanisms have been identified in yeast, migratory fibroblasts, epithelial cells, as well as in myoblasts to mediate local responses to internal or external cues (reviewed in Rodriguez et al., 2008). An underlying theme in all these examples is that local protein synthesis has evolved as a regulatory mechanism, which allows cells to carefully control the timing and localization of gene expression under circumstances where the canonical transcription, translation, and protein transport to the final destination are not sufficient. Indeed, in situ hybridization studies, for example, in Drosophila showed that 71% of mRNAs expressed in early embryos are subcellularly localized, suggesting that local protein synthesis is a major gene expression mechanism during development (Lecuyer et al., 2007). In mammalian cells, metabolic labeling coupled with mathematical modeling revealed that the cellular levels of mammalian proteins are predominantly controlled at the level of translation, and translation efficiency is the single best predictor of protein levels (Schwanhausser et al., 2011). These recent studies exemplify the critical need for the investigation of the role of translational control in development and disease, especially in cardiac muscle where this RNA regulatory mechanism remains significantly understudied.
Despite the early work of Fulton and L’Ecuyer, (1993) showing that local RNA translation is linked to sarcomere assembly (see later), the importance of RNA regulation in striated muscle is yet to come of age. Critical to this regulation are RNA-binding proteins that typically shuttle between the nucleus and the cytoplasm, associate directly with mRNAs, and control various aspects of RNA metabolism. Recent findings implicating the RNA-binding proteins RBM20 and muscleblind protein, splicing factors, and FXR1, a translational regulator in heart development and myopathy are reigniting attention to the magnitude of RNA-based mechanisms in the heart (Guo et al., 2012; Mientjes et al., 2004; Van’t Padje et al., 2009; Wang et al., 2012; Whitman et al., 2011). Mutations in RBM20 in rat and human lead to dilated cardiomyopathy (DCM) as a result of pathological, aberrant splicing of reportedly 30 transcripts encoding proteins such as the giant sarcomeric protein titin, as well as other sarcomeric proteins, and proteins linked to cardiomyopathy and ion homeostasis (Guo et al., 2012). While precise mechanisms leading to DCM in RBM20 mutants remain to be established, this recent report underscores both the importance and the complexity of RNA regulation in the heart. Here we focus on the role of translational control in the heart and highlight recent findings demonstrating that the RNA-binding protein, FXR1, the sole member of the Fragile X protein family to be expressed in striated muscle is required to regulate the translation of cytoskeletal assemblies including both costameric and junctional complex proteins during heart development (Whitman et al., 2011). We also discuss implications of RNA dysregulation with a focus on cardiovascular diseases.
Building a muscle: Localized translation and co-translational assembly
A large fraction of mRNAs (e.g., 71% in Drosophila epithelia) exhibit specific subcellular distributions, which appear to be facilitated by sequence-specific RNA-binding proteins via _cis_-regulatory elements located primarily within 3′ untranslated regions (UTRs) (Lecuyer et al., 2007). Localized translation of mRNAs is believed to be important for correct protein localization and assembly of protein complexes (Besse and Ephrussi, 2008; Lecuyer et al., 2007). Several lines of evidence suggest that localized translation and co-translational assembly of cytoskeletal components including integral sarcomeric and costameric proteins occurs in muscle. The first support for this model came in the 1960s from observations of electron micrographs, which revealed alignment of polyribosomes along myosin thick filaments in differentiating skeletal myofibrils, and biochemical isolation of polyribosomes that were shown to be involved in the synthesis of sarcomeric myosin (Allen and Terrence, 1968; Heywood et al., 1967). About 2 decades later, it was reported that hypertrophy led to an increase in polyribosomes within the A band (myosin thick filament location) of sarcomeres (Horne and Hesketh, 1990). Subsequent investigations led primarily by Fulton and colleagues provided additional data to support the notion that myosin and other cytoskeletal proteins may be integrated into myofibrils via co-translational assembly. First, mRNAs encoding several sarcomeric (titin, myosin, and nebulin) and costameric proteins (vimentin, desmin, and vinculin) were shown to colocalize with their cognate proteins in a striated pattern (reviewed in Fulton and L’Ecuyer, 1993), suggesting that they may be anchored and translated on site. Other studies revealed that mRNA localization is preceded by that of the cognate proteins, which may provide targeting and assembly cues for nascent peptides emerging from polyribosomes (reviewed in Fulton and L’Ecuyer, 1993). Titin, the largest protein identified in humans to date (up to 38,138 residues or a 4.2 MDa protein: Bang et al., 2001), provides a remarkable example of how local translation might solve the tremendous challenge to the protein trafficking machinery to transport the full-length protein within the myofibril and then to correctly assemble it in a highly structured contractile unit, the sarcomere. Second, pulse chase labeling followed by puromycin treatment showed that as nascent peptides encoding sarcomeric proteins emerge from polyribosomes, they are found in close proximity to their final destination where they associate with the preassembled fully translated protein product (Fulton and L’Ecuyer, 1993). Third, a combination of crosslinking and immunoprecipitation experiments showed that myosin and titin are within 15 Å of each other after only minutes of inhibiting protein synthesis with puromycin (Fulton and L’Ecuyer, 1993). Together, these observations are consistent with the assembly of contractile proteins within the constraints of sarcomeres and right off the polyribosomes hence the name of “co-translational assembly model” (Fig. 1).
Fig. 1.
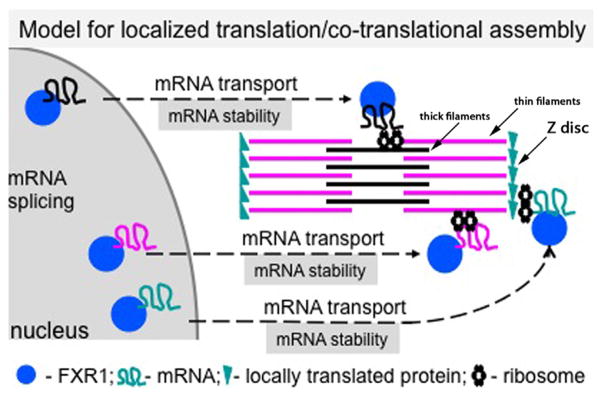
Model for localized translation/co-translational assembly in heart muscle. FXR1 (and other RNA-binding proteins, not shown) associate with target mRNAs in the nucleus. Protein-mRNA complexes are exported into the sarcoplasm and localized in the vicinity of local translation sites (marked by ribosomes). Regulatory mechanisms include mRNA transport and stability. mRNA anchoring may be controlled as well (not shown). Next, through the coupling of localization and translation processes, newly synthesized peptides assemble directly into complex cytoskeletal assemblies such as the sarcomere. (Color version of figure is available online.)
Kinetic arguments can also be made to support the localized co-translational assembly concept based on an observation that in fibroblasts the incorporation of the intermediate filament protein vimentin into the cytoskeleton occurs at a higher rate than its depletion from the soluble cytosolic pool (Fulton and Wan, 1983). Thus, there is now significant evidence mostly from the analysis of striated muscle cytoskeletal assemblies that cellular proteins can be assembled not only from a soluble cytoplasmic pool, but also from peptides translated and incorporated on site (Fig. 1). These mechanisms are not mutually exclusive; one may be chosen over the other in different developmental states or in response to insults that may be relevant in disease states. In fact, recent studies in yeast showed that a significant percentage (~40%) of proteins associate with mRNAs coding for their protein partners, which supports the notion that several protein complexes including multimeric membrane channels, some soluble proteins (p53 and Nf-κB), and the myosin-specific chaperone Rng3p assemble immediately following translation (Duncan and Mata, 2011). Thus, co-translational assembly appears to be a widespread mechanism and may be critical for the assembly of large protein complexes and/or might be important in instances where a protein is unstable on its own. Elucidating the underlying molecular mechanisms that dictate these choices is critical in understanding myogenesis in health and disease.
The requirements for post-transcriptional gene regulation in particular via RNA-binding proteins are significantly understudied in striated muscle, in comparison with other fields like neurobiology, inflammation, and cancer. However, several studies have in fact demonstrated the importance of translational control in striated muscle in health and disease, mostly in skeletal muscle. Most of the studies in skeletal muscle have focused on the ability to fine-tune protein synthesis in response to exercise and/or nutrient availability (Norton and Layman, 2006). Other reports demonstrate the need for precise regulation of RNA splicing during development and disease with the best studied of these examples being myotonic dystrophy (DM), the most common worldwide autosomal dominant muscular dystrophy (for recent review see Romeo, 2012). In fact, recent transcriptome-wide analysis uncovered a global role for muscleblind-like (Mbnl) protein in regulating not only the hundreds of pre-mRNA splicing events in muscle but also the localization of mRNAs to membranes in both mouse and Drosophila cells. These data are significant since they link Mbnl with the large percentage of aberrant splicing patterns associated with DM. An excellent recent review by Apponi et al., (2011) is focused on the roles of RNA-binding proteins in skeletal myogenesis, so this field will not be discussed here.
Fragile X-related protein 1 (FXR1): A member of the Fragile X family of RNA-binding proteins
The mammalian Fragile X (FraX) protein family is comprised of 3 highly homologous members, Fragile X Mental Retardation Protein (FMRP) and Fragile X-Related Proteins 1 and 2 (FXR1 and FXR2, respectively). FMRP has been extensively studied due to its linkage to the most common form of inherited mental retardation, Fragile X syndrome as well as autism (reviewed in Kim and Ceman, 2012). Although there are common structural and functional features among FraX family members, the development of isoform-specific antibodies and the identification of specific repertoires of associated mRNAs revealed clear differences between FraX family members, suggesting that they regulate their own specific set of targets and harbor specific as well as shared functions (Darnell et al., 2011).
The FraX proteins are highly conserved throughout evolution and consist of an Agenet domain, Nuclear Localization, and Nuclear Export Sequences (NLS and NES), a dimerization domain, a ribosomal binding site, and 2 types of RNA-binding domains: KH1,2 and RGG (Fig. 2) (Kim and Ceman, 2012). Systematic Evolution of Ligands by Exponential Enrichment (SELEX) approaches have identified sequences and structures specifically bound by FraX proteins in vitro: the KH2 domain binds RNAs that can fold into a structure known as a “kissing complex” while the more C-terminal “RGG” box binds to mRNAs that can form G-quartets (Brown et al., 2001; Darnell et al., 2005; Darnell et al., 2001). Recently, using a photo-activatable ribonucleoside-enhanced crosslinking and immunoprecipitation (PAR-CLIP) approach, the Tuschl group performed a genome-wide search for RNA recognition elements (RREs) bound in vivo by the FraX family of proteins (Ascano et al., 2012). Computational sequence analyses revealed that all FraX proteins bind 2 major RREs, namely ACUK and WGGA (in which K = G or U and W = A or U) that reside primarily within exons, particularly within coding and 3′ UTR regions. With these new findings in hand, experimentalists now have the tools to determine physiologically significant targets in developmental and functional contexts of interest.
Fig 2.
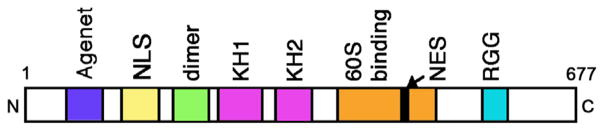
FXR1 protein structure. FXR1 is a nucleo-cytoplasmic shuttling protein and is comprised of 3 RNA-binding domains: KH1, KH2, and RGG (as shown). For more information on the other protein motifs, see text. (Color version of figure is available online.)
Biochemical purification and fractionation experiments have revealed that FXR1 and its family members associate with RiboNuclear Protein particles (RNPs) and polyribosomes, suggesting that FraX proteins regulate the transport and translation of target mRNAs (Feng et al., 1997; Khandjian et al., 1998). Indeed, live cell imaging experiments have confirmed the role of FraX proteins in RNA transport, while biochemical and genetic approaches have confirmed their role as both positive and negative regulators of translation (Darnell et al., 2011; Dictenberg et al., 2008; Estes et al., 2008). In this regard, FXR1 has been shown to bind with high-affinity AU-rich elements containing messages such as TNF-α, for which it acts as a translational activator (Vasudevan and Steitz, 2007). Furthermore, FXR1 was recently shown to be required for miRNA-mediated control of translation activation in a Xenopus oocyte heterologous system (Mortensen et al., 2011).
Like FMRP, FXR1 binds directly with its own 3′ UTR, suggesting that FXR1 may regulate its own expression (Ashley et al., 1993; Whitman et al., 2011). While not much else is known about the regulation of FXR1 itself, Ceman and colleagues have recently revealed a mechanism whereby FXR1 (but not FMRP) expression is controlled directly by the miRNA pathway. Specifically, using a conditional Dicer knockdown cell line, FXR1 (but not FMRP) was shown to be regulated via the miR-25/32/92/363/367 seed sequence in its 3′ UTR (Cheever et al., 2010). These studies together with findings that FXR1 (but not FMRP) regulates miR-9 and miR-124 in the brain (Xu et al., 2011), suggesting that FXR1 may act as a critical node for controlling gene expression by as well as through the miRNA pathway.
More studies are needed to establish the degree of similarity between FXR1 and FMRP as well as their unique targets, how they are each regulated, and their functional features. To date, there are only a few reports on the functional properties of FXR1 in striated muscle, and recent molecular evidence points to FXR1 as a negative regulator of translation (see later). The current data suggest that FXR1 is involved in heart-specific processes, especially in pathways involving key components of cytoskeletal structures unique to striated muscle, and that its misregulation leads to features of cardiomyopathy.
FXR1 in striated muscle
FXR1 is ubiquitously expressed during early mouse embryogenesis and becomes primarily expressed in skeletal and cardiac muscle toward the end of the gestation period (Bakker et al., 2000). Notably, FXR1 is the sole FraX family member that is highly enriched in skeletal and cardiac muscle (Dube et al., 2000; Khandjian et al., 1998; Mientjes et al., 2004). In cultured cells, the FXR1 locus encodes 7 different isoforms expressed at different levels depending on individual cell types (Khandjian et al., 1998). Most widely expressed are 2 short isoforms (70 and 74 kD) and 2 long isoforms (78 and 80 kD) (Khandjian et al., 1998). During myogenesis in culture, 2 “muscle-specific” isoforms have been detected (82 and 84 kD), which show a dynamic distribution and colocalize with the Z-disc protein α-actinin and the focal adhesion molecule, vinculin at Z-discs (Khandjian et al., 1998; Mientjes et al., 2004; Whitman et al., 2011).
Studies in animal models have shown that FXR1 is required for the structural integrity and function of skeletal and heart muscle. In Xenopus laevis, reduction of FXR1 altered MyoD expression and somite formation, while reintroduction of long and short FXR1 mRNA variants rescued these muscle-specific effects (Huot et al., 2005). In zebrafish, reduction of FXR1 protein levels by morpholino treatment results in muscular dystrophy and cardiomyopathy (Van’t Padje et al., 2009). In the absence of FXR1, the zebrafish heart exhibits abnormal looping, snapping of the atrium from the venous pole, as well as abnormal heartbeat (Van’t Padje et al., 2009). Furthermore, the kinase PAK1 [the first documented interacting partner for the KH(2) domain of FXR1] was found to phosphorylate FXR1, and this interaction was found to be essential for FXR1 function in striated muscle in zebrafish (Say et al., 2010). Studies in mice demonstrate that FXR1 expression is essential for postnatal viability; knockout of FXR1 (Fxr1 KO) leads to reduced skeletal muscle mass and perinatal lethality likely due to cardiac or respiratory failure (Mientjes et al., 2004). Although no definitive explanation exists for the lethal phenotype, FXR1’s localization at Z-discs and costameres suggests a model whereby it controls the assembly and maintenance of cytoskeletal assemblies linked to lateral force transmission and contraction in the heart (Mientjes et al., 2004). With respect to the consequences of FXR1 misregulation in humans, very little is known. However, FXR1 expression has been shown to be altered in myoblasts from patients with facioscapulohumeral muscular dystrophy (FSHD), suggesting that FXR1 may be involved in this human muscle disorder (Davidovic et al., 2008). Despite that collectively, these studies underscore the importance of FXR1 in striated muscle development and disease, the functional role of the protein and the identity of mRNA targets in striated muscle have remained unknown until recently.
FXR1 and its role in heart structure and function
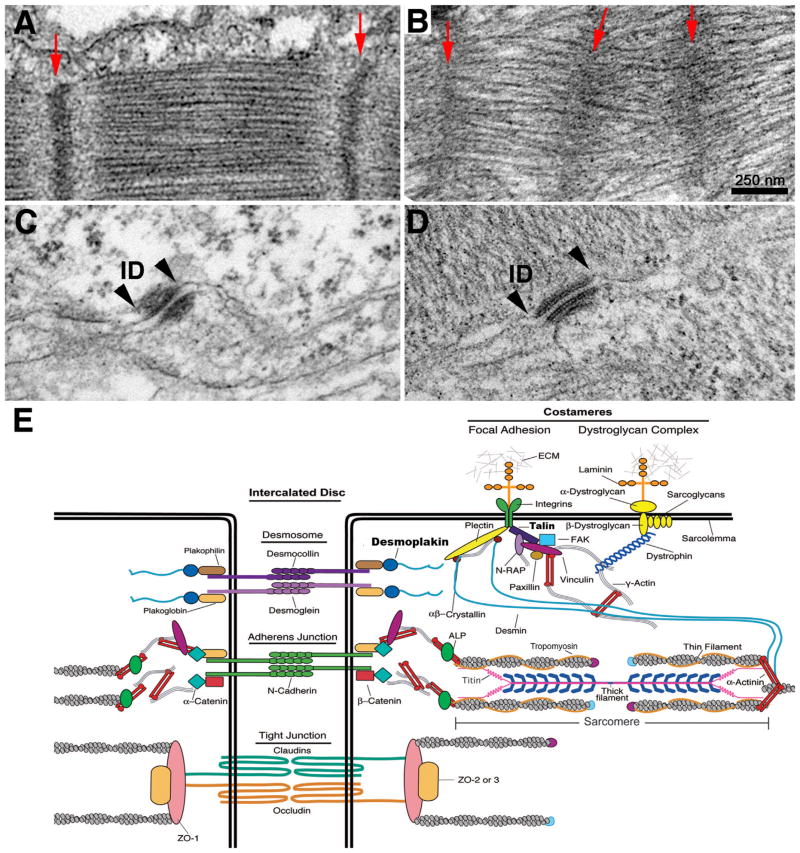
Recently, high-resolution ultrastructural phenotypic characterization of Fxr1 KO embryonic mouse hearts revealed significantly altered desmosomes, gap junctions, costameric architecture, sarcomere spacing, and myofibril alignment (Fig. 3A–D). Consistent with these observations, the first direct FXR1 mRNA targets in cardiac muscle were also discovered: the desmosome-associated protein, desmoplakin, and the costameric protein, talin 2. In vitro RNA-binding and luciferase assays demonstrated that the 3′ UTR regions of desmoplakin and talin 2 are directly bound by FXR1, which in turn represses their translation (Whitman et al., 2011). Given that the steady-state levels of desmoplakin and talin 2 mRNA remain unaltered in the absence of FXR1, these results support translational repression as a novel mechanism for regulating heart muscle development and function, in particular the assembly of specialized cytoskeletal structures. In addition, the distribution of FXR1 in a striated pattern at the Z-disc as well as in various other locations in heart muscle supports the notion that FXR1 may regulate multiple mRNA targets locally. This is consistent with the identification of several candidate mRNA targets that encode cytoskeletal components and provide additional support to the model of co-translational assembly (Fig. 1).
Fig. 3.
FXR1 is required for the structural integrity of junctional complexes and sarcomeres in the heart. (A and B) Electron micrographs of E18.5 mouse heart wild-type myofibrils (A) exhibit well-organized sarcomeres with tightly bundled filaments and parallel evenly spaced, narrow Z-discs (red arrows) compared with misaligned Fxr1 KO myofibrils (B), which contain shorter sarcomeres with broad Z-discs and less organized thick filaments (arrows denote Z-discs). (C and D) View of the inner dense plaque of desmosomes in wild-type (C) versus Fxr1 KO (D) E18.5 hearts. Note prominent electron dense material in wild-type (delineated by arrowheads), where intermediate filaments anchor to the desmosomal membrane compared with the inner reduced plaques in the KO hearts. (E) Schematic of cytoskeletal assemblies in heart muscle. Proteins in bold are reported targets of FXR1.
RNA-based mechanisms in muscle: An emerging view for muscle development and function
Large cytoskeletal assemblies are critical for the structure and function of all cells and consist of many cellular proteins whose functions must be tightly coordinated. However, little is known about how these assemblies form. One model that has support, mostly from striated muscle, is that proteins rather than being fully translated and released into the cytoplasm before finding their final destination, could interact as they are being translated. Recent studies on the FraX family member, FXR1, support translational repression as a novel mechanism for regulating heart muscle development and function, in particular the assembly of specialized cytoskeletal structures. Although to date, there are no reports of FXR1 mutations or truncations associated with human cardiac disease, the Fxr1 KO phenotypes identified in animal models demonstrate a critical role for FXR1 in the heart. Phenotypic analyses in mice and fish indicate that the absence of FXR1 leads to both developmental and functional cardiac abnormalities. Therefore, it will be important in the future to determine whether FXR1 may play a role in the etiology of human heart disease. There is precedent for RNA-binding proteins such as Rbm20, which regulates splicing to be linked to DCM (Guo et al., 2012). Another example is Inclusion body myopathy with early-onset Paget disease and frontotemporal dementia (IBMPFD), a myopathy accompanied by pathological inclusions that contain the RNA-binding protein TDP-43. Interestingly, TDP-43 and FXR1 associate in a complex (Freibaum et al., 2010), suggesting the possibility that FXR1 may share some pathological links to TDP-43 that remain to be explored. Furthermore, since miRNAs are intimately linked to myoblast proliferation, differentiation, and response to stress in the heart (reviewed in van Rooij et al., 2008) and since several muscular dystrophies, including Duchenne’s Muscular Dystrophy, Nemaline Myopathy, and others, have also been correlated with mis-expression of muscle-specific miRNAs (Eisenberg et al., 2007), it is tempting to speculate that FXR1 (like its FraX relative, FMRP) may be involved in the miRNA pathway and as a consequence, heart-specific miRNAs and their mRNA targets may be misexpressed. In summary, deciphering the functional roles of specific RNA-binding proteins will present insights not only into basic mechanisms regulating gene expression in muscle, but also into the etiology and pathology of muscle disease. This new knowledge about the involvement of RNA-binding proteins and their targets in striated muscle will provide novel future opportunities for therapeutic strategies.
Acknowledgments
The authors would like to thank Dr. Samantha Whitman for acquiring the electron microscopy images, and Drs. Miensheng Chu and Katie Bliss for preparing Fig. 3E. This work is funded by National Institutes of Health (to CG, HL108625), the American Heart Association (to DCZ, 0930170N), and Sarver Heart Center Grants (to DCZ, Schneider Family and Darlene and Kalidas Madhavpeddi Awards).
References
- Allen ER, Terrence CF. Immunochemical and ultrastructural studies of myosin synthesis. Proceedings of the National Academy of Sciences. 1968;60:1209–15. doi: 10.1073/pnas.60.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apponi LH, Corbett AH, Pavlath GK. RNA-binding proteins and gene regulation in myogenesis. Trends in Pharmacological Sciences. 2011;32:652–8. doi: 10.1016/j.tips.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascano M, Jr, Mukherjee N, Bandaru P, et al. FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature. 2012;492:382–6. doi: 10.1038/nature11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley CT, Jr, Wilkinson KD, Reines D, Warren ST. FMR1 protein: conserved RNP family domains and selective RNA binding. Science. 1993;262:563–6. doi: 10.1126/science.7692601. [DOI] [PubMed] [Google Scholar]
- Bakker CE, de Diego Otero Y, Bontekoe C, et al. Immunocytochemical and biochemical characterization of FMRP, FXR1P, and FXR2P in the mouse. Experimental Cell Research. 2000;258:162–70. doi: 10.1006/excr.2000.4932. [DOI] [PubMed] [Google Scholar]
- Bang ML, Centner T, Fornoff F, et al. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circulation Research. 2001;89:1065–72. doi: 10.1161/hh2301.100981. [DOI] [PubMed] [Google Scholar]
- Besse F, Ephrussi A. Translational control of localized mRNAs: restricting protein synthesis in space and time. Nature Reviews Molecular Cell Biology. 2008;9:971–80. doi: 10.1038/nrm2548. [DOI] [PubMed] [Google Scholar]
- Brown V, Jin P, Ceman S, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–87. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Cheever A, Blackwell E, Ceman S. Fragile X protein family member FXR1P is regulated by microRNAs. RNA. 2010;16:1530–9. doi: 10.1261/rna.2022210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Fraser CE, Mostovetsky O, et al. Kissing complex RNAs mediate interaction between the Fragile-X mental retardation protein KH2 domain and brain polyribosomes. Genes and Development. 2005;19:903–18. doi: 10.1101/gad.1276805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–99. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–61. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovic L, Sacconi S, Bechara EG, et al. Alteration of expression of muscle specific isoforms of the fragile X related protein 1 (FXR1P) in facioscapulohumeral muscular dystrophy patients. Journal of Medical Genetics. 2008;45:679–85. doi: 10.1136/jmg.2008.060541. [DOI] [PubMed] [Google Scholar]
- Dictenberg JB, Swanger SA, Antar LN, Singer RH, Bassell GJ. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Developmental Cell. 2008;14:926–39. doi: 10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube M, Huot M-E, Khandjian EW. Muscle specific fragile X related protein 1 isoform are sequestered in the nucleus of undifferentiated myoblasts. BMC Genetics. 2000;1:4. doi: 10.1186/1471-2156-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan CD, Mata J. Widespread cotranslational formation of protein complexes. PLoS Genetics. 2011;7:e1002398. doi: 10.1371/journal.pgen.1002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg I, Eran A, Nishino I, et al. Distinctive patterns of microRNA expression in primary muscular disorders. Proceedings of the National Academy of Sciences. 2007;104:17016–21. doi: 10.1073/pnas.0708115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes PS, O’Shea M, Clasen S, Zarnescu DC. Fragile X Protein controls the efficacy of mRNA transport in Drosophila neurons. Molecular and Cellular Neuroscience. 2008;39:170–9. doi: 10.1016/j.mcn.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersch SM. Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes. Journal of Neuroscience. 1997;17:1539–47. doi: 10.1523/JNEUROSCI.17-05-01539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freibaum BD, Chitta RK, High AA, Taylor JP. Global analysis of TDP-43 interacting proteins reveals strong association with RNA splicing and translation machinery. Journal of Proteome Research. 2010;9:1104–20. doi: 10.1021/pr901076y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton AB, L’Ecuyer TL. Cotranslational assembly of some cytoskeletal proteins: implications and prospects. Journal of Cell Science. 1993;105:867–71. doi: 10.1242/jcs.105.4.867. [DOI] [PubMed] [Google Scholar]
- Fulton AB, Wan KM. Many cytoskeletal proteins associate with the hela cytoskeleton during translation in vitro. Cell. 1983;32:619–25. doi: 10.1016/0092-8674(83)90481-6. [DOI] [PubMed] [Google Scholar]
- Guo W, Schafer S, Greaser ML, et al. RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing. Nature Medicine. 2012;18:766–73. doi: 10.1038/nm.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood SM, Dowben RM, Rich A. The identification of polyribosomes synthesizing myosin. Proceedings of the National Academy of Sciences. 1967;57:1002–9. doi: 10.1073/pnas.57.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne Z, Hesketh J. Increased association of ribosomes with myofibrils during the skeletal-muscle hypertrophy induced either by the beta-adrenoceptor agonist clenbuterol or by tenotomy. Biochemical Journal. 1990;272:831–3. doi: 10.1042/bj2720831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot ME, Bisson N, Davidovic L, et al. The RNA-binding protein fragile X-related 1 regulates somite formation in Xenopus laevis. Molecular Biology of the Cell. 2005;16:4350–61. doi: 10.1091/mbc.E05-04-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandjian EW, Bardoni B, Corbin F, et al. Novel isoforms of the fragile X related protein FXR1P are expressed during myogenesis. Human Molecular Genetics. 1998;7:2121–8. doi: 10.1093/hmg/7.13.2121. [DOI] [PubMed] [Google Scholar]
- Kim M, Ceman S. Fragile X mental retardation protein: past, present and future. Current Protein Peptide Science. 2012;13:358–71. doi: 10.2174/138920312801619420. [DOI] [PubMed] [Google Scholar]
- Lecuyer E, Yoshida H, Parthasarathy N, et al. Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell. 2007;131:174–87. doi: 10.1016/j.cell.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Martin KC, Zukin RS. RNA trafficking and local protein synthesis in dendrites: an overview. Journal of Neuroscience. 2006;26:7131–4. doi: 10.1523/JNEUROSCI.1801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mientjes EJ, Willemsen R, Kirkpatrick LL, et al. Fxr1 knockout mice show a striated muscle phenotype: implications for Fxr1p function in vivo. Human Molecular Genetics. 2004;13:1291–302. doi: 10.1093/hmg/ddh150. [DOI] [PubMed] [Google Scholar]
- Mortensen RD, Serra M, Steitz JA, Vasudevan S. Posttranscriptional activation of gene expression in Xenopus laevis oocytes by microRNA-protein complexes (microRNPs) Proceedings of the National Academy of Sciences. 2011;108:8281–6. doi: 10.1073/pnas.1105401108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton LE, Layman DK. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. Journal of Nutrition. 2006;136:533S–7S. doi: 10.1093/jn/136.2.533S. [DOI] [PubMed] [Google Scholar]
- Rodriguez AJ, Czaplinski K, Condeelis JS, Singer RH. Mechanisms and cellular roles of local protein synthesis in mammalian cells. Current Opinion in Cell Biology. 2008;20:144–9. doi: 10.1016/j.ceb.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo V. Myotonic dystrophy type 1 or Steinert’s disease. Advances in Experimental Medicine and Biology. 2012;724:239–57. doi: 10.1007/978-1-4614-0653-2_18. [DOI] [PubMed] [Google Scholar]
- Say E, Tay HG, Zhao ZS, et al. A functional requirement for PAK1 binding to the KH(2) domain of the fragile X protein-related FXR1. Molecular Cell. 2010;38:236–49. doi: 10.1016/j.molcel.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Schwanhausser B, Busse D, Li N, et al. Global quantification of mammalian gene expression control. Nature. 2011;473:337–42. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Liu N, Olson EN. MicroRNAs flex their muscles. Trends in Genetics. 2008;24:159–66. doi: 10.1016/j.tig.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Van’t Padje S, Chaudhry B, Severijnen LA, et al. Reduction in fragile X related 1 protein causes cardiomyopathy and muscular dystrophy in zebrafish. Journal of Experimental Biology. 2009;212:2564–70. doi: 10.1242/jeb.032532. [DOI] [PubMed] [Google Scholar]
- Vasudevan S, Steitz JA. AU-rich-element-mediated upregulation of translation by FXR1 and Argonaute 2. Cell. 2007;128:1105–18. doi: 10.1016/j.cell.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, Cody NA, Jog S, et al. Transcriptome-wide regulation of pre-mRNA splicing and mRNA localization by muscleblind proteins. Cell. 2012;150:710–24. doi: 10.1016/j.cell.2012.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman SA, Cover C, Yu L, Nelson DL, Zarnescu DC, Gregorio CC. Desmoplakin and talin2 are novel mRNA targets of fragile X-related protein-1 in cardiac muscle. Circulation Research. 2011;109:262–71. doi: 10.1161/CIRCRESAHA.111.244244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm JE, Smibert CA. Mechanisms of translational regulation in Drosophila. Biology of the Cell. 2005;97:235–52. doi: 10.1042/BC20040097. [DOI] [PubMed] [Google Scholar]
- Xu XL, Zong R, Li Z, et al. FXR1P but not FMRP regulates the levels of mammalian brain-specific microRNA-9 and microRNA-124. Journal of Neuroscience. 2011;31:13705–9. doi: 10.1523/JNEUROSCI.2827-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]