Disassembly of mitotic checkpoint complexes by the joint action of the AAA-ATPase TRIP13 and p31comet (original) (raw)
Significance
The mitotic checkpoint system has an important role to ensure accurate segregation of chromosomes in mitosis. This system regulates the activity of the ubiquitin ligase Anaphase-Promoting Complex/Cyclosome (APC/C) by the formation of a negatively acting Mitotic Checkpoint Complex (MCC). When the checkpoint is satisfied, MCC is disassembled, but the mechanisms of MCC disassembly are not well understood. We show here that the ATP-hydrolyzing enzyme Thyroid Receptor Interacting Protein 13 (TRIP13), along with the MCC-targeting protein p31comet, promote the disassembly of the mitotic checkpoint complexes and the inactivation of the mitotic checkpoint. The results reveal an important molecular mechanism in the regulation of APC/C by the mitotic checkpoint.
Keywords: mitosis, spindle checkpoint
Abstract
The mitotic (or spindle assembly) checkpoint system delays anaphase until all chromosomes are correctly attached to the mitotic spindle. When the checkpoint is active, a Mitotic Checkpoint Complex (MCC) assembles and inhibits the ubiquitin ligase Anaphase-Promoting Complex/Cyclosome (APC/C). MCC is composed of the checkpoint proteins Mad2, BubR1, and Bub3 associated with the APC/C activator Cdc20. When the checkpoint signal is turned off, MCC is disassembled and the checkpoint is inactivated. The mechanisms of the disassembly of MCC are not sufficiently understood. We have previously observed that ATP hydrolysis is required for the action of the Mad2-binding protein p31comet to disassemble MCC. We now show that HeLa cell extracts contain a factor that promotes ATP- and p31comet-dependent disassembly of a Cdc20–Mad2 subcomplex and identify it as Thyroid Receptor Interacting Protein 13 (TRIP13), an AAA-ATPase known to interact with p31comet. The joint action of TRIP13 and p31comet also promotes the release of Mad2 from MCC, participates in the complete disassembly of MCC and abrogates checkpoint inhibition of APC/C. We propose that TRIP13 plays centrally important roles in the sequence of events leading to MCC disassembly and checkpoint inactivation.
The mitotic (or spindle assembly) checkpoint is a surveillance system that prevents premature separation of sister chromatids in mitosis and thus ensures the fidelity of chromosome segregation. It monitors the existence of chromatids that are not attached yet correctly to the mitotic spindle through their kinetochores and delays anaphase until correct bipolar attachment is achieved. It acts by inhibiting the Anaphase Promoting Complex/Cyclosome (APC/C), a ubiquitin ligase that targets for degradation cyclin B and securin, an inhibitor of anaphase initiation. APC/C is inhibited by the Mitotic Checkpoint Complex (MCC), which consists of the checkpoint proteins Mad2, BubR1, and Bub3 associated with the APC/C activator Cdc20 (reviewed in refs. 1–4). The molecular mechanisms of the assembly of MCC when the checkpoint is turned on, and of its disassembly when the checkpoint is turned off, are not sufficiently understood. A key event in MCC formation appears to be a marked conformational transition in Mad2 promoted by the checkpoint signal. Mad2 exists in two conformations: an open inactive form (O-Mad2) and a closed active form (C-Mad2). C-Mad2 binds to Cdc20 in a structure in which the C-terminal tail of Mad2 is tightly wrapped around its partner (5, 6). It is thought that the active mitotic checkpoint stimulates the formation of the C-Mad2–Cdc20 subcomplex, which then combines with the constitutively present BubR1–Bub3 subcomplex to form the MCC (2, 3). The structure of MCC shows extensive binding interfaces between BubR1 and Cdc20, as well as between Cdc20 and C-Mad2 (7). In addition, C-Mad2 also binds to BubR1 (7, 8), thus stabilizing the structure of MCC.
We have been studying the mechanisms of the disassembly of MCC that are necessary for the inactivation of the mitotic checkpoint. We observed that ATP cleavage at the β−γ position was required for the disassembly of MCC and for the release of APC/C from checkpoint inhibition (9). It was furthermore found that ATP is also required for the action of p31comet to stimulate the disassembly of MCC (10). p31comet is a Mad2-binding protein involved in exit from mitosis (11). It preferentially associates with C-Mad2, to the same interface that also binds O-Mad2 or BubR1 (7, 12, 13). p31comet inactivates the mitotic checkpoint in vivo and in vitro in a manner that requires its interaction with Mad2 (12, 14). We found that p31comet stimulates the phosphorylation of Cdc20 in MCC by cyclin-dependent kinase (Cdk), a process that promotes MCC disassembly (15). However, this explained only a part of ATP requirement for p31comet action: We noted the existence of an additional pathway of MCC disassembly that required p31comet and ATP, but not phosphorylation (15). By the use of a purified in vitro system, we found that the phosphorylation-dependent MCC disassembly pathway promoted the dissociation of Cdc20 from BubR1, resulting in the release of a subcomplex in which Cdc20 was still bound to Mad2 (10).
The present study was initially undertaken to investigate the mechanism of the disassembly of the Cdc20–Mad2 subcomplex. An ATP-dependent disassembly factor was isolated and identified as Thyroid Receptor Interacting Protein 13 (TRIP13), an AAA-ATPase that had been found to interact with p31comet (16). Furthermore, we then found that the joint action of TRIP13 and p31comet also promotes MCC disassembly, releases APC/C from checkpoint inhibition, and inactivates the mitotic checkpoint.
Results
Isolation of an ATP-Requiring Factor that Disassembles Cdc20–Mad2 Complex and Its Identification as the TRIP13 AAA-ATPase.
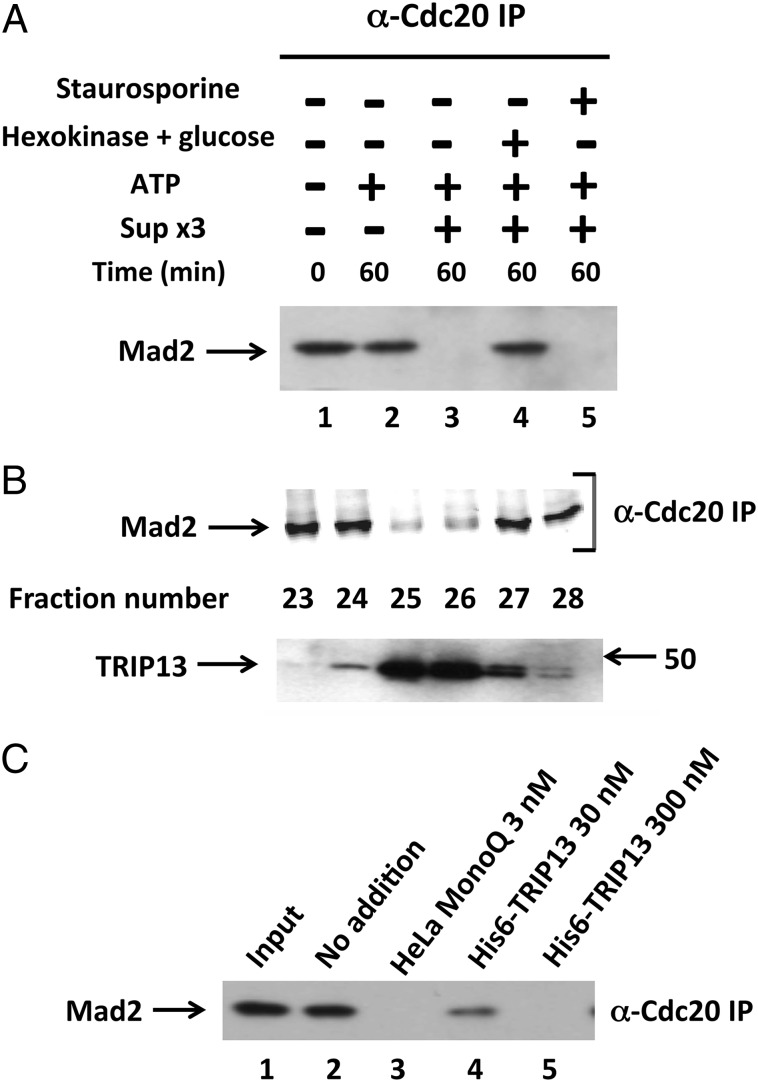
We have previously reported that a Cdc20–Mad2 subcomplex is an intermediary product in the disassembly of MCC (10). The initial aim of this study was to elucidate the process that disassembles this subcomplex. Because crystal structures showed a very tight binding of C-Mad2 to Cdc20 (1, 6), we suspected that an energy-dependent process may be involved in its dissociation. We searched for a factor that disassembles Cdc20–Mad2 in extracts from checkpoint-arrested HeLa cells. To prevent endogenous checkpoint proteins from interfering with the assay, extracts were depleted of MCC (free and bound to APC/C) and of Mad2 by three consecutive immunodepletions with anti-Cdc27, anti-BubR1, and anti-Mad2 (see Materials and Methods, “Sup ×3”). To generate Cdc20–Mad2 substrate for the assay, we used the subcomplex released from immunopurified MCC by incubation with p31comet, ATP, and Cdk, as described (10, 15). The disassembly of Cdc20–Mad2 was determined by immunoprecipitation with anti-Cdc20, followed by immunoblotting for Mad2. As shown in Fig. 1_A_, lane 1, without incubation, Mad2 in the subcomplex was coprecipitated with Cdc20 to which it was bound. Following incubation of Cdc20–Mad2 with ATP and Sup ×3 from HeLa cell extracts, Mad2 disappeared from anti-Cdc20 immunoprecipitate (Fig. 1_A_, lane 3), indicating disassembly of the subcomplex. The process required ATP, because disassembly was prevented by trapping ATP with hexokinase and glucose (Fig. 1_A_, lane 4). The disassembly of the subcomplex was not affected by the protein kinase inhibitor staurosporine (Fig. 1_A_, lane 5), indicating that ATP was not required for the action of a staurosporine-sensitive protein kinase such as Cdk.
Fig. 1.
Isolation from HeLa cell extracts of an ATP-requiring factor that disassembles Cdc20–Mad2 and its identification as TRIP13. (A) ATP-dependent disassembly of Cdc20–Mad2 by HeLa cell extract. Extract from checkpoint-arrested HeLa cells was subjected to three consecutive immunodepletions to remove endogenous MCC components, as described in Materials and Methods. The resulting supernatant (Sup ×3) was used as the enzyme source. The disassembly of Cdc20–Mad2 was determined as described in Materials and Methods, using material released from immunopurified MCC as the substrate. Where indicated, additions were as follows: Sup ×3, 1 μL; ATP, 1 mM, together with 10 mM phosphocreatine and 100 μg/mL creatine phosphokinase; hexokinase, 200 μg/mL, together with 20 mM 2-deoxyglucose; staurosporine, 10 μM. (B) Ion exchange chromatography of HeLa cell extract on MonoQ. See SI Materials and Methods for details. Samples of 5 μL of column fractions were assayed for Cdc20–Mad2 disassembly activity (Upper) and other 5-μL samples of the same column fractions were subjected to immunoblotting with affinity-purified rabbit anti-TRIP13 antibody (Lower). Arrow on the right indicates the position of marker protein (kDa). (C) Disassembly of Cdc20–Mad2 by recombinant TRIP13. The disassembly of the Cdc20–Mad2 subcomplex was determined as described in Materials and Methods, in the presence of either 5 μL of MonoQ-purified preparation from HeLa cell extract (lane 3) or of bacterially expressed, purified his6–TRIP13 (lanes 4 and 5) at the indicated concentrations.
We next tried to isolate, by chromatographic procedures, the factors responsible for the disassembly of the subcomplex. Sup ×3 from HeLa cell extracts was subjected to fractionation on various FPLC columns, and column fractions were assayed for activity of disassembly of Cdc20–Mad2 by the procedure described above. Following ion-exchange chromatography on MonoQ, activity of the disassembly factor eluted in a sharp peak at around 200 mM NaCl (Fig. 1_B_, Upper). On gel filtration chromatography on Superose-6, disassembly factor activity eluted at an apparent size of 50–60 kDa (Fig. S1). These findings showed the existence of a factor responsible for the ATP-dependent disassembly of the Cdc20–Mad2 subcomplex.
At this stage of partial purification, we tried to identify the factor by immunoblotting of fractions of the chromatographic separations described above with antibodies directed against suspected proteins. We suspected that the factor may be a molecular chaperone protein or an unfolding ATPase, as is the case with a variety of AAA-ATPases (reviewed in refs. 17–19). It has been reported that the AAA-ATPase TRIP13 is a kinetochore-associated protein that binds to p31comet (16). Because the Cdc20–Mad2 substrate used in our assay contained p31comet (necessary for the release of the subcomplex from MCC), TRIP13 seemed to be a likely candidate. Indeed, immunoblotting of MonoQ column fractions with anti-TRIP13 showed an exact coincidence of the peak of the elution of TRIP13 protein with that of Cdc20–Mad2 dissociating activity, with both centered in fractions 25–26 (Fig. 1_B_). Similarly, in fractions of Superose-6 gel filtration, the peak of TRIP13 protein, identified by immunoblotting, eluted in coincidence with that of Cdc20–Mad2 disassembly activity (Fig. S1). To further establish the identity of the disassembly factor as TRIP13, we next tested whether bacterially expressed his6–TRIP13 could promote the disassembly of Cdc20–Mad2 in our assay. As shown in Fig. 1_C_, lanes 4 and 5, recombinant TRIP13 indeed had activity to disassemble Cdc20–Mad2. It should be noted, however, that recombinant TRIP13 was >10-fold less active than that isolated from HeLa cells. Taken together, these findings identify the factor that disassembles Cdc20–Mad2 as the TRIP13 AAA-ATPase.
Role of TRIP13 in the Release of Mad2 from MCC.
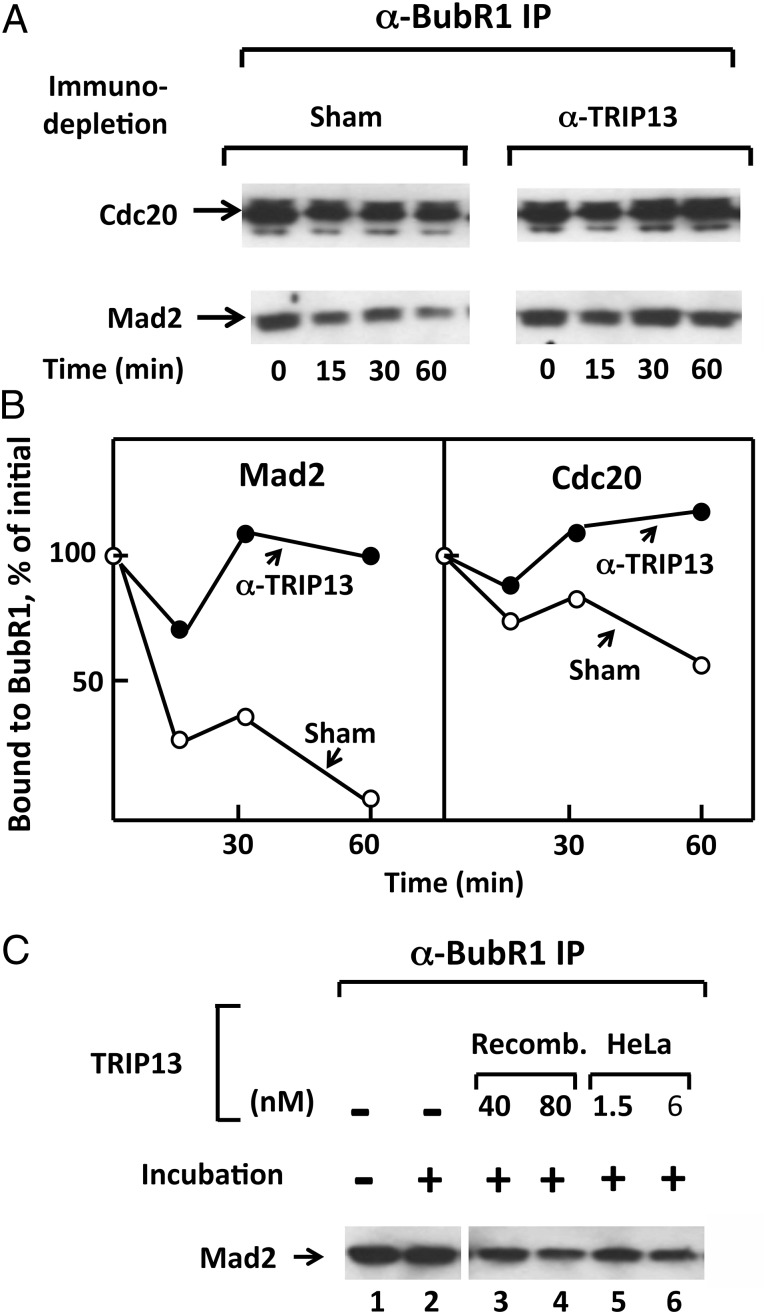
We next examined whether TRIP13 also plays a role in the release of Mad2 from MCC. This process can be observed upon incubation of checkpoint extracts with ATP (9, 10). To test the possible involvement of TRIP13 in this process, we have removed it from extracts by immunodepletion. Treatment with polyclonal anti-TRIP13 antibody depleted ∼95% of this protein (Fig. S2). TRIP13-depleted and sham-depleted extracts were incubated with ATP, and samples were taken at various times of incubation and were immunoprecipitated with anti-BubR1. Levels of Mad2 and Cdc20 associated with BubR1 in immunoprecipitates were estimated by immunoblotting. The results are shown in Fig. 2_A_, and their quantitation in Fig. 2_B_. As observed previously (9, 10), in sham-treated extracts, Mad2 was rapidly released from BubR1, whereas Cdc20 was released more slowly (Fig. 2_A_, Left). This may be due to the existence of different pathways of dissociation of Mad2 and Cdc20 from BubR1 (Discussion). Immunodepletion of TRIP13 markedly prevented the release of Mad2 from MCC (Fig. 2_A_, Right). This treatment also blocked the slower release of Cdc20 from BubR1 (Fig. 2 A and B).
Fig. 2.
TRIP13 promotes the release of Mad2 from MCC. (A) Influence of TRIP13 immunodepletion on the release of Mad2 and Cdc20 from MCC. The release of Mad2 and Cdc20 from endogenous MCC in checkpoint extracts subjected to the indicated immunodepletions was determined as described in SI Materials and Methods. Following incubation at 23 °C with ATP for the time periods indicated, samples were subjected to immunoprecipitation with anti-BubR1, followed by immunoblotting for Mad2 and Cdc20. (B) Quantification of results shown in A. Results are expressed as the percentage of values at time 0. (C) Supplementation of TRIP13 restores the release of Mad2 from MCC in checkpoint extracts depleted with anti-TRIP13. Extracts were subjected to immunodepletion with anti-TRIP13 and were incubated (23 °C, 1 h) with ATP, as described in Materials and Methods. Recombinant his6–TRIP13 (“Recomb.”) or MonoQ-purified TRIP13 from HeLa cells (“HeLa”) were supplemented at the concentrations indicated. Samples were precipitated with anti-BubR1 and were immunoblotted for Mad2.
It was possible that immunodepletion of TRIP13 codepleted an associated protein that was required for MCC disassembly. We therefore tested whether recombinant TRIP13 could restore the release of Mad2 from MCC in TRIP13-depleted extracts. As shown in Fig. 2_C_, both recombinant TRIP13 (lanes 3 and 4) and TRIP13 isolated from HeLa cells (lanes 5 and 6) stimulated the release of Mad2 from BubR1 in extracts depleted of TRIP13. As in the case of the dissociation of the Cdc20–Mad2 subcomplex, much higher concentrations of recombinant TRIP13 than of native protein from HeLa cells were required for MCC dissociation activity. This result may be due to the presence of incorrectly folded forms in the preparation of recombinant TRIP13, because most bacterially expressed TRIP13 was insoluble and recombinant TRIP13 was purified from the soluble fraction (SI Materials and Methods). It is also possible that recombinant TRIP13 misses an additional cofactor, although extracts used for these experiments did contain p31comet (Fig. S3). We concluded that the AAA-ATPase TRIP 13 has a role in the release of Mad2 from MCC. TRIP13 is also required for the slower release of Cdc20 from BubR1 (Fig. 2 A and B) and thus for the complete dissociation of MCC. However, the latter effect of TRIP13 may be indirect, due to the labilization of MCC structure by the prior release of Mad2 (Discussion).
Interrelationship Between Actions of TRIP13 and p31comet in the Dissociation of Cdc20–Mad2, Disassembly of MCC, and Release of APC/C from Checkpoint Inhibition.
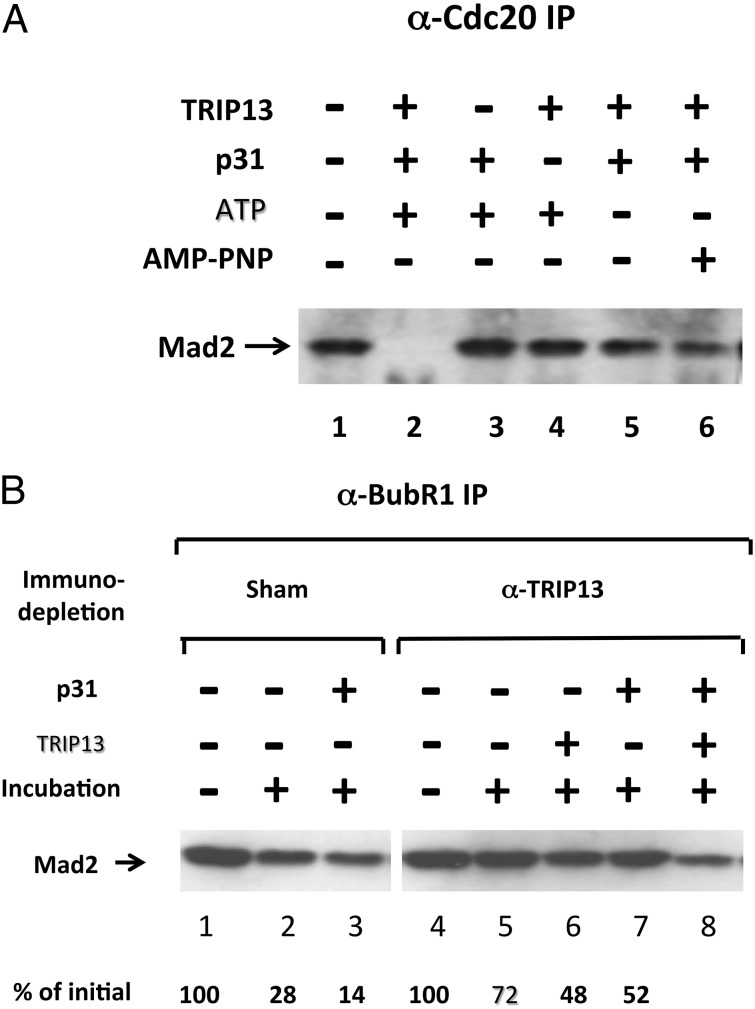
The Mad2-binding protein p31comet has important roles in checkpoint inactivation (11, 12, 14, 20–22) and MCC disassembly (10, 21), and has been reported to bind to TRIP13 (16). We therefore examined whether the actions of TRIP13 described above require the participation of p31comet. To examine this problem for the case of the dissociation of the Cdc20–Mad2 subcomplex, we had to prepare subcomplex free of p31comet, which was present in the initially used preparations (Materials and Methods). Recombinant Cdc20–Mad2 was prepared by coinfection of insect cells with baculoviruses expressing his6–Cdc20 and FLAG-Mad2. It was incubated with recombinant TRIP13 and p31comet in the presence of ATP, and the dissociation of the complex was estimated by immunoprecipitation with anti-Cdc20 followed by immunoblotting for Mad2. As shown in Fig. 3_A_, lane 2, recombinant Cdc20–Mad2 was effectively disassembled when all these components were present. Complex disassembly was prevented when either TRIP13 or p31comet were omitted (Fig. 3_A_, lanes 3 and 4, respectively). Disassembly was also prevented by the omission of ATP (Fig. 3_A_, lane 5) or by its replacement by the nonhydrolyzable analog adenosine-5′-(β, γ)-imidotriphosphate (AMP-PNP; Fig. 3_A_, lane 6), suggesting that the recombinant system faithfully reflects the process observed with endogenous proteins (compare Fig. 1_A_). The absolute dependence of the process on the presence of p31comet (Fig. 3_A_, lane 4) suggests that this Mad2-binding protein participates in the action of the AAA-ATPase TRIP13 to disassemble the Cdc20–Mad2 subcomplex.
Fig. 3.
Synergistic actions of TRIP13 and p31comet in the disassembly of Cdc20–Mad2 subcomplex and of MCC. (A) The disassembly of recombinant Cdc20–Mad2 complex requires TRIP13, p31comet, and hydrolyzable ATP. Recombinant Cdc20–Mad2 complex was expressed and purified as described in Materials and Methods and was added to the assay at 2 nM. The indicated additions were at the following concentrations: his6–TRIP13, 50 nM; his6–p31comet, 25 nM; ATP or AMP–PNP, 3 mM. Following incubation at 23 °C for 60 min, samples were subjected to immunoprecipitation with anti-Cdc20, followed by immunoblotting for Mad2. (B) Interrelationship between the actions of TRIP13 and p31comet on the release of Mad2 from MCC. Checkpoint extracts were subjected to immunodepletion with anti-TRIP13 or to sham immunodepletion, as specified. Where indicated, 80 nM his6–TRIP13 or 40 nM of his6–p31comet were supplemented. Following incubation at 23 °C for 1 h, the release of Mad2 from endogenous MCC was determined as described in Fig. 2_A_.
We have further examined whether a similar interrelationship between TRIP13 and p31comet exists in the release of Mad2 from MCC. As shown in Fig. 3_B_, the addition of recombinant p31comet to sham-depleted extract strongly stimulated the release of Mad2 from BubR1 (lane 3), in accordance with previous observations (10). By contrast, in TRIP13-depleted extract the addition of p31comet only slightly stimulated Mad2 release (Fig. 3_B_, lane 7 vs. 5). This result suggested that at least a part of the action of p31comet to stimulate Mad2 release from MCC required the involvement of TRIP13. Indeed, when p31comet was added along with recombinant TRIP13, a strong synergistic stimulation of Mad2 release from MCC could be seen (Fig. 3_B_, lane 8). These results support the notion that the joint action of p31comet and TRIP13 plays a role also in the release of Mad2 from MCC, an important step in MCC disassembly (Discussion).
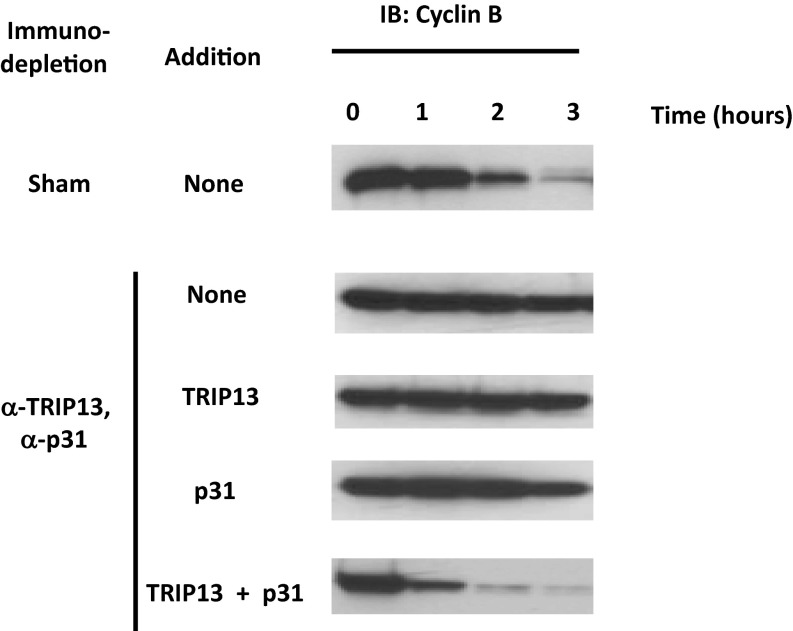
The disassembly of MCC has been correlated with the release of APC/C from checkpoint inhibition (9, 10). We have therefore examined the influences of TRIP13 and p31comet on APC/C activity in exit from mitotic checkpoint arrest. For this purpose, TRIP13-depleted extracts were subjected to further immunodepletion with anti-p31comet. This treatment effectively removed >90% of p31comet (Fig. S3). Sham-depleted and TRIP13, p31comet double-depleted extracts were incubated with ATP and recombinant proteins as indicated, and the activation of APC/C was determined by the degradation of its endogenous substrate, cyclin B. As shown in Fig. 4, in sham-depleted extract cyclin B was degraded after a lag, as described (9, 23). In TRIP13, p31comet double-depleted extract, there was little degradation of cyclin B after 3 h of incubation. The addition of TRIP13 had no influence, whereas the addition of p31comet only slightly stimulated cyclin B degradation. Most significantly, the joint supplementation of TRIP13 and p31comet to the double-depleted extract resulted in a strong synergistic stimulation of cyclin degradation, reflecting rapid release of APC/C from checkpoint inhibition (Fig. 4). The combined results thus indicate that, by affecting MCC disassembly, TRIP13 and p31comet act jointly to release APC/C from checkpoint inhibition and to inactivate the mitotic checkpoint.
Fig. 4.
The joint action of TRIP13 and p31comet abrogates checkpoint inhibition of APC/C. The degradation of endogenous cyclin B in sham-depleted extract or in TRIP13 and p31comet double-depleted extract was assayed as described in Materials and Methods. Where indicated, 80 nM of his6–TRIP13 or 40 nM of his6–p31comet were added. Samples were taken at the times indicated and were subjected to immunoblotting for cyclin B.
Discussion
We have previously observed that a Cdc20–Mad2 subcomplex is the intermediary product of MCC disassembly in a pathway that requires the action of p31comet and Cdc20 phosphorylation (10, 15). The initial aim of the present investigation was to explore the mechanisms by which this subcomplex is further dissociated into its individual components. It seemed reasonable to assume that the Cdc20–Mad2 subcomplex rapidly dissociates in checkpoint inactivation, preventing its possible reassociation with BubR1–Bub3 to form MCC. Another expectation was that the disassembly of the Cdc20–Mad2 subcomplex may require energy, because structural studies showed that, in such a complex, the C-terminal tail of Mad2 is tightly wrapped around its partner (1, 6). Therefore, it could be anticipated that an ATP-using process is needed to unfold such a tight interaction. We have indeed isolated a factor that carried out in ATP-dependent disassembly of the Cdc20–Mad2 subcomplex and identified it as the TRIP13 ATPase (Fig. 1 A_–_C).
TRIP13 was initially described as one of several proteins that interact with the thyroid hormone receptor and hence its name (Thyroid Receptor Interacting Protein; ref. 24). It belongs to the AAA (ATPases associated with diverse cellular activities) family of proteins, a large group whose members carry out a great number of cellular functions, including proteasome action, membrane trafficking, DNA replication, and movement of molecular motors (17–19). Most of these functions are executed by promoting conformational changes in target proteins, resulting in protein unfolding, disassembly of protein complexes, or generation of motion along tracks. In many cases, AAA proteins associate with specific adaptor proteins that target them to specific substrates. In some instances, an AAA-ATPase can bind to different adaptor proteins, thus changing its substrate specificities and cellular functions (19). In the case of the TRIP13 AAA-ATPase and of its homologs, a role in meiotic recombination has been reported in mice, yeast, worms, and plants (25–28). This function has been explained by their action to dislocate certain proteins from chromatin (26–28). A different function of TRIP13 has been suggested by a proteomic data mining study that identified it as a kinetochore protein that interacts with p31comet (16). The direct binding of TRIP13 to p31comet has been validated and the possibility has been raised that TRIP13 may facilitate p31comet-mediated checkpoint silencing (16).
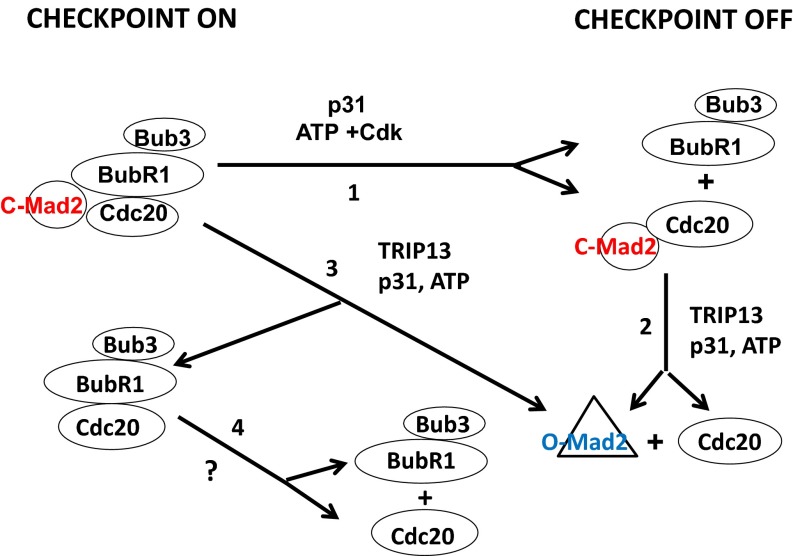
Our identification of the ATP-dependent factor that disassembles Cdc20–Mad2 subcomplex as TRIP13, along with the previously known interaction of TRIP13 with p31comet, raised the question of whether p31comet is required for the disassembling activity of this AAA-ATPase. We have indeed showed that TRIP13 action to disassemble the Cdc20–Mad2 complex requires the presence of p31comet (Fig. 3_A_). We furthermore found that the joint action of TRIP13 and p31comet is also required for the release of Mad2 from MCC, for the complete disassembly of MCC and for relieving APC/C from checkpoint inhibition (Figs. 3 and 4). Our proposed sequence of events in MCC disassembly and checkpoint inactivation is shown in Fig. 5. We propose that the Mad2-binding protein p31comet participates in two types of dissociation processes, which together lead to the compete disassembly of MCC. The first type releases Cdc20–Mad2 subcomplex from BubR1 (Fig. 5, Step 1). This process does not require TRIP13 and does not change C-Mad2 conformation. The role of p31comet here may be the destabilization of MCC structure as suggested (7), by disrupting the binding of BubR1 to C-Mad2 through competition of p31comet with BubR1 on a similar binding interface of C-Mad2. This dissociation is aided by Cdk-catalyzed phosphorylation of Cdc20 (15). The second type of dissociation process involves joint action of TRIP13 and p31comet and causes the release of free Mad2 from Cdc20–Mad2 (Fig. 5, Step 2) and from MCC (Fig. 5, Step 3). The rapid dissociation of Cdc20–Mad2 may prevent its possible reassociation with BubR1–Bub3 to form MCC. Thus, the dissociation of the Cdc20–Mad2 subcomplex (Fig. 5, Step 2) renders MCC dissociation to become irreversible. It appears likely that the AAA-ATPase TRIP13 promotes disassembly by converting C-Mad2 to O-Mad2, using the energy of ATP hydrolysis for the large conformational change involved. The role of p31comet in this process may be to bind to both TRIP13 and to C-Mad2 in the complexes, and thus to facilitate ATPase action on its specific target. The combined action of these two pathways may thus account for the complete and irreversible dissociation of MCC complexes, accompanied by the conversion of C-Mad2 to O-Mad2 and the release of APC/C from inhibition in checkpoint inactivation. Further studies are needed to test the above hypothesis, including direct assays testing the proposed role of the TRIP13 AAA-ATPase in the C-Mad2 to O-Mad2 conformational transition.
Fig. 5.
Proposed roles of TRIP13 ATPase and of p31comet Mad2-binding protein in pathways of disassembly of mitotic checkpoint complexes and in checkpoint inactivation. See Discussion. (Step 1) Dissociation of Cdc20–Mad2 from BubR1–Bub3 promoted by p31comet and Cdk-catalyzed phosphorylation of Cdc20. (Step 2) ATP-dependent disassembly of Cdc20–Mad2 subcomplex, carried out by the joint action of TRIP13 and p31comet. It is suggested that the release of Mad2 from Cdc20 may be promoted by its conversion from the closed to the open conformation. (Step 3) Release of Mad2 from MCC by a TRIP13- and p31comet-dependent process similar to that in Step 2. The suggested dissociation products are O-Mad2 and Mad2-free MCC. (Step 4) Mad2-free MCC is further disassembled with the release of Cdc20 from BubR1–Bub3 by an unknown mechanism. Cdk, cyclin-dependent kinase.
Further work is also required to gain more complete understanding of the pathways of MCC disassembly. The release of Mad2 from BubR1 is much more rapid than that of Cdc20 (refs. 9 and 10 and Fig. 2). This result suggests the intermediary formation of a Mad2-free MCC subcomplex (Fig. 5, Step 3), which is disassembled more slowly (Fig. 5, Step 4). The pathways of the disassembly of this Mad2-free MCC remain obscure. The slower release of Cdc20 from BubR1 is also stimulated by TRIP13 (Fig. 2), but this could be indirect and secondary to the rapid release of Mad2. Because C-Mad2 stabilizes the structure of MCC by binding to both Cdc20 and BubR1 (7), Mad2-free MCC may be less stable and more easily dissociated by some processes that remain to be elucidated. Other open problems include the details of the interactions of TRIP13 with its substrates, p31comet and ATP. The active form of AAA-ATPases is usually a hexamer (18, 19), whereas we found that the predominant form of TRIP13 in HeLa cell extracts is a monomer (Fig. S1). Hexamer formation is promoted in other cases by binding to ATP or to substrate (18, 19). It remains to be seen whether such oligomerization, or substrate binding, also affects the interaction of TRIP13 with p31comet. Of particular interest is the question of whether the interaction of TRIP13 with p31comet, or with specific C-Mad2-containing substrates, is regulated when the mitotic checkpoint is extinguished.
Materials and Methods
Immunodepletion of HeLa Cell Extracts.
Extracts from nocodazole-arrested HeLa cells were prepared as described (23). Details of immunodepletion procedures are described in SI Materials and Methods. To deplete extracts of MCC components that interfere with the assay of the disassembly of the Cdc20–Mad2 subcomplex (see below), they were subjected to three consecutive immunodepletions with α-Cdc27, α−BubR1, and α-Mad2 antibodies. These treatments were designed to remove APC/C-bound MCC, free MCC, and free Mad2, respectively. The final supernatant, designated Sup ×3, was depleted of ∼90% of BuBR1 and Mad2 and ∼85% of Cdc27.
Assay of the Disassembly of Cdc20–Mad2 Subcomplex.
As the substrate for this assay, we have initially used Cdc20–Mad2 released from immunopurified MCC from HeLa cells, by incubation of anti-BubR1 immunoprecipitates with p31comet, ATP, and Cdk2-cyclin A, as described (15), and collecting the supernatants. This preparation contained all reaction components used for the release of Cdc20 from MCC, including p31comet and ATP. Subsequently, we have also used recombinant Cdc20–Mad2 complex, produced by coinfection of SF9 insect cells with baculoviruses expressing his6–Cdc20 and Flag–Mad2. The complex was purified first with Ni-NTA agarose (Quiagen), and then by anti-Flag agarose (Sigma-Aldrich), according to the instructions of the manufacturers. Both preparations of Cdc20–Mad2 subcomplex were stored at −70 °C in small samples. The assay for the disassembly of Cdc20–Mad2 contained in a volume of 30 μL: 20 mM Tris⋅HCl (pH 7.6), 5 mM MgCl2, 1 mM DTT, 2 mg/mL BSA, 20% (vol/vol) glycerol, 1 mM ATP, 10 mM phosphocreatine, 100 μg/mL creatine phosphokinase, 10 μL of Cdc20–Mad2 subcomplex released from immunopurified HeLa cell MCC, or 2 nM of recombinant Cdc20–Mad2 complex, and enzyme source as specified in figure legends. Following incubation at 30 °C for 1 h, samples were mixed with 5 μL (packed volume) of anti-Cdc20 beads and were rotated at 4 °C for 1 h. The beads were then washed three times with 1-mL portions of a buffer consisting of 50 mM Tris⋅HCl (pH 7.2), 20% (vol/vol) glycerol, 300 mM NaCl, and 1% (vol/vol) Nonidet P-40. Immunoprecipitates were resolved by SDS/PAGE, transferred to PVDF membranes, and the amounts of Mad2 that remained bound to Cdc20 was estimated by immunoblotting with a monoclonal anti-Mad2 antibody (MBL Laboratories KO176).
Assays of the Disassembly of Endogenous MCC and of the Degradation of Cyclin B in Extracts.
To follow the release of Mad2 and of Cdc20 from endogenous MCC in checkpoint extracts, reaction mixtures contained in a volume of 20 μL: 15 μL of extract (following immunodepletion as described in the figure legends) and the following ingredients at concentrations similar to those described above for the assay of the disassembly of the Cdc20–Mad2 subcomplex: Tris buffer, MgCl2, DTT, ATP, phosphocreatine, and creatine phosphokinase. Where indicated, bacterially expressed, purified his6–p31comet or his6–TRIP13 were added. Following incubation at 23 °C for the time periods indicated, samples were subjected to immunoprecipitation with anti-BubR1 beads, followed by immunoblotting for Mad2, as described above. The immunoblots were also probed for Cdc20 using monoclonal antibody E7 (Santa Cruz Biotechnology, sc-13162). Immunoblots were quantified with Odyssey or C-Digit (Li-Cor) instruments. The degradation of endogenous cyclin B in checkpoint extracts was examined under similar incubation conditions, except that reaction mixtures also contained 50 μM ubiquitin (Sigma) and 250 nM purified recombinant his6–UbcH10. At various times of incubation, samples of 1 μL were withdrawn and subjected to SDS/PAGE and immunoblotting with anti-cyclin B monoclonal antibody (BD Transduction Laboratories 610220).
Supplementary Material
Supporting Information
Acknowledgments
This work was supported by grants from Israel Science Foundation and Israel Cancer Research Fund (to A.H.); National Institutes of Health Grant CA169706, Core Grant CA06927, Fox Chase Cancer Center Board of Associates, and an appropriation from the Commonwealth of Pennsylvania (to T.J.Y.); and National Science Foundation Grant MCB-1052413 and National Cancer Institute Grant R01CA169500 (to S.T.L.). Part of this work done during the stay of A.H. at Fox Chase Cancer Center, Philadelphia (on sabbatical leave).
Footnotes
The authors declare no conflict of interest.
References
- 1.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8(5):379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 2.Murray AW. A brief history of error. Nat Cell Biol. 2011;13(10):1178–1182. doi: 10.1038/ncb2348. [DOI] [PubMed] [Google Scholar]
- 3.Jia L, Kim S, Yu H. Tracking spindle checkpoint signals from kinetochores to APC/C. Trends Biochem Sci. 2013;38(6):302–311. doi: 10.1016/j.tibs.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Lara-Gonzalez P, Westhorpe FG, Taylor SS. The spindle assembly checkpoint. Curr Biol. 2012;22(22):R966–R980. doi: 10.1016/j.cub.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Sironi L, et al. Crystal structure of the tetrameric Mad1-Mad2 core complex: Implications of a ‘safety belt’ binding mechanism for the spindle checkpoint. EMBO J. 2002;21(10):2496–2506. doi: 10.1093/emboj/21.10.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo X, Yu H. Protein metamorphosis: The two-state behavior of Mad2. Structure. 2008;16(11):1616–1625. doi: 10.1016/j.str.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao WC, Kulkarni K, Zhang Z, Kong EH, Barford D. Structure of the mitotic checkpoint complex. Nature. 2012;484(7393):208–213. doi: 10.1038/nature10896. [DOI] [PubMed] [Google Scholar]
- 8.Tipton AR, et al. BUBR1 and closed MAD2 (C-MAD2) interact directly to assemble a functional mitotic checkpoint complex. J Biol Chem. 2011;286(24):21173–21179. doi: 10.1074/jbc.M111.238543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miniowitz-Shemtov S, Teichner A, Sitry-Shevah D, Hershko A. ATP is required for the release of the anaphase-promoting complex/cyclosome from inhibition by the mitotic checkpoint. Proc Natl Acad Sci USA. 2010;107(12):5351–5356. doi: 10.1073/pnas.1001875107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teichner A, et al. p31comet Promotes disassembly of the mitotic checkpoint complex in an ATP-dependent process. Proc Natl Acad Sci USA. 2011;108(8):3187–3192. doi: 10.1073/pnas.1100023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Habu T, Kim SH, Weinstein J, Matsumoto T. Identification of a MAD2-binding protein, CMT2, and its role in mitosis. EMBO J. 2002;21(23):6419–6428. doi: 10.1093/emboj/cdf659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang M, et al. p31comet blocks Mad2 activation through structural mimicry. Cell. 2007;131(4):744–755. doi: 10.1016/j.cell.2007.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mapelli M, et al. Determinants of conformational dimerization of Mad2 and its inhibition by p31comet. EMBO J. 2006;25(6):1273–1284. doi: 10.1038/sj.emboj.7601033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia G, et al. Conformation-specific binding of p31(comet) antagonizes the function of Mad2 in the spindle checkpoint. EMBO J. 2004;23(15):3133–3143. doi: 10.1038/sj.emboj.7600322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miniowitz-Shemtov S, et al. Role of phosphorylation of Cdc20 in p31(comet)-stimulated disassembly of the mitotic checkpoint complex. Proc Natl Acad Sci USA. 2012;109(21):8056–8060. doi: 10.1073/pnas.1204081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tipton AR, et al. Identification of novel mitosis regulators through data mining with human centromere/kinetochore proteins as group queries. BMC Cell Biol. 2012;13:15. doi: 10.1186/1471-2121-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vale RD. AAA proteins. Lords of the ring. J Cell Biol. 2000;150(1):F13–F19. doi: 10.1083/jcb.150.1.f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanson PI, Whiteheart SW. AAA+ proteins: Have engine, will work. Nat Rev Mol Cell Biol. 2005;6(7):519–529. doi: 10.1038/nrm1684. [DOI] [PubMed] [Google Scholar]
- 19.Meyer H, Bug M, Bremer S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat Cell Biol. 2012;14(2):117–123. doi: 10.1038/ncb2407. [DOI] [PubMed] [Google Scholar]
- 20.Hagan RS, et al. p31(comet) acts to ensure timely spindle checkpoint silencing subsequent to kinetochore attachment. Mol Biol Cell. 2011;22(22):4236–4246. doi: 10.1091/mbc.E11-03-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westhorpe FG, Tighe A, Lara-Gonzalez P, Taylor SS. p31comet-mediated extraction of Mad2 from the MCC promotes efficient mitotic exit. J Cell Sci. 2011;124(Pt 22):3905–3916. doi: 10.1242/jcs.093286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varetti G, Guida C, Santaguida S, Chiroli E, Musacchio A. Homeostatic control of mitotic arrest. Mol Cell. 2011;44(5):710–720. doi: 10.1016/j.molcel.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Braunstein I, Miniowitz S, Moshe Y, Hershko A. Inhibitory factors associated with anaphase-promoting complex/cylosome in mitotic checkpoint. Proc Natl Acad Sci USA. 2007;104(12):4870–4875. doi: 10.1073/pnas.0700523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JW, Choi HS, Gyuris J, Brent R, Moore DD. Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Mol Endocrinol. 1995;9(2):243–254. doi: 10.1210/mend.9.2.7776974. [DOI] [PubMed] [Google Scholar]
- 25.Li XC, Schimenti JC. Mouse pachytene checkpoint 2 (trip13) is required for completing meiotic recombination but not synapsis. PLoS Genet. 2007;3(8):e130. doi: 10.1371/journal.pgen.0030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wojtasz L, et al. Mouse HORMAD1 and HORMAD2, two conserved meiotic chromosomal proteins, are depleted from synapsed chromosome axes with the help of TRIP13 AAA-ATPase. PLoS Genet. 2009;5(10):e1000702. doi: 10.1371/journal.pgen.1000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miao C, et al. Central region component1, a novel synaptonemal complex component, is essential for meiotic recombination initiation in rice. Plant Cell. 2013;25(8):2998–3009. doi: 10.1105/tpc.113.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen C, Jomaa A, Ortega J, Alani EE. Pch2 is a hexameric ring ATPase that remodels the chromosome axis protein Hop1. Proc Natl Acad Sci USA. 2014;111(1):E44–E53. doi: 10.1073/pnas.1310755111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information