Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy (original) (raw)
Key Points
- Volasertib plus low-dose cytarabine increased the response rate and improved survival in AML patients ineligible for intensive treatment.
- Volasertib plus low-dose cytarabine resulted in responses across all AML genetic subgroups and had a clinically manageable safety profile.
Abstract
Treatment outcomes for older patients with acute myeloid leukemia (AML) have remained dismal. This randomized, phase 2 trial in AML patients not considered suitable for intensive induction therapy compared low-dose cytarabine (LDAC) with or without volasertib, a highly potent and selective inhibitor of polo-like kinases. Eighty-seven patients (median age 75 years) received LDAC 20 mg twice daily subcutaneously days 1-10 or LDAC + volasertib 350 mg IV days 1 + 15 every 4 weeks. Response rate (complete remission and complete remission with incomplete blood count recovery) was higher for LDAC + volasertib vs LDAC (31.0% vs 13.3%; odds ratio, 2.91; P = .052). Responses in the LDAC + volasertib arm were observed across all genetic groups, including 5 of 14 patients with adverse cytogenetics. Median event-free survival was significantly prolonged by LDAC + volasertib compared with LDAC (5.6 vs 2.3 months; hazard ratio, 0.57; 95% confidence interval, 0.35-0.92; P = .021); median overall survival was 8.0 vs 5.2 months, respectively (hazard ratio, 0.63; 95% confidence interval, 0.40-1.00; P = .047). LDAC + volasertib led to an increased frequency of adverse events that was most pronounced for neutropenic fever/infections and gastrointestinal events; there was no increase in the death rate at days 60 + 90. This study was registered at www.clinicaltrials.gov as #NCT00804856.
Introduction
Acute myeloid leukemia (AML) arises in all age groups, but it is mainly a disease of the older patient, with a median age at diagnosis that has reached 70 years.1,2 Age has a major impact on both the management and outcomes of patients with AML. Older patients frequently present with unfavorable prognostic factors that are related to patient characteristics, such as general health condition and specific comorbidities as well as those related to the leukemia cells, including a higher frequency of secondary AML arising from previous myelodysplastic syndrome and adverse genetics.3-5 The spectrum of treatment ranges from standard “3 + 7” induction therapy to palliation with supportive care (SC). No algorithm has ever entered clinical practice defining patients who are “ineligible” for intensive induction chemotherapy. In daily practice, age, performance status, comorbidities, disease features, and, importantly, the patient’s wishes and doctor’s view are major determinants for the decision-making process, an algorithm that is reflected in current recommendations for AML management.5
Many older patients will opt for a middle ground; that is, they wish for active leukemia treatment with the hope of prolonging life and preserving a high quality of life. One standard therapy that meets these criteria is the subcutaneous (s.c.) administration of low-dose cytarabine (LDAC) over 10 days in 4-week cycles, an approach that has been more widely accepted in Europe than in the United States.6 Attempts to improve upon this standard by novel drugs have so far failed.7
Polo-like kinases (Plks) are a family of 5 highly conserved serine/threonine protein kinases that have been shown to play a key role in mitotic checkpoint regulation and cell division, including entry into mitosis, centrosome maturation, assembly of the bipolar spindle, sister chromatid separation, and progression through anaphase and cytokinesis.8-10 Physiologically, Plk1 is expressed only in dividing cells with peak expression during G2/M phase. Plk1 has been shown to be overexpressed in a range of human cancers, including non-small cell lung cancer, prostate, ovarian, breast, and colorectal cancer as well as AML.8,11,12 In some tumor types, overexpression correlates with a worse prognosis.13 Inhibition of Plk1 results in G2/M arrest with an increase of phospho-histone H3 levels. Plk1 deficiency elicits a typical “Polo arrest” phenotype; that is, the formation of a monopolar or disorganized spindle with subsequent apoptotic cell death.
Volasertib (laboratory code: BI 6727) is a low-molecular-weight, adenosine triphosphate–competitive kinase inhibitor that potently inhibits Plk1 as well as the 2 closely related kinases, Plk2 and Plk3, with 50% inhibitory concentration values of 0.87, 5, and 56 nmol/L, respectively.14 Volasertib did not demonstrate inhibitory activity when screened against a panel of more than 50 other kinases at clinically relevant concentrations, confirming specificity for Plks. Volasertib has shown in vivo efficacy in multiple xenograft models, including AML. A predecessor compound, BI 2536,15,16 was previously evaluated in older patients with relapsed/refractory AML and provided a first proof-of-principle of the potential therapeutic value of targeting Plk in patients with AML.17 Clinical development of BI 2536 was discontinued in favor of volasertib, which has an improved pharmacokinetic profile.14 Other Plk inhibitors have shown efficacy in preclinical models of AML, but have not yet entered clinical development.18,19
This trial (BI 1230.4) was designed as a phase 1/2, open-label, multicenter trial of volasertib in patients with AML ineligible for intensive induction therapy. In the phase 1 part, dose escalation was performed in patients with relapsed/refractory AML. In combination with LDAC, a dose of 350 mg volasertib (given on days 1 + 15) was determined as the maximum tolerated dose (manuscript in preparation).20 Six of 32 patients responded, providing evidence for antileukemia activity. Objectives of the randomized, phase 2 part reported here were to evaluate efficacy and safety of LDAC + volasertib vs LDAC in patients with previously untreated AML not considered suitable for intensive therapy.
Patients and methods
Trial design and patients
In the phase 2 part of the trial, patients were randomized in a 1:1 ratio to receive LDAC 20 mg twice daily (bid) s.c. on days 1 through 10 alone vs the combination of LDAC + volasertib 350 mg IV over 1 hour on days 1 + 15. Randomization was not stratified for any patient or disease characteristics. Cycles were scheduled every 4 weeks until progression, relapse, intolerance, or patient/investigator requested discontinuation.
Inclusion criteria included previously untreated (except hydroxyurea during screening phase to control hyperleukocytosis) adult patients with AML who were considered unsuitable for intensive induction therapy, performance status ≤2, and signed written informed consent consistent with International Conference on Harmonization–Good Clinical Practice and local legislation. Ineligibility for intensive remission induction therapy was based on investigator assessment of disease characteristics such as AML genetics, type of AML (de novo or secondary), and patient characteristics such as age, performance score, concomitant diagnoses, and organ dysfunctions. Exclusion criteria included acute promyelocytic leukemia with t(15;17)(q22;q12), _PML_-RARA, presence of a second malignancy requiring treatment, known central nervous system leukemia, clinically relevant QT prolongation (ie, long QT syndrome; QTcF >470 ms), inadequate organ function (bilirubin >1.5 mg/dL, aspartate aminotransferase or alanine aminotransferase >2.5-fold the upper limit of normal, serum creatinine >2.0 mg/dL), concomitant intercurrent illness that could compromise the evaluation of efficacy or safety of the trial drug (eg, active severe infection, unstable angina pectoris, severe cardiac insufficiency), and psychiatric illness or social situation that would limit compliance with trial requirements.
Cytogenetics and NPM1, CEBPA, and FLT3 mutational studies as well as assignment to genetic groups according to the European LeukemiaNet (ELN) classification5 were performed centrally at Ulm University. Risk groups were assigned based on a validated scoring system.21 The plasma concentrations of volasertib and cytarabine were determined by validated liquid chromatography–tandem mass spectrometry and concentration-time data of volasertib and cytarabine in plasma were analyzed by noncompartmental methods using the software WinNonlin (Version 5.02, Pharsight).
First response assessment was performed at the end of the first cycle in all patients. Further assessments were dependent on leukemia activity: in the case of persistence of leukemic cells in the blood, no bone marrow aspiration had to be performed; if no leukemic cells were detected in the blood and other clinical signs of leukemia were absent, a bone marrow aspirate was obtained prior to the next cycle; in the case of complete remission (CR) and complete remission with incomplete blood count recovery (CRi), further bone marrow examinations were performed after every other cycle; and in the case of persistence of leukemia in the bone marrow, further examinations were done after each cycle. Finally, a bone marrow aspirate was obtained as soon as relapse was suspected. Patients not achieving a remission were permitted by protocol design to continue therapy as long as they were perceived not to suffer progression of disease and to derive benefit. Response to treatment was evaluated locally at the trial site according to standard criteria.5,22
The trial was conducted in accordance with the principles laid down by the Declaration of Helsinki, registered (www.clinicaltrials.gov #NCT00804856; EudraCT 2008-003617-27), and approved by the ethics committees of all participating centers.
Statistical analyses
The primary end point of the phase 2 part of the trial was objective response defined as CR and CRi; secondary end points included event-free survival (EFS), relapse-free survival (RFS), overall survival (OS), and incidence and intensity of adverse events (AEs) according to Common Terminology Criteria for Adverse Events, version 3.0, and pharmacokinetics. The data cutoff for this analysis was November 7, 2013. All analyses involved the treated patients only; the 2 patients randomized but never treated were excluded from the analysis.
The calculation of the phase 2 sample size was based on a stopping for futility approach,23,24 assuming that the actual phase 2 trial represented an interim analysis of a (hypothetical) phase 3 trial. The test decision (with respect to phase 2) was made by looking at the conditional power that volasertib would show superiority in the hypothetical phase 3 trial. It was assumed that the response rate in the control arm was 15%, whereas the response rate in the investigational arm was 30%. This led to a sample size of 86 patients for the (hypothetical) interim analysis; that is, 43 patients per treatment arm. These assumptions correspond to a power of 78.3% and a type I error (the critical P value) of 18.9% with a critical conditional power value of 23.6% (under the current trend hypothesis).23 The comparison of objective response rates between treatment arms was based on the odds ratio (OR).
EFS was defined as the time between the date of randomization and the date of progressive disease or death, whichever occurred first. OS was defined as the time between the date of randomization and the date of death. For patients with objective response, RFS was defined as the time between first date of objective response and date of relapse or death. Patients alive at their last contact date were censored for EFS, RFS (if without progression), and OS. In addition, patients receiving other antileukemia therapy before occurrence of progressive disease were censored for EFS and RFS, but not OS, at the date of last disease assessment before the new therapy was started. The Kaplan-Meier25 method was used to estimate the distribution of EFS, RFS, and OS; hazard ratios (HRs), including 95% confidence intervals (CIs) were estimated based on the Cox proportional hazards model; the treatment arms were compared using the log-rank test. Standard descriptive analyses were done for all other variables. All statistical analyses were performed on the set of patients receiving at least one dose of therapy with the statistical software SAS, version 9.2.
Results
Patient characteristics
Between October 2010 and September 2011, 89 patients were randomized at 27 sites in 7 countries, and 87 patients started treatment; 2 patients (both randomized to LDAC + volasertib) never commenced treatment because of a physical condition and a fatal AE in the screening period, respectively. Patient demographics and baseline disease characteristics were overall balanced between the treatment arms as listed in Table 1. The percentage of secondary AML was higher in the LDAC arm compared with the LDAC + volasertib arm. However, the validated Wheatley risk grouping that integrates important prognostic parameters (cytogenetics, white blood cell count, performance status, age, and AML type [de novo/secondary]) was well-balanced (Table 1), indicating that the imbalance in the number of secondary AML cases was outweighed by other factors.
Table 1.
Patient demographics and baseline disease characteristics
| LDAC | LDAC + volasertib | |
|---|---|---|
| No. of patients | 45 | 42 |
| Age (y), median (range) | 76 (57-86) | 75 (65-87) |
| No. of patients >75 y, n (%) | 23 (51) | 19 (45) |
| Gender (male), n (%) | 25 (56) | 23 (55) |
| ECOG performance status, n (%) | ||
| 0 | 16 (36) | 10 (24) |
| 1 | 20 (44) | 23 (55) |
| 2 | 9 (20) | 9 (21) |
| WBC, 109/L, median (range) | 5.2 (1.0-98.2) | 6.0 (0.7-104.9) |
| BM blasts, %, median (range) | 41 (20-95) | 50 (10-93) |
| Secondary AML, n (%) | 29 (64) | 17 (41) |
| Preceding MDS | 16 | 11 |
| Preceding MPN | 7 | 3 |
| Prior therapy | 4 | 1 |
| Missing | 2 | 2 |
| Genetic group (ELN classification), n (%) | ||
| Favorable | 3/42 (7) | 1/36 (3) |
| Intermediate I/II | 25/42 (60) | 21/36 (58) |
| Adverse | 14/42 (33) | 14/36 (39) |
| Missing | 3 | 6 |
| Gene mutations, n (%) | ||
| NPM1 mutation | 7/40 (18) | 7/33 (21) |
| NPM1 results missing | 5 | 9 |
| _FLT3_-ITD | 6/39 (15) | 5/35 (14) |
| _FLT3_-ITD results missing | 6 | 7 |
| Wheatley risk group, n (%) | ||
| Good | 1 (2) | 0 (0) |
| Standard | 14 (31) | 12 (29) |
| Poor | 30 (67) | 30 (71) |
Response to therapy and treatment exposure
Overall response.
The response rate (CR + CRi) was 13.3% (6 of 45 patients) for LDAC monotherapy and 31.0% (13 of 42 patients) for LDAC + volasertib (OR, 2.91; P = .052); the proportion of patients achieving CR vs CRi within each arm was comparable in both arms (Table 2). Time to response was similar in both arms, with a median of 63.5 days (range, 30-125 days) for LDAC and 71 days (range, 29-158 days) for LDAC + volasertib. There was no obvious correlation between response and presenting white blood cell counts or bone marrow blast percentages at presentation (Table 2).
Table 2.
Response to therapy and mortality
| LDAC | LDAC + volasertib | |
|---|---|---|
| No. of patients treated | 45 | 42 |
| Response to therapy, n (%) | ||
| Overall (CR + CRi)* | 6 (13.3) | 13 (31.0) |
| CR | 3 (6.7) | 6 (14.3) |
| CRi | 3 (6.7) | 7 (16.7) |
| Time to CR or CRi in days, median (range) | 64 (30-125) | 71 (29-158) |
| Death rate, cumulative events, n (%) | ||
| Day 0 | 0 (0) | 0 (0) |
| Day 30 | 4 (8.9) | 4 (9.5) |
| Day 60 | 8 (17.8) | 9 (21.4) |
| Day 90 | 15 (33.3) | 12 (28.6) |
| Response by ELN genetic group, n (%) | ||
| Favorable | 0/3 (0) | 1/1 (100) |
| Intermediate I/II | 5/25 (20) | 5/21 (24) |
| Adverse | 1/14 (7) | 5/14 (36) |
| Missing | 0/3 (0) | 2/6 (33) |
| Response by gene mutation, n (%) | ||
| NPM1 mutated | 4/7 (57) | 3/7 (43) |
| NPM1 wild-type | 2/33 (6) | 9/26 (35) |
| _FLT3_-ITD positive | 2/6 (33) | 1/5 (20) |
| _FLT3_-ITD negative | 4/33 (12) | 11/30 (37) |
| Response by Wheatley risk group, n (%) | ||
| Good | 0/1 (0) | 0/0 (0) |
| Standard | 3/14 (21) | 7/12 (58) |
| Poor | 3/30 (10) | 6/30 (20) |
| Response by WBC count, n (%) | ||
| <1 × 109/L | 0/2 (0) | 1/3 (33) |
| 1 × 109/L to <5 × 109/L | 3/22 (14) | 8/17 (47) |
| 5 × 109/L to 10 × 109/L | 1/5 (20) | 2/7 (29) |
| >10 × 109/L | 2/16 (13) | 2/15 (13) |
| Response by BM blast count, n (%) | ||
| <30% | 3/8 (38) | 3/8 (38) |
| 30-50% | 1/14 (7) | 3/11 (27) |
| >50% | 2/18 (11) | 7/20 (35) |
| Missing | 0/5 (0) | 0/3 (0) |
Response by genetics.
Of the 6 responders in the LDAC arm, 5 had intermediate I or II and 1 had adverse genetics according to the ELN recommendations;5 none of the 3 patients with favorable genetics responded. Of the 13 responders in the LDAC + volasertib arm, responses were seen across all genetic groups: 1 patient had favorable; 5 patients intermediate I/II; 5 patients had adverse genetics, and for 2 patients, the genetic group was missing. Responses by ELN genetic group, NPM1 mutational, and _FLT3_-ITD status as well as Wheatley risk group are given in Table 2.
Treatment exposure.
The median number of cycles initiated was similar in both treatment arms (Table 3): for responding patients, it was 8 (range, 2-22) for LDAC + volasertib, and 7 (range, 5-11) for LDAC; for nonresponding patients it was 2 (range, 1-10) and 2 (range, 1-12), respectively. On the other hand, the duration of treatment was longer in the combination arm: the median number of days on treatment in responding patients was 309 (range, 83-869) for LDAC + volasertib compared with only 214 (range, 153-350) for LDAC monotherapy.
Table 3.
Treatment exposure and subsequent therapy
| LDAC | LDAC + volasertib | |
|---|---|---|
| No. of patients treated | 45 | 42 |
| No. of cycles, median (range) | ||
| All patients | 2 (1-12) | 2 (1-22) |
| Patients with response | 7 (5-11) | 8 (2-22) |
| Patients without response | 2 (1-12) | 2 (1-10) |
| Days on treatment, median (range) | ||
| All patients | 64 (11-459) | 85 (12-869) |
| Patients with response | 214 (153-350) | 309 (83-869) |
| Patients without response | 56 (11-459) | 59 (12-355) |
| Patients discontinued study treatment, n | 45 | 40 |
| No other antileukemia therapy after discontinuation, n (%) | 17 (38) | 24 (60) |
| New antileukemia therapy after discontinuation, n (%) | 28 (62)* | 16 (40)* |
Survival analyses
At the time of analysis, 77 of 87 patients (88.5%) had died and the remaining patients were followed for a median of 28.2 months. One patient was lost to follow-up after 47 days and the remaining 9 living patients were followed for 23.7 to 34.1 months.
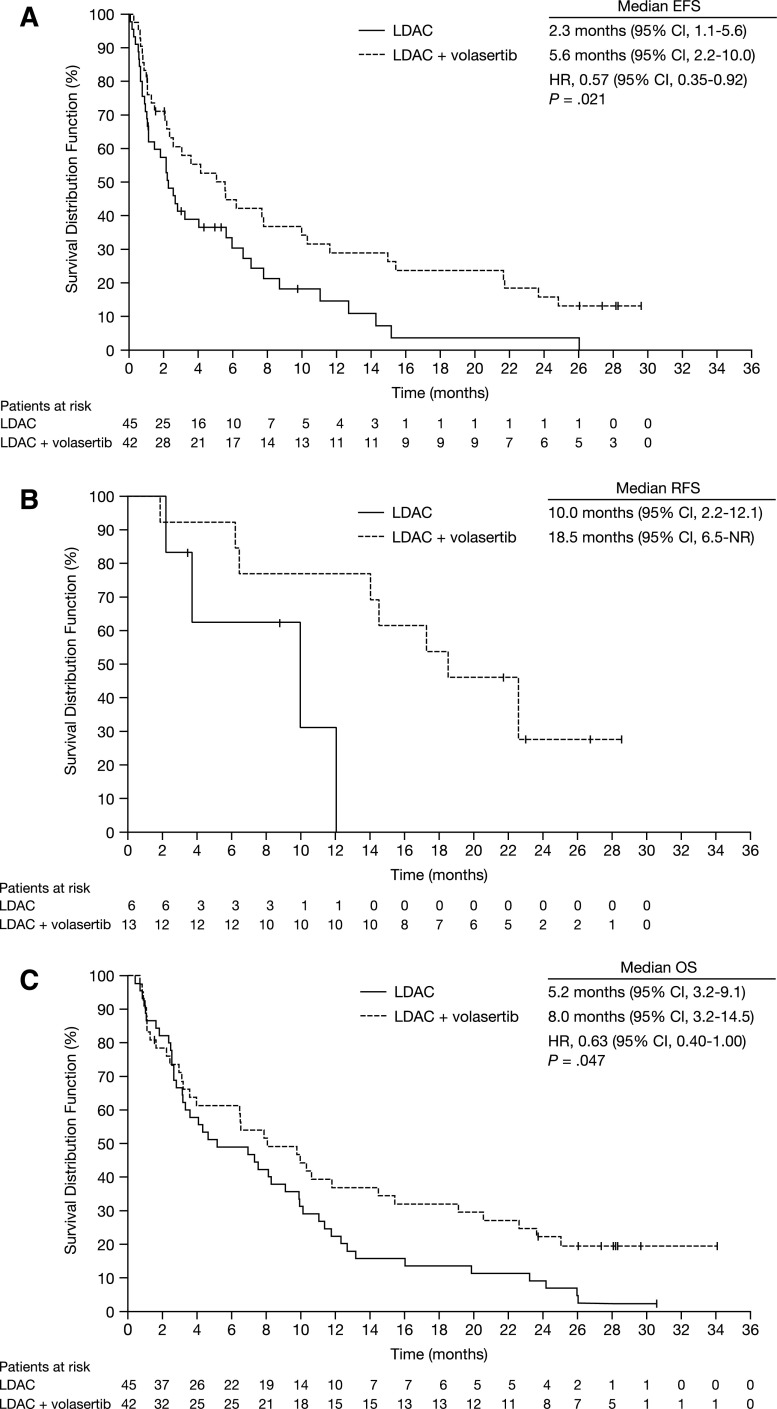
EFS of patients receiving LDAC + volasertib (n = 42) was significantly longer compared with patients receiving LDAC alone (n = 45; median EFS 5.6 vs 2.3 months; HR, 0.57; 95% CI, 0.35-0.92; P = .021; Figure 1A). Remissions achieved by the combination appeared to be more durable; the RFS was 18.5 vs 10.0 months for LDAC + volasertib (n = 13) and LDAC (n = 6), respectively (Figure 1B). In the combination arm, 4 patients are in sustained CR for 22 months or longer. The higher response rate and longer RFS translated into an improved OS for LDAC + volasertib vs LDAC (median OS 8.0 vs 5.2 months; HR, 0.63; 95% CI, 0.40-1.00; P = .047; Figure 1C).
Figure 1.
Kaplan-Meier survival estimates according to randomization. (A) Median EFS times for patients who received LDAC (n = 45) and LDAC + volasertib (n = 42) were 2.3 and 5.6 months, respectively (HR, 0.57; 95% CI, 0.35-0.92; P = .021); 1-year EFS was 14.6% and 29.0%. (B) Median RFS times were 10.0 and 18.5 months for LDAC (n = 6) and LDAC + volasertib (n = 13), respectively. (C) Median OS times for patients who received LDAC (n = 45) and LDAC + volasertib (n = 42) were 5.2 and 8.0 months, respectively (HR, 0.63; 95% CI, 0.40-1.00; P = .047); 1-year OS was 22.2% and 36.8%. Database snapshot November 7, 2013. NR, not reported.
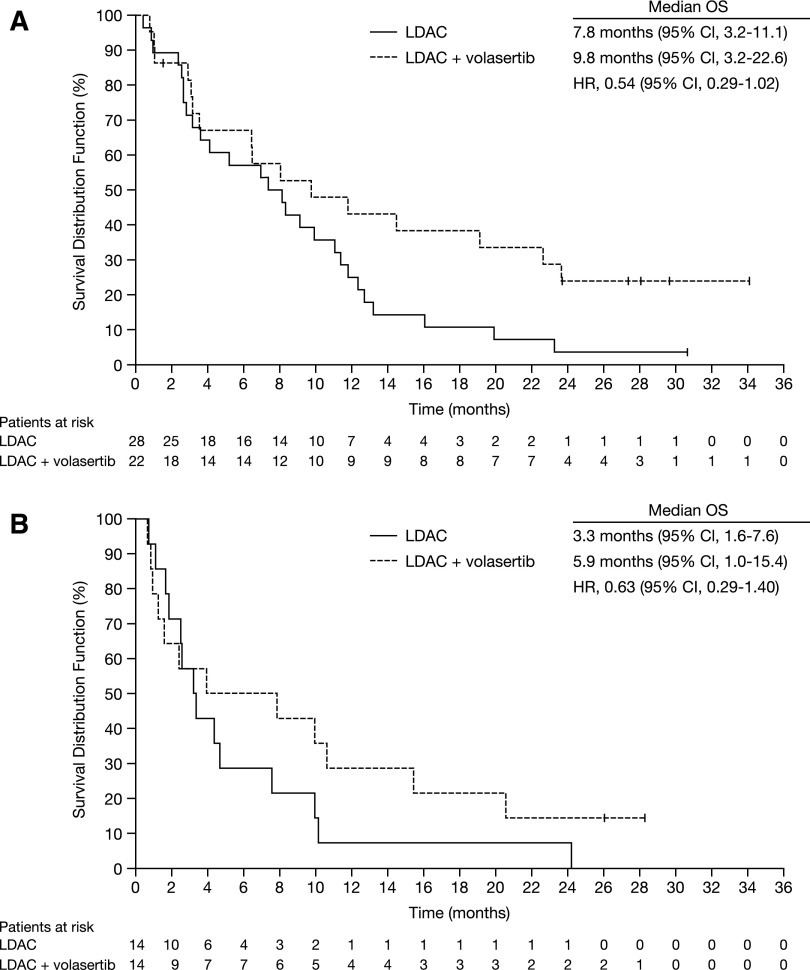
Exploratory analysis by genetic groups showed that patients with favorable, intermediate I, and intermediate II genetics combined as well as patients with adverse genetics appeared to benefit from the combination therapy. Median OS in the favorable, intermediate I and II groups were 9.8 vs 7.8 months for LDAC + volasertib and LDAC, respectively (HR, 0.54; 95% CI, 0.29-1.02; Figure 2A). Median OS in the adverse genetic group was 5.9 vs 3.3 months (HR, 0.63; 95% CI, 0.29-1.40; Figure 2B).
Figure 2.
OS estimates according to randomization and ELN genetic group.5 (A) Favorable and intermediate I/II groups. Median OS times for patients who received LDAC (n = 28) and LDAC + volasertib (n = 22) were 7.8 and 9.8 months, respectively (HR, 0.54; 95% CI 0.29-1.02). (B) Adverse group. Median OS times for patients who received LDAC (n = 14) and LDAC + volasertib (n = 14) were 3.3 and 5.9 months, respectively (HR, 0.63; 95% CI, 0.29-1.40). Database snapshot November 7, 2013.
Safety
In general, the combination of LDAC + volasertib showed a clinically acceptable safety profile. As mentioned previously, the median number of cycles administered in responding patients was similar for LDAC and LDAC + volasertib, although the duration on treatment was longer for the combination arm, giving indirect evidence for more profound myelosuppression and prolonged hematologic recovery. However, the number of red blood cell and platelet units transfused per month was comparable in both treatment arms (Table 4). There was an increase in nonhematologic AEs with LDAC + volasertib compared with LDAC, although AEs were clinically manageable (Table 5). The increased AE frequency in patients receiving LDAC + volasertib was most pronounced in gastrointestinal AEs grade 3 (21% vs 7%), febrile neutropenia grade 3 (38% vs 7%), and infections grade 3 (38% vs 7%). Notably, there was no difference in the death rates at days 30, 60, and 90 between the 2 treatment arms (Table 2).
Table 4.
Supportive care per month exposed to treatment
| Resource | LDAC | LDAC + volasertib |
|---|---|---|
| No. of red blood cell units transfused per month | 5.1 | 4.6 |
| No. of platelet units transfused per month | 5.6 | 5.9 |
| No. of days on antibiotics per month | 10.1 | 8.3 |
Table 5.
Nonhematologic adverse events grade ≥3 occurring by system organ class in >10% of patients in a treatment group, with preferred term displayed if reported in ≥2 patients in a treatment group
| System organ class Preferred term | LDAC, n (%) | LDAC + volasertib, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| CTCAE grade 3 | CTCAE grade 4 | CTCAE grade 5 | CTCAE grade 3-5 | CTCAE grade 3 | CTCAE grade 4 | CTCAE grade 5 | CTCAE grade 3-5 | |
| Febrile neutropenia | 3 (6.7) | 4 (8.9) | 0 | 7 (15.6) | 16 (38.1) | 5 (11.9) | 2 (4.8) | 23 (54.8) |
| Gastrointestinal | 3 (6.7) | 0 | 0 | 3 (6.7) | 9 (21.4) | 1 (2.4) | 0 | 10 (23.8) |
| Diarrhea | 1 (2.2) | 0 | 0 | 1 (2.2) | 4 (9.5) | 0 | 0 | 4 (9.5) |
| General disorders, administration site conditions | 8 (17.8) | 1 (2.2) | 0 | 9 (20.0) | 5 (11.1) | 1 (2.4) | 0 | 6 (14.3) |
| General health deterioration | 3 (6.7) | 1 (2.2) | 0 | 4 (8.9) | 2 (4.8) | 1 (2.4) | 0 | 3 (7.1) |
| Mucosal inflammation | 2 (4.4) | 0 | 0 | 2 (4.4) | 1 (2.4) | 0 | 0 | 1 (2.4) |
| Pyrexia | 1 (2.2) | 0 | 0 | 1 (2.2) | 3 (7.1) | 0 | 0 | 3 (7.1) |
| Infections and infestations | 3 (6.7) | 3 (6.7) | 4 (8.9) | 10 (22.2) | 16 (38.1) | 2 (4.8) | 2 (4.8) | 20 (47.6) |
| Cellulitis | 1 (2.2) | 2 (4.4) | 0 | 3 (6.7) | 2 (4.8) | 0 | 0 | 2 (4.8) |
| Clostridium difficile infection | 0 | 0 | 0 | 0 | 2 (4.8) | 1 (2.4) | 0 | 3 (7.1) |
| Escherichia sepsis | 0 | 0 | 0 | 0 | 2 (4.8) | 0 | 0 | 2 (4.8) |
| Lung infection | 0 | 0 | 0 | 0 | 2 (4.8) | 0 | 0 | 2 (4.8) |
| Pneumonia | 0 | 0 | 2 (4.4) | 2 (4.4) | 7 (16.7) | 0 | 2 (4.8) | 9 (21.4) |
| Sepsis | 0 | 0 | 1 (2.2) | 1 (2.2) | 3 (7.1) | 1 (2.4) | 0 | 4 (9.5) |
| Urinary tract infection | 1 (2.2) | 0 | 0 | 1 (2.2) | 2 (4.8) | 0 | 0 | 2 (4.8) |
| Investigations | 3 (6.7) | 2 (4.4) | 0 | 5 (11.1) | 3 (7.1) | 1 (2.4) | 0 | 4 (9.5) |
| CRP increased | 2 (4.4) | 0 | 0 | 2 (4.4) | 1 (2.4) | 0 | 0 | 1 (2.4) |
| Metabolism, nutrition disorders | 3 (6.7) | 0 | 0 | 3 (6.7) | 4 (9.5) | 3 (7.1) | 0 | 7 (16.7) |
| Hypoglycemia | 0 | 0 | 0 | 0 | 1 (2.4) | 1 (2.4) | 0 | 2 (4.8) |
| Hypokalemia | 1 (2.2) | 0 | 0 | 1 (2.2) | 2 (4.8) | 0 | 0 | 2 (4.8) |
| Respiratory, thoracic, mediastinal disorders | 5 (11.1) | 1 (2.2) | 0 | 6 (13.3) | 6 (14.3) | 1 (2.4) | 3 (7.1) | 10 (23.8) |
| Dyspnea | 4 (8.9) | 1 (2.2) | 0 | 5 (11.1) | 2 (4.8) | 0 | 0 | 2 (4.8) |
| Epistaxis | 0 | 0 | 0 | 0 | 2 (4.8) | 0 | 0 | 2 (4.8) |
| Hypoxia | 0 | 0 | 0 | 0 | 1 (2.4) | 0 | 1 (2.4) | 2 (4.8) |
| Pulmonary edema | 1 (2.2) | 0 | 0 | 1 (2.2) | 1 (2.4) | 1 (2.4) | 0 | 2 (4.8) |
| Respiratory failure | 1 (2.2) | 0 | 0 | 1 (2.2) | 0 | 0 | 2 (4.8) | 2 (4.8) |
Pharmacokinetics
The pharmacokinetics of volasertib were determined following a 1-hour IV infusion of 350 mg volasertib. The apparent half-life of volasertib was 116 hours. Volasertib showed a moderate plasma clearance (897 mL/min; geometric coefficient of variation 43%) and a large volume of distribution (6130 L; geometric coefficient of variation 42%). Volasertib pharmacokinetics in combination with LDAC were similar to those obtained earlier for volasertib monotherapy,26 suggesting no effect of cytarabine on the pharmacokinetics of volasertib. Further, the exposure to cytarabine following s.c. doses of 20 mg bid, as indicated by the maximum concentration and area under the curve values, were similar with or without coadministration of volasertib. Maximum concentration and area under the curve0-4h values were on average 53.8 ng/mL and 71.1 ng × h/mL, respectively.
Discussion
In this randomized, phase 2 study, we compared efficacy and safety of LDAC with and without volasertib, a highly potent and selective inhibitor of Plk, in previously untreated patients with AML considered unsuitable for intensive therapy. By adding volasertib to LDAC, the overall response was more than doubled, with 31% vs 13% for LDAC alone. More importantly, in contrast to most previous novel compounds tested in this patient population, the combination of LDAC + volasertib showed a signal for EFS and OS benefits. However, it should be kept in mind that the study was not powered to show an improvement in survival end points.
For the combination of LDAC + volasertib, responses were observed across all genetic subgroups of AML. Notably, 5 of 14 patients whose leukemic cells exhibited adverse genetics responded. The response rate in this genetic high-risk group was remarkable. In addition, LDAC + volasertib appeared to result in a similar increase in OS within the adverse and the other genetic groups, although survival was shorter in the adverse genetic group in both treatment arms (Figure 2). Preclinical data suggest that cancer cells with deficient p53, a hallmark of AML with complex karyotypes,27 may actually be more sensitive to Plk1 depletion than cancer cells with functional p53.28,29 Given the antimitotic mode of action of volasertib, we also evaluated response by presenting white blood cell counts and bone marrow blast percentages. However, there was no obvious correlation, and responses to volasertib were seen in patients with both low and high counts. Future studies should determine whether the level of PLK1 expression may predict response to volasertib.
Overall, the combination of LDAC + volasertib showed a clinically manageable safety profile. There was an increase in hematologic AEs, as expected, from the antimitotic mode of action and from data of the phase 1 study of volasertib in patients with advanced solid tumors.26 The time interval between cycles was somewhat longer in responding patients after LDAC + volasertib compared with those after LDAC alone, reflecting a more profound myelosuppression and prolonged hematologic recovery. However, patients in the LDAC + volasertib arm were on treatment longer; therefore, comparisons between treatment arms overestimate the difference. In contrast, there was no difference in the number of red blood cell and platelet transfusions per month on treatment between the treatment arms. With regard to nonhematologic toxicity, there was an increase in neutropenic fever, infections, and gastrointestinal toxicity that mostly did not exceed grade 3. This observation was also expected given that in the phase 1 part, the maximum tolerated dose for volasertib monotherapy was mainly determined by mucositis at doses of 400 mg and higher (manuscript in preparation).20 Of note, there was no increase in the death rate up to day 90, demonstrating that the increase in AEs did not result in an excess treatment-related mortality. Pharmacokinetic analyses suggest neither an effect of volasertib on the pharmacokinetics of cytarabine, nor an effect of cytarabine on the volasertib exposure.
In recent years, several attempts to improve upon the standard of care in older patients for who intensive induction therapy is not deemed appropriate have failed. Several novel agents have been tested in single-arm studies (eg, tipifarnib, laromustine, clofarabine), but very few have been validated in randomized, phase 3 trials. A phase 3 trial evaluated the efficacy and safety of tipifarnib compared with SC (including hydroxyurea) as first-line therapy in older patients.30 Tipifarnib resulted in a CR rate of only 8%, and it did not prolong survival, with median survival times of 107 and 109 days for the tipifarnib and SC arms, respectively. Clofarabine, a novel nucleoside analog, has shown encouraging activity in single-arm studies.31,32 However, in a recent phase 3 trial of 406 older patients comparing clofarabine 20 mg/m2 IV (days 1-5) with LDAC 20 mg bid s.c. (days 1-10), clofarabine doubled the CR/CRi rate (38% vs 20%), but did not result in an improvement of OS.33 Patients in the clofarabine arm experienced increased myelosuppression and more grade 3/4 nonhematologic toxicities. Of note, the 30- and 60-day mortality was higher in the clofarabine arm compared with LDAC (18% vs 13% and 32% vs 26%, respectively). This is in contrast to the findings in our phase 2 study, which did not suggest a difference in early death rates between the 2 treatment arms. Compared with clofarabine, LDAC + volasertib appears to be less toxic and may induce remission in a more gentle way.
Azacitidine was approved by US Food and Drug Administration and European Medicines Agency for AML with low bone marrow blast counts based on data from the pivotal myelodysplastic syndrome trial comparing azacitidine and conventional care regimens that included 113 patients classified as having AML under the current World Health Organization criteria (20% to 30% blasts).34 However, the difference in OS was only significant when comparing azacitidine with SC, but not with LDAC or intensive chemotherapy. A trial comparing azacitidine and conventional care regimens in AML with more than 30% blasts is under way (www.clinicaltrials.gov #NCT01074047). Based on the data from the DACO-016 trial, decitabine was recently approved by European Medicines Agency (but not the US Food and Drug Administration) for the treatment of older patients with AML.35 DACO-016 compared decitabine 20 mg/m2 on days 1 through 5 with either LDAC 20 mg/m2 administered once daily for 10 days + SC (88.5% of patients), or SC alone (11.5%), in 485 older (≥65 years) patients with AML and poor- or intermediate-risk cytogenetics. A significant survival benefit for the decitabine arm was only observed in an unplanned post hoc analysis. Furthermore, the response rate in the LDAC control arm was lower than reported in prior trials, and there was variation in efficacy among different geographic regions; for example, in Western Europe, where LDAC is used more widely, the response rate was higher for LDAC compared with decitabine and the HR for OS was 1.03 (95% CI, 0.62-1.72). The median RFS of CR patients in the DACO-016 trial was 8.3 months for the decitabine arm and 6.7 months for the control arm.36 In our study, the median RFS of CR and CRi patients was 18.5 months in the LDAC + volasertib arm and 10.0 months in the LDAC arm, suggesting that the LDAC + volasertib might provide benefit not only by increasing the remission rate, but also by prolonging RFS of responding patients. However, the limited sample size of this phase 2 trial was not sufficient to draw final conclusions regarding RFS differences.
In the current trial, the combination of LDAC + volasertib led to response (31% CR/CRi rate) and survival data (median survival 8.0 months; 1-year OS estimate 36.8%) that appear at least equivalent to data from previous trials conducted in similar patient populations. Based on these encouraging data, a randomized, placebo-controlled, phase 3 trial has been initiated comparing LDAC with and without volasertib.
Acknowledgments
The authors thank all participating patients and their families, the medical and nursing staff, and the other Study 1230.4 investigators for their participation in this study. A complete membership list appears in Appendix.
This study was funded by Boehringer Ingelheim Pharma GmbH & Co. KG. Editorial assistance, financially supported by Boehringer Ingelheim, was provided by GeoMed, part of KnowledgePoint360, an Ashfield Company, during the preparation of this manuscript. The sponsor, Boehringer Ingelheim, participated actively in designing the study, developing the protocol, and provided logistical support during the trial. Boehringer Ingelheim maintained the trial database, collected the data, monitored the study, and analyzed the data.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.D and F.V. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; H.D., O.O., F.V., and T.T. conceived and designed the study; H.D., M.L., W.F., L.F., A.H., J.B., S.L., O.R., P.T., O.O., C.M.-T., A.K., E.R., K.D, R.S., and J.M. enrolled the patients and collected the data_;_ H.D., M.L., S.L., O.O., C.M.-T., K.D., R.S., F.V., T.T., H.F., and J.M. analyzed and interpreted the data_;_ H.D., O.O., F.V., and T.T. drafted the manuscript; H.D., M.L., W.F., L.F., A.H., J.B., S.L., O.R., P.T., O.O., C.M.-T., A.K., E.R., K.D., R.S., F.V., T.T., H.F., and J.M. critically revised the manuscript for important intellectual content; F.V. statistically analyzed the data; H.D., M.L., O.O., A.K., E.R., K.D., R.S., and T.T. provided administrative, technical or material support; and H.F. performed pharmacokinetic evaluation.
Conflict-of-interest disclosure: H.D. and K.D. received funding from Boehringer Ingelheim (funding of central genetic diagnostics within BI 1230.4 trial). W.F. received financial support for research from Pfizer and Novartis and travel support to ASH 2013 from TEVA Pharmaceutical Industries Ltd. J.B. and P.T. received financial support for research from Boehringer Ingelheim for the current study. O.O. received consultation fees for advisory board participation. C.M.-T. received funding and financial support for research from Boehringer Ingelheim. F.V., T.T., and H.F. are or were employed by Boehringer Ingelheim Pharma GmbH & Co. The remaining authors declare no competing financial interests.
The current affiliation for L.F. is Centre Hospitalier General, Meaux, France.
The current affiliation for J.M.B. is Department of Medicine, University of Alberta, Edmonton, AB, Canada.
Correspondence: Hartmut Döhner, Department of Internal Medicine III, University Hospital Ulm, Albert-Einstein-Allee 23, 89081 Ulm, Germany; e-mail: hartmut.doehner@uniklinik-ulm.de.
References
- 1.Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113(18):4179–4187. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- SEER. Cancer Statistics Review 1975-2009. http://seer.cancer.gov.
- 3.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estey E. AML in older patients: are we making progress? Best Pract Res Clin Haematol. 2009;22(4):529–536. doi: 10.1016/j.beha.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Döhner H, Estey EH, Amadori S, et al. European LeukemiaNet. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 6.Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109(6):1114–1124. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- 7.Sekeres MA, Steensma DP. Boulevard of broken dreams: drug approval for older adults with acute myeloid leukemia. J Clin Oncol. 2012;30(33):4061–4063. doi: 10.1200/JCO.2012.44.2962. [DOI] [PubMed] [Google Scholar]
- 8.Schöffski P. Polo-like kinase (PLK) inhibitors in preclinical and early clinical development in oncology. Oncologist. 2009;14(6):559–570. doi: 10.1634/theoncologist.2009-0010. [DOI] [PubMed] [Google Scholar]
- 9.Strebhardt K. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat Rev Drug Discov. 2010;9(8):643–660. doi: 10.1038/nrd3184. [DOI] [PubMed] [Google Scholar]
- 10.Lens SM, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat Rev Cancer. 2010;10(12):825–841. doi: 10.1038/nrc2964. [DOI] [PubMed] [Google Scholar]
- 11.Degenhardt Y, Lampkin T. Targeting Polo-like kinase in cancer therapy. Clin Cancer Res. 2010;16(2):384–389. doi: 10.1158/1078-0432.CCR-09-1380. [DOI] [PubMed] [Google Scholar]
- 12.Renner AG, Dos Santos C, Recher C, et al. Polo-like kinase 1 is overexpressed in acute myeloid leukemia and its inhibition preferentially targets the proliferation of leukemic cells. Blood. 2009;114(3):659–662. doi: 10.1182/blood-2008-12-195867. [DOI] [PubMed] [Google Scholar]
- 13.Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6(4):321–330. doi: 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- 14.Rudolph D, Steegmaier M, Hoffmann M, et al. BI 6727, a Polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin Cancer Res. 2009;15(9):3094–3102. doi: 10.1158/1078-0432.CCR-08-2445. [DOI] [PubMed] [Google Scholar]
- 15.Steegmaier M, Hoffmann M, Baum A, et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr Biol. 2007;17(4):316–322. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 16.Hofheinz RD, Al-Batran SE, Hochhaus A, et al. An open-label, phase I study of the polo-like kinase-1 inhibitor, BI 2536, in patients with advanced solid tumors. Clin Cancer Res. 2010;16(18):4666–4674. doi: 10.1158/1078-0432.CCR-10-0318. [DOI] [PubMed] [Google Scholar]
- 17.Müller-Tidow C, Bug G, Lübbert M, et al. A randomized, open-label, phase I/II trial to investigate the maximum tolerated dose of the Polo-like kinase inhibitor BI 2536 in elderly patients with refractory/relapsed acute myeloid leukaemia. Br J Haematol. 2013;163(2):214–222. doi: 10.1111/bjh.12518. [DOI] [PubMed] [Google Scholar]
- 18.Hikichi Y, Honda K, Hikami K, et al. TAK-960, a novel, orally available, selective inhibitor of polo-like kinase 1, shows broad-spectrum preclinical antitumor activity in multiple dosing regimens. Mol Cancer Ther. 2012;11(3):700–709. doi: 10.1158/1535-7163.MCT-11-0762. [DOI] [PubMed] [Google Scholar]
- 19.Valsasina B, Beria I, Alli C, et al. NMS-P937, an orally available, specific small-molecule polo-like kinase 1 inhibitor with antitumor activity in solid and hematologic malignancies. Mol Cancer Ther. 2012;11(4):1006–1016. doi: 10.1158/1535-7163.MCT-11-0765. [DOI] [PubMed] [Google Scholar]
- 20.Bug G, Schlenk R, Müller-Tidow C, et al. Phase I/II study of BI 6727 (volasertib), an intravenous polo-like kinase-1 (PLK1) inhibitor, in patients with acute myeloid leukemia (AML): results of the dose finding for BI 6727 in combination with low-dose cytarabine [abstract]. Blood. 2010;116(suppl)
- 21.Wheatley K, Brookes CL, Howman AJ, et al. United Kingdom National Cancer Research Institute Haematological Oncology Clinical Studies Group and Acute Myeloid Leukaemia Subgroup. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol. 2009;145(5):598–605. doi: 10.1111/j.1365-2141.2009.07663.x. [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Bennett JM, Kopecky KJ, et al. International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia [published correction appears in J Clin Oncol. 2004;22(3):576]. J Clin Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Lachin JM. A review of methods for futility stopping based on conditional power. Stat Med. 2005;24(18):2747–2764. doi: 10.1002/sim.2151. [DOI] [PubMed] [Google Scholar]
- 24.Shih WJ, Quan H, Li G. Two-stage adaptive strategy for superiority and non-inferiority hypotheses in active controlled clinical trials. Stat Med. 2004;23(18):2781–2798. doi: 10.1002/sim.1877. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 26.Schöffski P, Awada A, Dumez H, et al. A phase I, dose-escalation study of the novel Polo-like kinase inhibitor volasertib (BI 6727) in patients with advanced solid tumours. Eur J Cancer. 2012;48(2):179–186. doi: 10.1016/j.ejca.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Rücker FG, Schlenk RF, Bullinger L, et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood. 2012;119(9):2114–2121. doi: 10.1182/blood-2011-08-375758. [DOI] [PubMed] [Google Scholar]
- 28.Guan R, Tapang P, Leverson JD, Albert D, Giranda VL, Luo Y. Small interfering RNA-mediated Polo-like kinase 1 depletion preferentially reduces the survival of p53-defective, oncogenic transformed cells and inhibits tumor growth in animals. Cancer Res. 2005;65(7):2698–2704. doi: 10.1158/0008-5472.CAN-04-2131. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Lei M, Erikson RL. Normal cells, but not cancer cells, survive severe Plk1 depletion. Mol Cell Biol. 2006;26(6):2093–2108. doi: 10.1128/MCB.26.6.2093-2108.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harousseau JL, Martinelli G, Jedrzejczak WW, et al. FIGHT-AML-301 Investigators. A randomized phase 3 study of tipifarnib compared with best supportive care, including hydroxyurea, in the treatment of newly diagnosed acute myeloid leukemia in patients 70 years or older. Blood. 2009;114(6):1166–1173. doi: 10.1182/blood-2009-01-198093. [DOI] [PubMed] [Google Scholar]
- 31.Kantarjian HM, Erba HP, Claxton D, et al. Phase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. J Clin Oncol. 2010;28(4):549–555. doi: 10.1200/JCO.2009.23.3130. [DOI] [PubMed] [Google Scholar]
- 32.Burnett AK, Russell NH, Kell J, et al. European development of clofarabine as treatment for older patients with acute myeloid leukemia considered unsuitable for intensive chemotherapy. J Clin Oncol. 2010;28(14):2389–2395. doi: 10.1200/JCO.2009.26.4242. [DOI] [PubMed] [Google Scholar]
- 33.Burnett AK, Russell NH, Hunter AE, et al. UK National Cancer Research Institute AML Working Group. Clofarabine doubles the response rate in older patients with acute myeloid leukemia but does not improve survival. Blood. 2013;122(8):1384–1394. doi: 10.1182/blood-2013-04-496596. [DOI] [PubMed] [Google Scholar]
- 34.Fenaux P, Mufti GJ, Hellström-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28(4):562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 35.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisai Inc. FDA ODAC briefing document NDA 021790/S-010: Dacogen (decitabine). Indication: acute myeloid leukemia. February 9, 2012. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM290512.pdf. Accessed April 7, 2014.