Effect Size of Memory Deficits in Mice with Adult-Onset P301L Tau Expression (original) (raw)
. Author manuscript; available in PMC: 2015 Oct 1.
Published in final edited form as: Behav Brain Res. 2014 Jul 6;272:181–195. doi: 10.1016/j.bbr.2014.06.057
Abstract
Transgenic mice expressing mutations in tau have yielded essential discoveries for Alzheimer’s disease. One of the most commonly used tau mouse models is the tet-off Tg(tauP301L)4510 model that expresses P301L human tau driven by the calcium-calmodulin kinase IIα (CaMKIIα) promoter system. Tau expression in this model is regulatable, allowing for suppression of mutant tau expression until adulthood and prevention of possible developmental alterations resulting from P301L tau expression during development. Here, we compared the effect and sample sizes needed for three learning and memory tasks in mice with adult-onset P301L tau expression. Our findings indicate that the Incremental Repeated Acquisition (IRA) and trace fear conditioning tasks, neither of which have previously been published with these mice, were highly sensitive to P301L tau expression, whereas the Morris water maze, the most commonly used task with this model, was the least sensitive. Memory deficits were observed at a time when tau pathology was subtle and prior to readily detectable neuronal loss. Thus, we provide essential information (effect and sample sizes needed) for establishing experimental designs at a time point when memory deficits are likely to go undetected if inadequate sample sizes are used. Our work also suggests the tet-off Tg4510 model provides a way to avoid mutant tau expression during the perinatal and early postnatal stages, thereby preventing possible developmental alterations unrelated to Alzheimer’s disease.
Keywords: Alzheimer’s Disease, Tau, Tg4510, Memory, Incremental Repeated Acquisition, Fear Conditioning
1. Introduction
Tauopathies are a group of neurodegenerative disorders involving tau, a microtubule-associated protein, and are characterized by intracellular aggregates of hyperphosphorylated tau [1]. To model human tauopathies, the Tg(tauP301L)4510 mouse is commonly used [2]. These mice express regulatable levels of human tau carrying the P301L mutation and exhibit age-dependent tau pathology, neurodegeneration, and progressive memory decline [3], similar to that observed in Alzheimer’s disease (AD), the most common tauopathy. Tau expression is limited to the forebrain in these mice [3], which restricts pathology to the brain regions most affected in AD. Forebrain tau expression also preserves motor function by avoiding tau expression in the brainstem, thereby permitting a clean interpretation of memory test results.
This model is frequently used to evaluate potential therapies for AD. An appropriate effect size is critical to guarantee that the dynamic range for a particular cognitive task is wide enough such that subtle treatment effects can be detected. Here, we examine the effect and sample sizes needed for three learning and memory tasks: (1) the most commonly used task with AD mouse models, the Morris water maze (MWM); (2) a task allowing for daily, longitudinal assessment, the Incremental Repeated Acquisition task; and (3) a rapid test requiring only two days, the trace fear conditioning task. Because of the recent recognition that mutant protein expression during developmental periods can result in phenotypes not related to AD (e.g., [4, 5]), we examined learning and memory in mice with adult-onset P301L tau expression.
In the Tg(tauP301L)4510 model, P301L human tau is driven by the calcium-calmodulin kinase IIα (CaMKIIα) promoter system. This model uses the tetracycline transactivator (TTA) expressed from the CaMKIIα promoter to produce a conditional model of tauP301L expression. In the absence of tetracycline, tau is expressed, whereas in the presence of tetracycline (or a tetracycline derivative, such as doxycycline), tau expression is suppressed (Figure 1A & 1B). The CaMKIIα promoter was believed to not produce transgene overexpression during the perinatal period [3, 6], yet in this model, tau expression can be detected as early as postnatal day 7 [3], a time when many neurogenic events take place [7]. Recent work with tet-off amyloid precursor protein (APP) transgenic mice suggests use of the CaMKIIα promotor system results in early postnatal APP overexpression, leading to severe locomotor hyperactivity; delaying transgene onset until adulthood attenuates locomotor hyperactivity, suggesting this phenotype is a developmental confound resulting from APP overexpression during brain development [4]. Similarly, a comparison of the effects of perinatal versus adult-onset P301L tau expression in the Tg(tauP301L)4510 mouse revealed differences in neuron loss, neurofibrillary tangles, and astrogliosis/microgliosis [5]. Because of these developmental effects of protein expression, more and more laboratories are using this adult-onset model to examine the effects of therapeutics and/or the mechanisms of neuropathology. Thus, we sought to provide critical information about the effect sizes needed to detect early and subtle memory deficits in mice with adult-onset P301L tau expression.
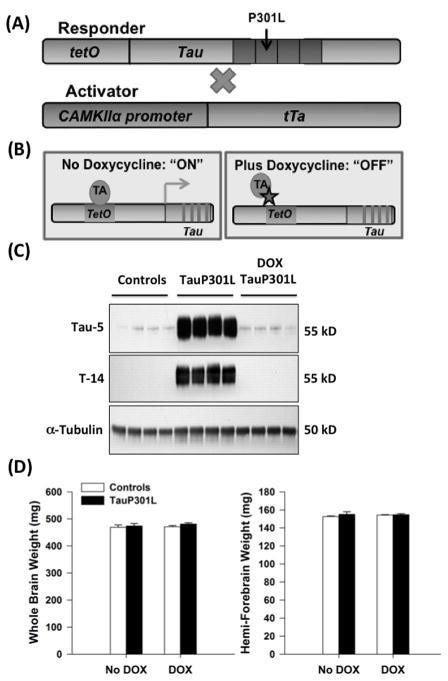
Figure 1.
Regulation of Tau Expression. (A) Both TauWT and TauP301L responder lines encode for human four repeat tau lacking the amino terminal sequences (4R0N). The activator line uses a forebrain specific promoter (CaMKIIα) to restrict tau expression to the forebrain. (B) The addition of doxycycline (DOX 40 ppm) to the drinking water prevents the transcription of tau by preferentially binding to the tetracycline transactivator (tTA) protein. In the absence of DOX, transcription occurs. (C) In the absence of DOX, TauP301L mice exhibit strong tau expression in the hippocampus at 3.5 months of age when examined using an antibody that recognizes both mouse and human tau (Tau-5), or just human tau (T-14). In the presence of DOX, tau expression is suppressed in tauP301L mice at 3.5 months of age. Equal loading was observed across the lanes (α-tubulin). (D) Whole and hemi-forebrain weights were similar for controls and tauP301L mice on or off DOX at 3.5 months of age.
One goal of the current study was to establish that memory deficits are dependent upon tau expression. Because the hippocampus is one of the first regions affected in AD [8], most studies examining memory deficits in tau mouse models have used the hippocampal-dependent Morris water maze (MWM) task [2, 3, 9]. To determine whether tau expression is required for memory deficits, tau was suppressed from conception until 3 months of age and memory was examined using the MWM at 2.5 months of age, before tau expression began. Previous work with tauP301L mice expressing tau during development suggests these mice exhibit memory deficits in the MWM after approximately 3 months of tau expression [2, 3]. To determine whether a similar duration of adult-onset P301L tau expression would result in memory deficits, we examined tauP301L mice at 6 months of age, after approximately 3 months of tau expression.
A second goal of this study was to identify a longitudinal task, as well as a high-throughput rapid memory task, sensitive to P301L tau expression. For longitudinal testing, we used the hippocampal-dependent incremental repeated acquisition (IRA) of response chains task [10–12]. In the IRA task, mice are required to make a sequence (or chain) of nose-poke responses for delivery of sweetened milk. Each session begins at a chain length of one (e.g., one right nosepoke response results in the delivery of milk). The chain length then increments by one (left then right result in milk delivery) after a criterion level of mastery in the previous chain is reached. That is, mice have to make three consecutive correct chains before another nose poke is added to the chain. The incremental nature of the task prevents floor performance by increasing the chain requirement when the mouse correctly performs. Likewise, because the task adjusts task difficulty based on the ability of each mouse, extinction (or a cessation of responding) due to a lack of milk delivery is prevented. Even though the chain length reached by the end of the session varies, the mice earn approximately equal numbers of milk deliveries, thereby preventing cessation of responding. The version of IRA used here is separated into a performance session, where the sequence of responses trained is always the same, and a learning session, where a different sequence of responses is trained in each session [12]. One session is run each day, and the two types of sessions alternate daily. Because a new sequence is presented during each learning session and advancement of the chain is based upon a mouse’s ability, the task remains difficult across time, allowing for longitudinal assessment of learning and memory.
For some situations, prolonged memory testing is not feasible or suitable to the experimental question, and thus, identification of a memory test capable of detecting deficits in tauP301L mice without prolonged training was sought. The trace fear conditioning paradigm takes only two days, one training and one testing day, thereby allowing for rapid, high-throughput testing of mice. To our knowledge, the trace fear conditioning paradigm has never been used with the tauP301L model. In the trace fear conditioning paradigm, mice are presented with a neutral stimulus (tone) paired with an aversive stimulus (shock), but the tone and shock are separated by a stimulus-free interval called the trace interval, which engages both the hippocampus [13] and prefrontal cortex [14]. This protocol allows for assessment of both contextual and cued conditioning. Here, we sought to determine whether the trace/contextual fear conditioning paradigm would serve as an rapid memory test for use with the adult-onset tauP301L mouse model.
A third goal was to report the effect and sample sizes needed for each of the behavioral tasks used. Essential in any study design is the inclusion of an adequate sample size to detect statistical differences. Properly powering studies is particularly important when the deficits are likely to be subtle, such as during early stages of the disease process. The recent increase in studies examining of mouse models before late-stage pathology (e.g., neuron loss) develops means properly powering studies is more important than ever. Failure to do so can result in underpowered studies and a misinterpretation of the results.
2. Materials and Methods
2.1. Subjects
Mice expressing P301L mutant human tau linked to a hereditary tauopathy [15] were created by crossing mice harboring a responder transgene with mice harboring an activator transgene (Figure 1A). Responder mice in an FVB background strain were initially generated by placing a tetracycline-responsive element (TRE) upstream of cDNA encoding human four-repeat tau with the P301L mutation, lacking both N-terminal inserts (4R0N tauP301L) [2]. The activator transgene in a second mouse line of a 129S6 background strain comprised a tet-off open reading frame placed downstream of a CaMKII promoter [3]. Heterozygous responder and activator mice were bred together to create bigenic progeny containing both transgenes; only the resulting +/+ FVB/129S6 mice, referred to as tauP301L mice, overexpress mutant P301L human tau. Littermate control mice do not express tau or exhibit behavioral impairments [2, 3]. The necessary mice to maintain activator and responder lines were generously donated by Dr. Karen Ashe at the University of Minnesota.
The use of the CaMKII promoter results in tau expression that is restricted to the forebrain [3, 16], the regions most affected in AD [17]. This responder/activator system also allows for the conditional expression of tau. In the tet-off system, the tetracycline transactivator (tTA) protein binds to the TRE in absence of doxycycline, a tetracycline derivative, resulting in transcription of tau (Figure 1B). In contrast, in the presence of doxycycline, tTA cannot bind the TRE, resulting in suppression of transcription [18]. Tau expression was suppressed during development using doxycycline hyclate (DOX 40 ppm). DOX was administered via water bottles to pregnant dams two weeks before mating, and DOX exposure continued in the experimental mice, both controls and tauP301L mice, until approximately 3.5 months ± 2 weeks of age. All mice were housed, between two and five per cage, in a temperature and humidity-controlled colony room with a 12:12 light/dark cycle.
To ensure 40 ppm DOX would suppress tau expression but not alter brain weights, ten mice (n = 5 tauP301L and n = 5 controls) were placed on 40 ppm DOX from conception until 3.5 months of age; tau expression, whole brain weight, and hemi forebrain weight were compared to 3.5 month mice (n = 5 tauP301L and n = 5 controls) not on DOX. Eighty-seven mice were (n = 41 tauP301L and n = 46 controls) tested in the Morris water maze (MWM) at 2.5 months, before removal of DOX from the drinking water at 3 months of age. At 6 months of age, after approximately 3 months of tau expression, the same mice were tested again in the MWM. Twenty mice (n = 9 tauP301L and n = 11 controls) began testing in the IRA procedure at approximately 3.5 months of age and continued until approximately 6.5 months of age. Weight was maintained between 85 and 90% of each mouse’s free feeding weight. Fear conditioning took place at approximately 6.5 months of age, after 3 months of tau expression; nineteen mice (n = 8 tauP301L and n = 11 control mice) were examined using a 10 s trace and twenty mice were (n = 7 tauP301L and n = 13 control mice) examined using a 30 s trace. Approximately half of the mice were female and half were male; sex differences were not observed in any behavioral task (_p_s > 0.05) and will not be described below. All experimental procedures were conducted in accordance with the standards of Institutional Animal Care and Use Committee (IACUC) and approved by West Virginia University’s IACUC.
2.2. Morris Water Maze (MWM)
Spatial reference memory was measured using the Morris water maze with a protocol tailored for the rapid learning of the 129FVBF1 mice and previously described in detail [19]. Mice were prehandled for 10 days during the 2 weeks preceding Morris water-maze testing. Prehandling consisted of a 20 s exposure to water at a depth of 1 cm and was designed to acclimate the mice to the introduction and removal from the testing pool. Mice received visible platform training for 3 days, 6 trials per day, followed by hidden platform training for 6 days, 4 trials per day. Four probe trials of 30 s duration were performed 20 h after 8, 12, 16, and 24 hidden training trials. The platform crossing index (PCI) for each of the four probe trials was calculated and analyzed, as well as the mean of the PCI across the four probe trials (Mean PCI). All trials were monitored using a computerized tracking system (Noldus EthoVision 3.0; Noldus Information Technology, Wageningen, The Netherlands), and performance measures were extracted using Wintrack (Wolfer et al., 2001).
2.3. Incremental Repeated Acquisition (IRA)
2.3.1. Apparatus
Testing took place in six standard model ENV-022 operant-conditioning chambers (Med Associates, St. Albans, VT) contained in sound-attenuating cubicles, dimensions 22″ W x 15″ H x 16″ D. An automated syringe and tubing system delivered 20 uL sweetened condensed milk, diluted 50:50 with water, into a constantly accessible holding cup as reinforcement. Chambers were equipped with nose-poke sensors to the left (L) and right (R) of the milk cup, and on the back wall across from the milk cup (B), which counted responses by interruption of a photobeam. Two sonalert tones, 2900 Hz (referred to as “low’) and 4500 Hz (referred to as “high”), functioned as discriminative auditory stimuli. Fans ran constantly for ventilation, and a house light above the back nose-poke turned on during the interval following an incorrect response. Programming was completed in MedState Notation and conducted with 10 ms temporal resolution in MED-PC IV (Med Associates, St. Albans, VT).
2.3.2. Training
Mice were removed from DOX the first day of training. All behavioral sessions were conducted six to seven days per week with each subject tested in the same chamber and at approximately the same time each day. Nose-poking was autoshaped in daily 1.5-hour sessions beginning on the left nose-poke hole. In the first component of the autoshaping procedure, noncontingent milk (i.e., no response was required for milk delivery) was delivered after a 5.5-min interval, during the last 30 s of which, the active nose-poke hole was illuminated and a tone sounded. Responses on the active nose-poke alternative also resulted in milk delivery (reinforcer). After 10 correct responses or 20 noncontingent milk deliveries, the mouse entered Component Two, in which noncontingent milk deliveries were no longer given. During Component 2, the active nose-poke hole was constantly illuminated and a response on the active nose-poke resulted in milk delivery. Sessions ended either after 40 reinforced nose-pokes or when 1.5 hours had passed, whichever occurred first. After entering Component Two, mice were not returned to Component One on subsequent days training the same nose-poke. Two consecutive days of 40 reinforced nose-pokes was the criterion used to determine response acquisition, at which point the mouse was placed into Component One on a new nose-poke hole during the next session. Training was complete when all mice acquired each of the three nose-poke responses. Mice were then returned to Component Two on each of the three nose-poke alternatives for an additional session to ensure retention of acquired responding. Training was completed in 12 – 13 sessions.
2.3.3. IRA Testing
The IRA procedure has been described in detail previously [20]. The IRA procedure consisted of daily 60-min sessions in which a maximum of a six-link chain could be reached by backward chaining. Thus, the performance chain of LRBLBR would be incremented as R > milk, B > R > milk, L > B > R > milk, etc. Six sequential correct responses were required to advance from a one-link chain, whereas all further chains required three consecutive correct chains to progress.
The three nose-poke sensors were illuminated at the start of each session. A correct chain resulted in a low tone and delivery of milk, followed by a 5-s inter-trial interval in which all lights were darkened and nose-pokes had no programmed consequences. Any incorrect response (1) illuminated the house light for a time out period of 5 s, during which responding had no programmed consequence, (2) turned off the LED in the active nose-poke hole, and (3) returned the current chain length to the response farthest from reinforcement. The consecutive number of correct chains completed was also reset. Responding in the last second of either the inter-trial interval or time out extended the period for an additional 2 s. A unique auditory discriminative stimulus was paired with each link; steady high, pulsing high, pulsing low, steady low, fast pulsing low, and fast pulsing high tones sounded in links one through six, respectively. IRA sessions could be terminated if a mouse earned 50 reinforcers in a six-link chain or the 1-h time limit elapsed, whichever occurred first.
The first 25 sessions were performance sessions, where the chain of responses being trained was always the same (LRBLBR). After the 25th session, sessions alternated between performance and learning sessions, where the response chain differed in each session. Learning sequences were presented in pseudorandom order without replacement, so that no two consecutive sequences (separated by a performance session) had the same initial nose-poke response. One of the following 17 sequences was trained on learning days: RLBRBL, LBRLBR, BRBLRL, RBLBRL, BLRBRL, LBRLRB, RBLRLB, BLBRLR, BLRBLR, LRBLRL, LRLBRB, RLRBLB, RLBLRB, RLBLBR, BRLBLR, BRBRLR, or LBRLBL. Sequences were generated such that no individual response was repeated twice in a row, and each sequence contained at least one response in each nose-poke hole. In addition, no learning sequence could include more than four nose pokes in the same position as in the performance sequence. The nose-poke sensors flashed during learning sessions instead of remaining continuously illuminated, and checkerboard patterns were placed on the window of each chamber to serve as a unique stimulus associated with a learning session.
Dependent measures included maximum chain length reached, total responses (i.e., nose pokes), total reinforcers, accuracy (total correct/(total errors + total correct) × 100), and a weighted average of earned reinforcers, called progress quotient (PQ). PQ is derived by the formula: PQ = [1(Rf1) + 2(Rf2) + 3(Rf3) + 4(Rf4) + 5(Rf5) + 6(Rf6)]/Total reinforcers, where Rfx signifies the number of reinforcers earned in chain length x [12, 21]. PQ is a more useful gauge of performance than overall accuracy in a mastery-based IRA, where each mouse may reach a different chain length on each day [20]. Using Pearson r correlations, we correlated total responses and total reinforcers during acquisition. Raw data was extracted to a Microsoft Excel spreadsheet using MED-PC to Excel (Med Associates, St. Albans, VT)
2.4. Contextual and Trace Fear Conditioning (FC)
2.4.1. Apparatus
Trace conditioning training occurred in a MED-VFC-NIR-M chamber (12″ H X 9.5″ W X 8.25″ H) from Med Associates. Through a connection from the PC to the chamber, the software (MED-SYST-VFC, Med Associates Inc.) controlled the test shock current and sound pressure. After a shock current was delivered, the program measured freezing behavior, defined as the absence of motion, except that needed for breathing, for 1 s. The software calculated the percent of freezing using data from the near infrared camera. Distinct contextual cues differentiated the training (Context A) and tone-retention testing (Context B) conditions. In the training condition (Context A), there was a grid floor and background noise from the ventilation system. Lights were turned on inside the chamber, while the room light was turned off, and a 95% ethyl alcohol solution was used to clean the chamber. In contrast, the tone-retention testing context (Context B) consisted of a plexiglass floor with curved inserts to change the shape of the chamber. The ventilation system and the lights inside the chamber were turned off, the room light kept on, and a 1% acetic acid cleaning solution was used to clean the chamber.
2.4.2. Training & Testing
On training day, the mouse was placed into a novel chamber (Context A). After three minutes of acclimation, mice were presented with a 15 s tone (conditioned stimulus (CS): 85 dB, 3 kHz tone) followed by a 2 s footshock (unconditioned stimulus (UCS): 0.65 mA) separated by a trace interval of either 10 s (group 1) or 30 s (group 2). After a 200 s inter-trial interval (ITI), the procedure was repeated four times, for a total of five tone-shock pairings. The mice remained in the chamber an additional 1 minute following the last ITI.
Twenty-hours after training, retention of the contextual CS:US memory was assessed by measuring freezing following placement into the original training chamber (Context A). Freezing behavior was evaluated for 5 minutes, and no stimuli were presented during this phase. Approximately four hours later, retention of the tone and trace CS:US memory was examined by placement into a novel context (Context B). Mice had a 3 min acclimation period to explore the chamber, during which baseline freezing behavior was scored. Immediately following this period, a 15 s tone was presented, and freezing behavior during this tone was scored. Freezing behavior for the 10 s (group 1) or 30 s (group 2) interval immediately following cessation of the tone was also measured and used to assess trace memory. A 210 s interval of no stimulus presentation followed the tone, and the mouse was removed upon completion of this period.
2.5. Western Blots
The ability of 40 ppm DOX to suppress tau expression was assessed by removing hippocampi and mixing 3 μg aliquots with loading buffer (450 mM Tris HCl, pH 8, 8% SDS, 24% glycerol, 5% β-mercaptoethanol, 0.1% bromophenol blue, 0.1% phenol red). Before loading, samples were heated to 100°C for 5 min and then separated on 10% Tris-HCl SDS-PAGE gels (Bio-Rad, Hercules, CA), and transferred onto 0.45 um polyvinylidene difluoride membranes (Millipore, Bedford, MA). Membrane blots were blocked for 1 h at RT in 5% BSA in 0.1% Tween 20/Tris-buffered saline (TTBS; 396 mM NaCl, 20 mM Trizma base, pH 7.4). After blocking, membranes were incubated with an antibody directed against mouse and human tau (Tau-5, 1:5000), human tau only (T-14, 1:500), or α-tubulin (1:5000) overnight at 4°C. The next day, membranes were incubated with extrAvidin peroxidase (1:5000) for 30 min at RT, followed by incubation with the appropriate biotinylated secondary antibody (1:60,000) for 60 min at RT. Immunoreactive bands were visualized by enhanced chemiluminescence (Pierce Chemical) on X-ray film. Membranes used to detect tau were first probed with Tau-5 or T-14, then stripped with Restore PLUS western blot stripping buffer (Pierce Chemical) and re-probed with the α-tubulin. All bands were normalized to α-tubulin.
To examine the effects of P301L tau expression on total cAMP response element-binding protein (CREB) and CREB phosphorylation, mice were decapitated and the prefrontal cortex (PFC) and hippocampi rapidly removed and stored at −70°C. The brain tissues were then lysed with RIPA lysis buffer (Upstate Chemicon, Temecula, CA) containing protease and phosphatase inhibitors (Pierce Biotechnology, Rockford, IL) and centrifuged at 13,000 rpm for 30 min at 4°C. The supernatant was assayed for total protein concentrations using BCA assay kit (Thermo Scientific, Rockford, IL). Samples were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, and the separated proteins were transferred onto polyvinylidene difluoride membranes. Blots were then incubated in blocking buffer for 2 h at room temperature, washing in tris-buffered saline with 0.1% Tween 20 (TBST), and incubated with the appropriate primary antibodies overnight at 4°C (anti-pCREBSer133, 1:1000; anti-CREB, 1:1000 and anti-β actin, 1:1000). After washing, the blots were incubated with the secondary antibodies (IRDye 800CW Gt Anti-Mouse IgG (H+L) or IRDye 700CW Gt Anti-Rabbit IgG (H+L), 1:10000) for 1 h at room temperature. The detection quantification of specific bands was carried out using a fluorescence scanner (Odyssey Infrared Imaging System, Li-COR Biotechnology, South San Francisco, CA) at 700 nm and 800 nm wavelengths.
2.6. Immunohistochemistry
Immunohistochemical detection of total and phosphorylated tau species in transgenic and control mice was performed as previously described [3, 9]. Briefly, hemibrains were immersion fixed in 10% formalin for 24–48 h and embedded in paraffin. Serial sections were cut at 5 μm using a microtome, mounted onto CapGap slides (Thermo-Fisher), and rehydrated according to standard protocols. Mounted slides were pretreated with a citrate buffer (pH 6.0) in a Black & Decker (Owings, MD) steamer for 30 min, with a 10 min cool down. Standard 2 d immunostaining procedures using peroxidase-labeled streptavidin and DAB chromagen on an automated TechMate 500 capillary gap immunostainer (Ventana Medical Systems, Tucson, AZ) were used with antibodies directed against (Table 1). Photomicrographs of hippocampal and cortical neurons were captured at three different magnifications (x5, x10 and x40) with a Zeiss Axioskop microscope coupled to a CCD camera and processed and assembled in Adobe Photoshop. No positive labeling was observed for pathological tau epitopes in nontransgenic mice.
Table 1.
Tau Antibodies.
| Antibody | Species | Specificity | Use | Dilution | Source |
|---|---|---|---|---|---|
| Tau-5 | Mouse | Tau; human/mouse specific | Tau marker | 1:5000 | Biosource |
| MC-1 | Mouse | Tau; conformational epitope, 7–9 and 326–330 aa | Conformation specific; early pathological change in AD | 1:8000 | P. Davies |
| CP-13 | Mouse | Tau; pSer202 | Phosphorylation specific; early pathological change in AD | 1:2000 | P. Davies |
| AT-8 | Mouse | Tau; pSer202/pThr205 | Pre-tangles; mid-stage | 1:4000 | Innogenetics |
| PG-5 | Mouse | Tau; pSer409 | Pre-tangles; mid-stage | 1:200 | P. Davies |
| NeuN | Mouse | NeuN | Neuronal marker | 1:4000 | Chemicon |
2.7. Statistical Analyses
Systat® 11 (Systat Software, Inc. Point Richmond, CA) was used for all statistical analyses described below. The Type 1 error rate (α) was set to 0.05 for all tests. For repeated measures ANOVAs, the Huynh-Feldt correction was used when necessary to adjust the degrees of freedom for the tests of within-subject effects. _F_-ratios, degrees of freedom, and p-values are reported for all RMANOVAs. All error bars display the standard error of the mean unless otherwise noted.
Effect size and sample size needed was calculated for (1) MWM: PIC for probe trial and the mean PIC, (2) IRA: PQ in the last 5 sessions of acquisition, the performance component, and the learning component, (3) FC: significant effects, including percent freezing in the novel context for the 10 and 30 s trace conditions, as well as the trace CS:US memory in the 30 s trace condition. Cohen’s d for t-tests was used to calculate effect sizes for two reasons. First, because of the popularity of this measure, inclusion in the current study allows for easier comparison to other published studies. Second, the use of categories to classify effect sizes (Cohen’s classification: .20—small, .50—medium, and .80—large [22]) facilitates comparisons of this experiment’s effect size results with known benchmarks. As previously reported [23], effect size was calculated by computing the standardized mean difference (SMD) as the difference between the controls and tauP301L divided by the pooled standard deviation. Effect sizes were computed as Cohen’s d where a positive effect size represents better performance for the controls when compared to tauP301L mice (Table 2). Sample size was determined by computing the SMD, setting the probability of a Type I error (α) and power at 0.05 and 0.80, respectively, and setting the alternative as not equal.
Table 2.
Effect and sample sizes of Control and TauP301L mice.
| Measure | Mean difference | Pooled standard deviation | Cohen’s d | N needed per group | |
|---|---|---|---|---|---|
| MWM | Probe 1 | 0.81 | 1.66 | 0.49 | 67 |
| Probe 2 | 0.89 | 1.86 | 0.48 | 70 | |
| Probe 3 | 1.56 | 1.87 | 0.84 | 24 | |
| Probe 4 | 2.36 | 1.64 | 1.44 | 9 | |
| Mean PCI | 1.41 | 1.17 | 1.20 | 12 | |
| IRA | Acquisition PQ | 0.80 | 0.51 | 1.57 | 8 |
| Performance PQ | 0.65 | 0.76 | 0.85 | 23 | |
| Learning PQ | 0.19 | 2.17 | 0.09 | 2047 | |
| FC | 10s Context | 28.86 | 14.91 | 1.94 | 6 |
| 30s Trace | 19.14 | 12.47 | 1.53 | 8 | |
| 30s Context | 17.18 | 10.91 | 1.58 | 8 |
d=M1-M2σpooledσpooled=[(n1-1)s12+(n2-1)s22n1+n2]
Key to symbols: d = Cohen’s d effect size; _M_1 = mean (average of controls); _M_2 = mean (average of tauP301L); s = standard deviation; n = number of subjects.
3. Results
3.1. Tau Suppression
Tau expression was compared using Tau-5, which recognizes both human and mouse tau, and T-14, specific for human tau, to determine whether 40 ppm DOX effectively suppressed tau expression (Figure 1C). TauP301L mice not exposed to DOX exhibited strong tau expression at 3.5 months of age, whereas tauP301L mice exposed to DOX from conception until 3.5 months did not. Neither tau expression nor DOX exposure altered whole brain weight (Tg: F(1)=1.19, _p_=.29; DOX: F(1)=0.40, _p_=.54; Tg*DOX: F(1)=0.16, _p_=.69). Because tau expression is limited to the forebrain [3], hemi-forebrain weight was also compared. Neither tau expression or DOX treatment affected hemi-forebrain weight (Tg: F(1)=0.9, _p_=.36; DOX: F(1)=0.19, _p_=.66; Tg*DOX: F(1)=0.48, _p_=.49).
3.2. Morris Water Maze
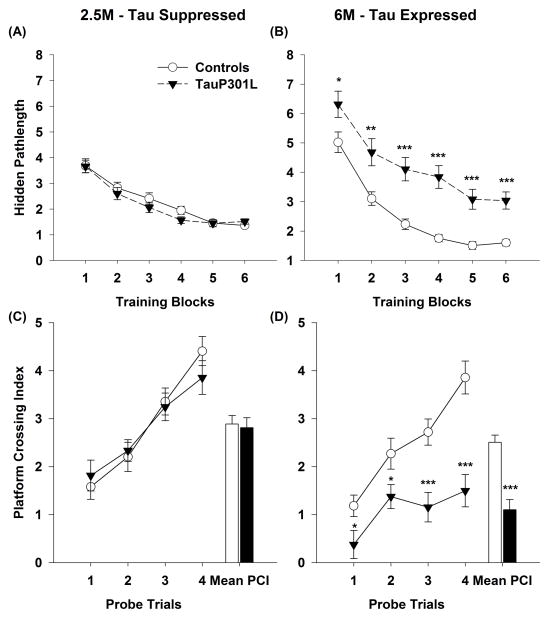
Pathlength in visible platform training did not differ between controls and tauP301L mice (Tg: F(1, 85)=0.86, _p_=0.36; Tg*Day: F(5,425)=302.5, _p_=.06). At 2.5 months of age, prior to the onset of tau expression, pathlength during hidden platform training did not differ across training blocks for controls and tauP301L mice (Figure 2A; Tg: F(1, 85)=1.21, _p_=0.28; Tg*Day: F(5,425)=0.71, _p_=.62). In contrast, at 6.5 months, after 3 months of tau expression, tauP301L mice demonstrated significantly longer pathlengths in hidden platform training across all training blocks (Figure 2B; Tg: F(1, 83)=41.96, p<.0001; Tg*Day: F(5,415)=.6141, _p_=.69). To determine the effects of tau expression on spatial reference memory, the mean PIC of probe trials was compared at 2.5 and 6.5 months of age. Comparing mean PIC revealed a transgene by age interaction (F(1,83)=15.14, _p_=.0002); tauP301L mice at 6.5 (p<.0001; Figure 2D) but not 2.5 (_p_=.78; Figure 2C) months displayed impaired retention of spatial reference memory.
Figure 2.
TauP301L mice exhibit spatial reference memory deficits after tau expression (6M) but not before tau expression (2.5M). At 2.5M, prior to expression of tau, there were no differences between controls or tauP301L mice in (A) pathlength during hidden platform training or (B) platform crossing index (PIC) during probe trials. In contrast, at 6M, after 3 months of tau expression, tauP301L mice exhibited (C) longer pathlengths during hidden platform training and (D) spatial reference memory deficits, as assessed by PCI. Months, M; Platform crossing index, PIC. *p<.05; **p<.01; ***p<.001.
Though significant differences in PIC were observed for all four probe trials (Figure 2D) at 6.5 months of age, effect and sample size analyses suggests that only the latter two probe trials (3 and 4) are sensitive enough to detect group differences using a realistic experiment design (Table 2). Probe trials 1 and 2 require many mice (67 and 70 mice per group, respectively) to achieve .80 power, whereas probe trials 3 and 4 require more practical sample sizes, 24 and 9 mice per group, respectively.
3.3. Incremental Repeated Acquisition
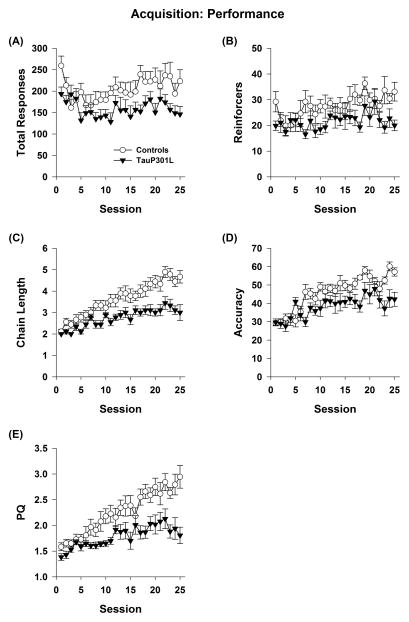
We next examined the sensitivity of a longitudinal behavioral task that allows for daily assessment. We observed no differences between controls and tauP301L for any measure during autoshaping of nose poke responses (_p_s>.05). For the IRA procedure, the first 25 sessions of training were performance sessions [12], where the chain of responses being trained was always the same (LRBLBR). For both total responses (Figure 3A) and reinforcers (Figure 3B), a main effect of transgene was observed such that tauP301L made fewer responses (Tg: F(1,16)=6.61, _p_=.021) and earned fewer reinforcers (Tg: F(1,16)=7.39, _p_=.015). This effect was observed across sessions for both responses (Tg*Session: F(24,384)=1.0489, _p_=.402) and reinforcers (Tg*Session: F(24,384)=1.08, _p_=.36). To assess whether the decreased number of reinforcers earned by tauP301L mice might be related to their lower rates of responding, the correlation between the two measures was assessed. Reinforcers and responses were positively correlated when all mice were included (Rein=5.7+0.1*Resp, _r_=.69, p<.0001), as well as when controls (Rein=6.9+0.1*Resp, _r_=.58, p<.0001 tauP301L) and tauP301L (Rein= 6.2+.0.1*Resp, _r_=.72, p<.0001 controls) mice were examined separately.
Figure 3.
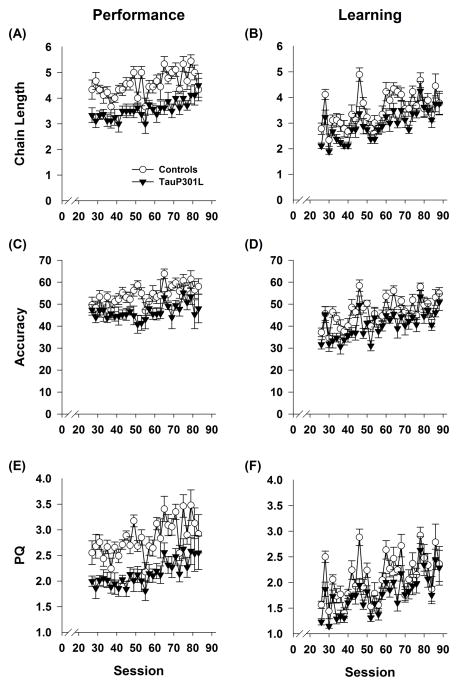
TauP301L mice exhibit deficits during acquisition of the performance chain. TauP301L mice made fewer total responses (A) and earned fewer reinforcers (B) across all sessions. In contrast, chain length (C), accuracy (D), and PQ (E) were similar during initial sessions, but diverged as training progressed.
For chain length, accuracy, and PQ (Figure 3 C–E), there was a significant interaction between transgene and session for all measures (Tg*Session: chain length F(24,384)=3.20, p<.0001; accuracy F(24,384)=2.39, _p_=.0003; PQ F(24,384)=3.42, p<.0001). Chain length, accuracy, and PQ were similar at the beginning of training, but controls improved at a faster rate as training progressed though both groups did improve across sessions for all measures (effect of session within each group; _p_s<.05).
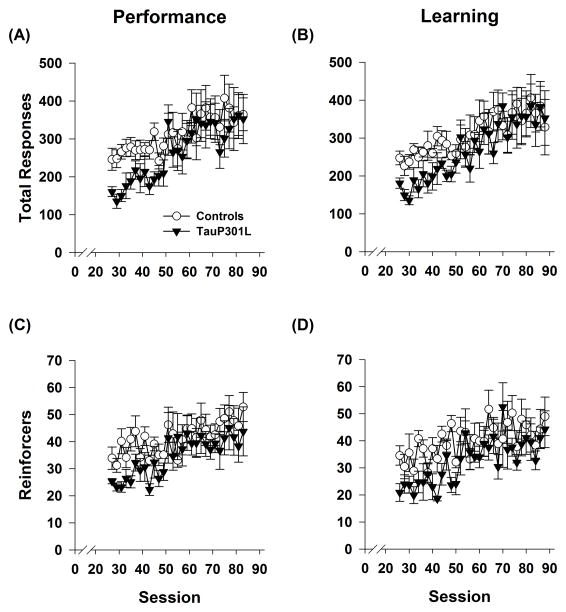
Starting with session 26, days alternated between learning and performance sessions and continued in this manner for a total of 90 sessions. Whereas tauP301L mice made fewer responses during acquisition, responding was similar between the groups for performance sessions after acquisition (Figure 4A; Tg: (F(1,15)=1.95, _p_=.18); Tg*Session: (F(28,420)=0.77, _p_=.79). Similarly, responding did not differ during learning sessions (Figure 4B; Tg: F(1,15)=1.22, _p_=.29); Tg*Session: F(31,465)=0.73, _p_=.85). TauP301L mice earned approximately the same number of reinforcers for performance sessions (Figure 4C; Tg: F(1,15)=3.37, _p_=.09); Tg*Session: F(28,420)=0.54, _p_=.98), but earned slightly fewer during learning sessions (Figure 4D; Tg: F(1,15)=7.13, _p_=.018); Tg*Session F(31,465)=1.018, _p_=.55).
Figure 4.
Responding and reinforcers earned in the IRA. Responding did not differ between tauP301L and controls for performance (A) or learning (B) sessions. Though reinforcers earned were similar in the performance sessions (C), tauP301L mice earned significantly fewer reinforcers during learning sessions (D).
For performance sessions, the maximum chain length reached by the end of each session was lower for tauP301L than controls (Figure 5A; Tg: F(1,15)=20.65, _p_=.0004), regardless of session (Tg*Session: F(28,420)=1.40, _p_=.086). For learning sessions (Figure 5B), a marginal effect of transgene was observed for chain length (Tg: F(1,15)=4.35, _p_=0.06) but there was no interaction between transgene and session (Tg*Session: F(31,465)=0.95, _p_=0.44). Accuracy in performance (Figure 5C) and learning (Figure 5D) sessions was significantly lower in tauP301L mice than controls (Tg: F(1,15)=11.96, _p_=0.0035 and F(1,15)=10.8, _p_=.005, respectively), an effect that did not change across sessions (Tg*Session: F(28,420)=0.95, _p_=0.54 and F(31,465)=0.81, _p_=0.76, respectively). Similarly PQ was significantly lower for tauP301L mice in both the performance (Figure 5E) and learning (Figure 5F) components (Tg: F(1,15)=20.29, _p_=0.0004 and F(1,15)=5.77, _p_=.03, respectively) and did not change across sessions (Tg*Session: F(28,420)=0.77, _p_=0.8 and F(31,465)=1.00, _p_=0.46, respectively).
Figure 5.
TauP301L mice are impaired in the IRA task. Maximum chain length reached (A,B), accuracy (C,D), and PQ (E,F) were lower in tauP301L mice compared to controls in both performance (left) and learning (right) sessions.
Effect sizes for PQ were determined using the last 5 sessions of acquisition, performance and learning sessions (Table 2). The effect size for acquisition was largest at 1.57. The effect size for performance sessions at the end of the study was reduced to 0.85. The effect size for learning was very small 0.09, suggesting this task is not very sensitive to effects of P301L tau expression.
3.4. Fear Conditioning
3.4.1. 10s Trace
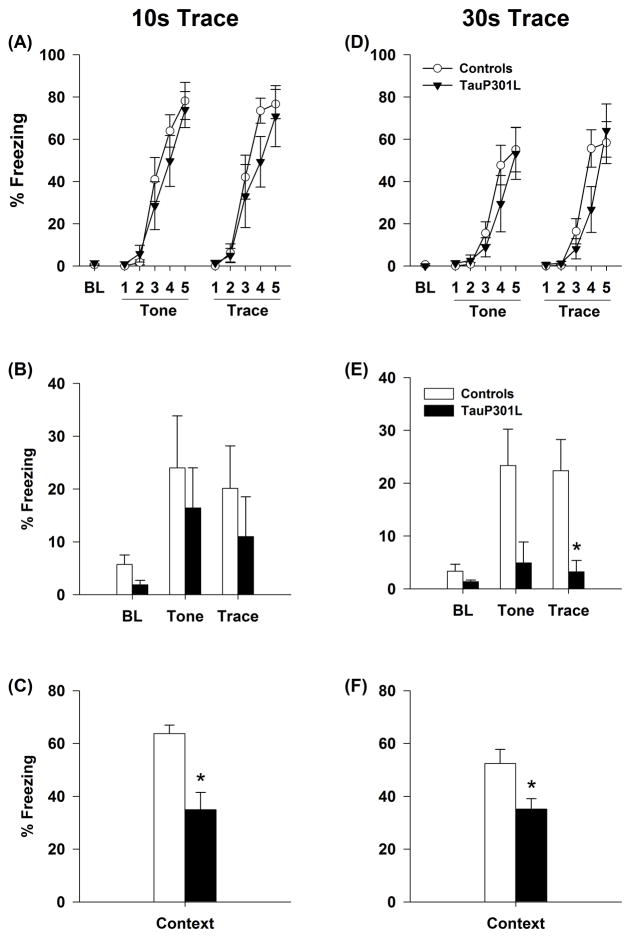
We first used a trace protocol [conditioned stimulus (CS): 15-s duration, 85 dB 3kHz; Trace: 10-s; unconditioned stimulus (US): footshock, 2 sec; Trials: 5] implemented by Taconic, as part of their comprehensive phenotypic data package [24] and pharmacologically validated using drugs capable of inducing memory deficits [25]. During training, no significant differences in percent time freezing between control and tauP301L mice were observed before the first CS presentation (baseline freezing; F(1,17)=0.74, _p_=0.40), indicating similar activity levels for the two groups. No effect of transgene was observed on the expression of freezing during the tone CS (Tg: F(1,17)=0.585, _p_=0.46; trial*Tg: F(4,68)=0.69, _p_=0.60) or trace (Tg: F(1,17)=1.69, _p_=0.21; trial*Tg: F(4,68)=0.751, _p_=0.56), suggesting similar expression of fear (measured as freezing) between the groups (Figure 6A). The following day, retention of the CS:US memory was assessed in a novel context (Context B). There were no differences in baseline freezing level (F(1,17)=3.063, _p_=0.10), conditional freezing in response to the tone CS (F(1,17)=0.326, _p_=0.58) or trace CS (F(1,17)=0.638, _p_=0.44) between the controls and tauP301L mice (Figure 6B), indicating retention for the CS:US memory following trace fear conditioning was intact for both groups. In contrast, when placed in the original training context (Context A), retention of contextual CS:US memory was significantly impaired for tauP301L mice (F(1,17)=20.54, p<0.0001; Figure 6C).
Figure 6.
TauP301L mice were impaired in trace and contextual fear conditioning. Baseline, tone CS, and trace CS freezing was similar between controls and tauP301L mice during training with a 10-s (A) and 30-s (D) trace interval. Baseline freezing and retention of the tone CS memory were similar in controls and tauP301L mice at the 10-s (B) and 30-s (E) trace intervals. Though trace fear retention was similar between controls and tauP301L mice in the 10-s trace group (B), tauP301L mice exhibited a significant decrease in mean percentage of freezing during trace fear retention testing after training with the 30-s trace (E). TauP301L mice exhibited a significant decrease in mean percentage of freezing during contextual fear retention for both the 10-s (C) and 30-s (F) traces. Baseline, BL; conditioned stimulus, CS. *p < 0.05.
3.4.2. 30s Trace
To determine whether a longer trace might be more sensitive to the hippocampal alterations produced by expression of mutant P301L human tau, a second cohort of age-matched mice were tested using a longer (30 s) trace interval, a duration at which a beta-amyloid mouse model of AD (5XFAD) was shown to exhibit trace fear conditioning deficits [26]. As with the shorter 10 s trace interval, there were no differences in baseline freezing (F(1,18)=1.33, _p_=0.26) or expression of freezing during the tone CS (Tg: F(1,18)=0.51, _p_=0.49; Trial*Tg: F(4,72)=0.69, _p_=0.60) and trace (Tg: F(1,18)=0.83, _p_=0.38; Trial*Tg: F(4,72)=2.20, _p_=0.08) during training (Figure 6D). When placed into the novel context (Context B), there were no differences in baseline freezing level (F(1,18)=3.32, _p_=0.09). Though graphically conditional freezing in response to the tone CS was lower in tauP301L mice, this difference was not statistically different using traditional levels of significance (F(1,18)=3.46, _p_=0.07). There was, however, a difference in conditional freezing in response to the trace CS (F(1,18)=5.31, _p_=0.03); tauP301L mice froze significantly less than Control mice, indicating a failure to retain the trace CS:US memory (Figure 6E). When placed in the original training context (Context A), tauP301L mice also exhibited deficits in the retention of contextual CS:US memory (F(1,18)=4.68, _p_=0.04; Figure 6F).
Comparison of effect size for the three significant effects obtained using trace conditioning (i.e., retention of CS:US memory in the 10 s and 30 s conditions, and trace memory in the 30 s condition) were very large, requiring only 6 – 8 for a power of .80 (Table 2).
3.5. CREB Activity & Pathological Tau Species in TauP301L Mice
To assess the effect of P301L expression on CREB, a transcription factor involved in both short- and long-term memory [27], total CREB and activated (phosphorylated at Ser133) CREB (pCREB) were compared in the hippocampi (Figure 7A) and PFC (Figure 7B) of tauP301L and control mice. Though total CREB levels did not differ (PFC: F(1,16)=0.02., _p_=0.90.; Hipp: F(1,16)=0.04, _p_=0.85), pCREB (PFC: F(1,16)=4.32, _p_=0.05; Hipp: F(1,16)=17.44, _p_=0.0007) and the ratio of pCREB to CREB (PFC: F(1,16)=5.33, _p_=0.03; Hipp: F(1,16)=22.70, _p_=0.0002) was down-regulated in TauP301L mice.
Figure 7.
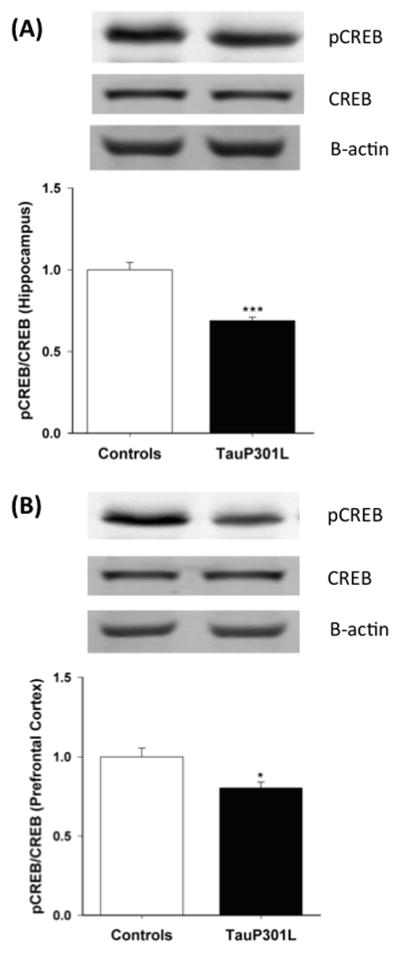
Western blot analysis of total CREB, pCREB, and the ratio of pCREB to CREB in the hippocampus and prefrontal cortex (PFC) of tauP301L mice compared to controls. Total CREB expression did not differ between controls and tauP301L mice in the hippocampus (A) or PFC (B), but pCREB and pCREB/CREB was decreased in the hippocampus (A) and PFC (B) of tauP301L mice. *p<.05; ***p<.001.
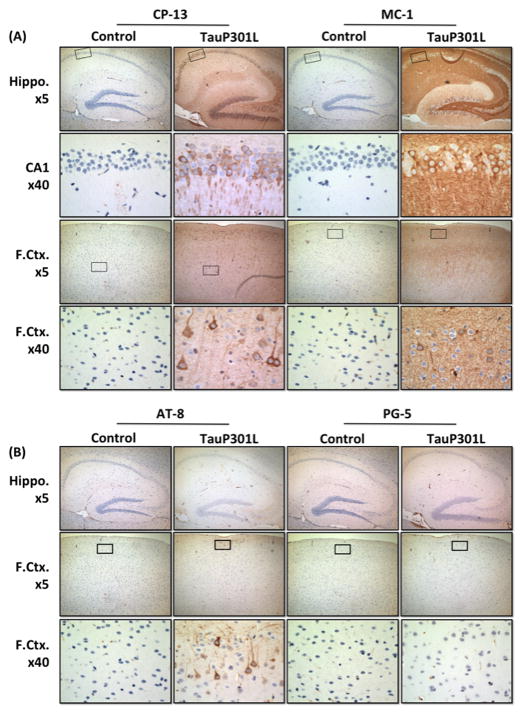
A panel of antibodies directed at biochemical changes in tau associated with AD was used to determine the extent of tau pathology at 6.5 months of age (Table 1). As previously reported [3, 28], the earliest positive labeling was identified using CP-13 and MC-1, which detect phosphorylation and conformation-specific changes, respectively (Figure 8A). CP-13 and MC-1 staining was observed throughout the hippocampus, but somatic neuronal staining was greater for CP-13 than MC-1. Similarly, CP-13 staining was found throughout the cortical layers, whereas MC-1 staining was observed only in the upper layers. Next, pretangles (accumulations of nonargyrophilic hyperphosphorylated tau in the neuronal cell body) were examined using AT-8 and PG-5 (Figure 8B). For AT-8, positive staining was observed in the upper layer of cortex but not the hippocampus, whereas for PG-5, no positive staining was observed in either region. To examine neuron loss, hematoxylin and eosin (H&E) stained tissue for tauP301L and controls were compared. To further supplement the examination of neuronal loss, tissue was also incubated with a neuron-specific antibody (NeuN). After 3 months of tau expression, there was no obvious cell loss in the hippocampus or cortex of tauP301L mice using either H&E or NeuN (Figure 9).
Figure 8.
Representative images of tau pathology in tauP301L mice. At 6.5 months of age, after 3 months of tau expression, IHC studies revealed abnormal tau conformation and phosphorylation when using the early pathological markers of AD, CP-13 and MC-1 (A) and the pretangle marker AT-8, but not PG-5 (B). Conformational changes determined using: MC-1, amino acids 7 to 9 and amino acids 326 to 300, and phosphorylation changes detected using: CP-13, pSer202; AT-8, pSer202/pThr205; PG-5, pSer409. No positive labeling was observed after parallel processing of control tissue (n = 4). Staining was consistent across all tauP301L mice (n = 4). Boxes indicate area shown at higher magnification. Original magnifications: x5 for hippocampus (Hippo.) and frontal cortex (F.Ctx.) and x40 for CA1 or F.Ctx.
Figure 9.
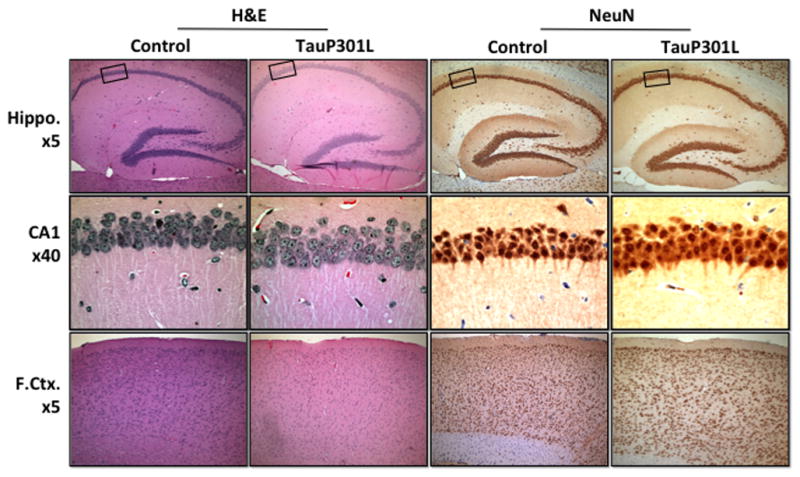
No readily detectable neuron loss was observed in tauP301L mice when compared using hematoxylin and eosin (H&E) or a neuron-specific antibody (NeuN). Boxes indicate area shown at higher magnification. Representative images shown and were consistent across all tauP301L mice (n = 4). Original magnifications: x5 for hippocampus (Hippo.) and frontal cortex (F.Ctx.) and x40 for CA1.
4. Discussion
Previously published work suggests perinatal P301L tau expression produces alterations not observed following adult-onset tau expression [5]. Such findings are not surprising given the importance of tau in brain development [29]. The presence of developmental phenotypes unrelated to AD can make results obtained from these mice difficult to interpret, particularly in the case of preclinical drug studies. For example, recent work with a tet-off amyloid precursor protein (APP) model using the same CaMKIIα examined here revealed that overexpression of APP during the early postnatal period produced severe locomotor hyperactivity, a phenotype attenuated by adult-onset APP expression. Such findings suggest overexpression of mutant proteins during the perinatal period can disrupt brain development, leading to long-lasting alterations unrelated to AD.
Fortunately, the tet-controllable nature of these mice offers a means of limiting the potential problems associated with perinatal mutant protein overexpression. Here, we provide evidence that short-term, adult-onset P301L tau expression results in behavioral deficits at a time when tau pathology is subtle and before readily detectable neuronal loss. Three months of adult-onset P301L tau expression resulted in early-stage tau pathology (CP-13 and MC-1) in both the hippocampus and cortex, and some mid-stage tau pathology (AT-8) in the cortex, but not hippocampus. As previously noted in the Tg4510 model [3], AT-8 positive staining was observed prior to the presence of PG-5 staining in the current study. The exact reason for this time-dependent pattern of staining is not clear, but it could be related to the kinases that phosphorylate these sites; phosphorylation of the PG-5 site has been reported to be PKA-dependent [30], whereas the AT8 site can be phosphorylated by either MAPK, GSK3, or CDK5 [31]. Based on a qualitative assessment, tauP301L mice did not exhibit readily detectable neuronal loss. However, it should be noted that subtle neuroanatomical differences might have occurred that were not easily revealed using a gross qualitative measure. Incorporation of more quantitative measures, such as unbiased stereology, in future studies may reveal differences not readily detectable by the qualitative assessment used here.
The MWM deficits observed in the current study are similar to those reported previously in tauP301L mice expressing tau during development until approximately 3 months of age [2, 3, 9]. Memory deficits were not observed when tau expression was suppressed. Though some labs have reported more severe memory deficits for female Tg(tauP301L)4510 mice than males in the MWM (e.g., [32]), we did not observe sex differences in any of the behavioral tasks used in the current study. There are a few differences between the two studies that may account for this discrepancy. First, the previous finding of sex differences in the MWM was observed after 5.5 months of tau expression [32], whereas mice in the present study expressed tau for only 3 months. It is possible sex differences would become apparent if the duration of tau expression in the adult-onset model used here was longer. Second, mice in the previous study expressed tau during development, and thus, it is possible the sex differences arose due to differential consequences of developmental tau expression in males versus females. Comparisons of males and females at later time points in the adult-onset P301L model would help address these issues.
To our knowledge, this is the first reported use of an IRA procedure with a transgenic tau model. This task allows for longitudinal, daily testing of the same mice. Because this procedure begins with a short chain (1 nose poke) and only increments to progressively longer chains if the behavior meets pre-set criteria, the IRA procedure effectively adjusts difficulty according to the mouse’s performance. This is in contrast to some procedures where the chain increase is determined by the investigator, irrespective of the mouse’s ability and performance (see [33] for review). The incremental, mastery-based version used here prevents the task from becoming so difficult that responding deteriorates due to a lack of reinforcers (milk deliveries). In fact, in the current study, both responding and the number of reinforcers earned (Figure 4) increased across sessions, suggesting this task was effective at maintaining behavior. The similarity in responding between controls and tauP301L mice in later sessions also suggests tauP301L mice do not exhibit motor deficits.
In the current study, tauP301L mice exhibited deficits in PQ after 7 sessions (Figure 3E). DOX was removed from the drinking water of these mice prior to autoshaping of nose poke responses, which took approximately two weeks. Thus, PQ deficits were observed after just 3 weeks of tau expression. In our previous work [3, 9], PCI in the MWM was decreased for tauP301L mice at 1.3 months of age (after 4–5 weeks of tau expression assuming PN day 7 is the first day of expression [3]), but this decrease was not statically significant. Thus, the IRA task might be more sensitive to the early effects of tau expression. Identification of the tau alterations (e.g., phosphorylation epitopes, tau cleavage products, etc.) present at this time could be of significance for determining the earliest change in tau responsible for memory deficits. However, one caveat of this tet-off model is that DOX efficacy is not 100%, but closer to 85–90% [2]. Consequently, the early deficits observed here might be due in part to a slow “leakage” of tau. The lack of deficits in pre-tau MWM testing suggests this is not the case, though the MWM may not be sensitive enough to detect such a mild effect.
Two surprising findings resulted from IRA testing. First, the effect size between the controls and tauP301L mice during acquisition, after approximately 5–6 weeks of tau expression, was greater than group differences at the end of the performance chain, after approximately 14 weeks of tau expression. One possible explanation is that overtraining occurred [34]. Previous work in the triple transgenic (3×Tg) mouse model of AD, which harbors two familial AD mutations, APP(Swe) and PS1(M146V), and the P301L tau mutation, reveals that use of 15 training trials, as opposed to 5 training trials, can attenuate group differences in the Barnes maze task [35]. However, the increasing disparity between groups observed in successive probe trials following additional training in the MWM (Figure 2D) weakens this argument, though differences in the task preclude definitive conclusions.
A second, and perhaps more likely, possibility is that a ceiling was reached for the controls. Several of the controls reached the maximum six-link chain programmed, and thus, the mean chainlength, accuracy, and PQ values could not increase for these control mice. In future studies, use of longer chains may allow for continued improvements in the controls, thereby increasing the dynamic range of this task. A second surprising finding was the small difference observed for PQ in the learning sessions. Previous use of an IRA task with Ts65Dn mice, a model of Down syndrome, revealed greater differences in learning, as opposed to performance, of chains [36]. However, several methodological differences were noted between these studies. In the current study, a mouse-driven, mastery-based advancement of chain length was used, whereas in the Ts65Dn study, an investigator-initiated advancement in the chain length was used. Greater differences may have been obtained in the learning sessions of the current study had we forced mice to accurately respond under a longer chain to earn reinforcers, but responding may have ultimately decreased in tauP301L mice if these mice failed to earn a similar number of reinforcers over the 90 sessions examined here. Second, for the Ts65Dn study [36], the performance sessions were used to ensure similar motivation and motoric abilities were observed between the groups, and therefore, the correct response in the performance sessions was indicated by illumination of the nose poke. By indicating the correct response, the need for memory was eliminated in the Ts65Dn study. In contrast, the performance sessions of the current study were used to examine reference memory, and therefore, the correct response was not indicated.
For fear conditioning, group differences in the trace CS:US memory were observed only in the 30 s trace paradigm. Lengthening the trace did not have an effect on the trace CS:US memory of controls; controls exhibited a similar degree of freezing when tone and trace memory were examined at 10 and 30 s. Instead, freezing for the tauP301L mice in the 30 s trace paradigm decreased compared to the freezing of tauP301L mice in the 10 s trace paradigm. Previous work suggests reliance on the hippocampus for trace fear memory increases when conditioned using longer trace intervals [37], which might explain the greater sensitivity of the 30 s trace paradigm. Deficits for the contextual CS:US memory in tauP301L mice were observed at both the 10 and 30 s trace interval, suggesting contextual conditioning might be particularly sensitive to P301L tau expression. Future studies are needed to determine whether delay conditioning might produce similar contextual deficits in this tau model.
One caveat of the fear conditioning studies is that graphically tauP301L mice appear to freeze less than controls in all situations except freezing prior to conditioning. This lower level of freezing could mean tauP301L mice are more hyperactive in general. Future studies are needed to address this possibility and should include unpaired groups in which the tone has no predictive value. If similar freezing levels are obtained between controls and tauP301L mice in the unpaired condition, hyperactivity could be ruled out as a possible explanation for contextual deficits. Though the current study cannot rule out hyperactivity, the lack of graphical differences prior to conditioning, along with previous findings that tauP301L mice have similar levels of exploratory behavior and anxiety-like behavior [38], suggests the differences obtained are not due solely to differences in hyperactivity.
Important in the design of any study is inclusion of enough subjects to reach statistical differences if differences exist. Failure to do can result in underpowered studies and a misinterpretation of the results. Here, we provide the effect and sample sizes needed for each of the behavioral tasks used. For the MWM, our results suggest deficits might not be readily detectable with a small sample size if only 1–2 probes are examined early in training. Because controls continued to improve with additional training, the dynamic range of subsequent probe trials increased, and differences were more readily observed in later probe trials. Researchers wanting to examine mice longitudinally could use relatively few mice and detect defects in the performance, but not learning, sessions of the IRA task. If a short protocol is needed, the two-day fear conditioning paradigm task requires very few mice to reach statistical significance.
5. Conclusions
In conclusion, we describe the effect and sample sizes needed for three behavioral tasks, including two tasks (IRA and trace fear conditioning) not previously used with the Tg(tauP301L)4510 mouse model. In addition, we provide evidence that the tet-off Tg4510 model provides a way to avoid mutant tau expression during the perinatal period, by suppressing tau expression until the brain is fully developed, and still produce readily observable memory deficits, as shown here in three behavioral tasks.
Highlights.
- Adult-onset P301L tau expression results in deficits in IRA task
- Adult-onset P301L tau expression produces deficits in trace fear conditioning
- Effect size of deficits are largest in a 30 s trace fear conditioning paradigm
Acknowledgments
Funding: National Institute of Health Training Grant (Reed - T32 DA022616-02); National Institute of General Medical Sciences (Reed - U54GM104942); and the Alzheimer’s Association (Reed - NIRG-12-242187).
This work was supported by funding to Karen H. Ashe by B. Grossman, and a post-doctoral training grant (Reed - T32 DA022616-02), the National Institute of General Medical Sciences (Reed - U54GM104942), and the Alzheimer’s Association (Reed - NIRG-12-242187). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or Alzheimer’s Association. We thank Linda Kotilinek and Tina Moroni for technical expertise and Dr. P. Davies for the generous gift of antibodies. A special thank you to Karen H. Ashe for providing the tauP301L mice and fear conditioning chamber.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iqbal K, del C, Alonso A, Chen S, Chohan MO, El-Akkad E, Gong C-X, et al. Tau pathology in Alzheimer disease and other tauopathies. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 2.SantaCruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–81. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsden M, Kotilinek L, Forster C, Paulson J, McGowan E, SantaCruz K, et al. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L) J Neurosci. 2005;25:10637–47. doi: 10.1523/JNEUROSCI.3279-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodgers SP, Born HA, Das P, Jankowsky JL. Transgenic APP expression during postnatal development causes persistent locomotor hyperactivity in the adult. Mol Neurodegener. 2012;7:28. doi: 10.1186/1750-1326-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caouette D, Xie Z, Milici A, Kuhn M, Bocan T, Yang D. Perinatal Suppression of Tau P301L Has a Long Lasting Preventive Effect against Neurodegeneration. International Journal of Neuropathology. 2013;1:53–69. [Google Scholar]
- 6.Paulson JB, Ramsden M, Forster C, Sherman MA, McGowan E, Ashe KH. Amyloid plaque and neurofibrillary tangle pathology in a regulatable mouse model of Alzheimer’s disease. Am J Pathol. 2008;173:762–72. doi: 10.2353/ajpath.2008.080175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–7. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braak H, Braak E. The human entorhinal cortex: normal morphology and lamina-specific pathology in various diseases. Neuroscience Research. 1992;15:6–31. doi: 10.1016/0168-0102(92)90014-4. [DOI] [PubMed] [Google Scholar]
- 9.Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68:1067–81. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks AI, Cory-Slechta DA, Murg SL, Federoff HJ. Repeated acquisition and performance chamber for mice: A paradigm for assessment of spatial learning and memory. Neurobiology of Learning and Memory. 2000;74:241–58. doi: 10.1006/nlme.1999.3951. [DOI] [PubMed] [Google Scholar]
- 11.Patel RC, Larson J. Impaired olfactory discrimination learning and decreased olfactory sensitivity in aged C57Bl/6 mice. Neurobiol Aging. 2009;30:829–37. doi: 10.1016/j.neurobiolaging.2007.08.007. Epub 2007 Sep 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson JM, Bailey JM, Johnson JE, Newland MC. Performance of BALB/c and C57BL/6 mice under an incremental repeated acquisition of behavioral chains procedure. Behavioural Processes. 2010;84:705–14. doi: 10.1016/j.beproc.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burman MA, Gewirtz JC. Hippocampal activity, but not plasticity, is required for early consolidation of fear conditioning with a short trace interval. Eur J Neurosci. 2007;25:2483–90. doi: 10.1111/j.1460-9568.2007.05493.x. [DOI] [PubMed] [Google Scholar]
- 14.Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav Neurosci. 2005;119:1496–510. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- 15.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–5. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 16.Mayford M, Bach ME, Huang Y-Y, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–83. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 17.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 18.Daniels RW, Miller BR, DiAntonio A. Increased vesicular glutamate transporter expression causes excitotoxic neurodegeneration. Neurobiol Dis. 2011;41:415–20. doi: 10.1016/j.nbd.2010.10.009. Epub 2010 Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, et al. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2002;22:1858–67. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey JM, Johnson JE, Newland MC. Mechanisms and performance measures in mastery-based incremental repeated acquisition: behavioral and pharmacological analyses. Psychopharmacology (Berl) 2010;209:331–41. doi: 10.1007/s00213-010-1801-3. [DOI] [PubMed] [Google Scholar]
- 21.Zempel H, Thies E, Mandelkow E, Mandelkow EM. Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci. 2010;30:11938–50. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen J. A power primer. Psychological Bulletin. 1992;112:155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 23.Reed MN, Liu P, Kotilinek LA, Ashe KH. Effect size of reference memory deficits in the Morris water maze in Tg2576 mice. Behavioural Brain Research. 2010;212:115–20. doi: 10.1016/j.bbr.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taconic. Neurological Disorders: Trace Conditioning. Comprehensive Phenotypic Data Packages. 2013. [Google Scholar]
- 25.Rudy JW. Scopolamine administered before and after training impairs both contextual and auditory-cue fear conditioning. Neurobiol Learn Mem. 1996;65:73–81. doi: 10.1006/nlme.1996.0008. [DOI] [PubMed] [Google Scholar]
- 26.Kaczorowski CC, Sametsky E, Shah S, Vassar R, Disterhoft JF. Mechanisms underlying basal and learning-related intrinsic excitability in a mouse model of Alzheimer’s disease. Neurobiology of Aging. 2011;32:1452–65. doi: 10.1016/j.neurobiolaging.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki A, Fukushima H, Mukawa T, Toyoda H, Wu LJ, Zhao MG, et al. Upregulation of CREB-mediated transcription enhances both short- and long-term memory. J Neurosci. 2011;31:8786–802. doi: 10.1523/JNEUROSCI.3257-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, et al. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86:582–90. doi: 10.1046/j.1471-4159.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang JZ, Liu F. Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog Neurobiol. 2008;85:148–75. doi: 10.1016/j.pneurobio.2008.03.002. Epub 2008 Mar 22. [DOI] [PubMed] [Google Scholar]
- 30.Jicha GA, Weaver C, Lane E, Vianna C, Kress Y, Rockwood J, et al. cAMP-dependent protein kinase phosphorylations on tau in Alzheimer’s disease. J Neurosci. 1999;19:7486–94. doi: 10.1523/JNEUROSCI.19-17-07486.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goedert M, Jakes R, Vanmechelen E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neuroscience Letters. 1995;189:167–70. doi: 10.1016/0304-3940(95)11484-e. [DOI] [PubMed] [Google Scholar]
- 32.Yue M, Hanna A, Wilson J, Roder H, Janus C. Sex difference in pathology and memory decline in rTg4510 mouse model of tauopathy. Neurobiology of Aging. 2011;32:590–603. doi: 10.1016/j.neurobiolaging.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Cohn J, Paule MG. Repeated acquisition of response sequences: the analysis of behavior in transition. Neuroscience and biobehavioral reviews. 1995;19:397–406. doi: 10.1016/0149-7634(94)00067-b. [DOI] [PubMed] [Google Scholar]
- 34.Morris RG, Schenk F, Tweedie F, Jarrard LE. Ibotenate Lesions of Hippocampus and/or Subiculum: Dissociating Components of Allocentric Spatial Learning. Eur J Neurosci. 1990;2:1016–28. doi: 10.1111/j.1460-9568.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 35.Attar A, Liu T, Chan WT, Hayes J, Nejad M, Lei K, et al. A shortened Barnes maze protocol reveals memory deficits at 4-months of age in the triple-transgenic mouse model of Alzheimer’s disease. PloS one. 2013;8:e80355. doi: 10.1371/journal.pone.0080355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders NC, Williams DK, Wenger GR. Does the learning deficit observed under an incremental repeated acquisition schedule of reinforcement in Ts65Dn mice, a model for Down syndrome, change as they age? Behav Brain Res. 2009;203:137–42. doi: 10.1016/j.bbr.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chowdhury N, Quinn JJ, Fanselow MS. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behav Neurosci. 2005;119:1396–402. doi: 10.1037/0735-7044.119.5.1396. [DOI] [PubMed] [Google Scholar]
- 38.O’Leary JC, 3rd, Li Q, Marinec P, Blair LJ, Congdon EE, Johnson AG, et al. Phenothiazine-mediated rescue of cognition in tau transgenic mice requires neuroprotection and reduced soluble tau burden. Mol Neurodegener. 2010;5:45. doi: 10.1186/750-326-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]