Therapy of Human Papillomavirus-Related Disease (original) (raw)
. Author manuscript; available in PMC: 2014 Sep 5.
Abstract
This chapter reviews the current treatment of chronic and neoplastic HPV-associated conditions and the development of novel therapeutic approaches. Surgical excision of HPV-associated lower genital tract neoplasia is very successful but largely depends on secondary prevention programmes for identification of disease. Only high-risk HPV-driven chronic, preneoplastic lesions and some very early cancers cannot be successfully treated by surgical procedures alone. Chemoradiation therapy of cervical cancer contributes to the 66–79% cervical cancer survival at 5 years. Outlook for those patients with persistent or recurrent cervical cancer following treatment is very poor. Topical agents such as imiquimod (immune response modifier), cidofovir (inhibition of viral replication; induction apoptosis) or photodynamic therapy (direct damage of tumour and augmentation of anti-tumour immunity) have all shown some useful efficacy (~50–60%) in treatment of high grade vulvar intraepithelial neoplasia. Provider administered treatments of genital warts include cryotherapy, trichloracetic acid, or surgical removal which has the highest primary clearance rate. Patient applied therapies include podophyllotoxin and imiquimod. Recurrence after “successful” treatment is 30–40%. Further improvements could derive from a rational combination of current therapy with new drugs targeting molecular pathways mediated by HPV in cancer. Small molecule inhibitors targeting the DNA binding activities of HPV E1/E2 or the anti-apoptotic consequences of E6/E7 oncogenes are in preclinical development. Proteasome and histone deacetylase inhibitors, which can enhance apoptosis in HPV positive tumour cells, are being tested in early clinical trials. Chronic high-risk HPV infection/neoplasia is characterised by systemic and/or local immune suppressive regulatory or escape factors. Recently two E6/E7 vaccines have shown some clinical efficacy in high grade VIN patients and this correlated with strong and broad systemic HPV-specific T cell response and modulation of key local immune factors. Treatments that can shift the balance of immune effectors locally in combination with vaccination are now being tested.
This article forms part of a special supplement entitled “_Opportunities for comprehensive control of HPV infections and related diseases_” Vaccine Volume 30, Supplement X, 2012.
Keywords: HPV-related disease therapy, Therapeutic HPV vaccines, HPV drug targets
1. Introduction
In the past decade, there have been remarkable advances in our understanding of the natural history of human papillomavirus (HPV) infection and its role through persistence as the major risk factor in the development of cervical and other anogenital cancers. Primary (vaccination) or secondary prevention programs (cervical screening) can impact decisively in preventing cancer but both these approaches are not available for many at greatest risk. All those with HPV-driven chronic or neoplastic lesions and cancers potentially require therapy. If surgical removal is not possible or is unsuccessful, then other approaches are necessitated. The purpose of this chapter is to review the current treatment of chronic and neoplastic HPV-associated conditions and the prospective clinical agenda driving the development of novel therapeutic approaches. These developments exploit knowledge of the molecular virology of infection and/or neoplasia and/or the potential for stimulation of the immune response to affect viral clearance or lesion elimination or ultimately cancer therapy.
2. Current treatment
2.1. Lower genital tract neoplasia
Lower genital tract neoplasia comprises cervical, vaginal, and vulvar intraepithelial neoplasia (VIN), which in a small proportion of cases, progresses to invasive cancer. Virtually 100% of cervical, ~43% of vulvar, and ~70% of vaginal tumors are attributable to human papillomavirus infection annually generating 530,000 cervical and 21,000 vulvar and vaginal cancers worldwide ([1] and see Forman D et al., Vaccine, this issue [2]). In the absence of a screening strategy, there has been an increase in the incidence of VIN and vulvar cancer in younger women [3]. Treatment standards for HPV-associated anogenital lesions have primarily been by surgical excision.
Since high-grade cervical intraepithelial neoplasia (CIN) affects mainly women of reproductive age, targeting the most clinically relevant CIN has clear clinical implications for young women. Current treatment strategies focus on eliminating the abnormal HPV-infected precancerous cells while minimizing harm to the cervical integrity. Common procedures for CIN treatment include a loop electrosurgical excision procedure (LEEP), cold knife cone biopsy, electrofulgaration, cold-coagulation and cryotherapy. Hysterectomy is unacceptable as primary therapy for high-grade CIN [4]. The decision to use one procedure over another is based on the provider, infrastructure, and clinical concerns. Due to the relatively inexpensive infrastructure needs and the ability to perform these procedures in an outpatient setting, a LEEP is one of the most commonly used procedures. If there are concerns about invasive disease or issues with the margins, typically a cold knife cone is the treatment standard due to the ability to resect the endocervical canal deeply and to avoid diathermy artefact at the margins.
Cryotherapy is a treatment widely used in many countries, since it is the only option available outside of surgical settings due to its ease of use. However, due to the lack of a specimen for histopathology, the diagnosis and visualization of the lesion must be certain prior to using cryotherapy to avoid missed cancers, such as those deep in the endocervical canal or in the case of glandular lesions. It is important to note, however, that while all of these extirpative procedures are very effective at treating CIN, none target the cause of these lesions, a persistent HPV infection. Additionally, all carry the same risk of increasing the risk of preterm birth [5,6], likely by weakening the cervical integrity.
2.2. Cervical cancer
Most cervical cancers identified in screening programmes are International Federation of Gynecology and Obstetrics (FIGO) stage 1a and these can be treated by conisation or radical hysterectomy with excellent survival. More advanced tumors are treated with concomitant chemoradiotherapy using cisplatin-based regimens. Survival is improved even in high-risk patients who have histologic evidence of metastatic disease to pelvic and/or para-aortic lymph nodes [7]. A Cochrane review of 19 randomized controlled trials (RCTs) in locally-advanced cervical cancer confirmed the improvement in survival with platinum–containing regimens with radiation therapy [7]. In patients who have persistent or recurrent cervical cancer after treatment, survival is poor. In addition to the mortality associated with this disease, advanced and recurrent cervical carcinoma is also associated with severe morbidities, including renal and liver toxicity and failure, complex fistulas, and painful bony metastases. Retreatment with platinum-based combination therapy offers some improvement in survival when compared to no therapy. A randomized trial of cisplatin versus the combination of cisplatin and paclitaxel demonstrated improved response rates for the combination, but no impact on survival, which remained in the 8–9 month range [9]. Recent trials [10] in this patient population include the addition of targeted therapies such as cetuximab (GOG207), taxanes (GOG240) or immunotherapies (GOG265). Improving the results of such trials will necessitate the use of predictive models and adaptive study designs to enrich the potential eligible patients for trials that might have more benefit. The lack of innovative treatments in persistent or relapsed disease will limit significant advances in prognosis.
2.3. VIN and vulvar cancer
The standard of care for treating VIN has been, and remains, surgical excision for unifocal disease and lesions suspicious for possible invasion. More problematic is multifocal disease, which can affect a large proportion of the vulva [11]. Excision of such a large area of vulvar skin can result in loss of vulvar contours and sexual function, which can have a profound effect on a woman’s self esteem and quality of life [12]. Treatment failure in VIN is not only common, but risks development of invasive disease, which only adds to the challenge. These difficulties mean that surgical excision is not an optimal means of treating multifocal disease. Laser ablation has the advantage of precise application and avoidance of skin loss, but it is associated with a high rate of treatment failure. These shortcomings in surgical treatment mean that effective non-surgical treatment is urgently needed. Vulvar cancer relies on surgery for localized disease and a combination of surgery and chemoradiation for nodal metastases. Only in very advanced disease, where surgery would necessitate defunctioning bowel or urinary tract, is chemoradiation preferred as sole therapy. The major development in vulvar cancer surgery in recent years has been sentinel node biopsy, which offers the means to avoid full groin dissection in sentinel node-negative women, thus reducing the risk of lower limb lymphoedema, which can be very disabling [13]. An effective topical treatment would greatly increase the scope for treatment, particularly in women who are reluctant to undergo surgery. Because it can be self-administered, it also has the potential advantage of reducing hospital visits. It might ultimately be used in combination with a systemic treatment such as vaccination.
2.4. Anal and penile cancer
HPV is associated with ~88% of the 24,000 worldwide annual cases (60% in women) of anal cancer (Forman D et al., Vaccine, this issue [2]). There is a disturbing trend of an increased incidence of anal cancer and anal precancerous lesions in the setting of human immunodeficiency virus (HIV) (Denny LA et al., Vaccine, this issue [14]). The dramatically increased relative risks of anal cancer are alarming; not only in men who have relative risks of anal cancer as high as 163 when compared to HIV-uninfected men (and in particular men who have sex with men), but in HIV-infected women who do not report anal receptive intercourse as well, where relative risks have been reported to be as high as 134 in the highly active antiretroviral treatment (HAART) era [15]. This epiphenomenon has likely been masked as men and women with acquired immunodeficiency syndrome (AIDS) historically died of other opportunistic infections or AIDS diseases before the onset of anal disease. Now that HIV-infected individuals are living longer on HAART, we are in the heart of the new leading edge of this clearly AIDS-related disease. In all HIV strata of disease control, HPV and abnormal anal cytology rates are exceedingly high, indeed higher in the anal canal than in the cervical-vaginal tract, suggesting synergistic effects of HPV-associated anal disease in the setting of HIV. Treatments are usually ablative, using electrofulgaration, infrared coagulation, or laser ablation. Excision is reserved for those high grade anal intraepithelial neoplasias (AIN) where there might be microinvasive disease or cancer. There have been encouraging results from clinical trials using topical therapies for the treatment of high-grade AIN such as 5-fluoruracil (5-FU), imiquimod, and topical cidofovir [16–18].
HPV is associated with ~50% of the 11,000 worldwide cases of penile cancer (see Forman D et al., Vaccine, this issue [2]). Surgical treatment for local disease remains the best option, but organ-preserving procedures provide good aesthetic and functional results with acceptable oncologic control. Regional disease is treated with radical inguinal lymphadenectomy if resectable. For nonresectable regional disease and metastases, neoadjuvant cisplatinum-based regimens are the best option. Topical chemotherapy agents (5-FU, imiquimod) are moderately effective first-line therapy in the treatment of penile carcinoma in situ (CIS). The issues of long-term surveillance and assessment of partial responders remain a challenge [19].
2.5. Head and neck cancers
Oncogenic HPV is recognized as a major risk factor for the development of head and neck (HN) squamous cell carcinomas (SCC) (Gillison ML et al., Vaccine, this issue [20] and [21]). HPV is mostly detected in tumors of the oropharynx (OP) and more specifically the tonsil, and this group displays clinical and molecular features distinct from other HNSCC. Inoperable OP SCC are treated by regimens using fractionated radiotherapy combined with cisplatin. The better relative survival of the HPV-positive patients is independent of the therapy. This favorable prognosis indicates that the patients may benefit from a less intense treatment regimen [22]. A current trial in HPV-positive OP patients (ECOG 1308) is assessing induction of responses using chemotherapy (paclitaxel; cisplatin; cetuximab) followed by concurrent radiation/cetuximab. Given the rising rates of HPV-related OP cancer [23,24], the use of new antiviral or immunotherapeutic approaches is certain to be of value.
2.6. Non-malignant HPV-associated anogenital warts and laryngeal papillomatosis (recurrent respiratory papillomatosis, RRP)
2.6.1. Genital warts (GW)
A recent survey in the UK synthesizing sexual health clinic and general practitioner data showed an incidence of new cases at 0.16% per annum (population survey, denominator all ages) and an incidence of recurrent cases at 0.13% per annum [25]. This highlights that recurrence of disease after successful clearance is a frequent problem in many cases. Recent clinical guidelines have remained broadly similar to those over the last 10 years [26,27]. Treatment is guided by patient preference in consultation with their health care provider, reflecting available resources and practitioner expertise. Treatments can be categorized as provider administered or patient applied. Provider administered therapies include cryotherapy, trichloracetic acid, or surgical removal. Surgical therapy has the highest primary clearance rates of any GW treatment [27], but requires substantial clinical training, additional equipment and a longer consultation time. Patient applied therapies include podophyllotoxin, which is available in a various formulations in different countries, and imiquimod. In general, podophyllotoxin is cheaper than imiquimod, whereas imiquimod 5% is associated with lower recurrence rates than podophyllotoxin [27,28]. There is only one published RCT of podophyllotoxin versus imiquimod which showed similar primary clearance rates, although the sample size was small and recurrence rates were not measured [27]. Recent UK sexual health clinic data show that ~40% of GW patients receive patient-applied therapy, in half of those, in combination with clinic treatment [28]. Furthermore 55% of men and 45% of women only attend for a single visit, during which they are issued with home treatment, arguing for the cost-effectiveness of such a strategy in suitable cases [28]. A new patient-applied therapy for GW containing green tea catechins (sinecatechins) has been developed, shown to be effective in RCTs against placebo, and approved [27]. To date, this is only available as a 15% ointment in the USA, and a 10% ointment in Germany and Austria. Low recurrence rates are seen after successful clearance with sinechatecins, similarly to imiquimod [29].
2.6.2. Recurrent respiratory papillomatosis
RRP is a rare serious disease, which usually shows a more refractory disease course in paediatric versus adult cases (Gillison ML et al. Vaccine, this issue) [20]. There is also potential for malignant transformation. Treatment is by surgical debridement, but many adjuvant therapies have been used with varying claims as to their effectiveness [30]. The desire to help these unfortunate patients has driven diverse studies, but no convincing long-term benefits have been shown with interferon-alpha, retinoids, methotrexate, 5-FU or acyclovir. Numerous other therapeutic approaches considered adjunctive to periodic surgery have been or are being tested, including the active ingredient in cabbage juice extracts (diindoylylmethane), anti-malarials (artemisinin, artesunate), direct injection of measles-mumpsrubella vaccine or anti-vascular agents (e.g., avastin after laser treatment). Unfortunately very few RCTs have been conducted in the treatment of RRP, except for a placebo-controlled trial of cidofovir, which showed no difference between arms, and a placebo-controlled trial of a cycloxgenase-2 inhibitor (COX-2), celecoxib, which is still recruiting [31,32].
3. Development of novel treatments
3.1. HPV specific immunotherapies
The immune system plays an important role in controlling the development of cancer [33].The HPV genome encodes two oncoproteins, E6 and E7, which are constitutively expressed in high-grade lesions and cancer, since they are required for the onset and maintenance of the malignant cellular phenotype [34]. The presence of these defined tumor-specific antigens forms an excellent basis for the development of strategies aiming to reinforce the immune response to combat cancer. It has long been known that the adaptive immune response, in particular T cells, confer protection against HPV-induced diseases [35,36]. However, it is likely that in some cases the natural immune response against these HPV proteins may have been compromised, e.g., in a chronic HPV infection or cancer by systemic and/or local immune regulatory or escape factors [37,38]. This presents challenges for therapeutic vaccination but strategies for further optimizing effective immunization to overcome these issues could lead to clearance of HPV infected cells, persistent lesions or cancer. Fig. 1 and 2 and Boxes 1 and 2 illustrate and summarize the mechanistic principles and challenges of antiviral immune therapies. Overall, HPV-induced progressive diseases are associated with a lack of a strong HPV-specific CD4+ and CD8+ T cell response and rather, are infested with immune suppressive cells. In some circumstances, the balance of positive and negative immune factors may be changed, leading to clearance of lesions. This is an area where therapeutic vaccines against HPV targets may be of value.
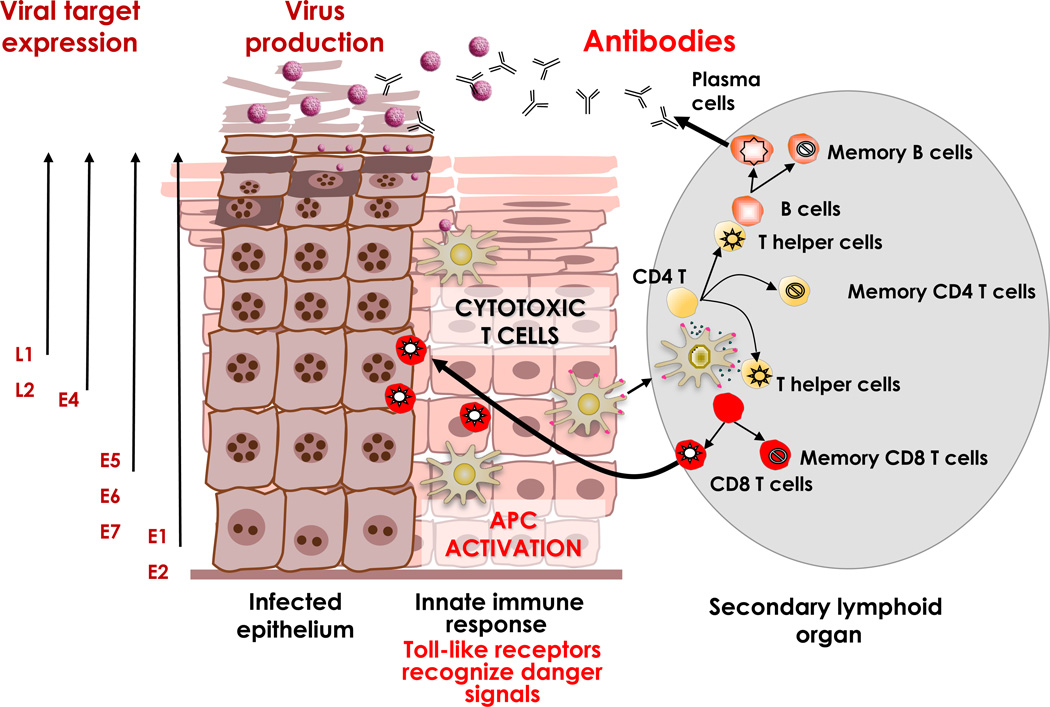
Figure 1.
Natural immune control of HPV infection. APC: Antigen-presenting cell. Taken with permission from: The Immunobiology of Human Papillomavirus Associated Oncogenesis; Stern PL, Einstein MH. In: HPV and Cervical Cancer: Achievements in Prevention and Future Prospects. Borrutto F, De Ridder M, editors. Dordrecht: Springer Science+Business Media B.V; 2012. See also Box 1.
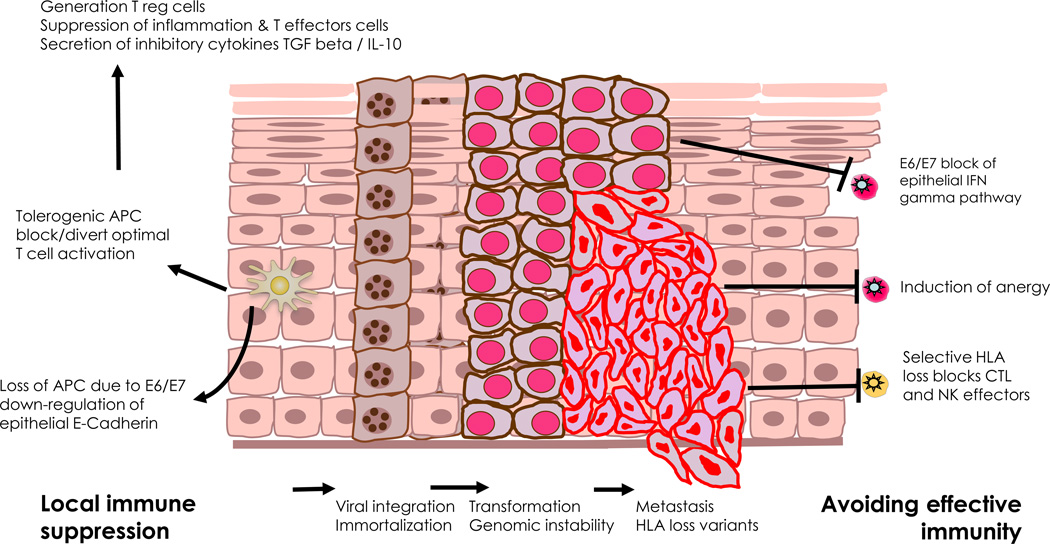
Figure 2.
Loss of immune control and escape in HPV-associated neoplasia. APC: Antigen-presenting cell; CTL: Cytotoxic T lymphocyte; HLA: Human leukocyte antigen; IFN: Interferon; IL-10: Interleukin 10; T reg: Regulatory T cell; TGF: Transforming growth factor. Taken with permission from: The Immunobiology of Human Papillomavirus Associated Oncogenesis; Stern PL, Einstein MH. In: HPV and Cervical Cancer: Achievements in Prevention and Future Prospects. Borrutto F, De Ridder M, editors. Dordrecht: Springer Science+Business Media B.V; 2012. See also Box 2.
BOX 1. Natural Immune control of HPV infection (Fig. 1).
- HPV lifecycle is associated with a normal desquamation processes, does not cause death of the host cell and has no viremia, and the infectious virus particles are shed from mucosal surfaces.
- Immune control of an infection is a derivative from the complex interplay of the innate (non-specific) and the adaptive mechanisms delivered by specific antibodies and cellular effectors (e.g., T cells). Importantly, the adaptive immune system is activated only if it gets the correct signals from the innate immune system.
- Detection of damage through innate immune system.
- Activation of immediate non-specific effectors; secretion of interferons.
- Proinflammatory cytokines & chemokines support optimal activation of local antigen presenting Langerhans’ cells, viral target antigen processing & migration to locoregional lymph nodes.
- Optimal activation of adaptive immunity and generation of specific CD4 T helper 1 type immunity supporting development of effector and memory CD8 cytotoxic T cells against viral E2, E6 and E7. Cell-mediated immunity is believed to be critical in clearance of virus in basal epithelial cells.
- T helper cells also support optimal activation of B cells secreting HPV capsid type specific neutralizing antibodies. Long-lived plasma cells providing high levels of antibodies can protect against a subsequent infection through transudation into the mucosal secretions or through serous exudation.
- T cells specific for HPV early antigens clear & confer protection against HPV-induced diseases
- HPV16-specific T cells in protected individuals revealed the presence of circulating HPV16-specific CD4+ T helper 1 (Th1; IFNγ-producing) and Th2 (IL-5-producing) cells and CD8+ cytotoxic T lymphocytes (CTLs) reactive to a broad array of Early and late antigens.
- Spontaneous regressions of HPV-induced lesions mainly occur in patients in whom the lesions are infiltrated with CD4+ and cytotoxic CD8+ T cells that outnumber CD25+ (regulatory) T cells and not by macrophages. •Spontaneous regression is also associated with the presence of circulating HPV early antigen-specific CD4+ and CD8+ T cells. Moreover, the presence of circulating HPV-specific CD4+ T cells is associated with stronger T-cell infiltration and a favorable clinical outcome in HSIL and cancer after treatment.
BOX 2. Loss of immune control and escape in HPV associated neoplasia (Fig. 2).
- Integration of virus with the host cellular genome blocks the productive life cell cycle, encourages immortalization and generates opportunity for acquisition of mutants which may offer additional means to escape immune control. There is a complex balance of immune system responses versus viral immune evasion mechanisms affects HPV persistence & likelihood of disease progression or regression.
- Virally mediated suppression of innate immunity; E6 and E7 mediated protection against interferons & inappropriate or reduction in activation of antigen presenting cells
- Immune activation is skewed toward T helper 2 response with cytokine balance supporting differentiation & infiltration of T and other immune regulatory cells which limit therapeutic T cell effector mechanisms either directly or by the production of cytokines such as IL10 and TGF beta.
- Progression of high grade lesions/ carcinoma is associated with a loss of local IFNγ and an increase in IL-10
- PCD4+ and CD8+ T-cell tumour infiltration can lack cytotoxicity and/or express co-inhibitory molecules Increased numbers of regulatory T cells (Tregs) and immunosuppressive myeloid cells are detected in HPV tumours. Tregs appear to be attracted by tumour-produced CXCL12 whereas myeloid cells are attracted by CCL2 (myeloid cells) to become immunosuppressive under the influence of tumour-produced mediators such as IL-6 and prostanglandin E2. A similar process occurs in tumour invaded lymph nodes.
- The ratio between tumour-infiltrating CD8+ T cells and Foxp3+ Tregs appears to be an independent prognostic factor in cervical carcinoma.
- The balance of local immune infiltration in persistent infection and chronic stimulation of the immune system with viral antigens can anergize controlling effector responses.
- Patients show either lack of or weak HPV early antigen-specific T cell reactivity in their blood and the response is often not associated with the production of Th1 or Th2 cytokines.
- HPV-specific T cells are detected in some HSIL, one third of the tumour-infiltrating lymphocytes (TILs) and in the majority of metastatic lymph nodes.
- Isolated CD4+ HPV-specific Tregs produced IL-10 and were highly capable of inhibiting the proliferation and cytokine (IFNγ and IL-2) production of activated naïve CD4+ T cells, Th1 cells and CTL.
- Frequent mutational events in neoplasia include HLA loss of expression make the tumour cells invisible to the CTL restricted by particular allelic genes but still resistant to NK cells. Moreover, the lowered expression of HLA or MICA reduces survival in patients whose tumours display low CD8/Treg ratios.
3.2. Therapeutic vaccines
Therapeutic vaccines aim to induce or boost HPV T cell adaptive immunity. Vaccines based on a wide range of recombinant viral vectors, recombinant proteins, naked DNA, antigen-pulsed dendritic cells (DC) and HLA-class I-restricted peptide-epitopes have all been tested for treatment of HPV16-driven diseases but without any significant clinical success (Table 1). This is likely the result of a failure to induce sufficient strength and breadth of HPV-specific CD4+ and CD8+ T cell responses [39]. However, two recent studies have reported successful treatment of about 50% of patients with HPV16-induced high-grade lesions of the vulva where there was a direct correlation with the vaccine-induced immune response. One vaccine was an adjuvanted set of synthetic long overlapping peptides of HPV16 E6 and E7 (HPV16 SLP) and the other a recombinant HPV16 E6E7L2 fusion protein (TA-CIN) [40–42]. Efficacy was associated with the induction of a strong HPV-specific CD4+ T cell response and included the induction of HPV16-specific CD8+ T cell activity. Importantly, the patients with unresponsive lesions showed reduced systemic vaccine responses but also increased numbers of lesion associated immune suppressive T regulatory cells (Tregs). The HPV16 SLP was also quite immunogenic in patients with cervix cancer but failed to induce tumor regression [43,44]. The failure to cure some premalignant lesions and the cancers appears to result from an unfavorable balance in effector T cells and Tregs.
Table 1.
Examples of immune and clinical responses to HPV vaccines.
| Reference | Vaccine strategy | Outcome/comment |
|---|---|---|
| Ressing ME et al. [127] Detection of T helper responses, but not of human papillomavirus-specific cytotoxic T lymphocyte responses, after peptide vaccination of patients with cervical carcinoma | Synthetic E7 peptides (2) restricted to HLA-A2 with pan HLA-DR epitope & adjuvant in 15 cervical cancer patients | 4/12 T helper but no CTL responses. Only 2 patients showed limited clinical responses with stable disease for one year. No correlation with vaccine responses |
| Baldwin PJ et al. [128] Vaccinia-expressed human papillomavirus 16 and 18 e6 and e7 as a therapeutic vaccination for vulval and vaginal intraepithelial neoplasia. | Live vaccinia virus encoding modified E6/E7 from HPV16/18 (TA-HPV) in 12 patients with high grade VIN | Serological & cell-mediated immune responses to vector in all patients. 6/10 patients showed increased IFN ELISPOT. No CTL responses. 50% patients show lesion reduction; no obvious correlation with immune responses |
| Smyth LJ et al. [129] Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. | Prime boost strategy of 3 x TA-CIN ( HPV 16 L2E6E7 fusion) followed by TA-HPV in 29 high grade AGIN patients | All patients showed responses to TA-CIN post vaccination by proliferation/IFN ELISPOT. 6 patients with significant lesion clinical responses, 19 stable & 4 showed progression. No simple relationship with vaccine immune responses |
| Trimble CL et al. [130] A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. | HPV16 DNA (Sig-E7(detox)-HSP70 fusion) x 3 with dose escalation in 15 patients with HPV 16+ CIN | Low frequency and activity of E7 specific T cells detectable after 2/3 immunizations. Regression rates of CIN about as expected so no correlation with induced immunity |
| Santin AD et al. [131] Human papillomavirus type 16 and 18 E7-pulsed dendritic cell vaccination of stage IB or IIA cervical cancer patients: a phase I escalating-dose trial. | Autologous DC from patients pulsed with HPV16/18 E7 antigen & KLH given 5 x sc prior to surgery of 10 early stage cervix cancer patients | CD4 & antibody responses detected. 8/10 CD8 IFN ELISPOT responses detected immediately after vaccination. DTH to intradermal E7 & KLH were detected in all patients. No means to assess immune contribution as patients cured by surgery |
| Frazer IH et al. [132] Phase 1 study of HPV16-specific immunotherapy with E6E7 fusion protein and ISCOMATRIX adjuvant in women with cervical intraepithelial neoplasia. | 31 CIN patients in randomized blinded placebo controlled dose ranging study of HPV16 E6E7fusion protein & ISCOMATRIX adjuvant vaccine | All vaccinated patients developed E6E7 immunity (antibody, DTH, cytokine release) CD8 T cell responses to E6 &E7 were greater than in placebo recipients. There was no correlation with clinical responses |
| Matijevic M et al. [133] Immunization with a poly (lactide co-glycolide) encapsulated plasmid DNA expressing antigenic regions of HPV 16 and 18 results in an increase in the precursor frequency of T cells that respond to epitopes from HPV 16, 18, 6 and 11. | Encapsulated plasmid DNA encoding antigenic parts of HPV 16/18 in 21 high-grade CIN patients | Subsets of HLA-A2+ subjects showed some evidence of CD8 T cell responses. No correlation with clinical responses |
| Brun JL et al. [134] Regression of high-grade cervical intraepithelial neoplasia with TG4001 targeted immunotherapy. | Modified Vaccinia Ankara (MVA) HPV 16 E6-E7-IL-2 in 21 high grade CIN patients | Promising clinical responses with 48% patients showing regression at 6 months but no data available for immunogenicity |
| Maciag PC et al. [135] The first clinical use of a live –attenyiated Listeria monocytogenes vaccine: a phase 1 safety study of LM-LLO-E7 in atients with advanced carcinoma of the cervix. | Lm-LLO-E7 attenuated Listeria monocytogenes with dose escalation given by iv infusion in 15 patients with late stage metastatic previously treated cervix cancer | Some adverse effects with one partial clinical response but no immunogenicity data available |
3.3. Modulating immune suppression
The future success of immunotherapies will need to address the means to alter this balance. Given the importance of the local microenvironment to the persistence of HPV-induced lesions and tumors, treatments that can alter the balance of immune effectors locally have shown effectiveness. Chemotherapy or radiotherapy can influence immune regulatory activity and in combination with vaccination can potentiate effective local HPV specific T cell immunity in mouse tumor models [45,46]. Successful treatment of large persistent low-risk HPV-induced lesions that are infested with Tregs has been achieved with low-dose cyclophosphamide which alters the local immune milieu [47]. Currently a randomized trial comparing chemotherapy alone versus a combination with HPV16 SLP in patients with metastatic cervical cancer is planned and a pilot study is ongoing (EudraCT2010-018841-76). Other approaches may use adoptive cell therapy (ACT) or therapeutic antibodies against the inhibitory molecules expressed by T cells, which have shown success in treating melanoma patients [48,49]. ACT with HPV-specific T cells used in combination with chemo- or radiotherapy and subsequent vaccination can potentiate tumour therapy in mouse models [50,51]. Early data suggest that it is possible to consistently obtain HPV-specific effector T cells from patients with cervical cancer under full GMP conditions (van Poelgeest and van der Burg, unpublished observations) and a trial is being planned. Given the importance of the local microenvironment in the persistence of HPV-induced lesions and tumors, treatments that can shift the balance of immune effectors locally, such as COX-2 inhibitors through prevention of the production of prostaglandin E2 or transforming growth factor β (TGFβ) type I receptor kinase inhibitors [52,53] as well as antibodies to interleukin (IL)-6 and/or IL-10 may be effective. The ultimate goal is to identify the optimal combination to generate a favorable T helper 1 type of microenvironment resulting in increased local immune cell trafficking providing for vaccine-induced immunity to effectively eradicate persistent lesions. A good example is the potential of imiquimod to prime the microenvironment for successful immune-mediated clearance of vulvar lesions.
3.4. Imiquimod
Imiquimod (an imidazoquinoline amine), is an immune response modifier licensed for the topical treatment of external genital and perianal warts. Imiquimod acts through a Toll-like receptor (TLR7) [54], which can stimulate not only local innate immunity with potent antitumor effects but also drive an adaptive immune response (e.g., specific T cell effectors) in secondary lymphoid tissues by activating tissue antigen presenting cells. Thus antiviral and antitumor effects derive from the stimulation of the innate immune response through induction of cytokines and the cellular arm of acquired immunity through interferons and interleukins. Fourteen studies of imiquimod VIN treatment have been reported, including two randomised double-blind placebo controlled trials, with clinical response rates varying from 30–90% [41,55,56] In responder lesions imiquimod produces a T helper 1 type inflammatory response facilitating significantly increased local cytotoxic T lymphocyte activity while in non-responder lesions, there is an increased density of Tregs pre and post treatment [41,55]. A significant correlation between the presence of circulating, pre-existing HPV specific T lymphocytes and regression of HPV positive lesions has also been observed [37,55]. The optimal therapeutic impact of treatment may depend both on local and systemic immune factors. Imiquimod as a topical VIN application has been used two or three times a week, usually for 16 weeks, which generally results in an acute and severe local inflammation and treatment tolerance can be an issue.
3.5. Cidofovir
Cidofovir (1-[(S)-3-hydroxy-2-(phosphonylmethoxy)-propyl]cytosine) is an acyclic nucleoside phosphonate. It is an antiviral licensed for intravenous use in the treatment of cytomegalovirus (CMV) retinitis in HIV patients [57]. Cidofovir has been shown to reduce E6 and E7 expression and to reduce the metastatic properties of HPV-positive tumor cells [58]. Cidofovir was first shown to have an effect in anogenital condyloma [59]. A study in CIN3 showed complete regression in seven out of 15 women (47%), after three treatments on alternate days with topical cidofovir, and a partial response was seen in five patients (33%) [60]. No effect was seen on the non-dysplastic tissue. In a pilot study, 12 women with high-grade VIN were treated with topical cidofovir, three times a week for 16 weeks. Cidofovir was applied to both the diseased vulvar skin and the surrounding healthy skin. There was an intense ulcerative reaction at the site of disease, which was sometimes painful. This, however, was limited to the site of disease, and no effect was seen on the neighboring healthy skin. Four of the 10 women who completed follow up had a complete regression of disease (40%), with resolution of long standing symptoms, and histological clearance [61]. Cidofovir is considerably more expensive than imiquimod and currently has to be individually formulated for patients. A randomized phase II multicenter trial of imiquimod and cidofovir topical treatments (a cream and gel) in women with biopsy-proven VIN is in progress (EUDRACT 2006-004327-11) to determine whether they are active, safe, and feasible to use. Interestingly, cidofovir selectively targets and inhibits the CMV DNA polymerase but HPV does not have a DNA polymerase. HPV uses the host enzyme and therefore the drug can only work in HPV transformed cells because these cells have compromised DNA repair which some believe is a somewhat risky strategy in patients with premalignant lesions [62]. The recent report documenting serious adverse reactions in RRP in off-label use of cidofovir is particularly worrying [63].
3.6. Photodynamic therapy (PDT)
PDT is an FDA-approved treatment option both for the elimination of early-stage malignancies and palliation of symptoms in patients with late stage tumors of skin, esophagus and lung [64,65]. A wide variety of non-oncological dermatological lesions, premalignant dysplasia and cutaneous malignancies have shown a favorable response to PDT [64]. With PDT treatment, a non-toxic photosensitizer is absorbed into the virus-infected neoplastic tissue, and activated by visible light tuned to the appropriate absorption wavelength of the photosensitizer molecule. This leads to production of reactive oxygen species resulting in directed tumor cell death. Additionally, secondary events including microvascular disruption and local acute inflammation are known to make a significant contribution to long-term tumor control. The initial cell death prompted by PDT treatment is believed to generate excess tumor antigens, and the inflammatory response activates antigen presenting cells which process the tumor antigens and produce IL-12 enabling the optimal activation, proliferation and differentiation of IFN secreting effector T cells necessary for effective anti-tumor immunity [66,67]. The characteristic feature of PDT, which is infiltration of immune cells into the treated tumor bed, provides a rational basis for treating HPV-associated neoplastic conditions of the lower genital tract. Cellular immunity clearly plays an important role in controlling high-risk HPV-associated lower genital tract neoplasia and PDT offers a means to alter the immunological balance in chronic VIN leading to lesion and viral clearance. The real benefit of PDT treatment lies in its ability to treat multifocal disease without tissue loss. Challenges include patient tolerance of treatment requiring regional or general anaesthesia, uniformly targeting the photosensitizer to the VIN lesions (cream, gel, solution, intravenous route, bioadhesive patch), and protecting normal tissues targeting the coherent light source [68,69]. There is a therefore a need to develop optimized and standardized methods of delivering photosensitizer PDT to the vulva. Even though initial treatment responses after PDT for VIN are encouraging, disease recurrences often occur with an overall clearance in the range of 40–60% [64]. In theory, combination with immunotherapy could prove superior to PDT alone by means of a two-pronged attack. The topical immune response modifier, imiquimod, and PDT are both easily available. Combination treatment has shown a 60% treatment response at week 52 [53] was sustained for up to 3 years in women who made a complete response to the treatment [39]. However, immune evasion strategies can ultimately influence outcome [70].
3.7. Strategies for drug targeting HPV genes
3.7.1. E1 and E2
Overall survival in cervical cancer patients treated by standard chemoradiation is 66– 79% at 5 years. Further improvements in treatment outcomes could derive from a rational combination of current therapy with new drugs targeting molecular pathways mediated by HPV in cancer. Targeting has included the viral E1 helicase, the E2 regulatory protein and the E6/E7 oncogenes [71–73]. E1 has ATPase and DNA helicase activities facilitating the unwinding of the viral DNA ahead of the replication fork. High throughput screening for inhibitors of E1 ATPase activity has identified some lead compounds but these have not been active in cell-based assays [74]. Viral DNA replication is initiated by recruitment of E1 by E2, to specific DNA sequences within the viral origin of replication [75]. Substantial efforts have been made to target the DNA-binding activities of E1 and E2 or prevent E1-E2 interaction with only a few compounds showing activity in vivo models [76,77]. However since expression of E1/E2 viral proteins are frequently lost during malignant transformation most effort has been directed at the HPV oncogene targets.
3.7.2. E6 and E7
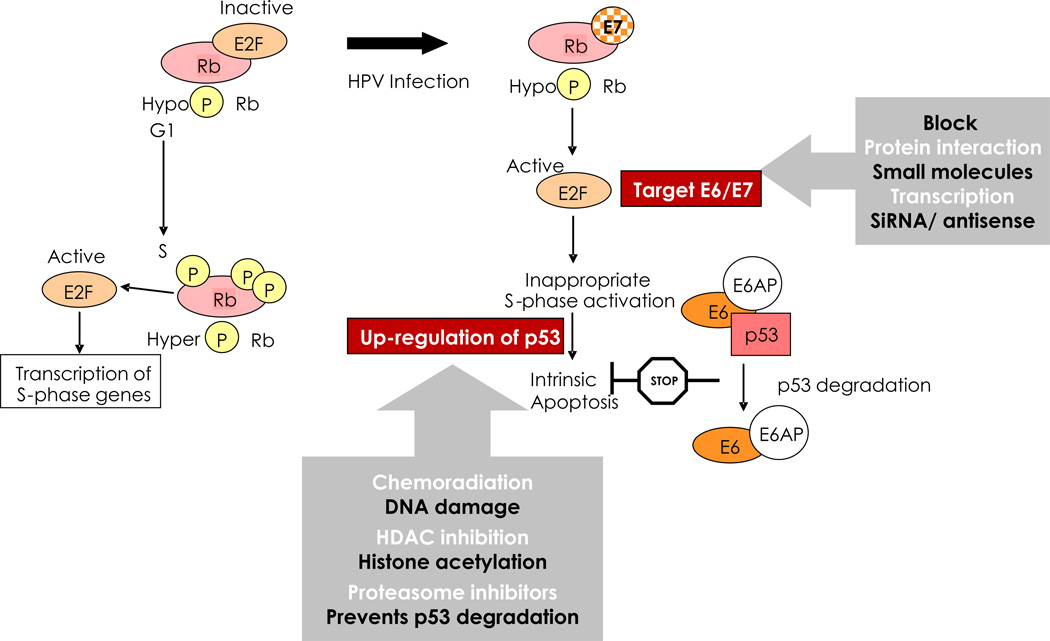
Chemoradiation causes DNA damage, ATM signalling, p53 activation and apoptosis (intrinsic pathway). However, HPV viral proteins E6 and E7 disrupt p53 and pRb functions reducing sensitivity to chemoradiation. The mechanisms whereby high-risk HPV E6 and E7 proteins subvert cellular proliferation and survival, promoting neoplasia, and potential drug strategies to exploit these processes are summarized in Fig. 3 and Box 3 (Doorbar J et al., Vaccine, this issue [78] and [34]). For example, short interfering RNA (siRNA), antisense oligodeoxynucleotides or ribozymes specific for E6 and E7 have shown some in vitro activity in sensitizing cervical cancer cells to chemoradiation [71,73]. Small molecule targeting of E6 interaction with E6AP represents an attractive therapeutic target since it can reactivate p53 function, leading to growth arrest and apoptosis in HPV-transformed cells [79]. Using sequence alignment, molecular modelling with virtual and actual screening has yielded some useful lead compounds [80–82]. Recently, a small peptide was identified that binds and degrades E7, thus restoring pRb regulatory control of E2F as shown by the G1-phase arrest and reduced proliferation of SiHa cells in vitro and tumorogenicity in vivo [83]. Generally, the relatively weak antiviral effects of small molecule inhibitors observed in vitro ensure that large improvements in potency and delivery will be required for clinically relevant use.
Figure 3.
Key HPV E6/E7 functions to control cell proliferation and survival. Strategies for drug targeting of HPV oncoproteins are shown. See also Box 3.
BOX 3. Key HPV E6/E7 functions to control cell proliferation and survival (Fig. 3).
Co-ordinated viral strategy providing access to the host’s replication machinery and the persistence of dividing cells necessary for the virus to amplify its own genome
- Retinoblastoma (Rb) protein regulates the activity of transcription factors like E2F which elicit the expression of proteins necessary for cycle cycle (G1 to S) progression and cellular genome replication.
- HPV-infected cells are forced into DNA replication regardless of cell cycle stage through E7 binding to Rb yielding hypophosphorylation and its inappropriate degradation, thereby releasing E2F.
- Such untimely DNA replication should trigger apoptosis, but this is prevented E6 directed destruction of the key proapoptotic protein p53. The E6 in complex with E6-AP enzymatically marks target proteins for degradation in the proteasome.
With persistent expression of E6 and E7, cells frequently acquire structural chromosomal changes such as translocations, deletions, and amplification, and numerical chromosomal imbalances (aneuploidy)
- The p53 pathway plays a critical role in sensing damage to the cell’s genome, HPV infection inhibits capacity to either leave the cell cycle for repair or to die by apoptosis and thereby facilitates the accumulation of secondary DNA mutations providing opportunity for cell immortalization and malignant conversion.
- E7 function also contributes to the genetic instability of HPV-infected cells by interfering with the normal replication of centrosomes.
- With CIN progression there is a high frequency of integration of the HPV genome into the host’s genome. This molecular event effectively deletes the viral E2 gene which limits the capacity of the virus to produce progeny virions, but importantly it eliminates E2 negative feedback on expression of E6 and E7 leading to increased cell proliferation. Proof that a loss of integrity of the E2-dependent regulation of E6 and E7 expression is inextricably associated with continuous cell proliferation has been shown by the reactivation of the p53 and Rb tumour suppressor growth inhibitory pathways in cervical cancer cells following reinstatement of E2 expression.
Many HPV types but only around 14 are associated with cancers. The difference in oncogenicity is delivered by the differential activity of E6 and E7 functions between high- and low-risk viruses
- Low-risk E6 proteins do not stimulate the degradation of p53 and activate telomerase while low-risk E7 or bind but less efficiently to Rb and do not induce its degradation and fail to disrupt centrosomes.
3.7.3. Proteasome inhibitors
Since HPV E6 targets p53 for degradation, proteasome inhibitors that act by preventing ubiquitin-proteasome protein degradation have also been tested in cervical carcinoma cells. The proteasome inhibitor MG132 increases p53 protein levels and transcriptional activity in human cervical cancer cell lines, providing for sensitization of the cells to TRAIL-receptor or Fas-mediated apoptosis [84] or radiosensitivity under hypoxic conditions [85]. Bortezomib (PS341, Velcade®, Millennium Pharmaceuticals, Cambridge, MA, USA), with specificity for the chymotrypsin-like proteasome activity [86], has been shown to sensitize SiHa to radiotherapy via inhibition of the NF-ĸB pathway, in tumor xenografts induces caspase 3 cleavage and under hypoxic conditions decreases vascular endothelial growth factor (VEGF) production, affecting tumor growth [87,88]. Recent clinical studies have shown that bortezomib combined with radiotherapy is well tolerated in patients with advanced cancers [89]. However, there are concerns over toxicity issues in relation to use of these proteasome inhibitors in patients with premalignant lesions. By contrast, lopinavir (Kaletra®, Abbott Laboratories, North Chicago, IL USA) is licensed for use in adult and paediatric HIV-positive patients, formulated for oral delivery. This drug showed dose-dependent upregulation of p53 in both HPV16 immortalized and transformed cells in vitro, leading to apoptosis, but normal keratinocytes were unaffected [90]. The mechanism is related to the ability to block viral proteasome activation and induce an upregulation of the antiviral protein RNASEL. These data provide support for further evaluation of the drug for local treatment of HPV-related neoplasia. However, effective dosing in vitro is 10 times that which is achievable by current oral delivery; therefore, trial design must focus on formulation for topical application. The manufacturers would need to make the drug available and the formulation development could be costly.
3.7.4. HDAC inhibitors
HAT (histone acetylase) and HDAC (histone deacetylase) activities generate the histone acetylation status that regulates the transcriptional activity of many genes [91]. In cancer, inhibition of HDAC can modulate the expression of tumor suppressor genes and potentiate the effects of other therapeutic modalities [92]. In HPV-positive cells, HDAC binds to E7 which prevents HDAC binding to the E2F promoter leading to upregulation of E2F and increased proliferation [93]. However, HDAC inhibitors (trichostatin A or Valpoerate) can compete with E6 for p53 binding which results in p53 hyperacetylation leading to increased apoptosis and early clinical trials have been performed [94]. The potential for combination with chemoradiation for treatment of cervical cancer has been investigated [95].
4. Barriers to progress
4.1. Immunotherapies
The challenge for immune- or antiviral-based therapies of HPV-associated conditions would be to safely provide a clear advantage over any existing treatments. For treating high grade CIN, this is likely to be extremely difficult given the current effectiveness of screening and treatment options. Nevertheless, an effective therapeutic vaccine targeting HPV16 and 18 oncogenes could provide for rapid viral clearance, prevention of latency and long-term protection against further infection relevant to all cancer risk at different sites. It would need to be very cheap and simple to deliver but, if efficacious therapeutically, might also be used prophylactically. It is likely that the proof of principle for therapeutic treatments will come from testing agents in the context of an unsatisfactory clinical management where there is room for improvement. Such conditions include high-grade VIN, recalcitrant GW, RRP or HPV-associated cancers. The increased understanding of the role of immune regulation in limiting effective anti-tumor responses, particularly lesion infiltration of effectors (Stanley M et al., Vaccine, this issue [96]) and the optimal use of adjuvants in vaccines [97] will drive the successful development of immunotherapeutic regimens in the next 5 years.
4.2. Drugs
A number of significant barriers exist, not least the limits to our insights in relation to the molecular mechanisms driving the pathology. Should a target be discovered, is there a drug available that can be used against this target? Even if there is, there are considerable complexities in obtaining access to drugs, particularly if they are not licensed for that purpose. Then, there is the expense of producing batches of drugs of sufficient grade for a clinical trial and all of the associated bureaucracy. If there were a candidate drug, can it be shown to be bioavailable to the basal layers of the epithelium and is there a rational basis for a regimen using the drug? Given the expense, time, and effort involved in all of this and the fact that the pharmaceutical industry would not consider premalignant disease a ‘blockbuster’ area, can sufficient resources be found to make progress in this area? Much of the above could be applicable to the cervix as nonsurgical treatment would be preferable to the current crude surgical methods for treating cervical pre-cancer; certainly, the frequency of the disease would be commercially more attractive in terms of developing new treatments. There is clearly a niche for HPV function-related drugs that can synergize with current treatment regimens to deliver improved clinical outcomes.
5. Conclusion
Progress will likely come from clinical trials testing treatment of low-grade lesions of the cervix with the aim of accelerated and sustained resolution following either HPV immune or drug treatments as the stepping stone for wider therapeutic application.
Highlights.
- Surgical excision of HPV-lower genital tract neoplasia is very successful.
- Chemoradiation therapy of cervical cancer contributes to 66–79% survival at 5yrs.
- Improvements in treatment aim to exploit drugs or immune targeting of HPV.
- Drugs targeting HPV action in DNA binding or apoptosis are in early development.
- Modulating local and/or systemic immunity has shown some efficacy in VIN.
Acknowledgments
Disclosed potential conflicts of interest
PLS: Has received support for Travel, Lectureships, (GlaxoSmithKline); Consultancy (GlaxoSmithKline, Oxford Biomedica); Meeting/Travel expenses (GlaxoSmithKline, Oxford Biomedica).
SHvdB: The Leiden University Medical Center (LUMC) holds a patent on the use of synthetic long peptides as vaccine (US 7.202.034). SHvdB is named as inventor on this patent. Note that the LUMC does not share the financial benefit from this patent with its employees.
AF: Has been a member of Advisory Boards (GlaxoSmithKline/Sanofi Pasteur MSD); received meeting/travel expenses (GlaxoSmithKline/Sanofi Pasteur MSD). She has been an investigator for studies sponsored and funded by GlaxoSmithKline/Sanofi Pasteur MSD.
CL: Has received support for travel and attending meetings form GlaxoSmithKline and SPMSD.
HCK: Has received a grant to his University to participate in the PATRICIA trial (funded by GSK). HCK is the Chair of the Advisory Committee for Cervical Screening in England, but any views are attributable to the author and in no way reflects that of the committee.
MHE: Has advised or participated in educational speaking activities, but do not receive an honorarium from any companies. In specific cases, my hospital, Montefiore Medical Center has received payment for my time spent for these activities from Merck, GSK, Roche, Bristol-Myers Squibb, Hologic, Advaxis, Aura Biosciences, Inovio, Photocure, Neodiagnostix, and PDS Biotechnologies. If travel is required for meetings with any industry, the company pays for MHE’s travel. Also, Montefiore has received grant funding for research related costs of clinical trials that I have been the Montefiore PI from Merck, GSK, Roche, Advaxis, Photocure, Inovio, and Hologic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
INH: no conflicts of interest.
References
- 1.Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res (Phila) 2012;5(1):18–23. doi: 10.1158/1940-6207.CAPR-11-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine, this issue. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 3.van de Nieuwenhof HP, Massuger LF, van der Avoort IA, Bekkers RL, Casparie M, Abma W, et al. Vulvar squamous cell carcinoma development after diagnosis of VIN increases with age. Eur J Cancer. 2009;45(5):851–856. doi: 10.1016/j.ejca.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 4.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197(4):346–355. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 5.Bruinsma F, Lumley J, Tan J, Quinn M. Precancerous changes in the cervix and risk of subsequent preterm birth. Bjog. 2007;114(1):70–80. doi: 10.1111/j.1471-0528.2006.01107.x. [DOI] [PubMed] [Google Scholar]
- 6.Kyrgiou M, Tsoumpou I, Vrekoussis T, Martin-Hirsch P, Arbyn M, Prendiville W, et al. The up-to-date evidence on colposcopy practice and treatment of cervical intraepithelial neoplasia: the Cochrane colposcopy & cervical cytopathology collaborative group (C5 group) approach. Cancer Treat Rev. 2006;32(7):516–523. doi: 10.1016/j.ctrv.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Rose PG. Combined-modality therapy of locally advanced cervical cancer. J Clin Oncol. 2003;21(10 Suppl):211s–217s. doi: 10.1200/JCO.2003.01.222. [DOI] [PubMed] [Google Scholar]
- 8.Green J, Kirwan J, Tierney J, Vale C, Symonds P, Fresco L, et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev. 2005;(3):CD002225. doi: 10.1002/14651858.CD002225.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore DH, Donnelly J, McGuire WP, Almadrones L, Cella DF, Herzog TJ, et al. Limited access trial using amifostine for protection against cisplatin- and three-hour paclitaxel-induced neurotoxicity: a phase II study of the Gynecologic Oncology Group. J Clin Oncol. 2003;21(22):4207–4213. doi: 10.1200/JCO.2003.02.086. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute. [last accessed May 2012];Clinical trials. Available at: http://www.cancer.gov/clinicaltrials/search/results?protocolsearchid=9718014.
- 11.Herod JJ, Shafi MI, Rollason TP, Jordan JA, Luesley DM. Vulvar intraepithelial neoplasia: long term follow up of treated and untreated women. Br J Obstet Gynaecol. 1996;103(5):446–452. doi: 10.1111/j.1471-0528.1996.tb09771.x. [DOI] [PubMed] [Google Scholar]
- 12.Shylasree TS, Karanjgaokar V, Tristram A, Wilkes AR, MacLean AB, Fiander AN. Contribution of demographic, psychological and disease-related factors to quality of life in women with high-grade vulval intraepithelial neoplasia. Gynecol Oncol. 2008;110(2):185–189. doi: 10.1016/j.ygyno.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 13.RCOG. The Management of Vulval Skin Disorders. Green-top Guideline. 2011;58 [Google Scholar]
- 14.Denny LA, Franceschi S, de Sanjose S, Heard I, Moscicki AB, Palefsky J. Human papillomavirus, human immunodeficiency virus and immunosuppression. Vaccine, this issue. doi: 10.1016/j.vaccine.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 15.Chiao EY, Krown SE, Stier EA, Schrag D. A population-based analysis of temporal trends in the incidence of squamous anal canal cancer in relation to the HIV epidemic. J Acquir Immune Defic Syndr. 2005;40(4):451–455. doi: 10.1097/01.qai.0000159669.80207.12. [DOI] [PubMed] [Google Scholar]
- 16.Kreuter A, Potthoff A, Brockmeyer NH, Gambichler T, Stucker M, Altmeyer P, et al. Imiquimod leads to a decrease of human papillomavirus DNA and to a sustained clearance of anal intraepithelial neoplasia in HIV-infected men. J Invest Dermatol. 2008;128(8):2078–2083. doi: 10.1038/jid.2008.24. [DOI] [PubMed] [Google Scholar]
- 17.Snyder SM, Siekas L, Aboulafia DM. Initial Experience with Topical Fluorouracil for Treatment of HIV-Associated Anal Intraepithelial Neoplasia. J Int Assoc Physicians AIDS Care (Chic) 2011;10(2):83–88. doi: 10.1177/1545109710382578. [DOI] [PubMed] [Google Scholar]
- 18.Stier EA, Goldstone SE, Einstein MH, Jay N, Berry JM, Wilkin T, et al. Phase IIA trial of 1% topical cidofovir for treatment of high-grade perianal squamous intraepithelial neoplasia in HIV-infected men and women (AMC046). 12th International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies (ICMAOI); USA Infect Agent Cancer; 2010 11 October; Bethesda, MD. 2010. [Google Scholar]
- 19.Alnajjar HM, Lam W, Bolgeri M, Rees RW, Perry MJ, Watkin NA. Treatment of carcinoma in situ of the glans penis with topical chemotherapy agents. Eur Urol. 2012 Mar 8; doi: 10.1016/j.eururo.2012.02.052. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Gillison ML, Alemany L, Snijders PJF, Chaturvedi A, Steinberg BM, Schwartz S, et al. Human papillomavirus and diseases of the upper airway: head and neck cancer and respiratory papillomatosis. Vaccine, this issue. doi: 10.1016/j.vaccine.2012.05.070. [DOI] [PubMed] [Google Scholar]
- 21.Olthof NC, Straetmans JM, Snoeck R, Ramaekers FC, Kremer B, Speel EJ. Next-generation treatment strategies for human papillomavirus-related head and neck squamous cell carcinoma: where do we go? Rev Med Virol. 2012;22(2):88–105. doi: 10.1002/rmv.714. [DOI] [PubMed] [Google Scholar]
- 22.Schache AG, Liloglou T, Risk JM, Filia A, Jones TM, Sheard J, et al. Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: sensitivity, specificity, and prognostic discrimination. Clin Cancer Res. 2011;17(19):6262–6271. doi: 10.1158/1078-0432.CCR-11-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown LM, Check DP, Devesa SS. Oropharyngeal cancer incidence trends: diminishing racial disparities. Cancer Causes Control. 2011;22(5):753–763. doi: 10.1007/s10552-011-9748-1. [DOI] [PubMed] [Google Scholar]
- 24.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desai S, Wetten S, Woodhall SC, Peters L, Hughes G, Soldan K. Genital warts and cost of care in England. Sex Transm Infect. 2011;87(6):464–468. doi: 10.1136/sti.2010.048421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1–110. [PubMed] [Google Scholar]
- 27.Lacey CJN, Woodhall SC, Wikstrom AJR. 2012 European guideline for the management of anogenital warts. J Eur Acad Dermatol Venereol. 2012 Mar 12; doi: 10.1111/j.1468-3083.2012.04493.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Woodhall SC, Jit M, Soldan K, Kinghorn G, Gilson R, Nathan M, et al. The impact of genital warts: loss of quality of life and cost of treatment in eight sexual health clinics in the UK. Sex Transm Infect. 2011;87(6):458–463. doi: 10.1136/sextrans-2011-050073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzellos TG, Sardeli C, Lallas A, Papazisis G, Chourdakis M, Kouvelas D. Efficacy, safety and tolerability of green tea catechins in the treatment of external anogenital warts: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2011;25(3):345–353. doi: 10.1111/j.1468-3083.2010.03796.x. [DOI] [PubMed] [Google Scholar]
- 30.Gallagher TQ, Derkay CS. Pharmacotherapy of recurrent respiratory papillomatosis: an expert opinion. Expert Opin Pharmacother. 2009;10(4):645–655. doi: 10.1517/14656560902793530. [DOI] [PubMed] [Google Scholar]
- 31.McMurray JS, Connor N, Ford CN. Cidofovir efficacy in recurrent respiratory papillomatosis: a randomized, double-blind, placebo-controlled study. Ann Otol Rhinol Laryngol. 2008;117(7):477–483. doi: 10.1177/000348940811700702. [DOI] [PubMed] [Google Scholar]
- 32.NCT. Study of Celebrex (Celecoxib) in Patients With Recurrent Respiratory Papillomatosis. clinicaltrials.gov. 2011 [Google Scholar]
- 33.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Roberts S, Young LS. Role of HPV in cervical carcinogenesis. In: Stern PL, Kitchener HC, editors. Vaccines for the prevention of cervical cancer. Oxford: Oxford University Press; 2008. [Google Scholar]
- 35.Stern PL, Einstein MH. From HPV infection to oncogenesis: A brief review of the complex immunobiological events. Curr Cancer Ther Rev. 2010:110–116. [Google Scholar]
- 36.van der Burg SH, Melief CJ. Therapeutic vaccination against human papilloma virus induced malignancies. Curr Opin Immunol. 2011;23(2):252–257. doi: 10.1016/j.coi.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 37.van Poelgeest MI, van Seters M, van Beurden M, Kwappenberg KM, Heijmans-Antonissen C, Drijfhout JW, et al. Detection of human papillomavirus (HPV) 16-specific CD4+ T-cell immunity in patients with persistent HPV16-induced vulvar intraepithelial neoplasia in relation to clinical impact of imiquimod treatment. Clin Cancer Res. 2005;11(14):5273–5280. doi: 10.1158/1078-0432.CCR-05-0616. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi A, Weinberg V, Darragh T, Smith-McCune K. Evolving immunosuppressive microenvironment during human cervical carcinogenesis. Mucosal Immunol. 2008;1(5):412–4120. doi: 10.1038/mi.2008.33. [DOI] [PubMed] [Google Scholar]
- 39.Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8(5):351–360. doi: 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- 40.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361(19):1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 41.Daayana S, Elkord E, Winters U, Pawlita M, Roden R, Stern PL, et al. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br J Cancer. 2010;102(7):1129–1136. doi: 10.1038/sj.bjc.6605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welters MJ, Kenter GG, de Vos van Steenwijk PJ, Lowik MJ, Berends-van der Meer DM, Essahsah F, et al. Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses. Proc Natl Acad Sci U S A. 2010;107(26):11895–11899. doi: 10.1073/pnas.1006500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res. 2008;14(1):169–177. doi: 10.1158/1078-0432.CCR-07-1881. [DOI] [PubMed] [Google Scholar]
- 44.Welters MJ, Kenter GG, Piersma SJ, Vloon AP, Lowik MJ, Berends-van der Meer DM, et al. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res. 2008;14(1):178–187. doi: 10.1158/1078-0432.CCR-07-1880. [DOI] [PubMed] [Google Scholar]
- 45.Tseng CW, Hung CF, Alvarez RD, Trimble C, Huh WK, Kim D, et al. Pretreatment with cisplatin enhances E7-specific CD8+ T-Cell-mediated antitumor immunity induced by DNA vaccination. Clin Cancer Res. 2008;14(10):3185–3192. doi: 10.1158/1078-0432.CCR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tseng CW, Trimble C, Zeng Q, Monie A, Alvarez RD, Huh WK, et al. Low-dose radiation enhances therapeutic HPV DNA vaccination in tumor-bearing hosts. Cancer Immunol Immunother. 2009;58(5):737–748. doi: 10.1007/s00262-008-0596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao Y, Zhao J, Yang Z, Cai Z, Zhang B, Zhou Y, et al. CD4+FOXP3+ regulatory T cell depletion by low-dose cyclophosphamide prevents recurrence in patients with large condylomata acuminata after laser therapy. Clin Immunol. 2010;136(1):21–29. doi: 10.1016/j.clim.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 48.Rosenberg SA. Cell transfer immunotherapy for metastatic solid cancer-what clinicians need to know. Nat Rev Clin Oncol. 2011;8(10):577–585. doi: 10.1038/nrclinonc.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bae SH, Park YJ, Park JB, Choi YS, Kim MS, Sin JI. Therapeutic synergy of human papillomavirus E7 subunit vaccines plus cisplatin in an animal tumor model: causal involvement of increased sensitivity of cisplatin-treated tumors to CTL-mediated killing in therapeutic synergy. Clin Cancer Res. 2007;13(1):341–349. doi: 10.1158/1078-0432.CCR-06-1838. [DOI] [PubMed] [Google Scholar]
- 51.Ly LV, Sluijter M, Versluis M, Luyten GP, van Stipdonk MJ, van der Burg SH, et al. Peptide vaccination after T-cell transfer causes massive clonal expansion, tumor eradication, and manageable cytokine storm. Cancer Res. 2010;70(21):8339–8346. doi: 10.1158/0008-5472.CAN-10-2288. [DOI] [PubMed] [Google Scholar]
- 52.Haas AR, Sun J, Vachani A, Wallace AF, Silverberg M, Kapoor V, et al. Cycloxygenase-2 inhibition augments the efficacy of a cancer vaccine. Clin Cancer Res. 2006;12(1):214–222. doi: 10.1158/1078-0432.CCR-05-1178. [DOI] [PubMed] [Google Scholar]
- 53.Kim S, Buchlis G, Fridlender ZG, Sun J, Kapoor V, Cheng G, et al. Systemic blockade of transforming growth factor-beta signaling augments the efficacy of immunogene therapy. Cancer Res. 2008;68(24):10247–10256. doi: 10.1158/0008-5472.CAN-08-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer T, Stockfleth E. Clinical investigations of Toll-like receptor agonists. Expert Opin Investig Drugs. 2008;17(7):1051–1065. doi: 10.1517/13543784.17.7.1051. [DOI] [PubMed] [Google Scholar]
- 55.Winters U, Daayana S, Lear JT, Tomlinson AE, Elkord E, Stern PL, et al. Clinical and immunologic results of a phase II trial of sequential imiquimod and photodynamic therapy for vulval intraepithelial neoplasia. Clin Cancer Res. 2008;14(16):5292–5299. doi: 10.1158/1078-0432.CCR-07-4760. [DOI] [PubMed] [Google Scholar]
- 56.van Seters M, van Beurden M, ten Kate FJ, Beckmann I, Ewing PC, Eijkemans MJ, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med. 2008;358(14):1465–1473. doi: 10.1056/NEJMoa072685. [DOI] [PubMed] [Google Scholar]
- 57.Andrei G, De Clercq E, Snoeck R. Drug targets in cytomegalovirus infection. Infect Disord Drug Targets. 2009;9(2):201–222. doi: 10.2174/187152609787847758. [DOI] [PubMed] [Google Scholar]
- 58.Amine A, Rivera S, Opolon P, Dekkal M, Biard DS, Bouamar H, et al. Novel anti-metastatic action of cidofovir mediated by inhibition of E6/E7, CXCR4 and Rho/ROCK signaling in HPV tumor cells. PLoS One. 2009;4(3):e5018. doi: 10.1371/journal.pone.0005018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toro JR, Sanchez S, Turiansky G, Blauvelt A. Topical cidofovir for the treatment of dermatologic conditions: verruca, condyloma, intraepithelial neoplasia, herpes simplex and its potential use in smallpox. Dermatol Clin. 2003;21(2):301–309. doi: 10.1016/s0733-8635(02)00116-x. [DOI] [PubMed] [Google Scholar]
- 60.Snoeck R, Noel JC, Muller C, De Clercq E, Bossens M. Cidofovir, a new approach for the treatment of cervix intraepithelial neoplasia grade III (CIN III) J Med Virol. 2000;60(2):205–209. doi: 10.1002/(sici)1096-9071(200002)60:2<205::aid-jmv16>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 61.Tristram A, Fiander A. Clinical responses to Cidofovir applied topically to women with high grade vulval intraepithelial neoplasia. Gynecol Oncol. 2005;99(3):652–655. doi: 10.1016/j.ygyno.2005.07.127. [DOI] [PubMed] [Google Scholar]
- 62.Donne AJ, Hampson L, He XT, Day PJ, Salway F, Rothera MP, et al. Potential risk factors associated with the use of cidofovir to treat benign human papillomavirus-related disease. Antivir Ther. 2009;14(7):939–952. doi: 10.3851/IMP1421. [DOI] [PubMed] [Google Scholar]
- 63.Tjon Pian Gi RE, Dietz A, Djukic V, Eckel HE, Friedrich G, Golusinski W, et al. Treatment of recurrent respiratory papillomatosis and adverse reactions following off-label use of cidofovir (Vistide®) Eur Arch Otorhinolaryngol. 2012;269(2):361–362. doi: 10.1007/s00405-011-1804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6(7):535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brackett CM, Gollnick SO. Photodynamic therapy enhancement of anti-tumor immunity. Photochem Photobiol Sci. 2011;10(5):649–652. doi: 10.1039/c0pp00354a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Korbelik M. Cancer vaccines generated by photodynamic therapy. Photochem Photobiol Sci. 2011;10(5):664–669. doi: 10.1039/c0pp00343c. [DOI] [PubMed] [Google Scholar]
- 67.St Denis TG, Aziz K, Waheed AA, Huang YY, Sharma SK, Mroz P, et al. Combination approaches to potentiate immune response after photodynamic therapy for cancer. Photochem Photobiol Sci. 2011;10(5):792–801. doi: 10.1039/c0pp00326c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kruijt B, van der Snoek EM, Sterenborg HJ, Amelink A, Robinson DJ. A dedicated applicator for light delivery and monitoring of PDT of intra-anal intraepithelial neoplasia. Photodiagnosis Photodyn Ther. 2010;7(1):3–9. doi: 10.1016/j.pdpdt.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 69.Zawislak A, Donnelly RF, McCluggage WG, Price JH, McClelland HR, Woolfson AD, et al. Clinical and immunohistochemical assessment of vulval intraepithelial neoplasia following photodynamic therapy using a novel bioadhesive patch-type system loaded with 5-aminolevulinic acid. Photodiagnosis Photodyn Ther. 2009;6(1):28–40. doi: 10.1016/j.pdpdt.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 70.Abdel-Hady ES, Martin-Hirsch P, Duggan-Keen M, Stern PL, Moore JV, Corbitt G, et al. Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer Res. 2001;61(1):192–196. [PubMed] [Google Scholar]
- 71.Bharti AC, Shukla S, Mahata S, Hedau S, Das BC. Anti-human papillomavirus therapeutics: facts & future. Indian J Med Res. 2009;130(3):296–310. [PubMed] [Google Scholar]
- 72.D'Abramo CM, Archambault J. Small molecule inhibitors of human papillomavirus protein - protein interactions. Open Virol J. 2011;5:80–95. doi: 10.2174/1874357901105010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tan S, De Vries EG, Van der Zee AG, De Jong S. Anticancer drugs aimed at E6 and E7 activity in HPV-positive cervical cancer. Curr Cancer Drug Targets. 2011;11(9) doi: 10.2174/156800912799095135. [DOI] [PubMed] [Google Scholar]
- 74.Faucher AM, White PW, Brochu C, Grand-Maitre C, Rancourt J, Fazal G. Discovery of small-molecule inhibitors of the ATPase activity of human papillomavirus E1 helicase. J Med Chem. 2004;47(1):18–21. doi: 10.1021/jm034206x. [DOI] [PubMed] [Google Scholar]
- 75.Berg M, Stenlund A. Functional interactions between papillomavirus E1 and E2 proteins. J Virol. 1997;71(5):3853–3863. doi: 10.1128/jvi.71.5.3853-3863.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fradet-Turcotte A, Archambault J. Recent advances in the search for antiviral agents against human papillomaviruses. Antivir Ther. 2007;12(4):431–451. [PMC free article] [PubMed] [Google Scholar]
- 77.White PW, Faucher AM, Goudreau N. Small molecule inhibitors of the human papillomavirus E1-E2 interaction. Curr Top Microbiol Immunol. 2011;348:61–88. doi: 10.1007/82_2010_92. [DOI] [PubMed] [Google Scholar]
- 78.Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, et al. The biology and life-cycle of human papillomaviruses. Vaccine, this issue. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 79.Massimi P, Shai A, Lambert P, Banks L. HPV E6 degradation of p53 and PDZ containing substrates in an E6AP null background. Oncogene. 2008;27(12):1800–1804. doi: 10.1038/sj.onc.1210810. [DOI] [PubMed] [Google Scholar]
- 80.Dymalla S, Scheffner M, Weber E, Sehr P, Lohrey C, Hoppe-Seyler F, et al. A novel peptide motif binding to and blocking the intracellular activity of the human papillomavirus E6 oncoprotein. J Mol Med (Berl) 2009;87(3):321–331. doi: 10.1007/s00109-008-0432-1. [DOI] [PubMed] [Google Scholar]
- 81.Nomine Y, Masson M, Charbonnier S, Zanier K, Ristriani T, Deryckere F, et al. Structural and functional analysis of E6 oncoprotein: insights in the molecular pathways of human papillomavirus-mediated pathogenesis. Mol Cell. 2006;21(5):665–678. doi: 10.1016/j.molcel.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 82.Sterlinko Grm H, Weber M, Elston R, McIntosh P, Griffin H, Banks L, et al. Inhibition of E6-induced degradation of its cellular substrates by novel blocking peptides. J Mol Biol. 2004;335(4):971–985. doi: 10.1016/j.jmb.2003.10.079. [DOI] [PubMed] [Google Scholar]
- 83.Guo CP, Liu KW, Luo HB, Chen HB, Zheng Y, Sun SN, et al. Potent anti-tumor effect generated by a novel human papillomavirus (HPV) antagonist peptide reactivating the pRb/E2F pathway. PLoS One. 2011;6(3):e17734. doi: 10.1371/journal.pone.0017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hougardy BM, Maduro JH, van der Zee AG, de Groot DJ, van den Heuvel FA, de Vries EG, et al. Proteasome inhibitor MG132 sensitizes HPV-positive human cervical cancer cells to rhTRAIL-induced apoptosis. Int J Cancer. 2006;118(8):1892–1900. doi: 10.1002/ijc.21580. [DOI] [PubMed] [Google Scholar]
- 85.Pajonk F, Grumann T, McBride WH. The proteasome inhibitor MG-132 protects hypoxic SiHa cervical carcinoma cells after cyclic hypoxia/reoxygenation from ionizing radiation. Neoplasia. 2006;8(12):1037–1041. doi: 10.1593/neo.06634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang Y, Kitagaki J, Wang H, Hou DX, Perantoni AO. Targeting the ubiquitin-proteasome system for cancer therapy. Cancer Sci. 2009;100(1):24–28. doi: 10.1111/j.1349-7006.2008.01013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Birle DC, Hedley DW. Suppression of the hypoxia-inducible factor-1 response in cervical carcinoma xenografts by proteasome inhibitors. Cancer Res. 2007;67(4):1735–1743. doi: 10.1158/0008-5472.CAN-06-2722. [DOI] [PubMed] [Google Scholar]
- 88.Kamer S, Ren Q, Dicker AP. Differential radiation sensitization of human cervical cancer cell lines by the proteasome inhibitor velcade (bortezomib, PS-341) Arch Gynecol Obstet. 2009;279(1):41–46. doi: 10.1007/s00404-008-0667-7. [DOI] [PubMed] [Google Scholar]
- 89.Pugh TJ, Chen C, Rabinovitch R, Eckhardt SG, Rusthoven KE, Swing R, et al. Phase I trial of bortezomib and concurrent external beam radiation in patients with advanced solid malignancies. Int J Radiat Oncol Biol Phys. 2010;78(2):521–526. doi: 10.1016/j.ijrobp.2009.07.1715. [DOI] [PubMed] [Google Scholar]
- 90.Batman G, Oliver AW, Zehbe I, Richard C, Hampson L, Hampson IN. Lopinavir up-regulates expression of the antiviral protein ribonuclease L in human papillomavirus-positive cervical carcinoma cells. Antivir Ther. 2011;16(4):515–525. doi: 10.3851/IMP1786. [DOI] [PubMed] [Google Scholar]
- 91.Selvi RB, Kundu TK. Reversible acetylation of chromatin: implication in regulation of gene expression, disease and therapeutics. Biotechnol J. 2009;4(3):375–390. doi: 10.1002/biot.200900032. [DOI] [PubMed] [Google Scholar]
- 92.Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J Clin Oncol. 2009;27(32):5459–5468. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- 93.Brehm A, Nielsen SJ, Miska EA, McCance DJ, Reid JL, Bannister AJ, et al. The E7 oncoprotein associates with Mi2 and histone deacetylase activity to promote cell growth. Embo J. 1999;18(9):2449–2458. doi: 10.1093/emboj/18.9.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de la Cruz-Hernandez E, Perez-Cardenas E, Contreras-Paredes A, Cantu D, Mohar A, Lizano M, et al. The effects of DNA methylation and histone deacetylase inhibitors on human papillomavirus early gene expression in cervical cancer, an in vitro and clinical study. Virol J. 2007;4:18. doi: 10.1186/1743-422X-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin Z, Bazzaro M, Wang MC, Chan KC, Peng S, Roden RB. Combination of proteasome and HDAC inhibitors for uterine cervical cancer treatment. Clin Cancer Res. 2009;15(2):570–577. doi: 10.1158/1078-0432.CCR-08-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stanley M, Pinto LA, Trimble C. Human papillomavirus vaccines – immune responses. Vaccine. doi: 10.1016/j.vaccine.2012.04.106. this issue. [DOI] [PubMed] [Google Scholar]
- 97.Garcon N, Stern PL, Cunningham AL, Stanberry LR. Perspectives in Vaccinology. 1. Vol. 1. Amsterdam: Elsevier B.V; 2012. [last accessed July 2012]. Understanding Modern Vaccines; pp. 1–200. Available at: http://www.sciencedirect.com/science/journal/22107622. [Google Scholar]
- 98.de Jong A, van Poelgeest MI, van der Hulst JM, Drijfhout JW, Fleuren GJ, Melief CJ, et al. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res. 2004 Aug 1;64(15):5449–5455. doi: 10.1158/0008-5472.CAN-04-0831. [DOI] [PubMed] [Google Scholar]
- 99.Nakagawa M, Stites DP, Farhat S, Sisler JR, Moss B, Kong F, et al. Cytotoxic T lymphocyte responses to E6 and E7 proteins of human papillomavirus type 16: relationship to cervical intraepithelial neoplasia. J Infect Dis. 1997 Apr;175(4):927–931. doi: 10.1086/513992. [DOI] [PubMed] [Google Scholar]
- 100.van den Hende M, van Poelgeest MI, van der Hulst JM, de Jong J, Drijfhout JW, Fleuren GJ, et al. Skin reactions to human papillomavirus (HPV) 16 specific antigens intradermally injected in healthy subjects and patients with cervical neoplasia. Int J Cancer. 2008 Jul 1;123(1):146–152. doi: 10.1002/ijc.23502. [DOI] [PubMed] [Google Scholar]
- 101.Kim KH, Greenfield WW, Cannon MJ, Coleman HN, Spencer HJ, Nakagawa M. CD4+ T-cell response against human papillomavirus type 16 E6 protein is associated with a favorable clinical trend. Cancer Immunol Immunother. 2012 Jan;61(1):63–70. doi: 10.1007/s00262-011-1092-5. Epub 2011 Aug 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Woo YL, Sterling J, Damay I, Coleman N, Crawford R, van der Burg SH, et al. Characterising the local immune responses in cervical intraepithelial neoplasia: a cross-sectional and longitudinal analysis. BJOG. 2008 Dec;115(13):1616–1621. doi: 10.1111/j.1471-0528.2008.01936.x. discussion 21-2. [DOI] [PubMed] [Google Scholar]
- 103.Trimble CL, Clark RA, Thoburn C, Hanson NC, Tassello J, Frosina D, et al. Human papillomavirus 16-associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium. J Immunol. 2010 Dec 1;185(11):7107–7114. doi: 10.4049/jimmunol.1002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Farhat S, Nakagawa M, Moscicki AB. Cell-mediated immune responses to human papillomavirus 16 E6 and E7 antigens as measured by interferon gamma enzyme-linked immunospot in women with cleared or persistent human papillomavirus infection. Int J Gynecol Cancer. 2009 May;19(4):508–512. doi: 10.1111/IGC.0b013e3181a388c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bourgault Villada I, Moyal Barracco M, Ziol M, Chaboissier A, Barget N, Berville S, et al. Spontaneous regression of grade 3 vulvar intraepithelial neoplasia associated with human papillomavirus-16-specific CD4(+) and CD8(+) T-cell responses. Cancer Res. 2004 Dec 1;64(23):8761–8766. doi: 10.1158/0008-5472.CAN-04-2455. [DOI] [PubMed] [Google Scholar]
- 106.Seresini S, Origoni M, Lillo F, Caputo L, Paganoni AM, Vantini S, et al. IFN-gamma produced by human papilloma virus-18 E6-specific CD4+ T cells predicts the clinical outcome after surgery in patients with high-grade cervical lesions. J Immunol. 2007 Nov 15;179(10):7176–7183. doi: 10.4049/jimmunol.179.10.7176. [DOI] [PubMed] [Google Scholar]
- 107.Heusinkveld M, Welters MJ, van Poelgeest MI, van der Hulst JM, Melief CJ, Fleuren GJ, et al. The detection of circulating human papillomavirus-specific T cells is associated with improved survival of patients with deeply infiltrating tumors. Int J Cancer. 2011 Jan 15;128(2):379–389. doi: 10.1002/ijc.25361. [DOI] [PubMed] [Google Scholar]
- 108.Sarkar AK, Tortolero-Luna G, Follen M, Sastry KJ. Inverse correlation of cellular immune responses specific to synthetic peptides from the E6 and E7 oncoproteins of HPV-16 with recurrence of cervical intraepithelial neoplasia in a cross-sectional study. Gynecol Oncol. 2005 Dec;99(3 Suppl 1):S251–S261. doi: 10.1016/j.ygyno.2005.07.099. [DOI] [PubMed] [Google Scholar]
- 109.Ovestad IT, Gudlaugsson E, Skaland I, Malpica A, Munk AC, Janssen EA, et al. The impact of epithelial biomarkers, local immune response and human papillomavirus genotype in the regression of cervical intraepithelial neoplasia grades 2–3. J Clin Pathol. 2011 Apr;64(4):303–307. doi: 10.1136/jcp.2010.083626. [DOI] [PubMed] [Google Scholar]
- 110.Scott ME, Ma Y, Kuzmich L, Moscicki AB. Diminished IFN-gamma and IL-10 and elevated Foxp3 mRNA expression in the cervix are associated with CIN 2 or 3. Int J Cancer. 2009 Mar 15;124(6):1379–1383. doi: 10.1002/ijc.24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.El-Sherif AM, Seth R, Tighe PJ, Jenkins D. Quantitative analysis of IL-10 and IFN-gamma mRNA levels in normal cervix and human papillomavirus type 16 associated cervical precancer. J Pathol. 2001 Sep;195(2):179–185. doi: 10.1002/path.929. [DOI] [PubMed] [Google Scholar]
- 112.Syrjanen S, Naud P, Sarian L, Derchain S, Roteli-Martins C, Longatto-Filho A, et al. Immunosuppressive cytokine Interleukin-10 (IL-10) is up-regulated in high-grade CIN but not associated with high-risk human papillomavirus (HPV) at baseline, outcomes of HR-HPV infections or incident CIN in the LAMS cohort. Virchows Arch. 2009 Dec;455(6):505–515. doi: 10.1007/s00428-009-0850-7. [DOI] [PubMed] [Google Scholar]
- 113.Bais AG, Beckmann I, Lindemans J, Ewing PC, Meijer CJ, Snijders PJ, et al. A shift to a peripheral Th2-type cytokine pattern during the carcinogenesis of cervical cancer becomes manifest in CIN III lesions. J Clin Pathol. 2005 Oct;58(10):1096–1100. doi: 10.1136/jcp.2004.025072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Piersma SJ, Jordanova ES, van Poelgeest MI, Kwappenberg KM, van der Hulst JM, Drijfhout JW, et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007 Jan 1;67(1):354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 115.Bontkes HJ, de Gruijl TD, Walboomers JM, van den Muysenberg AJ, Gunther AW, Scheper RJ, et al. Assessment of cytotoxic T-lymphocyte phenotype using the specific markers granzyme B and TIA-1 in cervical neoplastic lesions. Br J Cancer. 1997;76(10):1353–1360. doi: 10.1038/bjc.1997.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, et al. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res. 2009 Oct 15;15(20):6341–6347. doi: 10.1158/1078-0432.CCR-09-1652. [DOI] [PubMed] [Google Scholar]
- 117.Gooden M, Lampen M, Jordanova ES, Leffers N, Trimbos JB, van der Burg SH, et al. HLA-E expression by gynecological cancers restrains tumor-infiltrating CD8 T lymphocytes. Proc Natl Acad Sci U S A. 2011 Jun 28;108(26):10656–10661. doi: 10.1073/pnas.1100354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jaafar F, Righi E, Lindstrom V, Linton C, Nohadani M, Van Noorden S, et al. Correlation of CXCL12 expression and FoxP3+ cell infiltration with human papillomavirus infection and clinicopathological progression of cervical cancer. Am J Pathol. 2009 Oct;175(4):1525–1535. doi: 10.2353/ajpath.2009.090295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zijlmans HJ, Fleuren GJ, Baelde HJ, Eilers PH, Kenter GG, Gorter A. The absence of CCL2 expression in cervical carcinoma is associated with increased survival and loss of heterozygosity at 17q11.2. J Pathol. 2006 Mar;208(4):507–517. doi: 10.1002/path.1918. [DOI] [PubMed] [Google Scholar]
- 120.Heusinkveld M, de Vos van Steenwijk PJ, Goedemans R, Ramwadhdoebe TH, Gorter A, Welters MJ, et al. M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J Immunol. 2011 Aug 1;187(3):1157–1165. doi: 10.4049/jimmunol.1100889. [DOI] [PubMed] [Google Scholar]
- 121.Battaglia A, Buzzonetti A, Baranello C, Ferrandina G, Martinelli E, Fanfani F, et al. Metastatic tumour cells favour the generation of a tolerogenic milieu in tumour draining lymph node in patients with early cervical cancer. Cancer Immunol Immunother. 2009 Sep;58(9):1363–1373. doi: 10.1007/s00262-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jordanova ES, Gorter A, Ayachi O, Prins F, Durrant LG, Kenter GG, et al. Human leukocyte antigen class I, MHC class I chain-related molecule A, and CD8+/regulatory T-cell ratio: which variable determines survival of cervical cancer patients? Clin Cancer Res. 2008 Apr 1;14(7):2028–2035. doi: 10.1158/1078-0432.CCR-07-4554. [DOI] [PubMed] [Google Scholar]
- 123.Woo YL, van den Hende M, Sterling JC, Coleman N, Crawford RA, Kwappenberg KM, et al. A prospective study on the natural course of low-grade squamous intraepithelial lesions and the presence of HPV16 E2-, E6- and E7-specific T-cell responses. Int J Cancer. 2010 Jan 1;126(1):133–141. doi: 10.1002/ijc.24804. [DOI] [PubMed] [Google Scholar]
- 124.de Vos van Steenwijk PJ, Heusinkveld M, Ramwadhdoebe TH, Lowik MJ, van der Hulst JM, Goedemans R, et al. An unexpectedly large polyclonal repertoire of HPV-specific T cells is poised for action in patients with cervical cancer. Cancer Res. 2010 Apr 1;70(7):2707–2717. doi: 10.1158/0008-5472.CAN-09-4299. [DOI] [PubMed] [Google Scholar]
- 125.Piersma SJ, Welters MJ, van der Hulst JM, Kloth JN, Kwappenberg KM, Trimbos BJ, et al. Human papilloma virus specific T cells infiltrating cervical cancer and draining lymph nodes show remarkably frequent use of HLA-DQ and -DP as a restriction element. Int J Cancer. 2008 Feb 1;122(3):486–494. doi: 10.1002/ijc.23162. [DOI] [PubMed] [Google Scholar]
- 126.van der Burg SH, Piersma SJ, de Jong A, van der Hulst JM, Kwappenberg KM, van den Hende M, et al. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc Natl Acad Sci U S A. 2007 Jul 17;104(29):12087–12092. doi: 10.1073/pnas.0704672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ressing ME, van Driel WJ, Brandt RM, Kenter GG, de Jong JH, Bauknecht T, et al. Detection of T helper responses, but not of human papillomavirus-specific cytotoxic T lymphocyte responses, after peptide vaccination of patients with cervical carcinoma. J Immunother. 2000;23(2):255–266. doi: 10.1097/00002371-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 128.Baldwin PJ, van der Burg SH, Boswell CM, Offringa R, Hickling JK, Dobson J, et al. Vaccinia-expressed human papillomavirus 16 and 18 e6 and e7 as a therapeutic vaccination for vulval and vaginal intraepithelial neoplasia. Clin Cancer Res. 2003;9(14):5205–5213. [PubMed] [Google Scholar]
- 129.Smyth LJ, Van Poelgeest MI, Davidson EJ, Kwappenberg KM, Burt D, Sehr P, et al. Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clin Cancer Res. 2004 May 1;10(9):2954–2961. doi: 10.1158/1078-0432.ccr-03-0703. [DOI] [PubMed] [Google Scholar]
- 130.Trimble CL, Peng S, Kos F, Gravitt P, Viscidi R, Sugar E, et al. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin Cancer Res. 2009;15(1):361–367. doi: 10.1158/1078-0432.CCR-08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Santin AD, Bellone S, Palmieri M, Zanolini A, Ravaggi A, Siegel ER, et al. Human papillomavirus type 16 and 18 E7-pulsed dendritic cell vaccination of stage IB or IIA cervical cancer patients: a phase I escalating-dose trial. J Virol. 2008;82(4):1968–1979. doi: 10.1128/JVI.02343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Frazer IH, Quinn M, Nicklin JL, Tan J, Perrin LC, Ng P, et al. Phase 1 study of HPV16-specific immunotherapy with E6E7 fusion protein and ISCOMATRIX adjuvant in women with cervical intraepithelial neoplasia. Vaccine. 2004;23(2):172–181. doi: 10.1016/j.vaccine.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 133.Matijevic M, Hedley ML, Urban RG, Chicz RM, Lajoie C, Luby TM. Immunization with a poly (lactide co-glycolide) encapsulated plasmid DNA expressing antigenic regions of HPV 16 and 18 results in an increase in the precursor frequency of T cells that respond to epitopes from HPV 16, 18, 6 and 11. Cell Immunol. 2011;270(1):62–69. doi: 10.1016/j.cellimm.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brun JL, Dalstein V, Leveque J, Mathevet P, Raulic P, Baldauf JJ, et al. Regression of high-grade cervical intraepithelial neoplasia with TG4001 targeted immunotherapy. Am J Obstet Gynecol. 2011;204(2):169, e1–e8. doi: 10.1016/j.ajog.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 135.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009 Jun 19;27(30):3975–3983. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]