Clathrin-independent Endocytosis: A cargo-centric view (original) (raw)
. Author manuscript; available in PMC: 2014 Nov 1.
Published in final edited form as: Exp Cell Res. 2013 Aug 13;319(18):2759–2769. doi: 10.1016/j.yexcr.2013.08.008
Abstract
Clathrin-independent endocytosis occurs in all cells and interest in this mode of cellular entry has grown. Although this form of endocytosis was first described for entry of bacterial toxins, here we focus our attention on the endogenous cell surface “cargo” proteins that enter cells by this mechanism. The cargo proteins entering by this mechanism are varied and include nutrient transporters, ion channels, cell adhesion molecules and proteins associated with the immune system. Despite the apparent lack of selection at the cell surface, we provide some examples of specific sorting of these cargo proteins after entry, leading to distinct itineraries and cellular fates.
Introduction
Endocytosis is a fundamental cellular process that brings extracellular fluid and portions of the plasma membrane (PM) into the cell interior. This provides a mechanism for cells to take up nutrients and remove proteins from the cell surface. Once inside, however, membrane and content undergo a sorting process leading to transport to lysosomes, to the trans Golgi network or recycling endosomes that return the membrane back to the cell surface. The endocytic event and the subsequent sorting and trafficking of membrane and lipid are both important for the maintenance of PM protein and lipid composition and for cellular homeostasis.
There are two general categories of endocytosis distinguished by one requiring the coat protein clathrin (clathrin-mediated endocytosis or CME) and the other not requiring clathrin (clathrin-independent endocytosis or CIE) for vesicle formation and internalization. CME has been extensively studied and involves the selective recruitment and internalization of PM proteins that contain distinct cytoplasmic sorting sequences recognized by the adaptor proteins that are part of the clathrin coat. A complex set of machinery facilitates this event and this is the primary mechanism for endocytosis of transferrin and low density lipoprotein (LDL) receptors (TfR and LDLR) and signaling receptors after ligand stimulation [1, 2]. CIE, by contrast, does not appear to have a distinctive cytoplasmic coat nor apparent mechanism for selection of cell surface cargo proteins. There has been increased interest in CIE over the past ten years since it is the mode of entry for a number of bacterial toxins and other cell surface proteins.
Historically, CIE has been studied as the “other” pathway, a non-selective, bulk endocytic entry mechanism [1]. Some of the first descriptions of alternative endocytic portals of entry came from studies examining the entry of lipid-binding bacterial toxins [3]. It soon became clear that some toxins could enter through multiple endocytic mechanisms. Over the past decade descriptions of different CIE mechanisms have proliferated. In most cases these distinctions are based on the endocytic cargo being examined and the sensitivity to different chemical and genetic perturbations. Thus, one clathrin and dynamin-independent form of CIE is primarily involved in the endocytosis of proteins anchored to the membrane by glycosyl phosphatidylinositol (GPI) and is dependent upon PM cholesterol, and the G proteins Cdc42, and Arf1. The resulting endosome has been referred to as “CLIC” for clathrin-independent carrier”, which then joins with the “GEEC” for GPI-anchored protein (GPI-AP) early endosomal compartment [4–6]. Another clathrin and dynamin-independent form of CIE is also dependent upon PM cholesterol and is associated with Arf6 in that the activity of Arf6 can influence the subsequent trafficking of the endocytosed cargo proteins [7–9]. Other CIE forms include one dependent upon dynamin and Rho [10] and others dependent upon flotillins [11]. Although caveolae are important for trans-endothelial transport, they do not appear to be a mechanism of endocytosis in most cells; caveolin functions to organize PM domains [12]. This apparent proliferation of types of CIE may be a result of different cargo and cell types being examined. Additionally the use of bacterial toxins and overexpressed cargo proteins, often used to define different CIE mechanisms, might not faithfully reflect the entry and itinerary taken by endogenous cargo proteins.
The Arf6-associated CIE pathway has been built upon studying trafficking of endogenous cargo proteins. Although HeLa cells have proven to be a successful model system to study this form of CIE [8, 13, 14], it has been observed in a variety of human and mouse cell lines and in Caenorhabditis elegans, demonstrating that the Arf6-associated CIE pathway is highly conserved [15]. Many CIE cargo molecules, such as the major histocompatibility complex Class I (MHCI), the alpha-chain of the IL-2 receptor (Tac), β -integrins, and endogenous GPI-AP, such as CD55 and CD59, enter the cell through the CIE pathway associated with Arf6 [7, 9, 14–16]. Typically, CIE cargo proteins are internalized into vesicles that either fuse with or mature into endosomes associated with Rab5- and the early endosome-associated antigen 1 (EEA1). It is in this compartment where CIE cargo molecules meet with incoming transferrin receptor (TfR), a prototypical CME cargo protein (Figure 1) [8, 14]. From there, the cargo is delivered to late endosomes and lysosomes for degradation or recycled back to the PM through recycling tubular endosomes, which are a hallmark of this pathway in HeLa cells (Figure 1) [8, 14, 15].
Figure 1.
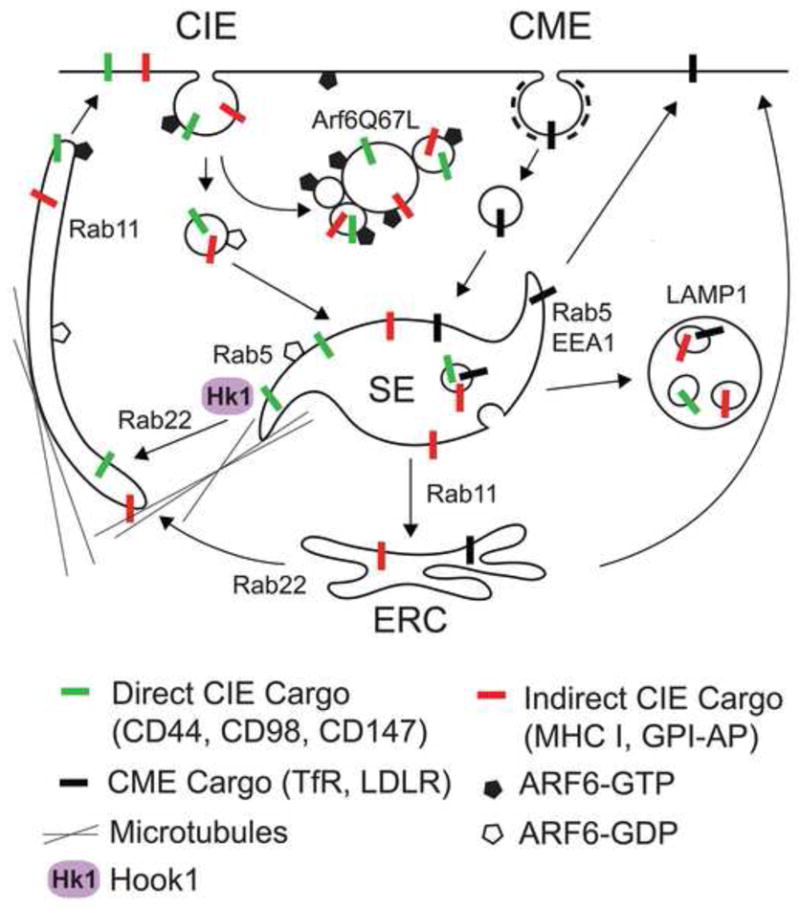
General model for CIE and CME and subsequent membrane trafficking. Itinerary followed by CIE and CME cargo proteins. CIE cargo (red and blue bars) enter separately from CME cargo (black bars) but then enter the common Sorting Endosome (SE) where cargo can be sorted and moved to different destinations. Some CIE cargo proteins (blue bars) take the Direct recycling pathway (via tubules) back to the PM while other CIE cargo proteins (red bars) take the Indirect pathway (via the Endocytic Recycling Compartment (ERC) and then tubules) back to the PM. Some of the Arf and Rab regulators are shown. Cargo entering by CME (black bars) can recycle back to the PM via Rab5 sorting endosome or Rab11 ERC. CME cargo and CIE cargo that take the indirect route (Red bars) also trafficking to Lamp1-positive late endosomes/lysosomes for degradation.
Although Arf6 activity is not required for the internalization of CIE cargo-containing vesicles, it does regulate the post-endocytic trafficking of CIE cargo proteins at two different stages in the pathway. Arf6 activates phosphatidylinositol 4-phoshate 5-kinase [7, 17], the enzyme that generates phosphatidylinositol (PI) 4,5-biphosphate (PI4,5P2). After internalization, inactivation of Arf6 (to Arf6-GDP) is required to decrease PI4,5P2 levels so that these endosomes can fuse with endosomes containing CME cargo proteins [8]. Expression of a constitutively active form of Arf6, Arf6Q67L, promotes CIE internalization, but the endosomes fuse with each other causing the accumulation of the cargo proteins in vacuolar-type structures, blocking further trafficking of the cargo to the Rab5/EEA1-early endosomes. These Arf6 Q67L vacuoles capture CIE cargo, but not CME cargo, such as TfR and LDLR [7, 8, 14]. On the other hand, Arf6 activation (to Arf6-GTP) is required for the subsequent recycling of CIE cargo, usually from the recycling tubular endosomes, back to the PM [9, 16]. Additionally, other regulatory factors have been shown to modulate and coordinate the recycling of CIE cargo including Rab5, Rab11, Rab22a, Rab35, the EPS15 homology domain-containing (EHD) proteins EHD1 and EHD3, ERK, ALX-1, Cdc42, and PAR3 [18–23]. Even though many of these proteins have been identified as important, it is not clear how they coordinate and regulate the trafficking of PM proteins through the CIE pathway.
The CIE trafficking itinerary described above is the trafficking route followed by prototypical CIE cargo proteins, such as, MHCI, Tac and CD59 (Figure 1) [8, 14, 15]. However, a proteomic analysis of the Arf6 Q67L vacuolar membranes uncovered a new set of CIE cargo proteins, including CD44, CD98 and CD147 (see Table 1) that exhibit an alternative trafficking itinerary that differs from the trafficking of MHCI/Tac/CD59 [24]. CD44, CD98 and CD147 are internalized in the same structures as MHCI, Tac and CD59, but are actively sorted away from the Rab5/EEA1-containing endosomes (Figure 1) [24]. Instead, CD44, CD98 and CD147 predominantly traffic directly to the recycling tubular endosomes, preventing their targeting to late endosomes. Consistent with this, these proteins have prolonged surface lifetimes in HeLa cells [25]. This post-endocytic separation of CIE cargo proteins on endosomes suggests that sorting mechanisms at the endosomal level regulate the trafficking itinerary of different groups of CIE cargo molecules and dictate their final destination.
Table 1.
CIE Cargo Proteins
| Immune Function | |
|---|---|
| MHCI | On all nucleated cells; presents endogenous peptides to T cells |
| MHCII | On the surface of antigen-presenting cells; presents exogenous peptides to T cells |
| CD1a | Presents lipid antigens to T cells |
| CD55 | Decay acceleration factor; protects from complement |
| CD59 | Protectin; protects cells from complement-mediated lysis |
| FBP | Folate receptor (GPI-AP); facilitates folate uptake |
| Ion & Nutrient Transport | |
| MCOLN2 | Mucolipin 2; cation channel member of the superfamily of transient receptor potential (TRP) channels |
| Kir3.4 | G protein activated inwardly rectifying potassium channel |
| Glut1 | Non-insulin regulated glucose transporter |
| Lat1(SLC7A5) | Neutral, bulky hydrophobic amino acid transporter |
| LOX-1 | Receptor for oxidized low-density lipoprotein (OxLDL) |
| MCT1(SLC16A1) | Monocarboxylic acid transporter |
| Growth Factor & GPCR (constitutive endocytosis) | |
| B2AR | B2-adrenergic receptor |
| M3R | Muscarinic receptor |
| mGluR7 | Metabotropic glutamate receptor 7 |
| EGFR | Epidermal growth factor receptor; internalized through CIE under high ligand concentration conditions |
| Met | Hepatocyte growth factor receptor |
| Cell Adhesion | |
| ICAM1(CD54) | Intracellular adhesion molecule 1; surface glycoprotein involved in cell-adhesion in endothelial and immune cells. |
| E-Cadherin (LCAM) | Epithelial cadherin; calcium-dependent cell-adhesion protein important for the regulation of cell-cell adhesion and cellular proliferation |
| Syndecans 1-4 | Cell surface heparan sulfate proteoglycans |
| β1-integrins | In association with α-integrins regulate cellular interactions with the extracellular matrix, cell motility and signaling. Also seen in CLIC/GEEC proteome. |
| CD44 | Hyaluronan receptor; also seen in CLIC/GEEC proteome. |
| CD98 (SLC3A2) | Facilitates the transport of neutral amino acids when associated with Lat1. Also seen in CLIC/GEEC proteome. |
| CD147 (Basigin) | Activates matrix metalloproteinase, associates with MCT1 and MCT4 for monocarboxylic acid transport |
In this review we aim to present CIE from the perspective of the endogenous cargo proteins that enter cells by this process, putting aside for the moment the specific mechanism of entry. We will describe the types of proteins that enter cells by CIE and describe what is known about their entry and subsequent itinerary within cells. What happens to the cargo after entry is of vital importance to the cell. CIE provides a means to sample the PM protein population for purposes of cargo redistribution or degradation. Finally, using information we have gleaned from our studies, we will discuss the ways in which these cargo proteins might be specifically sorted on endosomes after entry.
CIE Cargo Proteins
There are a wide variety of PM proteins that lack cytoplasmic sequences for facilitating endocytosis into cells by CME. Indeed, these proteins are supposed to remain at the PM to carry out their functions and so rapid clearance from the cell surface would be undesirable. One viewpoint is that CIE provides a means for the cell to sample the cell surface proteome and possibly, within the endosome, subject those proteins to a quality control monitoring system that will lead to either degradation or recycling of the protein back out to the cell surface. A number of different types of PM proteins have been demonstrated to enter cells via CIE and these are discussed below (Table 1). It is likely that many more will be identified in the future.
IMMUNE FUNCTION
A major group of CIE cargo proteins involved in immune surveillance consists of the structurally and functionally related proteins MHCI, MHC class II (MHCII) and CD1a. These proteins are responsible for presenting antigens from different origins to T-lymphocytes with the goal of preventing autoimmunity or initiating an immune response against infected cells. MHCI and MHCII molecules display self- and foreign peptides on the surface of cells, whereas CD1a molecules present self- and pathogenic lipids to T-cells.
MHCI is expressed at the surface of all nucleated cells and by presenting self-peptides protects host cells from being targeted by T-lymphocytes. However, in infected cells MHCI molecules present bacterial or viral peptides on the surface. MHCI is considered a prototypical CIE cargo protein. Its internalization, endosomal trafficking and recycling are regulated in part by Arf6, Rab22, Rab11, EHD1 and ubiquitination by E3 ligases of the MARCH family {Caplan, 2002 #25;Eyster, 2011 #1662;Naslavsky, 2003 #5;Naslavsky, 2004 #806;Weigert, 2004 #805}. Interestingly, the glycoprotein CD1a, which is structurally similar to MHCI, exhibits the same trafficking itinerary as MHCI [26]. CD1a is the only member of the CD1 family that does not have clathrin-internalization signals in its cytoplasmic tail and uses CIE for internalization. It has been proposed that other protein(s) may guide the trafficking of CD1a including Arl13b [27].
The role of MHCII is to present antigenic peptides on the surface of professional antigen presenting cells (APCs). MHCII is found in two different states at the PM: MHCII-newly synthesized or peptide free, and MHCII-loaded with antigenic peptides. The newly synthesized molecules are loaded in the endoplasmic reticulum with tri-peptides of invariant chain (Ii). These prevent self-peptides from binding prematurely to the antigenic peptide-binding site. Once at the PM, CME facilitates the internalization of peptide-free MHCII molecules [28, 29] as the clathrin adaptor protein AP-2 recognizes di-leucine internalization signals on the cytoplasmic sequence of invariant chain [29]. The internalized molecules reach acidic endosomes where the invariant chain is degraded and peptide loading takes place. Peptide-loaded MHC II is then recycled back to the PM for antigen presentation. Recently, it was shown that peptide-loaded MHC II enters cells by CIE. Peptide-loaded MHC II colocalizes with Arf6 on endosomes, accumulates in Arf6 Q67L vacuoles and has the same trafficking itinerary as MHCI [22]. Thus, the cell employs two different internalization mechanisms to regulate the trafficking of the same molecule in different states. This can provide the cell with an efficient way of modulating immunological responses by controlling the amount of peptide-loaded MHCII on the surface.
In addition to MHCI, all nucleated cells have cell surface proteins that are anchored to the membrane through a GPI moiety. These GPI-APs are enriched in cholesterol and glycolipid-rich domains of the PM and enter cells by CIE [14]. Among the surface-expressed GPI-APs are CD55 and CD59, also known as Decay Acceleration Factor and Protectin, respectively [14, 24]. CD55 and CD59 protect host cells from the action of the complement by binding to different subunits of the complement complex, preventing its assembly into an osmolytic pore on the PM of host cells. Loss in cellular capacity to synthesize GPI-AP in erythrocytes and lymphocytes results in a loss of CD55 and CD59 at the cell surface and is associated with autoimmune hemolytic anemia and thrombosis [30]. In addition to protecting from complement, CD59 plays a role in T-cell activation [31]. CD55 serves as a receptor for some viruses and other viruses integrate CD59 into their envelopes to avoid destruction by complement [32, 33]. Another GPI-AP is the folate receptor (FBP), shown to enter cells via the CLIC/GEEC pathway [6] and is thought to be involved in the cellular uptake of folate although the exact mechanism of transfer of folate into the cytoplasm is unclear [34].
ION AND NUTRIENT TRANSPORT
All cells in living organisms use ion channels to modulate the movement of ions across membranes. A number of ion channels use CIE as their mode of internalization and they recycle back to the PM in an Arf6 and Rab22-depedent manner. One example is Mucolipin 2, one of three mucolipins that belong to the superfamily of transient receptor potential (TRP) calcium channels. Mucolipin 2 travels along the Arf6-associated CIE pathway, meeting with MHCI and CD59 at the PM and in tubular recycling endosomes in HeLa cells [35]. Mucolipin 2 overexpression stimulates the activation of Arf6, while depletion of Mucolipin 2 or expression of a mutant inactive form of Mucolipin 2 decreased the recycling of CD59 back to the surface [35]. These studies revealed a novel function for Mucolipin 2, in the regulation of Arf6-dependent recycling of CIE cargo proteins.
Another ion channel associated with CIE endosomes is Kir3.4, a member of the G-protein-activated inwardly rectifying potassium channel family. These potassium channels allow the flow of potassium ions from the outside to the interior of the cells. The currents established by homomeric Kir3.4 complexes are required for keeping energy homeostasis, maintaining membrane excitability in neurons and regulating insulin release. Mutations on the Kir3.4 gene have been found to alter normal heart rate in congenital long QT syndrome patients due to defects in the trafficking of the channel [36]. Kir3.4 channel proteins can be observed in Arf6 Q67L vacuolar endosomes and localize with other CIE cargo molecules on tubular recycling endosomes [37]. Gong et al. identified a class of acidic cluster trafficking motifs in the cytoplasmic sequence of Kir3.4 responsible for its PM localization. Potentially, these motifs could mediate the endosomal sorting of Kir3.4 along the CIE pathway.
Cells need a constant supply of nutrients for growth, proliferation and survival. To overcome the PM barrier, cells use transporters to transport nutrients (glucose, lipids, vitamins and amino acids) across the membrane. In addition, there are receptors for nutrients, such as the transferrin and LDL receptors. Nutrient transporters reside and function at the PM and for the most part are endocytosed by CIE. Nutrient receptors for transferrin and LDL, on the other hand, are mostly internalized by CME.
GLUT1 belongs to the GLUT family of facilitative glucose transporters. Specifically, GLUT1 is a member of the class I subfamily of glucose transporters and is part of the solute-linked carrier gene family SLC2. The expression of GLUT1 is upregulated in cancer cells, which clearly correlates with the increased demand of nutrients that cancer cells have to maintain their highly proliferative state [38]. Since cancer cells rely mainly on anaerobic respiration for energy production, a rather inefficient pathway to produce energy when compared to aerobic respiration, higher levels of GLUT1 on the membrane is a very efficient way of increasing the levels of intracellular glucose. GLUT1 has the important role of maintaining the supply of glucose available for metabolism in the brain [39]. It is responsible for the transport of D-glucose across the blood-brain barrier. In humans, mutations in the GLUT1 gene are the cause of the glucose transporter type 1 deficiency syndrome (GLUT1-DS), an autosomal-dominant neurological disorder characterized by delayed development, ataxia, spasticity and other neurological defects [40].
LAT1 (SLC7A5) transports neutral bulky hydrophobic amino acids, such as phenylalanine, leucine, tryptophan and histidine, across cell membranes. This transporter is found at the surface of most cells in a complex with CD98 [41] and was identified as a CIE cargo protein [24]. LAT1 is the amino acid catalytic subunit, whereas CD98 function is required for proper assembly of the transport complex and delivery to the PM [42]. CD98 associates with other proteins at the PM but how these interactions modulate the function of the transporter complex remain to be elucidated. Similar to GLUT1, LAT1 is also a major transporter of nutrients across the blood-brain barrier and highly expressed in tumor cells. Uncovering the machinery and regulatory mechanisms required for LAT1 trafficking could provide valuable information for the potential use of LAT1 for drug delivery into the brain [43]. Finally, a recent study identified a critical role for Lat1 in the metabolic upregulation in activated T cells [44].
The lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) plays a pivotal role in the internalization of oxidized low-protein lipoprotein (OxLDL). This nutrient receptor is a member of the class E of scavenger receptors and is expressed in endothelial cells, macrophages, and platelets [45]. It also serves as a receptor for other modified lipoproteins, bacteria, fibronectin and phospholipids [46]. In recent years, the internalization of OxLDL by LOX-1 has been recognized as one of the major causes of vascular dysfunction and atherosclerosis. Therefore, understanding the function and elucidating the mechanism of regulation that control LOX-1 trafficking would reveal valuable information to design effective therapeutics to prevent vascular disease.
Using fluorescent-labeled OxLDL and multiple mutants of LOX-1, Murphy and colleagues demonstrated that LOX-1 internalizes through CIE [47], distinguishing it from the LDL receptor (LDLR), which enters by CME. Interestingly, mutation of residues DDL to AAA in the cytoplasmic domain blocks the internalization of LOX-1. The authors identified this acidic tri-peptide motif as critical for constitutive internalization of the LOX-1 receptor. After internalization, most of the OxLDL dissociates from the LOX-1 receptor. OxLDL traffics to late endosomes where it is degraded and LOX-1 presumably recycles back to the PM. Further studies are needed to elucidate the trafficking itinerary of LOX-1 and the impact of Rab proteins and Arf6 on it. As an interesting contrast to LOX-1, which enters by CIE, the LDLR normally enters cells by CME, however, in cells expressing IDOL, an E3 ligase that targets LDLR, the ubiquitinylated LDLR now enters cells via CIE and is routed to lysosomes for degradation [48].
GROWTH FACTOR AND G PROTEIN-COUPLED RECEPTORS - PATHWAY SWITCHING
Signaling receptors also may provide examples of PM proteins that alternate between CIE and CME, depending upon their state of activation. A number of G-protein coupled receptors (GPCRs) such as the β2-adrenergic receptor, M3-muscarinic receptor, and metabotropic glutamate receptor 7 (mGluR7) enter cells via CIE constitutively and then switch to CME upon ligand stimulation [49, 50]. An interesting, if controversial case of pathway switching, is the observations that the epidermal growth factor receptor (EGFR) is internalized by CME if a low concentration of EGF is present, while at high EGF concentrations, CIE is used to endocytose the EGFR [51]. This study also showed that when CIE was utilized, EGFR was routed to degradation [51], similar to what was observed for ubiquitinylated LDLR described above. In another example of a cell surface receptor using both pathways, the hepatocyte growth factor receptor Met upon activation has been shown to enter cells either by CIE or CME but the recycling of the receptor back to the cell surface is dependent upon activation of Arf6 [52], a requirement which is typically associated with recycling of CIE cargo proteins.
PROTEINS THAT FUNCTION IN CELL ADHESION
Intracellular adhesion molecule 1 (ICAM1, CD54) is a type-I glycoprotein of the immunoglobulin superfamily. This integrin-like protein is widely expressed but on endothelial cells and immune cells is involved in CD8+ T-cell activation, trans-endothelial migration of leukocytes, cell-cell adhesion, and endothelial cell activation [53]. ICAM1 is a ligand for lymphocyte function-associated antigen-1 (LFA-1) on leukocytes [54], as well as macrophage adhesion ligand-1 (Mac-1) on macrophages [55]. Studies of ICAM1 and platelet-endothelial cell adhesion molecule (PECAM1) in endothelial cells showed that they entered cells by CIE [56]. Interestingly, clustering of the CAM proteins was required for efficient endocytosis, as non-clustering anti-CAM antibodies did not internalize. ICAM1 is the surface receptor for rhinovirus, the major cause of the common cold [57, 58] and for poliovirus [59].
Epithelial cadherin (E-Cadherin, LCAM) is a calcium-dependent cell adhesion protein whose downregulation or dysfunction is a common feature of many invasive cancers. A type-I membrane glycoprotein, E-Cadherin traffics similar to MHCI in that it internalizes by a clathrin-independent mechanism, initially into transferrin-negative vesicles, but then traffics to early endosomes where it mixes with components of the CME pathway. Sustained Arf6 activity can further trap E-cadherin in large peripheral vacuoles devoid of transferrin [60]. E-cadherin binds p120 catenin at cell-cell junctions through its juxtamembrane domain, and through p120 catenin is complexed with microtubules. This complex suppresses E-cadherin endocytosis (reviewed in [61]).
Syndecan family proteins are abundant cell surface heparan sulfate proteoglycans (HSPG). Four type 1 membrane proteins belong to the syndecan family (syndecan-1, -2, -3, and -4) and act as receptors for the extracellular matrix, coreceptors for growth factors, mediators of cell-cell adhesion, and regulators of cell proliferation and migration [62]. Syndecan-1 (CD138) mediates apoE-VLDL uptake in human fibroblasts in a lipoprotein receptor-related protein (LPR)-independent manner, with endocytosis of syndecan-1 occuring via a clathrin-independent pathway [63]. Syndecan-1 clusters in detergent-insoluble rafts at the plasma membrane that are required for endocytosis [64]. Sydecan-1 appears to enter cells via Arf6-associated CIE as it becomes trapped in Arf6Q67L-induced vacuoles, losing the ability to recycle back to the plasma membrane [65]. Syndecans also influence the trafficking of integrins and a recent study showed how the phosphorylation state of Syndecan-4 regulates Arf6 activation and determines the recycling of different integrin complexes back to the PM [66]. Syndecans serve as receptors for herpes simplex virus, human papillomavirus, human immunodeficiency virus type-1, human T-lymphotropic virus type 1 [67], and for the attachment of hepatitis C virus to the surface of hepatocytes [68].
Integrins are type 1 integral membrane receptors involved in regulating cell shape, motility, adhesion to the extracellular matrix, and signaling. Integrins exist as heterodimers, composed of α and β subunits. Mammalian cells encode 18 α and 8 β subunit proteins, which pair in various combinations giving the heterodimers specificities for different matrix substances [69]. In epithelial cells, such as HeLa cells, β1 is a predominant integrin that pairs with multiple α subunits, and thus it is often studied as an integrin representative. β1-integrin enters cells via clathrin-independent endocytosis [7], and recycling back to the cell surface is dependent upon Arf6 [16] and the phosphorylation state of Syndecan-4 as mentioned above [66]. β1-integrin was also identified as being cargo associated with CLIC-GEEC pathway of CIE [4], suggesting that this endosomal system and mode of uptake is related to the Arf6-associate CIE pathway described here. Cellular integrins may also serve as entry receptors for human cytomegalovirus [70].
CD44 is a type-I membrane glycoprotein involved in matrix adhesion, organ development, neuronal axon guidance, lymphocyte activation, and lymph node homing. Encoded by a 20-exon gene, multiple CD44 isoforms are produced due to alternative splicing. CD44 is the primary cell surface receptor for hyaluronan [71], a major component of the extracellular matrix (ECM) especially of soft connective tissues, and facilitates the uptake and intracellular degradation of this glycosaminoglycan [72, 73]. The hyaluronic acid polysaccharide on the surface of Streptococcus pyogenes binds to CD44 on epithelial cells [74]. CD44 can also interact with other ligands besides hyaluronan, such as collagen, fibronectin, laminin, chondroitin sulfate, osteopontin, and matrix metalloproteinases [72]. CD44 is expressed on a wide variety of cell types, at low levels, but is absent on lymphatic vasculature [75]. CD44 can help regulate the activity of several growth factors such as fibroblast growth factor 2 and hepatocyte growth factor, by inhibiting their degradation [76, 77]. The small C-terminal cytoplasmic tail of CD44 associates with cytoskeletal organizing and signaling proteins including ankyrin, merlin, and ezrin-radixin-moesin proteins [78]. CD44 is palmitoylated on Cys286 and Cys295, a modification central to its ability to segregate into lipid rafts on the PM [79]. CD44 was also identified on CLIC-GEEC endosomes [4]. These acylation modifications of CD44 are required for both CD44 internalization, and concomitant hyaluronan cellular uptake.
CD98 (SLC3A2, 4F2HC, and CD98HC) is a type-II integral membrane glycoprotein that functions in amino acid transport, adhesion, and cell fusion at the PM [80]. Expressed in all cells except platelets, CD98 is known to covalently heterodimerize with one of several light chains (primarily LAT-1 or LAT-2) to facilitate amino acid transport primarily of large, neutral amino acids, reviewed in [81]. In intestinal epithelial Caco2-BBE cells, CD98/LAT-2 heterodimers were shown to interact basolaterally with ICAM-1, coupling signals from CD98 and ICAM-1 ligand binding to regulation of the transport activity of the LAT-2 light chain [82].
In addition to its role in amino acid transport, CD98 also can bind β1- and β3-integrins, and influence integrin trafficking and signaling [83]. Through its effects on integrin function, CD98 has been shown to participate in fibronectin matrix assembly, a process critical for development, wound healing, and tumorigenesis [84]; regulate keratinocyte adhesion and differentiation [85]; and stimulate adhesion of breast cancer cells to laminin [86]. CD98 also plays a role in controlling immune responses in the gut, one of the sites of highest CD98 expression in the body [87]. High surface expression of CD98 has been observed on proliferative normal tissue [88], as well as on the surface of tumor cells irrespective of the tissue of origin [89, 90]. Extracellular galectin-3, a secreted lectin, was demonstrated to bind the extracellular domain of CD98 and has been implicated in the fusion activity of CD98, with the galectin-3 inhibitor, lactose, reducing CD98 mediated cell fusion [91]. CD98 was also identified as cargo associated with CLIC-GEEC endosomes [4]. Finally, the endocytic entry of vaccinia virus into cells requires CD98 [92].
CD147 (Basigin, EMMPRIN) is a Type-1 integral membrane glycoprotein belonging to the immunoglobulin superfamily that is involved in tissue remodeling (via induction of matrix metalloproteinase), spermatogenesis, cell surface expression of monocarboxylate transporters, and thymocyte cycling [93]. CD147 can self-associate into homo-oligomers, which increases its avidity for substrates on the PM, or can interact with caveolin-1 in lipid raft areas, which inhibits its oligomerization capacity [94]. CD147 can interact with α3β1 and α6β1 integrins to affect cellular adhesion and spreading [95] and with Shrew-1, the E-cadherin binding protein on the basolateral surface of epithelial cells [96]. CD147 associates with the lactate transporters MCT1 and MCT4 and helps regulate their cell surface levels [97]. CD147 is a receptor for the extracellular inflammatory mediators cyclophilins A and B and binds to cyclophilin A on the surface of HIV-1 virions and stimulates entry of HIV into host cells [98]. Finally, CD147 serves as a receptor for Plasmodium falciparum, the parasite that causes malaria, and is required for its cellular entry into erythrocytes [99].
Although all of the above proteins have been shown to enter cells by CIE, we do not have much information on the post-endocytic trafficking of most of these cargo proteins. For a subset of these proteins, however, we do have some understanding as to their intracellular itinerary and have begun to identify amino acid sequences in these proteins that determine their sorting and to understand the cellular machinery involved in their trafficking.
Signals for Cargo Sorting and their Recognition
Sorting signals in the cytoplasmic tail of transmembrane proteins mediate routing of these proteins along endocytic trafficking pathways. Sorting signals traditionally consist of short peptide sequences of four to seven amino acid residues [100] that are recognized by the sorting machinery to direct cargo proteins to their final destination. A great deal is known about the sorting signals and interacting partners implicated in intracellular trafficking along CME [100]. In contrast, little is known about the sorting machinery that interacts with CIE cargo proteins to guide their trafficking to the intended compartments [15]. Although cytosolic sequences of CIE cargo proteins do not contain known sorting or trafficking motifs, the selective segregation of CIE cargo at the sorting endosome [24] suggests otherwise.
Recently, we identified sorting determinants in the cytoplasmic sequence of CD147 [101]. We found two acidic clusters in the cytoplasmic sequence that are conserved in CD147 from other vertebrates and are critical for specifically preventing the trafficking to EEA1- and late- endosomes. Deletion of the acidic residues containing region or site-directed mutagenesis of the acidic residues was sufficient to divert the trafficking of CD147 from the recycling route to the degradation route of the pathway. Furthermore, di-acidic clusters are also present in the cytoplasmic sequence of CD44 and CD98, which exhibit the same trafficking itinerary as CD147 (Figure 2) taking a direct route to recycling back to the cell surface. Interestingly, those cargo proteins that take the default route of the pathway to compartments associated with EEA1 and on to late endosomes and lysosomes (MHCI, Tac and GPI-APs) lack di-acidic or acidic cluster residues in their cytoplasmic regions. There have been reports of acidic residues playing a role in the trafficking of other CIE cargo proteins. Carboxyl-terminal acidic cluster residues, defined as the KAC motif, were reported to be important for maintaining surface levels of the rectifying K channel, Kir3.4 [37]. Moreover, Gong et al. observed that the Kir3.4 mutant lacking the KAC motif did not localize to the recycling tubular compartment but instead accumulated in internal structures. Since the acidic residues were essential for efficient recycling of the Kir3.4 channel similar to what we have observed for CD147, it is likely that this channel follows the same trafficking itinerary as CD44, CD98 and CD147. Interestingly, mutation of a conserved tri-peptide motif containing two acidic residues di-acidic motif (DDL) in the cytoplasmic sequence of the LOX-1 receptor prevents its internalization into HeLa cells. This finding suggests that in addition to serving as a sorting determinant, acidic residues may also play a role as internalization signals for CIE cargo [47]. Given that there is so much diversity in the cytoplasmic sequences of CIE cargo proteins, the idea of using particular arrangements of acidic residues to mediate the sorting of proteins gives the system the flexibility of handling multiple cargos without affecting specificity.
Figure 2.
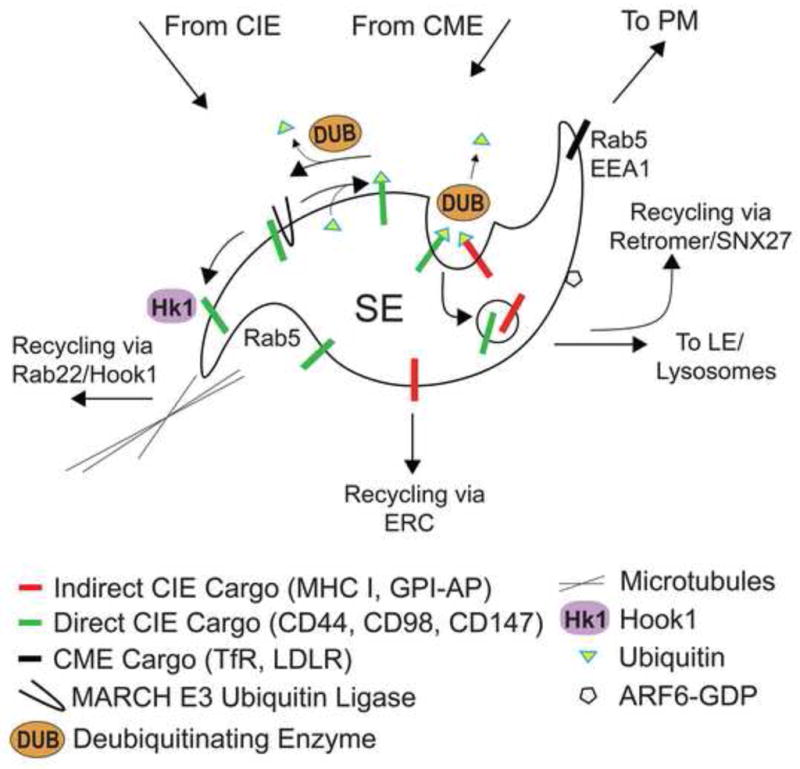
Factors influencing the sorting and trafficking of cargo on sorting endosomes. A closer look at the sorting events that occur on the sorting endosome (SE). Cargo that is ubiquitinylated (green triangle) by MARCH or other E3 ligases gets sorted into invaginating membrane forming intralumenal vesicles or multi-vesicular bodies (MVB). Deubiquitinating enzymes (DUBS) remove ubiquitin allowing cargo to escape degradation. Hook1, Rab22 and microtubules facilitate the sorting of Direct CIE cargo (blue bars) into recycling tubules. Indirect CIE cargo (red bars) is routed back to PM via ERC and tubules.
Additional motifs or domains could potentially be involved in the internalization or sorting of CIE cargo proteins either by themselves or working in coordination with acidic residues. CD44, Glut1 and the syndecans all harbor a PDZ-ligand sequence at their cytoplasmic carboxyl termini [65, 102]. The interaction between the PDZ-ligand domain in syndecan-2 and the PDZ domain-containing protein syntenin is required for the recycling of syndecan-2 back to the PM. Detailed examination of post-endocytic trafficking of CIE cargo proteins and their cytoplasmic sequence could lead to the discovery of new signals and sorting complexes devoted to CIE cargo trafficking.
In an attempt to discover cytoplasmic proteins that recognize CIE cargo proteins that take the direct tubular route back to the PM, we identified the tethering protein Hook1 [101]. Hook1 specifically binds to the cytoplasmic sequence of CD147 and CD98 through its carboxyl-terminal domain. The members of the Hook protein family function as tethering factors that connect cargo and membranes to microtubules [103]. The binding of Hook1 to the cargo is independent of its interaction with the microtubules. In the presence of the microtubule depolymerizing drug nocodazole, Hook1 and the cargo localize to the same intracellular structures and the recycling tubular endosomes disappear. Expression of a dominant negative mutant of Hook1, consisting of the carboxyl-terminal sequence of Hook1, prevents the binding of full length Hook1 to the cargo and alters the trafficking of the cargo to the Rab5/EEA1 compartment with a loss of the recycling tubular endosomes. Moreover, siRNA depletion of Hook1 specifically inhibits the recycling of CD98 but not of MHCI or TfR [101]. Our work demonstrates that Hook1 participates in the differential sorting of specific CIE cargo (CD44, CD98 and CD147) that need to be directly recycled back to the PM (Figure 2), presumably due to their role in cell spreading, migration, and interactions with the extracellular matrix.
GPI-APs represent another subset of CIE cargo proteins for which there appear to be distinct mechanisms for sorting and trafficking of these raft-partitioning proteins. CD55 and CD59 are two GPI-APs that colocalize on endosomal tubules with EHD1 and MICAL-L1; trafficking of these GPI-AP to the juxtanuclear recycling compartment, and into tubules appears to involve the concerted effort of these regulators plus the action of a phospholipase A2 to vesiculate the tubules [104,105]. In addition, myosin 1C associates with lipid rafts and is required for the trafficking of CD55 and CD59 into recycling tubules [106].
Protein post-translational modifications are commonly used to sort cargo proteins during their intracellular trafficking. Among those, ubiquitination of integral membrane proteins has been extensively characterized as a signal for sorting into multivesicular bodies (MVBs) and lysosomes for degradation [107,108]. The covalent addition of ubiquitin to lysine residues on the target protein is mediated by E3 ubiquitin ligases [109]. E3 ubiquitin ligases of the RING-CH (MARCH) family have been shown to downregulate the surface levels of a number of CIE cargo proteins [25]. Specifically, overexpression of MARCH8 redirects CD44 and CD98 to EEA1-associated endosomes and lysosomes, where the cargo is degraded (Figure 2). Normally, CD44 and CD98 begin to accumulate in late endosomes after 24 h of internalization. Remarkably, in the presence of MARCH proteins these CIE cargo proteins accumulate in late endosomes just 2 h post-endocytosis [25]. The site of ubiquitination of CIE cargo proteins by MARCH proteins is not clear. However, the fact that MARCH proteins are observed in the different compartments along the CIE pathway with the cargo suggests that MARCH proteins could potentially ubiquitinate the cargo at the PM or on endosomes. Presumably, the ubiquitination of CD98 abrogates the recognition of the acidic residues in CD98 by Hook1 protein thus leading to its routing to late endosomes and lysosomes for degradation (Figure 2).
Although MARCH E3 ligases can (upon overexpression) alter the half-life and traffic of CIE cargo proteins, we do not know which endogenous E3 ligases are responsible for the downregulation of these proteins normally and how this is regulated. Is there machinery that monitors protein quality control at the cell surface and how does it work? Also, it will be interesting to see whether ubiquitin-specific proteases or de-ubiquitinases (DUBs) might counter the effects of E3 ubiquitin ligases on the trafficking of CD44 and CD98 (Figure 2).
Regulators of Endosomal Sorting
Rab proteins recruit additional factors to endosomal membranes to specify the identity of membrane compartments and facilitate transport from one compartment to the next [110]. Rabs facilitate trafficking through their indirect interaction with coat components, motors and tethering proteins [110]. The communication between Rabs and their effectors is important to preserve the specificity of fusion events, scission events and attachment of motors to vesicles [110]. Given that Rab proteins participate in different steps along the CIE pathway [15], it is possible that CIE may use this machinery to establish unique domains on endosomal membranes where the cargo is delivered [111]. Our group demonstrated that Rab22a regulates the recycling of CIE cargo by controlling the formation of the tubular membranes and final fusion of the recycling membranes with the PM [23]. Expression of the GTP-binding-defective mutant form of Rab22a (Rab22a S19N) inhibits the recycling of CIE cargo. Interestingly, the dominant negative effect of Rab22 S19N can be rescued by overexpression of Hook1 [101]. Reciprocally, expression of Rab22a restores sorting of CIE cargo in Hook1 dominant negative expressing cells, suggesting that Hook1 and Rab22a work in coordination to mediate the recycling of CIE cargo proteins [101]. Activation of Rab11 is also required for MHCI recycling but not for the formation of the recycling tubular endosomes [23]. These observations suggested that both Rab22 and Rab11 work to control the recycling of CIE cargo proteins in the recycling tubular endosomes.
Rab5 regulates the fusion of endocytic vesicles with early endosomes and mediates the transport of early endosomes along microtubules [110]. Prototypical CIE cargo proteins (MHCI, Tac, CD59) meet with CME cargo proteins in Rab5/EEA1 early endosomes [8,14]. Although CD44, CD98 and CD147 do not reach the Rab5/EEA1 endosomes, they do reach Rab5-associated endosomes (Figure 1) [24]. It has been reported that Rab5 is associated with different types of sorting endosomes some of which lack EEA1 and instead are associated with the Rab5 effector APPL1 [112]. Based on these observations, we predict that the segregation of CIE cargo takes place in Rab5-associated endosomal membranes before the recruitment of EEA1 or at a separate membrane domain in the sorting endosomes that does not contain EEA1 (Figure 2). A study by Chen at al. suggested that different Rab5 isoforms might have distinct roles in endosomal recycling [113]. Therefore, it is possible that Rab5 and other endosomal Rabs may coordinate the segregation of the distinct CIE cargo proteins and promote the cross talk between CIE and CME pathways. The presence of distinct Rab proteins in different CIE compartments along the pathway and the recruitment of specific effectors to these membranes in a spatially and temporally regulated manner could determine the distribution and final destination of specific CIE cargo proteins [110, 111, 113]. This may be accomplished through the recruitment of specific sorting machinery to distinct sites in the pathway.
A recent study has uncovered a major role for the sorting nexin 27 (SNX27) and the retromer complex in the recycling of a wide variety of PM proteins [114]. Knockdown of either SNX27 or components of the retromer complex led to depletion of a number of CIE cargo proteins from the cell surface by routing these proteins to lysosomes instead of recycling. These included Glut1, CD147, MCT1, ICAM1 and various ion and amino acid transporters. Some of these cargo proteins (for example, Glut1) contain PDZ ligands that could interact with SNX27. Perhaps the most surprising finding is the role for the retromer complex, thought to be involved in trafficking from endosomes to the trans Golgi network, in recycling of internalized proteins back to the cell surface [114]. These findings open up new approaches to understanding how endosomal proteins are rescued from degradation and recycled back to the PM.
Summary and Perspective
We set out in this review to highlight the variety of cargo proteins that enter cells by CIE. These proteins serve vital functions at the cell surface as nutrient transporters, ion channels, adhesion proteins, and proteins that engage the immune system. By internalizing and recycling these cargo proteins, CIE and subsequent trafficking may provide the cells with the ability to remodel the plasma membrane in response to developmental cues, intracellular signals or changes in the cellular microenvironment. CIE also provides a means to sample the PM proteome and assess, perhaps on endosomes, whether the protein should be degraded or recycled. CIE plays an important role in cell spreading and migration in that recycling of CIE cargo proteins (such as CD44, D98 and CD147) back to the cell surface is required for cell spreading. Interestingly, some viruses use CIE cargo molecules as receptors to mediate their own internalization and infection. Elucidating the mechanisms regulating CIE cargo internalization and intracellular trafficking will provide valuable insights into the understanding of fundamental cellular processes, such as cell migration, nutrient metabolism and immune defense.
Many questions remain unanswered with regard to the internalization and sorting steps during CIE. For instance, little is known about the machinery implicated in the internalization step of the CIE pathway. It is also not clear whether cargo is actively selected at the plasma membrane and/or released from retention at the PM in order to get internalized. The extent to which CIE and CME occurs in whole animals has not been carefully examined although initial studies using intravital microscopy suggest that CIE is robust in stromal fibroblasts [115]. Future studies examining the trafficking of a range of cargo proteins in different cell and whole animal systems will reveal new insights into understanding this mode of endocytosis.
Highlights.
- CIE mediates the entry of PM proteins with a wide variety of functions.
- CIE cargo proteins are sorted on endosomes.
- Recruitment of Rab proteins to endosomes regulates CIE cargo trafficking.
- CIE is fundamental for cell migration, nutrient metabolism and immune defense.
Acknowledgments
We wish to thank N. Cole and J. Caviston for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 2.Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10:583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- 3.Sandvig K, Torgersen ML, Raa HA, van Deurs B. Clathrin-independent endocytosis: from nonexisting to an extreme degree of complexity. Histochem Cell Biol. 2008;129:267–276. doi: 10.1007/s00418-007-0376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howes MT, Kirkham M, Riches J, Cortese K, Walser PJ, Simpson F, Hill MM, Jones A, Lundmark R, Lindsay MR, Hernandez-Deviez DJ, Hadzic G, McCluskey A, Bashir R, Liu L, Pilch P, McMahon H, Robinson PJ, Hancock JF, Mayor S, Parton RG. Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. The Journal of cell biology. 2010;190:675–691. doi: 10.1083/jcb.201002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumari S, Mayor S. ARF1 is directly involved in dynamin-independent endocytosis. Nat Cell Biol. 2008;10:30–41. doi: 10.1038/ncb1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002;2:411–423. doi: 10.1016/s1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 7.Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J Cell Biol. 2001;154:1007–1017. doi: 10.1083/jcb.200103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naslavsky N, Weigert R, Donaldson JG. Convergence of Non-clathrin- and Clathrin-derived Endosomes Involves Arf6 Inactivation and Changes in Phosphoinositides. Mol Biol Cell. 2003;14:417–431. doi: 10.1091/mbc.02-04-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol. 1997;139:49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamaze C, Dujeancourt A, Baba T, Lo CG, Benmerah A, Dautry-Varsat A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol Cell. 2001;7:661–671. doi: 10.1016/s1097-2765(01)00212-x. [DOI] [PubMed] [Google Scholar]
- 11.Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nature cell biology. 2006;8:46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- 12.Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nature reviews. Molecular cell biology. 2013;14:98–112. doi: 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- 13.Donaldson JG, Porat-Shliom N, Cohen LA. Clathrin-independent endocytosis: a unique platform for cell signaling and PM remodeling. Cell Signal. 2009;21:1–6. doi: 10.1016/j.cellsig.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naslavsky N, Weigert R, Donaldson JG. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol Biol Cell. 2004;15:3542–3552. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powelka AM, Sun J, Li J, Gao M, Shaw LM, Sonnenberg A, Hsu VW. Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic. 2004;5:20–36. doi: 10.1111/j.1600-0854.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- 17.Aikawa Y, Martin TF. ADP-ribosylation factor 6 regulation of phosphatidylinositol-4,5-bisphosphate synthesis, endocytosis, and exocytosis. Methods Enzymol. 2005;404:422–431. doi: 10.1016/S0076-6879(05)04037-1. [DOI] [PubMed] [Google Scholar]
- 18.Balklava Z, Pant S, Fares H, Grant BD. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat Cell Biol. 2007;9:1066–1073. doi: 10.1038/ncb1627. [DOI] [PubMed] [Google Scholar]
- 19.Naslavsky N, Rahajeng J, Sharma M, Jovic M, Caplan S. Interactions between EHD proteins and Rab11-FIP2: a role for EHD3 in early endosomal transport. Mol Biol Cell. 2006;17:163–177. doi: 10.1091/mbc.E05-05-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson SE, Setty SR, Sitaram A, Marks MS, Lewis RE, Chou MM. Extracellular signal-regulated kinase regulates clathrin-independent endosomal trafficking. Mol Biol Cell. 2006;17:645–657. doi: 10.1091/mbc.E05-07-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi A, Pant S, Balklava Z, Chen CC, Figueroa V, Grant BD. A novel requirement for C. elegans Alix/ALX-1 in RME-1-mediated membrane transport. Curr Biol. 2007;17:1913–1924. doi: 10.1016/j.cub.2007.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walseng E, Bakke O, Roche PA. MHC class II-peptide complexes internalize using a clathrin- and dynamin-independent endocytosis pathway. J Biol Chem. 2008;283:14717–14727. doi: 10.1074/jbc.M801070200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weigert R, Yeung AC, Li J, Donaldson JG. Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol Biol Cell. 2004;15:3758–3770. doi: 10.1091/mbc.E04-04-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eyster CA, Higginson JD, Huebner R, Porat-Shliom N, Weigert R, Wu WW, Shen RF, Donaldson JG. Discovery of New Cargo Proteins that Enter Cells through Clathrin-independent Endocytosis. Traffic. 2009;10:590–599. doi: 10.1111/j.1600-0854.2009.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eyster CA, Cole NB, Petersen S, Viswanathan K, Fruh K, Donaldson JG. MARCH ubiquitin ligases alter the itinerary of clathrin-independent cargo from recycling to degradation. Molecular biology of the cell. 2011;22:3218–3230. doi: 10.1091/mbc.E10-11-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barral DC, Cavallari M, McCormick PJ, Garg S, Magee AI, Bonifacino JS, De Libero G, Brenner MB. CD1a and MHC Class I Follow a Similar Endocytic Recycling Pathway. Traffic. 2008;9:1446–1457. doi: 10.1111/j.1600-0854.2008.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barral DC, Garg S, Casalou C, Watts GF, Sandoval JL, Ramalho JS, Hsu VW, Brenner MB. Arl13b regulates endocytic recycling traffic. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:21354–21359. doi: 10.1073/pnas.1218272110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odorizzi CG, Trowbridge IS, Xue L, Hopkins CR, Davis CD, Collawn JF. Sorting signals in the MHC class II invariant chain cytoplasmic tail and transmembrane region determine trafficking to an endocytic processing compartment. The Journal of cell biology. 1994;126:317–330. doi: 10.1083/jcb.126.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dugast M, Toussaint H, Dousset C, Benaroch P. AP2 clathrin adaptor complex, but not AP1, controls the access of the major histocompatibility complex (MHC) class II to endosomes. The Journal of biological chemistry. 2005;280:19656–19664. doi: 10.1074/jbc.M501357200. [DOI] [PubMed] [Google Scholar]
- 30.Risitano AM. Paroxysmal nocturnal hemoglobinuria and other complement-mediated hematological disorders. Immunobiology. 2012;217:1080–1087. doi: 10.1016/j.imbio.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Kimberley FC, Sivasankar B, Paul Morgan B. Alternative roles for CD59. Mol Immunol. 2007;44:73–81. doi: 10.1016/j.molimm.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Patel KP, Coyne CB, Bergelson JM. Dynamin- and lipid raft-dependent entry of decay-accelerating factor (DAF)-binding and non-DAF-binding coxsackieviruses into nonpolarized cells. Journal of virology. 2009;83:11064–11077. doi: 10.1128/JVI.01016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riento K, Frick M, Schafer I, Nichols BJ. Endocytosis of flotillin-1 and flotillin-2 is regulated by Fyn kinase. Journal of cell science. 2009;122:912–918. doi: 10.1242/jcs.039024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antony AC. The biological chemistry of folate receptors. Blood. 1992;79:2807–2820. [PubMed] [Google Scholar]
- 35.Karacsonyi C, Miguel AS, Puertollano R. Mucolipin-2 Localizes to the Arf6-Associated Pathway and Regulates Recycling of GPI-APs. Traffic. 2007;8:1404–1414. doi: 10.1111/j.1600-0854.2007.00619.x. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Liang B, Liu J, Li J, Grunnet M, Olesen SP, Rasmussen HB, Ellinor PT, Gao L, Lin X, Li L, Wang L, Xiao J, Liu Y, Zhang S, Liang D, Peng L, Jespersen T, Chen YH. Identification of a Kir3.4 mutation in congenital long QT syndrome. American journal of human genetics. 2010;86:872–880. doi: 10.1016/j.ajhg.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong Q, Weide M, Huntsman C, Xu Z, Jan LY, Ma D. Identification and characterization of a new class of trafficking motifs for controlling clathrin-independent internalization and recycling. J Biol Chem. 2007;282:13087–13097. doi: 10.1074/jbc.M700767200. [DOI] [PubMed] [Google Scholar]
- 38.Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121:29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Augustin R. The protein family of glucose transport facilitators: It’s not only about glucose after all. IUBMB Life. 2010;62:315–333. doi: 10.1002/iub.315. [DOI] [PubMed] [Google Scholar]
- 40.Brockmann K. The expanding phenotype of GLUT1-deficiency syndrome. Brain Dev. 2009;31:545–552. doi: 10.1016/j.braindev.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) The Journal of biological chemistry. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 42.Mastroberardino L, Spindler B, Pfeiffer R, Skelly PJ, Loffing J, Shoemaker CB, Verrey F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288–291. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- 43.Rautio J, Gynther M, Laine K. LAT1-mediated prodrug uptake: a way to breach the blood-brain barrier? Ther Deliv. 2013;4:281–284. doi: 10.4155/tde.12.165. [DOI] [PubMed] [Google Scholar]
- 44.Sinclair LV, Rolf J, Emslie E, Shi YB, Taylor PM, Cantrell DA. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol. 2013;14:500–508. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Twigg MW, Freestone K, Homer-Vanniasinkam S, Ponnambalam S. The LOX-1 Scavenger Receptor and Its Implications in the Treatment of Vascular Disease. Cardiol Res Pract. 2012;2012:632408. doi: 10.1155/2012/632408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshimoto R, Fujita Y, Kakino A, Iwamoto S, Takaya T, Sawamura T. The discovery of LOX-1, its ligands and clinical significance. Cardiovasc Drugs Ther. 2011;25:379–391. doi: 10.1007/s10557-011-6324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy JE, Vohra RS, Dunn S, Holloway ZG, Monaco AP, Homer-Vanniasinkam S, Walker JH, Ponnambalam S. Oxidised LDL internalisation by the LOX-1 scavenger receptor is dependent on a novel cytoplasmic motif and is regulated by dynamin-2. Journal of cell science. 2008;121:2136–2147. doi: 10.1242/jcs.020917. [DOI] [PubMed] [Google Scholar]
- 48.Scotti E, Calamai M, Goulbourne CN, Zhang L, Hong C, Lin RR, Choi J, Pilch PF, Fong LG, Zou P, Ting AY, Pavone FS, Young SG, Tontonoz P. IDOL stimulates clathrin-independent endocytosis and multivesicular body-mediated lysosomal degradation of the low-density lipoprotein receptor. Molecular and cellular biology. 2013;33:1503–1514. doi: 10.1128/MCB.01716-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lavezzari G, Roche KW. Constitutive endocytosis of the metabotropic glutamate receptor mGluR7 is clathrin-independent. Neuropharmacology. 2007;52:100–107. doi: 10.1016/j.neuropharm.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 50.Scarselli M, Donaldson JG. Constitutive internalization of G protein-coupled receptors and G proteins via clathrin-independent endocytosis. J Biol Chem. 2009;284:3577–3585. doi: 10.1074/jbc.M806819200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell. 2008;15:209–219. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 52.Parachoniak CA, Luo Y, Abella JV, Keen JH, Park M. GGA3 functions as a switch to promote Met receptor recycling, essential for sustained ERK and cell migration. Developmental cell. 2011;20:751–763. doi: 10.1016/j.devcel.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Long EO. ICAM-1: getting a grip on leukocyte adhesion. J Immunol. 2011;186:5021–5023. doi: 10.4049/jimmunol.1100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51:813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 55.Smith CW, Marlin SD, Rothlein R, Toman C, Anderson DC. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989;83:2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. Journal of cell science. 2003;116:1599–1609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 57.Greve JM, Davis G, Meyer AM, Forte CP, Yost SC, Marlor CW, Kamarck ME, McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56:839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- 58.Staunton DE, Merluzzi VJ, Rothlein R, Barton R, Marlin SD, Springer TA. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989;56:849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- 59.Selinka HC, Zibert A, Wimmer E. Poliovirus can enter and infect mammalian cells by way of an intercellular adhesion molecule 1 pathway. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:3598–3602. doi: 10.1073/pnas.88.9.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paterson AD, Parton RG, Ferguson C, Stow JL, Yap AS. Characterization of E-cadherin Endocytosis in Isolated MCF-7 and Chinese Hamster Ovary Cells: THE INITIAL FATE OF UNBOUND E-CADHERIN. J Biol Chem. 2003;278:21050–21057. doi: 10.1074/jbc.M300082200. [DOI] [PubMed] [Google Scholar]
- 61.Kourtidis A, Ngok SP, Anastasiadis PZ. p120 catenin: an essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression. Prog Mol Biol Transl Sci. 2013;116:409–432. doi: 10.1016/B978-0-12-394311-8.00018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alexopoulou AN, Multhaupt HA, Couchman JR. Syndecans in wound healing, inflammation and vascular biology. Int J Biochem Cell Biol. 2007;39:505–528. doi: 10.1016/j.biocel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 63.Wilsie LC, Gonzales AM, Orlando RA. Syndecan-1 mediates internalization of apoE-VLDL through a low density lipoprotein receptor-related protein (LRP)-independent non-clathrin-mediated pathway. Lipids Health Dis. 2006;5:23. doi: 10.1186/1476-511X-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuki IV, Meyer ME, Williams KJ. Transmembrane and cytoplasmic domains of syndecan mediate a multi-step endocytic pathway involving detergent-insoluble membrane rafts. Biochem J. 2000;351(Pt 3):607–612. [PMC free article] [PubMed] [Google Scholar]
- 65.Zimmermann P, Zhang Z, Degeest G, Mortier E, Leenaerts I, Coomans C, Schulz J, N’Kuli F, Courtoy PJ, David G. Syndecan recycling is controlled by syntenin-PIP2 interaction and Arf6. Dev Cell. 2005;9:377–388. doi: 10.1016/j.devcel.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 66.Morgan MR, Hamidi H, Bass MD, Warwood S, Ballestrem C, Humphries MJ. Syndecan-4 phosphorylation is a control point for integrin recycling. Developmental cell. 2013;24:472–485. doi: 10.1016/j.devcel.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bobardt MD, Chatterji U, Schaffer L, de Witte L, Gallay PA. Syndecan-Fc hybrid molecule as a potent in vitro microbicidal anti-HIV-1 agent. Antimicrobial agents and chemotherapy. 2010;54:2753–2766. doi: 10.1128/AAC.01606-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi Q, Jiang J, Luo G. Syndecan-1 serves as the major receptor for attachment of hepatitis C virus to the surfaces of hepatocytes. Journal of virology. 2013;87:6866–6875. doi: 10.1128/JVI.03475-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 70.Feire AL, Koss H, Compton T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15470–15475. doi: 10.1073/pnas.0406821101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 72.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nature reviews. Molecular cell biology. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 73.Toole BP. Hyaluronan-CD44 Interactions in Cancer: Paradoxes and Possibilities. Clin Cancer Res. 2009;15:7462–7468. doi: 10.1158/1078-0432.CCR-09-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schrager HM, Alberti S, Cywes C, Dougherty GJ, Wessels MR. Hyaluronic acid capsule modulates M protein-mediated adherence and acts as a ligand for attachment of group A Streptococcus to CD44 on human keratinocytes. J Clin Invest. 1998;101:1708–1716. doi: 10.1172/JCI2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mackay CR, Terpe HJ, Stauder R, Marston WL, Stark H, Gunthert U. Expression and modulation of CD44 variant isoforms in humans. The Journal of cell biology. 1994;124:71–82. doi: 10.1083/jcb.124.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jones M, Tussey L, Athanasou N, Jackson DG. Heparan sulfate proteoglycan isoforms of the CD44 hyaluronan receptor induced in human inflammatory macrophages can function as paracrine regulators of fibroblast growth factor action. The Journal of biological chemistry. 2000;275:7964–7974. doi: 10.1074/jbc.275.11.7964. [DOI] [PubMed] [Google Scholar]
- 77.Saksela O, Moscatelli D, Sommer A, Rifkin DB. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. The Journal of cell biology. 1988;107:743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsukita S, Oishi K, Sato N, Sagara J, Kawai A. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. The Journal of cell biology. 1994;126:391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thankamony SP, Knudson W. Acylation of CD44 and its association with lipid rafts are required for receptor and hyaluronan endocytosis. The Journal of biological chemistry. 2006;281:34601–34609. doi: 10.1074/jbc.M601530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cantor JM, Ginsberg MH. CD98 at the crossroads of adaptive immunity and cancer. Journal of cell science. 2012;125:1373–1382. doi: 10.1242/jcs.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nguyen HT, Merlin D. Homeostatic and innate immune responses: role of the transmembrane glycoprotein CD98. Cell Mol Life Sci. 2012;69:3015–3026. doi: 10.1007/s00018-012-0963-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu X, Charrier L, Gewirtz A, Sitaraman S, Merlin D. CD98 and intracellular adhesion molecule I regulate the activity of amino acid transporter LAT-2 in polarized intestinal epithelia. The Journal of biological chemistry. 2003;278:23672–23677. doi: 10.1074/jbc.M302777200. [DOI] [PubMed] [Google Scholar]
- 83.Zent R, Fenczik CA, Calderwood DA, Liu S, Dellos M, Ginsberg MH. Class- and splice variant-specific association of CD98 with integrin beta cytoplasmic domains. The Journal of biological chemistry. 2000;275:5059–5064. doi: 10.1074/jbc.275.7.5059. [DOI] [PubMed] [Google Scholar]
- 84.Feral CC, Zijlstra A, Tkachenko E, Prager G, Gardel ML, Slepak M, Ginsberg MH. CD98hc (SLC3A2) participates in fibronectin matrix assembly by mediating integrin signaling. The Journal of cell biology. 2007;178:701–711. doi: 10.1083/jcb.200705090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lemaitre G, Stella A, Feteira J, Baldeschi C, Vaigot P, Martin MT, Monsarrat B, Waksman G. CD98hc (SLC3A2) is a key regulator of keratinocyte adhesion. J Dermatol Sci. 2011;61:169–179. doi: 10.1016/j.jdermsci.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 86.Chandrasekaran S, Guo NH, Rodrigues RG, Kaiser J, Roberts DD. Pro-adhesive and chemotactic activities of thrombospondin-1 for breast carcinoma cells are mediated by alpha3beta1 integrin and regulated by insulin-like growth factor-1 and CD98. The Journal of biological chemistry. 1999;274:11408–11416. doi: 10.1074/jbc.274.16.11408. [DOI] [PubMed] [Google Scholar]
- 87.Yan Y, Vasudevan S, Nguyen HT, Merlin D. Intestinal epithelial CD98: an oligomeric and multifunctional protein. Biochimica et biophysica acta. 2008;1780:1087–1092. doi: 10.1016/j.bbagen.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parmacek MS, Karpinski BA, Gottesdiener KM, Thompson CB, Leiden JM. Structure, expression and regulation of the murine 4F2 heavy chain. Nucleic Acids Res. 1989;17:1915–1931. doi: 10.1093/nar/17.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bellone G, Alloatti G, Levi R, Geuna M, Tetta C, Peruzzi L, Letarte M, Malavasi F. Identification of a new epitope of the 4F2/44D7 molecular complex present on sarcolemma and isolated cardiac fibers. Eur J Immunol. 1989;19:1–8. doi: 10.1002/eji.1830190102. [DOI] [PubMed] [Google Scholar]
- 90.Dixon WT, Sikora LK, Demetrick DJ, Jerry LM. Isolation and characterization of a heterodimeric surface antigen on human melanoma cells and evidence that it is the 4F2 cell activation/proliferation molecule. Int J Cancer. 1990;45:59–68. doi: 10.1002/ijc.2910450113. [DOI] [PubMed] [Google Scholar]
- 91.Dalton P, Christian HC, Redman CW, Sargent IL, Boyd CA. Membrane trafficking of CD98 and its ligand galectin 3 in BeWo cells--implication for placental cell fusion. Febs J. 2007;274:2715–2727. doi: 10.1111/j.1742-4658.2007.05806.x. [DOI] [PubMed] [Google Scholar]
- 92.Schroeder N, Chung CS, Chen CH, Liao CL, Chang W. The lipid raft-associated protein CD98 is required for vaccinia virus endocytosis. Journal of virology. 2012;86:4868–4882. doi: 10.1128/JVI.06610-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huet E, Gabison EE, Mourah S, Menashi S. Role of emmprin/CD147 in tissue remodeling. Connect Tissue Res. 2008;49:175–179. doi: 10.1080/03008200802151722. [DOI] [PubMed] [Google Scholar]
- 94.Tang W, Hemler ME. Caveolin-1 regulates matrix metalloproteinases-1 induction and CD147/EMMPRIN cell surface clustering. The Journal of biological chemistry. 2004;279:11112–11118. doi: 10.1074/jbc.M312947200. [DOI] [PubMed] [Google Scholar]
- 95.Berditchevski F, Chang S, Bodorova J, Hemler ME. Generation of monoclonal antibodies to integrin-associated proteins. Evidence that alpha3beta1 complexes with EMMPRIN/basigin/OX47/M6. The Journal of biological chemistry. 1997;272:29174–29180. doi: 10.1074/jbc.272.46.29174. [DOI] [PubMed] [Google Scholar]
- 96.Schreiner A, Ruonala M, Jakob V, Suthaus J, Boles E, Wouters F, Starzinski-Powitz A. Junction protein shrew-1 influences cell invasion and interacts with invasion-promoting protein CD147. Molecular biology of the cell. 2007;18:1272–1281. doi: 10.1091/mbc.E06-07-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. The EMBO journal. 2000;19:3896–3904. doi: 10.1093/emboj/19.15.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pushkarsky T, Zybarth G, Dubrovsky L, Yurchenko V, Tang H, Guo H, Toole B, Sherry B, Bukrinsky M. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6360–6365. doi: 10.1073/pnas.111583198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Crosnier C, Bustamante LY, Bartholdson SJ, Bei AK, Theron M, Uchikawa M, Mboup S, Ndir O, Kwiatkowski DP, Duraisingh MT, Rayner JC, Wright GJ. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480:534–537. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bonifacino JS, Lippincott-Schwartz J. Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol. 2003;4:409–414. doi: 10.1038/nrm1099. [DOI] [PubMed] [Google Scholar]
- 101.Maldonado-Baez L, Cole NB, Kramer H, Donaldson JG. Microtubule-dependent endosomal sorting of clathrin-independent cargo by Hook1. The Journal of cell biology. 2013;201:233–247. doi: 10.1083/jcb.201208172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thorne RF, Legg JW, Isacke CM. The role of the CD44 transmembrane and cytoplasmic domains in co-ordinating adhesive and signalling events. Journal of cell science. 2004;117:373–380. doi: 10.1242/jcs.00954. [DOI] [PubMed] [Google Scholar]
- 103.Walenta JH, Didier AJ, Liu X, Kramer H. The Golgi-associated hook3 protein is a member of a novel family of microtubule-binding proteins. The Journal of cell biology. 2001;152:923–934. doi: 10.1083/jcb.152.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cai B, Caplan S, Naslavsky N. cPLA2alpha, EHD1 interact, regulate the vesiculation of cholesterol-rich, GPI-anchored, protein-containing endosomes. Molecular biology of the cell. 2012;23:1874–1888. doi: 10.1091/mbc.E11-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cai B, Katafiasz D, Horejsi V, Naslavsky N. Pre-sorting endosomal transport of the GPI-anchored protein, CD59 is regulated by EHD1. Traffic. 2011;12:102–120. doi: 10.1111/j.1600-0854.2010.01135.x. [DOI] [PubMed] [Google Scholar]
- 106.Brandstaetter H, Kendrick-Jones J, Buss F. Myo1c regulates lipid raft recycling to control cell spreading, migration and Salmonella invasion. Journal of cell science. 2012;125:1991–2003. doi: 10.1242/jcs.097212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 109.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 110.Zerial M, McBride H. Rab proteins as membrane organizers. Nature reviews. Molecular cell biology. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 111.Allaire PD, Marat AL, Dall’Armi C, Di Paolo G, McPherson PS, Ritter B. The Connecdenn DENN domain: a GEF for Rab35 mediating cargo-specific exit from early endosomes. Mol Cell. 2010;37:370–382. doi: 10.1016/j.molcel.2009.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton RG, Zerial M. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004;116:445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- 113.Chen PI, Kong C, Su X, Stahl PD. Rab5 isoforms differentially regulate the trafficking and degradation of epidermal growth factor receptors. J Biol Chem. 2009;284:30328–30338. doi: 10.1074/jbc.M109.034546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Steinberg F, Gallon M, Winfield M, Thomas EC, Bell AJ, Heesom KJ, Tavare JM, Cullen PJ. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nature cell biology. 2013;15:461–471. doi: 10.1038/ncb2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Masedunskas A, Weigert R. Intravital two-photon microscopy for studying the uptake and trafficking of fluorescently conjugated molecules in live rodents. Traffic. 2008;9:1801–1810. doi: 10.1111/j.1600-0854.2008.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]