Two Phase 3 Trials of Bapineuzumab in Mild-to-Moderate Alzheimer’s Disease (original) (raw)
. Author manuscript; available in PMC: 2014 Sep 10.
Published in final edited form as: N Engl J Med. 2014 Jan 23;370(4):322–333. doi: 10.1056/NEJMoa1304839
Abstract
BACKGROUND
Bapineuzumab, a humanized anti–amyloid-beta monoclonal antibody, is in clinical development for the treatment of Alzheimer’s disease.
METHODS
We conducted two double-blind, randomized, placebo-controlled, phase 3 trials involving patients with mild-to-moderate Alzheimer’s disease — one involving 1121 carriers of the apolipoprotein E (APOE) ε4 allele and the other involving 1331 noncarriers. Bapineuzumab or placebo, with doses varying by study, was administered by intravenous infusion every 13 weeks for 78 weeks. The primary outcome measures were scores on the 11-item cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-cog11, with scores ranging from 0 to 70 and higher scores indicating greater impairment) and the Disability Assessment for Dementia (DAD, with scores ranging from 0 to 100 and higher scores indicating less impairment). A total of 1090 carriers and 1114 noncarriers were included in the efficacy analysis. Secondary outcome measures included findings on positron-emission tomographic amyloid imaging with the use of Pittsburgh compound B (PIB-PET) and cerebrospinal fluid phosphorylated tau (phospho-tau) concentrations.
RESULTS
There were no significant between-group differences in the primary outcomes. At week 78, the between-group differences in the change from baseline in the ADAS-cog11 and DAD scores (bapineuzumab group minus placebo group) were −0.2 (P = 0.80) and −1.2 (P = 0.34), respectively, in the carrier study; the corresponding differences in the noncarrier study were −0.3 (P = 0.64) and 2.8 (P = 0.07) with the 0.5-mg-per-kilogram dose of bapineuzumab and 0.4 (P = 0.62) and 0.9 (P = 0.55) with the 1.0-mg-per-kilogram dose. The major safety finding was amyloid-related imaging abnormalities with edema among patients receiving bapineuzumab, which increased with bapineuzumab dose and APOE ε4 allele number and which led to discontinuation of the 2.0-mg-per-kilogram dose. Between-group differences were observed with respect to PIB-PET and cerebrospinal fluid phospho-tau concentrations in APOE ε4 allele carriers but not in noncarriers.
CONCLUSIONS
Bapineuzumab did not improve clinical outcomes in patients with Alzheimer’s disease, despite treatment differences in biomarkers observed in APOE ε4 carriers. (Funded by Janssen Alzheimer Immunotherapy and Pfizer; Bapineuzumab 301 and 302 ClinicalTrials.gov numbers, NCT00575055 and NCT00574132, and EudraCT number, 2009-012748-17.)
Alzheimer’s disease, a neurodegenerative disease resulting in progressive dementia, is characterized by neuropathological changes that include intraneuronal neurofibrillary tangles and extracellular neuritic plaques. The predominant component of plaques is the amyloid-beta (A_β_) peptide. Multiple lines of evidence indicate that aberrant A_β_ production or clearance is an early component in the pathogenesis of Alzheimer’s disease.1–3 Bapineuzumab is a humanized N-terminal–specific anti-A_β_ monoclonal antibody in clinical development for the treatment of Alzheimer’s disease. In preclinical studies, the murine form of the antibody (3D6) was shown to bind to fibrillar, oligomeric, and monomeric forms of A_β_, reduce the amount of A_β_ in the brain, and improve memory in transgenic mice that overproduced A_β_.4–8 In phase 2 clinical studies involving patients with mild-to-moderate Alzheimer’s disease, patients who received bapineuzumab, as compared with those who received placebo, had a greater reduction in amyloid on positron-emission tomographic amyloid imaging with the use of Pittsburgh compound B (PIB-PET) and reduced cerebrospinal fluid phosphorylated tau (phospho-tau), suggesting target engagement and attenuated neurodegeneration.9,10 Owing to differences in the incidence of amyloid-related imaging abnormalities with effusion or edema11,12 and potential efficacy13 between apolipoprotein E (APOE) ε4 carriers and noncarriers in the phase 2 study, we conducted separate phase 3 clinical trials among APOE ε4 carriers and noncarriers. The primary study objective was to determine the efficacy of intravenous bapineuzumab as compared with placebo in patients with mild-to-moderate dementia associated with Alzheimer’s disease.
METHODS
STUDY SITES AND PATIENTS
We performed two separate clinical trials in the phase 3 program of bapineuzumab for the treatment of mild-to-moderate Alzheimer’s disease to determine the efficacy and safety of bapineuzumab and key biomarker results. One trial involved carriers of the APOE ε4 allele and the other involved APOE ε4 noncarriers. Both were multi-center, randomized, double-blind, placebo-controlled, parallel-group studies. The carrier study was conducted at 170 sites in the United States from December 2007 through April 2012, and the noncarrier study was conducted at 218 sites in the United States (195 sites), Canada (17), Germany (4), and Austria (2) from December 2007 through June 2012.
Eligible patients were 50 to 88 years of age, met the criteria for probable Alzheimer’s disease of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association,14 and had a magnetic resonance imaging (MRI) scan that showed results consistent with Alzheimer’s disease, a score on the Mini–Mental State Examination (MMSE) of 16 to 2615 (with scores ranging from 0 to 30 and higher scores indicating less impairment), and a score on the Hachinski Ischemic scale, as modified by Rosen et al., of 4 or lower16 (with scores ranging from 0 to 12 and higher scores indicating greater degrees of ischemia). Exclusion criteria were neurologic disease other than Alzheimer’s disease; a screening brain MRI scan that showed evidence of an abnormality (two or more microhemorrhages, a prior hemorrhage larger than 1 cm3, two or more lacunar infarcts, a prior infarct larger than 1 cm3, or space-occupying lesions); a major psychiatric disorder; a history of stroke or seizures; and treatment with cognitive enhancers other than stable doses of acetylcholinesterase inhibitors or memantine.
STUDY OVERSIGHT
The studies were approved by the institutional review board at each participating site; written informed consent was obtained from the patients or their legally authorized representatives or caregivers. The sponsor designed the studies in consultation with the academic authors. Data were gathered by the study investigators, analyzed by the sponsor, and interpreted by the sponsors in collaboration with the academic authors. All the authors were involved in the development and approval of the manuscript; the first draft was written by the first author. The academic authors had full access to the study data, and vouch for the accuracy and integrity of the data and the fidelity of this report to the study protocols, which are available with the full text of this article at NEJM.org.
STUDY DESIGN AND TREATMENT
Our primary objective was to evaluate the efficacy of intravenous bapineuzumab, as compared with placebo, by measuring the change from baseline to week 78 on the 11-item cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-cog11, with scores ranging from 0 to 70 and higher scores indicating greater impairment)17 and the Disability Assessment for Dementia (DAD, with scores ranging from 0 to 100 and higher scores indicating less impairment).18 Key secondary objectives were assessments of changes from baseline to week 71 in substudies of three disease biomarkers: brain amyloid burden, cerebrospinal fluid phospho-tau concentrations, and whole-brain volume.
The planned sample size of the carrier study was approximately 1000 participants, who would be randomly assigned in a 3:2 ratio to 0.5 mg of bapineuzumab per kilogram of body weight or to placebo; for the noncarrier study, the planned sample size was approximately 1300 participants, who would be randomly assigned in a 3:3:4 ratio to 0.5 mg of bapineuzumab per kilogram, 1.0 mg of bapineuzumab per kilogram, or placebo. We had initially planned the sample size for the non-carrier study to be approximately 1450 participants, who would be randomly assigned in a 1:1:1:2 ratio to 0.5 mg of bapineuzumab per kilogram, 1.0 mg of bapineuzumab per kilogram, 2.0 mg of bapineuzumab per kilogram, or placebo. However, the sponsor discontinued the 2.0-mg-per-kilogram dose early in the trial on the recommendation of the independent, external data and safety monitoring committee because of a high rate of clinically symptomatic amyloid-related imaging abnormalities with effusion or edema. Participants who had initially been randomly assigned to receive 2.0 mg of bapineuzumab per kilogram (141 participants) were reassigned to the 1.0-mg-per-kilogram group and were included in the safety, but not efficacy, analyses.
Randomization was stratified according to the use or nonuse of a cholinesterase inhibitor or memantine, baseline MMSE total score (16 to 21 vs. 22 to 26), participation in a substudy, and, in the carrier study, APOE ε4 copy number (1 vs. 2). Patients received the study drug as a 1-hour intravenous infusion every 13 weeks for up to six infusions. The final clinical assessment was performed at week 78.
OUTCOME MEASURES
The coprimary outcome measures, the ADAS-cog11 and DAD scores, were assessed at baseline, at treatment visits, and at week 78. Other cognitive and functional outcome measures included scores on the Neuropsychological Test Battery (which was scored on a standardized z scale, with higher scores indicating less impairment),19 the Clinical Dementia Rating–Sum of Boxes (with scores ranging from 0 to 18 and higher scores indicating greater impairment),20 the MMSE, and the Dependence Scale (with scores ranging from 0 to 15 and higher scores indicating greater need for assistance).21
Participants in the PIB-PET substudy underwent PET scanning at baseline, week 45, and week 71. The PIB-PET global cortical average was calculated as the average standardized uptake value ratio (SUVR) of five cortical regions of interest: anterior cingulate cortex, posterior cingulate cortex or precuneus, frontal cortex, lateral temporal cortex, and parietal cortex. Participants in the substudy assessing cerebrospinal fluid phospho-tau concentrations underwent a lumbar puncture at baseline and at week 71. Tau phosphorylated at threonine 181 was measured with the use of a sandwich enzyme-linked immunosorbent assay (Innogenetics).22 Participants in the substudy assessing whole-brain volume underwent MRI scanning at baseline and week 6 and at 13-week intervals through week 71. The primary MRI volumetric outcome was the change in whole-brain volume from baseline to week 71, as measured by the brain boundary-shift-integral method.23
POPULATIONS
The safety population included all participants who received at least one infusion of a study drug. The modified intention-to-treat population included participants who received at least one dose of a study drug and underwent evaluation of the coprimary efficacy end points at baseline and at least once after baseline. The population in the PET substudy included participants who received at least one dose of a study drug, had a scan that was positive for amyloid at baseline (defined as an SUVR ≥1.35), and underwent at least one PET assessment after baseline. The population in the substudy assessing cerebrospinal fluid phospho-tau concentrations included participants who received at least one dose of a study drug and underwent measurement of cerebrospinal fluid phospho-tau at baseline and at least once on or after week 52. The population in the substudy assessing whole-brain volume included participants who received at least one dose of a study drug and underwent volumetric MRI at baseline and at least once after baseline.
SAFETY MONITORING
Safety was evaluated by means of reports of adverse events, clinical laboratory testing (hematologic and serum chemical testing and urinalysis), assessment of vital signs, physical and neurologic examinations, electrocardiography, and brain MRI, with scans reviewed both locally and by a central neuroradiologist. MRI assessments documented amyloid-related imaging abnormalities with effusion or edema.12 An independent, external data and safety monitoring committee, whose members were aware of the study assignments, reviewed the safety data.
STATISTICAL ANALYSIS
The sample sizes were calculated so that the study would have 90% power, with the use of a two-sided test and an alpha level of 0.05, to detect the following differences between the bapineuzumab groups and the placebo groups: in the carrier study, 2.21 units on the ADAS-cog11 and 5.39 units on the DAD; and in the noncarrier study, 2.65 units on the ADAS-cog11 and 6.56 units on the DAD.
The primary analyses of estimated between-group differences in the change in clinical end points from baseline to week 78 were performed on data from the modified intention-to-treat population. The changes from baseline in scores on the ADAS-cog11 and DAD were estimated with the use of a mixed model for repeated measures. The model included assigned study drug (in the carrier study, 0.5 mg per kilogram of bapineuzumab and placebo, and in the non-carrier study, 0.5 mg and 1.0 mg of bapineuzumab per kilogram and placebo), scheduled visit (a categorical factor), interaction between treatment and visit, baseline ADAS-cog11 or DAD score, interaction between baseline score and visit, baseline score on the MMSE (≤21 vs. ≥22), APOE ε4 copy number (for the carrier study), use or nonuse of a cholinesterase inhibitor or memantine at baseline, and age at baseline. We also performed prespecified subgroup analyses according to baseline severity of Alzheimer’s disease (mild or moderate), categorized on the basis of MMSE scores (see Section 3 in the Supplementary Appendix, available at NEJM.org).
The relationship of amyloid-related imaging abnormalities with effusion or edema to the dose of bapineuzumab and the number of APOE ε4 alleles was analyzed with the use of the Cochran–Armitage trend test. Statistical analyses of the change in PIB-PET SUVR and brain-volume boundary-shift integral were performed with the use of a mixed model for repeated measures similar to that used in the analysis of the clinical end points; the change in phospho-tau concentration at week 71 was analyzed by means of analysis of covariance (see Section 3 in the Supplementary Appendix for more detail). In the noncarrier study, the prespecified primary analysis of PIB-PET and cerebrospinal fluid phospho-tau concentration (but not brain-volume boundary-shift integral) was a comparison of the placebo group with the pooled bapineuzumab groups. Prespecified exploratory efficacy analyses were also conducted according to individual dose groups (see Section 4 in the Supplementary Appendix).
All analyses were performed with the use of SAS software, version 9.2. A prespecified statistical testing hierarchy was used to control for multiple comparisons among the coprimary and key secondary end points; in this article, nominal P values are presented. See Section 3 in the Supplementary Appendix for additional details regarding the statistical analyses.
RESULTS
DISPOSITION AND BASELINE CHARACTERISTICS
The screening, randomization, and follow-up of patients are summarized in Figure S1 in the Supplementary Appendix. In the carrier study, 1121 patients underwent randomization and received at least one dose of a study drug: 673 were randomly assigned to 0.5 mg of bapineuzumab per kilogram, and 448 to placebo; in the noncarrier study, 1331 patients underwent randomization and received at least one dose of a study drug: 807 were randomly assigned to bapineuzumab (337 to a dose of 0.5 mg per kilogram, 329 to a dose of 1.0 mg per kilogram, and 141 to a dose of 2.0 mg per kilogram), and 524 to placebo. The modified intention-to-treat population included 1090 patients in the carrier study (658 in the group that received 0.5 mg of bapineuzumab per kilogram and 432 in the placebo group), and 1114 patients in the noncarrier study (314 in the group that received 0.5 mg of bapineuzumab per kilogram, 307 in the group that received 1.0 mg of bapineuzumab per kilogram, and 493 in the placebo group).
The demographic characteristics of the modified intention-to-treat population were generally similar in the bapineuzumab and placebo groups in both studies (Table 1). Table S1 in the Supplementary Appendix shows comparable data for the safety population.
Table 1.
Baseline Characteristics of the Patients and Total Number of Study-Drug Infusions Received.*
| Characteristic | Carriers (N = 1090) | Noncarriers (N = 1114) | |||
|---|---|---|---|---|---|
| Placebo(N = 432) | Bapineuzumab, 0.5 mg/kg(N = 658) | Placebo(N = 493) | Bapineuzumab, 0.5 mg/kg(N = 314) | Bapineuzumab, 1.0 mg/kg(N = 307) | |
| Age — yr | 72.3±8.4 | 72.0±8.0 | 71.9±10.1 | 73.1±9.3 | 73.5±9.1 |
| Female sex — no. (%) | 242 (56.0) | 358 (54.4) | 248 (50.3) | 165 (52.5) | 175 (57.0) |
| White race — no. (%)† | 420 (97.2) | 624 (94.8) | 469 (95.1) | 298 (94.9) | 292 (95.1) |
| APOE ε4 status — no. (%) | |||||
| ε4 Heterozygote | 325 (75.2) | 495 (75.2) | |||
| ε4 Homozygote | 107 (24.8) | 163 (24.8) | |||
| Use of acetylcholinesterase inhibitor or memantine — no. (%) | 400 (92.6) | 606 (92.1) | 442 (89.7) | 281 (89.5) | 278 (90.6) |
| MMSE total score‡ | 20.7±3.2 | 20.8±3.1 | 21.2±3.2 | 21.2±3.4 | 21.2±3.3 |
| ADAS-cog11 total score§ | 23.9±9.5 | 23.5±9.4 | 22.2±10.1 | 22.4±9.7 | 22.2±10.0 |
| DAD total score¶ | 79.4±18.9 | 80.9±17.3 | 80.5±19.2 | 80.0±18.1 | 80.4±18.8 |
| Total infusions received — no. (%) | |||||
| 1 | 9 (2.1) | 29 (4.4) | 13 (2.6) | 6 (1.9) | 8 (2.6) |
| 2 | 32 (7.4) | 42 (6.4) | 44 (8.9) | 13 (4.1) | 25 (8.1) |
| 3 | 17 (3.9) | 37 (5.6) | 26 (5.3) | 23 (7.3) | 22 (7.2) |
| 4 | 17 (3.9) | 48 (7.3) | 18 (3.7) | 26 (8.3) | 16 (5.2) |
| 5 | 31 (7.2) | 82 (12.5) | 26 (5.3) | 23 (7.3) | 37 (12.1) |
| 6 | 326 (75.5) | 420 (63.8) | 366 (74.2) | 223 (71.0) | 199 (64.8) |
In the carrier study, 75.4% of the patients in the placebo group and 69.5% in the bapineuzumab group completed the study. In the noncarrier study, 71.2% of the patients in the placebo group and 70.6%, 68.7%, and 67.4% in the 0.5-mg-per-kilogram, 1.0-mg-per-kilogram, and 2.0-mg-per-kilogram bapineuzumab groups, respectively, completed the study (after the patients in the 2.0-mg-per-kilogram group were reassigned to the 1.0-mg-per-kilogram group). More patients in the bapineuzumab groups than in the placebo groups withdrew from the study because of adverse events (11.0% vs. 7.6% in the carrier study; 9.5%, 8.8%, and 13.5%, in the 0.5-mg-per-kilogram, 1.0-mg-per-kilogram, and 2.0-mg-per-kilogram bapineuzumab groups, respectively, vs. 7.6% in the noncarrier study).
EFFICACY
No significant differences with respect to the coprimary outcomes were observed between the bapineuzumab groups and the placebo groups in the carrier study or in the noncarrier study (Fig. 1 and Table 2). No significant between-group differences were observed at week 78 in the scores on the Neuropsychological Test Battery, Clinical Dementia Rating–Sum of Boxes, MMSE, or Dependence Scale in either study (Table 2).
Figure 1. Primary Outcome.
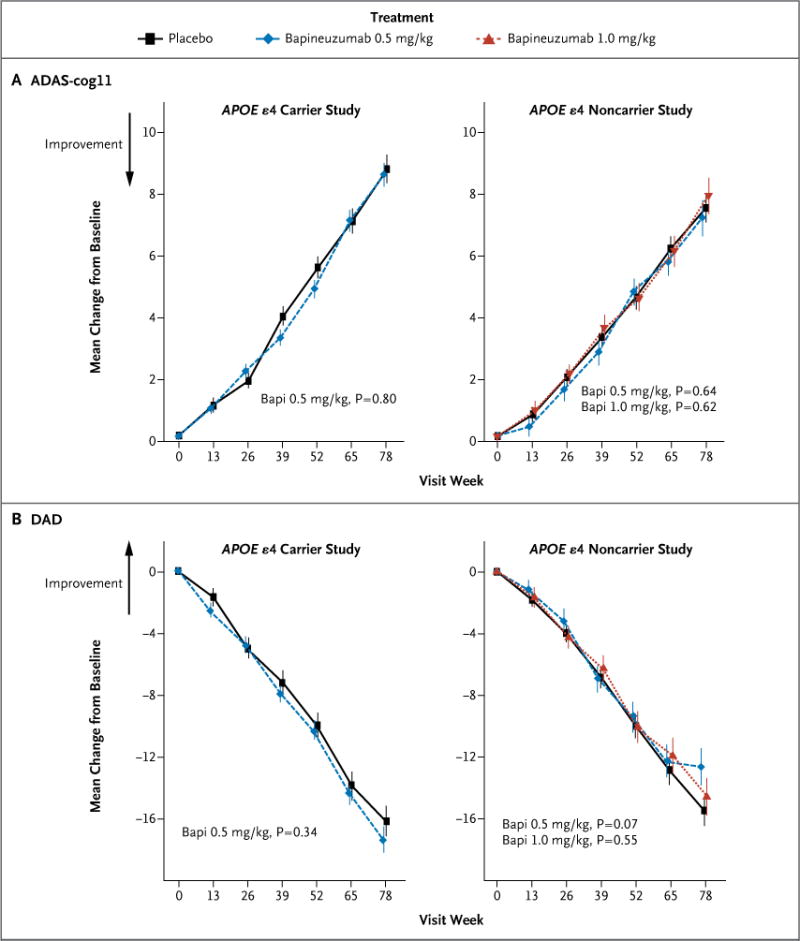
Panel A shows the estimated mean change from baseline to week 78 in scores on the 11-item cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-cog11, with scores ranging from 0 to 70 and higher scores indicating greater impairment), and Panel B the estimated mean change from baseline to week 78 in the Disability Assessment for Dementia (DAD, with scores ranging from 0 to 100 and higher scores indicating less impairment) among APOE ε4 carriers and noncarriers, according to study regimen. The P values that are shown are unadjusted. Bapi denotes bapineuzumab.
Table 2.
Changes in Clinical End Points from Baseline to Week 78.*
| End Point | Placebo | Bapineuzumab, 0.5 mg/kg | Bapineuzumab, 1.0 mg/kg | ||||
|---|---|---|---|---|---|---|---|
| Change | Change | Least Squares Mean Difference from Placebo (95% CI) | P Value | Change | Least Squares Mean Difference from Placebo (95% CI) | P Value | |
| Carrier study† | |||||||
| ADAS-cog11 total score | 8.7±0.5 | 8.5±0.4 | −0.2 (−1.4 to 1.0) | 0.80 | |||
| DAD total score | −16.2±1.0 | −17.4±0.8 | −1.2 (−3.8 to 1.3) | 0.34 | |||
| Clinical Dementia Rating Scale–Sum of Boxes total score‡ | 3.0±0.2 | 3.3±0.1 | 0.2 (−0.2 to 0.6) | 0.25 | |||
| Neuropsychological Test Battery total score§ | −0.204±0.029 | −0.213±0.024 | −0.009 (−0.082 to 0.065) | 0.82 | |||
| MMSE total score | −4.5±0.2 | −4.7±0.2 | −0.2 (−0.9 to 0.4) | 0.50 | |||
| Dependence Scale¶ | 1.4±0.1 | 1.6±0.1 | 0.2 (−0.1 to 0.5) | 0.30 | |||
| Noncarrier study‖ | |||||||
| ADAS-cog11 total score | 7.4±0.5 | 7.1±0.6 | −0.3 (−1.8 to 1.1) | 0.64 | 7.8±0.6 | 0.4 (−1.1 to 1.8) | 0.62 |
| DAD total score | −15.5±1.0 | −12.7±1.2 | 2.8 (−0.2 to 5.8) | 0.07 | −14.6±1.2 | 0.9 (−2.1 to 4.0) | 0.55 |
| Clinical Dementia Rating Scale–Sum of Boxes total score‡ | 2.6±0.2 | 2.6±0.2 | 0.0 (−0.5 to 0.5) | 0.97 | 2.8±0.2 | 0.2 (−0.3 to 0.7) | 0.42 |
| Neuropsychological Test Battery total score§ | −0.111±0.025 | −0.143±0.031 | −0.032 (−0.109 to 0.045) | 0.42 | −0.069±0.032 | 0.042 (−0.036 to 0.121) | 0.29 |
| MMSE total score | −3.9±0.2 | −3.5±0.3 | 0.4 (−0.3 to 1.2) | 0.29 | −3.7±0.3 | 0.2 (−0.6 to 0.9) | 0.66 |
| Dependence Scale¶ | 1.4±0.1 | 1.3±0.1 | −0.1 (−0.4 to 0.3) | 0.74 | 1.5±0.2 | 0.1 (−0.2 to 0.5) | 0.46 |
The main prespecified exploratory efficacy analyses that were performed on data from patients with mild Alzheimer’s disease (an MMSE score of 21 or higher) revealed no significant differences with respect to the coprimary end points in either study; however, when a prespecified alternative MMSE threshold of 20 or higher was used to define mild Alzheimer’s disease, potential differences (P<0.05) in scores on the DAD in favor of bapineuzumab were observed among patients with mild Alzheimer’s disease in both the 0.5-mg-per-kilogram group and the 1.0-mg-per-kilogram group in the noncarrier study. No significant differences were observed with respect to the ADAS-cog11 score at any prespecified MMSE cutoff points that we tested (Table S2 in the Supplementary Appendix).
KEY BIOMARKER OUTCOMES
PIB-PET
In the carrier study, the analysis of PIB-PET included 75 patients in the bapineuzumab group and 40 in the placebo group; in the noncarrier study, this analysis included 12 patients in the 0.5-mg-per-kilogram bapineuzumab group, 12 in the 1.0-mg-per-kilogram bapineuzumab group, and 15 in the placebo group (Fig. 2, and Table S3 in the Supplementary Appendix). A total of 6.5% of carriers and 36.1% of noncarriers did not reach the threshold SUVR of 1.35 and were not included in the analysis. Among carriers, the mean (±SE) SUVR increased in the placebo group (by 0.102±0.026), but remained almost unchanged in the bapineuzumab group (an increase of only 0.001±0.021) over the course of 71 weeks (difference in change, bapineuzumab minus placebo, −0.101; P = 0.004). Among noncarriers, no increase in the SUVR was observed in the placebo group and no significant differences were observed at week 71 between the 0.5-mg-per-kilogram or 1.0-mg-per-kilogram bapineuzumab group and the placebo group, either in pooled or separate analyses.
Figure 2. Key Biomarkers.
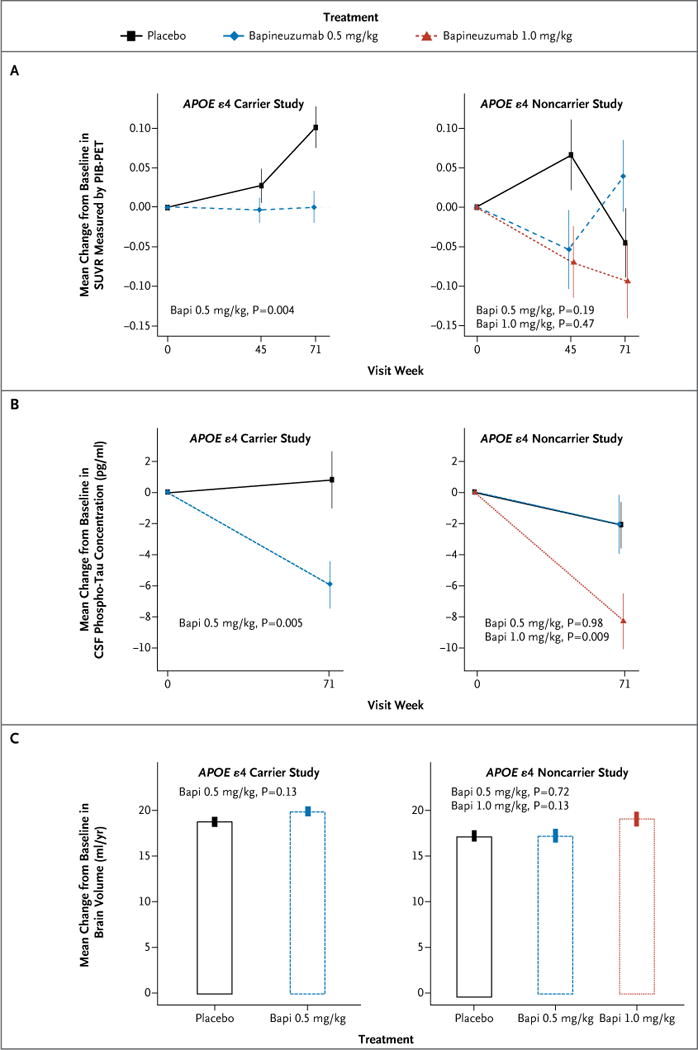
Shown are the changes from baseline to week 71 in brain amyloid burden, as measured by the average standardized uptake value ratio (SUVR) of five cortical regions of interest (anterior cingulate cortex, posterior cingulate cortex or precuneus, frontal cortex, lateral temporal cortex, and parietal cortex), assessed by means of positron-emission tomographic amyloid imaging with the use of Pittsburgh compound B (PIB-PET) (Panel A); cerebrospinal fluid (CSF) phosphorylated tau (phospho-tau) concentration (Panel B); and brain volume on MRI, calculated with the use of the brain boundary-shift-integral method23 (Panel C), among carriers of the apolipoprotein E (APOE) ε4 allele and noncarriers, according to study regimen. In the primary analysis of cerebrospinal fluid phospho-tau concentration among noncarriers, the 0.5-mg-per-kilogram and the 1.0-mg-per-kilogram bapineuzumab groups were pooled, and the difference in the reduction in cerebrospinal fluid phospho-tau concentration between the pooled results and placebo was not significant (P = 0.11) (Table S3b in the Supplementary Appendix). The P values that are shown are unadjusted.
Cerebrospinal Fluid Phospho-Tau Concentration
In the carrier study, the analysis of cerebrospinal fluid phospho-tau concentrations included 127 patients in the bapineuzumab group and 85 in the placebo group; in the noncarrier study, this analysis included 47 patients in the 0.5-mg-per-kilogram bapineuzumab group, 54 in the 1.0-mg-per-kilogram bapineuzumab group, and 77 in the placebo group (Fig. 2, and Table S3 in the Supplementary Appendix). Among carriers, a significant reduction in the cerebrospinal fluid phospho-tau concentration was observed with bapineuzumab by week 71 (−5.80±1.49 pg per milliliter), whereas there was an increase with placebo (0.95±1.83 pg per milliliter), representing a significant between-group difference of −6.75 pg per milliliter (P = 0.005). Among noncarriers, no significant between-group difference was observed between the pooled 0.5-mg-per-kilogram and 1.0-mg-per-kilogram bapineuzumab group and the placebo group (between-group difference, −3.30 pg per milliliter; P = 0.11). Dose-specific analyses (prespecified exploratory analyses) showed a reduction in the cerebrospinal fluid phospho-tau concentration at week 71 in the 1.0-mg-per-kilogram bapineuzumab group relative to the placebo group (decrease of −8.17±1.80 pg per milliliter vs. −1.98±1.48 pg per milliliter; between-group difference, −6.19 pg per milliliter; P = 0.009) but not in the 0.5-mg-per-kilogram bapineuzumab group relative to the placebo group (between-group difference, 0.05 pg per milliliter; P = 0.98).
Volumetric MRI
In the carrier study, volumetric MRI analysis included 352 patients in the bapineuzumab group and 238 in the placebo group; in the noncarrier study, this analysis included 169 patients in the 0.5-mg-per-kilogram bapineuzumab group, 146 in the 1.0-mg-per-kilogram bapineuzumab group, and 244 in the placebo group (Fig. 2, and Table S3 in the Supplementary Appendix). Among carriers, there was no significant difference in the annual rate of loss of brain volume between the bapineuzumab group and the placebo group (19.9±0.50 ml per year and 18.7±0.59 ml per year, respectively; between-group difference, 1.2 ml per year; P = 0.13). Similarly, no significant differences were observed among noncarriers: 17.5±0.61 ml per year in the placebo group, as compared with 17.2±0.73 ml per year in the 0.5-mg-per-kilogram bapineuzumab group (difference in change, bapineuzumab minus placebo, −0.3 ml per year; P = 0.73) and 19.0±0.79 ml per year in the 1.0-mg-per-kilogram group (difference in change, bapineuzumab minus placebo, 1.5 ml per year; P = 0.13).
SAFETY
In both the carrier and noncarrier studies, the incidence of adverse events occurring during the study period was similar in the placebo and bapineuzumab groups. In the carrier study, 92.6% of the patients in the bapineuzumab group and 88.8% of those in the placebo group had an adverse event; in the noncarrier study: 88.7%,88.8%, and 90.8% of the patients in the 0.5-mg-per-kilogram, 1.0-mg-per-kilogram, and 2.0-mg-per-kilogram bapineuzumab groups, respectively, and 88.7% of the patients in the placebo group had an adverse event. Adverse events occurring in more than 10% of the patients in any group are shown in Table 3, and those occurring in more than 5% in any group are shown in Table S4 in the Supplementary Appendix; all serious adverse events are shown in Table S6 in the Supplementary Appendix. In both the carrier and noncarrier studies, amyloid-related imaging abnormalities with effusion or edema were the most notable adverse events in the safety population; this abnormality was identified, with the use of MRI performed during the course of the study, in 103 of the 673 carriers who received bapineuzumab (15.3%), including 58 of the 508 APOEε4 heterozygotes (11.4%) and 45 of the 165 APOEε4 homozygotes (27.3%). In the noncarrier study, amyloid-related imaging abnormalities with effusion or edema were identified in 14 of the 337 patients in the 0.5-mg-per-kilogram bapineuzumab group (4.2%), 31 of the 329 patients in the 1.0-mg-per-kilogram group (9.4%), and 20 of the 141 patients in the 2.0-mg-per-kilogram group (14.2%) (Fig. S2 in the Supplementary Appendix). One patient in the placebo group in each study was identified as having amyloid-related imaging abnormalities with effusion or edema (0.2%). This abnormality tended to occur early in the course of bapineuzumab treatment (with most occurring between the first and third infusions). A total of 84.5% of carriers and 64.3%, 83.9% and 45.0% of the noncarriers in the 0.5-mg-per-kilogram, 1.0-mg-per-kilogram, and 2.0-mg-per-kilogram groups, respectively, were asymptomatic for this abnormality. In addition, a retrospective central review was performed of amyloid-related imaging abnormalities with effusion or edema from all MRI study scans, and this review identified a larger number of cases than had been identified previously (Table S5 in the Supplementary Appendix).
Table 3.
Adverse Events.*
| Adverse Event | Placebo | Bapineuzumab, 0.5 mg/kg | Bapineuzumab, 1.0 mg/kg | Bapineuzumab, 2.0 mg/kg |
|---|---|---|---|---|
| number of patients (percent) | ||||
| Carrier study† | ||||
| Amyloid-related imaging abnormalities with edema | 1 (0.2) | 103 (15.3) | ||
| Fall | 64 (14.3) | 100 (14.9) | ||
| Headache | 48 (10.7) | 78 (11.6) | ||
| Noncarrier study‡ | ||||
| Amyloid-related imaging abnormalities with edema | 1 (0.2) | 14 (4.2) | 31 (9.4) | 20 (14.2) |
| Fall | 73 (13.9) | 43 (12.8) | 43 (13.1) | 23 (16.3) |
| Urinary tract infection | 59 (11.3) | 40 (11.9) | 42 (12.8) | 15 (10.6) |
| Anxiety | 43 (8.2) | 19 (5.6) | 39 (11.9) | 11 (7.8) |
| Headache | 49 (9.4) | 30 (8.9) | 34 (10.3) | 16 (11.3) |
| Agitation | 37 (7.1) | 26 (7.7) | 15 (4.6) | 16 (11.3) |
Among carriers, 20 patients — 5 (1.1%) in the placebo group and 15 (2.2%) in the bapineuzumab group — had a fatal adverse event that occurred during treatment. Neoplasm was the most common adverse event occurring during treatment that led to death (6 cases [0.9%], all in the bapineuzumab group). No specific tumor type was observed with increased frequency. Among noncarriers, 23 patients had fatal adverse events that occurred during treatment: 7 patients in the placebo group (1.3%), 4 in the 0.5-mg-per-kilogram bapineuzumab group (1.2%), 7 in the 1.0-mg-per-kilogram bapineuzumab group (2.1%), and 5 in the 2.0-mg-per-kilogram bapineuzumab group (3.5%); a total of 5 noncarriers (0.4%) had neoplasms leading to death (2 in the placebo group [0.4%] and 3 in the bapineuzumab groups [0.4%]) (Tables S6 and S7 in the Supplementary Appendix).
DISCUSSION
We found no significant differences between the bapineuzumab groups and the placebo groups with respect to the primary end points (scores on the ADAS-cog11 and DAD) or other clinical end points (scores on the Clinical Dementia Rating Scale–Sum of Boxes, Neuropsychological Test Battery, MMSE, or Dependence Scale) among carriers or noncarriers. The lack of clinical efficacy that we observed could be due to a number of factors. The doses of bapineuzumab used in these studies were limited because of higher rates of amyloid-related imaging abnormalities with effusion or edema at higher doses. The greater incidence of these abnormalities with increasing bapineuzumab dose and number of APOEε4 alleles is consistent with phase 2 findings.11,13 Recent research in transgenic mice suggests that these abnormalities may be related to antibody effects on amyloid in cerebral arterioles.11,24 Studies with alternative dosing regimens for bapineuzumab may lead to a decrease in the rates of amyloid-related abnormalities on imaging. It is also possible that A_β_ may not be the best target for therapeutic intervention, that too little amyloid was removed, that an important species of A_β_ was insufficiently affected, or that other aspects of neurodegeneration were inadequately affected.
Among APOE ε4 carriers, bapineuzumab was associated with reduced cerebrospinal fluid phospho-tau concentrations, a marker of neurodegeneration; this finding is consistent with phase 2 results.9,10 Among noncarriers, bapineuzumab was not associated with a significant lowering of cerebrospinal fluid phospho-tau concentrations in the analysis of pooled data from the 0.5-mg-per-kilogram and 1.0-mg-per-kilogram groups — the prespecified primary analysis for this outcome. However, in prespecified exploratory analyses, a reduction in cerebrospinal fluid phospho-tau concentrations was observed with the 1.0-mg-per-kilogram dose, but not the 0.5-mg-per-kilogram dose, relative to placebo.
A decreased rate of accumulation of amyloid in the brain on PIB-PET was seen in APOE ε4 carriers who received bapineuzumab, but the difference was smaller than that seen in phase 2 studies, which included the 2.0-mg-per-kilogram dose.10 Significant between-group differences in amyloid on PIB-PET were not seen in the noncarrier study, but the relatively small number of noncarriers who could be evaluated limits the conclusions that can be drawn regarding such changes. The negative scans for amyloid in 36% of APOE ε4 noncarriers at baseline raises concern about the reliability of diagnoses of Alzheimer’s disease among noncarriers and suggests the possible usefulness of incorporating amyloid thresholds into eligibility criteria in future trials of anti-amyloid therapy.
Although biomarker results in the carrier study suggest that bapineuzumab may modify A_β_ accumulation and a downstream biomarker (phospho-tau), neither trial showed a benefit of bapineuzumab with respect to clinical outcomes. As reported elsewhere in this issue of the Journal, two recent phase 3 clinical trials with solanezumab, another anti-A_β_ monoclonal antibody, also did not show benefit with respect to the primary clinical outcomes in patients with mild-to-moderate Alzheimer’s disease.25 Amyloid accumulation probably starts many years before the onset of symptoms,2,26,27 and initiation of anti-amyloid treatment only after dementia develops may be too late to affect the clinical course of the disease.28
Supplementary Material
supplement
Acknowledgments
Supported by Janssen Alzheimer Immunotherapy Research and Development and Pfizer.
We thank all the patients with Alzheimer’s disease and their caregivers who participated in these studies. Acknowledgment of specific persons who assisted with the study can be found in the Supplementary Appendix.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [Erratum, Science 2002;297:2209.] [DOI] [PubMed] [Google Scholar]
- 2.Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [Erratum, N Engl J Med 2012;367:780.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golde TE, Schneider LS, Koo EH. Anti-aβ therapeutics in Alzheimer’s disease: the need for a paradigm shift. Neuron. 2011;69:203–13. doi: 10.1016/j.neuron.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bard F, Cannon C, Barbour R, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–9. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 5.Bard F, Barbour R, Cannon C, et al. Epitope and isotype specificities of antibodies to beta-amyloid peptide for protection against Alzheimer’s disease-like neuropathology. Proc Natl Acad Sci U S A. 2003;100:2023–8. doi: 10.1073/pnas.0436286100. [Erratum, Proc Natl Acad Sci U S A 2004;101:11526.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buttini M, Masliah E, Barbour R, et al. Beta-amyloid immunotherapy prevents synaptic degeneration in a mouse model of Alzheimer’s disease. J Neurosci. 2005;25:9096–101. doi: 10.1523/JNEUROSCI.1697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankar GM, Li S, Mehta TH, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zago W, Buttini M, Comery TA, et al. Neutralization of soluble, synaptotoxic amyloid β species by antibodies is epitope specific. J Neurosci. 2012;32:2696–702. doi: 10.1523/JNEUROSCI.1676-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blennow K, Zetterberg H, Rinne JO, et al. Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate Alzheimer disease. Arch Neurol. 2012;69:1002–10. doi: 10.1001/archneurol.2012.90. [DOI] [PubMed] [Google Scholar]
- 10.Rinne JO, Brooks DJ, Rossor MN, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9:363–72. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- 11.Sperling R, Salloway S, Brooks DJ, et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11:241–9. doi: 10.1016/S1474-4422(12)70015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sperling RA, Jack CR, Jr, Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Work-group. Alzheimers Dement. 2011;7:367–85. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salloway S, Sperling R, Gilman S, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–70. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7:486–8. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 17.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–64. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 18.Gauthier S, Gélinas I, Gauthier L. Functional disability in Alzheimer’s disease. Int Psychogeriatr. 1997;9(Suppl 1):163–5. doi: 10.1017/s1041610297004857. [DOI] [PubMed] [Google Scholar]
- 19.Harrison J, Minassian SL, Jenkins L, Black RS, Koller M, Grundman M. A neuropsychological test battery for use in Alzheimer disease clinical trials. Arch Neurol. 2007;64:1323–9. doi: 10.1001/archneur.64.9.1323. [DOI] [PubMed] [Google Scholar]
- 20.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 21.Stern Y, Albert SM, Sano M, et al. Assessing patient dependence in Alzheimer’s disease. J Gerontol. 1994;49:M216–M222. doi: 10.1093/geronj/49.5.m216. [DOI] [PubMed] [Google Scholar]
- 22.Vanmechelen E, Vanderstichele H, Davidsson P, et al. Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: a sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci Lett. 2000;285:49–52. doi: 10.1016/s0304-3940(00)01036-3. [DOI] [PubMed] [Google Scholar]
- 23.Fox NC, Cousens S, Scahill R, Harvey RJ, Rossor MN. Using serial registered brain magnetic resonance imaging to measure disease progression in Alzheimer disease: power calculations and estimates of sample size to detect treatment effects. Arch Neurol. 2000;57:339–44. doi: 10.1001/archneur.57.3.339. [DOI] [PubMed] [Google Scholar]
- 24.Zago W, Schroeter S, Guido T, et al. Vascular alterations in PDAPP mice after anti-Aβ immunotherapy: implications for amyloid-related imaging abnormalities. Alzheimers Dement. 2013 Apr 11; doi: 10.1016/j.jalz.2012.11.010. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 25.Doody RS, Thomas RG, Farlow M, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:311–21. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 26.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12:357–67. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 28.Sperling RA, Jack CR, Jr, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3:111cm33. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplement