Estimated Glomerular Filtration Rate versus Albuminuria in the Assessment of Kidney Function: What’s More Important? (original) (raw)
Abstract
Clinical practice guidelines state that any evaluation of kidney disease requires the assessment of (1) kidney function in the form of the estimated glomerular filtration rate (eGFR) and (2) kidney damage by a quantitative assessment of proteinuria, preferably by the determination of the urine albumin-to-creatinine ratio. This review discusses the relative merits of each measurement, focusing on the strengths of each measurement in relationship to all-cause and cardiovascular mortality risk prediction as well as the prediction of kidney disease progression with loss of kidney function over time and the progression to end-stage kidney disease treated by dialysis or kidney transplantation.
Introduction
Kidney disease is a major public health problem and is associated with significant morbidity and mortality.1,2 Independent of the aetiology of the underlying kidney disease, laboratory assessment of kidney function involves calculation of the estimated glomerular filtration rate (eGFR) from the serum creatinine and assessment of kidney damage by measurement of proteinuria. This review summarises the current state of knowledge in the relationship of laboratory measures of kidney function (eGFR and proteinuria) to mortality and cardiovascular risk, the diagnosis of kidney disease and the progression of established kidney disease. Here we focus on the relative strengths of each measurement and discuss the interrelationship between them, highlighting the recent advances in the epidemiology of kidney disease seen in the last 10 years.
Kidney Disease and Cardiovascular Risk in the General Population
Estimated Glomerular Filtration Rate
While it had long been recognised that subjects with reduced kidney function had high rates of cardiovascular disease,3 it was not until 2004 that the link between kidney function and cardiovascular disease was first assessed in large epidemiological studies. This occurred in part due to the development and validation of the glomerular filtration rate estimating (eGFR) equations, and specifically, the modification of diet in renal disease (MDRD) study equation.4 In a landmark study published in the New England Journal of Medicine,5 Go and colleagues assessed the relationship between moderate and severe kidney dysfunction, defined as eGFR <60 mL/min, and cardiovascular disease, in more than one million subjects from a large US medical insurance database. They demonstrated a large exponential increase in the age-standardised rate for all-cause mortality and cardiovascular events over a three year period in subjects with kidney function reduced below 60 mL/min (Figure 1). After adjustment for multiple confounding factors, reduced kidney function remained independently associated with increased risk of death, cardiovascular events and hospitalisation, in a graded fashion compared to subjects with eGFR >60 mL/min. This study was important for several reasons. Firstly, it was the first study to demonstrate conclusively that reduced kidney function was an independent risk factor for mortality and cardiovascular events. Secondly it focused the nephrology and public health community on the importance of kidney function in the broader sense. It has helped lead a large body of work assessing the clinical epidemiology of mild to moderate kidney dysfunction in both community and general population cohorts, where previously the emphasis had been on severe kidney dysfunction, and in particular, on those with end-stage kidney failure treated with dialysis and/or kidney transplantation.6
Figure 1.
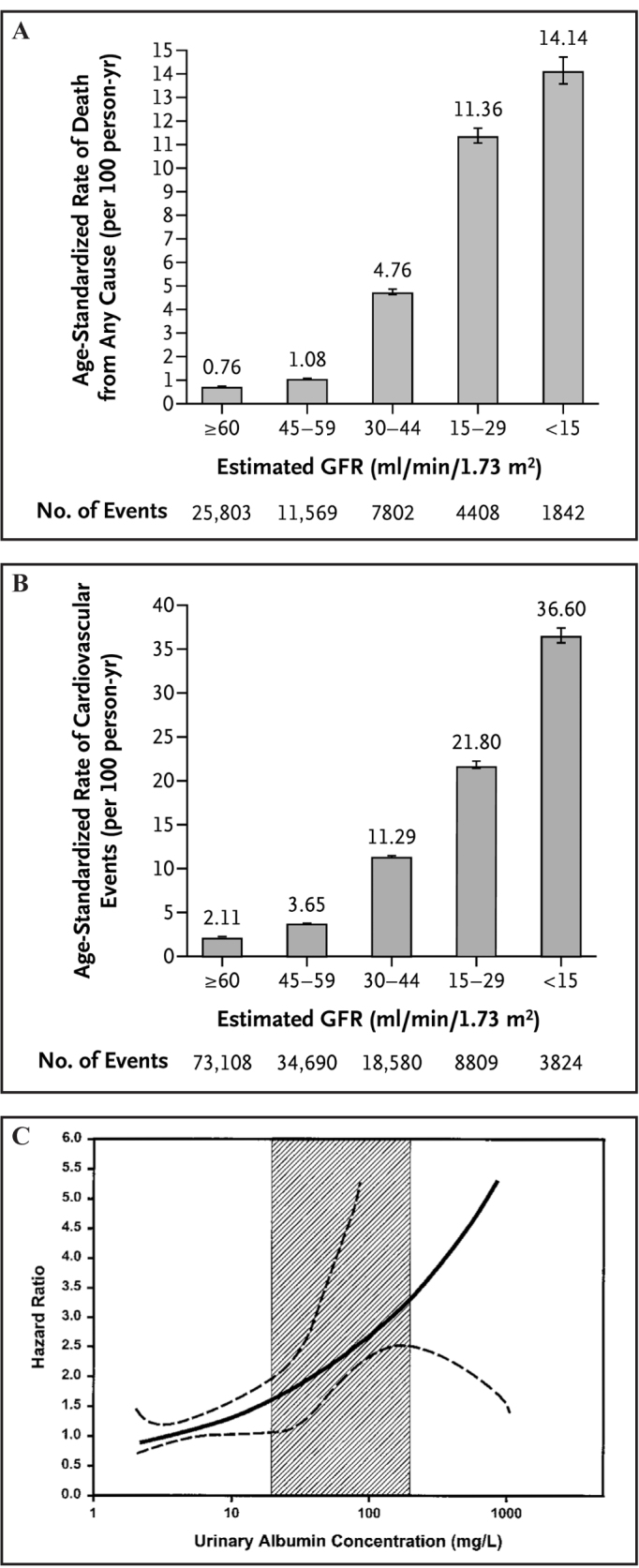
Age-standardised rates for death (A) and cardiovascular events (B) by level of eGFR and hazard ratio for cardiovascular death by urinary albumin. (C), Adjusted effect of urine albumin concentration on hazard function; dotted lines are 95% confidence limits. Reproduced with permission from Go et al 5 and Hillege et al.14
Following the work of Go et al,5 numerous investigators set out to confirm the findings and explore the relationship more comprehensively - in particular, assessing the exact eGFR threshold of the mortality effect. Go and colleagues chose to assess level of kidney function with the comparison group >60 mL/min. This was likely for two reasons – (1) the MDRD eGFR equation used at the time was known to be less accurate at levels of eGFR greater than 60 mL/min tending to underestimate true GFR,7 and, (2) clinical practice guidelines at the time, defined chronic kidney disease (CKD) as an eGFR below 60 mL/min and thus this threshold was seen as an important clinical cut-point. However eGFR is a continuous variable and investigators remained interested in whether milder forms of kidney dysfunction also conferred an elevated risk for mortality and cardiovascular disease.
Both issues above were subject to vigorous debate in the nephrology community8 and drove further research through collaboration of large research groups. The first data to provide a more definitive answer on the mortality effects of mild levels of kidney dysfunction came from the CKD Prognosis Consortium.9 The CKD Prognosis Consortium10 was established in 2009 to provide comprehensive evidence about the prognostic impact of eGFR and albuminuria on mortality and kidney outcomes. The Consortium consists of 46 cohorts with data on 2.1 million subjects from Asia, Europe, North and South America and Australasia. The consortium’s first major paper assessed the relationship between MDRD eGFR and all-cause and cardiovascular mortality. It definitively demonstrated that subjects with milder forms of kidney dysfunction (eGFR 60 mL/min) had elevated risks of death compared to those with normal function (eGFR 95 mL/min). This data was important as it was derived from a wide group of subjects from a number of different populations including community cohorts as well as those with cardiovascular disease risk factors.
In concert with the formation of the CKD Prognosis Consortium, the CKD Epidemiology Collaboration developed and validated a new creatinine-based eGFR estimating equation to try and address concerns regarding the underestimation of true GFR in those subjects with GFR levels equal and above 60 mL/min. The new equation - the ‘CKD-Epi equation’,11 using the same coefficients (creatinine, gender, age and race), improved the bias (the mean or median difference between true and eGFR) in subjects with GFR levels over 60 mL/min when compared to the MDRD equation. In addition there was no reduction in bias on subjects with GFR below 60 mL/min. The precision (the variability of the differences between the two measures around the average difference) of the equation remained unchanged from the MDRD formula. Compared to the MDRD equation, the new CKD-Epi equation reduces the estimated prevalence of CKD (eGFR<60 mL/min) in the general population, and improves risk prediction by more accurately categorising subjects within risk categories for mortality and end-stage renal disease.12,13
Proteinuria
In addition to eGFR, proteinuria, either measured as total urinary protein or as urine albumin, is a potent predictor of mortality and cardiovascular risk. The first major population-based study demonstrated a linear increase in the risk for cardiovascular events with the risk increasing well within the normal range for urine albumin concentration. (Figure 1c)14 This work has been replicated in other cohorts assessing the urine albumin-to-creatinine ratio,15 urine protein-to-creatinine ratio, as well as assessment by urine protein dipstick.16 Again the CKD Prognosis Consortium has been able to confirm and extend these findings by demonstrating the linear increase in the risk of all-cause and cardiovascular mortality as urine albumin-to-creatinine ratio increases.9 This increase in risk is independent of GFR such that there is an additive effect of proteinuria on the risk of death or events at any level or stage of GFR. The additional assessment of proteinuria (assessed either quantitatively or qualitatively) provides improved stratification of risk with specific eGFR staging.17
Predicting Cardiovascular Disease Risk
While both eGFR and albuminuria independently associate with increased risk of cardiovascular disease, a key question for the practising clinician is whether they add anything to improve cardiovascular risk prediction in an individual patient over and above the known traditional risk factors for cardiovascular disease such as age, hypertension or diabetes? Surprisingly few studies have attempted to address this specific question with resulting conflicting data.
Data from the Hunt II study, a general population cohort, assessed the addition of both kidney measures to improve 10 year cardiovascular mortality risk prediction over a model including traditional cardiovascular risk factors.18 Both the traditional and non-traditional model including kidney measures, classified 76.6% of the cohort at low risk. Overall, only 6.6% of the cohort were classified differently by adding the kidney measures to the traditional model. However improvements in risk prediction were seen in those subjects classified in the intermediate risk category (5–10% 10 yr cardiovascular mortality risk). Of the intermediate risk subjects, 25% and 10% were reclassified to either low risk or high risk respectively with the addition of the kidney measures. Those subjects in each reclassified group risk had significantly reduced or increased mortality risk compared to those subjects that remained within the intermediate risk category by both risk equations.
Clase et al19 assessed 27,000 patients in the TRANSCEND and ONTARGET randomised clinical trial who were at high cardiovascular risk. Unlike the previous study, the addition of eGFR and albuminuria did not amount to a reduction in the number of subjects classified into the intermediate risk group (31% versus 32% with kidney measures). Finally the PREVEND study group20 assessed the value of kidney measure to predict a composite endpoint of all-cause and cardiovascular mortality as well as incident cardiovascular events. In this study, both eGFR and albuminuria were assessed separately against a model using Framingham cardiovascular risk factors. Albuminuria but not eGFR was associated with improved risk prediction as evidenced by a significant improvement in the net reclassification index (7.2%, 95% confidence interval (CI) 3.3 to 11.0%, compared to −1.2%, 95% CI 5.1 to −2.7%) when added to a model containing the “Framingham” cardiovascular risk factors.
While it is clear that measures of kidney function are associated with increased risk for mortality and cardiovascular disease, the addition of these factors to risk prediction models suggests at best only modest improvement. Further work will be necessary to determine the utility of these measures in classifying patients at risk and whether other measures, such as cystatin C, for example,21 will prove more useful.
Assessment of Kidney Disease
Kidney Disease Diagnosis
The assessment of both albuminuria and eGFR are critical to the assessment and diagnosis of CKD, as emphasised in the most recent update to the clinical practice guidelines for the classification of CKD.22 Initial guidelines had emphasised the staging of CKD based on eGFR with a partial consideration of ‘markers of kidney damage’ (albuminuria, haematuria or structural abnormalities) only for levels of eGFR above 60 mL/min.23 The major change in the most recent update has been to add consideration of proteinuria (using the assessment of urine albumin) to all levels of eGFR, emphasising the important relationship seen in both mortality and renal risk depending on the presence or absence of proteinuria (as discussed above).
Kidney Prognosis and Risk of Progression
Once kidney function and damage have been determined, the kidney prognosis and overall risk of progressive kidney dysfunction are the next important clinical problems. However it remains important to emphasise that although subjects with CKD are more likely to develop end-stage kidney failure compared to those without kidney disease, work in a general CKD population as well as those with diabetes demonstrates that subjects with CKD are more likely to die from cardiovascular events rather than survive to reach end-stage kidney disease (ESKD) treated with dialysis.24,25
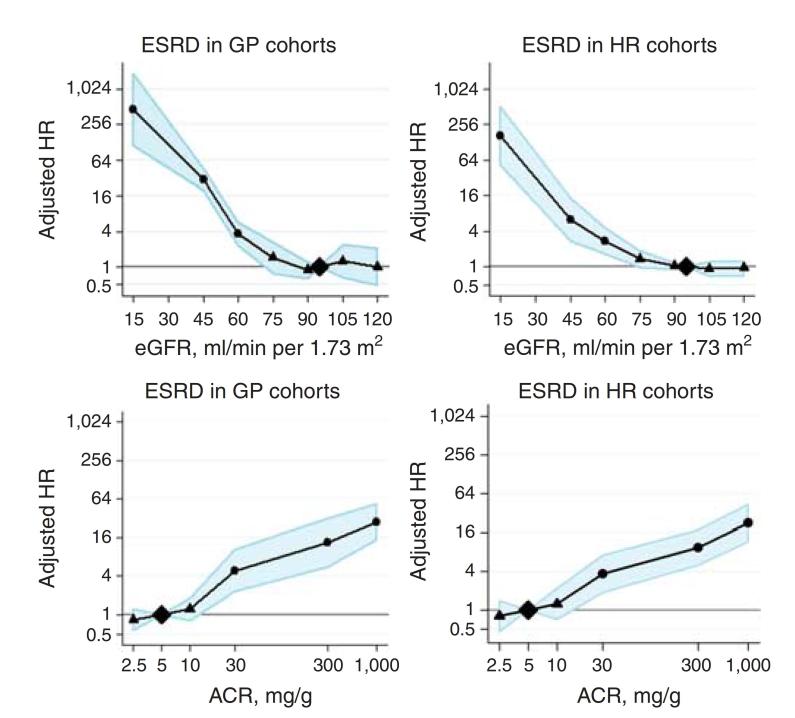
Reductions in eGFR are associated with an increased risk of the development of ESKD in the general population and ‘high risk’ populations (Figure 2). The risk increases in an exponential fashion once baseline GFR falls below 75 mL/min.15,26,27 The relationship is also seen when eGFR is estimated using the newer CKD-Epi formula with improved risk prediction for ESKD events when compared to eGFR estimated using the MDRD formula.13,27 Similarly, increasing levels of albuminuria are also associated with increased risk for the progression and development of ESKD.26 This relationship is seen in both general population studies as well as those ‘high risk’ cohorts.
Figure 2.
Hazard ratios for end-stage renal disease (ESRD) according to spline eGFR and urine albumin-to-creatinine ratio (ACR) in general population (GP) and high risk (HR) cohorts. . Data from CKD Prognosis Consortium, reproduced with permission from Ganevoort et al.26
Predicting Kidney Disease Progression
Kidney function and albuminuria are both significantly associated with increased risk of kidney disease progression and the occurrence of ESKD. A natural extension of the data is to develop a risk prediction model that would allow the practising clinician to predict the likelihood of CKD progression in a particular individual, similar to models predicting future with cardiovascular disease events. On a practical level the common question that patients with CKD ask is – “What is the chance of my needing dialysis and how long have I got?”
There have been a number of studies and a recent systematic review on this subject. Here I review three of the more important studies, all of which use quite different approaches aimed at different populations. In the first study, Hippisley-Cox and Coupland28 developed a predication equation (the ‘QKidney score’) using more than 1.5 million subjects in a United Kingdom general practice database. This study used patient demographics and known medical comorbidity to produce an equation to predict the percentage risk of new, moderate to severe CKD, defined as any of the following: kidney transplant; kidney dialysis; diagnosis of nephropathy; persistent proteinuria; or glomerular filtration rate of <45 mL/min. They also developed a risk equation to assess ‘end-stage kidney failure’ defined as either a recorded kidney transplant, dialysis or eGFR <15mL/min. This study is interesting as the investigators had no measures of kidney function or damage in the database and therefore the developed models were without any baseline kidney measures. The overall risk of ESKD was very low, with the top decile having a 1% five year risk of ESKD. Overall the models performed generally well in predicting five year risk, with receiver operating characteristic (ROC) statistic of 0.875 for women and 0.876 for men in the validation cohort. However the role of such equations in clinical practice is unclear given the general low background risk of the population.
Kshirsagar et al29 studied two well defined general population cohorts but assessed incident CKD defined as an eGFR of <60 mL/min as opposed to a more clinically severe or important outcome of ESKD. Similar to the UK General Practice Study, the predictive model did not include any measure of kidney function and utilised a combination of medical and demographic characteristics and the presence of anaemia and hypercholesterolaemia. However discrimination was only modest (ROC statistic ranging from 0.69 to 0.70) and given the use of a less severe outcome, the utility of such a model is low.
A more useful approach for the practising clinician is an assessment of a patient with CKD and predicting their risk of progression to ESKD. Tangri and colleagues30 developed prediction models in patients with moderate to severe (stage 3 to 5, eGFR 10 to 60 mL/min) CKD assessing risk of progression to ESKD over a three year period. ESKD was defined as the need for dialysis or pre-emptive transplantation. Six different models were developed with increasing levels of complexity. As perhaps might be expected, the more complex models performed better in both the development and validation datasets, although the most complicated model which included patient demographics, measures of blood pressure, BMI and laboratory measures did not perform better than a simpler model including only demographic and laboratory measures. While the predictive model that included age, gender, eGFR and albuminuria performed very well (ROC statistic 0.91), the addition of other laboratory values (serum albumin, phosphate, bicarbonate, and calcium) significantly improved risk classification (net reclassification index 8% for those with stage 3 CKD and 4.1% for those with stage 4 CKD). The developed equation can be easily applied in the clinic to estimate the percentage risk for progression over the three year period and has been incorporated into simple applications available on smart cellular phones.
Finally the recent systematic review of risk prediction models in CKD patients highlights the need for further large and well designed clinical studies. Of the eight studies assessing kidney failure prediction, only two reported reclassification indices and one used clinically relevant categories that could ‘affect diagnostic or therapeutic decision making.’31
Conclusion
Assessment of kidney disease involves assessment of eGFR as well as quantification of any proteinuria, preferably through the assessment of the spot urine albumin-to-creatinine ratio. Both markers are pivotal for the assessment of cardiovascular risk including mortality and cardiovascular events, the diagnosis and assessment of CKD and in determining the overall prognosis of CKD. Whether one is more important that the other is largely dependent on the clinical context, however in practice the assessment of any individual subject should include both.
Footnotes
Competing Interests: None declared.
References
- 1.Eckardt K-U, Coresh J, Devuyst O, Johnson RJ, Köttgen A, Levey AS, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158–69. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 2.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–72. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 3.Lindner A, Charra B, Sherrard DJ, Scribner BH. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med. 1974;290:697–701. doi: 10.1056/NEJM197403282901301. [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 6.Polkinghorne KR, Chadban SJ. A decade after the KDOQI CKD guidelines: a perspective from Australia. Am J Kidney Dis. 2012;60:725–6. doi: 10.1053/j.ajkd.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Stevens LA, Coresh J, Feldman HI, Greene T, Lash JP, Nelson RG, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18:2749–57. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- 8.Glassock RJ, Winearls CG. Routine reporting of estimated glomerular filtration rate: not ready for prime time. Nat Clin Pract Nephrol. 2008;4:422–3. doi: 10.1038/ncpneph0860. [DOI] [PubMed] [Google Scholar]
- 9.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Chronic Kidney Disease Prognosis Consortium Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–81. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsushita K, Ballew SH, Astor BC, Jong PE, Gansevoort RT, Hemmelgarn BR, et al. Cohort profile: the Chronic Kidney Disease Prognosis Consortium. Int J Epidemiol. 2013;46:1660–8. doi: 10.1093/ije/dys173. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White SL, Polkinghorne KR, Atkins RC, Chadban SJ. Comparison of the prevalence and mortality risk of CKD in Australia using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR estimating equations: the AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. Am J Kidney Dis. 2010;55:660–70. doi: 10.1053/j.ajkd.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, et al. Chronic Kidney Disease Prognosis Consortium Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307:1941–51. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, et al. Prevention of Renal and Vascular End-stage Disease (PREVEND) Study Group Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–82. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 15.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, et al. Alberta Kidney Disease Network Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–9. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 16.Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371:2173–82. doi: 10.1016/S0140-6736(08)60952-6. [DOI] [PubMed] [Google Scholar]
- 17.Tonelli M, Muntner P, Lloyd A, Manns BJ, James MT, Klarenbach S, et al. Alberta Kidney Disease Network Using proteinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: a cohort study. Ann Intern Med. 2011;154:12–21. doi: 10.7326/0003-4819-154-1-201101040-00003. [DOI] [PubMed] [Google Scholar]
- 18.Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: The HUNT II Study. Arch Intern Med. 2007;167:2490–6. doi: 10.1001/archinte.167.22.2490. [DOI] [PubMed] [Google Scholar]
- 19.Clase CM, Gao P, Tobe SW, McQueen MJ, Grosshennig A, Teo KK, et al. ONTARGET (ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial) and TRANSCEND (Telmisartan Randomized Assessment Study in Angiotensin-Converting-Enzyme-Inhibitor Intolerant Subjects with Cardiovascular Disease) Estimated glomerular filtration rate and albuminuria as predictors of outcomes in patients with high cardiovascular risk: a cohort study. Ann Intern Med. 2011;154:310–8. doi: 10.7326/0003-4819-154-5-201103010-00005. [DOI] [PubMed] [Google Scholar]
- 20.Smink PA, Lambers Heerspink HJ, Gansevoort RT, de Jong PE, Hillege HL, Bakker SJ, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis. 2012;60:804–11. doi: 10.1053/j.ajkd.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Shlipak MG, Matsushita K, Ärnlöv J, Inker LA, Katz R, Polkinghorne KR, et al. CKD Prognosis Consortium Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932–43. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 23.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–47. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 24.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–63. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 25.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR, UKPDS GROUP Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63:225–32. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 26.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. Chronic Kidney Disease Prognosis Consortium Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80:93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsushita K, Selvin E, Bash LD, Astor BC, Coresh J. Risk implications of the new CKD Epidemiology Collaboration (CKD-EPI) equation compared with the MDRD Study equation for estimated GFR: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2010;55:648–59. doi: 10.1053/j.ajkd.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hippisley-Cox J, Coupland C. Predicting the risk of chronic Kidney Disease in men and women in England and Wales: prospective derivation and external validation of the QKidney Scores. BMC Fam Pract. 2010;11:49. doi: 10.1186/1471-2296-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kshirsagar AV, Bang H, Bomback AS, Vupputuri S, Shoham DA, Kern LM, et al. A simple algorithm to predict incident kidney disease. Arch Intern Med. 2008;168:2466–73. doi: 10.1001/archinte.168.22.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305:1553–9. doi: 10.1001/jama.2011.451. [DOI] [PubMed] [Google Scholar]
- 31.Tangri N, Kitsios GD, Inker LA, Griffith J, Naimark DM, Walker S, et al. Risk prediction models for patients with chronic kidney disease: a systematic review. Ann Intern Med. 2013;158:596–603. doi: 10.7326/0003-4819-158-8-201304160-00004. [DOI] [PubMed] [Google Scholar]