Cloning of 16S rRNA genes amplified from normal and disturbed vaginal microflora suggests a strong association between Atopobium vaginae, Gardnerella vaginalis and bacterial vaginosis (original) (raw)
Abstract
Background
The pathogenesis of bacterial vaginosis remains largely elusive, although some microorganisms, including Gardnerella vaginalis, are suspected of playing a role in the etiology of this disorder. Recently culture-independent analysis of microbial ecosystems has proven its efficacy in characterizing the diversity of bacterial populations. Here, we report on the results obtained by combining culture and PCR-based methods to characterize the normal and disturbed vaginal microflora.
Results
A total of 150 vaginal swab samples from healthy women (115 pregnant and 35 non-pregnant) were categorized on the basis of Gram stain of direct smear as grade I (n = 112), grade II (n = 26), grade III (n = 9) or grade IV (n = 3). The composition of the vaginal microbial community of eight of these vaginal swabs (three grade I, two grade II and three grade III), all from non-pregnant women, were studied by culture and by cloning of the 16S rRNA genes obtained after direct amplification. Forty-six cultured isolates were identified by tDNA-PCR, 854 cloned 16S rRNA gene fragments were analysed of which 156 by sequencing, yielding a total of 38 species, including 9 presumptively novel species with at least five species that have not been isolated previously from vaginal samples. Interestingly, cloning revealed that Atopobium vaginae was abundant in four out of the five non-grade I specimens. Finally, species specific PCR for A. vaginae and Gardnerella vaginalis pointed to a statistically significant co-occurrence of both species in the bacterial vaginosis samples.
Conclusions
Although historically the literature regarding bacterial vaginosis has largely focused on G. vaginalis in particular, several findings of this study – like the abundance of A. vaginae in disturbed vaginal microflora and the presence of several novel species – indicate that much is to be learned about the composition of the vaginal microflora and its relation to the etiology of BV.
Background
Bacterial vaginosis (BV) is considered to be the most common cause of vaginal inflammation among both pregnant and non-pregnant women and prevalences between 4.9% and 36.0% have been reported from European and American studies [1]. The etiology of this condition remains largely unknown.
Nonetheless, the abundant literature, addressing bacterial vaginosis and ascending genital tract infection, has largely focused on the bacterial component and, in particular, on a few microorganisms, which are thought to play a pivotal role in the pathology of bacterial vaginosis. Although probably rather complex microbial community dynamics are involved, culture-dependent studies indicate that a limited number of bacterial species, including Mobiluncus spp., Gardnerella vaginalis, Bacteroides spp., Prevotella spp. and Mycoplasma hominis, along with the relative absence of Lactobacillus spp., can be used as a sensitive index for bacterial vaginosis. Consequently, according to Nugent et al. [2], the standard diagnosis of BV relies on the quantification of only three cellular types (Lactobacillus, Gardnerella, and Mobiluncus) on a Gram stained vaginal smear.
While conventional microbiological techniques are useful as screening tools to identify women with BV, they do not enable prediction of the clinical burden associated with bacterial vaginosis. It has therefore been stated that better understanding of the composition and dynamics of the vaginal microflora along with the factors associated with the pathology of bacterial vaginosis is essential to improve our predictive abilities [1].
The analysis of complex bacterial communities has been hampered by conventional culture-dependent methods and by biochemical identification methods, since it leaves many bacteria uncultured and unidentified. This may prove especially true for vaginal microflora, both under normal circumstances as well as in the setting of bacterial vaginosis, a condition primarily characterized by overgrowth of anaerobic and fastidious microorganisms. For example, until recently, several important species, like Lactobacillus crispatus, L. gasseri and L. iners, were all lumped together into the L. acidophilus complex, while present molecular techniques make it possible to differentiate between these closely related species [3-9]. Here we combined culture with tDNA-PCR [4,10,11] and sequencing of the 16S rRNA-gene for the identification of cultured organisms.
Detailed information of complex microbial communities can also be acquired from the phylogenetic analysis of 16S rDNA sequences obtained directly from samples by PCR amplification, cloning, and sequencing, so that uncultivable species are also included [12,13], although this approach may be biased as well [14,15], as becomes also apparent from this study.
Here we report on the cloning and sequencing of 16S rRNA gene fragments, amplified directly from vaginal swabs, to examine the microbial diversity in the vaginal fluid of healthy women with different grades of vaginal microflora patterns as defined previously [16,17]. Finally, the data obtained by anaerobic culture and with culture independent techniques, lead us to carry out species specific PCR for A. vaginae and G. vaginalis, such that the presence of both species in differently graded samples could be established.
Results
Grading of the Gram stained smears of the vaginal samples for which cloning was carried out, according to the criteria of Ison and Hay [17] assigned three specimens (W1-W3) to grade I, two (W4-5) to grade II and three (W6-8) to grade III. Table 1 lists the species identified by cloning and sequencing of cloned bacterial 16S rDNA-fragments, as well as by bacterial culture and tDNA-PCR from the vaginal microflora of these 8 healthy women.
Table 1.
Cloning and culturea results for 8 healthy females with different grades of vaginal microflora.
| Grade | I | I | I | II | II | III | III | III |
|---|---|---|---|---|---|---|---|---|
| Sample designation | BVS30 | BVS62 | BVS63 | BVS36 | BVS34 | BVS59 | BVS61 | BVS44 |
| Subject code | W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 |
| Age | 51 | 34 | 38 | 49 | 41 | 46 | 28 | 44 |
| Number of clones | 124 | 118 | 107 | 69 | 72 | 125 | 169 | 70 |
| Species | ||||||||
| Lactobacillus crispatus [AF257097]b | 66.1c | 99.1 | ||||||
| Lactobacillus gasseri [AF243144] | 18.5 | 99.2 | ||||||
| Lactobacillus jensenii [AF243159] | 0.9 | |||||||
| Lactobacillus vaginalis [AF243177] | 0.8 | |||||||
| Atopobium vaginae [AF325325] | 1.4 | 41.7 | 36.0 | 80.5 | ||||
| Atopobium vaginae 97.6%d [AF325325] | 0.0 | |||||||
| _Gardnerella vaginalis_e [M58744] | 0.0 | 0.0 | 4.1 | |||||
| Lactobacillus iners [Y16329] | 84.1 | 1.4 | 12.4 | |||||
| Mobiluncus mulieris [AJ427625] | 5.6 | 3.0 | ||||||
| Peptostreptococcus anaerobius [L04168] | 0.0 | 1.6 | 68.6 | |||||
| Peptoniphilus sp. [D14147] | 0.0 | 14.3 | ||||||
| Prevotella bivia 91% [L16475] | 7.2 | 22.2 | ||||||
| Prevotella buccalis 96.6% [L16476] | 1.6 | 22.2 | 31.2 | |||||
| Sneathia (Leptotrichia) sanguinegens [L37789] | 1.4 | 1.4 | 6.4 | |||||
| Uncultured Megasphaera sp. clone [AY271937] | 1.4 | 4.8 | ||||||
| Uncultured Actinobacteridae clone 86% [AB089070] | 2.9 | 0.8 | ||||||
| Unidentified clone 1f [AY207059] | 6.9 | 4.0 | ||||||
| Atopobium rimae [AF292371] | 0.8 | |||||||
| Fusobacterium nucleatum [AJ006964] | 6.5 | |||||||
| Peptostreptococcus sp. [AJ277208] | 0.8 | |||||||
| Pseudoramibacter alactolyticus [AB036761] | 0.8 | |||||||
| Streptococcus anginosus group [AF104676] | 0.0 | |||||||
| Treponema sp. clone [AF023055] | 0.8 | |||||||
| Porphyromonas levi clone 94% [L16493] | 1.6 | |||||||
| Unidentified clone 2 [AF371910] | 2.4 | |||||||
| Aerococcus christensenii [Y17318] | 3.2 | 0.0 | ||||||
| Anaerococcus tetradius [AF542234] | 0.0 | |||||||
| Anaerococcus vaginalis [AF542229] | 1.4 | |||||||
| Bacteroides ureolyticus [L04321] | 0.0 | |||||||
| Bifidobacterium biavatii (urinalis) [AJ278695] | 1.4 | |||||||
| Dialister sp. [AF473837] | 2.9 | |||||||
| Enterococcus faecalis [AJ420803] | 1.4 | |||||||
| [Leptotrichia amnionii] [AY078425] | 1.4 | |||||||
| Prevotella bivia [L16475] | 0.0 | 0.0 | ||||||
| Prevotella ruminicola 87% [L16476] | 4.3 | |||||||
| Streptococcus sp. oral strain [AY005041] | 8.6 | |||||||
| Unidentified clone 3 [AF371693] | 6.4 | |||||||
| Ureaplasma urealyticum [AF073455] | 1.4 |
Identification of cultured isolates from 8 healthy women by tDNA-PCR and 16S rRNA gene sequencing
Forty six isolates were obtained after anaerobic culture of the vaginal fluid from the 8 women in the cloning part of the study. Twenty nine isolates, belonging to the species Bacteroides ureolyticus, Enterococcus faecalis, Lactobacillus crispatus, L. jensenii, L. gasseri, L. vaginalis, Peptoniphilus sp., Prevotella bivia, Streptococcus anginosus group and S. mitis group were identified by tDNA-PCR using a library containing tDNA-PCR fingerprints of well-identified strains. Six isolates belonging to the species Aerococcus christensenii, Anaerococcus tetradius, Anaerococcus vaginalis, Lactobacillus iners, Mobiluncus mulieris and Peptostreptococcus anaerobius were identified by sequencing because they produced a tDNA pattern that initially was not present in the database. These species were characterized by a specific tDNA-PCR pattern that allowed unambiguous identification after the new patterns were added to the database. In summary, 40 of 46 isolates (87.0%) were correctly identified by tDNA-PCR to the species level, including the species within the L. acidophilus complex. Of the six isolates that could not be identified, one isolate, with a sequence that was 99% identical to that of a Peptostreptococcus sp. strain (CCUG 42997, AJ277208), could not be identified unambiguously by tDNA-PCR because its pattern was identical to that of P. micros, one isolate – that was identified by sequencing as Atopobium vaginae – did not yield a tDNA-pattern and another four isolates (8.7%) were tDNA-PCR negative and also 16S PCR negative.
Identification of 16S rRNA gene clones from 8 healthy women by sequencing and culture results
The ARDRA pattern of 854 clones was analysed and enabled to establish the relative frequency of the different species present (Table 1). For 130 clones, belonging to 33 ARDRA types, on average 447 bp of the 5' end of the 16S rRNA gene was sequenced. In case of grade I vaginal smears, the most predominant species were L. crispatus and L. gasseri. Smaller numbers of L. jensenii and L. vaginalis were also present. All females with grade I smears (W1-W3) were colonized by two Lactobacillus species. W2 (age 34) and W3 (age 38) were colonized by Lactobacillus species only, with one species – respectively L. gasseri and L. crispatus – represented by more than 99% of the clones. In the vaginal fluid of W1 (age 51) the most abundant species were L. crispatus (66.1%), L. gasseri (18.6%) and Fusobacterium nucleatum (6.5%). An additional 8 species were found in low numbers, six only by cloning and one only by culture. Three of these species, representing 5.6% of the clones, showed less than 98% similarity with previously published sequences. Culture results coincided largely with cloning for the grade I samples, except for the non Lactobacillus species of W1 of which only two were cultured.
For the two grade II smears (W4 and W5) cloning revealed four species, A. vaginae (resp. 1.5% and 41.6%), L. iners (resp. 84.1% and 1.4%), a _P. bivia_-like species (resp. 7.3% and 22.2%) and Sneathia sanguinegens (both 1.4%), that were present in both samples. P. bivia was also present in both grade II microflora but it was found by culture only. Several other species were found by cloning only in either W4 or W5. For example, [Leptotrichia amnionii] was found only in W5. G. vaginalis and Peptostreptococcus anaerobius were found respectively in W4 and W5 by culture only and two species, Anaerococcus vaginalis and Enterococcus faecalis, were found by both cloning and culture in the microflora of W5.
Two women (W6 and W7) with a grade III vaginal smear were colonized predominantly by A. vaginae (resp. 36.0 and 80.5%), L. iners (absent and 12.4%), G. vaginalis (absent and 4.1%), M. mulieris (5.6 and 3.0%) and a _Prevotella buccalis_-like species (31.2% and absent), according to cloning. As for grade II samples, there was limited correspondence with culture. For W6, an _A. vaginae_-like organism was cultured, but the sequence of this isolate showed less than 98% similarity to the sequence of the clones. Furthermore, a large number of colonies of G. vaginalis and Peptoniphilus sp. were present after anaerobic culture but no clones were obtained.
The most abundant species obtained by cloning the third grade III vaginal sample (W8) were Peptostreptococcus anaerobius (68.6%)(also cultured), Peptoniphilus sp. (14.3%) and an unidentified Streptococcus sp. (8.6%)(also cultured).
Total number of species identified and comparison between cloning and culture
Of the 38 species, 18 were discovered by cloning only, 5 by culture only, and 8 by both cloning and culture. Three species were shown by both culture and cloning in some samples, but only by culture in the remaining samples, another three species were shown by both cloning and culture in some samples, but only by cloning in the remaining samples and one species was found once by culture only and once by cloning only. The presence of all four grade I Lactobacillus sp. was shown in grade I samples by both cloning and culture, whereas L. iners was found only in three non-grade I samples, in all three by cloning – even abundantly in W4, but only in W7 by culture. G. vaginalis was cultured three times, but was found by cloning only once. A. vaginae was shown in four samples by cloning, but cultured only once. The _P. bivia_-like species and _P. buccalis_-like species were found in respectively two and three samples by cloning but not by culture.
Possible novel species and genera
Of the 38 species that were distinguished by culture and cloning in this study, only 76.3% demonstrated more than 98% identity with previously known bacterial species (Table 1). Five of the eight vaginal samples contained previously unidentified species.
Anaerobic culture of the microflora of W6 revealed an Atopobium species that showed only 97.6% similarity with previously reported A. vaginae sequences (see below).
Two presumptively novel species within the genus Prevotella were found. For W4 and W5 (grade II), respectively 7.3% and 22.2% of the clones had 91% similarity with a P. bivia sequence. For W1 (grade I), W5 and W6 (grade III), respectively 1.6%, 22.2% and 31.2% of the clones showed 96.6% similarity with a P. buccalis sequence. Respectively 2.9% and 0.8% of the clones of W4 (grade II) and W6 (grade III) were identical and had only 86% similarity with an uncultured termite Actinobacteridae bacterium (accession number AB089070).
Variation in Atopobium sequences
Sixteen A. vaginae sequences are present in GenBank, one from a clinical isolate [18], one from the type strain [19] and 14 from cloned 16S rDNA fragments (Zhou, unpublished) (Figure 1). Another 17 sequences were obtained in this study, one from an isolate from patient W6, two from isolates from other patients (BVS38 and PB9) and 14 from clones from four different patients, i.e. W4 (grade II), W5 (II), W6 (III) and W7 (III) (Figure 1).
Figure 1.
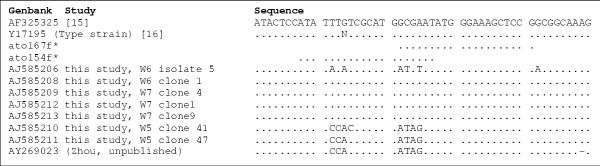
Atopobium vaginae 16S rDNA sequences * Designations of the forward primers specific for A. vaginae and their position in the 16S rRNA gene.
The sequence of all 7 clones from W5 was different from that of all other isolates and from the clones and the Genbank sequences, except from one Genbank clone sequence (AY269023). The sequence of all clones from the three other patients and from the two isolates from samples BVS38 and PB9 were identical to the previously published clinical isolate [18] and the type strain [19]. The sequence of the cultured isolate from patient W6 was somewhat intermediate between both above sequences, although the sequence of the clones of the same patient were identical to that of the type strain. Specific amplification with both the ato167f primer and the ato154f primer gave positive signals for the vaginal sample of W6, indicating that indeed both types of sequence were present.
Species-specific PCR for A. vaginae and G. vaginalis
To substantiate the results obtained by cloning, an additional series of 142 vaginal samples obtained by swab from healthy pregnant (n = 115) and non-pregnant (n = 27) women, attending our out-patient clinic, were selected for culture and for PCR with 16S rRNA gene based primers specific for A. vaginae and G. vaginalis (Table 2). Of the 150 subjects in total, 38 (of which 5 non-pregnant) presented with non-grade I microflora, of which 26 were assigned grade II, 9 grade III and 3 grade IV.
Table 2.
Amplification results with A. vaginae and G. vaginalis specific primers obtained for 150 vaginal samples of different grades
| Grade | n | A+a GOY+/GZ+ | A+ GOY-/GZ- | A- GOY+/GZ+ | A- GOY-/GZ- |
|---|---|---|---|---|---|
| I | 112 | 2/11 | 20/11 | 3/21 | 87/69 |
| II | 26 | 3/4 | 6/5 | 5/9 | 12/8 |
| III | 9 | 7/7 | 0/0 | 0/1 | 2/1 |
| IV | 3 | 0/0 | 0/0 | 0/1 | 3/2 |
| total | 150 | 12/22 | 26/16 | 8/32 | 104/80 |
After amplification with the ato167f A. vaginae primer set, respectively 19.6% of grade I, 34.6% of grade II, 77.8% of grade III and 0.0% of grade IV samples showed an amplicon (Table 2). Of the 37 samples that showed an amplicon after amplification with the ato167f primer only 23 were positive after amplification with ato154f. A. vaginae was cultured only three times from these 150 samples.
The number of positive samples for G. vaginalis specific PCR varied depending on the primer set that was used. The difference was greatest for grade I samples, with respectively 4.5% of grade I samples that were positive after amplification with the GOY primer set and 28.6% with the GZ primer set (Table 2).
Comparative ROC analysis of the four indicators (ato154f, ato167f, GZ, and GOY respectively) in discriminating normal and disturbed vaginal microflora showed that the overall discriminative value of qualitative PCR-based detection of A. vaginae or G. vaginalis as such is actually rather low with AUCs of 0.627 (95%CI: 0.544–0.704), 0.625 (95%CI: 0.543–0.703), 0.633 (95%CI: 0.551–0.711), and 0.675 (95%CI: 0.594–0.749).
Detection of the simultaneous presence of A. vaginae (ato167f) and G. vaginalis (GOY) in a vaginal swab specimen with the purpose of assessing true bacterial vaginosis (grade III) had an accuracy of 87.8% (AUC = 0.878, 95%CI = 0.714, 1.041, p < 0.001), a sensitivity of 0.78 (95%CI = 0.40, 0.96), a specificity of 0.98 (95%CI = 0.91, 1.00), a positive predictive value of 0.78 (95%CI = 0.40, 0.96), and a negative predictive value of 0.98 (95%CI = 0.91, 1.00). When the co-existence of A. vaginae (ato167f) and G. vaginalis was assessed using the GZ-primer set, the overall performance of the assay was significantly lower.
Discussion
To our knowledge, no cloning study addressing the composition of the microflora of vaginal samples and bacterial vaginosis samples has been published thus far. By means of culture and by cloning and sequencing of 16S rRNA genes, amplified directly from the vaginal samples, we studied the vaginal microflora of 8 healthy women with different grades of bacterial vaginosis according to Nugent [2], as modified by Ison and Hay [16,17].
The frequency of the different clones could be assessed by performing ARDRA of the cloned 16S rRNA genes and counting the number of clones belonging to each ARDRA type. Representative clones for each ARDRA type were subsequently sequenced to determine the species identity. Five bacterial species, i.e. Atopobium rimae, Bifidobacterium biavatii (urinalis), Dialister sp., [Leptotrichia amnionii] and Sneathia sanguinegens, had never been recovered from the vagina. Twenty two percent of the clones, belonging to 9 putative species, showed less than 98% homology to any of the 16S rRNA gene sequences present in GenBank, indicating that several bacterial species, indigeneous to the female genital tract, remain to be characterized.
In this study, cloning confirmed that the vaginal microflora of healthy women is dominated by a limited number of Lactobacillus species. For the women with grade I microflora, the two species L. crispatus and L. gasseri, alone or in combination, accounted for 85–99% of the clones. The two younger women, 34 and 38 years, had almost pure cultures of L. crispatus resp. L. gasseri, in association with low numbers of resp. L. jensenii (< 1%) and L. vaginalis (< 1%) and without any other bacteria. In our ongoing studies we find this to be the case for 61.5% of the grade I samples (unpublished data). The finding of monocultures of L. crispatus and L. gasseri is also in correspondence with recent reports, which point to the predominance of only three to four Lactobacillus species in normal vaginal microflora [7,20]. Antonio et al. [3] considered L. crispatus, L. jensenii and L. gasseri as the indicator species for normal vaginal microflora, also because most strains of these species are hydroxyperoxide producers in opposition to other Lactobacillus spp. It should be noted in this context that another group could not substantiate any protective effect of hydroxyperoxide producing lactobacilli [21].
In the 51-year-old woman (W1), with a combination of both L. crispatus and L. gasseri, some additional non Lactobacillus species were present in low numbers, with Fusobacterium nucleatum as the most predominant (i.e. 6.5%). This may be in accordance with another observation, namely that postmenopausal healthy women frequently present with BV-like microscopy [5,22].
Of the five women with non-grade I microflora (two with grade II and three with grade III), one presented with predominant Peptostreptococcus clones (68%), a picture that was very different from that observed in the other women. However, most striking was the observation that for the other four patients between 1.5% and 80.0% of the clones were identified as Atopobium vaginae, a species previously known only from a single isolate from the vagina of a healthy woman [19] and from a clinically important isolate, described as the causative agent in a case of a pelvic inflammatory disease (PID) following transvaginal oocyte recruitment [18]. Interestingly, Zhou et al. [20] reported dominating Atopobium sp. in a woman, judged to carry a normal vaginal microflora. The authors concluded that Atopobium may be present as part of the normal microflora of the vagina [23]. This warrants a more detailed discussion of this recently described species.
The genus Atopobium was introduced to accommodate the species Lactobacillus minutus, Lactobacillus rimae and Streptococcus parvulus [24]. Atopobium species have been described to produce major amounts of lactic acid [19], a characteristic reminiscent of lactobacilli, although no special reference to this property was made in the description of A. vaginae [19]. Atopobium species are anaerobic, Gram-positive elliptical cocci or rod-shaped organisms occurring singly, in pairs or as short chains. The variable cell morphology of A. vaginae makes that this species may reside perfectly camouflaged and as a consequence undetectable among the mixture of other species present in grade II and III bacterial communities. Also the fact that A. vaginae is fastidious and forms small pinhead colonies can explain why this species was not yet established as part of the vaginal microflora, when using classical microbiology. In this study, we could culture the species from only three out of 150 vaginal specimens.
Based on our findings with A. vaginae specific PCR, which recovered this species in 19.6% of the 112 grade I specimens, it appears as if A. vaginae may be a constituent – presumably in low numbers – of the human vagina, possibly attaining replicative dominance in association with decreasing lactobacillary grading. This hypothesis could be substantiated with quantitative PCR and the development of selective culture media.
The striking fact that Gardnerella vaginalis, predominantly present in bacterial vaginosis samples [e.g. [25,26]], was not found by cloning as carried out in this study, can be explained as the consequence of a methodological bias, because the forward primer used for cloning (10f) contained 3 mismatches for G. vaginalis. On the other hand, the use of these non G. vaginalis 'universal' primers may have facilitated to establish the high prevalence of A. vaginae. To assess the relative importance of both species, cloning should be carried out with different sets of primers. Amplification of grade I, grade II and grade III samples with species specific primers indicated that G. vaginalis was present in respectively 28.6%, 50.0 % and 88.9% of the samples, while Atopobium was present in respectively 19.6%, 34.6% and 77.8% of the vaginal microflora.
Since any pairwise comparison of the four ROC plots did not show any significant differences between the AUCs, it is also apparent that each of the four indicators under study (ato154f, ato167f, GZ, and GOY respectively) presents with a comparably limited accuracy in discriminating normal and disturbed vaginal microflora. This may be explained by the fact that, although both species are present in about 80% of the true bacterial vaginosis samples, their presence in grade I samples is not uncommon. This lack of accuracy for G. vaginalis was also previously assessed in culture-dependent studies [27,28].
The co-existence of A. vaginae and G. vaginalis in women with grade III microflora together with their simultaneous absence in grade I samples (Table 2) is striking. The simultaneous presence of both species therefore is highly predictive for BV.
Lactobacillus iners, was present among the clones in three of the five non-grade I samples, even in large numbers, and absent from the three grade I samples. This species was only recently recognized as a separate Lactobacillus species [29]. In the original description of this relatively asaccharolytic Lactobacillus species, the only vaginal isolate among the 9 strains from females came from vaginal discharge [29]. This species may have been largely overlooked in culture based studies [30-32] since it does not grow on Lactobacillus selective media, including MRS and Rogosa-Sharp medium [29]. Using molecular techniques L. iners was reported as one of the most frequently encountered vaginal Lactobacillus species [8,9] present in normal and BV microflora [5].
Leptotrichia sp. are slow-growing, gram-negative anaerobic organisms of the oral cavity and genital tract. [_L. amnionii_] is an extremely fastidious organism, which most likely explains why this organism was hitherto virtually undetectable by conventional culture-based microbiological techniques. Of interest is that this particular Leptotrichia species was isolated by Shukla et al. [33] from the amniotic fluid of a woman with second trimester fetal loss, which led the authors to conclude that [L. amnionii] is presumably indigenous to the genital tract and acts as an opportunist under particular clinical conditions, including pregnancy. This study demonstrates for the first time the presence of [L. amnionii] in the vagina.
Sneathia sanguinegens (formerly Leptotrichia sanguinegens) was found in this study to be present in three patients with non-grade I vaginal microflora, in moderate numbers. S. sanguinegens has been isolated from human blood and amniotic fluid [34] and has been associated with several cases of pregnancy-associated bacteraemia, i.e. postpartum fever in four patients and neonatal sepsis in two patients [35]. This study demonstrates the presence of S. sanguinegens in the vagina for the first time.
One strain from W6 and 14.3% of the clones from W8 were identified as Peptoniphilus indolicus based on more than 98% similarity with a Genbank sequence with accession number D14147 corresponding with the type strain CCUG 17639 of this species. This type strain however has two Genbank entries (also AY153430) with low similarity [36]. Unfortunately our isolate was not stored so further phenotypic identification was not possible. Because of the ambiguous Genbank sequences, we chose to designate our isolate and clones as Peptoniphilus sp.
Thus far, identification of organisms cultured from complex microbial biota like the vagina, especially under the condition of vaginosis, has also been hampered by the limitations of conventional biochemical and phenotypic identification methods. However, using a DNA-based method, namely tDNA-PCR [11], it is convenient to appropriately identify most of these organisms, once a library, based on tDNA-PCR of well characterized organisms, has been constructed. Also, newly unknowns can be first identified by 16S rRNA gene sequence determination, whereafter it is possible to use the corresponding tDNA fingerprint for future identification of the organisms of the same species.
tDNA-PCR, which consists of the amplification of the spacer regions between tRNA genes, has been shown to yield mainly species-specific DNA fingerprints [10]. Combined with capillary electrophoresis, tDNA-PCR has been shown to be a semi-automated, digital DNA-fingerprinting method that enables rapid and discriminatory identification of species from very diverse phylogenetic groups, including Lactobacillus [4,11].
Conclusions
In summary, the use of tDNA-PCR based identification of cultured organisms in combination with cloning of 16S rRNA genes, amplified directly from vaginal swabs, enabled us to characterize the vaginal microflora in a detailed manner, and to compare the composition of the normal vaginal microflora with the bacterial vaginosis microflora. The presence of A. vaginae in 4 of the 5 women with bacterial vaginosis grade II-III microflora is an unexpected and previously not reported finding, which may shed new light on the etiology of this condition. There is also the ambiguous position of L. iners, which apparently is not indicative of a normal microflora, but may point to some intermediate condition.
Furthermore, our findings warrant more detailed studies of the ability of species like L. iners and A. vaginae to produce lactic acid, hydrogen peroxide and of their cellular and colonial morphology and biochemical characteristics, as well as their interaction with lactobacilli and G. vaginalis. A selective medium for these organisms might be a welcome tool for further studies, given the fact that until now they have been largely overlooked by culture methods.
Finally, the discrepancies between culture, cloning and specific PCR, together with the diversity of the recovered species, their unequal distribution over different vaginal samples, the elucidation of several species – including presumptively new species – previously not associated with the vagina, indicate that much is to be learned about the composition of the vaginal microflora and its relation to the etiology of BV.
Methods
Study population, sample collection and grading
For a total of 150 healthy women of reproductive age, attending our out-patient clinic, of which 115 were pregnant, the health condition with regard to the composition of the vaginal bacterial community was assessed microscopically after Gram stain, according to the criteria of Hay and Ison [17] and vaginal samples were taken. Briefly, specimens were considered grade I (normal) when only Lactobacillus morphotypes were present, grade II (intermediate) when both Lactobacillus and other morphotypes were present, grade III (BV) when only non Lactobacillus morphotypes were seen and grade IV when only Gram positive cocci were seen.
Sampling was carried out as follows. After placement of a non-lubrificated speculum two sterile cotton swabs were inserted into the vaginal vault. The swabs were rotated against the vaginal wall at the midportion of the vault and were carefully removed to prevent contamination with the vulva and introitus microflora. One swab was returned to a sterile tube (dry swab) (Copan, Brescia, Italy), for the purpose of DNA-extraction. The other swab was placed into Amies transport medium (Nuova Aptaca, Canelli, Italy) and was used for making a smear for the purpose of grading according to the Hay and Ison criteria [17] and for anaerobic culture. Both swabs were processed in the microbiology laboratory within 4 h.
Based on smear results, eight non-pregnant women (mean age 41.4 years, range 28–51 years) from the studied population, attending our out-patient clinic for a routine gynaecological visit, were selected for cloning of the 16S rRNA genes present in the vaginal microflora. The culture results are reported only for these eight women.
To substantiate the results obtained by cloning and culture, PCR with species specific primers for A. vaginae and G. vaginalis and culture were carried out for the complete population.
Culture and identification of cultured isolates by tDNA-PCR
For eight non-pregnant women, the swab on Amies transport medium was streaked onto tryptic soy agar supplemented with 5% sheep blood (Becton Dickinson, Franklin Lakes, NJ) and incubated anaerobically at 37°C upon arrival at the microbiology laboratory. After 4 days of incubation, all the isolates with different colony morphology were selected for identification. DNA was extracted by simple alkaline lysis: one colony was suspended in 20 μl of 0.25% sodium dodecyl sulfate-0.05 N NaOH, heated at 95°C for 15 min and diluted with 180 μl of distilled water. tDNA-PCR and capillary electrophoresis were carried out as described previously [4,11]. The species to which each isolate belonged was determined by comparing the tDNA-PCR fingerprint obtained from each isolate with a library of tDNA-PCR fingerprints obtained from reference strains, using an in-house software program [11]. The library of tDNA-PCR fingerprints and the software are available on request.
DNA extraction of vaginal swab samples
For DNA extraction from the dry vaginal swabs, the QIAamp DNA mini kit (Qiagen, Hilden, Germany) was used according to the manufacturer's recommendations, with minor modifications. The dry swab specimen from each patient was swirled for 15 s in 400 μl of lysis buffer (20 mM Tris-HCl, pH 8.0; 2 mM EDTA; 1.2% Triton). Fifty units of mutanolysin (25 U/μl) (Sigma, Bornem, Belgium) were added and the samples were incubated for 30 min at 37°C. After the addition of 20 μl Proteinase K (20 mg/ml) and 200 μl AL buffer (Qiagen), samples were incubated for 30 min at 56°C. Next, 200 μl of ethanol was added and DNA was purified by adding the lysate to the Qiagen columns as described by the manufacturer. Finally, the total bacterial DNA was eluted with 100 μl of AE buffer (Qiagen). DNA-extracts were stored at -20°C and were used for the purpose of cloning experiments and species specific PCR.
Cloning of amplified mixtures of 16S rDNA
To amplify the 5' part of the bacterial 16S rRNA genes by PCR, primers 10f (5' AGTTTGATCCTGGCTCAG) and 534r (5' ATTACCGCGGCTGCTGG) [37], which target the domain Bacteria, were used. It should be noted that the forward primer contains mismatches for G. vaginalis at positions 1 (A/G), 5 (T/C) and 9 (C/T). A 50 μl PCR mixture contained 0.1 μM of each primer, 25 μl of Promega master mix (Promega, Madison, WI), 5 μl of DNA extract and distilled water. Thermal cycling consisted of an initial denaturation of 5 min at 94°C, followed by three cycles of 1 min at 94°C, 2 min at 50°C and 1 min at 72°C, followed by 35 cycles of 20 sec at 94°C, 1 min at 50°C and 1 min 72°C, with a final extension of 10 min at 72°C, and cooling to 10°C. PCR products were purified using the QIAquick PCR Purification Kit (Qiagen). Cloning was done using the Qiagen PCR Cloning Kit (UA-cloning, Qiagen), whereby the purified amplicons were ligated into the pDrive cloning vector and transformed into Qiagen EZ Competent E. coli Cells, as specified by the manufacturer.
Screening for clones with different 16S rRNA gene inserts by ARDRA
From each sample, 100 to 200 ampicillin-resistant transformants, recognizable as white colonies on Luria-Bertani (LB) agar containing IPTG (Roche, Basel, Switzerland), X-Gal (Roche) and 100 μg ampicillin/ml after overnight incubation at 37°C, were selected for further analysis. Single colonies were picked from the agar plates and transferred with sterile tips to the wells of a 96 well plate filled with LB Broth supplemented with 100 μg ampicillin/ml and incubated for 3 h. To avoid sequencing of all clones, the 16S rRNA gene inserts were differentiated initially from each other by means of Amplified rDNA Restriction Analysis (ARDRA)[38]. One μl of bacterial culture was added to a final volume of 20 μl PCR mix, containing 0.2 μM of two plasmid-targeted primers, QF (5'TACGTATCGGATCCAGAATTC) and QR (5'CGAGAAGCTTGTCGACGAATT), 10 μl of Promega master mix and distilled water. The position of primers QF and QR was chosen as such that their distance towards the insert was the same in order to result in the same ARDRA pattern regardless the orientation of the insert in the cloning vector. Cycling conditions were the same as described above for the 16S rRNA gene. Ten microliter of the amplified products were digested with 10 U of the restriction endonuclease _Bst_UI in the appropriate enzyme buffer and incubated for 3 h at 60°C. The DNA restriction fragments were separated in a 2.5% agarose electrophoresis gel, containing 2% Methaphor (FMC Bioproducts, Rockland, ME) and 0.5% MP agarose (Roche, Basel, Switzerland) in the presence of ethidium bromide (50 ng/ml). The gels were photographed and the obtained ARDRA fingerprints were compared visually. For each ARDRA type, the 16S rRNA gene insert of at least one representative clone was sequenced, thus minimizing the number of sequence reactions that had to be carried out.
Sequencing of 16S rRNA genes from isolates and clones
For 8 healthy women, the 16S rRNA gene was amplified and sequenced from selected clones and from cultured isolates that could not be identified by tDNA-PCR, using primers 10f and 534r, as described above. For the clones, 1 μl of the QF-QR amplified PCR product was diluted 1000 times with distilled water and re-amplification of the 16S rRNA gene was done with the primers 10f and 534r. For the cultured isolates, a 5 μl aliquot of the alkaline lysate was added to a 50 μl PCR mixture. The amplification products were then purified with the Qiaquick PCR purification kit, according to the manufacturer's instructions. Sequencing was done using the ABI Big Dye cycle sequencing reaction kit with Ampli_Taq_ FS DNA polymerase (Applied Biosystems, Foster City, CA.) with primer 534r. Sequencing reaction products were analyzed on an ABI 310 genetic analyzer (Applied Biosystems). Inspection of the electropherograms was done with Chromas http://www.technelysium.com.au/chromas14x.html and with the BioEdit package [39]. Comparison of the sequences of the inserts and isolates to the 16S rRNA gene sequences in GenBank was done using the BLAST software [40]. Clones or isolates with DNA sequences sharing more than 98% identity with known sequences were assigned to that phylotype.
Species specific PCR for Gardnerella vaginalis
G. vaginalis species-specific primers as designed by Zariffard et al. (GZ)[26] and Obata-Yasuoka et al. (GOY) [25] were used. Briefly, a 20 μl PCR mixture contained respectively 0.05 and 0.4 μM primers, 10 μl of Promega master mix (Promega, Madison, WI), 2 μl of Qiagen DNA-extract of the samples and distilled water. Thermal cycling with GZ primers consisted of an initial denaturation of 10 min at 94°C, followed by 50 cycles of 5 s at 94°C, 45 s at 55°C and 45 s at 72°C, and a final extension of 10 min at 72°C. Thermal cycling with the GOY primers was performed by an initial denaturation of 1 min at 94°C was followed by 40 cycles of 1 min at 94°C, 1 min at 60°C and 1 min at 70°C and a final extension of 7 min at 72°C. During the first ten cycles the annealing temperature was lowered by 0.5°C per cycle. Five microliter of the amplified product of each PCR was visualized on a 2% agarose gel.
Species specific PCR for Atopobium vaginae
Two primer sets that allowed amplification of the 16S rRNA gene of A. vaginae and that lacked homology with non-target bacteria as determined by searching the Gene Bank database using BLAST software [40], were designed. The selected primers, ato167f 5' (GCGAATATGGGAAAGCTCCG), ato154f 5' (ATATTTGTCGCATGGCGAAT) and ato587r 5' (GAGCGGATAGGGGTTGAGC), were analysed for secondary structures using NetPrimer (Premier Biosoft International, Palo Alto, CA). A 20 μl PCR mixture contained 0.2 μM of primers (respectively ato167f or ato154f and ato587r), 10 μl of Promega master mix (Promega, Madison, WI), 2 μl of Qiagen DNA-extract of the samples and distilled water. Thermal cycling consisted of an initial denaturation of 5 min at 94°C, followed by three cycles of 1 min at 94°C, 2 min at 58°C and 1 min at 72°C, followed by 35 cycles of 20 sec at 94°C, 1 min at 58°C and 1 min 72°C, with a final extension of 10 min at 72°C, and cooling to 10°C. Five microliter of the amplified product was visualized on a 2% agarose gel. The primers amplified a DNA-fragment of respectively 420 and 433 basepairs from A. vaginae and showed no cross reactivity to other organisms, including A. rimae (data not presented).
Statistics
To assess the relative accuracy of PCR-based detection of G. vaginalis or A. vaginae, we applied comparative receiver-operating-characteristic (ROC) analysis under the non-parametric assumption and compared the accuracies of the isolated markers (amplification with ato154f, ato167f, GOY and GZ) in allocating subjects to a particular Gram stain category (grades I to IV).
Subsequently, we compared the accuracy of PCR-based combined detection of G. vaginalis (using the two different primer sets designated GZand GOY) and A. vaginae (using ato167f) to assess the vaginal microflora status (according to Gram stain category) by non-parametric ROC-analysis.
Accuracy in these analyses is expressed as the area-under-the-curve (AUC) in the ROC-plot, the 95% confidence interval (CI) to the AUC, and the p-value to the 95% CI. We also calculated the estimated sensitivity, specificity, positive and negative predictive values (PPV and NPV) and the 95% confidence intervals to these measures. Statistical significance was accepted at the α = 0.05-level.
Analyses were carried out using the statistical software packages EpiCalc2000 v.1.02 and SPSS v.11.0.
Nucleotide sequence accession numbers
Out of 156 16S rRNA gene sequences, obtained from 26 isolates and from 130 clones, two sequences of A. vaginae (-like) species and 23 sequences of uncultured bacterium clones were submitted to GenBank and were assigned accession no. AJ585206 to AJ585213 and no. AJ619698 to AJ619714.
Authors' contributions
RV, GC, GV and MV participated in the development of the study design, the analysis of the study samples, the collection, analysis and interpretation of the data, and in the writing of the report. HV and MT participated in the development of the study design, the collection of the study samples, the collection, analysis and interpretation of the data, and in the writing of the report. JD participated in the analysis and interpretation of the data and in the writing of the report. LVS and CDG participated in the analysis of the study samples. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This work was supported through a research grant by the Marguerite-Marie Delacroix Foundation. As the main funding source, the Marguerite-Marie Delacroix Foundation was not involved in the development of the study design, the collection, analysis, and interpretation of the data, in the writing of the report nor in the decision to submit the paper for publication.
Contributor Information
Rita Verhelst, Email: Rita.Verhelst@UGent.be.
Hans Verstraelen, Email: Hans.Verstraelen@UGent.be.
Geert Claeys, Email: Geert.Claeys@UGent.be.
Gerda Verschraegen, Email: Gerda.Verschraegen@UGent.be.
Joris Delanghe, Email: Joris.Delanghe@UGent.be.
Leen Van Simaey, Email: Leen.VanSimaey@UGent.be.
Catharine De Ganck, Email: Catharine_De_Ganck@yahoo.com.
Marleen Temmerman, Email: Marleen.Temmerman@UGent.be.
Mario Vaneechoutte, Email: Mario.Vaneechoutte@UGent.be.
References
- Morris M, Nicoll A, Simms I, Wilson J, Catchpole M. Bacterial vaginosis: a public health review. Br J Obstet Gynaecol. 2001;108:439–450. doi: 10.1016/S0306-5456(00)00124-8. [DOI] [PubMed] [Google Scholar]
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio MA, Hawes SE, Hillier SL. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis. 1999;180:1950–1956. doi: 10.1086/315109. [DOI] [PubMed] [Google Scholar]
- Baele M, Vaneechoutte M, Verhelst R, Vancanneyt M, Devriese LA, Haesebrouck F. Identification of Lactobacillus species using tDNA-PCR. J Microbiol Methods. 2002;50:263–271. doi: 10.1016/S0167-7012(02)00035-0. [DOI] [PubMed] [Google Scholar]
- Burton JP, Reid G. Evaluation of the bacterial vaginal flora of 20 postmenopausal women by direct (Nugent score) and molecular (polymerase chain reaction and denaturing gradient gel electrophoresis) techniques. J Infect Dis. 2002;186:1770–1780. doi: 10.1086/345761. [DOI] [PubMed] [Google Scholar]
- Burton JP, McCormick JK, Cadieux PA, Reid G. Digoxigenin-labelled peptide nucleic acid to detect lactobacilli PCR amplicons immobilized on membranes from denaturing gradient gel electrophoresis. Lett Appl Microbiol. 2003;36:145–149. doi: 10.1046/j.1472-765X.2003.01281.x. [DOI] [PubMed] [Google Scholar]
- Pavlova SI, Kilic AO, Kilic SS, So JS, Nader-Macias ME, Simoes JA, Tao L. Genetic diversity of vaginal lactobacilli from women in different countries based on 16S rRNA gene sequences. J Appl Microbiol. 2002;92:451–459. doi: 10.1046/j.1365-2672.2002.01547.x. [DOI] [PubMed] [Google Scholar]
- Tarnberg M, Jakobsson T, Jonasson J, Forsum U. Identification of randomly selected colonies of lactobacilli from normal vaginal fluid by pyrosequencing of the 16S rDNA variable V1 and V3 regions. APMIS. 2002;110:802–810. doi: 10.1034/j.1600-0463.2002.1101106.x. [DOI] [PubMed] [Google Scholar]
- Vasquez A, Jakobsson T, Ahrne S, Forsum U, Molin G. Vaginal Lactobacillus flora of healthy Swedish women. J Clin Microbiol. 2002;40:2746–2749. doi: 10.1128/JCM.40.8.2746-2749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J, McClelland M. Genomic fingerprints produced by PCR with consensus tRNA gene primers. Nucleic Acids Res. 1991;19:861–866. doi: 10.1093/nar/19.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baele M, Baele P, Vaneechoutte M, Storms V, Butaye P, Devriese LA, Verschraegen G, Gillis M, Haesebrouck F. Application of tDNA-PCR for the identification of enterococci. J Clin Microbiol. 2000;38:4201–4207. doi: 10.1128/jcm.38.11.4201-4207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suau A, Bonnet R, Sutren M, Godon JJ, Gibson GR, Collins MD, Dore J. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol. 1999;65:4799–807. doi: 10.1128/aem.65.11.4799-4807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, Sahasrabudhe A, Dewhirst FE. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–83. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtzt P, Huber T. Chimeric 16S rDNA sequences of diverse origin are accumulating in the public databases. Int J Syst Evol Microbiol. 2003;53:289–293. doi: 10.1099/ijs.0.02441-0. [DOI] [PubMed] [Google Scholar]
- von Wintzingerode F, Gobel UB, Stackebrandt E. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol Rev. 1997;21:213–29. doi: 10.1016/S0168-6445(97)00057-0. [DOI] [PubMed] [Google Scholar]
- Hay PE, Taylor-Robinson D, Lamont RF. Diagnosis of bacterial vaginosis in a gynaecology clinic. Br J Obstet Gynaecol. 1992;99:63–66. doi: 10.1111/j.1471-0528.1992.tb14395.x. [DOI] [PubMed] [Google Scholar]
- Ison CA, Hay PE. Validation of a simplified grading of Gram stained vaginal smears for use in genitourinary medicine clinics. Sex Transm Infect. 2002;78:413–415. doi: 10.1136/sti.78.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiβdörfer W, Bohmer C, Pelz K, Schoerner C, Frobenius W, Bogdan C. Tuboovarian abscess caused by Atopobium vaginae following transvaginal oocyte recovery. J Clin Microbiol. 2003;41:2788–2790. doi: 10.1128/JCM.41.6.2788-2790.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Jovita M, Collins MD, Sjödén B, Falsen E. Characterization of a novel Atopobium isolate from the human vagina: description of Atopobium vaginae sp. nov. Int J Syst Bacteriol. 1999;49:1573–1576. doi: 10.1099/00207713-49-4-1573. [DOI] [PubMed] [Google Scholar]
- Zhou X, Bent SJ, Schneider MG, Davis CC, Islam MR, Forney LJ. 103rd ASM General Meeting. Washington D.C; Characterization of vaginal microbial communities based on terminal restriction fragment length polymorphisms (T-RFLP) and sequencing of 16S rRNA genes.http://www.asmusa.org/memonly/abstracts/AbstractView.asp?AbstractID=81240 18–22 May 2003. [Google Scholar]
- Rosenstein IJ, Fontaine EA, Morgan DJ, Sheehan M, Lamont RF, Taylor-Robinson D. Relationship between hydrogyen peroxide-producing strains of lactobacilli and vaginosis-associated bacterial species in pregnant women. Eur J Clin Microbiol Infect Dis. 1997;16:517–522. doi: 10.1007/BF01708235. [DOI] [PubMed] [Google Scholar]
- Cauci S, Driussi S, De Santo D, Penacchioni P, Iannicelli T, Lanzafame P, De Seta F, Quadrifoglio F, de Aloysio D, Guaschino S. Prevalence of bacterial vaginosis and vaginal flora changes in peri- and postmenopausal women. J Clin Microbiol. 2002;40:2147–2152. doi: 10.1128/JCM.40.6.2147-2152.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Bent SJ, Schneider MG, Davis CC, Forney LJ. 103rd ASM General Meeting. Washington D.C; Is Atopobium vaginae a predominant member in vaginal microbial communities of healthy women.http://www.asmusa.org/memonly/abstracts/AbstractView.asp?AbstractID=81143 18–22 May 2003. [Google Scholar]
- Collins MD, Wallbanks S. Comparative sequence analyses of the 16S rRNA genes of Lactobacillus minutus, Lactobacillus rimae and Streptococcus parvulus: proposal for the creation of a new genus Atopobium. FEMS Microbiol Lett. 1992;74:235–2340. doi: 10.1016/0378-1097(92)90435-q. [DOI] [PubMed] [Google Scholar]
- Obata-Yasuoka M, Ba-Thein W, Hamada H, Hayashi H. A multiplex polymerase chain reaction-based diagnostic method for bacterial vaginosis. Obstet Gynecol. 2002;100:759–764. doi: 10.1016/S0029-7844(02)02201-9. [DOI] [PubMed] [Google Scholar]
- Zariffard MR, Saifuddin M, Sha BE, Spear GT. Detection of bacterial vaginosis-related organisms by real-time PCR for lactobacilli, Gardnerella vaginalis and Mycoplasma hominis. FEMS Immunol Med Microbiol. 2002;34:277–281. doi: 10.1016/S0928-8244(02)00397-8. [DOI] [PubMed] [Google Scholar]
- Hillier SL. Diagnostic microbiology of bacterial vaginosis. Am J Obstet Gynecol. 1993;169:455–459. doi: 10.1016/0002-9378(93)90340-o. [DOI] [PubMed] [Google Scholar]
- Aroutcheva AA, Simoes JA, Behbakht K, Faro S. Gardnerella vaginalis isolated from patients with bacterial vaginosis and from patients with healthy vaginal ecosystems. Clin Infect Dis. 2001;33:1022–1027. doi: 10.1086/323030. [DOI] [PubMed] [Google Scholar]
- Falsen E, Pascual C, Sjoden B, Collins MD. Phenotypic and phylogenetic characterization of a novel Lactobacillus species from human sources: description of Lactobacillus iners sp. nov. Int J Syst Bacteriol. 1999;49:217–221. doi: 10.1099/00207713-49-1-217. [DOI] [PubMed] [Google Scholar]
- Reid G, McGroarty JA, Tomeczek L, Bruce AW. Identification and plasmid profiles of Lactobacillus species from the vagina of 100 healthy women. FEMS Immunol Med Microbiol. 1996;15:23–26. doi: 10.1016/0928-8244(96)00039-9. [DOI] [PubMed] [Google Scholar]
- Eschenbach DA, Davick PR, Williams BL, Klebanoff SJ, Young-Smith K, Critchlow CM, Holmes KK. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol. 1989;27:251–256. doi: 10.1128/jcm.27.2.251-256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YL, Kato N, Matsumiya Y, Liu CX, Kato H, Watanabe K. Identification of and hydrogen peroxide production by fecal and vaginal lactobacilli isolated from Japanese women and newborn infants. J Clin Microbiol. 1999;37:3062–3064. doi: 10.1128/jcm.37.9.3062-3064.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla SK, Meier PR, Mitchell PD, Frank DN, Reed KD. Leptotrichia amnionii sp. nov., a novel bacterium isolated from the amniotic fluid of a woman after intrauterine fetal demise. J Clin Microbiol. 2002;40:3346–3349. doi: 10.1128/JCM.40.9.3346-3349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MD, Hoyles L, Tornqvist E, von Essen R, Falsen E. Characterization of some strains from human clinical sources which resemble "Leptotrichia sanguinegens": description of Sneathia sanguinegens sp. nov., gen. nov. Syst Appl Microbiol. 2001;24:358–361. doi: 10.1078/0723-2020-00047. [DOI] [PubMed] [Google Scholar]
- Hanff PA, Rosol-Donoghue JA, Spiegel CA, Wilson KH, Moore LH. Leptotrichia sanguinegens sp. nov., a new agent of postpartum and neonatal bacteremia. Clin Infect Dis. 1995;20 Suppl 2:S237–S239. doi: 10.1093/clinids/20.supplement_2.s237. [DOI] [PubMed] [Google Scholar]
- Song Y, Liu C, McTeague M, Finegold SM. 16S ribosomal DNA sequence-based analysis of clinically significant gram-positive anaerobic cocci. J Clin Microbiol. 2003;41:1363–9. doi: 10.1128/JCM.41.4.1363-1369.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyzer G, de Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaneechoutte M, De Beenhouwer H, Claeys G, Verschraegen G, De Rouck A, Paepe N, Elaichouni A, Portaels F. Identification of Mycobacterium species by using amplified ribosomal DNA restriction analysis. J Clin Microbiol. 1993;31:2061–2065. doi: 10.1128/jcm.31.8.2061-2065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 98/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1006/jmbi.1990.9999. [DOI] [PubMed] [Google Scholar]